Nonvolatile Voltage Controlled Molecular Spin-State Switching for Memory Applications
Abstract
1. Introduction
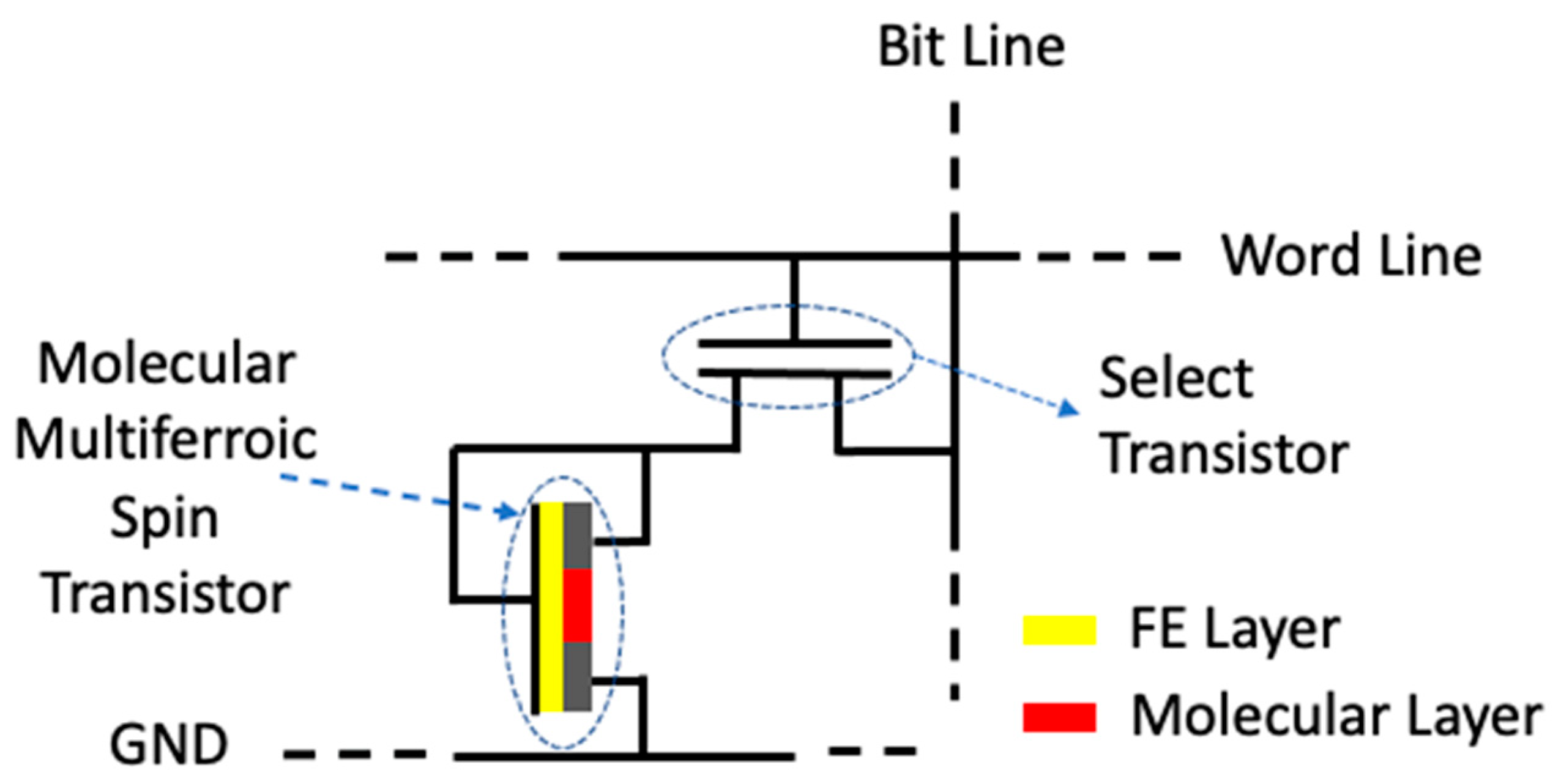
- A device implementation scheme;
- The ability to make a thin film;
- The ability to “lock” the spin state (i.e., nonvolatility);
- The ability to isothermally “unlock” and switch the spin state, ideally with voltage;
- A low coercive voltage (ideally less than 1 V) and low write peak currents (ideally 104 A/cm2);
- A conductance change with spin state;
- Room-temperature operation and at temperatures well above room temperature;
- A device on/off ratio > 10;
- An on-state device resistivity less than 1 Ω·cm;
- A device fast switching speed, ideally less than 100 ps;
- Device reproducibility;
- Device endurance, ideally 1016 switches without degradation;
- The ability of having a nonvolatile device with a transistor channel width of 40 nm or below.
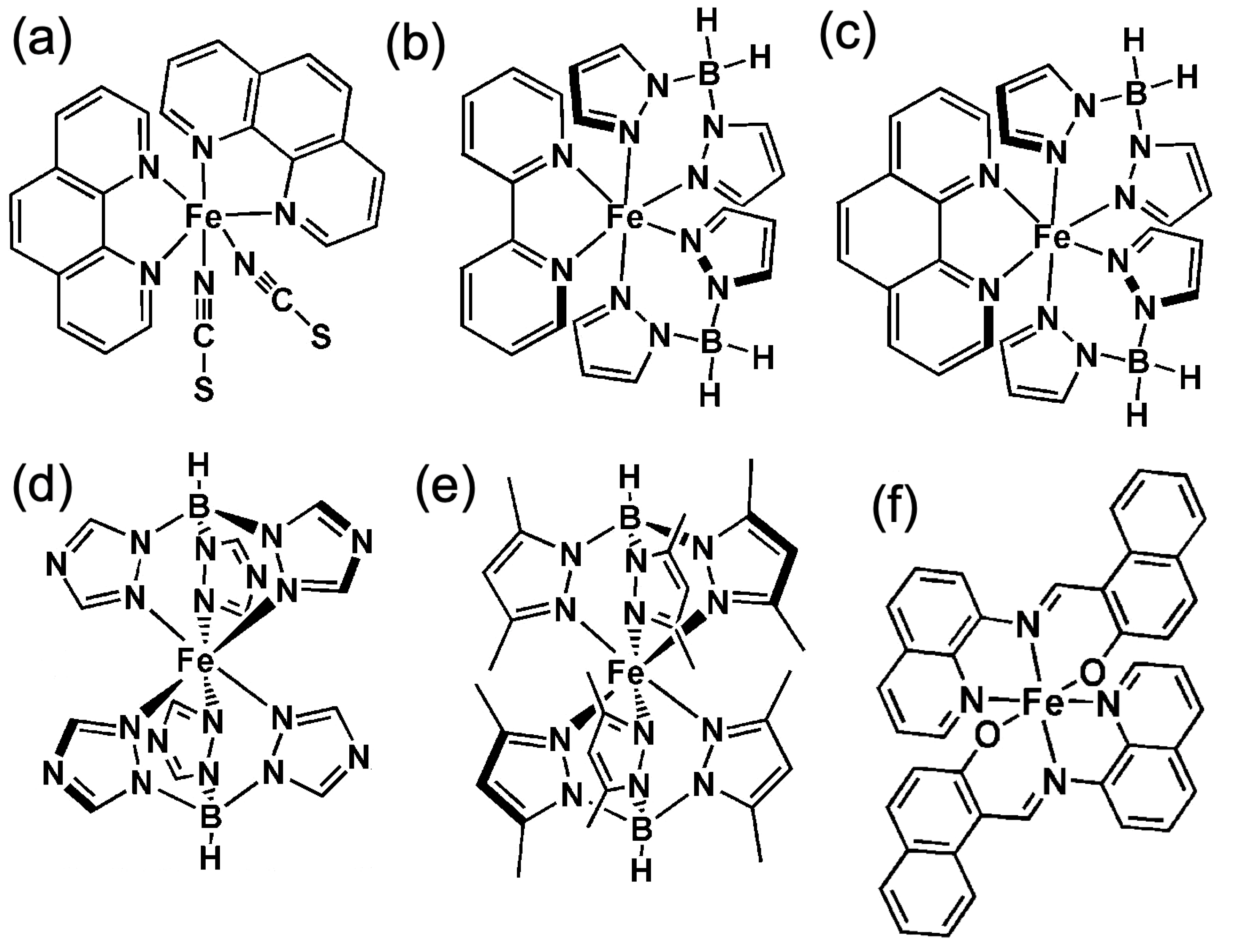
2. Making a Spin Crossover Molecular Thin Film
3. Locking the Molecular Spin State
4. Isothermal Molecular Spin-State Switching
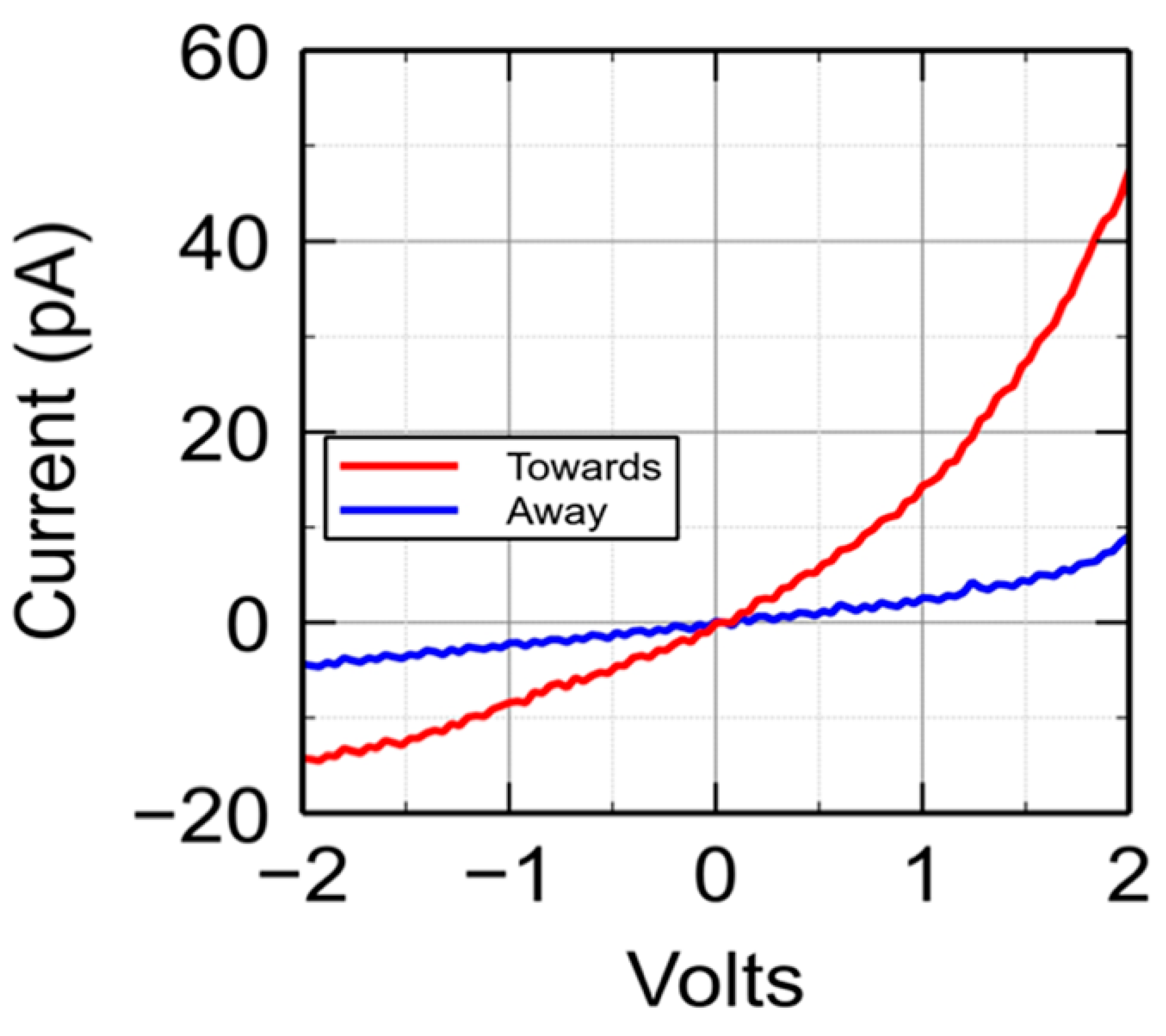
5. The Search for a Low Coercive Voltage and Low Peak Write Currents
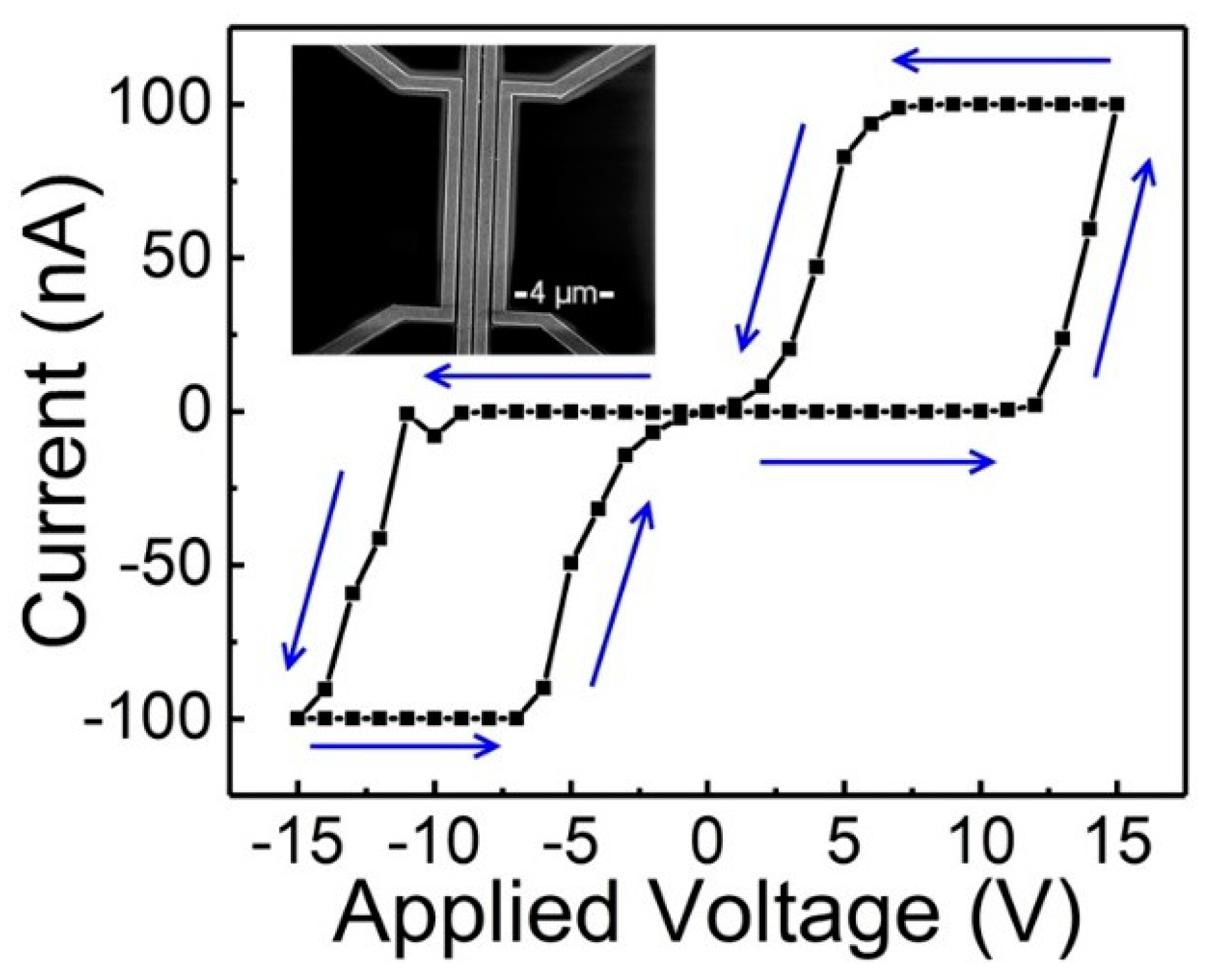
6. Isothermal Switching of Conductance in a Nonvolatile Spin Crossover Molecular Device
7. Room-Temperature Operation of a Nonvolatile Spin Crossover Molecular Device
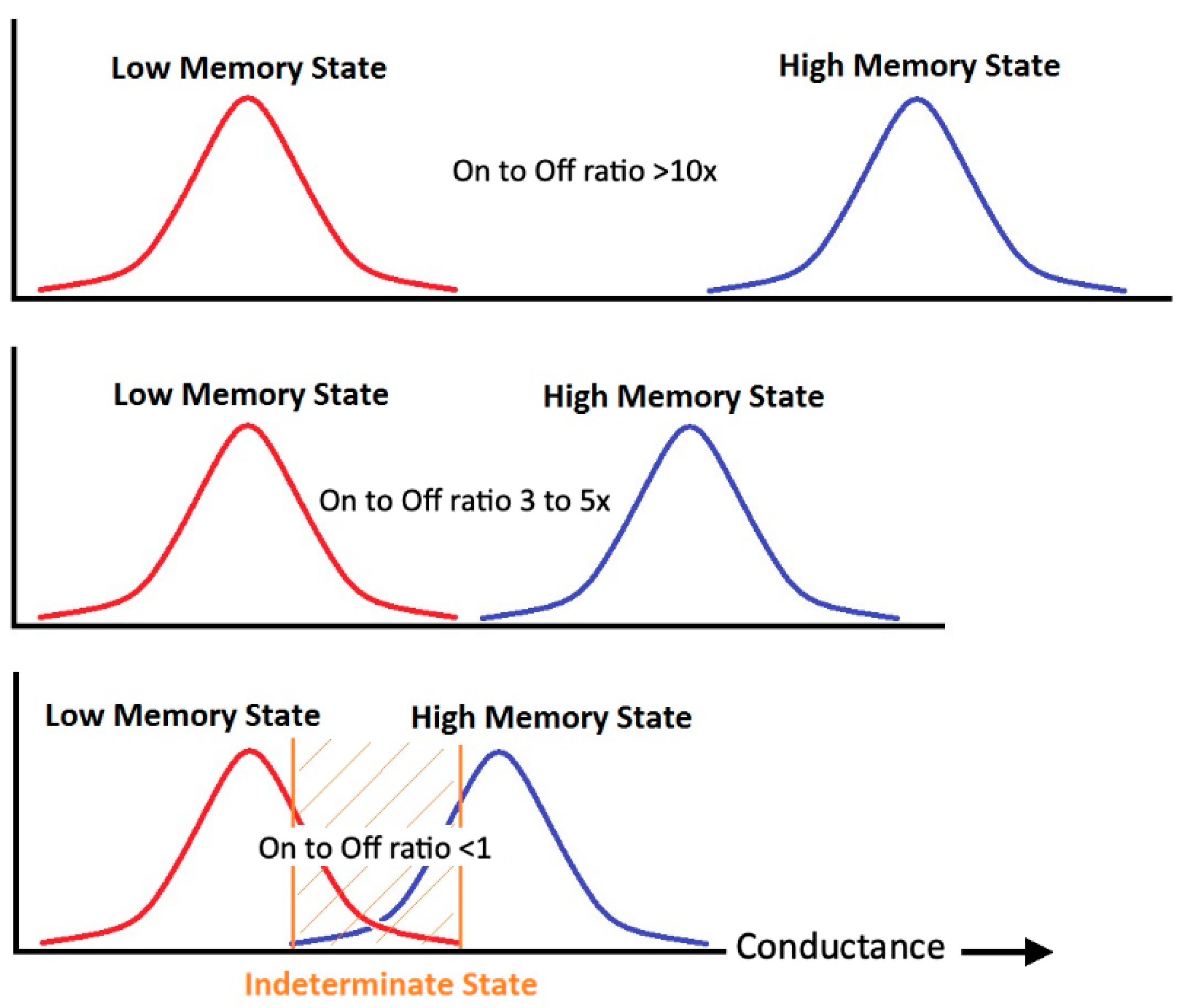
8. Improving the Molecular Nonvolatile Multiferroic Device On/Off Ratio
9. Reducing the On-State Resistance of a Spin Crossover Molecular Device
10. Faster Device Switching Speeds for a Nonvolatile Spin Crossover Molecular Device
11. Reproducibility for Nonvolatile Spin Crossover Molecular Devices
12. Nonvolatile Spin Crossover Molecular Device Endurance, Without Degradation
13. The Potential for Transistor Widths on the Tens of Nanometer Scale or Less
14. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kahn, O.; Martinez, C.J. Spin-Transition Polymers: From Molecular Materials toward Memory Devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular Spin Crossover Phenomenon: Recent Achievements and Prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Demont, P.; Menegotto, J. Observation of a Thermal Hysteresis Loop in the Dielectric Constant of Spin Crossover Complexes: Towards Molecular Memory Devices. J. Mater. Chem. 2003, 13, 2069–2071. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ruben, M. Emerging Trends in Spin Crossover (SCO) Based Functional Materials and Devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Molnár, G.; Rat, S.; Salmon, L.; Nicolazzi, W.; Bousseksou, A. Spin Crossover Nanomaterials: From Fundamental Concepts to Devices. Adv. Mater. 2018, 30, 17003862. [Google Scholar] [CrossRef]
- Rotaru, A.; Dugay, J.; Tan, R.P.; Guralskiy, I.A.; Salmon, L.; Demont, P.; Carrey, J.; Molnár, G.; Respaud, M.; Bousseksou, A. Nano-electromanipulation of Spin Crossover Nanorods: Towards Switchable Nanoelectronic Devices. Adv. Mater. 2013, 25, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Lefter, C.; Davesne, V.; Salmon, L.; Molnár, G.; Demont, P.; Rotaru, A.; Bousseksou, A. Charge Transport and Electrical Properties of Spin Crossover Materials: Towards Nanoelectronic and Spintronic Devices. Magnetochemistry 2016, 2, 18. [Google Scholar] [CrossRef]
- Kumar, K.S.; Ruben, M. Sublimable Spin-crossover Complexes: From Spin-State Switching to Molecular Devices. Angew. Chem. Int. Ed. 2020, 59, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Lefter, C.; Tan, R.; Dugay, J.; Tricard, S.; Molnár, G.; Salmon, L.; Carrey, J.; Nicolazzi, W.; Rotaru, A.; Bousseksou, A. Unidirectional Electric Field-Induced Spin-State Switching in Spin Crossover Based Microelectronic Devices. Chem. Phys. Lett. 2016, 644, 138–141. [Google Scholar] [CrossRef]
- Konstantinov, N.; Tauzin, A.; Noumbe, U.N.; Dragoe, D.; Kundys, B.; Majjad, H.; Brosseau, A.; Lenertz, M.; Singh, A.; Berciaud, S.; et al. Electrical read-out of light-induced spin transition in thin film spin crossover/graphene heterostructures. J. Mater. Chem. C 2021, 9, 2712–2720. [Google Scholar] [CrossRef]
- Mahfoud, T.; Molnár, G.; Cobo, S.; Salmon, L.; Thibault, C.; Vieu, C.; Demont, P.; Bousseksou, A. Electrical Properties and Non-Volatile Memory Effect of the [Fe(HB(pz)3)2] Spin Crossover Complex Integrated in a Microelectrode Device. Appl. Phys. Lett. 2011, 99, 053307. [Google Scholar] [CrossRef]
- Hao, G.; Cheng, R.; Dowben, P.A. The Emergence of the Local Moment Molecular Spin Transistor. J. Phys. Condens. Matter 2020, 32, 234002. [Google Scholar] [CrossRef]
- Hao, G.; Mosey, A.; Jiang, X.; Yost, A.J.; Sapkota, K.R.; Wang, G.T.; Zhang, X.; Zhang, J.; N’Diaye, A.T.; Cheng, R.; et al. Nonvolatile Voltage Controlled Molecular Spin State Switching. Appl. Phys. Lett. 2019, 114, 032901. [Google Scholar] [CrossRef]
- Mosey, A.; Dale, A.S.; Hao, G.; N’Diaye, A.T.; Dowben, P.A.; Cheng, R. Quantitative Study of Energy Change in Voltage-controlled Spin Crossover Molecules for Molecular Spintronics. J. Phys. Chem. Lett. 2020, 11, 8231–8237. [Google Scholar] [CrossRef]
- Khusniyarov, M.M. How to Switch Spin-Crossover Metal Complexes at Constant Room Temperature. Chem. Eur. J. 2016, 22, 15178–15191. [Google Scholar] [CrossRef]
- Cambi, L.; Cagnasso, A. Iron dithiocarbamates and nitrosodithiocarbamates. Atti. R. Accad. Naz. Lincei 1931, 13, 809. [Google Scholar]
- Cambi, L.; Szegö, L. Über die magnetische Susceptibilität der komplexen Verbindungen. Ber. Deutsch. Chem. Ges. B 1931, 64, 2591–2598. [Google Scholar] [CrossRef]
- Cambi, L.; Szegö, L.; Cagnasso, A. The Magnetic Susceptibility of Complexes. IV. Atti. R. Accad. Naz. Lincei 1932, 15, 266. [Google Scholar]
- Cambi, L.; Szegö, L.; Cagnasso, A. The magnetic susceptibility of complexes. V. Iron dibutyldithio-carbamates. Atti. R. Accad. Naz. Lincei 1932, 15, 329–335. [Google Scholar]
- Cambi, L.; Szegö, L. Über die magnetische Susceptibilität der komplexen Verbindungen (II. Mitteil.). Ber. Deutsch. Chem. Ges. B 1933, 66, 656–661. [Google Scholar] [CrossRef]
- Cambi, L.; Malatesta, L. Magnetismus und polymorphie innerer komplexsalze: Eisensalze der dithiocarbamidsäuren. Ber. Deutsch. Chem. Ges. B 1937, 70, 2067–2078. [Google Scholar] [CrossRef]
- Prins, F.; Monrabal-Capilla, M.; Osorio, E.A.; Coronado, E.; van der Zant, H.S.J. Room-Temperature Electrical Addressing of a Bistable Spin-Crossover Molecular System. Adv. Mater. 2011, 23, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Dowben, P.A. Preface to the JPCM special issue on molecular magnetism. J. Phys. Condens. Matter 2020, 32, 440201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.J. Magnetoresistive Random Access Memory: The Path to Competitiveness and Scalability. Proc. IEEE 2008, 96, 1786–1798. [Google Scholar] [CrossRef]
- Yu, S.; Chen, P.-Y. Emerging Memory Technologies. IEEE Solid State Circuits Mag. 2016, 8, 43–56. [Google Scholar] [CrossRef]
- Senni, S.; Torres, L.; Sassatelli, G.; Gamatie, A.; Mussard, B. Exploring MRAM Technologies for Energy Efficient Systems-on-Chip. IEEE J. Emerg. Sel. Top. Circuits Syst. 2016, 6, 279–292. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Pan, C.; Naeemi, A. Benchmarking and Optimization of Spintronic Memory Arrays. IEEE J. Explor. Solid-State Computat. Devices Circuits 2020, 6, 9–17. [Google Scholar] [CrossRef]
- Everspin 256Mb STT-MRAM (ST-DDR3). Available online: https://www.everspin.com/family/emd3d256m?npath=3557 (accessed on 15 November 2020).
- Everspin 1Gb STT-MRAM (ST-DDR4). Available online: https://www.everspin.com/family/emd4e001g?npath=3557 (accessed on 15 November 2020).
- Avalanche Technology Discrete MRAM, 50MHz Clock Rate in 1Mb and 4Mb Density Chips. Available online: https://www.avalanche-technology.com/products/discrete-mram/ (accessed on 15 November 2020).
- Kitagawa, E.; Fujita, S. TT-MRAM Cuts Power Use by 80%. In Proceedings of the 2013 IEEE International Electron Devices Meeting (IEDM 2013), Washington, DC, USA, 9–11 December 2013; Available online: http://www.eetimes.com/document.asp?doc_id=1280753 (accessed on 15 November 2020).
- Wang, K.L.; Alzate, J.G.; Amiri, P.K. Low-Power Non-Volatile Spintronic Memory: STT-RAM and Beyond. J. Phys. D Appl. Phys. 2013, 46, 074003. [Google Scholar] [CrossRef]
- Zhang, K.; Cao, K.; Zhang, Y.; Huang, Z.; Cai, W.; Wang, J.; Nan, J.; Wang, G.; Zheng, Z.; Chen, L.; et al. Rectified Tunnel Magnetoresistance Device With High On/Off Ratio for In-Memory Computing. IEEE Electron. Device Lett. 2020, 41, 928–931. [Google Scholar] [CrossRef]
- Patel, R.; Ipek, E.; Friedman, E.G. 2T–1R STT-MRAM memory cells for enhanced on/off current ratio. Microelectron. J. 2014, 45, 133–143. [Google Scholar] [CrossRef]
- Ohsawa, T.; Ikeda, S.; Hanyu, T.; Ohno, H.; Endoh, T. Trend of tunnel magnetoresistance and variation in threshold voltage for keeping data load robustness of metal–oxide–semiconductor/magnetic tunnel junction hybrid latches. J. Appl. Phys. 2014, 115, 17C728. [Google Scholar] [CrossRef]
- Cobo, S.; Molnár, G.; Real, J.A.; Bousseksou, A. Multilayer Sequential Assembly of Thin Films that Display Room Temperature Spin Crossover with Hysteresis. Angew. Chem. Int. Ed. 2006, 45, 5786–5789. [Google Scholar] [CrossRef]
- Molnár, G.; Cobo, S.; Real, J.A.; Carcenac, F.; Daran, E.; Vieu, C.; Bousseksou, A. A Combined Top-Down/Bottom-Up Approach for the Nanoscale Patterning of Spin-Crossover Coordination Polymers. Adv. Mater. 2007, 19, 2163–2167. [Google Scholar] [CrossRef]
- Miyamachi, T.; Gruber, M.; Davesne, V.; Bowen, M.; Boukari, S.; Scheurer, L.; Joly, F.; Rogez, G.; Yamada, T.K.; Ohresser, P.; et al. Robust Spin Crossover and Memristance Across a Single Molecule. Nat. Commun. 2012, 3, 938. [Google Scholar] [CrossRef]
- Briois, V.; Moulin, C.C.D.; Sainctavit, P.; Brouder, C.; Flank, A.-M. Full Multiple Scattering and Crystal Field Multiplet Calculations Performed on the Spin Transition FeII(phen)2(NCS)2 Complex at the Iron K and L2,3 X-ray Absorption Edges. J. Am. Chem. Soc. 1995, 117, 1019–1026. [Google Scholar] [CrossRef]
- Gruber, M.; Miyamachi, T.; Davesne, V.; Bowen, M.; Boukari, S.; Wulfhekel, W.; Alouani, M.; Beaurepaire, E. Spin crossover in Fe(phen)2(NCS)2 Complexes on Metallic Surfaces. J. Chem. Phys. 2017, 146, 092312. [Google Scholar] [CrossRef]
- Gruber, M.; Davesne, V.; Bowen, M.; Boukari, S.; Beaurepaire, E.; Wulfhekel, W.; Miyamachi, T. Spin State of Spin-Crossover Complexes: From Single Molecules to Ultrathin Films. Phys. Rev. B 2014, 89, 195415. [Google Scholar] [CrossRef]
- Ellingsworth, E.C.; Turner, B.; Szulczewski, G. Thermal Conversion of [Fe(phen)3](SCN)2 Thin Films into the Spin Crossover Complex Fe(phen)2(NCS)2. RSC Adv. 2013, 3, 3745–3754. [Google Scholar] [CrossRef]
- Beniwal, S.; Sarkar, S.; Baier, F.; Weber, B.; Dowben, P.A.; Enders, A. Site Selective Adsorption of the Spin Crossover Complex Fe(phen)2(NCS)2 on Au(111). J. Phys. Condens. Matter 2020, 32, 324003. [Google Scholar] [CrossRef]
- Shi, S.; Schmerber, G.; Arabski, J.; Beaufrand, J.-B.; Kim, D.J.; Boukari, S.; Bowen, M.; Kemp, N.T.; Viart, N.; Rogez, G.; et al. Study of molecular spin-crossover complex Fe(phen)2(NCS)2 thin films. Appl. Phys. Lett. 2009, 95, 043303. [Google Scholar] [CrossRef]
- Gopakumar, T.G.; Bernien, M.; Naggert, H.; Matino, F.; Hermanns, C.F.; Bannwarth, A.; Mühlenberend, S.; Krüger, A.; Krüger, D.; Nickel, F.; et al. Spin-Crossover Complex on Au(111): Structural and Electronic Differences Between Mono- and Multilayers. Chem. Eur. J. 2013, 19, 15702–15709. [Google Scholar] [CrossRef] [PubMed]
- Poggini, L.; Gonidec, M.; Balasubramanyam, R.K.; Squillantini, L.; Pecastaings, G.; Caneschi, A.; Rosa, P. Temperature-Induced Transport Changes in Molecular Junctions Based on a Spin Crossover Complex. J. Mater. Chem. C 2019, 7, 5343–5347. [Google Scholar] [CrossRef]
- Lefter, C.; Rat, S.; Sánchez Costa, J.; Manrique-Juárez, M.D.; Quintero, C.M.; Salmon, L.; Séguy, I.; Leichle, T.; Nicu, L.; Demont, P.; et al. Current Switching Coupled to Molecular Spin-States in Large-Area Junctions. Adv. Mater. 2016, 28, 7508–7514. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, E.; Naggert, H.; Kalläne, M.; Rohlf, S.; Kröger, E.; Bannwarth, A.; Quer, A.; Rossnagel, K.; Kipp, L.; Tuczek, F. Iron(II) Spin-Crossover Complexes in Ultrathin Films: Electronic Structure and Spin-State Switching by Visible and Vacuum-UV Light. Angew. Chem. Int. Ed. 2014, 53, 3019–3023. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, F.; Studniarek, M.; Kumar, K.S.; Urbain, E.; Katcko, K.; Chen, J.; Frauhammer, T.; Hervé, M.; Halisdemir, U.; Kandpal, L.M.; et al. Linking Electronic Transport through a Spin Crossover Thin Film to the Molecular Spin State Using X-ray Absorption Spectroscopy Operando Techniques. ACS Appl. Mater. Interfaces 2018, 10, 31580–31585. [Google Scholar] [CrossRef]
- Bernien, M.; Naggert, H.; Arruda, L.M.; Kipgen, L.; Nickel, F.; Miguel, J.; Hermanns, C.F.; Krüger, A.; Krüger, D.; Schierle, E.; et al. Highly Efficient Thermal and Light-Induced Spin-State Switching of an Fe(II) Complex in Direct Contact with a Solid Surface. ACS Nano 2015, 9, 8960–8966. [Google Scholar] [CrossRef] [PubMed]
- Gopakumar, T.G.; Matino, F.; Naggert, H.; Bannwarth, A.; Tuczek, F.; Berndt, R. Electron-Induced Spin Crossover of Single Molecules in a Bilayer on Gold. Angew. Chem. Int. Ed. 2012, 51, 6262–6266. [Google Scholar] [CrossRef]
- Poggini, L.; Gonidec, M.; González-Estefan, J.H.; Pecastaings, G.; Gobaut, B.; Rosa, P. Vertical Tunnel Junction Embedding a Spin Crossover Molecular Film. Adv. Electron. Mater. 2018, 4, 1800204. [Google Scholar] [CrossRef]
- Shalabaeva, V.; Ridier, K.; Rat, S.; Manrique-Juarez, M.D.; Salmon, L.; Séguy, I.; Rotaru, A.; Molnár, G.; Bousseksou, A. Room Temperature Current Modulation in Large Area Electronic Junctions of Spin Crossover Thin Films. Appl. Phys. Lett. 2018, 112, 013301. [Google Scholar] [CrossRef]
- Zhang, Y.; Séguy, I.; Ridier, K.; Shalabaeva, V.; Piedrahita-Bello, M.; Rotaru, A.; Salmon, L.; Molnár, G.; Bousseksou, A. Resistance Switching in Large-Area Vertical Junctions of the Molecular Spin Crossover Complex [Fe(HB(tz)3)2]: ON/OFF Ratios and Device Stability. J. Phys. Condens. Matter 2020, 32, 214010. [Google Scholar] [CrossRef] [PubMed]
- Ridier, K.; Bas, A.C.; Shalabaeva, V.; Nicolazzi, W.; Salmon, L.; Molnár, G.; Bousseksou, A.; Lorenc, M.; Bertoni, R.; Collet, E.; et al. Finite Size Effects on the Switching Dynamics of Spin-Crossover Thin Films Photoexcited by a Femtosecond Laser Pulse. Adv. Mater. 2019, 31, 1901361. [Google Scholar] [CrossRef] [PubMed]
- Ridier, K.; Bas, A.C.; Zhang, Y.; Routaboul, L.; Salmon, L.; Molnár, G.; Bergaud, C.; Bousseksou, A. Unprecedented Switching Endurance Affords for High-Resolution Surface Temperature Mapping Using a Spin-Crossover Film. Nat. Commun. 2020, 11, 3611. [Google Scholar] [CrossRef]
- Zhang, L.; Tong, Y.; Kelai, M.; Bellec, A.; Lagoute, J.; Chacon, C.; Girard, Y.; Rousset, S.; Boillot, M.-L.; Rivière, E.; et al. Anomalous Light-Induced Spin-State Switching for Iron(II) Spin-Crossover Molecules in Direct Contact with Metal Surfaces. Angew. Chem. Int. Ed. 2020, 59, 13341–13346. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, K.; Iasco, O.; Bellec, A.; Kartsev, A.; Li, D.; Lagoute, J.; Chacon, C.; Girard, Y.; Rousset, S.; Miserque, F.; et al. Molecular-Scale Dynamics of Light-Induced Spin crossover in a Two-Dimensional layer. Nat. Commun. 2016, 7, 12212. [Google Scholar] [CrossRef]
- Bairagi, K.; Bellec, A.; Fourmental, C.; Iasco, O.; Lagoute, J.; Chacon, C.; Girard, Y.; Rousset, S.; Choueikani, F.; Otero, E.; et al. Temperature-, Light-, and Soft X-ray-Induced Spin Crossover in a Single Layer of FeII-Pyrazolylborate Molecules in Direct Contact with Gold. J. Phys. Chem. C 2018, 122, 727–731. [Google Scholar] [CrossRef]
- Iasco, O.; Boillot, M.-L.; Bellec, A.; Guillot, R.; Rivière, E.; Mazerat, S.; Nowak, S.; Morineau, D.; Brosseau, A.; Miserque, F.; et al. The Disentangling of Hysteretic Spin Transition, Polymorphism and Metastability in Bistable Thin Films Formed by Sublimation of Bis(scorpionate) Fe(II) Molecules. J. Mater. Chem. C 2017, 5, 11067–11075. [Google Scholar] [CrossRef]
- Fourmental, C.; Mondal, S.; Banerjee, R.; Bellec, A.; Garreau, Y.; Coati, A.; Chacon, C.; Girard, Y.; Lagoute, J.; Rousset, S.; et al. Importance of Epitaxial Strain at a Spin-Crossover Molecule−Metal Interface. J. Phys. Chem. Lett. 2019, 10, 4103–4109. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Poggini, L.; Squillantini, L.; Cortigiani, B.; Gonidec, M.; Bencok, P.; Sessoli, R.; Mannini, M. Thermal and Light-Induced Spin Transition in a Nanometric Film of a New High-Vacuum Processable Spin Crossover Complex. J. Mater. Chem. C 2018, 6, 8885–8889. [Google Scholar] [CrossRef]
- Zhao, T.; Dong, M.; Zhao, Y. A novel fabrication of [Fe(HB(pz)3)2]@MIL-101 hybrid material via diffusion and the lower temperature shift on its spin transition behavior. Appl. Phys. A 2019, 125, 670. [Google Scholar] [CrossRef]
- Warner, B.; Oberg, J.C.; Gill, T.G.; El Hallak, F.; Hirjibehedin, C.F.; Serri, M.; Heutz, S.; Arrio, M.A.; Sainctavit, P.; Mannini, M.; et al. Temperature- and Light-Induced Spin Crossover Observed by X-ray Spectroscopy on Isolated Fe(II) Complexes on Gold. J. Phys. Chem. Lett. 2013, 4, 1546–1552. [Google Scholar] [CrossRef]
- Pronschinske, A.; Bruce, R.C.; Lewis, G.; Chen, Y.; Calzolari, A.; Buongiorno-Nardelli, M.; Shultz, D.A.; You, W.; Dougherty, D.B. Iron(II) Spin Crossover Films on Au(111): Scanning Probe Microscopy and Photoelectron Spectroscopy. Chem. Commun. 2013, 49, 10446–10452. [Google Scholar] [CrossRef]
- Pronschinske, A.; Chen, Y.; Lewis, G.F.; Shultz, D.A.; Calzolari, A.; Buongiorno Nardelli, M.; Dougherty, D.B. Modification of Molecular Spin Crossover in Ultrathin Films. Nano Lett. 2013, 13, 1429. [Google Scholar] [CrossRef]
- Zhang, X.; Palamarciuc, T.; Rosa, P.; Létard, J.F.; Doudin, B.; Zhang, Z.; Wang, J.; Dowben, P.A. The Electronic Structure of a Spin Crossover Molecular Adsorbate. J. Phys. Chem. C 2012, 116, 23291–23296. [Google Scholar] [CrossRef]
- Beniwal, S.; Zhang, X.; Mu, S.; Naim, A.; Rosa, P.; Chastanet, G.; Létard, J.F.; Liu, J.; Sterbinsky, G.E.; Arena, D.A.; et al. Substrate-Induced Spin State Locking of the [Fe(H2B(pz)2)2(bipy)] Spin Crossover Complex. J. Phys. Condens. Matter 2016, 28, 206002. [Google Scholar] [CrossRef] [PubMed]
- Palamarciuc, T.; Oberg, J.C.; El Hallak, F.; Hirjibehedin, C.F.; Serri, M.; Heutz, S.; Letard, J.-F.; Patrick Rosa, P. Spin crossover materials evaporated under clean high vacuum and ultra-high vacuum conditions: From thin films to single molecules. J. Mater. Chem. 2012, 22, 9690. [Google Scholar] [CrossRef]
- Naggert, H.; Bannwarth, A.; Chemnitz, S.; von Hofe, T.; Quandt, E.; Tuczek, F. First Observation of Light-Induced Spin Change in Vacuum Deposited Thin Films of Iron Spin Crossover Complexes. Dalton Trans. 2011, 40, 6364–6366. [Google Scholar] [CrossRef]
- Jiang, X.; Hao, G.; Wang, X.; Mosey, A.; Zhang, X.; Yu, L.; Yost, A.J.; DiChiara, A.D.; Cheng, X.; Zhang, J.; et al. Tunable Spin-State Bistability in a Spin Crossover Molecular Complex. J. Phys. Condens. Matter 2019, 31, 315401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Palamarciuc, T.; Létard, J.F.; Rosa, P.; Lozada, E.V.; Torres, F.; Rosa, L.G.; Doudin, B.; Dowben, P.A. Spin State of a Molecular Adsorbate Driven by the Ferroelectric Substrate Polarization. Chem. Commun. 2014, 50, 2255. [Google Scholar] [CrossRef]
- Zhang, X.; Costa, P.S.; Hooper, J.; Miller, D.P.; N’Diaye, A.T.; Beniwal, S.; Jiang, X.; Yin, Y.; Rosa, P.; Routaboul, L.; et al. Locking and Unlocking the Molecular Spin Cross-Over Transition. Adv. Mater. 2017, 29, 1702257. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Zhang, X.; Yin, Y.; Chen, X.; Hong, X.; Xu, X.; Dowben, P.A. Indications of Magnetic Coupling Effects in Spin Cross-over Molecular Thin Films. Chem. Commun. 2018, 54, 944–947. [Google Scholar] [CrossRef]
- Kipgen, L.; Bernien, M.; Ossinger, S.; Nickel, F.; Britton, A.J.; Arruda, L.M.; Naggert, H.; Luo, C.; Lotze, C.; Ryll, H.; et al. Evolution of Cooperativity in the Spin Transition of an Iron(II) Complex on a Graphite Surface. Nat. Commun. 2018, 9, 2984. [Google Scholar] [CrossRef]
- Kipgen, L.; Bernien, M.; Nickel, F.; Naggert, H.; Britton, A.J.; Arruda, L.M.; Schierle, E.; Weschke, E.; Tuczek, F.; Kuch, W. Soft-x-ray-induced spin-state switching of an adsorbed Fe(II) spin-crossover complex. J. Phys. Condens. Matter 2017, 29, 394003. [Google Scholar] [CrossRef]
- Waäckerlin, C.; Donati, F.; Singha, A.; Baltic, R.; Decurtins, S.; Liu, S.-X.; Rusponi, S.; Dreiser, J. Excited Spin-State Trapping in Spin Crossover Complexes on Ferroelectric Substrates. J. Phys. Chem. C 2018, 122, 8202–8208. [Google Scholar] [CrossRef]
- Hernández, E.M.; Quintero, C.M.; Kraieva, O.; Thibault, C.; Bergaud, C.; Salmon, L.; Molnár, G.; Bousseksou, A. AFM Imaging of Molecular Spin-State Changes through Quantitative Thermomechanical Measurements. Adv. Mater. 2014, 26, 2889–2893. [Google Scholar] [CrossRef]
- Bas, A.-C.; Shalabaeva, V.; Thompson, X.; Vendier, L.; Salmon, L.; Thibault, C.; Molnár, G.; Routaboul, L.; Bousseksou, A. Effects of Solvent Vapor Annealing on the Crystallinity and Spin Crossover Properties of Thin Films of [Fe(HB(tz)3)2]. Comptes Rendus Chim. 2019, 22, 525–533. [Google Scholar] [CrossRef]
- Rat, S.; Ridier, K.; Vendier, L.; Molnár, G.; Salmon, L.; Bousseksou, A. Solvatomorphism and Structural-Spin Crossover Property Relationship in Bis[hydrotris(1,2,4-triazol-1-yl)borate]iron(II). Cryst. Eng. Comm. 2017, 19, 3271–3280. [Google Scholar] [CrossRef]
- Costa, P.; Hao, G.; Routaboul, L.; Braunstein, P.; Zhang, X.; Zhang, J.; Doudin, B.; Enders, A.; Dowben, P.A. Perturbing the Spin Crossover Transition Activation Energies in Fe(H2B(pz)2)2(bipy) with Zwitterionic Additions. J. Phys. Condens. Matter 2018, 30, 305503. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; N’Diaye, A.T.; Jiang, X.; Ekanayaka, T.K.; Mellinger, C.; Zhang, J.; Xu, X.; Dowben, P.A. Magnetic Field Perturbations to a Soft X-ray Activated Fe (II) Molecular Spin State Transition. J. Phys. Chem. C 2021, submitted. [Google Scholar]
- Guillaume, F.; Tobon, Y.A.; Bonhommeau, S.; Létard, J.-F.; Moulet, L.; Freysz, E. Photoswitching of the spin crossover polymeric material [Fe(Htrz)2(trz)](BF4) under continuous laser irradiation in a Raman scattering experiment. Chem. Phys. Lett. 2014, 604, 105–109. [Google Scholar] [CrossRef]
- Etrillard, C.; Faramarzi, V.; Dayen, J.-F.; Letard, J.-F.; Doudin, B. Photoconduction in [Fe(Htrz)2(trz)](BF4)-H2O nanocrystals. Chem. Commun. 2011, 47, 9663–9665. [Google Scholar] [CrossRef]
- Rösner, B.; Milek, M.; Witt, A.; Gobaut, B.; Torelli, P.; Fink, R.H.; Khusniyarov, M.M. Reversible Photoswitching of a Spin-Crossover Molecular Complex in the Solid State at Room Temperature. Angew. Chem. 2015, 127, 13168–13172. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kume, S.; Nishihara, H. Reversible light-induced magnetization change in an azobenzene-attached pyridylbenzimidazole complex of iron(II) at room temperature. Dalton Trans. 2009, 280–284. [Google Scholar] [CrossRef]
- Poggini, L.; Milek, M.; Londi, G.; Naim, A.; Poneti, G.; Squillantini, L.; Magnani, A.; Totti, F.; Rosa, P.; Khusniyarov, M.M.; et al. Room temperature control of spin states in a thin film of a photochromic iron(II) complex. Mater. Horiz. 2018, 5, 506–513. [Google Scholar] [CrossRef]
- Takahashi, K.; Hasegawa, Y.; Sakamoto, R.; Nishikawa, M.; Kume, S.; Nishibori, E.; Nishihara, H. Solid-State Ligand-Driven Light-Induced Spin Change at Ambient Temperatures in Bis(dipyrazolylstyrylpyridine)iron(II) Complexes. Inorg. Chem. 2012, 51, 5188–5198. [Google Scholar] [CrossRef]
- Milek, M.; Heinemann, F.W.; Khusniyarov, M.M. Spin Crossover Meets Diarylethenes: Efficient Photoswitching of Magnetic Properties in Solution at Room Temperature. Inorg. Chem. 2013, 52, 11585–11592. [Google Scholar] [CrossRef]
- Bune, A.V.; Fridkin, V.M.; Ducharme, S.; Blinov, L.M.; Palto, S.P.; Sorokin, A.V.; Yudin, S.G.; Zlatkin, A. Two-Dimensional Ferroelectric Films. Nature 1998, 391, 874–877. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, H.; Yin, Y.; Zhang, X.; Wang, X.; Yu, L.; Ahmadi, Z.; Costa, P.S.; DiChiara, A.D.; Cheng, X.; et al. Room Temperature Ferroelectricity in Continuous Croconic Acid Thin Films. Appl. Phys. Lett. 2016, 109, 102902. [Google Scholar] [CrossRef]
- Blinov, L.M.; Fridkin, V.M.; Palto, S.P.; Bune, A.V.; Dowben, P.A.; Ducharme, S. Two-Dimensional Ferroelectrics. Uspekhi Fizicheskikh Nauk [Russian edition vol.] 2000, 170, 247–262. [Google Scholar] [CrossRef]
- Kimura, K.; Ohigashi, H. Polarization Behavior in Vinylidene Fluoride-Trifluoroethylene Copolymer Thin Films. Jpn. J. Appl. Phys. 1986, 25, 383–387. [Google Scholar] [CrossRef]
- Ducharme, S.; Fridkin, V.M.; Bune, A.V.; Palto, S.P.; Blinov, L.M.; Petukhova, N.N.; Yudin, S.G. Intrinsic Ferroelectric Coercive Field. Phys. Rev. Lett. 2000, 84, 175. [Google Scholar] [CrossRef]
- Carvell, J.; Cheng, R. Study of Electrical Polarization Hysteresis in Ferroelectric Polyvinylidene Fluoride Films. Mater. Lett. 2010, 64, 1992–1995. [Google Scholar] [CrossRef]
- Qu, H.; Yao, W.; Garcia, T.; Zhang, J.; Sorokin, A.V.; Ducharme, S.; Dowben, P.A.; Fridkin, V.M. Nanoscale Polarization Manipulation and Conductance Switching in Ultra Thin Ferroelectric Copolymer Films: P(VDF-TrFE). Appl. Phys. Lett. 2003, 82, 4322. [Google Scholar] [CrossRef]
- Chu, B.; Zhou, X.; Ren, K.; Neese, B.; Lin, M.; Wang, Q.; Bauer, F.; Zhang, Q. A Dielectric Polymer with High Electric Energy Density and Fast Discharge Speed. Science 2006, 313, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, S.; Tokunaga, Y.; Giovannetti, G.; Picozzi, S.; Itoh, H.; Shimano, R.; Kumai, R.; Tokura, Y. Above-Room-Temperature Ferroelectricity in a Single-Component Molecular Crystal. Nature 2010, 463, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.; Bibes, M. Ferroelectric Tunnel Junctions for Information Storage and Processing. Nat. Commun. 2014, 5, 4289. [Google Scholar] [CrossRef]
- Dawber, M.; Rabe, K.M.; Scott, J.F. Physics of Thin-Film Ferroelectric Oxides. Rev. Mod. Phys. 2005, 77, 1083–1130. [Google Scholar] [CrossRef]
- Chen, J.; Lu, H.; Liu, H.J.; Chu, Y.H.; Dunn, S.; Ostrikov, K.; Gruverman, A.; Valanoor, N. Interface Control of Surface Photochemical Reactivity in Ultrathin Epitaxial Ferroelectric Films. Appl. Phys. Lett. 2013, 102, 182904. [Google Scholar] [CrossRef]
- Kholkin, A.L.; Brooks, K.G.; Taylor, D.V.; Hiboux, S.; Setter, N. Self-polarization Effect in Pb(Zr,Ti)O3 Thin Films. Integr. Feroelectr. 1988, 22, 525–533. [Google Scholar] [CrossRef]
- Duan, C.G.; Mei, W.N.; Yin, W.G.; Liu, J.; Hardy, J.R.; Ducharme, S.; Dowben, P.A. Simulations of Ferroelectric Polymer Film Polarization: The Role of Dipole Interactions. Phys. Rev. B 2004, 69, 235106. [Google Scholar] [CrossRef]
- Aravena, D.; Ruiz, E. Coherent Transport through Spin-Crossover Single Molecules. J. Am. Chem. Soc. 2012, 134, 777–779. [Google Scholar] [CrossRef] [PubMed]
- Mahfoud, T.; Molnár, G.; Bonhommeau, S.; Cobo, S.; Salmon, L.; Demont, P.; Tokoro, H.; Ohkoshi, S.I.; Boukheddaden, K.; Bousseksou, A. Electric-Field-Induced Charge-Transfer Phase Transition: A Promising Approach Toward Electrically Switchable Devices. J. Am. Chem. Soc. 2009, 131, 15049–15054. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Cui, H.B.; Okano, Y.; Kobayashi, H.; Einaga, Y.; Sato, O. Electrical Conductivity Modulation Coupled to a High-Spin−Low-Spin Conversion in the Molecular System [FeIII(qsal)2][Ni(dmit)2]3·CH3CN·H2O. Inorg. Chem. 2006, 45, 5739–5741. [Google Scholar] [CrossRef]
- Phan, H.; Benjamin, S.M.; Steven, E.; Brooks, J.S.; Shatruk, M. Photomagnetic Response in Highly Conductive Iron(II) Spin-Crossover Complexes with TCNQ Radicals. Angew. Chem. Int. Ed. 2015, 54, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Dorbes, S.; Valade, L.; Real, J.A.; Faulmann, C. [Fe(sal2-trien)][Ni(dmit)2]: Towards Switchable Spin Crossover Molecular Conductors. Chem. Commun. 2005, 69–71. [Google Scholar] [CrossRef]
- Van Geest, E.P.; Shakouri, K.; Fu, W.; Robert, V.; Tudor, V.; Bonnet, S.; Schneider, G.F. Contactless Spin Switch Sensing by Chemo-Electric Gating of Graphene. Adv. Mater. 2020, 32, 1903575. [Google Scholar] [CrossRef]
- Dugay, J.; Aarts, M.; Giménez-Marqués, M.; Kozlova, T.; Zandbergen, H.W.; Coronado, E.; Van der Zant, H.S.J. Phase Transitions in Spin-Crossover Thin Films Probed by Graphene Transport Measurements. Nano Lett. 2017, 17, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Mucciolo, E.R.; Lewenkopf, C.H. Disorder and Electronic Transport in Graphene. J. Phys. Condens. Matter 2010, 22, 273201. [Google Scholar] [CrossRef]
- Wurm, J.; Richter, K.; Adagideli, İ. Edge effects in graphene nanostructures: From multiple reflection expansion to density of states. Phys. Rev. B 2011, 84, 075468. [Google Scholar] [CrossRef]
- Wimmer, M.; Adagideli, İ.; Berber, S.; Tománek, D.; Richter, K. Spin Currents in Rough Graphene Nanoribbons: Universal Fluctuations and Spin Injection. Phys. Rev. Lett. 2008, 100, 177207. [Google Scholar] [CrossRef] [PubMed]
- Han, M.Y.; Brant, J.C.; Kim, P. Electron Transport in Disordered Graphene Nanoribbons. Phys. Rev. Lett. 2010, 104, 056801. [Google Scholar] [CrossRef]
- Shi, Y.-J.; Lan, J.; Ye, E.-J.; Sui, W.-Q.; Zhao, X. Four-terminal impedance of a graphene nanoribbon based structure. Eur. Phys. J. B 2014, 87, 251. [Google Scholar] [CrossRef]
- Tanaka, D.; Aketa, N.; Tanaka, H.; Horike, S.; Fukumori, M.; Tamaki, T.; Inose, T.; Akai, T.; Toyama, H.; Sakata, O.; et al. Facile preparation of hybrid thin films composed of spin-crossover nanoparticles and carbon nanotubes for electrical memory devices. Dalton Trans. 2019, 48, 7074–7079. [Google Scholar] [CrossRef]
- Hauser, A. Intersystem Crossing in Fe (II) Coordination Compounds. Coord. Chem. Rev. 1991, 111, 275–290. [Google Scholar] [CrossRef]
- Horiuchi, S.; Kagawa, F.; Hatahara, K.; Kobayashi, K.; Kumai, R.; Murakami, Y.; Tokura, Y. Above-Room-Temperature Ferroelectricity and Antiferroelectricity in Benzimidazoles. Nat. Commun. 2012, 3, 1308. [Google Scholar] [CrossRef]
- Horiuchi, S.; Ishibashi, S.; Inada, S.; Aoyagi, S. Hydrogen-Bonded Architectures and Field-Induced Polarization Switching in Bridged Bis(benzimidazole) Crystals. Cryst. Growth Des. 2019, 19, 328–335. [Google Scholar] [CrossRef]
- Yamane, H.; Tanaka, Y.; Kawabe, E.; Kanai, K.; Seki, K. Elucidation and Control of Energy Level Alignment at Organic/Metal Interfaces. Hyomen Kagaku 2008, 29, 99–104. [Google Scholar] [CrossRef][Green Version]
- Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Energy Level Alignment and Interfacial Electronic Structures at Organic/Metal and Organic/Organic Interfaces. Adv. Mater. 1999, 11, 605–625. [Google Scholar] [CrossRef]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-Level Alignment at Organic/Metal and Organic/Organic Interfaces. Adv. Mater. 2009, 21, 1450–1472. [Google Scholar] [CrossRef]
- Tokarev, A.; Salmon, L.; Guari, Y.; Nicolazzi, W.; Molnár, G.; Bousseksou, A. Cooperative Spin Crossover phenomena in [Fe(NH2trz)3](tosylate)2 Nanoparticles. Chem. Commun. 2010, 46, 8011–8013. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Lee, H.; Amiri, P.K. Magnetoelectric Random Access Memory-Based Circuit Design by Using Voltage-Controlled Magnetic Anisotropy in Magnetic Tunnel Junctions. IEEE Trans. Nanotechnol. 2015, 14, 992–997. [Google Scholar] [CrossRef]
- George, S.; Ma, K.; Aziz, A.; Li, X.; Khan, A.; Salahuddin, S.; Chang, M.F.; Datta, S.; Sampson, J.; Gupta, S.; et al. Nonvolatile memory design based on ferroelectric FETs. In Proceedings of the 53nd Annual Design Automation Conference (DAC), Austin, TX, USA, 5–9 June 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Peng, X.; Huang, S.; Luo, Y.; Sun, X.; Yu, S. DNN+NeuroSim: An End-to-End Benchmarking Framework for Compute-in-Memory Accelerators with Versatile Device Technologies. In Proceedings of the IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 7–11 December 2019. [Google Scholar] [CrossRef]
- Fernandes, C.; Santa, A.; Santos, A.; Bahubalindruni, P.; Deuermeier, J.; Martins, R.; Fortunato, E.; Barquinha, P. A Sustainable Approach to Flexible Electronics with Zinc-Tin Oxide Thin-Film Transistors. Adv. Electron. Mater. 2018, 1800032. [Google Scholar] [CrossRef]
- Kamiya, T.; Nomura, K.; Hosono, H. Present status of amorphous In–Ga–Zn–O thin-film transistors. Sci. Technol. Adv. Mater. 2010, 11, 044305. [Google Scholar] [CrossRef] [PubMed]



| Molecular Multiferroic FETs | ||
|---|---|---|
| Possibilities | Demonstrated in a Device | |
| Minimum size | 3.6 ± 0.8 nm [122] | - |
| Switching speed | <1 ps [55,108] | - |
| On state resistance | 0.5 Ω·cm [108] | 103 Ω·cm * |
| On/off ratio | 104 * | 4 [13,14] |
| Number of switches | 107 [56] | - |
| Coercive voltage | 50 mV [95], <0.2 V [91] | 25 V [13,14] |
| Upper bound operational temperature | 400 K [8] | 350 K [14] |
| Peak current density | 3.6 × 105 A.cm−2 * | - |
| Write energy/bit | 0.7 fJ * | - |
| Silicon compatible | yes | yes [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekanayaka, T.K.; Hao, G.; Mosey, A.; Dale, A.S.; Jiang, X.; Yost, A.J.; Sapkota, K.R.; Wang, G.T.; Zhang, J.; N’Diaye, A.T.; et al. Nonvolatile Voltage Controlled Molecular Spin-State Switching for Memory Applications. Magnetochemistry 2021, 7, 37. https://doi.org/10.3390/magnetochemistry7030037
Ekanayaka TK, Hao G, Mosey A, Dale AS, Jiang X, Yost AJ, Sapkota KR, Wang GT, Zhang J, N’Diaye AT, et al. Nonvolatile Voltage Controlled Molecular Spin-State Switching for Memory Applications. Magnetochemistry. 2021; 7(3):37. https://doi.org/10.3390/magnetochemistry7030037
Chicago/Turabian StyleEkanayaka, Thilini K., Guanhua Hao, Aaron Mosey, Ashley S. Dale, Xuanyuan Jiang, Andrew J. Yost, Keshab R. Sapkota, George T. Wang, Jian Zhang, Alpha T. N’Diaye, and et al. 2021. "Nonvolatile Voltage Controlled Molecular Spin-State Switching for Memory Applications" Magnetochemistry 7, no. 3: 37. https://doi.org/10.3390/magnetochemistry7030037
APA StyleEkanayaka, T. K., Hao, G., Mosey, A., Dale, A. S., Jiang, X., Yost, A. J., Sapkota, K. R., Wang, G. T., Zhang, J., N’Diaye, A. T., Marshall, A., Cheng, R., Naeemi, A., Xu, X., & Dowben, P. A. (2021). Nonvolatile Voltage Controlled Molecular Spin-State Switching for Memory Applications. Magnetochemistry, 7(3), 37. https://doi.org/10.3390/magnetochemistry7030037







