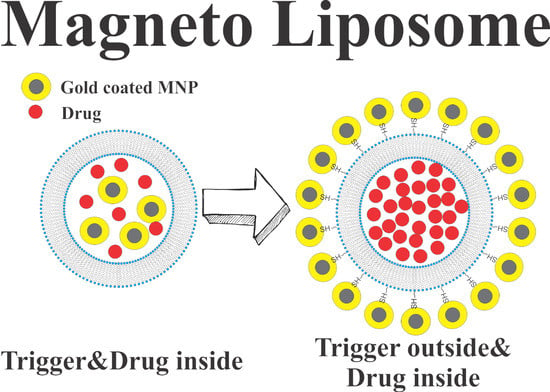
Pulse Magnetic Fields Induced Drug Release from Gold Coated Magnetic Nanoparticle Decorated Liposomes
Abstract
1. Introduction
The Role of Magnetic Field Duration in Triggering Magnetic Fields in Magneto-Liposome Experiments
2. Results
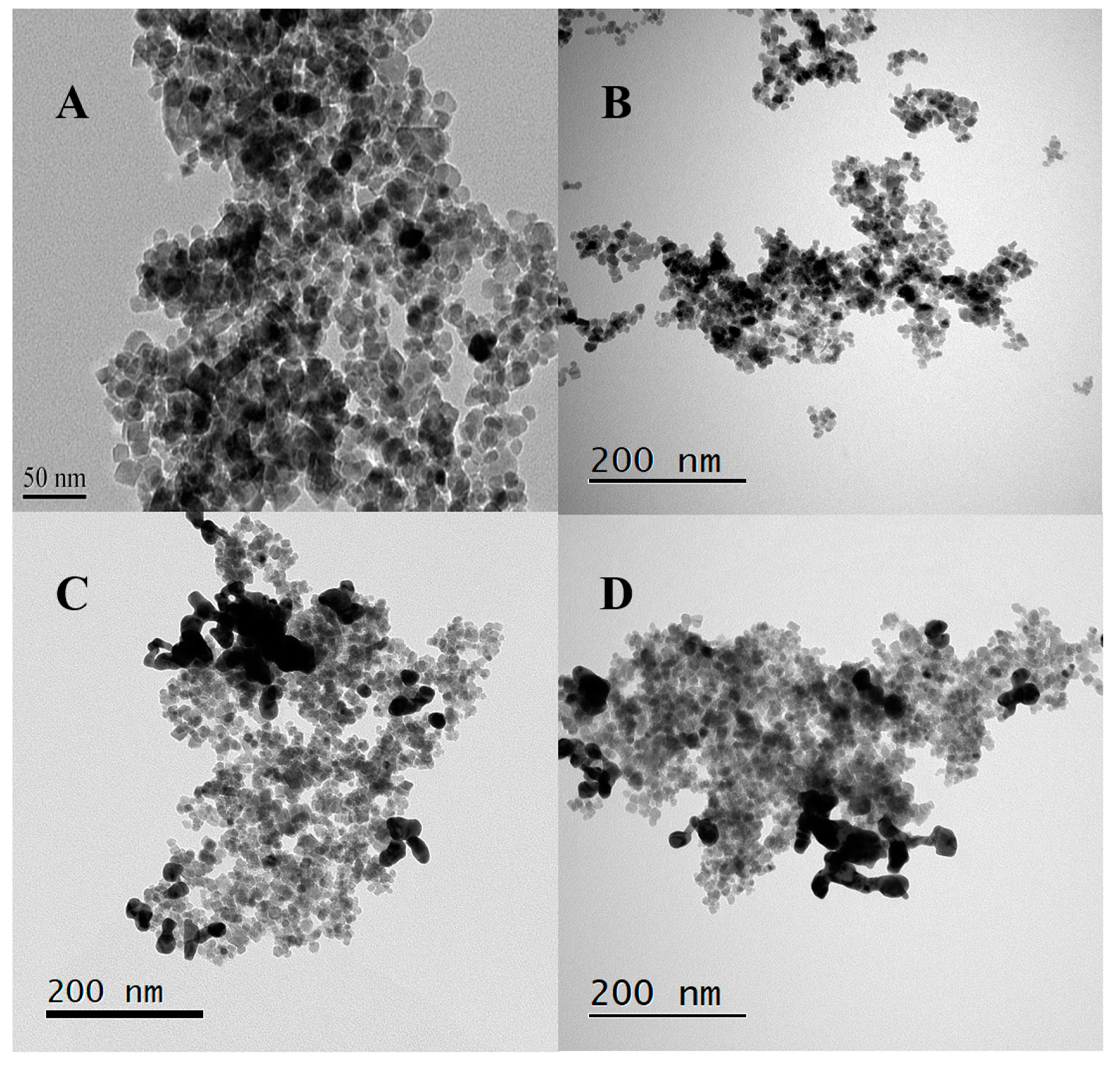
2.1. Characterizations of Nanoparticles
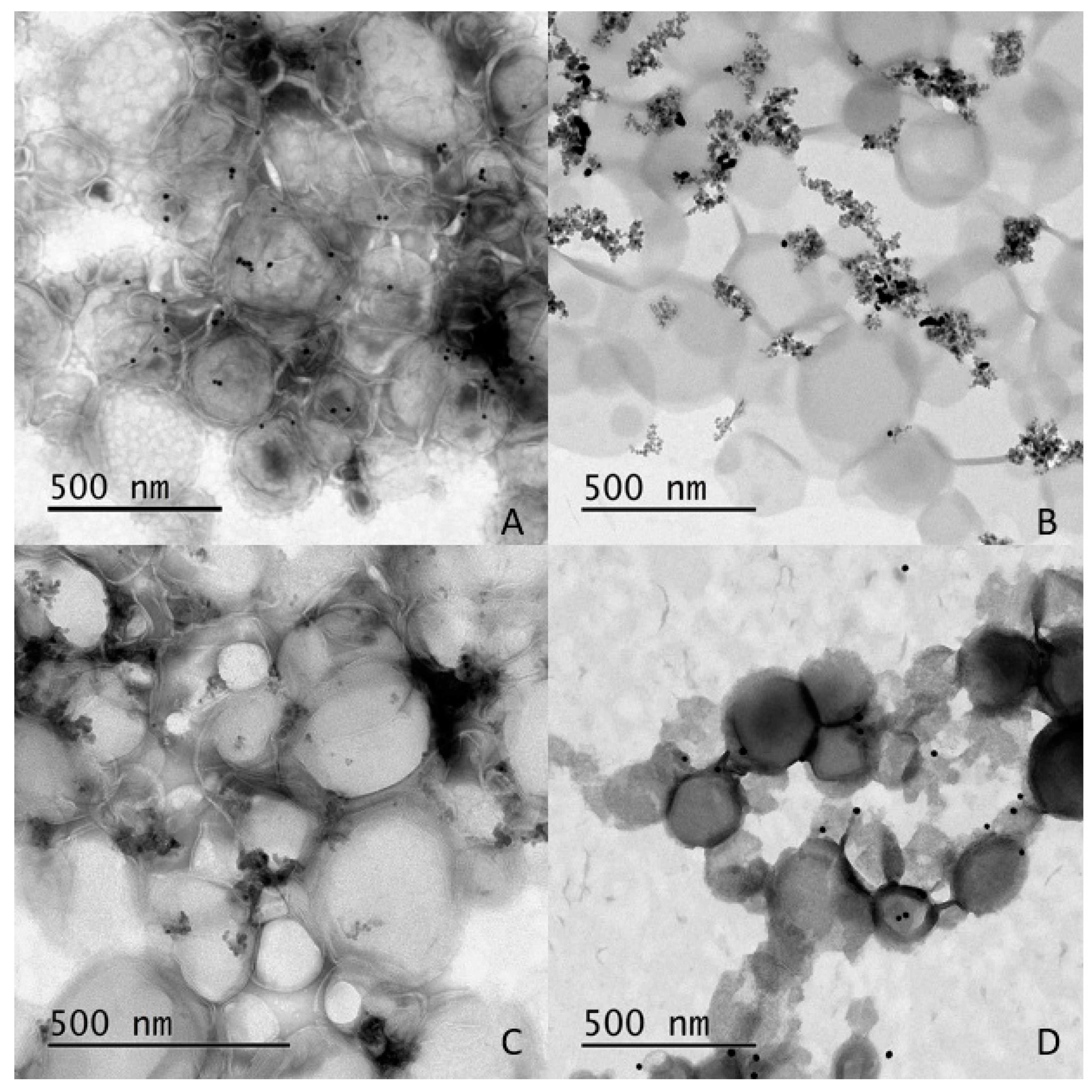
2.2. Characterization of Liposomes—Nanoparticle Composites
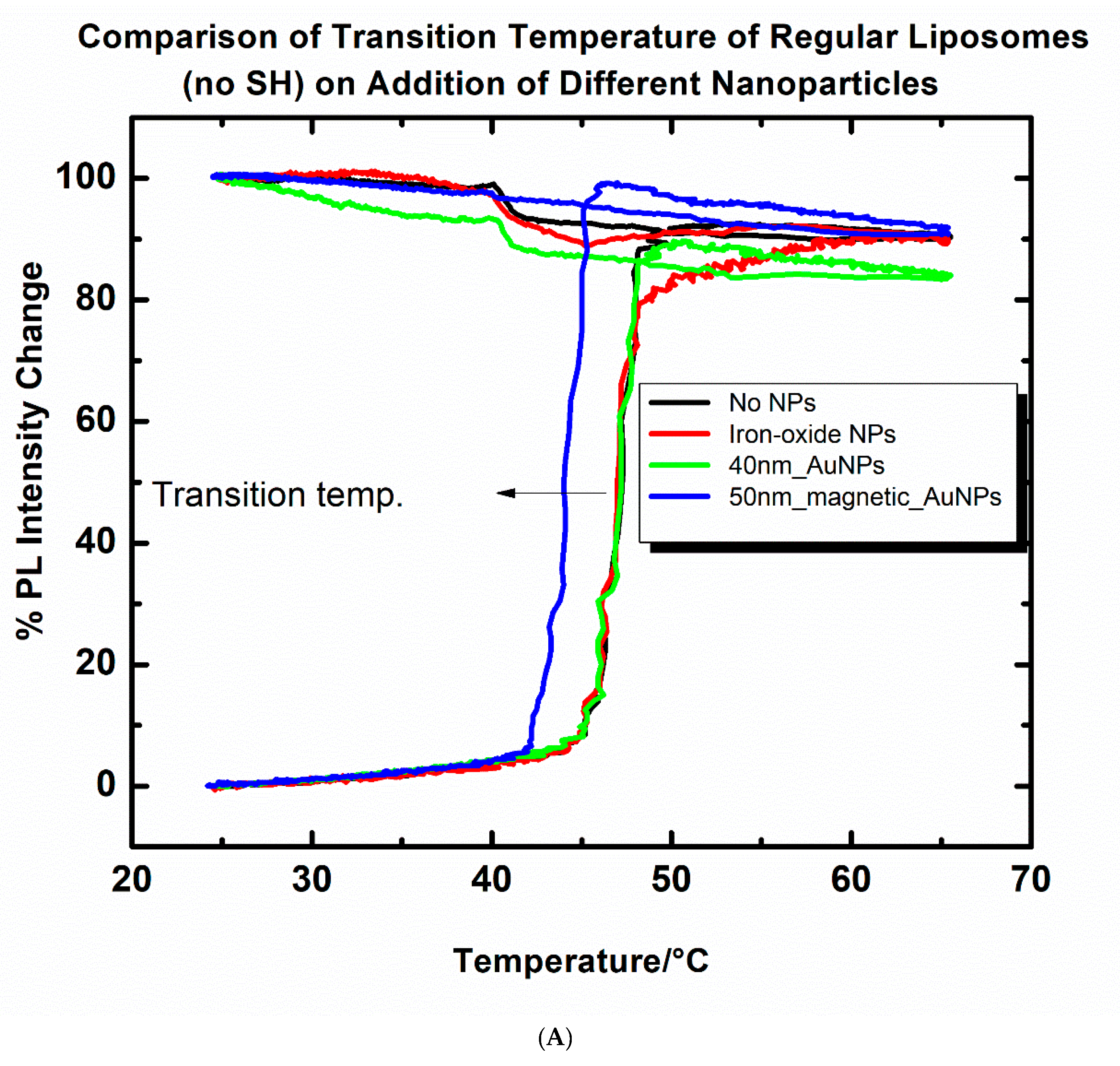
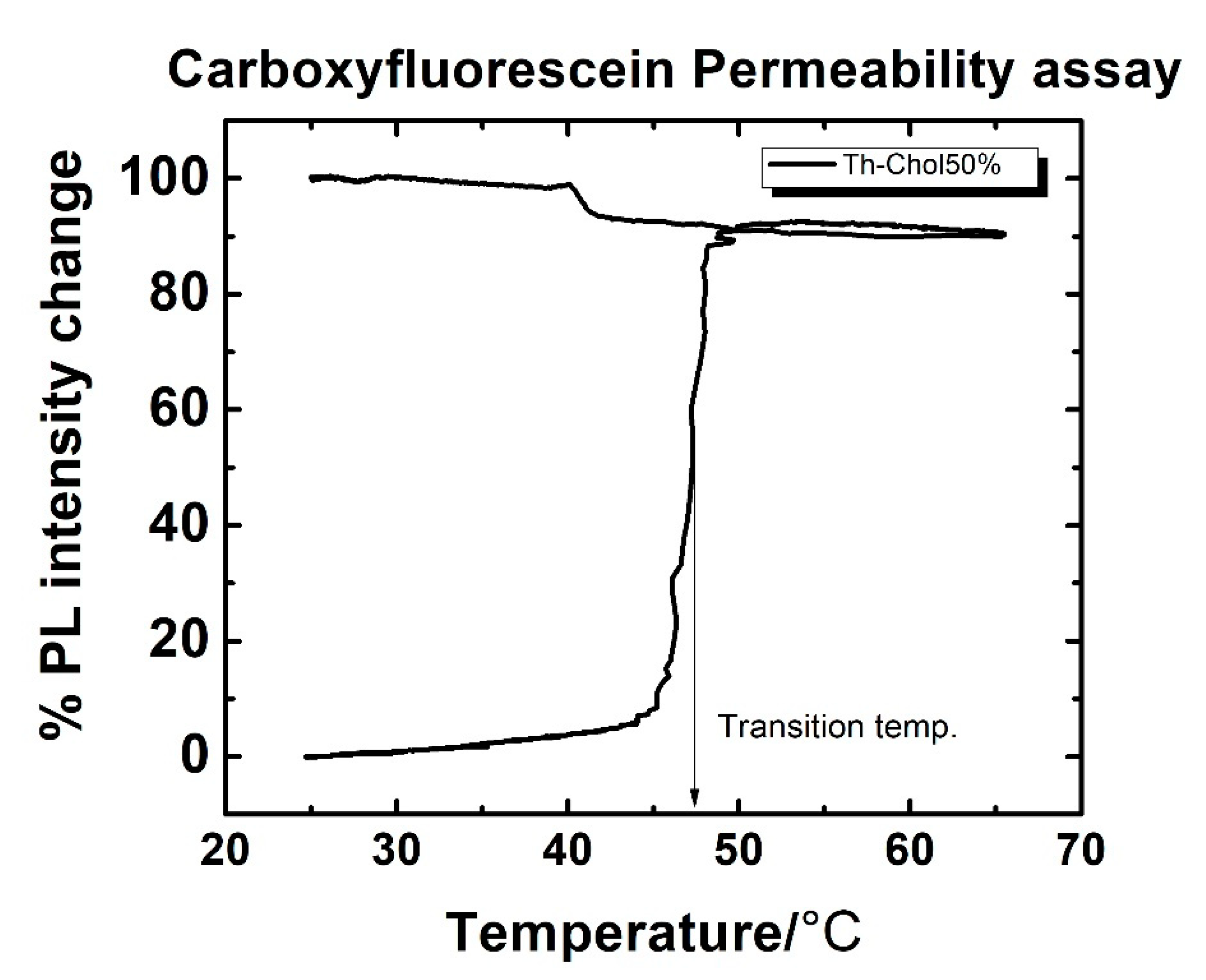
2.3. Changes in the Transition Temperature of the Liposomes upon Addition of Nanoparticles
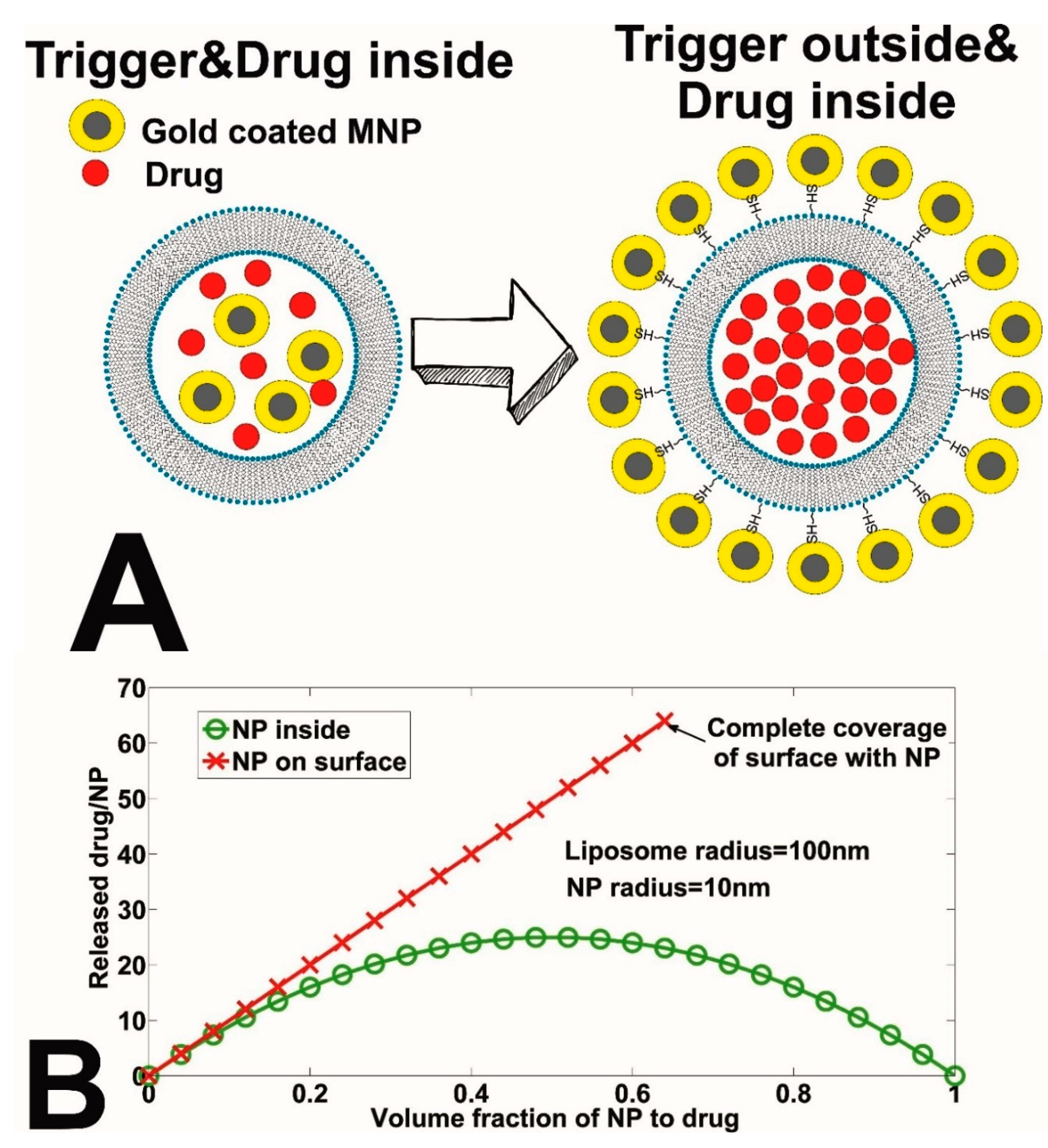
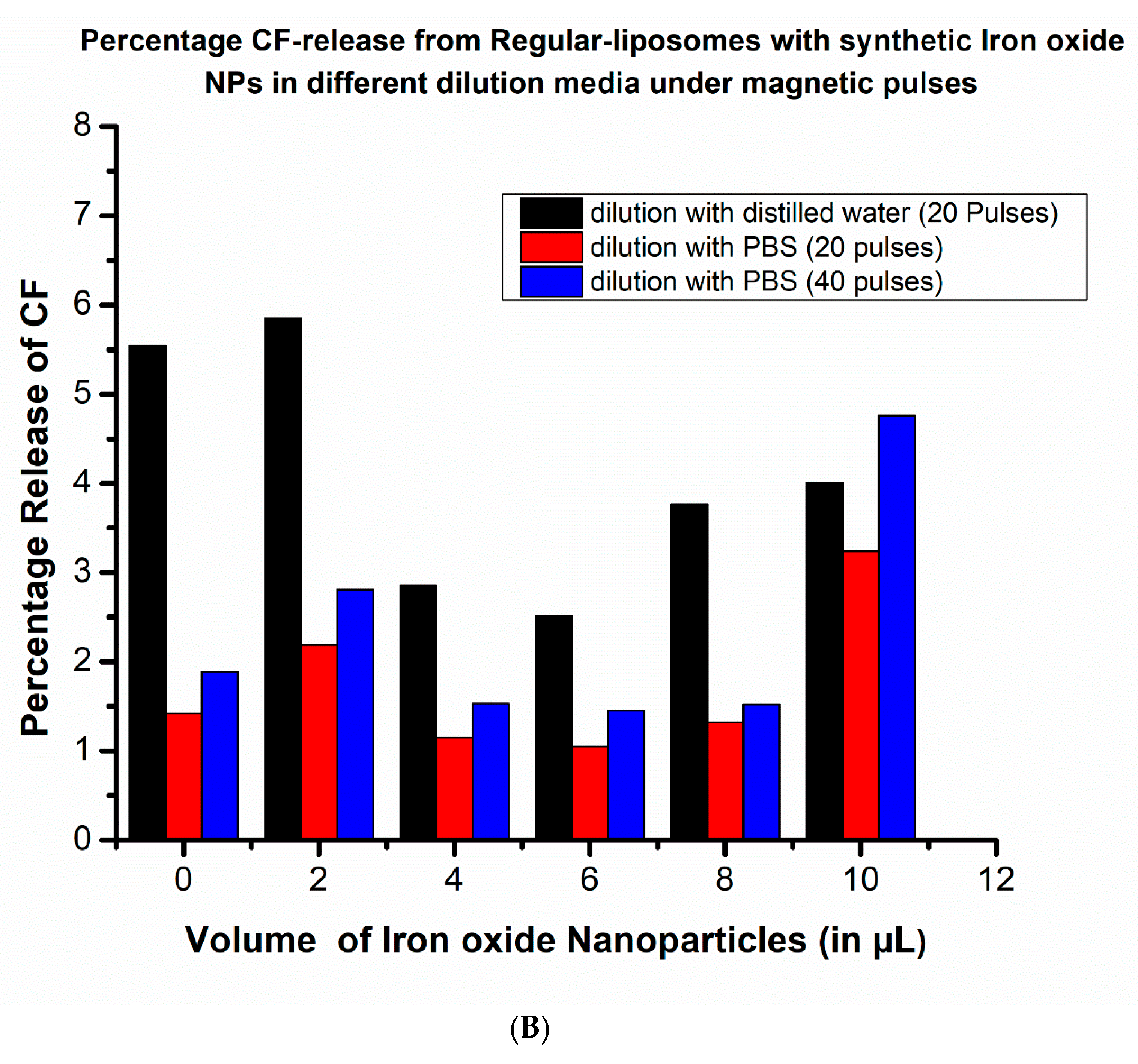
2.4. Pulsed Magnetic Field Triggered Drug Release from MNP-Coated Liposomes: The Impact of Dilution (Osmotic Pressure)
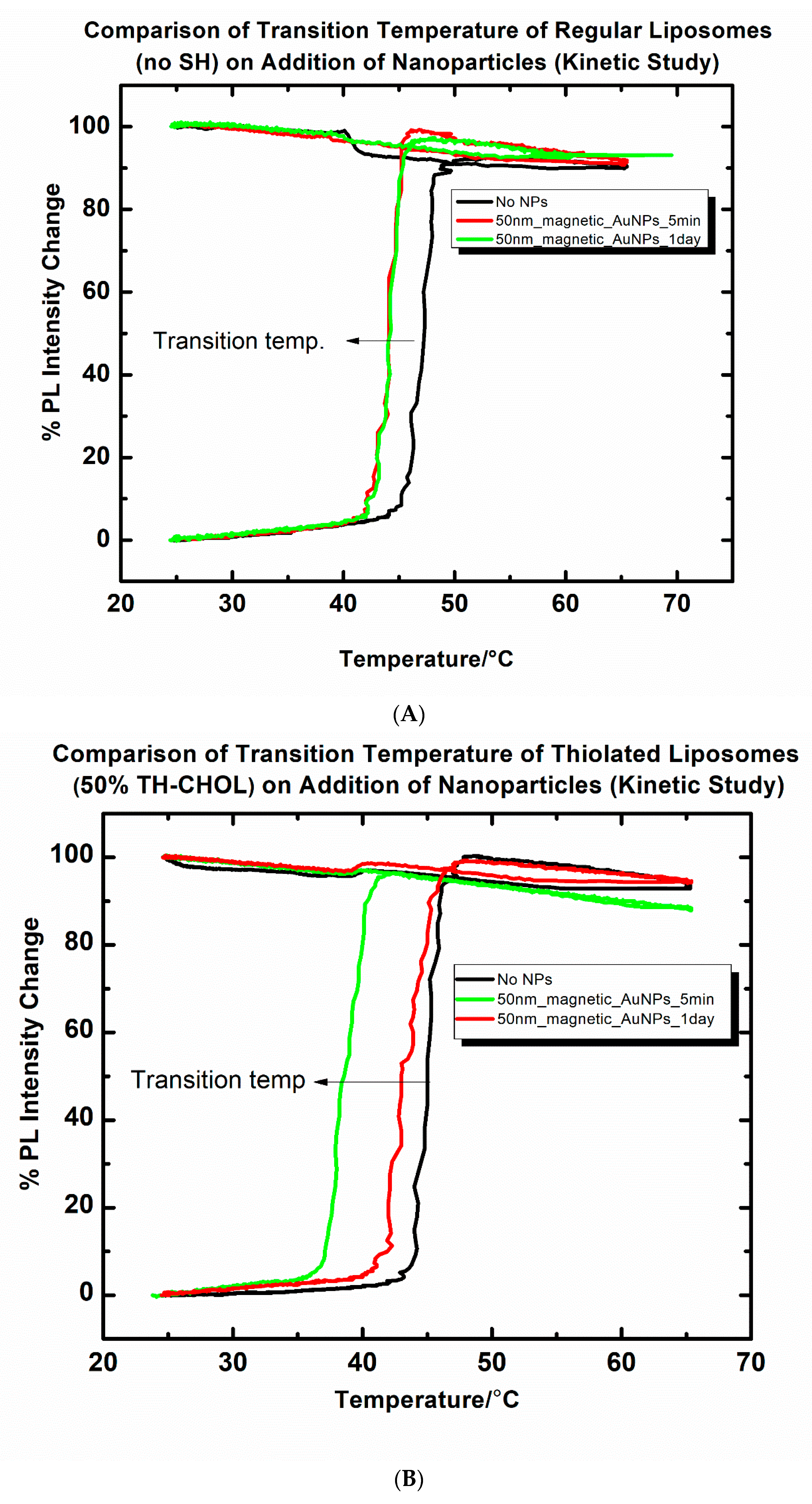
2.5. Pulsed Magnetic Field Triggered Drug Release from MNP-Coated Liposomes: Comparison of Adding Thiolated and Non-Thiolated Liposomes
3. Discussions
3.1. Effect of Nanoparticles on Liposomal Transition Temperature
3.2. Effect of Osmotic Pressure on Drug Release
3.3. Comparative Drug (Cf) Release Study from Thiolated and Non-Thiolated Liposomes with MNP Coatings under a Pulsed Magnetic Field
4. Materials and Methods
4.1. Preparation of Liposomes with Carboxyfluorescein (CF) Encapsulation
4.2. Synthesis of Iron-Oxide Core/Gold Shell Nanoparticles
4.2.1. Method I: Gold Coating on Commercial Iron Oxide Nanoparticles
4.2.2. Method II: Gold Coating on Lab Synthesized Iron Oxide Nanoparticles
- (A)
- Synthesis of iron oxide nanoparticles
- (B)
- Preparation of Au–Fe oxide composite nanoparticles
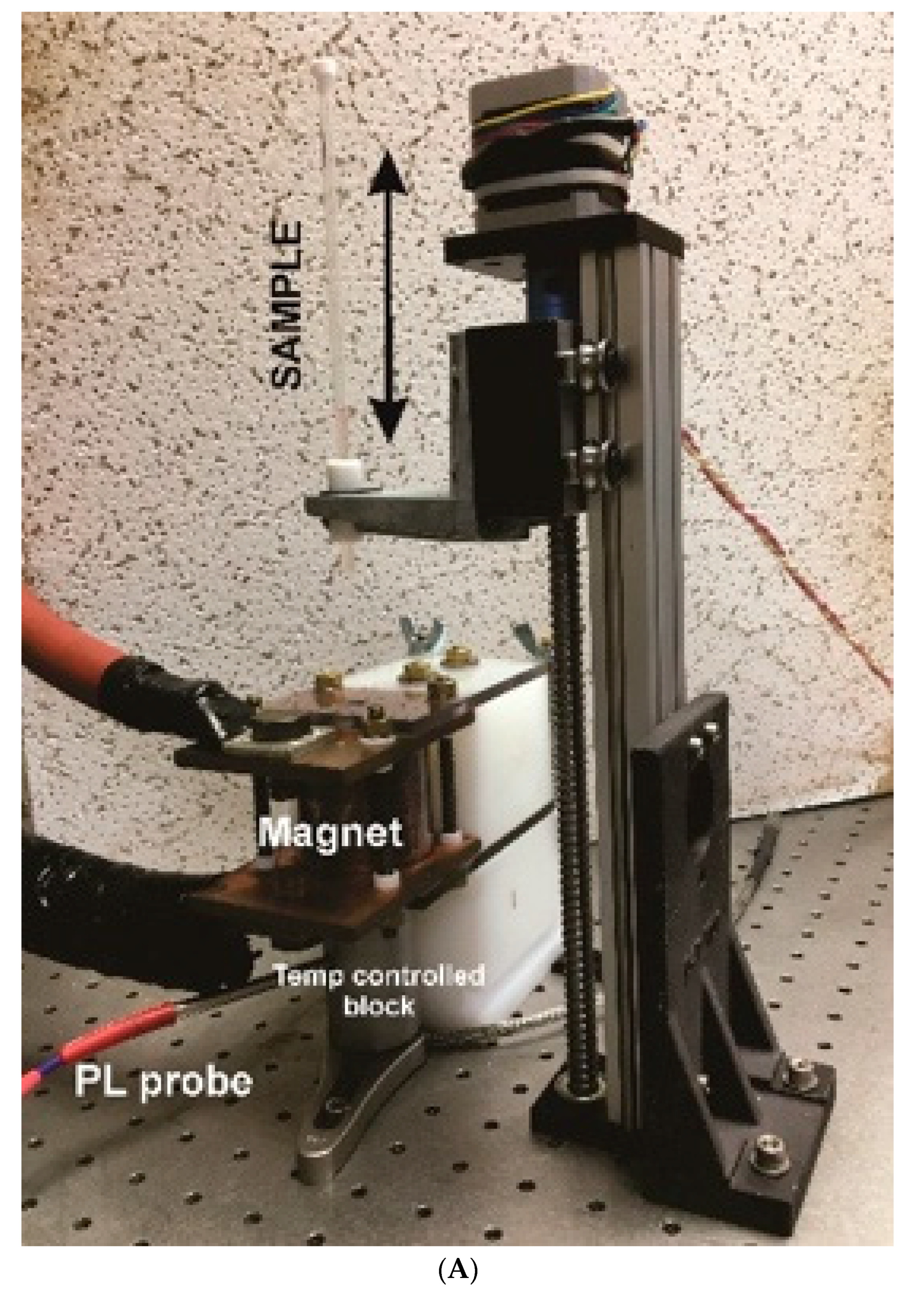
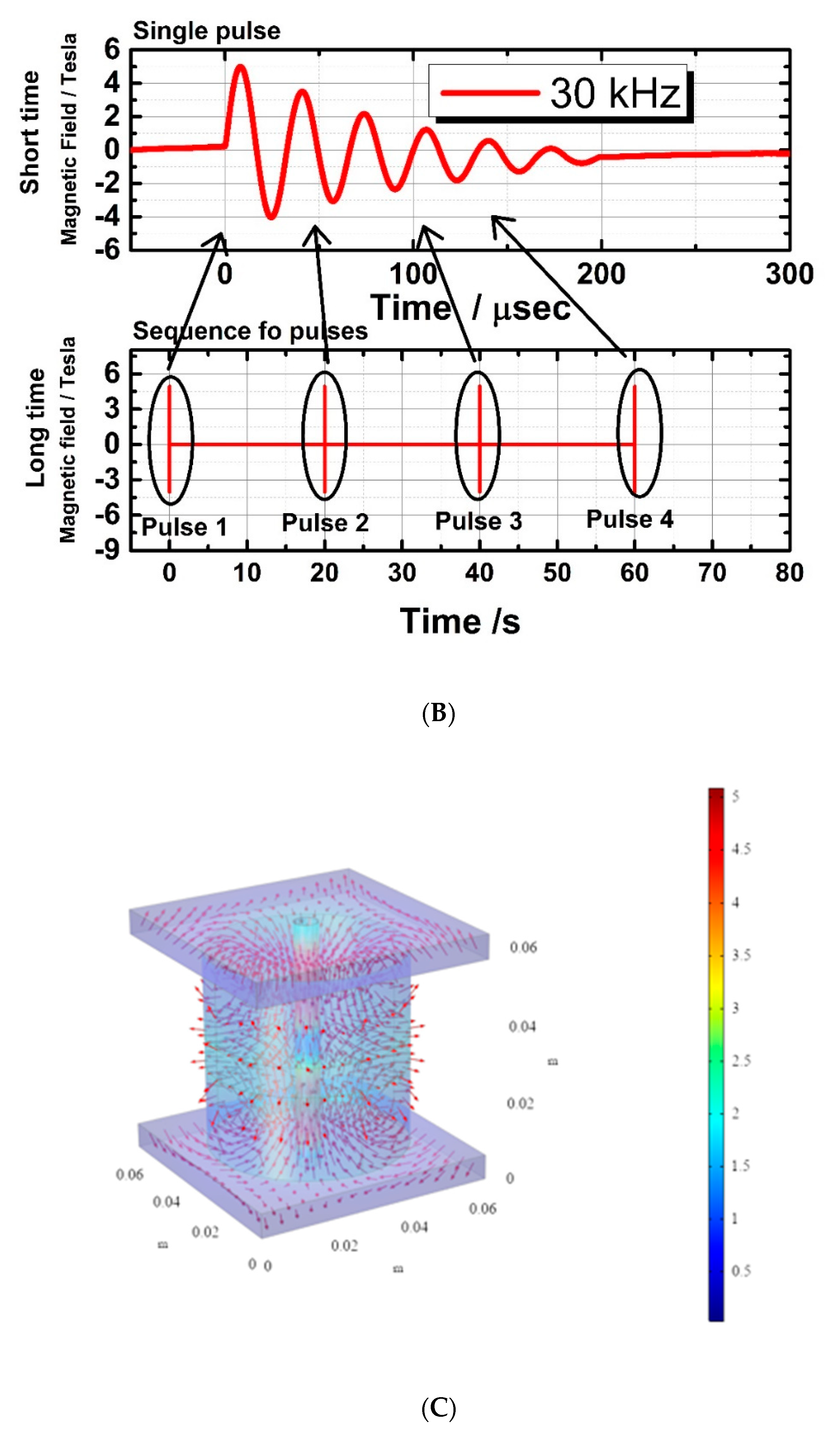
4.3. Description of Pulsed Magnetic Field for Ultrasound Generation
4.4. Carboxyfluorescein Permeability Assay
- fd = fluorescence data points;
- fi = initial background fluorescence data point;
- fmax = maximum value of fluorescence release factor;
- 1st fluorescence data point/initial background fluorescence = 1 (normalized).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Woodle, M.C. Sterically stabilized liposome therapeutics. Adv. Drug Del. Rev. 1995, 16, 249–265. [Google Scholar] [CrossRef]
- Charrois, G.J.R.; Allen, T.M. Rate of biodistribution of STEALTH liposomes to tumor and skin: Influence of liposome diameter and implications for toxicity and therapeutic activity. Biochim. Biophys. Acta 2003, 1609, 102–108. [Google Scholar] [CrossRef]
- Nappini, S.; Bombelli, F.B.; Bonini, M.; Nordèn, B.; Baglioni, P. Magnetoliposomes for controlled drug release in the presence of low-frequency magnetic field. Soft Matter 2010, 6, 154–162. [Google Scholar] [CrossRef]
- Al-Jamal, W.T.; Al-Jamal, K.T.; Bomans, P.H.; Frederik, P.M.; Kostarelos, K. Functionalized quantum-dot-liposome hybrids as multimodal nanoparticles for cancer. Small 2008, 4, 1406–1415. [Google Scholar] [CrossRef]
- Nappini, S.; Bonini, M.; Bombelli, F.B.; Pineider, F.; Sangregorio, C.; Baglioni, P.; Norden, B. Controlled drug release under a low frequency magnetic field: Effect of the citrate coating on magnetoliposomes stability. Soft Matter 2011, 7, 1025–1037. [Google Scholar] [CrossRef]
- Kloepfer, J.A.; Cohen, N.; Nadeau, J.L. FRET Between CdSe Quantum Dots in lipid vesicles and Water- and Lipid- soluble dyes. J. Phys. Chem. B 2004, 108, 17042–17049. [Google Scholar] [CrossRef]
- Nappini, S.; Bonini, M.; Ridi, F.; Baglioni, P. Structure and permeability of magnetoliposomes loaded with hydrophobic magnetic nanoparticles in the presence of a low frequency magnetic field. Soft Matter 2011, 7, 4801–4811. [Google Scholar] [CrossRef]
- Needham, D.; Dewhirstb, M.W. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv. Drug Delivery Rev. 2001, 53, 285. [Google Scholar] [CrossRef]
- Amstad, E.; Kohlbrecher, J.; Muller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered Release from Liposomes through Magnetic Actuation of Iron Oxide Nanoparticle Containing Membranes. Nano Lett. 2011, 11, 1664–1670. [Google Scholar] [CrossRef]
- Masaharu, U.; Shoshin, Y.; Isamu, H. Characteristics of the Membrane Permeability of Temperature-Sensitive Liposome. Bull. Chem. Soc. Jpn. 1991, 64, 1588–1593. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Hegh, D.Y.; Mackay, S.M.; Tan, E.W. Pulsatile release from pH triggered imidazoline switchable surfactant liposomes. RSC Adv. 2016, 6, 56859–56866. [Google Scholar] [CrossRef]
- Wu, G.; Mikhailovsky, A.; Khant, H.A.; Fu, C.; Chiu, W.; Zasadzinski, J.A. Remotely Triggered Liposome Release by Near-Infrared Light Absorption via Hollow Gold Nanoshells. J. Am. Chem. Soc. 2008, 130, 8175–8177. [Google Scholar] [CrossRef]
- Schroeder, A.; Kost, J.; Barenholz, Y. Ultrasound, liposomes, and drug delivery: Principles for using ultrasound to control the release of drugs from liposomes. Chem. Phys. Lipids 2009, 162, 1–16. [Google Scholar] [CrossRef]
- Malekar, S.A.; Sarode, A.L.; Bach II, A.C.; Bose, A.; Bothun, G.; Worthen, D.R. Radio Frequency-Activated Nanoliposomes for Controlled Combination Drug Delivery. AAPS PharmSciTech 2015, 16, 1335–1343. [Google Scholar] [CrossRef]
- Tai, L.A.; Tsai, P.J.; Wang, Y.C.; Wang, Y.J.; Lo, L.W.; Yang, C.S. Thermosensitive liposomes entrapping iron oxide nanoparticles for controllable drug release. Nanotechnology 2009, 20, 135101. [Google Scholar] [CrossRef]
- Nardoni, M.; Valle, E.D.; Liberti, M.; Relucenti, M.; Casadei, M.A.; Paolicelli, P.; Apollonio, F.; Petralito, S. Can Pulsed Electromagnetic Fields Trigger On-Demand Drug Release from High-Tm Magnetoliposomes? Nanomaterials 2018, 8, 196. [Google Scholar] [CrossRef]
- Shaghasemi, B.S.; Virk, M.M.; Reimhult, E. Optimization of Magneto-thermally Controlled Release Kinetics by Tuning of Magnetoliposome Composition and Structure. Sci. Rep. 2017, 7, 7474. [Google Scholar] [CrossRef]
- Wong, D.W.; Gan, W.L.; Liu, N.; Lew, W.S. Magneto-actuated cell apoptosis by biaxial pulsed magnetic field. Sci. Rep. 2017, 7, 10919. [Google Scholar] [CrossRef]
- Goya, G.F.; Grazu, V.; Ibarra, M.R. Magnetic Nanoparticles for Cancer Therapy. Curr. Nanosci. 2008, 4, 1–16. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Ahmed, S.E.; Moussa, H.G.; Martins, A.M.; Al-Sayah, M.H.; Husseini, G.A. Effect of pH, ultrasound frequency and power density on the release of calcein rom stealth liposomes. Eur. J. Nanomed. 2016, 8, 31–43. [Google Scholar] [CrossRef]
- Steinberg, Y.; Schroeder, A.; Talmon, Y.; Schmidt, J.; Khalfin, R.L.; Cohen, Y.; Devoisselle, J.-M.; Begu, S.; Avnir, D. Triggered Release of Aqueous Content from Liposome-Derived Sol-Gel Nanocapsules. Langmuir 2007, 23, 12024–12031. [Google Scholar] [CrossRef]
- Jozefczak, A.; Kaczmarek, K.; Hornowski, T.; Kubovcikova, M.; Rozynek, Z.; Timko, M.; Skumeil, A. Magnetic nanoparticles for enhancing the effectiveness of ultrasonic hyperthermia. Appl. Phys. Lett. 2016, 108, 263701. [Google Scholar] [CrossRef]
- Carrey, J.; Connord, V.; Respaud, M. Ultrasound generation and high-frequency motion of magnetic nanoparticles in an alternating magnetic field: Toward intracellular ultrasound therapy? Appl. Phys. Lett. 2013, 102, 232404. [Google Scholar] [CrossRef]
- Mehrmohammadi, M.; Qu, M.; Ma, L.L.; Romanovicz, D.K.; Johnston, K.P.; Sokolov, K.V.; Emelianov, S.Y. Pulsed magneto-motive ultrasound imaging to detect intracellular trafficking of magnetic nanoparticles. Nanotechnology 2011, 22, 415105. [Google Scholar] [CrossRef]
- Podaru, G.V.; Prakash, P.; Chikan, V. Magnetic Field Induced Ultrasound from Colloidal Superparamagnetic Nanoparticles. J. Phys. Chem. C 2016, 120, 2386–2391. [Google Scholar] [CrossRef]
- Hu, G.; He, B. Magnetoacoustic Imaging of Magnetic Iron Oxide Nanoparticles Embedded in Biological Tissues with Microsecond Magnetic Stimulation. Appl. Phys. Lett. 2012, 100, 013704. [Google Scholar] [CrossRef] [PubMed]
- Callen, E.; Callen, H.B. Magnetostriction Forced Magnetostriction and Anomalous Thermal Expansion in Ferromagnets. Phys. Rev. 1965, 139, A455. [Google Scholar] [CrossRef]
- Podaru, G.; Ogden, S.; Baxter, A.; Shrestha, T.; Ren, S.; Thapa, P.; Dani, R.K.; Wang, H.; Basel, M.T.; Prakash, P.; et al. Pulsed Magnetic Field Induced Fast Drug Release from Magneto Liposomes Via Ultrasound Generation. J. Phys. Chem. B 2014, 118, 11715–11722. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Nappini, S.; Fogli, S.; Castroflorio, B.; Bonini, M.; Bombelli, F.B.; Baglioni, P. Magnetic field responsive drug release from magnetoliposomes in biological fluids. J. Mater. Chem. B 2016, 4, 716–725. [Google Scholar] [CrossRef]
- Das, M.; Dahal, U.; Mesele, O.; Liang, D.; Cui, Q. Molecular Dynamics Simulation of Interaction between Functionalized Nanoparticles with Lipid Membranes: Analysis of Coarse-Grained Models. J. Phys. Chem. B 2019, 123, 10547–10561. [Google Scholar] [CrossRef]
- Chakraborty, S.; Abbasi, A.; Bothun, G.D.; Nagao, M.; Kitchens, C.L. Phospholipid Bilayer Softening Due to Hydrophobic Gold Nanoparticle Inclusions. Langmuir 2018, 34, 13416–13425. [Google Scholar] [CrossRef]
- Häffner, S.M.; Malmsten, M. Membrane interactions and antimicrobial effects of inorganic nanoparticles. Adv. Colloid Interface Sci. 2017, 248, 105–128. [Google Scholar] [CrossRef]
- Liburdy, R.P.; Tenforde, T.S.; Magin, R.L. Magnetic Field-Induced Drug Permeability in Liposome Vesicles. Radiat. Res. 1986, 108, 102–111. [Google Scholar] [CrossRef]
- Liburdy, R.P.; Tenforde, T.S. Magnetic Deformation of Phospholipid Bilayers: Effects on Liposome Shape and Solute Permeability at Prephase Transition Temperatures. J. Theor. Biol. 1988, 133, 385–396. [Google Scholar] [CrossRef]
- Liu, J.F.; Neel, N.; Dang, P.; Lamb, M.; McKenna, J.; Rodgers, L.; Litt, B.; Cheng, Z.; Tsourkas, A.; Issadore, D. Radiofrequency-Triggered Drug Release from Nanoliposomes with Millimeter-Scale Resolution Using a Superimposed Static Gating Field. Small 2018, 14, e1802563. [Google Scholar] [CrossRef]
- Hulangamuwa, W.; Acharya, B.; Chikan, V.; Rafferty, R.J. Triggering Passive Molecular Transport into Cells with a Combination of Inhomogeneous Magnetic Fields and Magnetic Nanoparticles. ACS Appl. Nano Mater. 2020, 3, 2414–2420. [Google Scholar] [CrossRef]
- Sharma, P.; Holliger, N.; Pfromm, P.H.; Liu, B.; Chikan, V. Size-Controlled Synthesis of Iron and Iron Oxide Nanoparticles by the Rapid Inductive Heating Method. ACS Omega 2020, 5, 19853–19860. [Google Scholar] [CrossRef]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic Core/Shell Fe3O4/Au and Fe3O4/Au/Ag Nanoparticles with Tunable Plasmonic Properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef]
- Lyon, J.L.; Fleming, D.A.; Stone, M.B.; Schiffer, P.; Williams, M.E. Synthesis of Fe Oxide Core/Au Shell Nanoparticles by Iterative Hydroxylamine Seeding. Nano Lett. 2004, 4, 719–723. [Google Scholar] [CrossRef]
- Brown, K.R.; Walter, D.G.; Natan, M.J. Seeding of Colloidal Au Nanoparticle Solutions. 2. Improved Control of Particle Size and Shape. Chem. Mater. 2000, 12, 306–313. [Google Scholar] [CrossRef]


| Ref | Liposome/NP Formulation | Location of Trigger | Max Release (%) | Application Time (min) | Duty Cycle of Magnet (%) | Intensity of the Magnetic Field (mT) | Unit Time Release (% Release/s) |
|---|---|---|---|---|---|---|---|
| [8] | PC/CoFe2O4 | Bilayer | 90 | 50 | 100 | 330 | 0.03 |
| [10] | DSPC/PEG/IONPs | Bilayer | 180 | 30 | 100 | - | 0.1 |
| [19] | MPPC/SPION | Bilayer | 90 | 6 | 100 | 94.5 | 0.25 |
| [18] | HSPC/Fe3O4 | Core | 20 | 180 | 9.77 | 1.5 | 0.00185 |
| [31] | DPPC/FePt | Core | 8.4 | 3.3 × 10−5 | 0.001 | 3000 | 248,000 |
| Element | Weight % | Atomic % | Uncertainty % | Detector Correction |
|---|---|---|---|---|
| C | 77.87 | 90.10 | 1.32 | 0.28 |
| O | 7.08 | 6.15 | 0.33 | 0.51 |
| Fe | 15.04 | 3.74 | 0.33 | 0.99 |
| Element | Weight % | Atomic % | Uncertainty % | Detector Correction |
|---|---|---|---|---|
| O | 22.60 | 76.61 | 2.73 | 0.51 |
| Fe | 2.98 | 2.89 | 1.38 | 0.99 |
| Au | 74.41 | 20.48 | 5.78 | 0.99 |
| Element | Weight % | Atomic % | Uncertainty % | Detector Correction |
|---|---|---|---|---|
| O | 23.36 | 75.79 | 1.10 | 0.51 |
| Fe | 6.02 | 5.59 | 0.50 | 0.99 |
| Au | 70.60 | 18.60 | 2.40 | 0.99 |
| Volumes of NPs (µL) | Commercial Iron Oxide NPs | Gold coated Commercial IONPs | Synthetic Iron Oxide | Gold Coated Synthetic IONPs |
|---|---|---|---|---|
| 0 | 5.5% | 5.2% | 5.0% | 5.4% |
| 2 | 2.1% | 3.4% | 5.8% | 2.7% |
| 4 | 2.6% | 10.8% | 2.9% | 3.0% |
| 6 | 1.2% | 9.8% | 2.5% | 5.8% |
| 8 | 1.3% | 9.3% | 3.7% | 2.8% |
| 10 | 0.9% | 6.3% | 4.0% | 3.6% |
| Volumes of NPs (µL) | Commercial Iron Oxide NPs | Gold coated Commercial IO NPs | Synthetic Iron Oxide | Gold Coated Synthetic IO NPs |
|---|---|---|---|---|
| 0 | 4.8% | 4.5% | 4.7% | 4.6% |
| 2 | 5.0% | 7.5% | 10.5% | 6.5% |
| 4 | 3.6% | 14.2% | 6.6% | 7.5% |
| 6 | 9.5% | 17.6% | 7.5% | 9.9% |
| 8 | 3.9% | 20.5% | 5.6% | 7.6% |
| 10 | 3.8% | 14.6% | 7.4% | 6.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acharya, B.; Chikan, V. Pulse Magnetic Fields Induced Drug Release from Gold Coated Magnetic Nanoparticle Decorated Liposomes. Magnetochemistry 2020, 6, 52. https://doi.org/10.3390/magnetochemistry6040052
Acharya B, Chikan V. Pulse Magnetic Fields Induced Drug Release from Gold Coated Magnetic Nanoparticle Decorated Liposomes. Magnetochemistry. 2020; 6(4):52. https://doi.org/10.3390/magnetochemistry6040052
Chicago/Turabian StyleAcharya, Basanta, and Viktor Chikan. 2020. "Pulse Magnetic Fields Induced Drug Release from Gold Coated Magnetic Nanoparticle Decorated Liposomes" Magnetochemistry 6, no. 4: 52. https://doi.org/10.3390/magnetochemistry6040052
APA StyleAcharya, B., & Chikan, V. (2020). Pulse Magnetic Fields Induced Drug Release from Gold Coated Magnetic Nanoparticle Decorated Liposomes. Magnetochemistry, 6(4), 52. https://doi.org/10.3390/magnetochemistry6040052