Iron(III) Azadiphenolate Compounds in a New Family of Spin Crossover Iron(II)–Iron(III) Mixed-Valent Complexes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Precursor Materials
2.2. Attempted Preparation of ‘Double Spin Crossover’ Materials
2.3. Structural Analysis for Anionic Precursor Materials (Compound 1)
2.4. Structural Analyses of Cationic Precursor Materials [FeII{(pz)3CH}2][ClO4]2 (Compound 2) and [FeII(TPPZ)2][(FeIIICl3)2O]∙2MeCN (Compound 3)
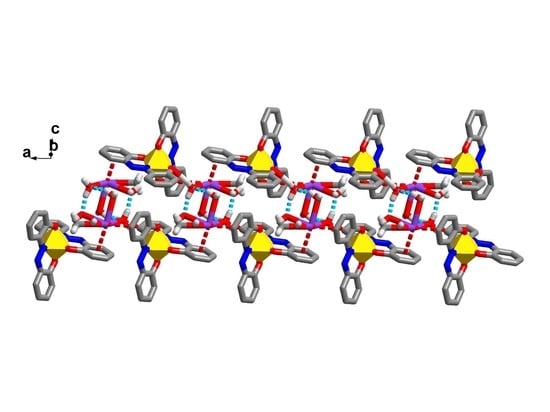
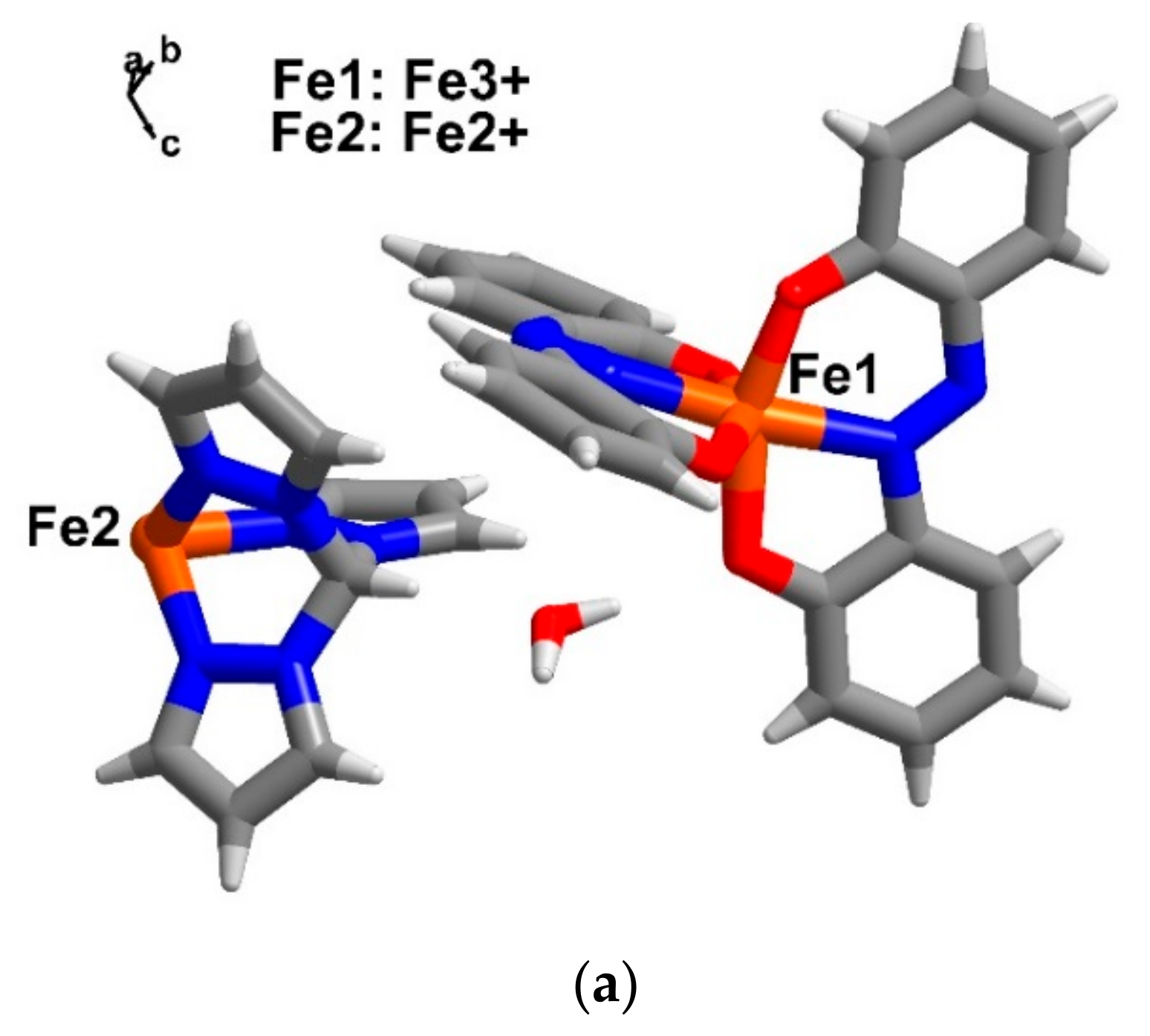
2.5. Structural Analysis for Double Spin Crossover Materials (Compound 4–6)
2.6. Magnetic Susceptibility Results
3. Materials and Methods
3.1. General
3.2. Synthesis of K[FeIII(azp)2]∙(H2O)3∙MeOH, 1
3.3. Synthesis of [FeII{(pz)3CH}2][ClO4]2, 2
3.4. Synthesis of [FeII(TPPZ)2][(FeIIICl3)2O]∙2MeCN, 3
3.5. Synthesis of [FeII{(pz)3CH}2][FeIII(azp)2]2∙2H2O, 4
3.6. Synthesis of [FeII(TPPZ)2][FeIII(azp)2]2∙H2O, 5
3.7. Synthesis of [FeII(TPPZ)2][FeIII(azp)2]2∙H2O∙3MeCN, 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harding, D.J.; Harding, P.; Phonsri, W. Spin crossover in iron(III) complexes. Coord. Chem. Rev. 2016, 313, 38–61. [Google Scholar] [CrossRef]
- Harding, D.J.; Phonsri, W.; Harding, P.; Murray, K.S.; Moubaraki, B.; Jameson, G.N.L. Abrupt two-step and symmetry breaking spin crossover in an iron(III) complex: An exceptionally wide [LS-HS] plateau. Dalton Trans. 2015, 44, 15079–15082. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Garcia, Y.; Goodwin, H.A. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar] [CrossRef]
- Spin Crossover in Transition Metal Compounds I–III; Gütlich, P.; Goodwin, H.A. (Eds.) Springer: Berlin/Heidelberg, Germany, 2004; Volumes 232–235. [Google Scholar]
- Floquet, S.; Guillou, N.; Negrier, P.; Riviere, E.; Boillot, M.-L. The crystallographic phase transition for a ferric thiosemicarbazone spin crossover complex studied by X-ray powder diffraction. New J. Chem. 2006, 30, 1621–1627. [Google Scholar] [CrossRef] [Green Version]
- Zelentsov, V.V.; Bogdanova, L.G.; Ablov, A.V.; Gerbeleu, N.V.; Dyatlova, C.V. Russ J. Inorg. Chem. (Engl. Trans.) 1973, 18, 1410.
- Li, Z.-Y.; Dai, J.-W.; Shiota, Y.; Yoshizawa, K.; Kanegawa, S.; Sato, O. Multi-step spin crossover accompanied by symmetry breaking in an FeIII complex: Crystallographic evidence and dft studies. Chem. Eur. J. 2013, 19, 12948–12952. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Takahashi, K.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Shiota, Y.; Yoshizawa, K. The role of coulomb interactions for spin crossover behaviors and crystal structural transformation in novel anionic Fe(III) complexes from a π-extended ONO ligand. Crystals 2016, 6, 49. [Google Scholar] [CrossRef]
- Takahashi, K.; Kawamukai, K.; Okai, M.; Mochida, T.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Shiota, Y.; Yoshizawa, K. A new family of anionic FeIII spin crossover complexes featuring a weak-field N2O4 coordination octahedron. Chem. Eur. J. 2016, 22, 1253–1257. [Google Scholar] [CrossRef]
- Kazuyuki, T.; HengBo, C.; Hayao, K.; Yasuaki, E.; Osamu, S. The light-induced excited spin state trapping effect on Ni(dmit)2 salt with an Fe(III) spin-crossover cation: [Fe(qsal)2][Ni(dmit)2]·2CH3CN. Chem. Lett. 2005, 34, 1240–1241. [Google Scholar]
- Vieira, B.J.C.; Coutinho, J.T.; Dias, J.C.; Nunes, J.C.; Santos, I.C.; Pereira, L.C.J.; da Gama, V.; Waerenborgh, J.C. Crystal structure and spin crossover behavior of the [Fe(5-Cl-qsal)2][Ni(dmit)2]·2CH3CN complex. Polyhedron 2015, 85, 643–651. [Google Scholar] [CrossRef]
- Faulmann, C.; Dorbes, S.; Lampert, S.; Jacob, K.; Garreau de Bonneval, B.; Molnár, G.; Bousseksou, A.; Real, J.A.; Valade, L. Crystal structure, magnetic properties and mössbauer studies of [Fe(qsal)2][Ni(dmit)2]. Inorg. Chim. Acta 2007, 360, 3870–3878. [Google Scholar] [CrossRef]
- Takahashi, K.; Sakurai, T.; Zhang, W.-M.; Okubo, S.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Mori, H. Spin-singlet transition in the magnetic hybrid compound from a spin-crossover Fe(III) cation and π-radical anion. Inorganics 2017, 5, 54. [Google Scholar] [CrossRef]
- Murray, K.S.; Fallon, G.D.; Hockless, D.C.R.; Lu, K.D.; Moubaraki, B.; Van Langenberg, K. Molecular magnetic materials and small clusters containing n-donor chelated metal species combined with hexacyanometallate, tris-oxalatometallate, and related bridging groups. In Molecule-Based Magnetic Materials; Turnbull, M.M., Sugimoto, T., Thompson, L.K., Eds.; American Chemical Society: Washington, DC, USA, 1996; Volume 644, pp. 201–215. [Google Scholar]
- Kuramochi, S.; Shiga, T.; Cameron, J.M.; Newton, G.N.; Oshio, H. Synthesis, crystal structures and magnetic properties of composites incorporating an Fe(II) spin crossover complex and polyoxometalates. Inorganics 2017, 5, 48. [Google Scholar] [CrossRef]
- Phonsri, W.; Davies, C.G.; Jameson, G.N.L.; Moubaraki, B.; Murray, K.S. Spin crossover, polymorphism and porosity to liquid solvent in heteroleptic iron(III) {quinolylsalicylaldimine/thiosemicarbazone-salicylaldimine} complexes. Chem. Eur. J. 2016, 22, 1322–1333. [Google Scholar] [CrossRef]
- Sunatsuki, Y.; Ikuta, Y.; Matsumoto, N.; Ohta, H.; Kojima, M.; Iijima, S.; Hayami, S.; Maeda, Y.; Kaizaki, S.; Dahan, F.; et al. An unprecedented homochiral mixed-valence spin-crossover compound. Angew. Chem. Int. Ed. 2003, 42, 1614–1618. [Google Scholar] [CrossRef] [PubMed]
- Uhrecký, R.; Svoboda, I.; Růžičková, Z.; Koman, M.; Dlháň, Ľ.; Pavlik, J.; Moncol, J.; Boča, R. Synthesis, structure and magnetism of manganese and iron dipicolinates with N,N’-donor ligands. Inorg. Chim. Acta 2015, 425, 134–144. [Google Scholar] [CrossRef]
- Aghabozorg, H.; Mohamad Panah, F.; Sadr-Khanlou, E. Crystal structure of [Fe(bpy)3][Fe(pydc)2]2(pydch2)1/2 6.5H2O complex (bpy = 2,2′-bipyridine, pydc = pyridine-2,6-dicarboxylate). Anal. Sci. X-ray Struct. Anal. Online 2007, 23, x139–x140. [Google Scholar] [CrossRef]
- Laine, P.; Gourdon, A.; Launay, J.P. Chemistry of iron with dipicolinic acid. 4. Mixed-ligand complexes of iron(III) and related compounds. Inorg. Chem. 1995, 34, 5156–5165. [Google Scholar] [CrossRef]
- Sunatsuki, Y.; Ohta, H.; Kojima, M.; Ikuta, Y.; Goto, Y.; Matsumoto, N.; Iijima, S.; Akashi, H.; Kaizaki, S.; Dahan, F.; et al. Supramolecular spin-crossover iron complexes based on imidazole–imidazolate hydrogen bonds. Inorg. Chem. 2004, 43, 4154–4171. [Google Scholar] [CrossRef]
- Brewer, C.T.; Brewer, G.; Butcher, R.J.; Carpenter, E.E.; Schmiedekamp, A.M.; Schmiedekamp, C.; Straka, A.; Viragh, C.; Yuzefpolskiy, Y.; Zavalij, P. Synthesis and characterization of homo- and heterodinuclear M(II)-M′(III) (M(II) = Mn or Fe, M′(III) = Fe or Co) mixed-valence supramolecular pseudo-dimers. The effect of hydrogen bonding on spin state selection of M(II). Dalton Trans. 2011, 40, 181–194. [Google Scholar] [CrossRef]
- Ikuta, Y.; Ooidemizu, M.; Yamahata, Y.; Yamada, M.; Osa, S.; Matsumoto, N.; Iijima, S.; Sunatsuki, Y.; Kojima, M.; Dahan, F.; et al. A new family of spin crossover complexes with a tripod ligand containing three imidazoles: Synthesis, characterization, and magnetic properties of [FeIIH3LMe](NO3)2·1.5H2O, [FeIIILMe]·3.5H2O, [FeIIH3LMe][FeIILMe]NO3, and [FeIIH3LMe][FeIIILMe](NO3)2 (H3LMe = tris[2-(((2-methylimidazol-4-yl)methylidene)amino)ethyl]amine). Inorg. Chem. 2003, 42, 7001–7017. [Google Scholar] [PubMed]
- Brewer, C.T.; Brewer, G.; Butcher, R.J.; Carpenter, E.E.; Schmiedekamp, A.M.; Viragh, C. Synthesis and characterization of a spin crossover iron(II)–iron(III) mixed valence supramolecular pseudo-dimer exhibiting chiral recognition, hydrogen bonding, and π–π interactions. Dalton Trans. 2007, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Lavrenova, L.G. Spin crossover in homo- and heteroligand iron(II) complexes with tris (pyrazol-1-y1)methane derivates. Russ. Chem. Bull. Int. Ed. 2018, 67, 1142–1152. [Google Scholar] [CrossRef]
- Paulsen, H.; Duelund, L.; Zimmermann, A.; Averseng, F.; Gerdan, M.; Winkler, H.; Toftlund, H.; Trautwein, A.X. Substituent effects on the spin-transition temperature in complexes with tris(pyrazolyl) ligands. Monatsh. Chem. 2003, 134, 295–306. [Google Scholar] [CrossRef]
- McGarvey, J.J.; Toftlund, H.; Al-Obaidi, A.H.R.; Taylor, K.P.; Bell, S.E.J. Photoperturbation of the 1A → 5T spin equilibrium in an iron(II) complex in solution via ligand field excitation. Inorg. Chem. 1993, 32, 2469–2472. [Google Scholar] [CrossRef]
- Anderson, P.A.; Astley, T.; Hitchman, M.A.; Keene, F.R.; Moubaraki, B.; Murray, K.S.; Skelton, B.W.; Tiekink, E.R.T.; Toftlund, H.; White, A.H. Structures and spectra of bis-tripodal iron(II) chelates, [FeL2]2+, where L = tris(pyrazol-1-yl)methane, tris(pyridin-2-yl)methane, bis(pyrazol-1-yl)(pyridin-2-yl)methane and tris(pyridin-2-yl)phosphine oxide. Magnetism and spin crossover in the (pz)3CH case. J. Chem. Soc. Dalton Trans. 2000, 3505–3512. [Google Scholar]
- Reger, D.L.; Little, C.A.; Rheingold, A.L.; Lam, M.; Liable-Sands, L.M.; Rhagitan, B.; Concolino, T.; Mohan, A.; Long, G.J.; Briois, V.; et al. A synthetic, structural, magnetic, and spectral study of several {Fe[tris(pyrazolyl)methane]2}(BF4)2 complexes: Observation of an unusual spin-state crossover. Inorg. Chem. 2001, 40, 1508–1520. [Google Scholar] [CrossRef]
- Campos-Fernández, C.S.; Smucker, B.W.; Clérac, R.; Dunbar, K.R. Reactivity studies of 2,3,5,6-tetra(2-pyridyl) pyrazine (tppz) with first-row transition metal ions. Isr. J. Chem. 2001, 41, 207–218. [Google Scholar] [CrossRef]
- Toma, L.M.; Armentano, D.; De Munno, G.; Sletten, J.; Lloret, F.; Julve, M. 2,3,5,6-tetrakis(2-pyridyl)pyrazine (tppz)-containing iron(II) complexes: Syntheses and crystal structures. Polyhedron 2007, 26, 5263–5270. [Google Scholar] [CrossRef]
- Halcrow, M.A. The synthesis and coordination chemistry of 2,6-bis(pyrazolyl)pyridines and related ligands—Versatile terpyridine analogues. Coord. Chem. Rev. 2005, 249, 2880–2908. [Google Scholar] [CrossRef]
- Pritchard, R.; Kilner, C.A.; Halcrow, M.A. Iron(II) complexes with a terpyridine embrace packing motif show remarkably consistent cooperative spin-transitions. Chem. Commun. 2007, 577–579. [Google Scholar] [CrossRef] [PubMed]
- McCusker, J.K.; Rheingold, A.L.; Hendrickson, D.N. Variable-temperature studies of laser-initiated 5T2 → 1A1 intersystem crossing in spin-crossover complexes: Empirical correlations between activation parameters and ligand structure in a series of polypyridyl ferrous complexes. Inorg. Chem. 1996, 35, 2100–2112. [Google Scholar] [CrossRef]
- Marchivie, M.; Guionneau, P.; Letard, J.-F.; Chasseau, D. Photo-induced spin-transition: The role of the iron(II) environment distortion. Acta Crystallogr. Sect. B Struct. Sci. 2005, 61, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Abibat Salaudeen, A.; Kilner, C.A.; Halcrow, M.A. Mononuclear and dinuclear iron thiocyanate and selenocyanate complexes of tris-pyrazolylmethane ligands. Polyhedron 2008, 27, 2569–2576. [Google Scholar] [CrossRef]
- Haselhorst, G.; Wieghardt, K.; Keller, S.; Schrader, B. The (μ-oxo)bis[trichloroferrate(III)] dianion revisited. Inorg. Chem. 1993, 32, 520–525. [Google Scholar] [CrossRef]
- Padgett, C.W.; Pennington, W.T.; Hanks, T.W. Conformations and binding modes of 2,3,5,6-tetra(2′-pyridyl)pyrazine. Cryst. Growth Des. 2005, 5, 737–744. [Google Scholar] [CrossRef]
- Behnamfar, M.T.; Hadadzadeh, H.; Simpson, J.; Darabi, F.; Shahpiri, A.; Khayamian, T.; Ebrahimi, M.; Amiri Rudbari, H.; Salimi, M. Experimental and molecular modeling studies of the interaction of the polypyridyl Fe(II) and Fe(III) complexes with DNA and bsa. Spectrochim. Acta Part A 2015, 134, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Russell, V.; Scudder, M.; Dance, I. The crystal supramolecularity of metal phenanthroline complexes. J. Chem. Soc. Dalton Trans. 2001, 789–799. [Google Scholar] [CrossRef]
- Hu, X.; Guo, J.; Wang, Y.; Liu, C. Synthesis, infrared spectra, thermal analyses and structural studies of half-sandwich Fe(III)/Fe(II) complex containing pyridine-2,6-dicarboxylate and 1,10-phenanthroline. Spectrochim. Acta Part A 2009, 74, 48–51. [Google Scholar] [CrossRef] [PubMed]
- McPhillips, T.M.; McPhillips, S.E.; Chiu, H.-J.; Cohen, A.E.; Deacon, A.M.; Ellis, P.J.; Garman, E.; Gonzalez, A.; Sauter, N.K.; Phizackerley, R.P.; et al. Blu-ice and the distributed control system: Software for data acquisition and instrument control at macromolecular crystallography beamlines. J. Synchrotron Radiat. 2002, 9, 401–406. [Google Scholar] [CrossRef]
- Kabsch, W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 1993, 26, 795–800. [Google Scholar] [CrossRef]
- Langenberg, K.V. Molecular-Based Magnetic Materials Incorporating Bis-and Tris(pyrazolyl)methane transition Metal Complexes. Hons. B.Sc. Honours Thesis, Monash University, Melbourne, Australia, 1993. [Google Scholar]
- Cowieson, N.P.; Aragao, D.; Clift, M.; Ericsson, D.J.; Gee, C.; Harrop, S.J.; Mudie, N.; Panjikar, S.; Price, J.R.; Riboldi-Tunnicliffe, A.; et al. Mx1: A bending-magnet crystallography beamline serving both chemical and macromolecular crystallography communities at the australian synchrotron. J. Synchrotron Radiat. 2015, 22, 187–190. [Google Scholar] [CrossRef]










| 4 | 5 | 6 | ||||
|---|---|---|---|---|---|---|
| 100 K | 300 K | 100 K | 300 K | 100 K | 300 K | |
| Formula | C68H56Fe3N20O10 | C68H56Fe3N20O10 | C96H66Fe3N20O9 | C96H66Fe3N20O9 | C102H75Fe3N23O9 | C102H74Fe3N23O8.50 |
| Molecular weight/gmol−1 | 1480.87 | 1480.87 | 1811.23 | 1811.23 | 1934.40 | 1925.39 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Monoclinic | Monoclinic | Monoclinic |
| Space group | P | P | P21/n | P21/n | P21/n | P21/n |
| a/Å | 11.860 (2) | 11.9211 (2) | 12.650(3) | 12.760 (3) | 12.075 (2) | 12.212 (2) |
| b/Å | 12.400 (3) | 12.5340 (2) | 18.010(4) | 18.130 (4) | 25.936 (5) | 26.134 (5) |
| c/Å | 13.420 (3) | 13.5051 (2) | 35.000(7) | 35.270 (7) | 28.191 (6) | 28.394 (6) |
| α/o | 109.64 (3) | 109.674 (2) | 90 | 90 | 90 | 90 |
| β/o | 114.74 (3) | 114.771 (2) | 99.97(3) | 99.86 (3) | 94.67 (3) | 94.57 (3) |
| γ/o | 93.63 (3) | 93.838 (2) | 90 | 90 | 90 | 90 |
| Cell volume/Å3 | 1639.4 (7) | 1673.46 (6) | 7854(3) | 8039 (3) | 8799 (3) | 9033 (3) |
| Z | 1 | 1 | 4 | 4 | 4 | 4 |
| Absorption coefficient/mm−1 | 0.732 | 5.775 | 0.626 | 0.612 | 0.565 | 0.550 |
| Reflections collected | 25901 | 33332 | 157439 | 169560 | 107312 | 110214 |
| Independent reflections, Rint | 6379, 0.0395 | 6931, 0.0880 | 23792, 0.0448 | 24544, 0.0497 | 24937, 0.0488 | 25677, 0.0536 |
| Max. and min. transmission | 0.985 and 0.978 | 1.00000 and 0.55733 | 0.994 and 0.978 | 0.978 and 0.994 | 0.989 and 0.973 | 0.989 and 0.974 |
| Restraints/parameters | 2/446 | 0/479 | 0/1161 | 2/1161 | 0/1273 | 0/1210 |
| Final R indices [I > 2σ(I)]: R1, wR2 | 0.0532, 0.1472 | 0.0570, 0.1469 | 0.0422, 0.1118 | 0.0490, 0.1408 | 0.0580, 0.1534 | 0.0536, 0.1562 |
| CCDC number | 1905262 | 1905258 | 1905264 | 1905266 | 1905263 | 1905265 |
| 4 | 5 | 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 100 Κ | 300 K | 100 Κ | 300 Κ | 100 K | 300 K | ||||
| Fe(III) | Fe1-O1/Å | 1.948 (2) | 1.948 (3) | Fe(III) | Fe1—O1/Å | 1.9699 (12) | 1.9706 (16) | 1.9772 (19) | 1.971 (2) |
| Fe1-O2/Å | 1.9409 (13) | 1.950 (3) | Fe1—O2/Å | 1.9494 (12) | 1.9481 (17) | 1.9558 (19) | 1.947 (2) | ||
| Fe1-O3/Å | 2.003 (2) | 2.006 (3) | Fe1—O3/Å | 1.9962 (12) | 1.9936 (16) | 1.9860 (19) | 1.982 (2) | ||
| Fe1-O4/Å | 1.916 (2) | 1.926 (3) | Fe1—O4/Å | 1.9563 (13) | 1.9521 (18) | 1.9590 (18) | 1.954 (2) | ||
| Fe1-N1 Fe1-N1’/Å | 2.152 (4) 2.099 (11) | 2.149 (5) 2.229 (15) | Fe1—N1/Å | 2.1901 (13) | 2.1907 (16) | 2.174 (2) | 2.175 (2) | ||
| Fe1-N3/Å | 2.1368 (17) | 2.162 (3) | Fe1—N3/Å | 2.1638 (13) | 2.1648 (16) | 2.188 (2) | 2.183 (2) | ||
| Fe(II) | Fe2—N5/Å | 1.9739 (12) | 1.968 (3) | Fe(III) | Fe2—O5/Å | 1.9135 (13) | 1.9156 (18) | 1.910 (2) | 1.915 (2) |
| Fe2—N5i/Å | 1.9739 (12) | 1.968 (3) | Fe2—O6/Å | 1.8813 (13) | 1.8945 (17) | 1.892 (2) | 1.886 (2) | ||
| Fe2—N6/Å | 1.9735 (12) | 1.975 (3) | Fe2—O7/Å | 1.9259 (12) | 1.9253 (17) | 1.8835 (19) | 1.879 (2) | ||
| Fe2—N6i/Å | 1.9736 (12) | 1.975 (3) | Fe2—O8/Å | 1.8868 (12) | 1.8959 (16) | 1.9016 (19) | 1.9085 (19) | ||
| Fe2—N7/Å | 1.9611 (16) | 1.963 (3) | Fe2—N5/Å | 1.9253 (15) | 1.963 (2) | 1.908 (2) | 1.932 (2) | ||
| Fe2—N7i/Å | 1.9610 (16) | 1.963 (3) | Fe2—N7/Å | 1.9107 (13) | 1.9516 (18) | 1.911 (2) | 1.934 (2) | ||
| Σ/° (Fe1, Fe2) | 100, 25 | 103, 27 | Fe(II) | Fe3—N9/Å | 1.9802 (13) | 1.9840 (15) | 1.956 (2) | 1.9555 (18) | |
| Θ/° (Fe1, Fe2) | 293, 31 | 308, 32 | Fe3—N10/Å | 1.8861 (12) | 1.8846 (14) | 1.885 (2) | 1.8825 (19) | ||
| Fe3—N11/Å | 1.9699 (13) | 1.9721 (16) | 1.971 (2) | 1.9666 (19) | |||||
| Fe3—N12/Å | 1.9600 (13) | 1.9596 (15) | 1.972 (2) | 1.977 (2) | |||||
| Fe3—N13/Å | 1.8838 (12) | 1.8833 (14) | 1.880 (2) | 1.8804 (19) | |||||
| Fe3—N14/Å | 1.9652 (13) | 1.9657 (15) | 1.977 (2) | 1.9812 (19) | |||||
| Σ/° (Fe1, Fe2, Fe3) | 103, 36, 79 | 100, 37, 79 | 106, 26, 78 | 101, 26, 78 | |||||
| Θ/° (Fe1, Fe2, Fe3) | 314, 49, 255 | 302, 71, 255 | 332, 44, 258 | 319, 52,260 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phonsri, W.; Macedo, D.S.; Lewis, B.A.I.; Wain, D.F.; Murray, K.S. Iron(III) Azadiphenolate Compounds in a New Family of Spin Crossover Iron(II)–Iron(III) Mixed-Valent Complexes. Magnetochemistry 2019, 5, 37. https://doi.org/10.3390/magnetochemistry5020037
Phonsri W, Macedo DS, Lewis BAI, Wain DF, Murray KS. Iron(III) Azadiphenolate Compounds in a New Family of Spin Crossover Iron(II)–Iron(III) Mixed-Valent Complexes. Magnetochemistry. 2019; 5(2):37. https://doi.org/10.3390/magnetochemistry5020037
Chicago/Turabian StylePhonsri, Wasinee, David S. Macedo, Barnaby A. I. Lewis, Declan F. Wain, and Keith S. Murray. 2019. "Iron(III) Azadiphenolate Compounds in a New Family of Spin Crossover Iron(II)–Iron(III) Mixed-Valent Complexes" Magnetochemistry 5, no. 2: 37. https://doi.org/10.3390/magnetochemistry5020037
APA StylePhonsri, W., Macedo, D. S., Lewis, B. A. I., Wain, D. F., & Murray, K. S. (2019). Iron(III) Azadiphenolate Compounds in a New Family of Spin Crossover Iron(II)–Iron(III) Mixed-Valent Complexes. Magnetochemistry, 5(2), 37. https://doi.org/10.3390/magnetochemistry5020037