Successes and Challenges: Inhaled Treatment Approaches Using Magnetic Nanoparticles in Cystic Fibrosis
Abstract
1. Introduction
1.1. Cystic Fibrosis
1.1.1. An Introduction to the Disease
1.1.2. Current Treatments
CFTR Modulators
Antibiotics
- Tobramycin
- Aztreonam
- Colistinmethate Sodium
Mucolytics and Osmotic Agents
1.2. Magnetic Nanoparticles
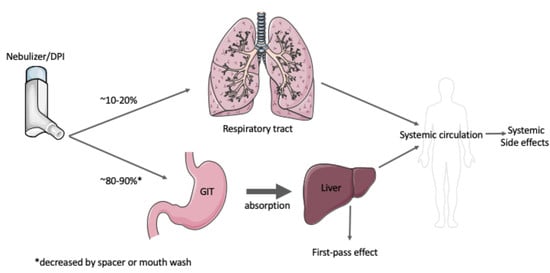
1.3. Inhaled Therapeutics
1.4. Approved Inhaled Antibiotics for the Treatment of Lung Infections in CF
1.5. Approved Inhaled Mucolytics and Osmotic Agents
1.6. Inhaled Antibiotics in Clinical Trials
1.7. Gene Delivery
1.7.1. Barriers to Gene Delivery
1.7.2. Mucus-Penetrating Magnetic Nanoparticles (MNPs)
1.7.3. Non-Viral Vectors for Gene Delivery
1.7.4. Clinical Trials in CF Using NPs
1.8. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Andrade, F.; Rafael, D.; Videira, M.; Ferreira, D.; Sosnik, A.; Sarmento, B. Nanotechnology and pulmonary delivery to overcome resistance in infectious diseases. Adv. Drug Deliv. Rev. 2013, 65, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Worldwide CF. 2018. Available online: https://www.cfww.org (accessed on 23 April 2020).
- De Boeck, K.; Amaral, M.D. Progress in therapies for cystic fibrosis. Lancet Respir. Med. 2016, 4, 662–674. [Google Scholar] [CrossRef]
- Saint-Criq, V.; Gray, M.A. Role of CFTR in epithelial physiology. Cell Mol. Life Sci. 2017, 74, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Döring, G.; Flume, P.; Heijerman, H.; Elborn, J.S. Treatment of lung infection in patients with cystic fibrosis: Current and future strategies. J. Cyst. Fibros. 2012, 11, 461–479. [Google Scholar] [CrossRef]
- Schneider-Futschik, E.K.; Paulin, O.K.A.; Hoyer, D.; Roberts, K.D.; Ziogas, J.; Baker, M.A.; Karas, J.; Li, J.; Velkov, T. Sputum Active Polymyxin Lipopeptides: Activity against Cystic Fibrosis Pseudomonas aeruginosa Isolates and Their Interactions with Sputum Biomolecules. ACS Infect. Dis. 2018, 4, 646–655. [Google Scholar] [CrossRef]
- D’Angelo, I.; Conte, C.; La Rotonda, M.I.; Miro, A.; Quaglia, F.; Ungaro, F. Improving the efficacy of inhaled drugs in cystic fibrosis: Challenges and emerging drug delivery strategies. Adv. Drug Deliv. Rev. 2014, 75, 92–111. [Google Scholar] [CrossRef]
- Velino, C.; Carella, F.; Adamiano, A.; Sanguinetti, M.; Vitali, A.; Catalucci, D.; Bugli, F.; Iafisco, M. Nanomedicine Approaches for the Pulmonary Treatment of Cystic Fibrosis. Front. Bioeng. Biotechnol. 2019, 7, 406. [Google Scholar] [CrossRef]
- Ong, V.; Mei, V.; Cao, L.; Lee, K.; Chung, E.J. Nanomedicine for Cystic Fibrosis. Slas Technol. 2019, 24, 169–180. [Google Scholar] [CrossRef]
- Schneider, E.K.; Reyes-Ortega, F.; Li, J.; Velkov, T. Can Cystic Fibrosis Patients Finally Catch a Breath with Lumacaftor/Ivacaftor? Clin. Pharmacol. Ther. 2017, 101, 130–141. [Google Scholar] [CrossRef]
- Davies, J.C.; Cunningham, S.; Harris, W.T.; Lapey, A.; Regelmann, W.E.; Sawicki, G.S.; Southern, K.W.; Robertson, S.; Green, Y.; Cooke, J.; et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2-5 years with cystic fibrosis and a CFTR gating mutation (KIWI): An open-label, single-arm study. Lancet Respir. Med. 2016, 4, 107–115. [Google Scholar] [CrossRef]
- Schneider-Futschik, E.K. Beyond cystic fibrosis transmembrane conductance regulator therapy: A perspective on gene therapy and small molecule treatment for cystic fibrosis. Gene 2019, 26, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Ghelani, D.P.; Schneider-Futschik, E.K. Emerging Cystic Fibrosis Transmembrane Conductance Regulator Modulators as New Drugs for Cystic Fibrosis: A Portrait of in Vitro Pharmacology and Clinical Translation. ACS Pharmacol. Transl. Sci. 2020, 3, 4–10. [Google Scholar] [CrossRef]
- Schneider, E.K.; Azad, M.A.; Han, M.L.; Tony Zhou, Q.; Wang, J.; Huang, J.X.; Cooper, M.A.; Doi, Y.; Baker, M.A.; Bergen, P.J.; et al. An "Unlikely" Pair: The Antimicrobial Synergy of Polymyxin B in Combination with the Cystic Fibrosis Transmembrane Conductance Regulator Drugs KALYDECO and ORKAMBI. ACS Infect. Dis. 2016, 2, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Hamed, K.; Conti, V.; Tian, H.; Loefroth, E. Adherence to tobramycin inhaled powder vs inhaled solution in patients with cystic fibrosis: Analysis of US insurance claims data. Patient Prefer. Adherence 2017, 11, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Konstan, M.W.; Flume, P.A.; Kappler, M.; Chiron, R.; Higgins, M.; Brockhaus, F.; Zhang, J.; Angyalosi, G.; He, E.; Geller, D.E. Safety, efficacy and convenience of tobramycin inhalation powder in cystic fibrosis patients: The EAGER trial. J. Cyst. Fibros. 2011, 10, 54–61. [Google Scholar] [CrossRef]
- Brandt, Y.I.; Armijo, L.M.; Rivera, A.C.; Plumley, J.B.; Cook, N.C.; Smolyakov, G.A.; Smyth, H.D.; Osiński, M. Effectiveness of Tobramycin Conjugated to Iron Oxide Nanoparticles in Treating Infection in Cystic Fibrosis. In Proceedings of the SPIE BiOS, San Francisco, CA, USA, 2–3 February 2013. [Google Scholar]
- Armijo, L.; Kopciuch, M.; Olszόwka, Z.; Wawrzyniec, S.; Rivera, A.; Plumley, J.; Cook, N.; Brandt, Y.; Huber, D.; Smolyakov, G.; et al. Delivery of Tobramycin Coupled to Iron Oxide Nanoparticles Across the Biofilm of Mucoidal Pseudonomas aeruginosa and Investigation of its Efficacy; SPIE: Bellingham, WA, USA, 2014; Volume 8955. [Google Scholar]
- Kirkby, S.; Novak, K.; McCoy, K. Aztreonam (for inhalation solution) for the treatment of chronic lung infections in patients with cystic fibrosis: An evidence-based review. Core Evid. 2011, 6, 59–66. [Google Scholar] [CrossRef]
- Koerner-Rettberg, C.; Ballmann, M. Colistimethate sodium for the treatment of chronic pulmonary infection in cystic fibrosis: An evidence-based review of its place in therapy. Core Evid. 2014, 9, 99–112. [Google Scholar] [CrossRef]
- Velkov, T.; Abdul Rahim, N.; Zhou, Q.T.; Chan, H.K.; Li, J. Inhaled anti-infective chemotherapy for respiratory tract infections: Successes, challenges and the road ahead. Adv. Drug Deliv. Rev. 2015, 85, 65–82. [Google Scholar] [CrossRef]
- Rogers, D.F. Mucoactive agents for airway mucus hypersecretory diseases. Respir. Care 2007, 52, 1176–1193; discussion 1193–1197. [Google Scholar]
- Elkins, M.R.; Robinson, M.; Rose, B.R.; Harbour, C.; Moriarty, C.P.; Marks, G.B.; Belousova, E.G.; Xuan, W.; Bye, P.T. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N. Engl. J. Med. 2006, 354, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Burness, C.B.; Keating, G.M. Mannitol dry powder for inhalation: In patients with cystic fibrosis. Drugs 2012, 72, 1411–1421. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Delgado, A.V.; Schneider, E.K.; Checa Fernandez, B.L.; Iglesias, G.R. Magnetic Nanoparticles Coated with a Thermosensitive Polymer with Hyperthermia Properties. Polymer 2017, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, C.; Tindall, J.M.; Hong, J.S.; Sorscher, E.J. Making precision medicine personal for cystic fibrosis. Science 2019, 365, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.P.; De Boeck, K. A new era in the treatment of cystic fibrosis: Correction of the underlying CFTR defect. Lancet Respir. Med. 2013, 1, 158–163. [Google Scholar] [CrossRef]
- Brogden, R.N.; Heel, R.C. Aztreonam. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1986, 31, 96–130. [Google Scholar] [CrossRef]
- Allobawi, R.; Ghelani, D.P.; Schneider-Futschik, E.K. Metabolomic description of ivacaftor elevating polymyxin B mediated antibacterial activity in cystic fibrosis Pseudomonas aeruginosa. ACS Pharmacol. Transl. Sci. 2020. [Google Scholar] [CrossRef]
- Jiang, L.; Patel, D.J. Solution structure of the tobramycin-RNA aptamer complex. Nat. Struct. Biol. 1998, 5, 769–774. [Google Scholar] [CrossRef]
- LeBel, M. Ciprofloxacin: Chemistry, mechanism of action, resistance, antimicrobial spectrum, pharmacokinetics, clinical trials, and adverse reactions. Pharmacotherapy 1988, 8, 3–33. [Google Scholar] [CrossRef]
- Croom, K.F.; Goa, K.L. Levofloxacin: A review of its use in the treatment of bacterial infections in the United States. Drugs 2003, 63, 2769–2802. [Google Scholar] [CrossRef]
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the Effects of N-Acetylcysteine in Neurodegenerative Diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef]
- Lazarus, R.A.; Wagener, J.S. Recombinant Human Deoxyribonuclease I. In Pharmaceutical Biotechnology; Crommelin, D., Sindelar, R., Meibohm, B., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Moreno, R.; Poyser, S.; Meilak, D.; Meo, A.; Jenkins, S.; Lazarov, V.K.; Vallejo-Fernandez, G.; Majetich, S.; Evans, R.F.L. The role of faceting and elongation on the magnetic anisotropy of magnetite Fe3O4 nanocrystals. Sci. Rep. 2020, 10, 2722. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sotomayor, R.; Ahualli, S.; Viota, J.L.; Rudzka, K.; Delgado, A.V. Iron/Magnetite Nanoparticles as Magnetic Delivery Systems for Antitumor Drugs. J. Nanosci. Nanotechnol. 2015, 15, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Roca, X.; Karginov, F.V. RNA biology in a test tube—An overview of in vitro systems/assays. Wiley Interdiscip. Rev. RNA 2012, 3, 509–527. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Yiu, H.H.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar] [CrossRef]
- Ansari, S.; Ficiara, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Functionalization for Biomedical Applications in the Central Nervous System. Material 2019, 12, 465. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Checa Fernandez, B.L.; Delgado, A.V.; Iglesias, G.R. Hyperthermia-Triggered Doxorubicin Release from Polymer-Coated Magnetic Nanorods. Pharmaceutics 2019, 11, 517. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Martinez, L.M.; Khurshid, H.; Garaio, E.; Garcia, J.A.; Srikanth, H. Enhanced Magnetic Hyperthermia in Iron Oxide Nano-Octopods: Size and Anisotropy Effects. J. Phys. Chem. C 2016, 120, 8370–8379. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M.; Elbaz, N.M.; Sedki, M.; Elgammal, A.; Yacoub, M.H. Magnetic nanoparticles-based drug and gene delivery systems for the treatment of pulmonary diseases. Nanomedicine 2017, 12, 387–402. [Google Scholar] [CrossRef]
- Sadhukha, T.; Wiedmann, T.S.; Panyam, J. Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials 2013, 34, 5163–5171. [Google Scholar] [CrossRef] [PubMed]
- Cristallini, C.; Barbani, N.; Ventrelli, L.; Summa, C.; Filippi, S.; Capelôa, T.; Vitale, E.; Albera, C.; Messore, B.; Giachino, C. Biodegradable microparticles designed to efficiently reach and act on cystic fibrosis mucus barrier. Mater. Sci. Eng. C 2018, 95. [Google Scholar] [CrossRef] [PubMed]
- Klinger-Strobel, M.; Lautenschlager, C.; Fischer, D.; Mainz, J.G.; Bruns, T.; Tuchscherr, L.; Pletz, M.W.; Makarewicz, O. Aspects of pulmonary drug delivery strategies for infections in cystic fibrosis—where do we stand? Expert Opin. Drug Deliv. 2015, 12, 1351–1374. [Google Scholar] [CrossRef] [PubMed]
- Leuba, K.D.; Durmus, N.G.; Taylor, E.N.; Webster, T.J. Short communication: Carboxylate functionalized superparamagnetic iron oxide nanoparticles (SPION) for the reduction of S. aureus growth post biofilm formation. Int. J. Nanomed. 2013, 8, 731–736. [Google Scholar] [CrossRef][Green Version]
- Usmani, O.S. Choosing the right inhaler for your asthma or COPD patient. Clin. Risk Manag. 2019, 15, 461–472. [Google Scholar] [CrossRef]
- Konstan, M.W.; Geller, D.E.; Minic, P.; Brockhaus, F.; Zhang, J.; Angyalosi, G. Tobramycin inhalation powder for P. aeruginosa infection in cystic fibrosis: The EVOLVE trial. Pediatr. Pulmonol. 2011, 46, 230–238. [Google Scholar] [CrossRef]
- Elson, E.C.; Mermis, J.; Polineni, D.; Oermann, C.M. Aztreonam Lysine Inhalation Solution in Cystic Fibrosis. Clin. Med. Insights Circ. Respir. Pulm. Med. 2019, 13. [Google Scholar] [CrossRef]
- Schuster, A.; Haliburn, C.; Doring, G.; Goldman, M.H.; Freedom Study, G. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: A randomised study. Thorax 2013, 68, 344–350. [Google Scholar] [CrossRef]
- Bilton, D.; Bellon, G.; Charlton, B.; Cooper, P.; De Boeck, K.; Flume, P.A.; Fox, H.G.; Gallagher, C.G.; Geller, D.E.; Haarman, E.G.; et al. Pooled analysis of two large randomised phase III inhaled mannitol studies in cystic fibrosis. J. Cyst. Fibros. 2013, 12, 367–376. [Google Scholar] [CrossRef]
- Aitken, M.L.; Bellon, G.; De Boeck, K.; Flume, P.A.; Fox, H.G.; Geller, D.E.; Haarman, E.G.; Hebestreit, H.U.; Lapey, A.; Schou, I.M.; et al. Long-Term Inhaled Dry Powder Mannitol in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2012, 185, 645–652. [Google Scholar] [CrossRef]
- Dorkin, H.L.; Staab, D.; Operschall, E.; Alder, J.; Criollo, M. Ciprofloxacin DPI: A randomised, placebo-controlled, phase IIb efficacy and safety study on cystic fibrosis. BMJ Open Respir. Res. 2015, 2, e000100. [Google Scholar] [CrossRef] [PubMed]
- Flume, P.A.; VanDevanter, D.R.; Morgan, E.E.; Dudley, M.N.; Loutit, J.S.; Bell, S.C.; Kerem, E.; Fischer, R.; Smyth, A.R.; Aaron, S.D.; et al. A phase 3, multi-center, multinational, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT-1026) in stable cystic fibrosis patients. J. Cyst. Fibros. 2016, 15, 495–502. [Google Scholar] [CrossRef]
- Stuart Elborn, J.; Geller, D.E.; Conrad, D.; Aaron, S.D.; Smyth, A.R.; Fischer, R.; Kerem, E.; Bell, S.C.; Loutit, J.S.; Dudley, M.N.; et al. A phase 3, open-label, randomized trial to evaluate the safety and efficacy of levofloxacin inhalation solution (APT-1026) versus tobramycin inhalation solution in stable cystic fibrosis patients. J. Cyst. Fibros. 2015, 14, 507–514. [Google Scholar] [CrossRef]
- Elborn, J.S. Ciprofloxacin dry powder inhaler in cystic fibrosis. BMJ Open Respir. Res. 2016, 3, e000125. [Google Scholar] [CrossRef][Green Version]
- Hua, X.; Tan, S.; Bandara, H.M.; Fu, Y.; Liu, S.; Smyth, H.D. Externally controlled triggered-release of drug from PLGA micro and nanoparticles. PLoS ONE 2014, 9, e114271. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.E.; Flume, P.A.; Staab, D.; Fischer, R.; Loutit, J.S.; Conrad, D.J.; Mpex 204 Study, G. Levofloxacin inhalation solution (MP-376) in patients with cystic fibrosis with Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 2011, 183, 1510–1516. [Google Scholar] [CrossRef]
- Griesenbach, U.; Pytel, K.M.; Alton, E.W. Cystic Fibrosis Gene Therapy in the UK and Elsewhere. Hum. Gene 2015, 26, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Griesenbach, U.; Alton, E.W. Moving forward: Cystic fibrosis gene therapy. Hum. Mol. Genet. 2013, 22, R52–R58. [Google Scholar] [CrossRef]
- Griesenbach, U.; Alton, E.W. Current status and future directions of gene and cell therapy for cystic fibrosis. BioDrugs 2011, 25, 77–88. [Google Scholar] [CrossRef]
- Griesenbach, U.; Geddes, D.M.; Alton, E.W. Advances in cystic fibrosis gene therapy. Curr. Opin. Pulm. Med. 2004, 10, 542–546. [Google Scholar] [CrossRef]
- Stern, M.; Ulrich, K.; Geddes, D.M.; Alton, E.W. Poly (D, L-lactide-co-glycolide)/DNA microspheres to facilitate prolonged transgene expression in airway epithelium in vitro, ex vivo and in vivo. Gene 2003, 10, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Schuster, B.S.; Kim, A.J.; Kays, J.C.; Kanzawa, M.M.; Guggino, W.B.; Boyle, M.P.; Rowe, S.M.; Muzyczka, N.; Suk, J.S.; Hanes, J. Overcoming the cystic fibrosis sputum barrier to leading adeno-associated virus gene therapy vectors. Mol. Ther. 2014, 22, 1484–1493. [Google Scholar] [CrossRef] [PubMed]
- Xia, E.; Munegowda, M.A.; Cao, H.; Hu, J. Lung gene therapy-How to capture illumination from the light already present in the tunnel. Genes Dis. 2014, 1, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Yonemitsu, Y.; Kitson, C.; Ferrari, S.; Farley, R.; Griesenbach, U.; Judd, D.; Steel, R.; Scheid, P.; Zhu, J.; Jeffery, P.K.; et al. Efficient gene transfer to airway epithelium using recombinant Sendai virus. Nat. Biotechnol. 2000, 18, 970–973. [Google Scholar] [CrossRef]
- Wilson, B.R.; Bogdan, A.R.; Miyazawa, M.; Hashimoto, K.; Tsuji, Y. Siderophores in Iron Metabolism: From Mechanism to Therapy Potential. Trends Mol. Med. 2016, 22, 1077–1090. [Google Scholar] [CrossRef]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Kruger, A.; Gansbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene 2002, 9, 102–109. [Google Scholar] [CrossRef]
- Morishita, N.; Nakagami, H.; Morishita, R.; Takeda, S.; Mishima, F.; Terazono, B.; Nishijima, S.; Kaneda, Y.; Tanaka, N. Magnetic nanoparticles with surface modification enhanced gene delivery of HVJ-E vector. Biochem. Biophys. Res. Commun. 2005, 334, 1121–1126. [Google Scholar] [CrossRef]
- Abdulkarim, M.; Agullo, N.; Cattoz, B.; Griffiths, P.; Bernkop-Schnurch, A.; Borros, S.G.; Gumbleton, M. Nanoparticle diffusion within intestinal mucus: Three-dimensional response analysis dissecting the impact of particle surface charge, size and heterogeneity across polyelectrolyte, pegylated and viral particles. Eur. J. Pharm. Biopharm. 2015, 97 Pt A, 230–238. [Google Scholar] [CrossRef]
- Suk, J.S.; Lai, S.K.; Boylan, N.J.; Dawson, M.R.; Boyle, M.P.; Hanes, J. Rapid transport of muco-inert nanoparticles in cystic fibrosis sputum treated with N-acetyl cysteine. Nanomedecine 2011, 6, 365–375. [Google Scholar] [CrossRef]
- Craparo, E.F.; Porsio, B.; Sardo, C.; Giammona, G.; Cavallaro, G. Pegylated Polyaspartamide-Polylactide-Based Nanoparticles Penetrating Cystic Fibrosis Artificial Mucus. Biomacromolecules 2016, 17, 767–777. [Google Scholar] [CrossRef]
- Porsio, B.; Craparo, E.F.; Mauro, N.; Giammona, G.; Cavallaro, G. Mucus and Cell-Penetrating Nanoparticles Embedded in Nano-into-Micro Formulations for Pulmonary Delivery of Ivacaftor in Patients with Cystic Fibrosis. ACS Appl. Mater. Interfaces 2018, 10, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Matthews, D.A.; Blair, G.E. Novel molecular approaches to cystic fibrosis gene therapy. Biochem. J. 2005, 387 Pt 1, 1–15. [Google Scholar] [CrossRef]
- Xenariou, S.; Griesenbach, U.; Ferrari, S.; Dean, P.; Scheule, R.K.; Cheng, S.H.; Geddes, D.M.; Plank, C.; Alton, E.W. Using magnetic forces to enhance non-viral gene transfer to airway epithelium in vivo. Gene 2006, 13, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhou, Y.; Sun, H.; Li, D.; Zhou, S. Biodegradable magnetic calcium phosphate nanoformulation for cancer therapy. Eur. J. Pharm. Biopharm. 2014, 87, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Reul, R.; Betz, T.; Dayyoub, E.; Schmehl, T.; Gessler, T.; Bakowsky, U.; Seeger, W.; Kissel, T. Nanocomposites of lung surfactant and biodegradable cationic nanoparticles improve transfection efficiency to lung cells. J. Control. Release 2009, 140, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Dames, P.; Gleich, B.; Flemmer, A.; Hajek, K.; Seidl, N.; Wiekhorst, F.; Eberbeck, D.; Bittmann, I.; Bergemann, C.; Weyh, T.; et al. Targeted delivery of magnetic aerosol droplets to the lung. Nat. Nanotechnol. 2007, 2, 495–499. [Google Scholar] [CrossRef]
- Yuan, C.; An, Y.; Zhang, J.; Li, H.; Zhang, H.; Wang, L.; Zhang, D. Magnetic nanoparticles for targeted therapeutic gene delivery and magnetic-inducing heating on hepatoma. Nanotechnology 2014, 25, 345101. [Google Scholar] [CrossRef][Green Version]
- Deacon, J.; Abdelghany, S.M.; Quinn, D.J.; Schmid, D.; Megaw, J.; Donnelly, R.F.; Jones, D.S.; Kissenpfennig, A.; Elborn, J.S.; Gilmore, B.F.; et al. Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: Formulation, characterisation and functionalisation with dornase alfa (DNase). J. Control. Release 2015, 198, 55–61. [Google Scholar] [CrossRef]
- Liu, M.Z.J.; Shan, W. Developments of Mucus Penetrating Nanoparticles. Asian J. Pharm. Sci. 2015, 10, 275–282. [Google Scholar] [CrossRef]
- Jasim, R.; Schneider, E.K.; Han, M.; Azad, M.A.K.; Hussein, M.; Nowell, C.; Baker, M.A.; Wang, J.; Li, J.; Velkov, T. A Fresh Shine onCystic Fibrosis Inhalation Therapy: Antimicrobial Synergy of Polymyxin B in Combination with Silver Nanoparticles. J. Biomed. Nanotechnol. 2017, 13, 447–457. [Google Scholar] [CrossRef]
- Maclachlan, T.K.; Lukason, M.; Collins, M.; Munger, R.; Isenberger, E.; Rogers, C.; Malatos, S.; Dufresne, E.; Morris, J.; Calcedo, R.; et al. Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol. Ther. 2011, 19, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, F.E.; Clancy, J.P.; Perricone, M.A.; Bebok, Z.; Hong, J.S.; Cheng, S.H.; Meeker, D.P.; Young, K.R.; Schoumacher, R.A.; Weatherly, M.R.; et al. A clinical inflammatory syndrome attributable to aerosolized lipid-DNA administration in cystic fibrosis. Hum. Gene 2001, 12, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Hyde, S.C.; Pringle, I.A.; Abdullah, S.; Lawton, A.E.; Davies, L.A.; Varathalingam, A.; Nunez-Alonso, G.; Green, A.M.; Bazzani, R.P.; Sumner-Jones, S.G.; et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat. Biotechnol. 2008, 26, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Alton, E.W.; Boyd, A.C.; Porteous, D.J.; Davies, G.; Davies, J.C.; Griesenbach, U.; Higgins, T.E.; Gill, D.R.; Hyde, S.C.; Innes, J.A.; et al. A Phase I/IIa Safety and Efficacy Study of Nebulized Liposome-mediated Gene Therapy for Cystic Fibrosis Supports a Multidose Trial. Am. J. Respir. Crit. Care Med. 2015, 192, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Alton, E.; Armstrong, D.K.; Ashby, D.; Bayfield, K.J.; Bilton, D.; Bloomfield, E.V.; Boyd, A.C.; Brand, J.; Buchan, R.; Calcedo, R.; et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015, 3, 684–691. [Google Scholar] [CrossRef]


| CFTR Class | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| CFTR defect | Protein synthesis | Maturation processing | Ion channel gating | Ion channel conductance | Reduced protein | Reduced membrane stability |
| Type of mutations | Nonsense; frameshift; canonical splice | Missense; amino acid deletion | Missense; amino acid change | Missense; amino acid change | Splicing defect; missense | Missense; amino acid change |
| CFTR protein effect | Complete loss of protein function | Defective regulation processing | Defective protein regulation | Defective protein conductance | Reduced protein synthesis | Impacted surface retention |
| Cellular compartment where defect occurs | Ribosome | Golgi/endoplasmic reticulum | Plasma membrane CFTR | Plasma membrane CFTR | Plasma membrane, spliceosome | Plasma membrane, post-endoplasmic reticulum compartments |
| Example mutations | 3659delC; 621 + 1G→T 1078delT; 1717-1G→A; | R560T, N1303K G85E, F508del, I507del | G551S, G970R, G1244E, S1251N, G178R, S549N, S549R, G551D, S1255P, G1349D | R347P, R334W, R117H | A455E, 2789 + 5G→A, 3849 + 10KbC→T | 120del23, N287Y |
| Prevalence within CF cohort * | 22% | 88% | 6% | 6% | 5% | 0.5% |
| Patients with modulator approved genotype | <0.5% | 39.2% | 4.6% | 2.6% | 3.5% | - |
| Therapeutic strategies | Read-through synthesis | Correctors and potentiators | Potentiators | Potentiators | Amplifiers, splicing modulators, potentiators | Stabilizers |
| Name | Mode of Action | Structure | Ref. |
|---|---|---|---|
| CFTR modulator | |||
| Ivacaftor | Potentiator | 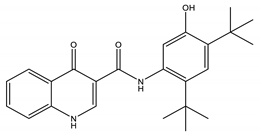 | [14] |
| Lumacaftor | Corrector |  | [14] |
| Tezacaftor | Corrector |  | [14] |
| Elexacaftor | Corrector | 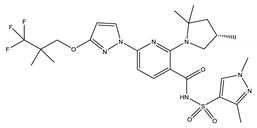 | [14] |
| Inhaled Antibiotics | |||
| Aztreonam lysine | β-lactam | 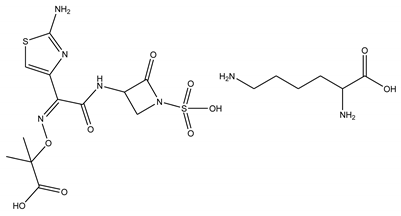 | [29] |
| Colistin | Lipopeptide | 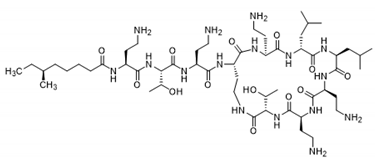 | [30] |
| Tobramycin | Aminoglycoside | 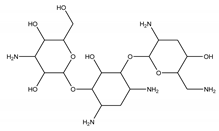 | [31] |
| Ciprofloxacin | Fluoroquinolone | 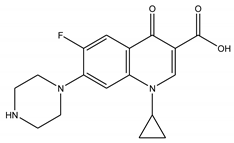 | [32] |
| Levofloxacin | Fluoroquinolone |  | [33] |
| Mucolytics | |||
| N-acetyl cysteine (NAC) and | reduces disulphide bonds | 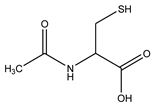 | [34] |
| Recombinant human deoxyribonuclease I (rhDNase) | Cleaves extracellular DNA | 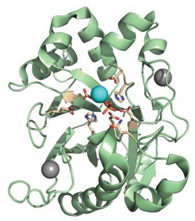 | [35] |
| Inhalation Device | ||
|---|---|---|
| Nebuliser | Dry Powder Inhaler (DPI) | |
| Mechanism | Nebulisation by air-jet | Dry powder |
| Characteristics | Vibrating mesh technology or aerosol droplets generated from liquids | High stability and sterility |
| Advantages | Little training for correct use required Ability to deliver large dosages Usage is independent on age | Short administration Small and portable Breath-actuated Little coordination required |
| Disadvantages | Requires regular maintenance Frequent administration Long inhalation times | Use in children limited Efficiency requires high inspiratory effort Optimal dosage requires proper dose preparation and loading |
| Inhaled Therapy | Status | Dosage | Delivery Method | Target | Potential Side Effects | Reference |
|---|---|---|---|---|---|---|
| Mucociliary clearance | ||||||
| Mannitol | Phase III completed | 400 mg (in 10 capsules), twice daily | Dry powder | Thick viscous mucous | Cough | [53,54] |
| Hypertonic saline | - | 7% saline (active), twice daily | Nebuliser | Increase hydration of airway surface liquid | Cough | [24] |
| Anti-infective | ||||||
| Aztreonam Lysine | FDA approved | 75 mg, thrice daily | Nebuliser | P. aeruginosa colonization | Fever Cough Bronchospasm Throat and chest discomfort Nasal congestion Headache | [51] |
| Ciprofloxacin | Phase II completed | 32.5 mg twice daily | Dry powder | P. aeruginosa colonization | Sunlight sensitivity rash Cartilage toxicity Emergence of antibiotic resistance | [8,55] |
| Colistimethate sodium salt | Phase III completed | 125 mg, twice daily | Dry powder | P. aeruginosa colonization | Cough Throat irritation Chest tightness Bronchospasm | [52] |
| Levofloxacin (approved for use in Europe & Canada) | Phase III completed in USA; | 240 mg, twice daily | Nebuliser | P. aeruginosa colonization | Dysgeusia Cough Nausea Pyrexia Hemoptysis | [56,57] |
| Tobramycin (TIS) | FDA approved | 300 mg/5 mL vial, twice daily | Nebuliser | P. aeruginosa colonization | Cough Bronchospasm Dyspnoea Dysphonia Haemoptysis Transient tinnitus Voice alteration | [17,50] |
| Tobramycin (TIP) | FDA approved | 112 mg, twice daily | Dry powder | |||
| Size | Surface Charge | Material | Composition | ||||
|---|---|---|---|---|---|---|---|
| MNP | 100–200 nm | Positive Charge e.g., Chitosan | Neutral Charge, e.g., Low Molecular Weight PEG | Lipophilic Material, e.g., PGLA | PGLA *, Chitosan | Silver | Alginate |
| Benefit | Epithelial cell uptake | Targets negatively charged mucus | Reduced electrostatic hindrance resulting in improved epithelial cell penetration PEG coating allows longer circulation | Penetration reduced by hydrophobic mucus | Biocompatible, low toxicity | Antimicrobial properties | High loading efficiency due to negative charge |
| Reference | [73] | [82] | [83] | [83] | [82] | [84] | [82] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, M.; Reyes-Ortega, F.; Schneider-Futschik, E.K. Successes and Challenges: Inhaled Treatment Approaches Using Magnetic Nanoparticles in Cystic Fibrosis. Magnetochemistry 2020, 6, 25. https://doi.org/10.3390/magnetochemistry6020025
Tan M, Reyes-Ortega F, Schneider-Futschik EK. Successes and Challenges: Inhaled Treatment Approaches Using Magnetic Nanoparticles in Cystic Fibrosis. Magnetochemistry. 2020; 6(2):25. https://doi.org/10.3390/magnetochemistry6020025
Chicago/Turabian StyleTan, Marsha, Felisa Reyes-Ortega, and Elena K. Schneider-Futschik. 2020. "Successes and Challenges: Inhaled Treatment Approaches Using Magnetic Nanoparticles in Cystic Fibrosis" Magnetochemistry 6, no. 2: 25. https://doi.org/10.3390/magnetochemistry6020025
APA StyleTan, M., Reyes-Ortega, F., & Schneider-Futschik, E. K. (2020). Successes and Challenges: Inhaled Treatment Approaches Using Magnetic Nanoparticles in Cystic Fibrosis. Magnetochemistry, 6(2), 25. https://doi.org/10.3390/magnetochemistry6020025