Exploring the Effects of Synthetic and Postsynthetic Grinding on the Properties of the Spin Crossover Material [Fe(atrz)3](BF4)2 (atrz = 4-Amino-4H-1,2,4-Triazole)
Abstract
:1. Introduction
2. Experimental
3. Instrumentation
4. Results and Discussion
4.1. Synthetic Grinding
4.2. PostSynthetic Grinding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanchez Costa, J. Macroscopic methods: Magnetic, optical, and calorimetric techniques. Comptes Rendus Chim. 2018, 21, 1121–1132. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Molnár, G.; Rotaru, A.; Shepherd, H.J. Pressure effect investigations on spin-crossover coordination compounds. Comptes Rendus Chim. 2018, 21, 1095–1120. [Google Scholar] [CrossRef]
- Chastanet, G.; Lorenc, M.; Bertoni, R.; Desplanches, C. Light-induced spin crossover—Solution and solid-state processes. Comptes Rendus Chim. 2018, 21, 1075–1094. [Google Scholar] [CrossRef]
- Zenere, K.A.; Duyker, S.G.; Trzop, E.; Collet, E.; Chan, B.; Doheny, P.W.; Kepert, C.J.; Neville, S.M. Increasing spin crossover cooperativity in 2D Hofmann-type materials with guest molecule removal. Chem. Sci. 2018, 9, 5623–5629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.C.; Liew, E.; Hooley, R.J. Colorimetric barbiturate sensing with hybrid spin crossover assemblies. Chem. Commun. 2014, 50, 5043–5045. [Google Scholar] [CrossRef] [PubMed]
- Tissot, A.; Kesse, X.; Giannopoulou, S.; Stenger, I.; Binet, L.; Rivière, E.; Serre, C. A spin crossover porous hybrid architecture for potential sensing applications. Chem. Commun. 2019, 55, 194–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senthil Kumar, K.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Manrique-Juárez, M.D.; Rat, S.; Salmon, L.; Molnár, G.; Quintero, C.M.; Nicu, L.; Shepherd, H.J.; Bousseksou, A. Switchable molecule-based materials for micro-and nanoscale actuating applications: Achievements and prospects. Coord. Chem. Rev. 2016, 308, 395–408. [Google Scholar] [CrossRef]
- Molnár, G.; Rat, S.; Salmon, L.; Nicolazzi, W.; Bousseksou, A. Spin crossover nanomaterials: From fundamental concepts to devices. Adv. Mater. 2018, 30, 1703862. [Google Scholar] [CrossRef]
- Molnár, G.; Salmon, L.; Nicolazzi, W.; Terki, F.; Bousseksou, A. Emerging properties and applications of spin crossover nanomaterials. J. Mater. Chem. C 2014, 2, 1360–1366. [Google Scholar] [CrossRef]
- Dugay, J.; Giménez-Marqués, M.; Kozlova, T.; Zandbergen, H.W.; Coronado, E.; Van Der Zant, H.S.J. Spin Switching in Electronic Devices Based on 2D Assemblies of Spin-Crossover Nanoparticles. Adv. Mater. 2015, 27, 1288–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and optical switching of iron(II) complexes. Angew. Chem. Int. Ed. Engl. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Matouzenko, G.S.; Bousseksou, A.; Borshch, S.A.; Perrin, M.; Zein, S.; Salmon, L.; Molnar, G.; Lecocq, S. Cooperative Spin Crossover and Order−Disorder Phenomena in a Mononuclear Compound [Fe (DAPP)(abpt)](ClO4) 2 [DAPP=[Bis (3-aminopropyl)(2-pyridylmethyl) amine], abpt= 4-Amino-3, 5-bis (pyridin-2-yl)-1, 2, 4-triazole]. Inorg. Chem. 2004, 43, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, H.; Chakraborty, S.; Saha-Dasgupta, T. Design and control of cooperativity in spin-crossover in metal–organic complexes: A theoretical overview. Inorganics 2017, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Haddad, M.S.; Federer, W.D.; Lynch, M.W.; Hendrickson, D.N. An explanation of unusual properties of spin-crossover ferric complexes. J. Am. Chem. Soc. 1980, 102, 1468–1470. [Google Scholar] [CrossRef]
- Haddad, M.S.; Federer, W.D.; Lynch, M.W.; Hendrickson, D.N. Spin-crossover ferric complexes: Unusual effects of grinding and doping solids. Inorg. Chem. 1981, 20, 131–139. [Google Scholar] [CrossRef]
- Goodwin, H.A. Spin crossover—An overall perspective. Spin Crossover Transit. Met. Compd. I 2004, 233, 59–90. [Google Scholar]
- Nieto-Castro, D.; Garcés-Pineda, F.A.; Moneo-Corcuera, A.; Pato-Doldan, B.; Gispert-Guirado, F.; Benet-Buchholz, J.; Galán-Mascarós, J.R. Effect of Mechanochemical Recrystallization on the Thermal Hysteresis of 1D FeII-triazole Spin Crossover Polymers. Inorg. Chem. 2020, 59, 7953–7959. [Google Scholar] [CrossRef]
- Roubeau, O. Triazole-based one-dimensional spin-crossover coordination polymers. Chem. A Eur. J. 2012, 18, 15230–15244. [Google Scholar] [CrossRef]
- Jonas, K.; Jean-Paul, A.; Renée, C.; Epiphane, C.; Olivier, K.; Haasnoot, J.G.; Françoise, G.; Charlotte, J.; Bousseksou, A.; Jorge, L.; et al. Spin transitions and thermal hysteresis in the molecular-based materials [Fe (Htrz) 2 (trz)](BF4) and [Fe (Htrz) 3](BF4) 2. cntdot. H2O (Htrz= 1, 2, 4-4H-triazole; trz= 1, 2, 4-triazolato). Chem. Mater. 1994, 6, 1404–1412. [Google Scholar]
- Durand, P.; Pillet, S.; Bendeif, E.-E.; Carteret, C.; Bouazaoui, M.; El Hamzaoui, H.; Capoen, B.; Salmon, L.; Hébert, S.; Ghanbaja, J.; et al. Room temperature bistability with wide thermal hysteresis in a spin crossover silica nanocomposite. J. Mater. Chem. C 2013, 1, 1933. [Google Scholar] [CrossRef]
- Goodwin, H.A. Spin Crossover—An Overall Perspective. In Spin Crossover in Transition Metal Compounds I; Springer: Berlin, Germany, 2004; pp. 1–47. [Google Scholar]
- Kahn, O.; Kröber, J.; Jay, C. Spin transition molecular materials for displays and data recording. Adv. Mater. 1992, 4, 718–728. [Google Scholar] [CrossRef]
- Varnek, V.A.; Lavrenova, L.G. Mossbauer study of the influence of ligands and anions of the second coordination sphere in Fe(II) complexes with 1,2,4-triazole and 4-amino-1,2,4-triazole on the temperature of the 1A1 ↔ 5T2 spin transitions. J. Struct. Chem. 1995, 36, 104–111. [Google Scholar] [CrossRef]
- Daro, N.; Moulet, L.; Penin, N.; Paradis, N.; Létard, J.F.; Lebraud, E.; Buffière, S.; Chastanet, G.; Guionneau, P. Spray-drying to get spin-crossover materials. Materials 2017, 10, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, O.; Martinez, C.J. Spin-transition polymers: From molecular materials toward memory devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Grosjean, A. Matériaux Polymériques 1D à Transition de Spin: Investigations Structurales Multi-Echelles. Ph.D. Thesis, L’université Bordeaux, Bordeaux, France, 2013. [Google Scholar]
- Askew, J.H.; Shepherd, H.J. Mechanochemical synthesis of cooperative spin crossover materials. Chem. Commun. 2018, 54, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askew, J.H.; Shepherd, H.J. Post-synthetic anion exchange in iron (ii) 1, 2, 4-triazole based spin crossover materials via mechanochemistry. Dalt. Trans. 2020, 49, 2966–2971. [Google Scholar] [CrossRef]
- Michalowicz, A.; Moscovici, J.; Ducourant, B.; Cracco, D.; Kahn, O. EXAFS and X-ray powder diffraction studies of the spin transition molecular materials [Fe(Htrz)2(trz)](BF4) and [Fe(Htrz)3](BF4)2.H2O (Htrz= 1, 2, 4-4H-triazole; trz= 1, 2, 4-triazolato). Chem. Mater. 1995, 7, 1833–1842. [Google Scholar] [CrossRef]
- Michalowicz, A.; Moscovici, J.; Kahn, O. Polymerie Fe(II) Spin Cross Over Compounds: XAS Structural Results. Le J. Phys. IV 2009, 7, C2-633–C2-635. [Google Scholar] [CrossRef]
- Suleimanov, I.; Sánchez Costa, J.; Molnár, G.; Salmon, L.; Bousseksou, A. The photo-thermal plasmonic effect in spin crossover@ silica–gold nanocomposites. Chem. Commun. 2014, 50, 13015–13018. [Google Scholar] [CrossRef]
- Michen, B.; Geers, C.; Vanhecke, D.; Endes, C.; Rothen-Rutishauser, B.; Balog, S.; Petri-Fink, A. Avoiding drying-artifacts in transmission electron microscopy: Characterizing the size and colloidal state of nanoparticles. Sci. Rep. 2015, 5, 9793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
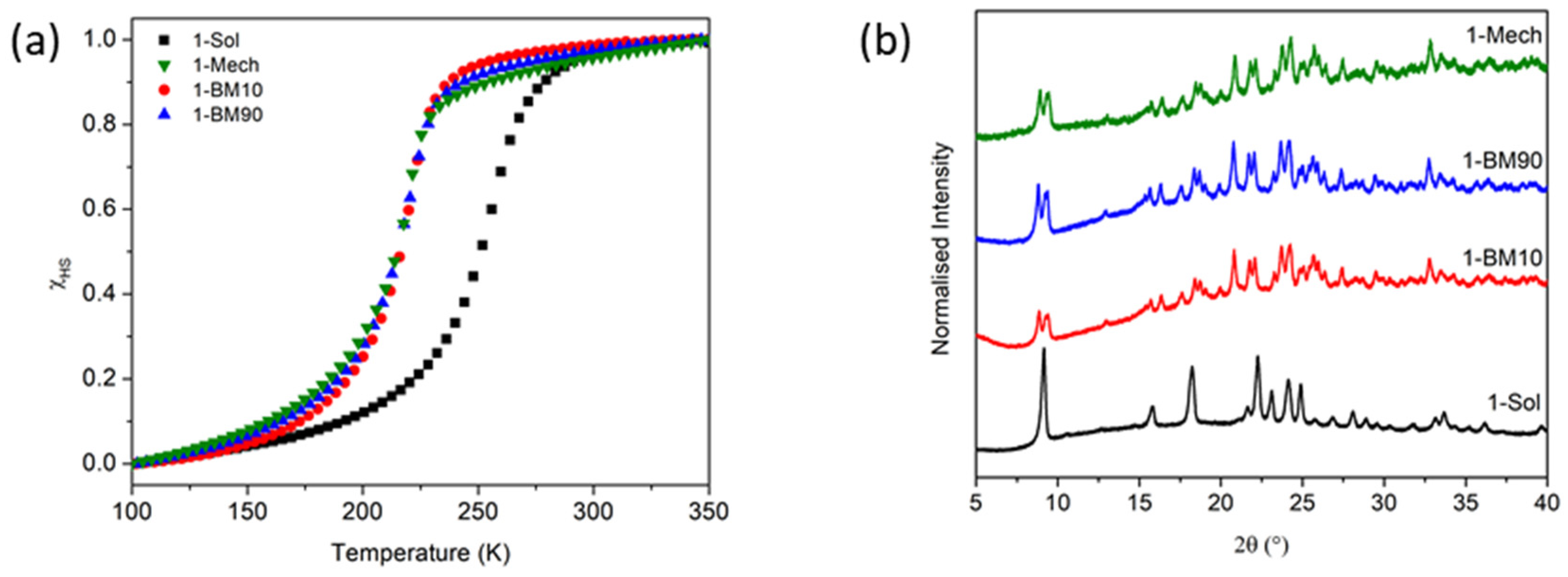
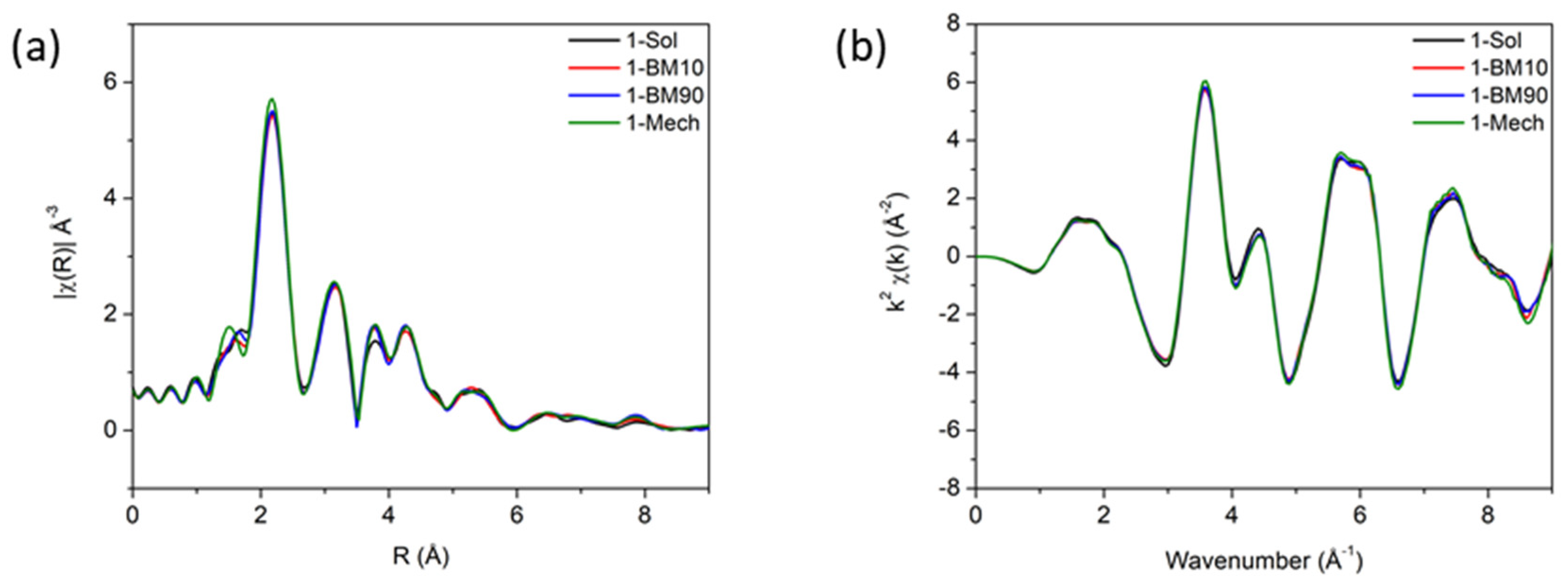
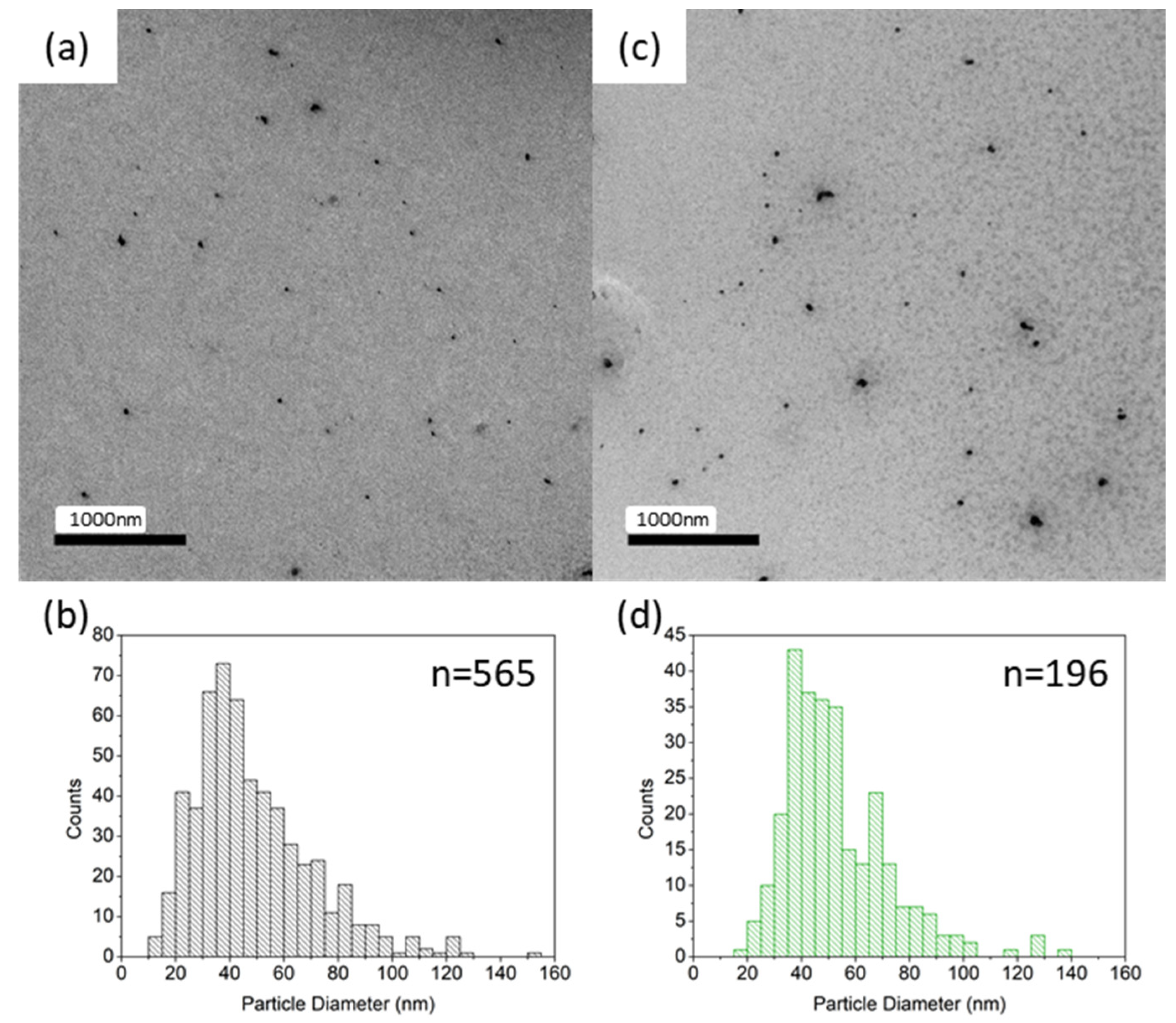



| Sample | T1/2↑ (K) | T1/2↓ (K) | ∆T (K) | ‘Smoothness’ |
|---|---|---|---|---|
| 1-Sol | 251 | 250 | 1 | 39 |
| 1-Mech | 215 | 212 | 3 | 31 |
| 1-BM10 | 216 | 212 | 4 | 30 |
| 1-BM90 | 215 | 211 | 4 | 30 |
| Sample | Mass Loss (%) | Stoichiometric Ratio of Water |
|---|---|---|
| 1-Mech | 2.3 | 0.6 |
| 1-BM10 | 0.9 | 0.2 |
| 1-BM90 | 2.6 | 0.7 |
| 1-Sol | 1.5 | 0.4 |
| 1-Sol-10 | 3.5 | 0.9 |
| 1-Sol-30 | 4.2 | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Askew, J.H.; Pickup, D.M.; Lloyd, G.O.; Chadwick, A.V.; Shepherd, H.J. Exploring the Effects of Synthetic and Postsynthetic Grinding on the Properties of the Spin Crossover Material [Fe(atrz)3](BF4)2 (atrz = 4-Amino-4H-1,2,4-Triazole). Magnetochemistry 2020, 6, 44. https://doi.org/10.3390/magnetochemistry6030044
Askew JH, Pickup DM, Lloyd GO, Chadwick AV, Shepherd HJ. Exploring the Effects of Synthetic and Postsynthetic Grinding on the Properties of the Spin Crossover Material [Fe(atrz)3](BF4)2 (atrz = 4-Amino-4H-1,2,4-Triazole). Magnetochemistry. 2020; 6(3):44. https://doi.org/10.3390/magnetochemistry6030044
Chicago/Turabian StyleAskew, Jed H., David M. Pickup, Gareth O. Lloyd, Alan V. Chadwick, and Helena J. Shepherd. 2020. "Exploring the Effects of Synthetic and Postsynthetic Grinding on the Properties of the Spin Crossover Material [Fe(atrz)3](BF4)2 (atrz = 4-Amino-4H-1,2,4-Triazole)" Magnetochemistry 6, no. 3: 44. https://doi.org/10.3390/magnetochemistry6030044
APA StyleAskew, J. H., Pickup, D. M., Lloyd, G. O., Chadwick, A. V., & Shepherd, H. J. (2020). Exploring the Effects of Synthetic and Postsynthetic Grinding on the Properties of the Spin Crossover Material [Fe(atrz)3](BF4)2 (atrz = 4-Amino-4H-1,2,4-Triazole). Magnetochemistry, 6(3), 44. https://doi.org/10.3390/magnetochemistry6030044





