Fluctuation Effects of Magnetohydrodynamic Micro-Vortices on Odd Chirality in Magnetoelectrolysis
Abstract
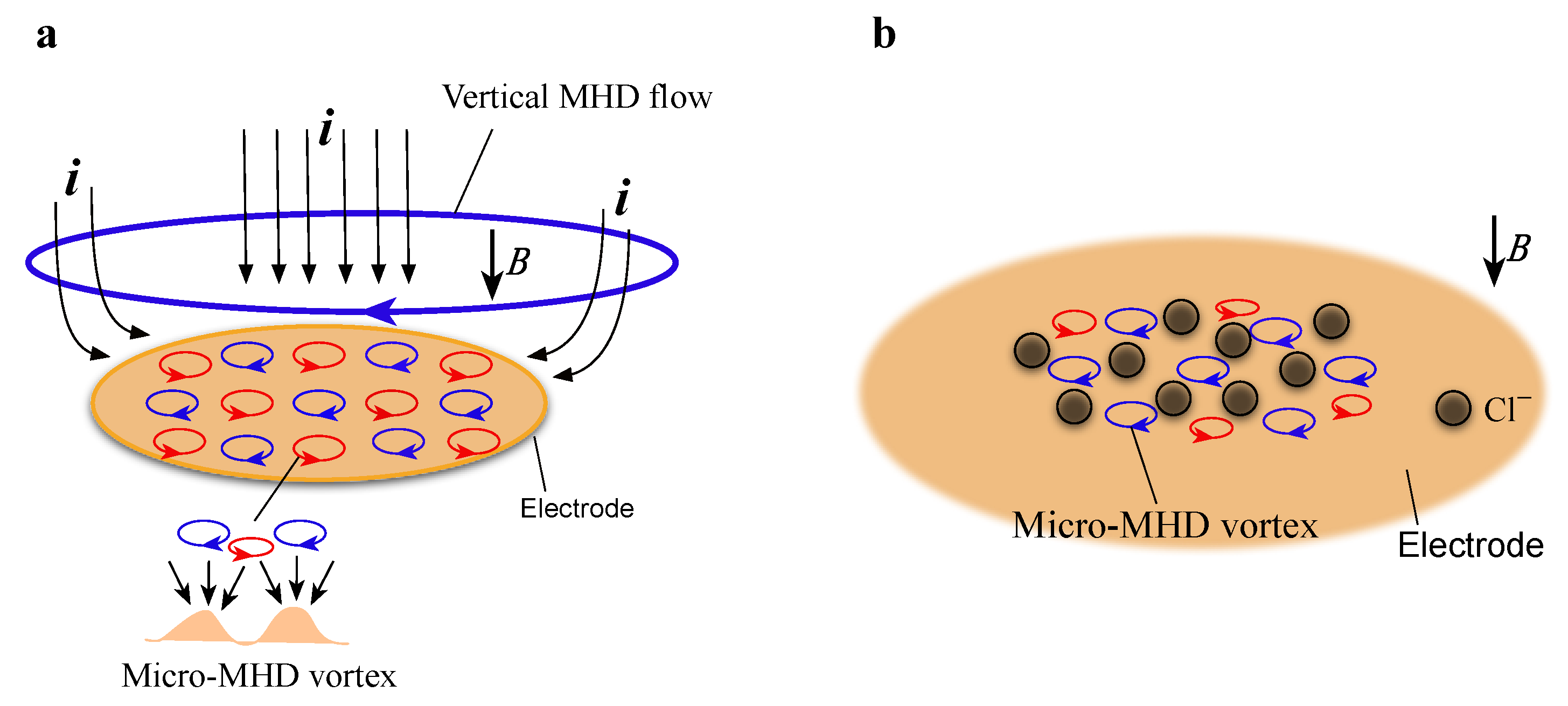
1. Introduction
2. Results and Discussion
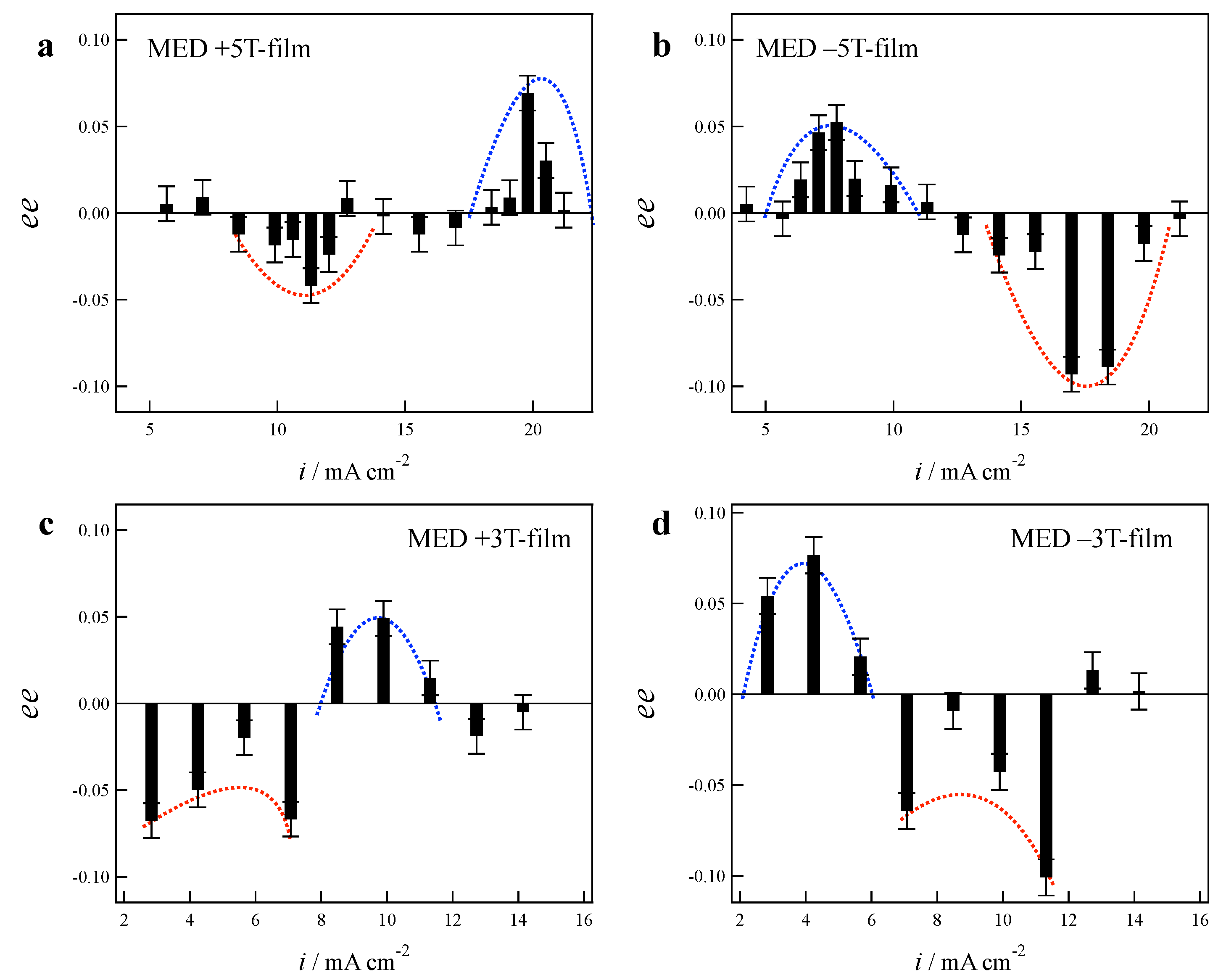
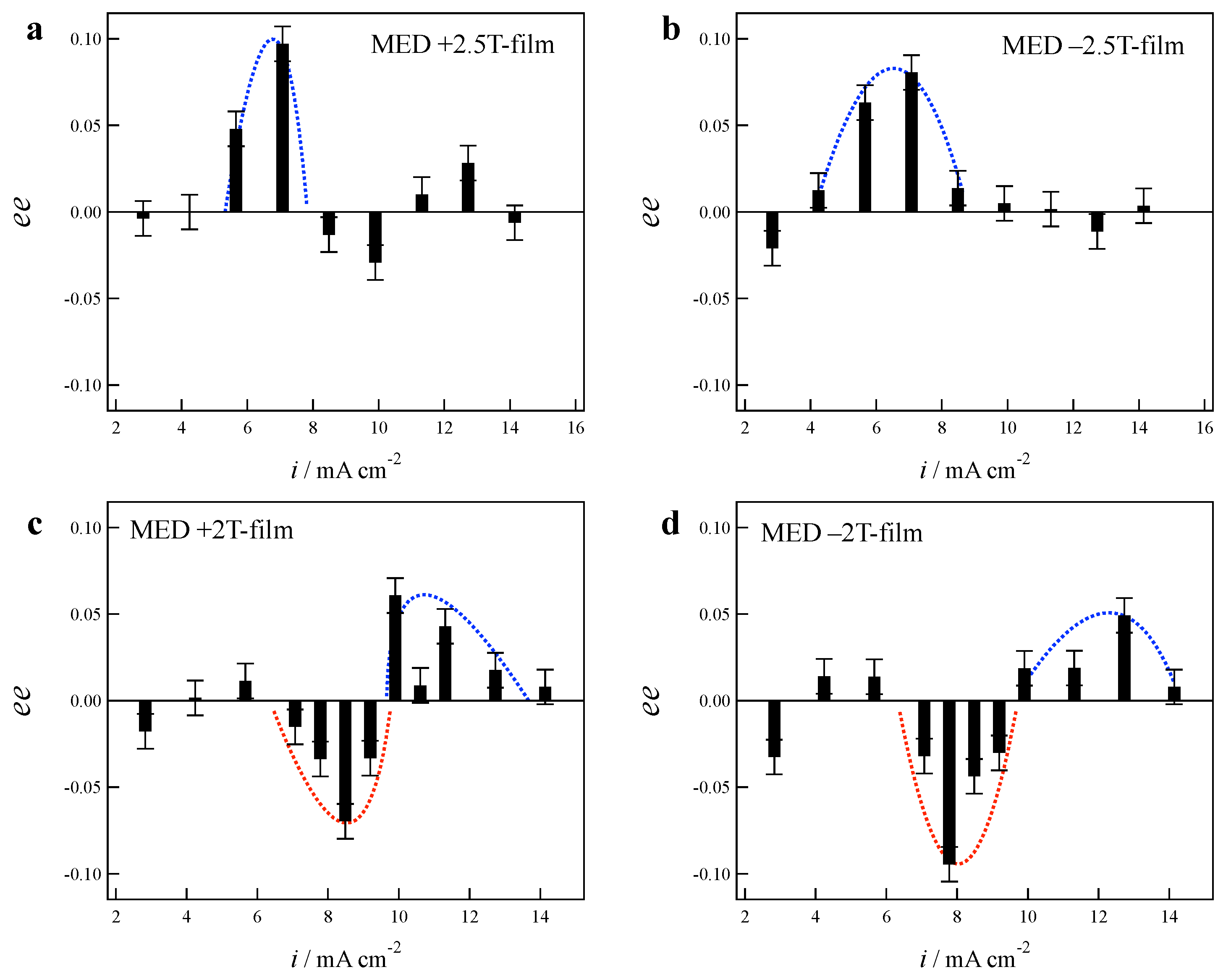
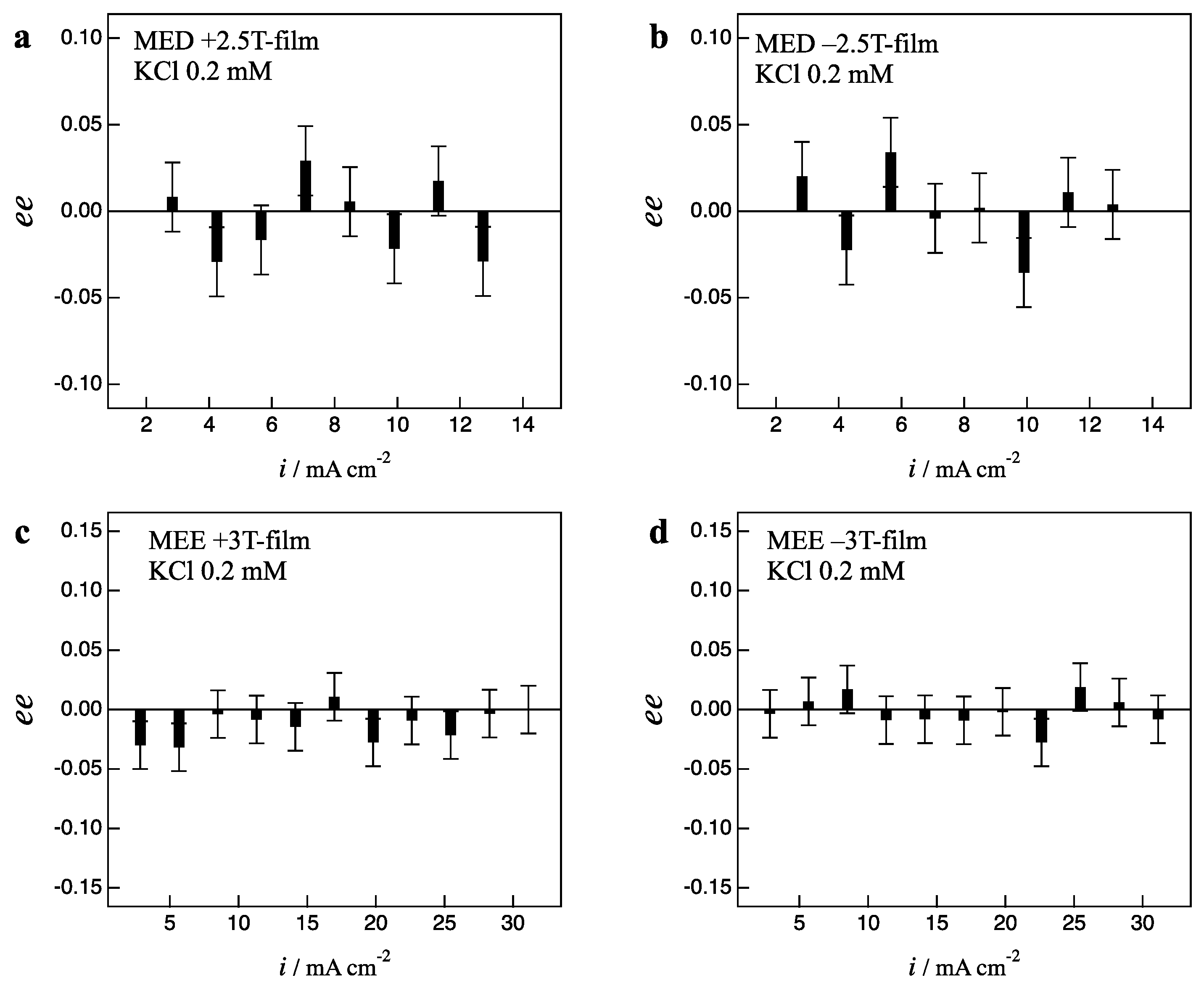
2.1. Effects of Low Magnetic Fields on MED
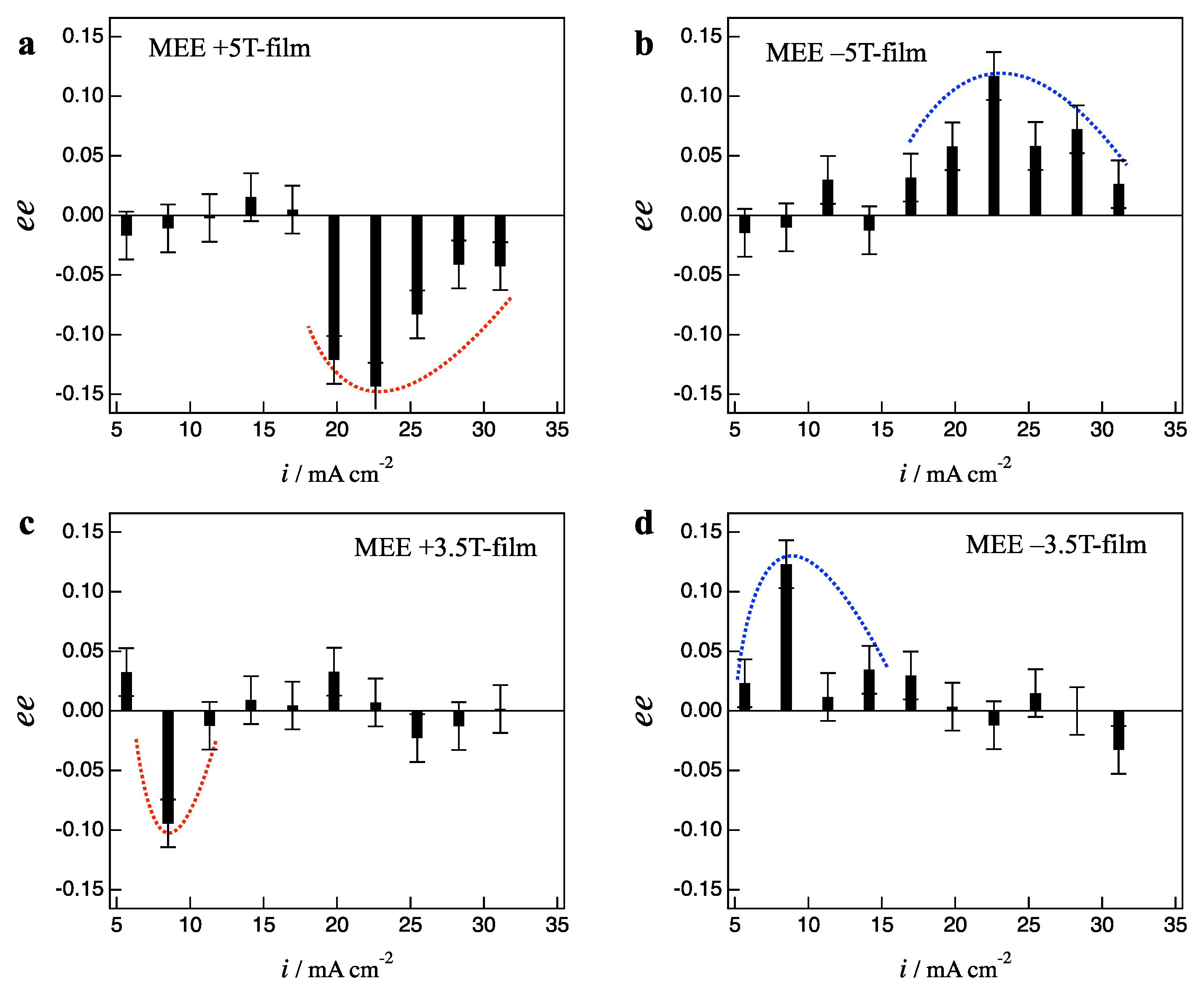
2.2. Effects of Low Magnetic Fields on MEE
2.3. Superimposed Effects of Low Magnetic Fields and Specific Adsorption
3. Materials and Methods
3.1. Electrodeposition and Electrochemical Etching
3.2. MED and MEE Procedures
3.3. Estimation of Surface Chirality
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wachterchauser, G. Before Enzyme and Templates: Theory of Surface Metabolism. Microbiol. Rev. 1988, 52, 452–484. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nakamura, R.; Kasaya, T.; Kumagai, H.; Suzuki, K.; Takai, K. Spontaneous and Widespread Electricity Generation in Natural Deep-Sea Hydrothermal Fields. Angew. Chem. Int. Ed. 2017, 56, 5725–5728. [Google Scholar] [CrossRef] [PubMed]
- Mogi, I.; Watanabe, K. Chiral Electrode Behavior of Magneto-Electrodeposited Silver Films. ISIJ Int. 2007, 47, 585–587. [Google Scholar] [CrossRef]
- Mogi, I.; Watanabe, K. Chiral Recognition of Amino Acids by Magnetoelectrodeposited Cu Film Electrodes. Int. J. Electrochem. 2011, 239637. [Google Scholar] [CrossRef]
- Mogi, I.; Morimoto, R.; Aogaki, R. Surface Chirality Effects Induced by Magnetic Fields. Curr. Opin. Electrochem. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Gazzotti, M.; Arnaboldi, S.; Grecchi, S.; Giovanardi, R.; Cannio, M.; Pasquali, L.; Giacomino, A.; Abollino, O.; Fontanesi, C. Spin-Dependent Electrochemistry: Enantio-Selectivity Driven by Chiral-Induced Spin Selectivity Effect. Electrochim. Acta 2018, 286, 271–278. [Google Scholar] [CrossRef]
- Kumar, A.; Mondal, P.C.; Fontanesi, C. Chiral Magneto-Electrochemistry. Magnetochemistry 2018, 4, 36. [Google Scholar] [CrossRef]
- Aogaki, R. Micro-MHD Effect on Electrodeposition in Vertical Magnetic Field. Magnetohydrodynamics 2003, 4, 453–460. [Google Scholar]
- Aogaki, R.; Morimoto, R. Nonequilibrium Fluctuations in Micro-MHD Effects on Electrodeposition. In Heat and Mass Transfer: Modeling and Simulation; Hossain, M., Ed.; InTech: London, UK, 2011; pp. 189–216. [Google Scholar]
- Yanson, Y.I.; Rost, M.J. Structural Accelerating Effect of Chloride on Copper Electrodeposition. Angew. Chem. Int. Ed. 2013, 52, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Sommeria, J.; Meyers, S.D.; Swinney, H.L. Laboratory Simulation of Jupiter’s Great Red Spot. Nature 1988, 331, 689–693. [Google Scholar] [CrossRef]
- Marcus, S.M. Numerical Simulation of Jupiter’s Great Red Spot. Nature 1988, 331, 693–696. [Google Scholar] [CrossRef]
- Rikken, G.L.J.A.; Folling, J.; Wyder, P. Electrical Magnetochiral Anisotropy. Phys. Rev. Lett. 2001, 87, 236602. [Google Scholar] [CrossRef] [PubMed]
- Mogi, I.; Aogaki, R.; Watanabe, K. Chiral Surface Formation of Copper Films by Magnetoelectrochemical Etching. Magnetohydrodynamics 2015, 51, 361–368. [Google Scholar] [CrossRef]
- Mogi, I.; Aogaki, R.; Takahashi, K. Chiral Symmetry Breaking in Magnetoelectrochemical Etching with Chloride Additives. Molecules 2018, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Mogi, I.; Aogaki, R.; Watanabe, K. Tailoring of Surface Chirality by Micro-Vortices and Specific Adsorption in Magnetoelectrodeposition. Bull. Chem. Soc. Jpn. 2015, 88, 1479–1485. [Google Scholar] [CrossRef]
- Luo, P.; Zhang, F.; Baldwin, R.P. Constant Potential Amperometric Detection of Underivatized Amino Acids and Peptides at A Copper Electrode. Anal. Chem. 1991, 63, 1702–1707. [Google Scholar] [CrossRef]
- Mogi, I.; Aogaki, R.; Takahashi, K. Chiral Surface Formation in Magnetoelectrolysis on Micro-Electrodes. Magnetohydrodynamics 2017, 53, 321–328. [Google Scholar] [CrossRef]
- Mogi, I.; Morimoto, R.; Aogaki, R.; Takahashi, K. Surface Chirality in Rotational Magnetoelectrodeposition of Copper Films. Magnetochemistry 2019, 5, 53. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogi, I.; Aogaki, R.; Takahashi, K. Fluctuation Effects of Magnetohydrodynamic Micro-Vortices on Odd Chirality in Magnetoelectrolysis. Magnetochemistry 2020, 6, 43. https://doi.org/10.3390/magnetochemistry6030043
Mogi I, Aogaki R, Takahashi K. Fluctuation Effects of Magnetohydrodynamic Micro-Vortices on Odd Chirality in Magnetoelectrolysis. Magnetochemistry. 2020; 6(3):43. https://doi.org/10.3390/magnetochemistry6030043
Chicago/Turabian StyleMogi, Iwao, Ryoichi Aogaki, and Kohki Takahashi. 2020. "Fluctuation Effects of Magnetohydrodynamic Micro-Vortices on Odd Chirality in Magnetoelectrolysis" Magnetochemistry 6, no. 3: 43. https://doi.org/10.3390/magnetochemistry6030043
APA StyleMogi, I., Aogaki, R., & Takahashi, K. (2020). Fluctuation Effects of Magnetohydrodynamic Micro-Vortices on Odd Chirality in Magnetoelectrolysis. Magnetochemistry, 6(3), 43. https://doi.org/10.3390/magnetochemistry6030043




