Electrocaloric Cooling: A Review of the Thermodynamic Cycles, Materials, Models, and Devices
Abstract
1. Introduction
- Section 2 is devoted to the thermodynamics of the electrocaloric effect and to the possible thermodynamic cycles that an electrocaloric device for cooling or heat pumping could be based on.
- In Section 3, a wide overview of the materials showing an electrocaloric effect is provided. The electrocaloric materials are discussed and reviewed based on multiple possibilities of classification.
- Section 4 aims to underline the importance of numerical modelling to opportunely tune the working conditions to operate with optimized device, in terms of energy performances.
- Section 5 provides a review of the experimental prototypes of electrocaloric coolers built up to now.
- In Section 6, the main conclusions are drawn and an analysis of the future perspectives for the electrocaloric research is done.
2. Thermodynamics and Cycles
2.1. Thermodynamics of the Electrocaloric Effect
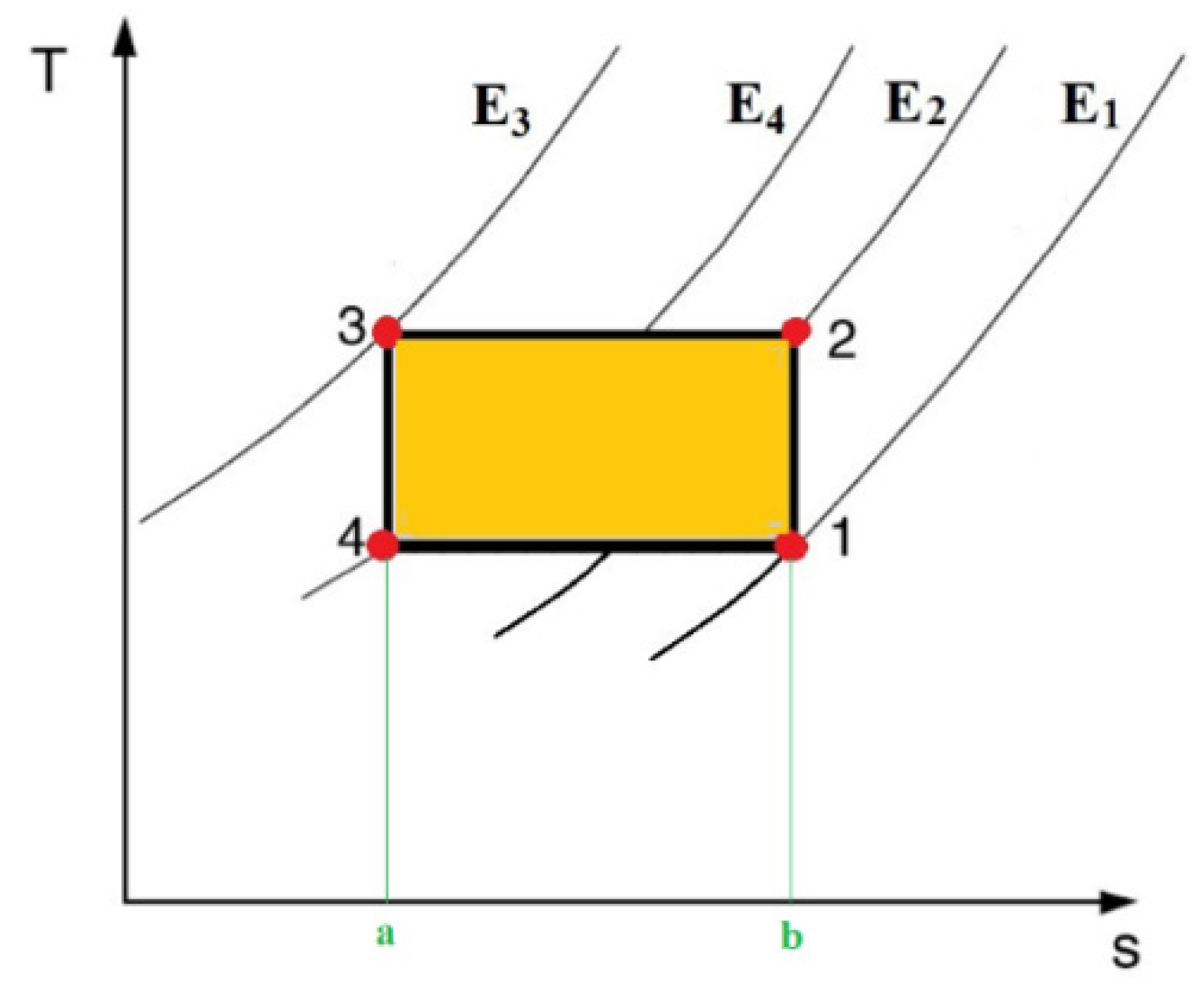
2.2. Thermodynamic Cycles
2.2.1. Carnot Cycle
2.2.2. Brayton Cycle
2.2.3. Ericsson Cycle
2.2.4. Stirling Cycle
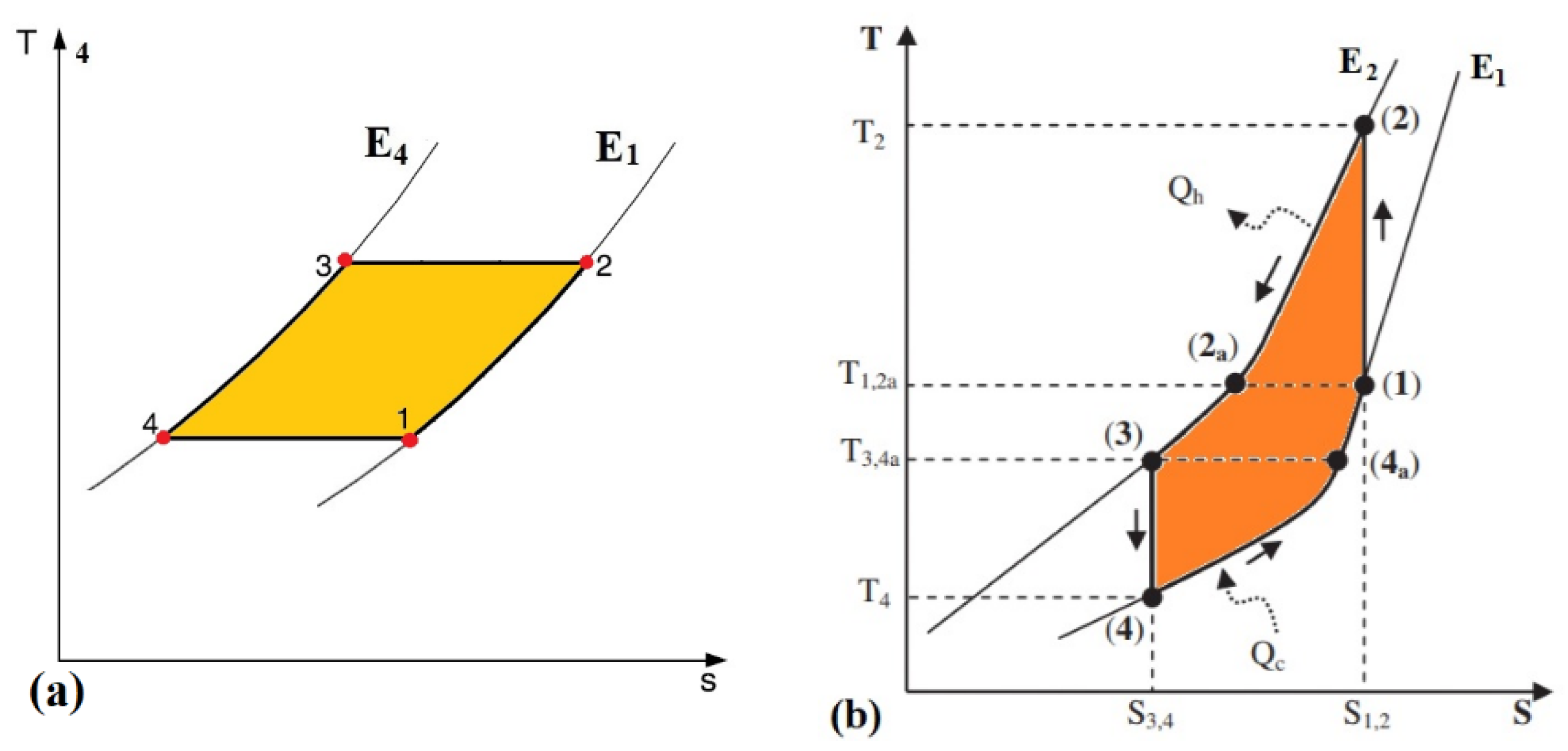
2.3. The Concept of Active Regeneration and the Active Electrocaloric Regenerative Refrigeration (AER) Cycle
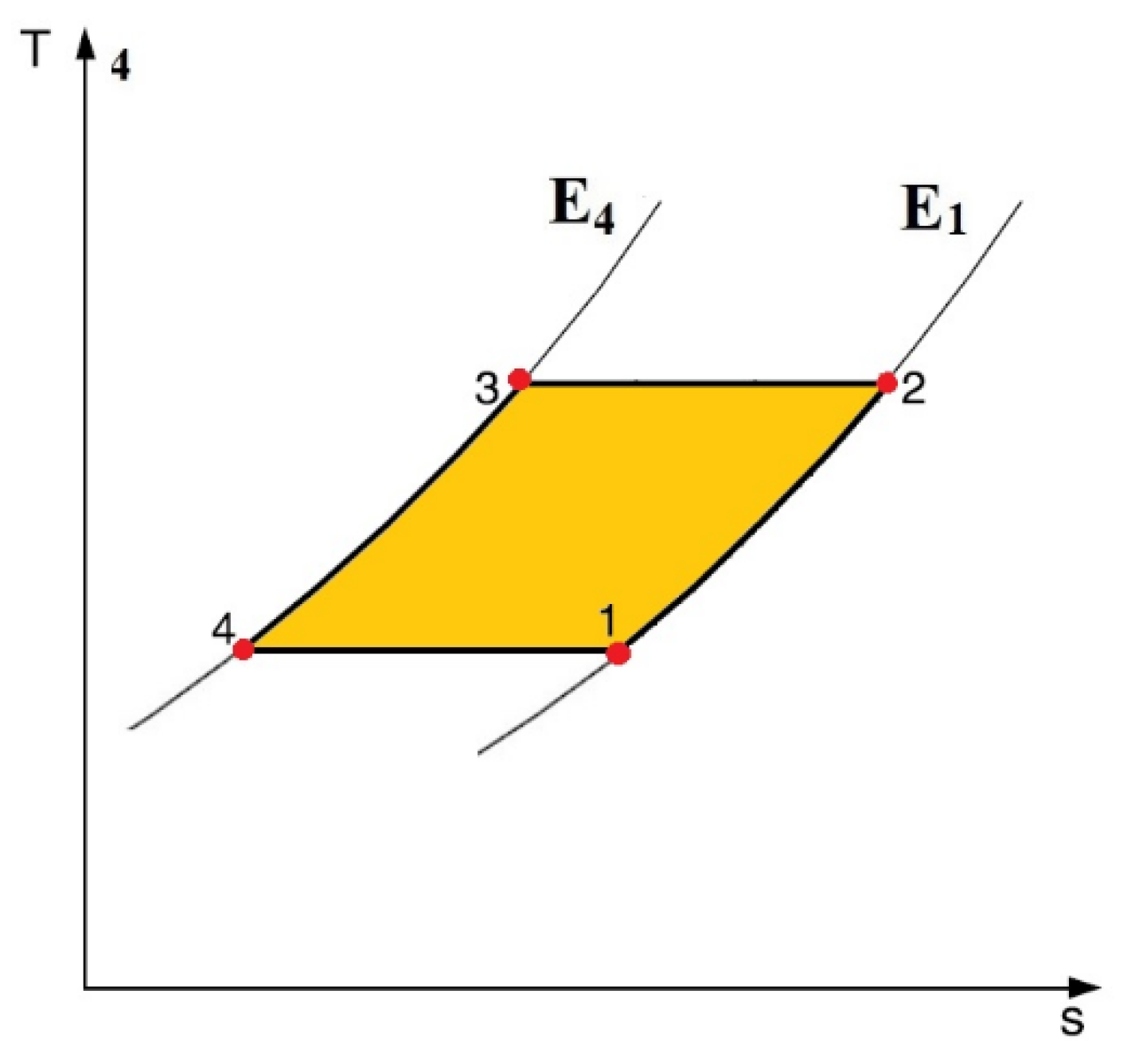
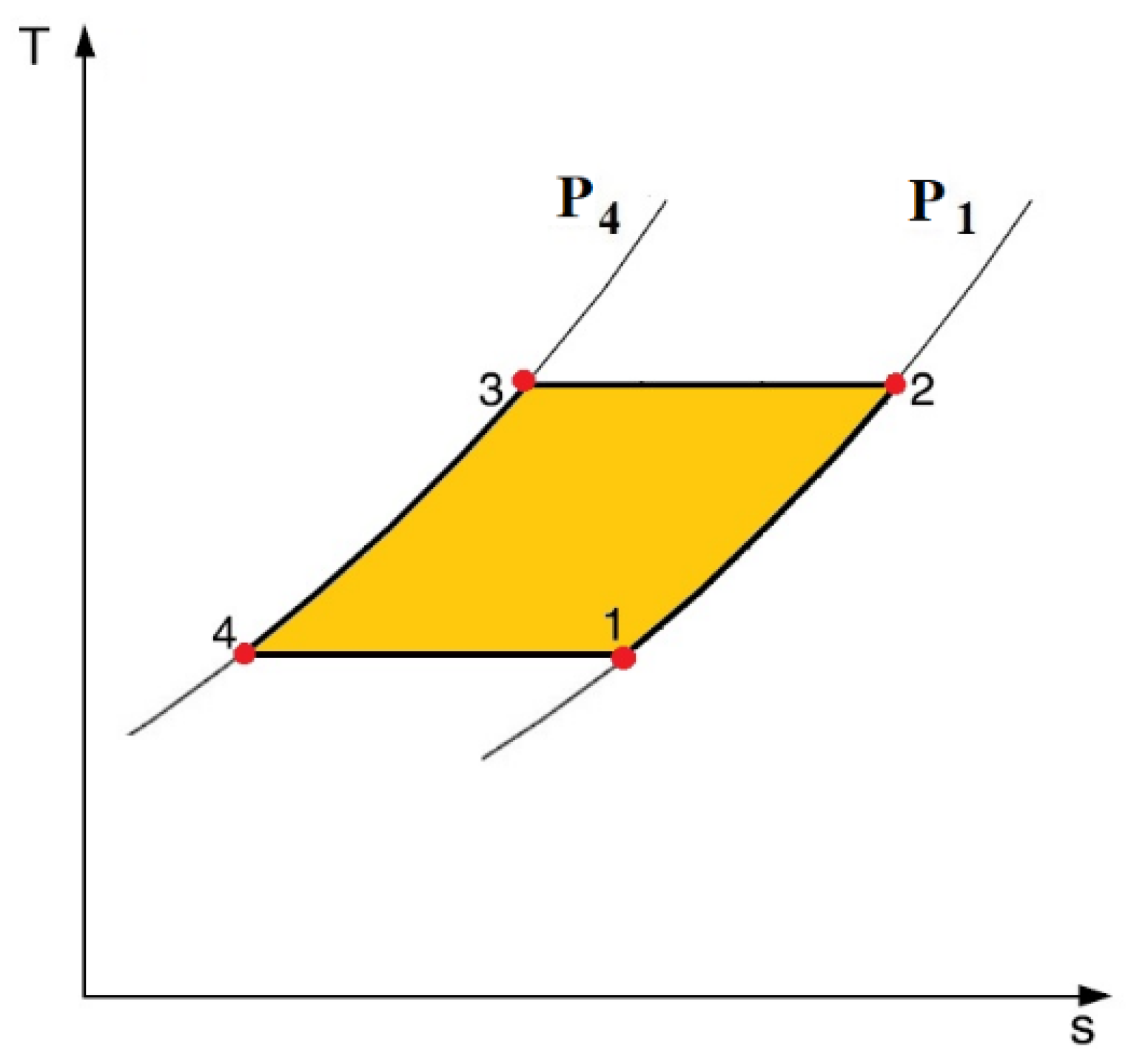
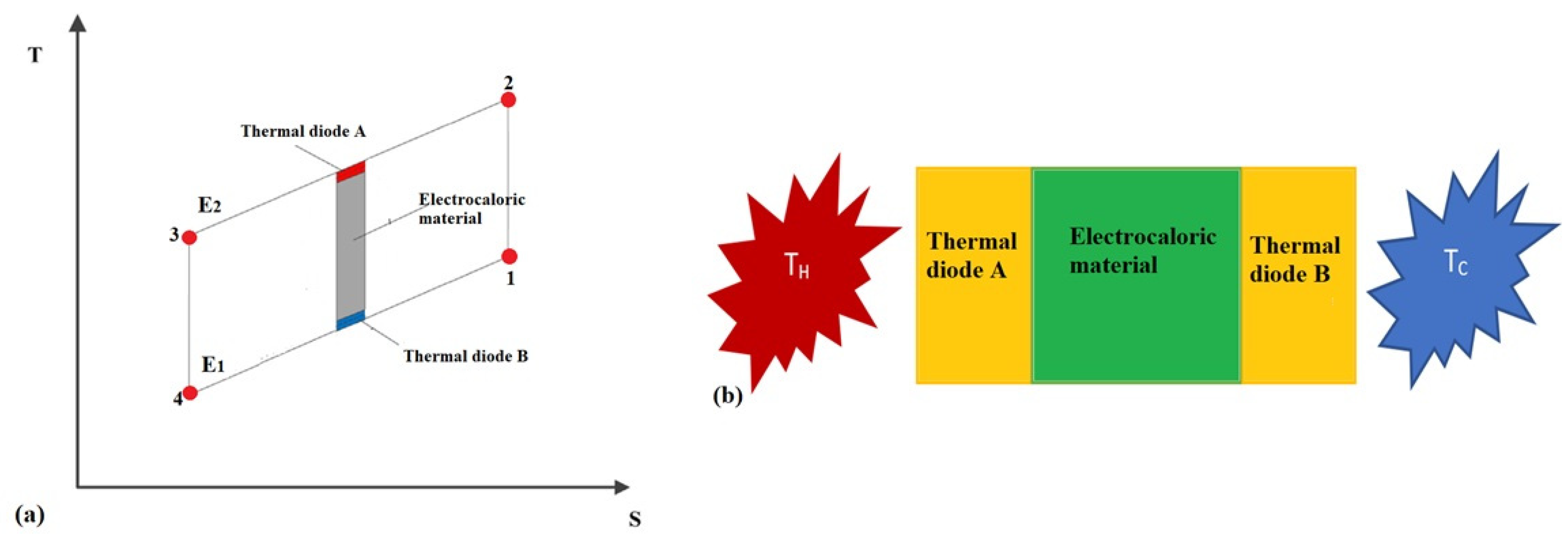
2.4. Thermal Diodes-Based Electrocaloric Cooler
3. Materials
- The detection of a pronounced electrocaloric effect at room temperature, under relatively small intensities of the electric field;
- To be toxic-free and chemically stable;
- To present low economic costs;
- The resistance to corrosion (by the heat transfer fluid or by the contact with other materials);
- The easiness to be reproduced (in terms of the number of composing elements, the compatibility with large-scale production);
- To show slight thermal hysteresis;
- To present little values of specific heat to enhance the adiabatic temperature changes as well as a rapid response, in terms of electrocaloric effect, to the electric field variations;
- The possibility of being polarized by high electric fields (indeed electrical robustness to avoid the breakdown phenomenon);
- Small electrical conductivity to prevent the instauration of leakage currents that provoke the undesired heating (due to Joule heating), which could conflict with the cooling phases of the cycle and high thermal conductivity to efficiently manage heat fluxes as fast as possible [41].
3.1. Order of Phase Transition
3.2. Structural Composition and Shape Classification
- Monocrystals;
- Ceramics;
- Polymers.
- Bulk samples;
- Thick films;
- Thin films.
3.2.1. Monocrystals
3.2.2. Ceramics
3.2.3. Polymers
4. Numerical Models
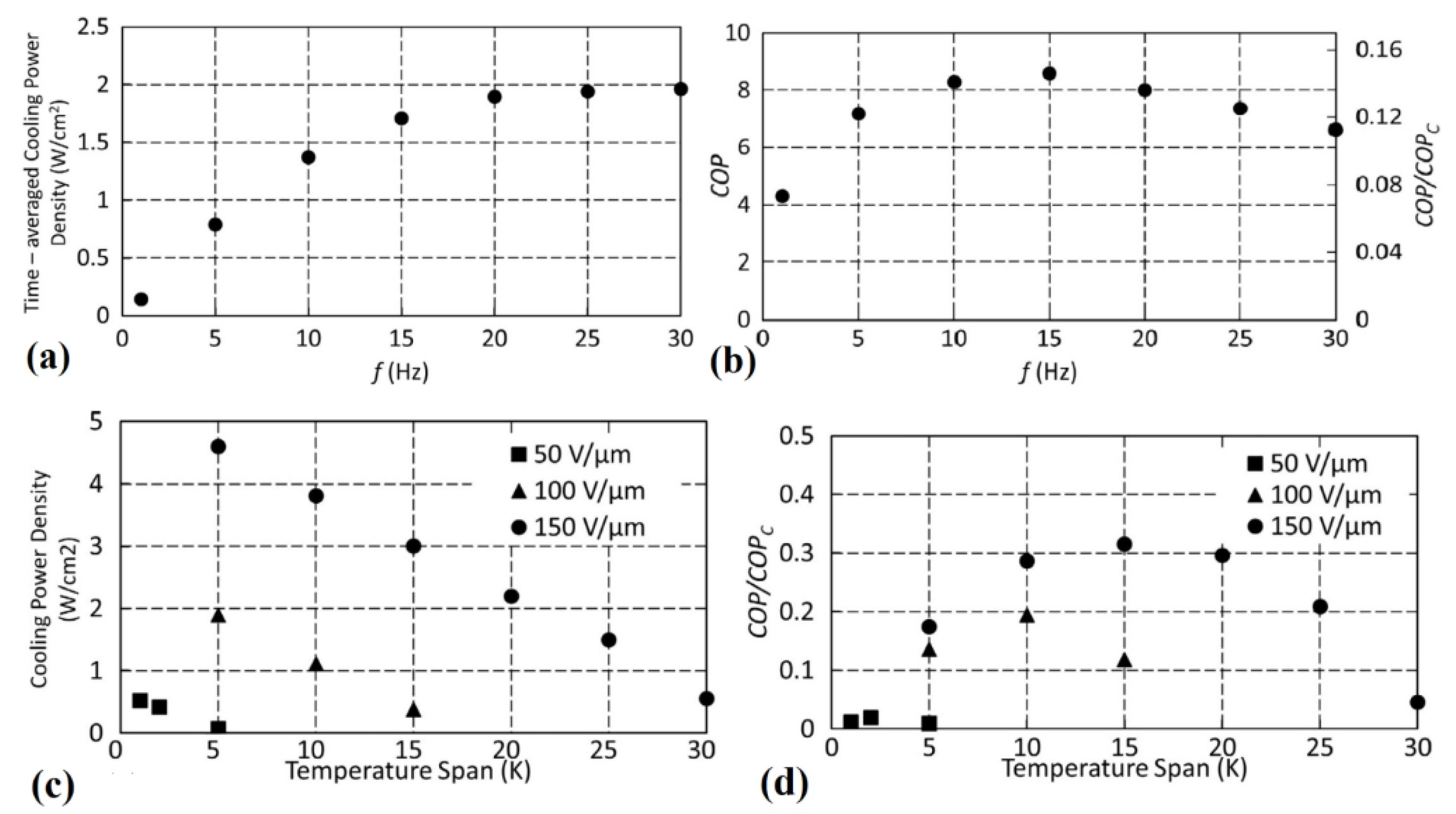
- The frequency of the electrocaloric cycle that varies from 0 to 30 Hz;
- The electric field that was varies between 0–150 MV m−1;
- The hot source temperature was set at 300.15 K;
- The temperature span was varied from 0 to 30 K.
- The wider temperature range over which the COP remains large was noticed for P(VDF-TrFE-CFE);
- 0.2 Hz is the frequency ensuring the larger COPs;
- Due to heat losses, the best COP calculated is only 60% of the Carnot COP and it was detected in the copolymer P(VDF-TrFE).
- Hot side temperature = 293 K;
- The temperature span varied in 5–15 K;
- The fluid flow rate changes in 0.003–0.2 kg s−1,
- The investigated frequencies of the AER are: 0.25, 0.5, 1, 2 and 3 Hz.
- (1)
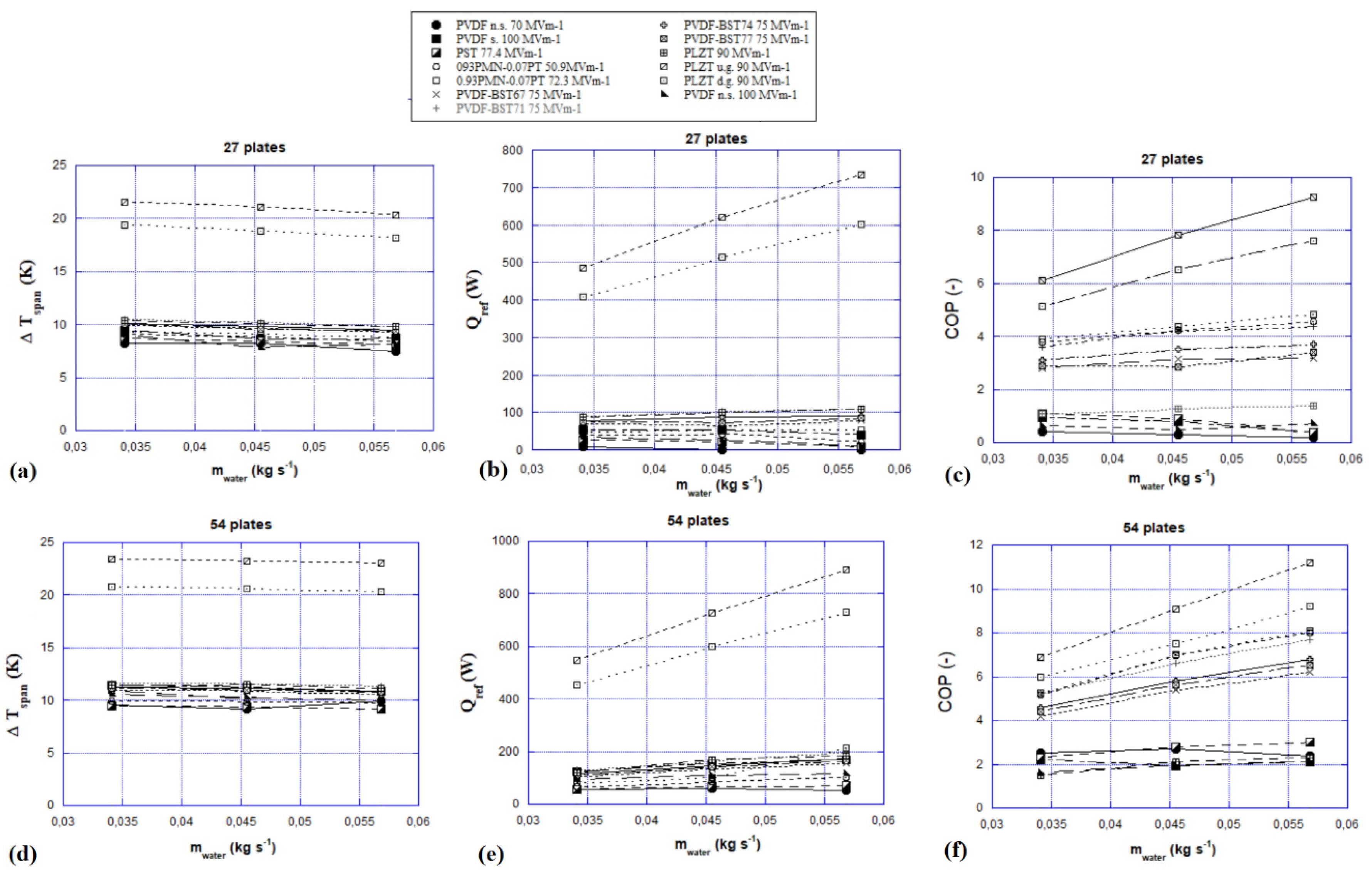
- 27 plates of 0.5 mm thick;
- (2)
- 54 plates of 0.25 mm thick.
- The frequency of the electrocaloric cycle was fixed at 1.25 Hz;
- The fluid flow rate was varied in 0.03–0.06 kg s−1;
- The temperatures of the hot and cold source were set at 300 K and 292 K, respectively;
- The electric field was changed adiabatically according to the literature data of each electrocaloric material [38].
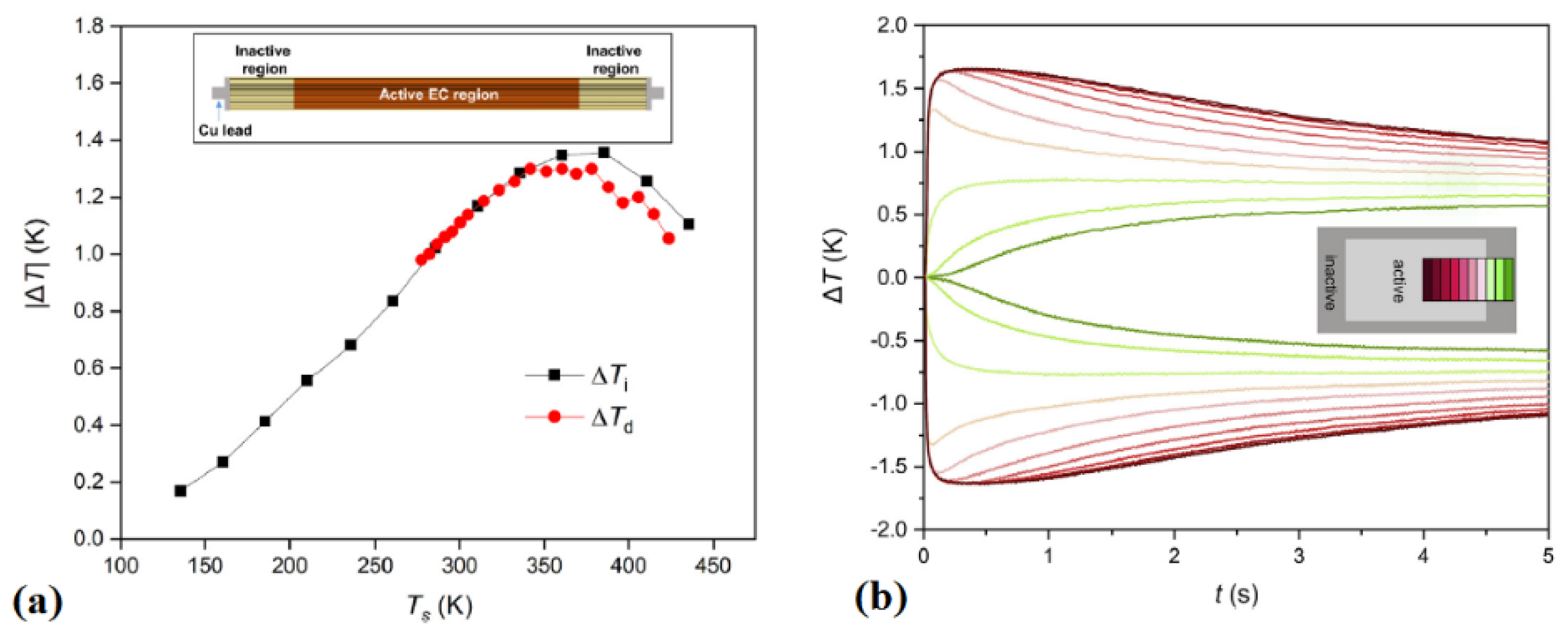
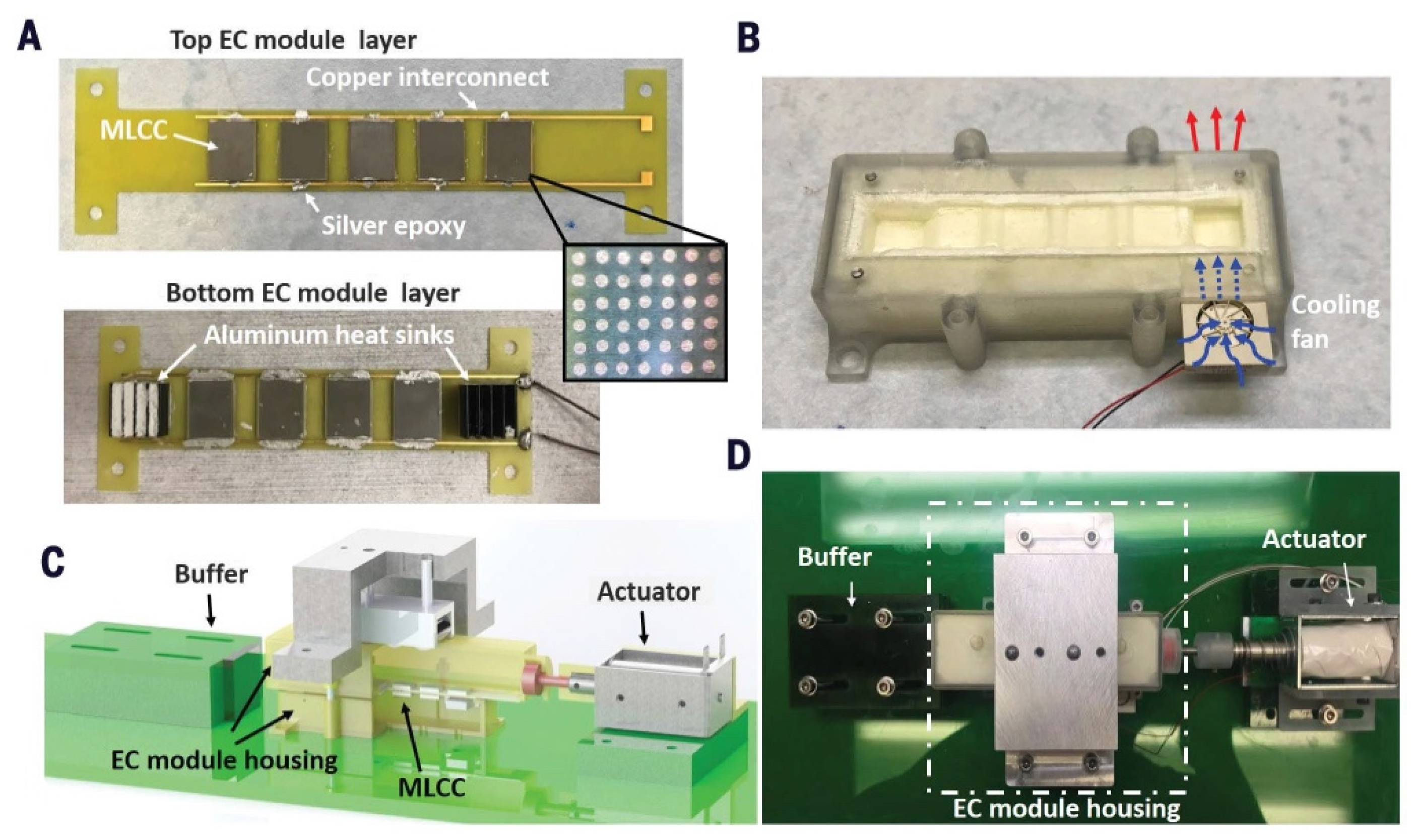
5. Experimental Devices
- 300 K is the hot side temperature;
- 5 Hz is the AER frequencies;
- 0.6 MV m−1 is the electric field change applied to the material.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Roman symbols | |
| A | area, m2 |
| B | magnetic field induction, T |
| c | specific heat capacity, J kg−1 K−1 |
| D | electric displacement, C m−2 |
| d | diameter, |
| E | electric field, V m−1 |
| f | frequency, Hz |
| h | heat transfer coefficient, W m−2 |
| k | thermal conductivity, W m−1 K−1 |
| identity vector | |
| flow rate, kg s−1 | |
| P | electric polarization, C m−2 |
| p | pressure, Pa |
| Q | power, W |
| power density, w m−3 | |
| s | entropy, J kg−1 K−1 |
| T | temperature, K |
| t | time, s |
| velocity vector, m s−1 | |
| Greek symbols | |
| β | temperature derivative of the reciprocal permittivity, m F−1 K−1 |
| electric permittivity, F m−1 | |
| µ | dynamic viscosity, Pa s |
| ρ | density, kg m−3 |
| porosity, - | |
| Curie constant, K A T−1. m−1 | |
| χ | electric susceptibility, - |
| ψ | volume fraction, - |
| Subscripts | |
| 0 | vacuum |
| ab | absorbed |
| ad | adiabatic |
| Curie | at the Curie point |
| E | constant electric field |
| e | due to interactions with an electric field |
| ECM | ElectroCaloric Material |
| eff | effective |
| el | due to electrons agitation |
| electrodes | electrodes |
| f | fluid |
| fin | final |
| fr | frontal |
| gen | generated |
| h | hydraulic |
| ht | heat transfer |
| i | initial |
| irr | irreversible |
| JH | Joule Heating |
| l | due to lattice vibrations |
| max | maximum |
| P | constant polarization |
| r | proper of the material |
| s | solid |
| st | structural |
| T | constant temperature |
| TDM | Thermal Diode Material |
| x | x-axes component |
References
- Kitanovski, A.; Plaznik, U.; Tomc, U.; Poredoš, A. Present and future caloric refrigeration and heat-pump technologies. Int. J. Refrig. 2015, 57, 288–298. [Google Scholar] [CrossRef]
- Fähler, S. Caloric effects in ferroic materials: New concepts for cooling. Energy Technol. 2018, 6, 1394–1396. [Google Scholar] [CrossRef]
- Qian, S.; Nasuta, D.; Rhoads, A.; Wang, Y.; Geng, Y.; Hwang, Y.; Radermacher, R.; Takeuchi, I. Not-in-kind cooling technologies: A quantitative comparison of refrigerants and system performance. Int. J. Refrig. 2016, 62, 177–192. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. The environmental impact of solid-state materials working in an active caloric refrigerator compared to a vapor compression cooler. Int. J. Heat Technol. 2018, 36, 1155–1162. [Google Scholar] [CrossRef]
- Greco, A.; Vanoli, G.P. Experimental two-phase pressure gradients during evaporation of pure and mixed refrigerants in a smooth horizontal tube. Comparison with correlations. Heat Mass Transf. 2006, 42, 709–725. [Google Scholar] [CrossRef]
- Greco, A.; Vanoli, G.P. Flow boiling heat transfer with HFC mixtures in a smooth horizontal tube. Part II: Assessment of predictive methods. Exp. Therm. Fluid Sci. 2005, 29, 199–208. [Google Scholar] [CrossRef]
- Greco, A.; Mastrullo, R.; Palombo, A. R407C as an alternative to R22 in vapour compression plant: An experimental study. Int. J. Energy Res. 1997, 21, 1087–1098. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. The drop-in of HFC134a with HFO1234ze in a household refrigerator. Int. J. Therm. Sci. 2018, 127, 117–125. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A. The application of a desiccant wheel to increase the energetic performances of a transcritical cycle. Energy Convers. Manag. 2015, 89, 222–230. [Google Scholar] [CrossRef]
- Moya, X.; Kar-Narayan, S.; Mathur, N.D. Caloric materials near ferroic phase transitions. Nat. Mater. 2014, 13, 439–450. [Google Scholar] [CrossRef]
- Ožbolt, M.; Kitanovski, A.; Tušek, J.; Poredoš, A. Electrocaloric refrigeration: Thermodynamics, state of the art and future perspectives. Int. J. Refrig. 2014, 40, 174–188. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. The use of barocaloric effect for energy saving in a domestic refrigerator with ethylene-glycol based nanofluids: A numerical analysis and a comparison with a vapor compression cooler. Energy 2020, 190, 116404. [Google Scholar] [CrossRef]
- Qian, S.; Yuan, L.; Yu, J.; Yan, G. Numerical modeling of an active elastocaloric regenerator refrigerator with phase transformation kinetics and the matching principle for materials selection. Energy 2017, 141, 744–756. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. Analyzing the energetic performances of AMR regenerator working with different magnetocaloric materials: Investigations and viewpoints. Int. J. Heat Technol. 2017, 35, S383–S390. [Google Scholar] [CrossRef]
- Kobeko, P.; Kurtschatov, J. Dielektrische eigenschaften der seignettesalzkristalle. Z. Phys. 1930, 66, 192–205. [Google Scholar] [CrossRef]
- Hautzenlaub, J.F. Electric and Dielectric Behavior Potassium Dihydrogen Phosphate. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1943. [Google Scholar]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. Electrocaloric refrigeration: An innovative, emerging, eco-friendly refrigeration technique. J. Phys. Conf. Ser. 2017, 796, 012019. [Google Scholar] [CrossRef]
- Mischenko, A.S.; Zhang, Q.; Scott, J.F.; Whatmore, R.W.; Mathur, N.D. Giant electrocaloric effect in thin-film PbZr0.95Ti0.05O3. Science 2006, 311, 1270–1271. [Google Scholar] [CrossRef]
- Valant, M. Electrocaloric materials for future solid-state refrigeration technologies. Prog. Mater. Sci. 2012, 57, 980–1009. [Google Scholar] [CrossRef]
- Lu, S.G.; Zhang, Q. Electrocaloric materials for solid-state refrigeration. Adv. Mater. 2009, 21, 1983–1987. [Google Scholar] [CrossRef]
- Scott, J.F. Electrocaloric materials. Annu. Rev. Mater. Res. 2011, 41, 229–240. [Google Scholar] [CrossRef]
- Kar-Narayan, S.; Mathur, N.D. Electrocaloric materials for cooling applications. Ferroelectrics 2012, 433, 107–110. [Google Scholar] [CrossRef]
- Crossley, S. Electrocaloric Materials and Devices. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2013. [Google Scholar]
- Shi, J.; Han, D.; Li, Z.; Yang, L.; Lu, S.G.; Zhong, Z.; Chen, J.; Zhang, Q.M.; Qian, X. Electrocaloric cooling materials and devices for zero-global-warming-potential, high-efficiency refrigeration. Joule 2019, 3, 1200–1225. [Google Scholar] [CrossRef]
- Greco, A.; Aprea, C.; Maiorino, A.; Masselli, C. A review of the state of the art of solid-state caloric cooling processes at room-temperature before 2019. Int. J. Refrig. 2019, 106, 66–88. [Google Scholar] [CrossRef]
- Kitanovski, A.; Egolf, P.W. Thermodynamics of magnetic refrigeration. Int. J. Refrig. 2006, 29, 3–21. [Google Scholar] [CrossRef]
- Gómez, J.R.; Garcia, R.F.; Catoira, A.D.M.; Gómez, M.R. Magnetocaloric effect: A review of the thermodynamic cycles in magnetic refrigeration. Renew. Sustain. Energy Rev. 2013, 17, 74–82. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Zhou, Y.; Wang, J.T. Regenerative characteristics of electrocaloric Stirling or Ericsson refrigeration cycles. Energy Convers. Manag. 2002, 43, 2319–2327. [Google Scholar] [CrossRef]
- Plaznik, U.; Vrabelj, M.; Kutnjak, Z.; Malič, B.; Rožič, B.; Poredoš, A.; Kitanovski, A. Numerical modelling and experimental validation of a regenerative electrocaloric cooler. Int. J. Refrig. 2019, 98, 139–149. [Google Scholar] [CrossRef]
- Plaznik, U.; Vrabelj, M.; Kutnjak, Z.; Malič, B.; Poredoš, A.; Kitanovski, A. Electrocaloric cooling: The importance of electric-energy recovery and heat regeneration. Europhys. Lett. 2015, 111, 57009. [Google Scholar] [CrossRef]
- Defay, E.; Faye, R.; Despesse, G.; Strozyk, H.; Sette, D.; Crossley, S.; Moya, X.; Mathur, N.D. Enhanced electrocaloric efficiency via energy recovery. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Gu, H.; Craven, B.; Qian, X.; Li, X.; Cheng, A.; Zhang, Q.M. Simulation of chip-size electrocaloric refrigerator with high cooling-power density. Appl. Phys. Lett. 2013, 102, 112901. [Google Scholar] [CrossRef]
- Gu, H.; Qian, X.S.; Ye, H.J.; Zhang, Q.M. An electrocaloric refrigerator without external regenerator. Appl. Phys. Lett. 2014, 105, 162905. [Google Scholar] [CrossRef]
- Epstein, R.I.; Malloy, K.J. Electrocaloric devices based on thin-film heat switches. J. Appl. Phys. 2009, 106, 064509. [Google Scholar] [CrossRef]
- Guo, D.; Gao, J.; Yu, Y.J.; Santhanam, S.; Slippey, A.; Fedder, G.K.; McGaughey, A.J.H.; Yao, S.C. Design and modeling of a fluid-based micro-scale electrocaloric refrigeration system. Int. J. Heat Mass Transf. 2014, 72, 559–564. [Google Scholar] [CrossRef]
- Kitanovski, A.; Egolf, P.W. Innovative ideas for future research on magnetocaloric technologies. Int. J. Refrig. 2010, 33, 449–464. [Google Scholar] [CrossRef]
- Tishin, A.M.; Spichkin, Y.I. The Magnetocaloric Effect and Its Applications; Series in Condensed Matter Physics; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. A comparison between different materials in an active electrocaloric regenerative cycle with a 2D numerical model. Int. J. Refrig. 2016, 69, 369–382. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. Solid-state refrigeration: A comparison of the energy performances of caloric materials operating in an active caloric regenerator. Energy 2018, 165, 439–455. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. The employment of caloric-effect materials for solid-state heat pumping. Int. J. Refrig. 2020, 109, 1–11. [Google Scholar] [CrossRef]
- Zhang, G.; Weng, L.; Hu, Z.; Liu, Y.; Bao, R.; Zhao, P.; Feng, H.; Yang, N.; Li, M.Y.; Zhang, S.; et al. Nanoconfinement-Induced Giant Electrocaloric Effect in Ferroelectric Polymer Nanowire Array Integrated with Aluminum Oxide Membrane to Exhibit Record Cooling Power Density. Adv. Mater. 2019, 31, 1806642. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. A comparison between rare earth and transition metals working as magnetic materials in an AMR refrigerator in the room temperature range. Appl. Therm. Eng. 2015, 91, 767–777. [Google Scholar] [CrossRef]
- Correia, T.; Zhang, Q. Electrocaloric Materials: New Generation of Coolers; Springer: Berlin/Heidelberg, Germany, 2014; Volume 34. [Google Scholar]
- Wang, J.J.; Fortino, D.; Wang, B.; Zhao, X.; Chen, L.Q. Extraordinarily Large Electrocaloric Strength of Metal-Free Perovskites. Adv. Mater. 2020, 32, 1906224. [Google Scholar] [CrossRef]
- Huang, F.; Tian, H.; Meng, X.; Tan, P.; Cao, X.; Bai, Y.; Hu, C.; Zhou, Z. Large room temperature electrocaloric effect in KTa1−xNbxO3 single crystal. Phys. Status Solidi RRL Rapid Res. Lett. 2019, 13, 1800515. [Google Scholar] [CrossRef]
- Jankowska-Sumara, I.; Gwizd, P.; Majchrowski, A.; Soszyński, A. Electrocaloric effect in pure and Cr doped lead germanate single crystals. Mater. Chem. Phys. 2020, 242, 122494. [Google Scholar] [CrossRef]
- Zhang, T.F.; Tang, X.G.; Ge, P.Z.; Liu, Q.X.; Jiang, Y.P. Orientation related electrocaloric effect and dielectric phase transitions of relaxor PMN-PT single crystals. Ceram. Int. 2017, 43, 16300–16305. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, L.; Wang, H.; Guo, M.; Lou, X.; Wang, D. Composition-driven inverse-to-conventional transformation of electrocaloric effect and large energy storage density in strontium modified Ba (Zr0.1Ti0.9) O3 thin films. J. Mater. Chem. C 2020, 8, 1366–1373. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, X.; Huang, H.; Wang, J.; Li, Q.; Chen, L.Q.; Wang, Q. Toward wearable cooling devices: Highly flexible electrocaloric Ba0.67Sr0.33TiO3 nanowire arrays. Adv. Mater. 2016, 28, 4811–4816. [Google Scholar] [CrossRef]
- Bai, Y.; Zheng, G.P.; Ding, K.; Qiao, L.; Shi, S.Q.; Guo, D. The giant electrocaloric effect and high effective cooling power near room temperature for BaTiO3 thick film. J. Appl. Phys. 2011, 110, 094103. [Google Scholar]
- Qian, X.S.; Ye, H.J.; Zhang, Y.T.; Gu, H.; Li, X.; Randall, C.A.; Zhang, Q.M. Giant electrocaloric response over a broad temperature range in modified BaTiO3 ceramics. Adv. Funct. Mater. 2014, 24, 1300–1305. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, C.; Zheng, T.; Wu, J. Large electrocaloric effect in (Bi0.5Na0.5) TiO3-based relaxor ferroelectrics. ACS Appl. Mater. Interfaces 2020, 12, 33934–33940. [Google Scholar] [CrossRef]
- Shirsath, S.E.; Cazorla, C.; Lu, T.; Zhang, L.; Tay, Y.Y.; Lou, X.; Liu, Y.; Li, S.; Wang, D. Interface-charge induced giant electrocaloric effect in lead free ferroelectric thin-film bilayers. Nano Lett. 2019, 20, 1262–1271. [Google Scholar] [CrossRef]
- Peng, B.; Fan, H.; Zhang, Q. A giant electrocaloric effect in nanoscale antiferroelectric and ferroelectric phases coexisting in a relaxor Pb0.8Ba0.2ZrO3 thin film at room temperature. Adv. Funct. Mater. 2013, 23, 2987–2992. [Google Scholar] [CrossRef]
- Shen, B.Z.; Li, Y.; Hao, X. Multifunctional all-inorganic flexible capacitor for energy storage and electrocaloric refrigeration over a broad temperature range based on PLZT 9/65/35 thick films. ACS Appl. Mater. Interfaces 2019, 11, 34117–34127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hao, X.; Zhang, Q. A giant electrocaloric effect of a Pb0.97La0.02(Zr0.75Sn0.18Ti0.07)O3 antiferroelectric thick film at room temperature. J. Mater. Chem. C 2015, 3, 1694–1699. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, X.; Zhang, Q. Enhanced energy-storage performance and electrocaloric effect in compositionally graded Pb (1− 3x/2) LaxZr0.85Ti0.15O3 antiferroelectric thick films. Ceram. Int. 2016, 42, 1679–1687. [Google Scholar] [CrossRef][Green Version]
- Correia, T.M.; Young, J.S.; Whatmore, R.W.; Scott, J.F.; Mathur, N.D.; Zhang, Q. Investigation of the electrocaloric effect in a PbMg2/3Nb1/3 O3-PbTiO3 relaxor thin film. Appl. Phys. Lett. 2009, 95, 182904. [Google Scholar] [CrossRef]
- Hamad, M.A. Theoretical investigations on electrocaloric properties of relaxor ferroelectric 0.9PbMg1/3Nb2/3O3–0.1PbTiO3 thin film. J. Comput. Electron. 2012, 11, 344–348. [Google Scholar] [CrossRef]
- Rožič, B.; Kosec, M.; Uršič, H.; Holc, J.; Malič, B.; Zhang, Q.M.; Blinc, R.; Pirc, R.; Kutnjak, Z. Influence of the critical point on the electrocaloric response of relaxor ferroelectrics. J. Appl. Phys. 2011, 110, 064118. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, G.; Zhang, X.; Jiang, S.; Zeng, Y.; Wang, Q. Relaxor ferroelectric-based electrocaloric polymer nanocomposites with a broad operating temperature range and high cooling energy. Adv. Mater. 2015, 27, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, X.; Yang, T.; Li, Q.; Chen, L.Q.; Jiang, S.; Wang, Q. Colossal room-temperature electrocaloric effect in ferroelectric polymer nanocomposites using nanostructured barium strontium titanates. ACS Nano 2015, 9, 7164–7174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Q.; Gu, H.; Jiang, S.; Han, K.; Gadinski, M.R.; Haque, M.A.; Zhang, Q.; Wang, Q. Ferroelectric polymer nanocomposites for room-temperature electrocaloric refrigeration. Adv. Mater. 2015, 27, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; McGaughey, A.J. Device-level thermodynamic model for an electrocaloric cooler. Int. J. Energy Res. 2020, 44, 5343–5359. [Google Scholar] [CrossRef]
- Feng, D.; Yao, S.C.; Zhang, T.; Zhang, Q. Modeling of a smart heat pump made of laminated thermoelectric and electrocaloric materials. J. Electron. Packag. 2016, 138. [Google Scholar] [CrossRef]
- Shi, J.; Li, Q.; Gao, T.; Han, D.; Li, Y.; Chen, J.; Qian, X. Numerical evaluation of a kilowatt-level rotary electrocaloric refrigeration system. Int. J. Refrig. 2020, in press. [Google Scholar] [CrossRef]
- Hirasawa, S.; Kawanami, T.; Shirai, K. Electrocaloric Refrigeration using Multi-Layers of Electrocaloric Material Films and Thermal Switches. Heat Transf. Eng. 2018, 39, 1091–1099. [Google Scholar] [CrossRef]
- Ožbolt, M.; Kitanovski, A.; Tušek, J.; Poredoš, A. Electrocaloric vs. magnetocaloric energy conversion. Int. J. Refrig. 2014, 37, 162–167. [Google Scholar] [CrossRef]
- Plaznik, U.; Kitanovski, A.; Rožič, B.; Malič, B.; Uršič, H.; Drnovšek, S.; Cilenšek, J.; Vrabelj, M.; Poredoš, A.; Kutnjak, Z. Bulk relaxor ferroelectric ceramics as a working body for an electrocaloric cooling device. Appl. Phys. Lett. 2015, 106, 043903. [Google Scholar] [CrossRef]
- Faye, R.; Usui, T.; Torello, A.; Dkhil, B.; Moya, X.; Mathur, N.D.; Hirose, S.; Defay, E. Heat flow in electrocaloric multilayer capacitors. J. Alloy. Compd. 2020, 834, 155042. [Google Scholar] [CrossRef]
- Li, X.; Qian, X.S.; Lu, S.G.; Cheng, J.; Fang, Z.; Zhang, Q.M. Tunable temperature dependence of electrocaloric effect in ferroelectric relaxor poly(vinylidene fluoride-trifluoroethylene-chlorofluoroethylene terpolymer. Appl. Phys. Lett. 2011, 99, 052907. [Google Scholar] [CrossRef]
- Tušek, J.; Kitanovski, A.; Prebil, I.; Poredoš, A. Dynamic operation of an active magnetic regenerator (AMR): Numerical optimization of a packed-bed AMR. Int. J. Refrig. 2011, 34, 1507–1517. [Google Scholar] [CrossRef]
- Aprea, C.; Greco, A.; Maiorino, A.; Masselli, C. Energy performances and numerical investigation of solid-state magnetocaloric materials used as refrigerant in an active magnetic regenerator. Therm. Sci. Eng. Prog. 2018, 6, 370–379. [Google Scholar] [CrossRef]
- Aprea, C.; Cardillo, G.; Greco, A.; Maiorino, A.; Masselli, C. A rotary permanent magnet magnetic refrigerator based on AMR cycle. Appl. Therm. Eng. 2016, 101, 699–703. [Google Scholar] [CrossRef]
- Blumenthal, P.; Raatz, A. Design Methodology for Electrocaloric Cooling Systems. Energy Technol. 2018, 6, 1560–1566. [Google Scholar] [CrossRef]
- Radebaugh, R.; Lawless, W.N.; Siegwarth, J.D.; Morrow, A.J. Feasibility of electrocaloric refrigeration for the 4–15 K temperature range. Cryogenics 1979, 19, 187–208. [Google Scholar] [CrossRef]
- Sinyavsky, Y.V.; Pashkov, N.D.; Gorovoy, Y.M.; Lugansky, G.E.; Shebanov, L. The optical ferroelectric ceramic as working body for electrocaloric refrigeration. Ferroelectrics 1989, 90, 213–217. [Google Scholar] [CrossRef]
- Sinyavsky, Y.V.; Brodyansky, V.M. Experimental testing of electrocaloric cooling with transparent ferroelectric ceramic as a working body. Ferroelectrics 1992, 131, 321–325. [Google Scholar] [CrossRef]
- Lawless, W.N. Electrocaloric Device and Thermal Transfer Systems Employing the Same. U.S. Patent No. 6,877,325, 12 March 2005. [Google Scholar]
- Plaznik, U.; Kitanovski, A.; Malič, B.; Kutnjak, Z.; Poredoš, A. Small scale electrocaloric cooling device with an active heat regenerator. In Proceedings of the Thermag VI, 6th IIR/IIF International Conference on Magnetic Refrigeration 2014, Victoria, BC, Canada, 7–10 September 2014; International Institute of Refrigeration: Paris, France, 2014. [Google Scholar]
- Blumenthal, P.; Molin, C.; Gebhardt, S.; Raatz, A. Active electrocaloric demonstrator for direct comparison of PMN-PT bulk and multilayer samples. Ferroelectrics 2016, 497, 1–8. [Google Scholar] [CrossRef]
- Gu, H.; Qian, X.; Li, X.; Craven, B.; Zhu, W.; Cheng, A.; Yao, S.C.; Zhang, Q.M. A chip scale electrocaloric effect based cooling device. Appl. Phys. Lett. 2013, 102, 122904. [Google Scholar] [CrossRef]
- Zhang, T.; Qian, X.S.; Gu, H.; Hou, Y.; Zhang, Q.M. An electrocaloric refrigerator with direct solid to solid regeneration. Appl. Phys. Lett. 2017, 110, 243503. [Google Scholar] [CrossRef]
- Wang, Y.; Schwartz, D.; Kalb, J.; Lee, J. A self-regenerating electrocaloric cooler. In Proceedings of the Thermag VIII, 8th IIR/IIF International Conference on Caloric Cooling, Darmstadt, Germany, 16–20 September 2018; International Institute of Refrigeration: Paris, France, 2018. [Google Scholar]
- Wang, Y.; Zhang, Z.; Usui, T.; Benedict, M.; Hirose, S.; Lee, J.; Kalb, J.; Schwartz, D. A high-performance solid-state electrocaloric cooling system. Science 2020, 370, 129–133. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, Z.; Tong, K.; Huber, D.; Kornbluh, R.; Ju, Y.S.; Pei, Q. Highly efficient electrocaloric cooling with electrostatic actuation. Science 2017, 357, 1130–1134. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Z.; Wu, H.; Wu, R.; Wu, J.; Wang, H.; Pei, Q. A cascade electrocaloric cooling device for large temperature lift. Nat. Energy 2020, 10. [Google Scholar] [CrossRef]




| Material | Shape | Tpeak [K] | ΔTad 1 [K] | ΔsT 1 [J kg−1 K−1] | ΔE 1 [MV m−1] | Reference |
|---|---|---|---|---|---|---|
| BaTiO3 | Bulk | 413 | 1.6 | / | 1 | [24] |
| KCl:OH | Bulk | 0.15 | 0.15 | / | 2.6 | [11] |
| KH2PO4 | Bulk | / | 1 | 0.99 | 1.1 | [11] |
| KTaO3 | Bulk | 13 | 0.25 | / | 1.56 | [11] |
| KTa0.57Nb0.43O3 | Bulk | 326 | 0.76 | / | 0.15 | [45] |
| KTa0.61Nb0.39O3 | Bulk | 296 | 0.57 | / | 0.15 | [45] |
| [MDABCO](NH4)I3 | Bulk | 448 | 16 | 36 | 2 | [44] |
| NaKC4H4O64H2O | Bulk | 295 | 0.004 | / | 0.12 | [11] |
| (NH2CH2COOH)2HNO3 | Bulk | 206 | 0.054 | / | 0.4 | [11] |
| (NH2CH2COOH)2H2SO4 | Bulk | 323 | 0.11 | / | 0.16 | [11] |
| NH4HSO4 | Bulk | / | 0.025 | / | 0.15 | [11] |
| Pb5Ge3O11 | Bulk | 453 | 1.25 | / | 5.3 | [46] |
| Pb5Ge3O11 + 0.2%Cr | Bulk | 431 | 1.50 | / | 6 | [46] |
| (Pb,La)(Zr,Sn,Ti)O3 | Bulk | 398 | -3.6 | / | 12 | [24] |
| Pb(Mg1/3Nb2/3)0.75Ti0.25O3 | Bulk | 383 | 1.1 | / | 2.5 | [24] |
| PMN | Bulk | 274 | 0.11 | / | 1 | [11] |
| 0.72PMN-0.28PT | Bulk | 403 | 2.7 | / | 1.2 | [19] |
| 0.75PMN-0.25PT | Bulk | 373 | 0.78 | / | 1 | [19] |
| 0.76PMN-0.24PT | Bulk | 400 | 0.80 | 0.66 | 0.2 | [47] |
| 0.8PMN-0.2PT | Bulk | 414 | 0.74 | / | 1.5 | [11] |
| 0.90PMN-0.1PT | Bulk | 328 | 1 | / | 4 | [19] |
| 0.92PZN-0.08PT | Bulk | 453 | 0.25 | / | 1.2 | [11] |
| Rb0.33(NH4)0.67 | Bulk | 270 | 0.4 | / | 0.34 | [11] |
| Rochelle salt | Bulk | 295 | 0.0036 | 0.0156 | 0.14 | [11] |
| Sr0.75Ba0.25Nb2O6 | Bulk | 353 | 0.4 | / | 1 | [24] |
| Material | Shape | Tpeak [K] | ΔTad 1 [K] | ΔsT 1 J kg−1 K−1] | ΔE 1 [MV m−1] | Reference |
|---|---|---|---|---|---|---|
| Ba0.65Sr0.35TiO3 | Film | 423 | 12.5 | 14.3 | 156.25 | [48] |
| Ba0.67Sr0.33TiO3 | Bulk | 298 | 0.45 | / | 1.33 | [11] |
| Ba0.67Sr0.33TiO3 | Film | 303 | 10.1 | 16.3 | 60 | [49] |
| BaTiO3 | Film | 353 | 7.1 | 10.1 | 3679 | [50] |
| Ba(Zr0.2Ti0.8)O3 | Bulk | 312 | 4.5 | 7.8 | 8.6 | [51] |
| (Bi0.5Na0.5)TiO3 | Bulk | 357 | 2.51 | 3.3 | 6.5 | [52] |
| BNBT-BCZT | Film | 370 | 23 | 26.1 | 62 | [53] |
| BNT-0.3BT | Bulk | 423 | −2.1 | / | 5 | [11] |
| BST | Film | / | 15 | / | 50 | [11] |
| BT | Bulk | 380 | 2.8 | / | 3 | [11] |
| BT | Film | 333 | 7.1 | 10.1 | 80 | [11] |
| (Cd0.83Pb0.17)2Nb2O7 | Film | 94 | 0.8 | / | 10 | [19] |
| CdTiO3 | Bulk | 60 | 0.02 | / | 1.1 | [11] |
| NBT | Bulk | 413 | −0.34 | -0.43 | 5 | [11] |
| NBT-0.08BT | Bulk | 370 | 0.19 | 0.26 | 4 | [11] |
| Pb0.8Ba0.2ZrO3 | Film | 290 | 45 | / | 0.6 | [54] |
| Pb0.91La0.09(Zr0.65Ti0.35)0.9775O3 | Film | 353 | 18 | 15 | 82 | [55] |
| Pb0.97La0.02(Zr0.75Sn0.18Ti0.07)O3 | Film | 278 | 54 | / | 90 | [56] |
| PbZr0.95Ti0.05O3 | Bulk | 499 | 12 | 8 | 48 | [18] |
| PLZT 8/65/35 | Bulk | 385 | 2.2 | / | 8.8 | [11] |
| PLZT 8/65/35 | Film | 318 | 40 | 50 | 120 | [11] |
| PLZT 11/85/15 | Film | 111 | 12 | 10 | 90 | [57] |
| PLZT down-graded | Film | / | 20 | 23 | 90 | [57] |
| PLZT up-graded | Film | / | 28 | 33 | 90 | [57] |
| PMN | Bulk | 451 | 2.6 | / | 9 | [11] |
| PMN-0.07 PT | Film | 298 | 13 | 14 | 72.3 | [58] |
| PMN-0.08 PT | Bulk | 296 | 1.4 | / | 1.5 | [11] |
| PMN-0.1 PT | Bulk | 301 | 1.25 | / | 1.5 | [19] |
| PMN-0.1 PT | Film | 348 | 7.75 | 8.07 | 89.5 | [59] |
| PMN-0.13 PT | Bulk | 343 | 0.558 | / | 2.4 | [19] |
| PMN-0.15 PT | Bulk | 291 | 1.71 | / | 1.6 | [19] |
| PMN-0.25 PT | Bulk | 307 | 0.4 | / | 1.5 | [19] |
| PMN-0.3 PT | Bulk | 429 | 2.7 | 2.3 | 9 | [60] |
| PMN-0.35 PT | Film | 413 | 31 | / | 74.7 | [11] |
| PMN-0.85 PT | Bulk | 291 | 1.7 | / | 1.6 | [11] |
| PNZST | Bulk | 443 | 2.6 | / | 3 | [11] |
| PSN | Bulk | 370 | 0.9 | / | 2 | [11] |
| PST | Bulk | / | 2.3 | / | 3 | [11] |
| PST | Film | 341 | −6.2 | −6.3 | 77.4 | [11] |
| PST-0.2 PSN | Bulk | 273 | 1.25 | / | 2.5 | [11] |
| PST doped with Co,Sb | Bulk | 291 | 2.3 | / | n.s 2 | [11] |
| PST doped with Co,Sb | Film | 343 | 0.5 | / | 30 | [19] |
| PZ-0.29BT | Bulk | 298 | 0.15 | / | 2 | [11] |
| PZST | Bulk | 311 | 0.6 | / | 3 | [11] |
| PZ | Film | 508 | 11.4 | / | 40 | [11] |
| PZN-0.08 PT | Bulk | 453 | 0.23 | / | 1.2 | [19] |
| PZT | Bulk | / | 2.7 | 1.8 | n.s 2 | [11] |
| PZT-PT | Film | / | 4.1 | / | 40 | [11] |
| SrBi2Ta2O9 | Film | 561 | 4.93 | / | 60 | [11] |
| ST | Bulk | 4 | 1.0 | 0.007 | 0.54 | [11] |
| Material | Shape | Tpeak [K] | ΔTad 1 [K] | ΔsT 1 [J kg−1 K−1] | ΔE 1 [MV m−1] | Reference |
|---|---|---|---|---|---|---|
| P(VDF-TrFE 55/45) | Film | 340 | 12 | 70 | 120 | [11,19] |
| P(VDF-TrFE 68/32) | Film | 489 | 2.3 | / | 49 | [11,19] |
| P(VDF-TrFE 68/32) 2 | Film | 306 | 20 | 95 | 160 | [11,19] |
| P(VDF-TrFE 70/30) | Film | 390 | 21.2 | / | 300 | [11,19] |
| P(VDF-TrFE-CFE) (56.2/36.3/7.6) | Film | 350 | 21.6 | / | 350 | [11,19] |
| P(VDF-TrFE-CFE) (56.2/36.3/7.6) 3 | Film | 318 | 9 | 47 | 300 | [11,19] |
| P(VDF-TrFE-CFE) (59.2/33.6/7.2) | Film | 328 | 12 | 55 | 307 | [11,19] |
| P(VDF-TrFE-CFE) (59.4/33.4/7.2)/ PMN–PT (different % filling) | Film | 308 | 25 | 80 | 75 | [61] |
| P(VDF–TrFE–CFE)0.9–P(VDF–TrFE–CTFE)0.1 | Film | 317 | 6 | 25 | 116 | [11,19] |
| P(VDF–TrFE–CFE)/BST12.5 | Film | n.s 4 | 14 | 130 | 75 | [62] |
| P(VDF–TrFE–CFE) (62.3/29.9/7.8) /BST67 | Film | 311 | 9.2 | 80 | 75 | [63] |
| P(VDF–TrFE–CFE) (62.3/29.9/7.8) /BST71 | Film | 322 | 9.4 | 79 | 75 | [63] |
| P(VDF–TrFE–CFE) (62.3/29.9/7.8) /BST74 | Film | 331 | 9.7 | 78.5 | 75 | [63] |
| P(VDF–TrFE–CFE) (62.3/29.9/7.8) /BST77 | Film | 337 | 9.9 | 78 | 75 | [63] |
| Research Group and Location | Year | AER/TD 1/Other | Dimension | EC Refrigerant | HTF/TD 2 Material | Reference |
|---|---|---|---|---|---|---|
| Carnegie Mellon University Pittsburgh (USA) | 2014 | AER | 3D | P(VDF–TrFE–CFE) | HTF HT-70 | [35] |
| 2020 | TD | 1D | P(VDF–TrFE) P(VDF–TrFE–CFE) | n.s 3 | [64] | |
| Carnegie Mellon University Pittsburgh (USA) and Pennsylvania State University, University Park (USA) | 2016 | TD | 3D | BZT | TD Bi2Te3 | [65] |
| Institute of Refrigeration and Cryogenics - Shanghai Jiao Tong University | 2020 | AER | quasi-3D | P(VDF–TrFE–CFE) | HTF HT-70 | [66] |
| Kobe University Kobe (Japan) | 2018 | TD | 1D | BaTiO3 | TD Aluminium-based heat storage material | [67] |
| Multilayer PMN-PT | ||||||
| Ljubljana University Ljubljana (Slovenia) | 2014 | AER | 1D | PST | HTF water | [68] |
| 2019 | AER | 2D | PMN-0.1PT | HTF silicone oil | [69] | |
| Luxembourg Institute of Science and Technology Belvaux (Luxembourg) + University of Cambridge Cambridge (UK) | 2020 | Multi-layer capacitor | 3D | Multilayer PMN | TD Palladium | [70] |
| University of Salerno + University of Naples Federico (Italy) | 2016 | AER | 2D | ceramics and polymers | HTF water | [38] |
| 2018 | [39] |
| Research Group and Location | Year | AER/TD 1/other | ∆Tspan,max [K] | ECM | HTF/TD 2 Material | Reference |
|---|---|---|---|---|---|---|
| National Bureau of Standards; Corning Glass Works, USA | 1979 | TD | 0.3 | SrTiO3 ceramics (parallel-plates) | TD magnetothermal-mechanical heat switches | [76] |
| Moscow Power Engineering Institute, URSS | 1989 | AER | 5.0 | PST multilayer | HTF Liquid pentane | [77,78] |
| CeramPhysics, Columbus, OH 43081, USA | 2005 | n.s3 | / | multilayer of x(PbMg0.33Nb0.67O3) + y(PbSc0.5Nb0.5O3) or x(PbMg0.33Nb0.67O3) + y(PbTiO3) + z(SrTiO3) | n.s 3 | [79] |
| Pennsylvania State University, USA | 2013 | TD | 6.0 | PVDF-TrFE 68/32 | n.s 3 | [83] |
| 2017 | TD | 2.0 | Y5V multilayer capacitors | n.s 3 | [84] | |
| Ljubljana University Ljubljana (Slovenia) | 2014 | AER | 3.3 | PMN-10PT (bulk samples/multilayer structure) | TD silicone oil | [80] |
| 2019 | AER | 3.3 | [29] | |||
| Leibniz Universitat Hannover; Fraunhofer Institute for Ceramic Technologies and Systems, Germany | 2016 | AER | 0.17 | PMN-PT | TD silicone oil | [81] |
| Parc, a Xerox, USA | 2018 | TD | 2.5 | BaTiO3 multilayer capacitors | n.s | [84,85] |
| University of California, USA | 2017 | Electrostatic actuation | 1.4 | P(VDF-TrFE-CFE) | / | [86] |
| 2020 | 8.7 | P(VDF-TrFE-CFE) (60/32/8 mol %) | / | [87] | ||
| Luxembourg Institute of Science and Technology + University of Cambdrige, UK et al. | 2018 | TD | 0.26 | BaTiO3 Multilayer capacitors | TD and Inductor | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, A.; Masselli, C. Electrocaloric Cooling: A Review of the Thermodynamic Cycles, Materials, Models, and Devices. Magnetochemistry 2020, 6, 67. https://doi.org/10.3390/magnetochemistry6040067
Greco A, Masselli C. Electrocaloric Cooling: A Review of the Thermodynamic Cycles, Materials, Models, and Devices. Magnetochemistry. 2020; 6(4):67. https://doi.org/10.3390/magnetochemistry6040067
Chicago/Turabian StyleGreco, Adriana, and Claudia Masselli. 2020. "Electrocaloric Cooling: A Review of the Thermodynamic Cycles, Materials, Models, and Devices" Magnetochemistry 6, no. 4: 67. https://doi.org/10.3390/magnetochemistry6040067
APA StyleGreco, A., & Masselli, C. (2020). Electrocaloric Cooling: A Review of the Thermodynamic Cycles, Materials, Models, and Devices. Magnetochemistry, 6(4), 67. https://doi.org/10.3390/magnetochemistry6040067






