Theory of Chiral Electrodeposition by Chiral Micro-Nano-Vortices under a Vertical Magnetic Field -1: 2D Nucleation by Micro-Vortices
Abstract
:1. Introduction
2. Theory
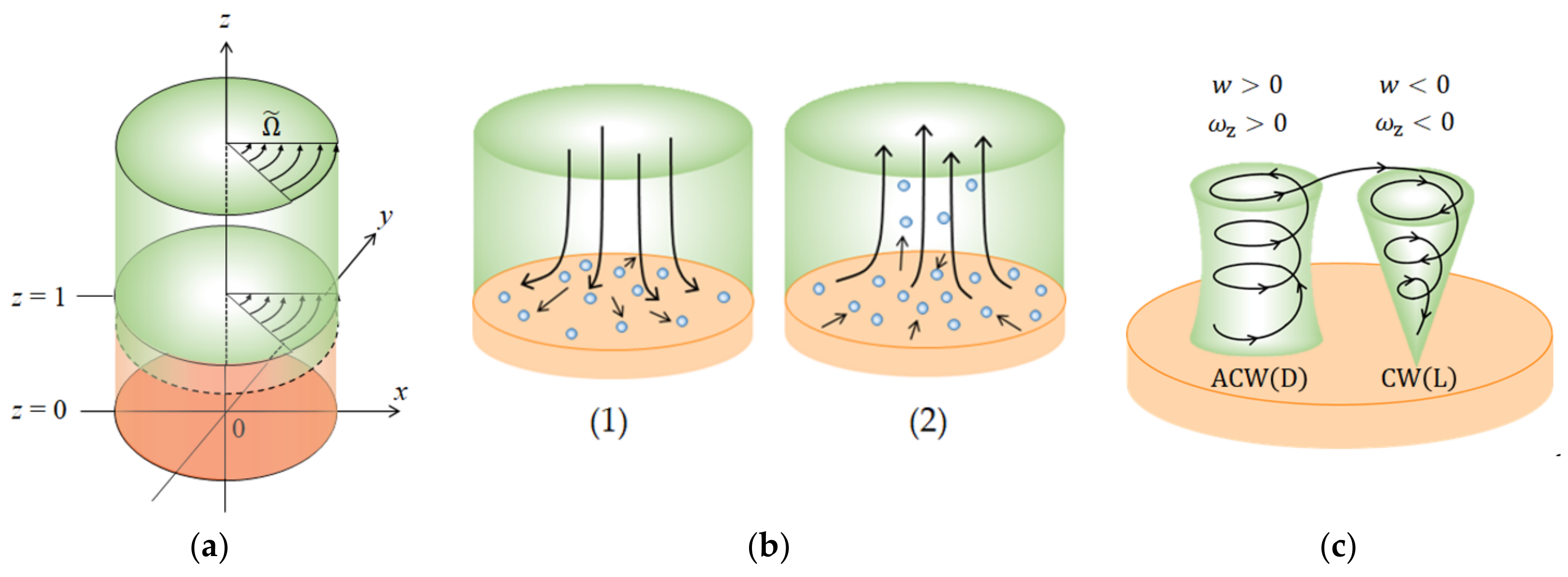
2.1. Vortex Motions in the Stationary Lower Layer
2.2. Amplitude Equations of the Fluctuations in the Lower Layer
2.3. Vortex Motions Induced in the Rotating Upper Layer
2.4. Boundary Conditions
2.4.1. Hydrodynamic Conditions
- (a)
- For the rigid surfaces:
- (b)
- For the free surfaces:
- (c)
- For the upper boundary between the lower and upper layers:
2.4.2. Mass Transfer Conditions
2.5. Solutions of and in the Lower Layer
- (a)
- For the rigid surface vortices:
- (b)
- For the free surface vortices:
2.6. Determination of the Velocity Coefficients and
- (a)
- For the rigid and free surface vortices at the upper boundary:
- (b)
- For the rigid surface vortices in the lower layer:
- (c)
- For the free surface vortices in the lower layer:
2.7. The Solution of and at the Electrode Surface
- For the rigid surface vortices:
- (b)
- For the free surface vortices:
3. 2D Nucleation
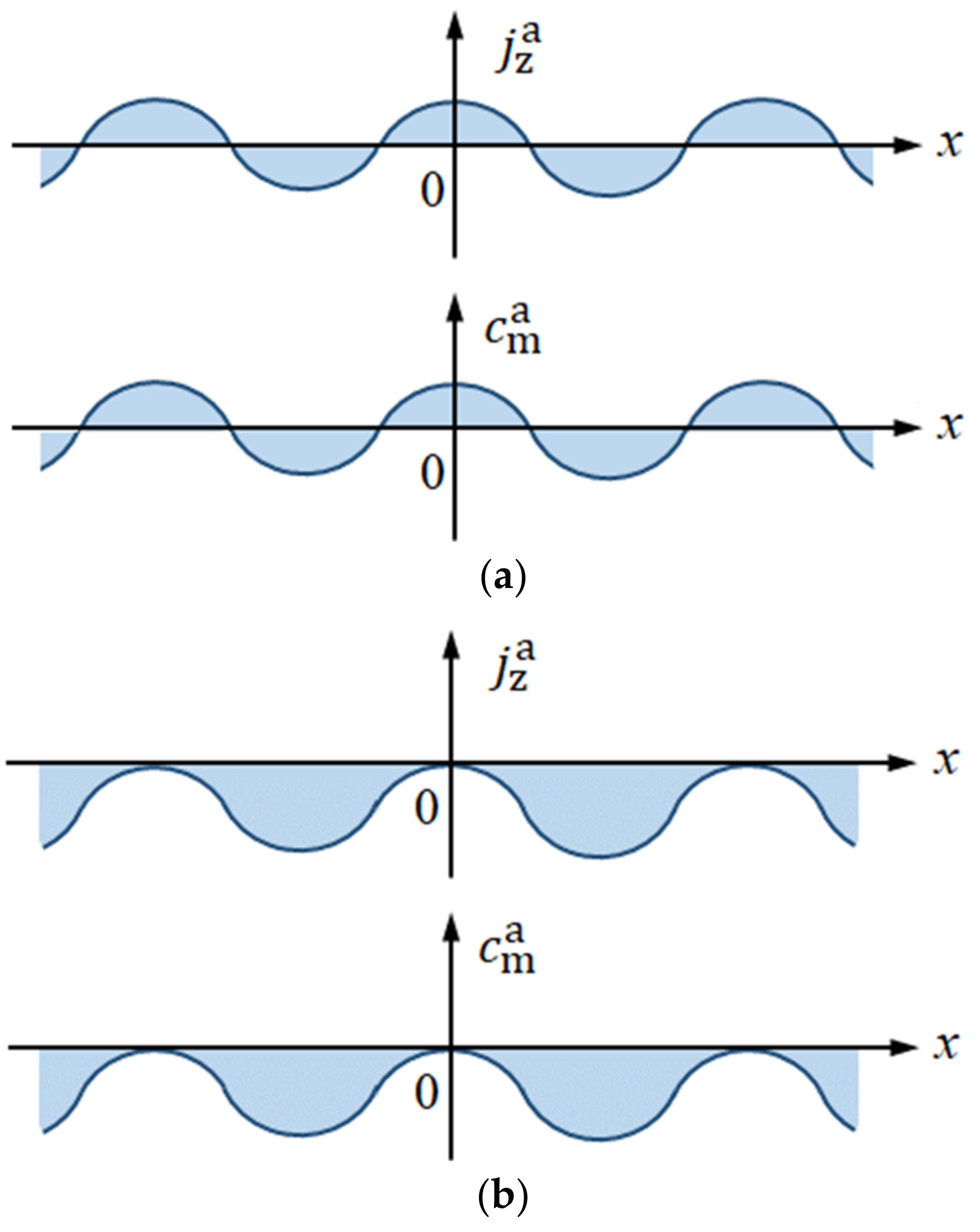
3.1. Asymmetrical Fluctuations in 2D Nucleation Process
3.2. Characteristic Equations of the Vorticity Coefficients and
- (a)
- For the rigid surface vortices:
- (b)
- For the free surface vortices:
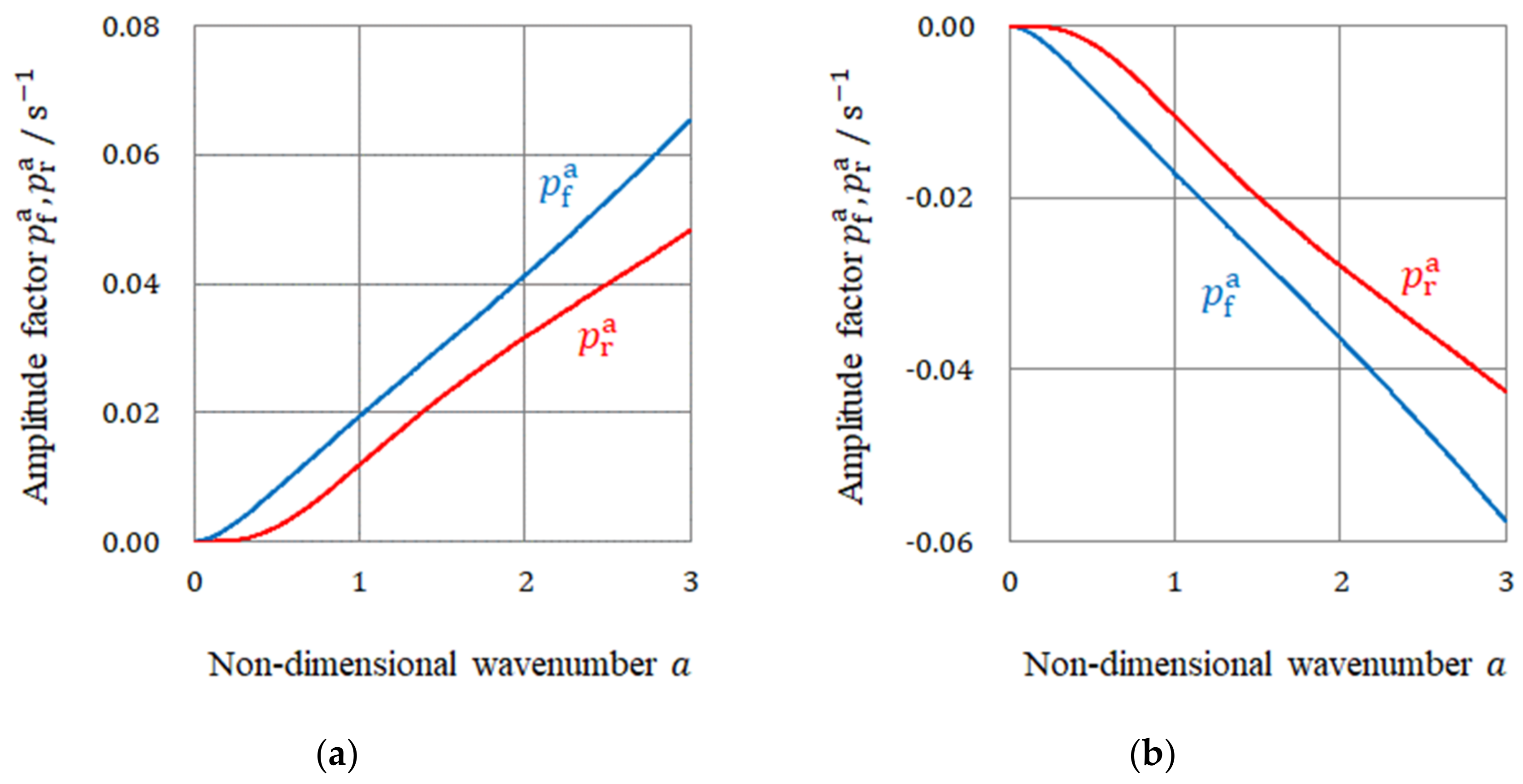
3.3. Nucleation by the Rigid and Free Surface Vortices
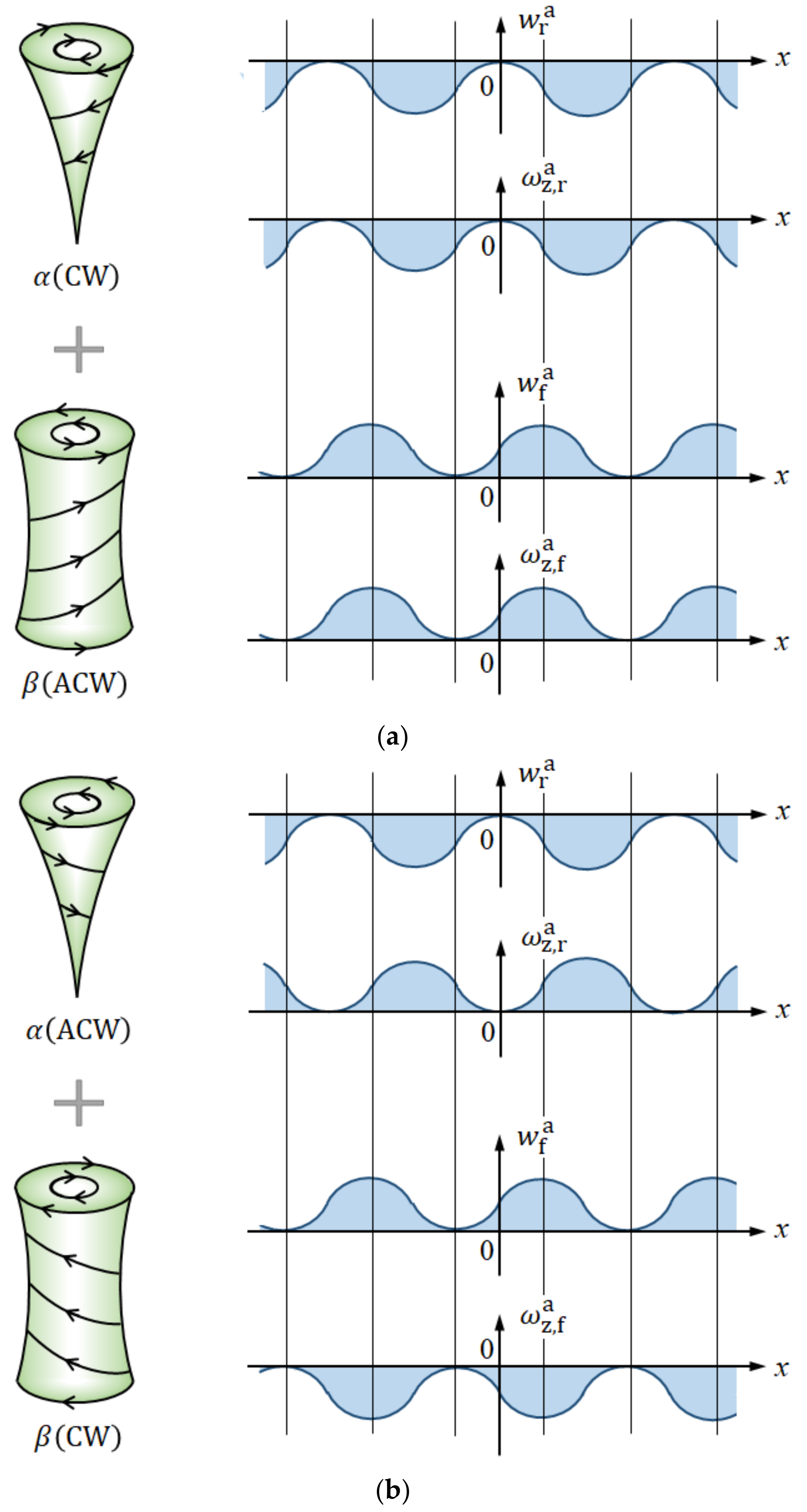
3.4. The Rotational Directions of the Micro-MHD Flows on the Rigid and Free Surfaces
- (a)
- For the rigid surfaces:
- (b)
- For the free surfaces:
4. Results and Discussion
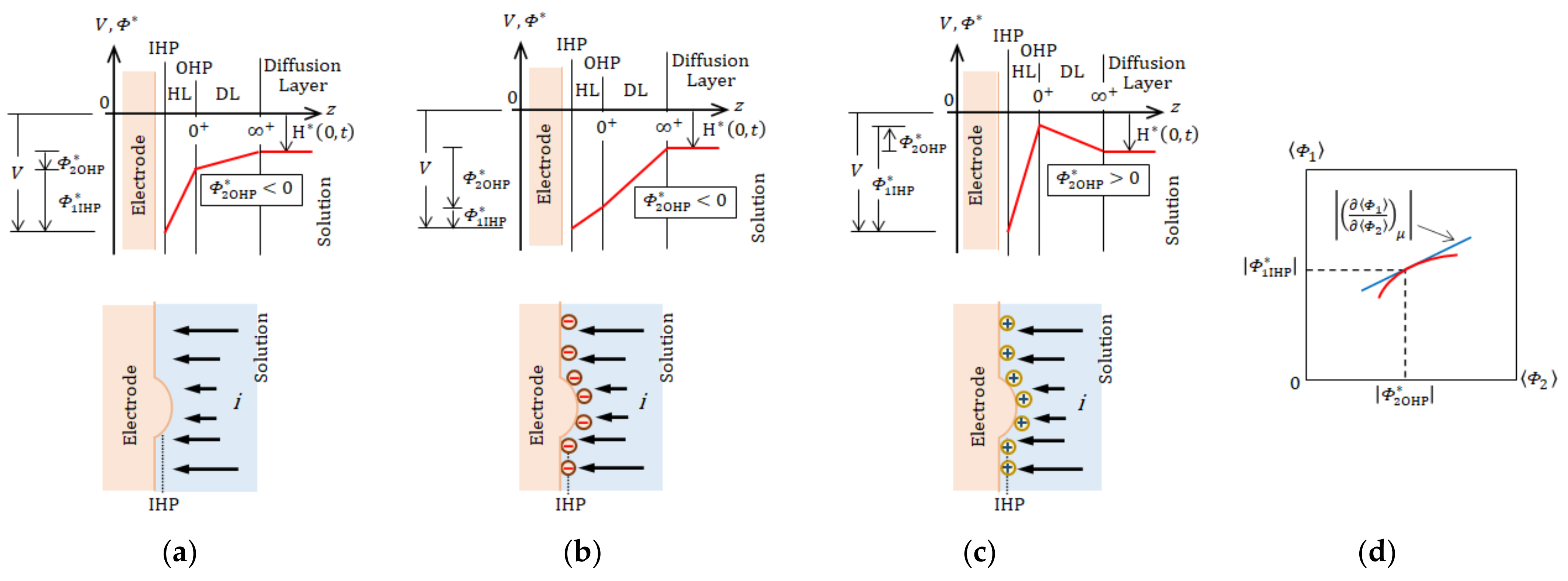
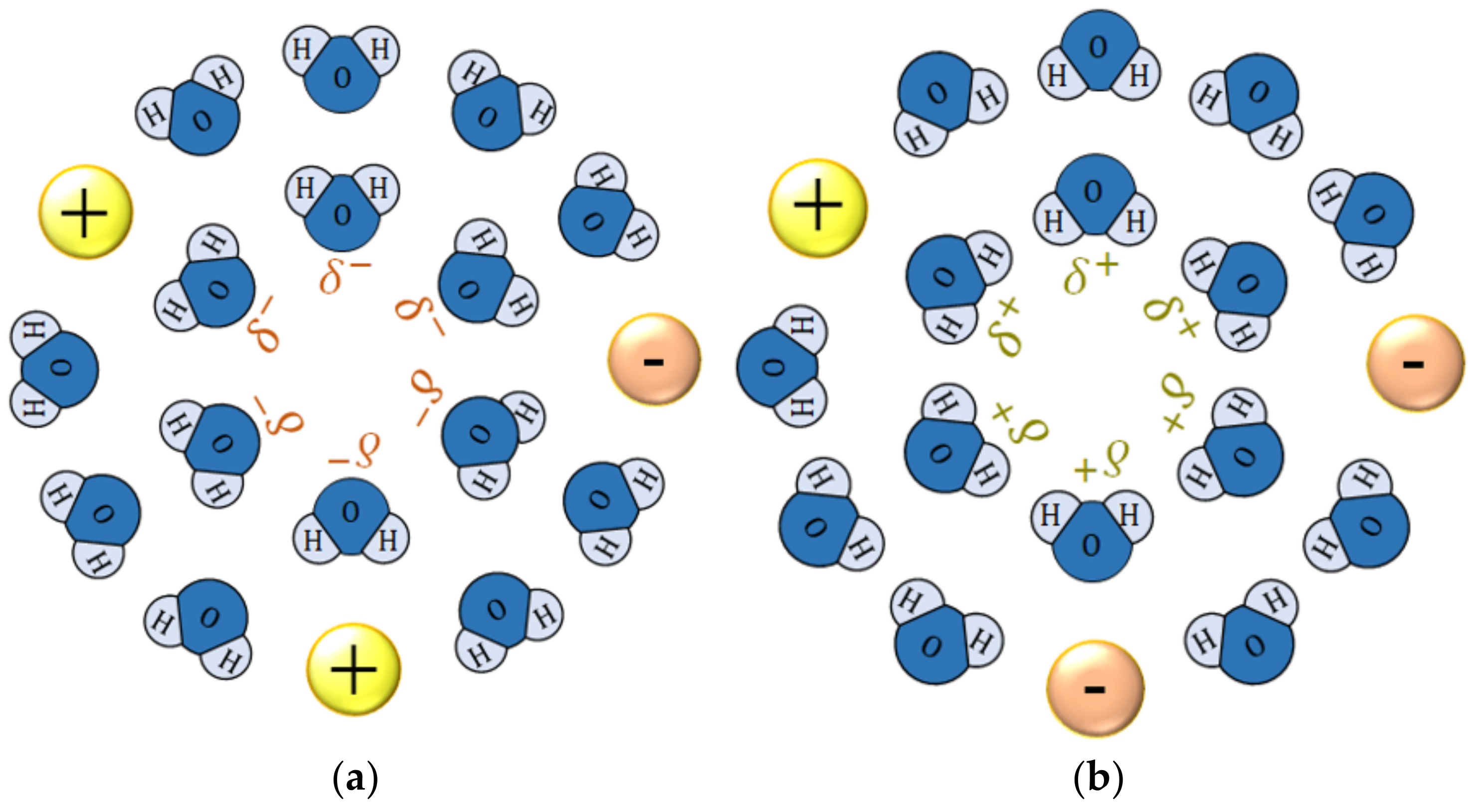
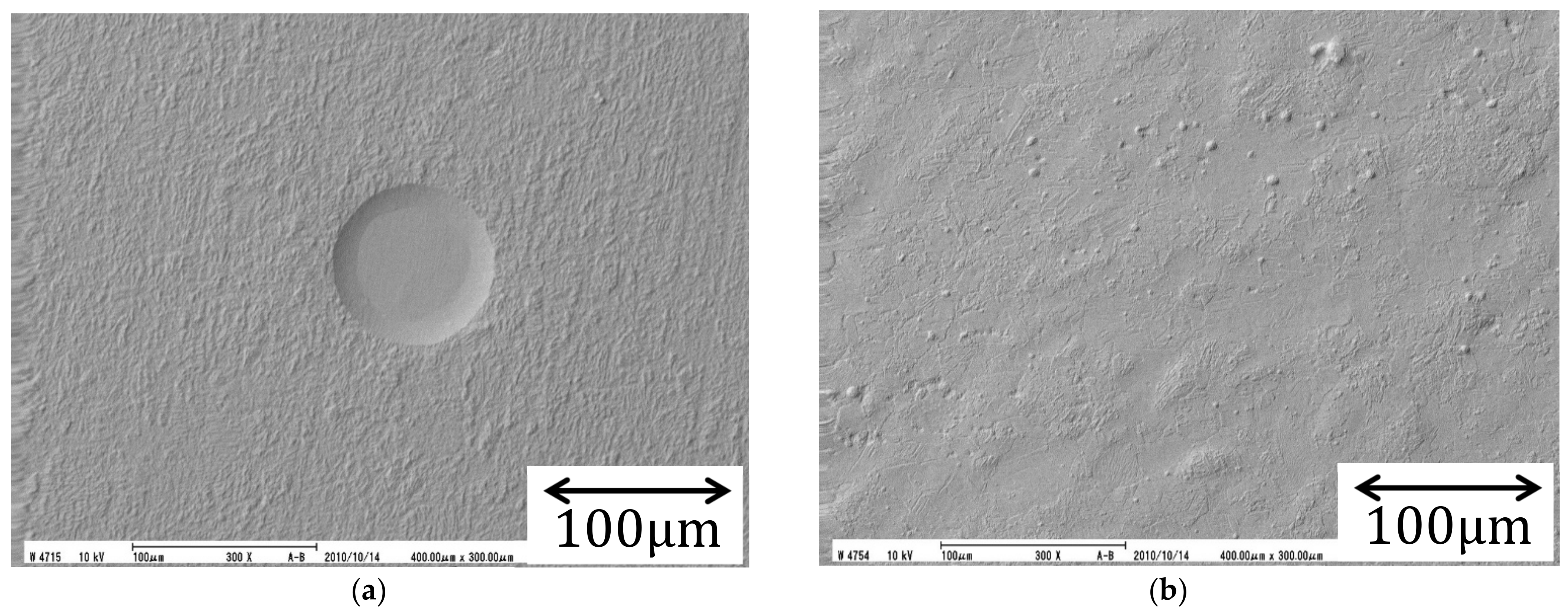
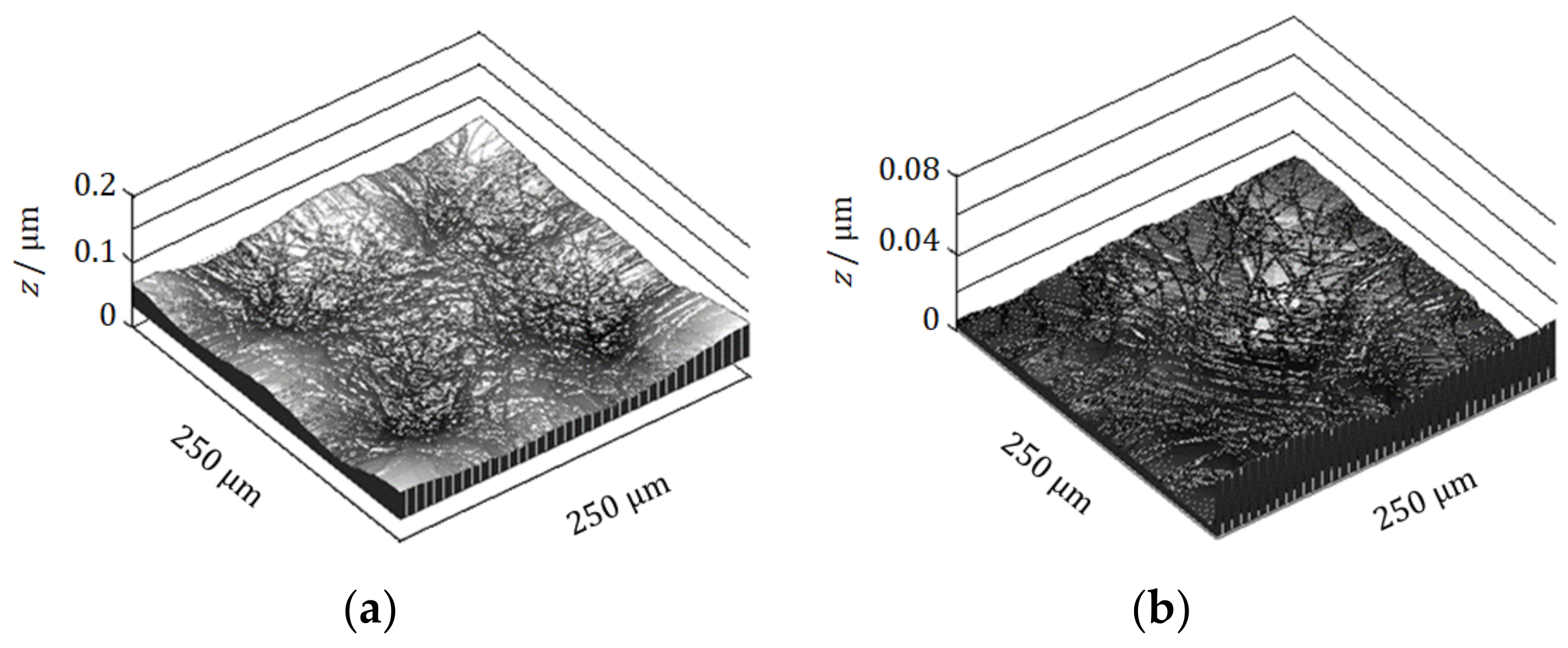
4.1. Micro-Mystery Circles Formed by the Non-Specific Adsorption of Ions
4.2. Inversion of Chirality by the Specific Adsorption of Chloride Ions
5. Materials and Methods
6. Conclusions
- 1.
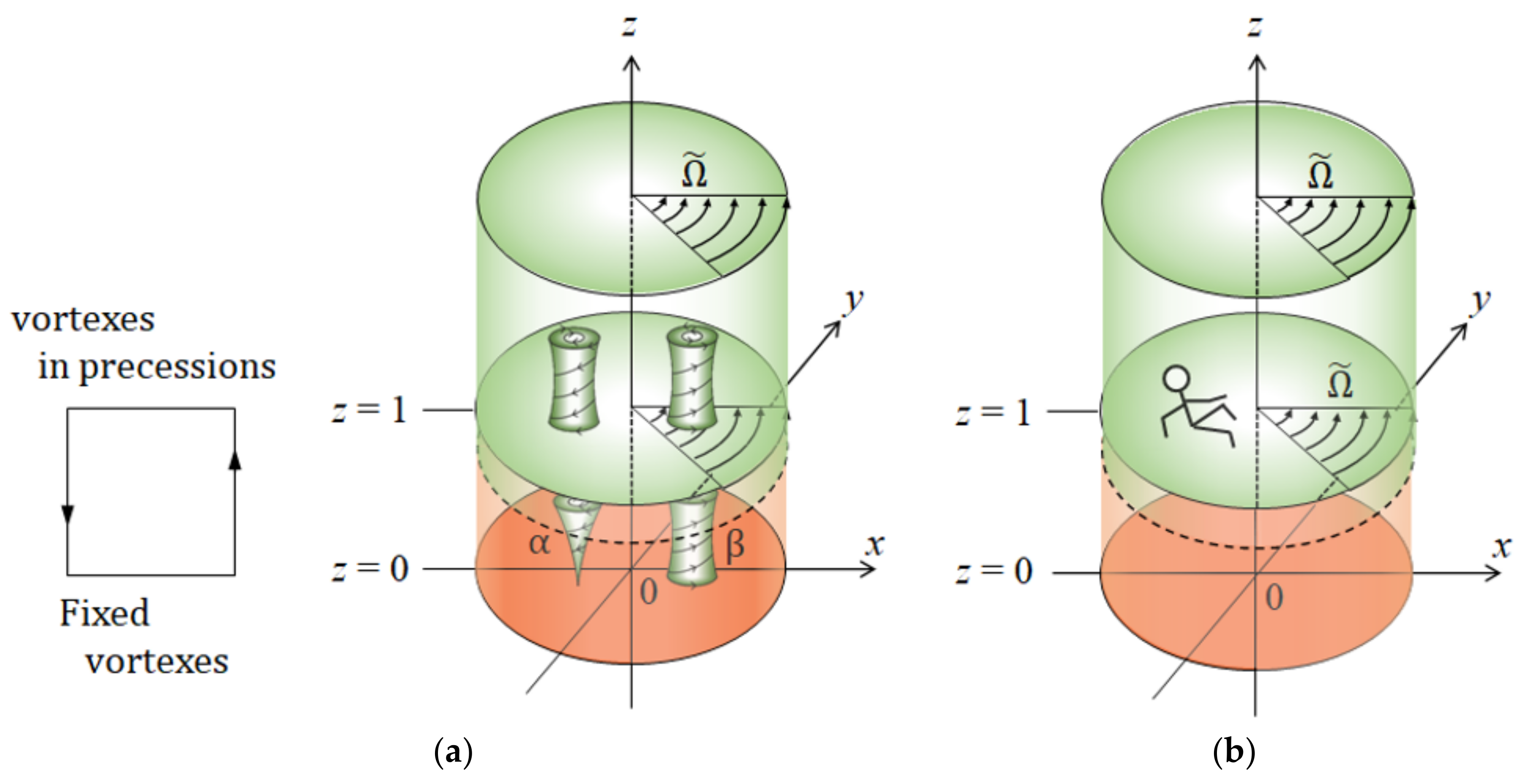
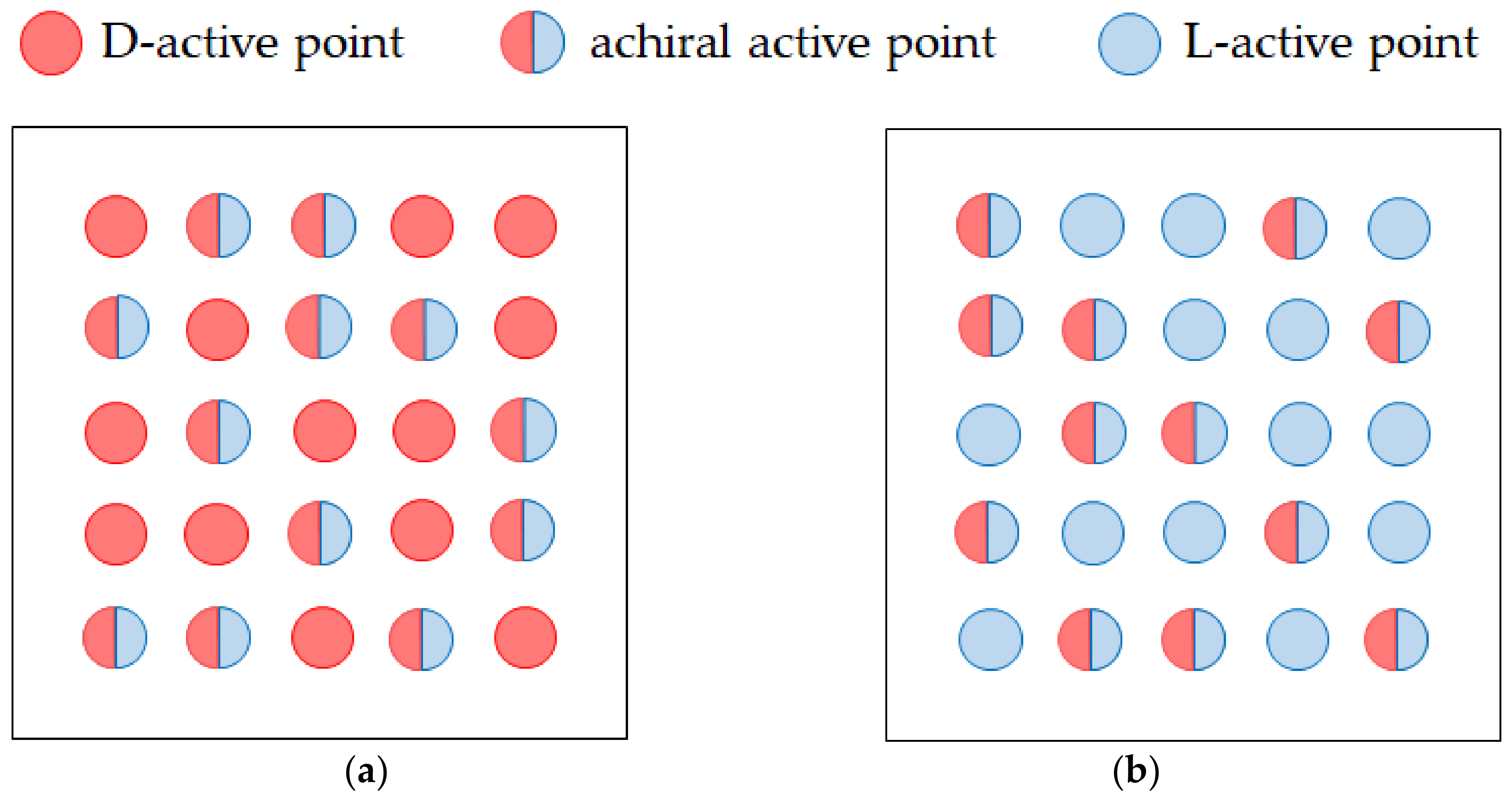
- Chiral screw dislocations under a VMHDF arise from the three generations of chiral nuclei, which constitute nesting boxes. Namely, chiral 2D nuclei are formed by the chiral micro-MHD vortices with rigid surfaces. Then, chiral 3D nuclei are created by the chiral nano-MHD vortices with rigid surfaces on a chiral 2D nucleus. Finally, chiral screw dislocations grow by chiral ultra-micro MHD vortices with rigid surfaces on a chiral 3D nucleus. Such a structure was validated by the fact that the observed enantiomeric excess (ee) ratios are always smaller than .
- 2.
- The chiral nucleation system is composed of a rotating upper layer and a stationary lower layer so that vortices in the lower layer can receive the precessions from the upper layer and raise chiral nuclei at fixed places.
- 3.
- For chirality to emerge, two types of vortices are necessary, having rigid surfaces with friction and free surfaces covered with ionic vacancies. Due to the rigid surface with friction, the rigid surface vortices not only work as pins to stop the migration of the vortices in the lower layer but also create chiral nuclei at fixed positions. Which vortex receives the precession depends on whether the growth mode is unstable or stable. Free surface vortices unstably grow faster than the rigid surface vortices, whereas, under stable conditions, rigid surface vortices activated dwindle with time more slowly than free surface vortices. Therefore, when unstable, free surface vortices have the priority of precession, and in stable cases, the precessions are donated to rigid surface vortices.
- 4.
- Due to fluid and vortex continuities, a pair of adjoining vortices are composed of rigid and free surface vortices with opposite rotations. To raise nuclei fixed to a solid surface, chiral nucleation must occur only under the rigid surface vortices. Since in a CuSO4 + H2SO4 solution, simple non-specific adsorption takes place, unstable copper nucleation proceeds. As a result, the rotation of a VMHDF transfers to the free surface vortices as the precessions, so that 2D nuclei with reverse chirality are formed under rigid surface vortices in the rotation opposite to that of the VMHDF. Though this result does not directly explain the chiral activity of the electrode, we can understand the mechanism of the emergence of the opposite chirality to the VMHDF. In accordance with the three-generation model, if such a nucleation process were repeated three times, the opposite chirality would be realized.
- 5.
- When a chloride additive is added to a CuSO4 + H2SO4 solution, specific adsorption of the chloride ions takes place, leading to stable nucleation. In this case, the rotation of a VMHDF is bestowed on the rigid surface vortices as precessions. Therefore, 2D nuclei growing under the rigid surface vortices have the same chirality as that of the VMHDF. Namely, due to the stability of the specific adsorption of chloride ions, we can expect a change in the chiral activity of the electrode. However, if the differences between both amplitude factors and their values themselves were sufficiently small, the breakdown would also take place.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| , , | Cartesian coordinates corresponding to , , (m) |
| , , | non-dimensional coordinates normalized by |
| position vector (m) | |
| d | representative length (m) |
| representative length of asymmetrical fluctuations in 2D nucleation (m) | |
| , | wavenumber components in the - and -directions (m−1) |
| wavenumber defined by (m−1) | |
| , | wavenumber components of in the - and -directions |
| non-dimensional wavenumber () | |
| autocorrelation distance of the fluctuation, i.e., the average size of the vortices (m) | |
| velocity which an observer feels (m s−1) | |
| i-component of the main flow velocity of the rotation (m s−1) | |
| velocity (m s−1) | |
| i-component of (i = 1, 2, 3) (m s−1) | |
| -component of the velocity, (m s−1) | |
| -component of the velocity, (m s−1) | |
| w | -component of the velocity, (m s−1) |
| i-component of the vorticity (s−1) | |
| -component of the vorticity (s−1) | |
| -component of stream function (m s−1) | |
| -component of stream function (m s−1) | |
| Gaussian-type power spectrum defined by Equation (F5) | |
| viscous stress tensor defined in Equation (7a) (N m−2) | |
| viscous stress tensor defined in Equation (7b) (N m−2) | |
| density of solution (kg m−3) | |
| viscosity of solution (N s m−2) | |
| kinematic viscosity (m2 s−1) | |
| kinematic viscosity of bulk solution in 2D nucleation | |
| molar volume of deposit metal (m3 mol−1) | |
| P | pressure (N m−2) |
| magnetic permeability (4π × 107 N A−2) | |
| resistivity defined by Equation (B14) | |
| dielectric constant of water (6.95 × 10−10 J−1 C2 m−1, 25 °C) | |
| universal gas constant (8.31 J K−1 mol−1) | |
| absolute temperature (K) | |
| Faraday constant (96,500 C mol−1) | |
| magnetic flux density (T) | |
| i-component of (T) | |
| external magnetic flux density in the absence of reactions (T) | |
| j-component of (T) | |
| -component of with sign (T) | |
| fluctuation of by reactions (T) | |
| i-component of (T) | |
| -component of (T) | |
| electric field (V m−1) | |
| current density (A m−2) | |
| i-component of the current density fluctuation (A m−2) | |
| -component of the current density fluctuation (A m−2) | |
| asymmetrical fluctuation of at the electrode (A m−2) | |
| electrical conductivity (S m−1) | |
| charge number of ionic species i including sign | |
| charge number of the metallic ion | |
| mobility of ionic species i (m2 V−1 s−1) | |
| -component of unit normal vector | |
| concentration of ionic species (mol m−3) | |
| diffusion coefficient of ionic species i (m2 s−1) | |
| diffusion coefficient of the metallic ion (m2 s−1) | |
| Lorentz force per unit volume (N m−3) | |
| i-component of (N m−3) | |
| acceleration which an observer feels in a frame of reference rotation with the same angular velocity as the upper layer (N m−3) | |
| i-component of the fluctuation of (N m−3) | |
| i-component of the fluctuation of the Lorentz force (N m−3) | |
| concentration of the metallic ion (mol m−3) | |
| concentration of the metallic ion in the absence of fluctuation (mol m−3) | |
| concentration fluctuation of the metallic ion (mol m−3) | |
| asymmetrical fluctuation of the concentration of the metallic ion (mol m−3) | |
| at OHP (mol m−3) | |
| surface concentration of the metallic ion outside the double layer (mol m−3) | |
| bulk concentration of the metallic ion (mol m−3) | |
| bulk concentration of ionic species j (mol m−3) | |
| average concentration gradient in the diffusion layer defined by Equation (C8) (mol m−4) | |
| concentration difference between the bulk and the surface (mol m−3) | |
| average thickness of a diffusion layer (m) | |
| amplitude of u (m s−1) | |
| amplitude of v (m s−1) | |
| amplitude of w (m s−1) | |
| amplitude of (s−1) | |
| real amplitude without i (m s−1) | |
| real amplitude without i (s−1) | |
| amplitudes of the stream functions (m s−1) | |
| amplitudes of the stream functions (m s−1) | |
| amplitude of (T) | |
| amplitude of (A m−2) | |
| amplitude of (mol m−3) | |
| amplitude of w of the rigid surface vortices (m s−1) | |
| amplitude of w of the free surface vortices (m s−1) | |
| in 2D nucleation (m s−1) | |
| in 2D nucleation (m s−1) | |
| amplitude of of the rigid surface vortices (s−1) | |
| amplitude of of the free surface vortices (s−1) | |
| in 2D nucleation (s−1) | |
| in 2D nucleation (s−1) | |
| amplitude of at the rigid surface (mol m−3) | |
| amplitude of at the free surface (mol m−3) | |
| magneto-induction coefficient defined by Equation (D4c) | |
| non-dimensional magneto-induction coefficient defined by Equation (D5a) | |
| angular velocity vector (s−1) | |
| angular velocity of the upper layer corresponding to VMHDF (s−1) | |
| representative angular velocity of the rigid surface vortices (s−1) | |
| rotation coefficient defined by Equation (E20c) (m−1) | |
| mass transfer coefficient defined by Equation (J2b) (mol m−4 s) | |
| magneto-viscosity coefficient defined by Equation (J9b) (m2 A−1 s−1) | |
| in 2D nucleation defined by Equation (G5b) (mol m−4 s) | |
| in 2D nucleation defined by Equation (G5c) | |
| in 2D nucleation defined by Equation (G5d) (m−1) | |
| in 2D nucleation defined by Equation (G5e) (m2 A−1 s−1) | |
| amplitude factor of the rigid surface vortices in 2D nucleation defined by Equation (46b) (s−1) | |
| amplitude factor of the free-surface vortices in 2D nucleation defined by Equation (48b) (s−1) | |
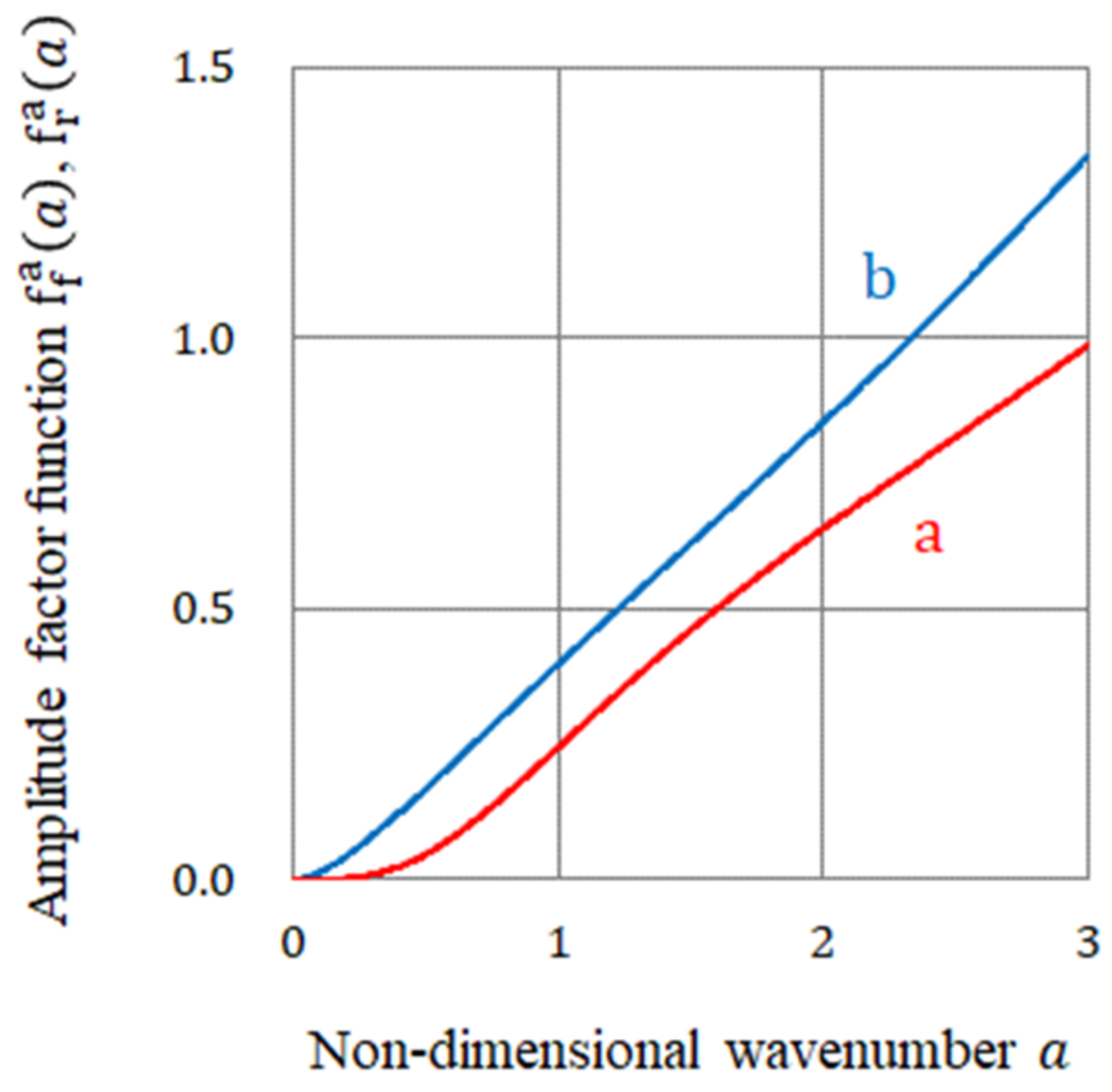
| amplitude factor function of the rigid surface vortices in 2D nucleation defined by Equation (45b) | |
| amplitude factor function of the free surface vortices in 2D nucleation defined by Equation (47b) | |
| chemical potential of the ad-atom (J mol−1) | |
| surface morphology of 2D nuclei by the asymmetrical fluctuations (m) | |
| shortened expression of (m) | |
| electrochemical potential of the metallic ion (J mol−1) | |
| electrochemical potential of the free electron (J mol−1) | |
| asymmetrical fluctuation of (J mol−1) | |
| asymmetrical fluctuation of (J mol−1) | |
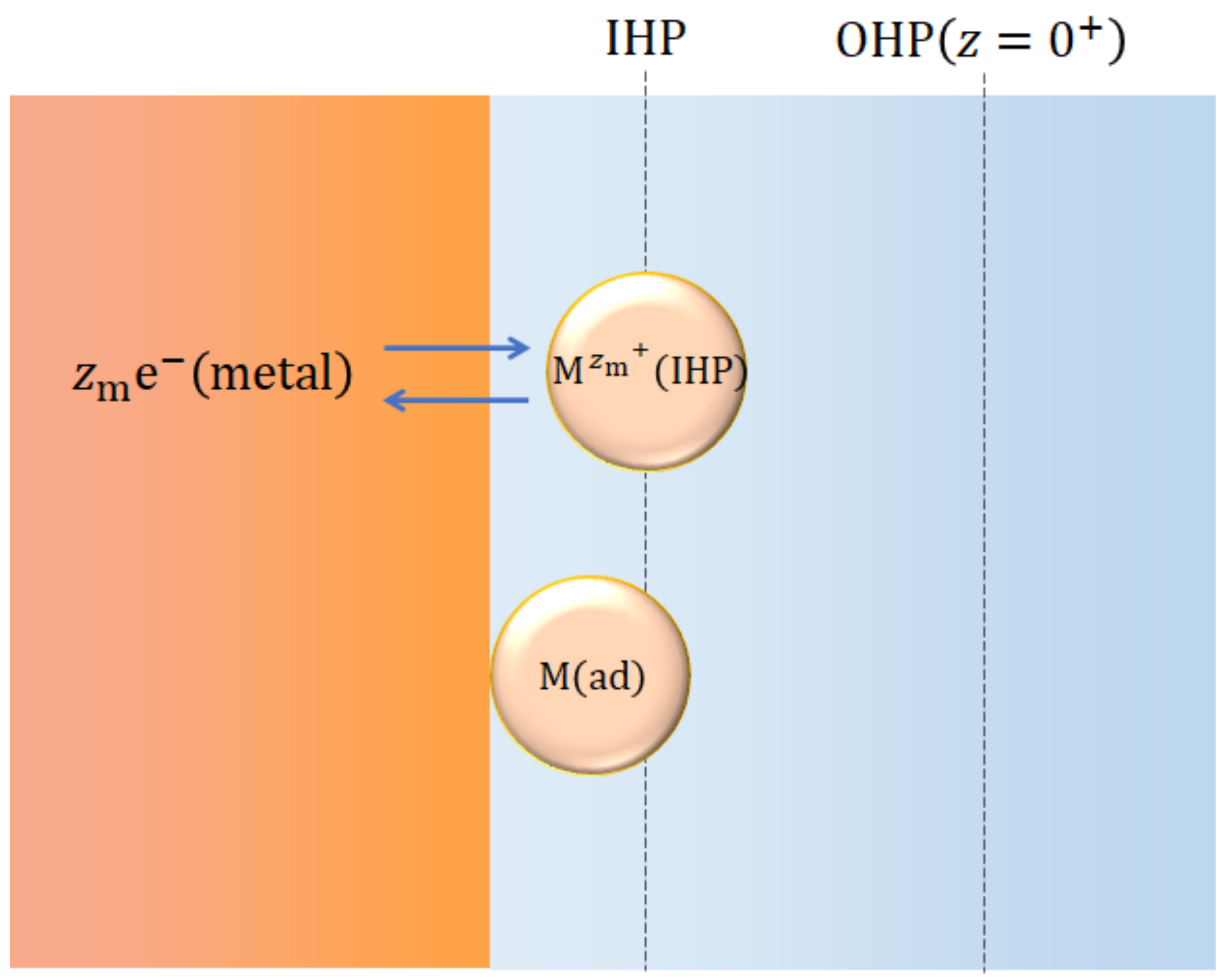
| IHP | inner Helmholtz plane |
| OHP | outer Helmholtz plane |
| -coordinate of the outer Helmholtz plane (OHP) | |
| overpotential at IHP (V) | |
| asymmetrical fluctuation of (V) | |
| overpotential at the flat OHP without 2D nuclei measured from the outer boundary of the diffuse layer (V) | |
| overpotential of the diffuse layer (V) | |
| asymmetrical fluctuation of (V) | |
| asymmetrical fluctuation of at OHP (V) | |
| asymmetrical fluctuation at the surface of 2D nuclei in the diffuse layer (V) | |
| gradient of the electrostatic overpotential in the diffuse layer defined by Equation (35b) (V m−1) | |
| Debye length equalized to the average diffuse layer thickness defined by Equation (35c) (m) | |
| average concentration gradient of the metallic ion in the diffuse layer defined by Equation (36b) (mol m−4) | |
| differential potential coefficient | |
| adsorption coefficient defined by Equation (43b) (s−1) | |
| uniform random number between 0 and 2 | |
| 2D random number defined by Equation (49) | |
| initial ratio of the rigid surface component to the total concentration fluctuation | |
| initial ratio of the free surface component to the total concentration fluctuation | |
| constant of the vorticity coefficient of the free surface vortex in 2D nucleation defined by Equation (G10b) (s−1) | |
| constant of the vorticity coefficient of the rigid surface vortex in 2D nucleation defined by Equation (G7b) (s−1) | |
| probability that the chiral screw dislocations emerge from all the active points | |
| total current of an electrode covered with only achiral active points (A) | |
| total current of the electrode active for either of D- and L-reagents (A) | |
| total current of the electrode for the other reagent (A) | |
| enantiomeric excess (ee) ratio | |
| electric charge stored in the Helmholtz layer of an electric double layer (A) | |
| electric charge stored in the diffuse layer of an electric double layer (A) | |
| differential charge coefficient | |
| electric capacity of the Helmholtz layer (F m−2) | |
| transposition of tensor | |
| operator defined by or non-dimensional operator defined by Equation (D5b) | |
| operator to embed the odd and even functions into a complex space defined by Equation (53a) or Equation (53b) | |
| rms | operator defining the root mean square value |
| function of a defined by Equation (47c) | |
| function of defined by Equation (47d) | |
| function of a defined by Equation (47e) | |
| function of a defined by Equation (45c) | |
| function of a defined by Equation (45d) | |
| function of a defined by Equation (45e) | |
| , | arbitrary constants of the z-velocity component of vortices (m s−1) |
| , | arbitrary constants of the z-velocity component of vortices (m s−1) |
| velocity coefficient of the rigid surface vortices defined by Equation (21b) (m) | |
| velocity coefficient of the rigid surface vortices defined by Equation (22b) (m) | |
| velocity coefficient of the free surface vortices defined by Equation (25b) (m) | |
| velocity coefficient of the free surface vortices defined by Equation (26b) (m) | |
| vorticity coefficient of the free surface vortices (s−1) | |
| vorticity coefficient of the rigid surface vortices (s−1) | |
| vorticity coefficient of the free surface vortices in 2D nucleation (s−1) | |
| vorticity coefficient of the rigid surface vortices in 2D nucleation (s−1) | |
| Superscript ‘a’ | implies asymmetrical fluctuation |
| Subscripts ‘r’ and ‘f’ | mean rigid surface and free surface components, respectively |
| Subscripts ‘1′ and ‘2′ | imply the Helmholtz and diffuse layers, respectively |
Appendix A. Stability by the Non-Specific and Specific Adsorption in 2D Nucleation
Appendix B. Basic MHD Equations in the Stationary Lower Layer
Appendix C. Non-Equilibrium Fluctuations Activated in the Stationary Lower Layer
Appendix D. Derivation of the Amplitude Equations of the Fluctuations in the Stationary Lower Layer
Appendix E. Microscopic Vortices Induced in the Rotating Upper Layer
Appendix F. Intrinsic Spectrum of the Asymmetrical Fluctuations in 2D Nucleation
Appendix G. Amplitudes of the Asymmetrical Concentration and Concentration Gradient Fluctuations in 2D Nucleation
- (a)
- For a rigid surface:
- (b)
- For a free surface:
Appendix H. Amplitude Equations of - and -Components of the Velocity Fluctuation
Appendix I. Solutions of the Amplitudes and of the Fluctuations of Velocity and Vorticity in the Lower Layer
Appendix J. Solution of the Amplitude of the Concentration Fluctuation in the Lower Layer
Appendix K. Derivation of the - and -Components of the Velocity in the Lower Layer in 2D Nucleation
- (a)
- For the rigid surface:
- (b)
- For the free surface:
References
- Aogaki, R. Theory of stable formation of ionic vacancy in a liquid solution. Electrochemistry 2008, 76, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Aogaki, R.; Sugiyama, A.; Miura, M.; Oshikiri, Y.; Miura, M.; Morimoto, R.; Takagi, S.; Mogi, I.; Yamauchi, Y. Origin of nanobubbles electrochemically formed in a magnetic field: Ionic vacancy production in electrode reaction. Sci. Rep. 2016, 6, 28297. [Google Scholar] [CrossRef] [PubMed]
- Aogaki, R.; Motomura, K.; Sugiyama, A.; Morimoto, R.; Mogi, I.; Miura, M.; Asanuma, M.; Oshikiri, Y. Measurement of the lifetime of ionic vacancy by the cyclotron-MHD electrode. Magnetohydrodynamics 2012, 48, 289–297. [Google Scholar] [CrossRef]
- Sugiyama, A.; Morimoto, R.; Osaka, T.; Mogi, I.; Asanuma, M.; Miura, M.; Oshikiri, Y.; Yamauchi, Y.; Aogaki, R. Lifetime of ionic vacancy created in redox electrode reaction measured by cyclotron MHD electrode. Sci. Rep. 2016, 6, 19795. [Google Scholar] [CrossRef]
- Takagi, S.; Asada, T.; Oshikiri, Y.; Miura, M.; Morimoto, R.; Sugiyama, A.; Mogi, I.; Aogaki, R. Nanobubble formation from ionic vacancies in an electrode reaction on a fringed electrode under a uniform vertical magnetic field -1. Formation process in a vertical magnetohydrodynamic (MHD) flow. J. Electroanal. Chem. 2022, 914, 116291. [Google Scholar] [CrossRef]
- Takagi, S.; Asada, T.; Oshikiri, Y.; Miura, M.; Morimoto, R.; Sugiyama, A.; Mogi, I.; Aogaki, R. Nanobubble formation from ionic vacancies in an electrode reaction on a fringed electrode under a uniform vertical magnetic field -2. Measurement of the angular velocity of a vertical magnetohydrodynamic (MHD) flow by the microbubbles originating from ionic vacancies. J. Electroanal. Chem. 2022, 916, 116375. [Google Scholar] [CrossRef]
- Mogi, I.; Watanabe, K. Chiral recognition of amino acids magnetoelectrodeposited Cu film electrodes. Int. J. Electrochem. 2011, 2011, 239637. [Google Scholar] [CrossRef] [Green Version]
- Mogi, I.; Morimoto, R.; Aogaki, R.; Watanabe, K. Surface chirality induced by rotational electrodeposition in magnetic fields. Sci. Rep. 2013, 3, 2574. [Google Scholar] [CrossRef] [Green Version]
- Mogi, I.; Aogaki, R.; Watanabe, K. Chiral surface formation of copper films by magnetoelectrochemical etching. Magnetohydrodynamics 2015, 51, 361–368. [Google Scholar] [CrossRef]
- Mogi, I.; Aogaki, R.; Watanabe, K. Tailoring of surface chirality by micro-vortices and specific adsorption in magnetoelectrodeposition. Bull. Chem. Soc. Jpn. 2015, 88, 1479–1485. [Google Scholar] [CrossRef] [Green Version]
- Mogi, I.; Aogaki, R.; Takahashi, K. Chiral surface formation in magnetoelectrolysis on micro-electrodes. Magnetohydrodynamics 2017, 53, 321–328. [Google Scholar] [CrossRef]
- Mogi, I.; Morimoto, R.; Aogaki, R. Surface chirality effects induced by magnetic fields. Curr. Opin. Electrochem. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Mogi, I.; Aogaki, R.; Takahashi, K. Chiral symmetry breaking in magnetoelectrochemical etching with chloride additives. Molecules 2018, 23, 19. [Google Scholar] [CrossRef] [Green Version]
- Mogi, I.; Morimoto, R.; Aogaki, R.; Takahashi, K. Effects of vertical magnetohydrodynamic flows on chiral surface formation in magnetoelectrolysis. Magnetochemistry 2018, 4, 40. [Google Scholar] [CrossRef] [Green Version]
- Mogi, I.; Morimoto, R.; Aogaki, R.; Takahashi, K. Effects of vertical MHD flows and cell rotation on surface chirality in magnetoelectrodeposition. In Proceedings of the 9th International Symposium on Electromagnetic Processing of Materials (EPM2018), Hyogo, Japan, 14–18 October 2018; Volume 424, p. 012024. [Google Scholar] [CrossRef] [Green Version]
- Mogi, I.; Morimoto, R.; Aogaki, R.; Takahashi, K. Surface chirality in rotational magnetoelectrodeposition of copper films. Magnetochemistry 2019, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Mogi, I.; Iwasaka, M.; Aogaki, R.; Takahashi, K. Visualization of magnetohydrodynamic micro-vortices with guanine micro-crystals. J. Electrochem. Soc. 2017, 164, H584–H586. [Google Scholar] [CrossRef]
- Mogi, I.; Aogaki, R.; Takahashi, K. Fluctuation effects of magnetohydrodynamic micro-vortices on odd chirality in magnetoelectrolysis. Magnetochemistry 2020, 6, 43. [Google Scholar] [CrossRef]
- Aogaki, R.; Morimoto, R. Nonequilibrium fluctuation in micro-MHD effects on electrodeposition. In Heat and Mass Transfer—Modelling and Simulation; Hossain, M., Ed.; Intech: Rijeka, Croatia, 2011; pp. 189–216. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, R.; Miura, M.; Sugiyama, A.; Miura, M.; Oshikiri, Y.; Mogi, I.; Takagi, S.; Yamauchi, Y.; Aogaki, R. Theory of microscopic electrodeposition under a uniform parallel magnetic field -1. Nonequilibrium fluctuations of magnetohydrodynamic (MHD) flow. J. Electroanal. Chem. 2019, 848, 113254. [Google Scholar] [CrossRef]
- Morimoto, R.; Miura, M.; Sugiyama, A.; Miura, M.; Oshikiri, Y.; Mogi, I.; Takagi, S.; Yamauchi, Y.; Aogaki, R. Theory of microscopic electrodeposition under a uniform parallel magnetic field -2. Suppression of 3D nucleation by micro-MHD flow. J. Electroanal. Chem. 2019, 847, 113255. [Google Scholar] [CrossRef]
- Morimoto, R.; Miura, M.; Sugiyama, A.; Miura, M.; Oshikiri, Y.; Kim, Y.; Mogi, I.; Takagi, S.; Yamauchi, Y.; Aogaki, R. Long-Term Electrodeposition under a Uniform Parallel Magnetic Field. 1. Instability of Two-Dimensional Nucleation in an Electric Double Layer. J. Phys. Chem. B 2020, 124, 11854–11869. [Google Scholar] [CrossRef]
- Morimoto, R.; Miura, M.; Sugiyama, A.; Miura, M.; Oshikiri, Y.; Kim, Y.; Mogi, I.; Takagi, S.; Yamauchi, Y.; Aogaki, R. Long-Term Electrodeposition under a Uniform Parallel Magnetic Field. 2. Flow-Mode Transition from Laminar MHD Flow to Convection Cells with Two-Dimensional (2D) Nucleation. J. Phys. Chem. B 2020, 124, 11870–11881. [Google Scholar] [CrossRef]
- Fahidy, T.Z. The effect of magnetic fields on electrochemical processes. In Modern Aspects of Electrochemistry No. 32; Conway, B.E., Bockris, J.M., White, R.E., Eds.; Springer: Boston, MA, USA, 2002; pp. 333–354. [Google Scholar] [CrossRef]
- Alemany, A.; Chopart, J.P. An Outline of Magnetoelectrochemistry. In Magnetohydrodynamics—Historical Evolution and Trends; Molokov, S., Moreau, R., Moffatt, K., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 391–407. [Google Scholar] [CrossRef]
- Monzon, L.M.A.; Coey, J.M.D. Magnetic field in electrochemistry: The Lorentz force. A mini-review. Electrochem. Commun. 2014, 42, 38–41. [Google Scholar] [CrossRef]
- Fahidy, T.Z. Wave phenomena in magnetoelectrolytic systems. Electrochim. Acta 1976, 21, 21–24. [Google Scholar] [CrossRef]
- Mohanta, S.; Fahidy, T.Z. The hydrodynamics of a magnetoelectrolytic cell. J. Appl. Electrochem. 1976, 6, 211–220. [Google Scholar] [CrossRef]
- Fahidy, T.Z. Magnetoelectrolysis. J. Appl. Electrochem. 1983, 13, 553–563. [Google Scholar] [CrossRef]
- Aaboubi, O.; Chopart, J.P.; Douglade, J.; Olivier, A.; Gabrielli, C.; Tribollet, B. Magnetic field effects on mass transport. J. Electrochem. Soc. 1990, 137, 1796–1804. [Google Scholar] [CrossRef]
- Devos, O.; Aaboubi, O.; Chopart, J.P.; Merienne, E.; Olivier, A.R. Magnetic impedance method: The MHD transfer function. Electrochemistry 1999, 67, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Aaboubi, O.; Amblard, J.; Chopart, J.P.; Olivier, A. Magnetohydrodynamic analysis of silver electrocrystallization from a nitric and tartaric solution. J. Electrochem. Soc. 2004, 151, C112–C118. [Google Scholar] [CrossRef]
- Aogaki, R.; Fueki, K.; Mukaibo, T. Application of magnetohydrodynamic effect to the analysis of electrochemical reactions -1. MHD flow of an electrolyte solution in an electrode-cell with a short rectangular channel. Denki Kagaku (Presently Electrochem.) 1975, 43, 504–508. [Google Scholar] [CrossRef]
- Aogaki, R.; Fueki, K.; Mukaibo, T. Application of magnetohydrodynamic effect to the analysis of electrochemical reactions -2. Diffusion process in MHD forced flow of electrolyte solutions. Denki Kagaku (Presently Electrochem.) 1975, 43, 509–514. [Google Scholar] [CrossRef]
- Aogaki, R.; Fueki, K.; Mukaibo, T. Diffusion process in viscous-flow of electrolyte solution in magnetohydrodynamic pump electrodes. Denki Kagaku (Presently Electrochem.) 1976, 44, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Mutschke, G.; Bund, A. On the 3D character of the magnetohydrodynamic effect during metal electrodeposition in cuboid cells. Electrochem. Commun. 2008, 10, 597–601. [Google Scholar] [CrossRef]
- Mühlenhoff, S.; Mutschke, G.; Koschichow, D.; Yang, X.; Bund, A.; Fröhlich, J.; Odenbach, S.; Eckert, K. Lorentz-force-driven convection during copper magnetoelectrolysis in the presence of a supporting buoyancy force. Electrochim. Acta 2012, 69, 209–219. [Google Scholar] [CrossRef]
- Devos, O.O.; Olivier, A.; Chopart, J.P.; Aaboubi, O.; Maurin, G. Magnetic field effects on nickel electrodeposition. J. Electrochem. Soc. 1998, 145, 401–405. [Google Scholar] [CrossRef]
- Olivas, P.; Alemany, A.; Bark, F.H. Electromagnetic control of electroplating of a cylinder in forced convection. J. Appl. Electrochem. 2004, 34, 19–30. [Google Scholar] [CrossRef]
- Daltin, A.L.; Bohr, F.; Chopart, J.P. Kinetics of Cu2O electrocrystallization under magnetic fields. Electrochim. Acta 2009, 54, 5813–5817. [Google Scholar] [CrossRef]
- Fernández, D.; Coey, J.M.D. Inhomogeneous electrodeposition of copper in a magnetic field. Electrochem. Commun. 2009, 11, 379–382. [Google Scholar] [CrossRef]
- Msellak, K.; Chopart, J.P.; Jbara, O.; Aaboubi, O.; Amblard, J. Magnetic field effect on Ni-Fe alloys codeposition. J. Magn. Magn. Mater. 2004, 281, 295–304. [Google Scholar] [CrossRef]
- Żabiński, P.R.; Mech, K.; Kowalik, R. Co-Mo and Co-Mo-C alloys deposited in a magnetic field of high intensity and their electrocatalytic properties. Arch. Metall. Mater. 2012, 57, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Żabiński, P.R.; Franczak, K.; Kowalik, R. Electrocatalytically active Ni-Re binary alloys electrodeposited with superimposed magnetic field. Arch. Metall. Mater. 2012, 57, 495–501. [Google Scholar] [CrossRef]
- Żabiński, P.R.; Mech, K.; Kowalik, R. Electrocalalytically active Co-W and Co-W-C alloys electrodeposited in a magnetic field. Electrochim. Acta 2013, 104, 542–548. [Google Scholar] [CrossRef]
- Chouchane, S.; Levesque, A.; Żabiński, P.R.; Rehamnia, R.; Chopart, J.P. Electrochemical corrosion behavior in NaCl medium of zinc-nickel alloys electrodeposited under applied magnetic field. J. Alloy. Compd. 2010, 506, 575–580. [Google Scholar] [CrossRef]
- Kishioka, S.; Yamada, A.; Aogaki, R. Analysis of gas dissolution rate into liquid phase under magnetic field gradient. Phys. Chem. Chem. Phys. 2000, 2, 4179–4183. [Google Scholar] [CrossRef]
- Devos, O.; Aogaki, R. Transport of paramagnetic liquids under nonuniform high magnetic field. Anal. Chem. 2000, 72, 2835–2840. [Google Scholar] [CrossRef]
- Sugiyama, A.; Morisaki, S.; Aogaki, R. Mass transfer process by magneto-convection at a solid-liquid interface in a heterogeneous vertical magnetic field. Jpn. J. Appl. Phys. 2003, 42, 5322–5329. [Google Scholar] [CrossRef]
- Tschulik, K.; Koza, J.A.; Uhlemann, M.; Gebert, A.; Schultz, L. Effects of well-defined magnetic field gradients on the electrodeposition of copper and bismuth. Electrochem. Commun. 2009, 11, 2241–2244. [Google Scholar] [CrossRef]
- Dunne, P.; Mazza, L.; Coey, J.M.D. Magnetic structuring of electrodeposits. Phys. Rev. Lett. 2011, 107, 024501. [Google Scholar] [CrossRef]
- Tschulik, K.; Yang, X.; Mutschke, G.; Uhlemann, M.; Eckert, K.; Sueptitz, R.; Schultz, L.; Gebert, A. How to obtain structural deposits from diamagnetic ions in magnetic gradient fields? Electrochem. Commun. 2011, 13, 946–950. [Google Scholar] [CrossRef]
- Tschulik, K.; Sueptitz, R.; Koza, J.; Uhlemann, M.; Mutschke, G.; Weier, T.; Gebert, A.; Schultz, L. Studies on the patterning effect of copper deposits in magnetic gradient fields. Electrochim. Acta 2010, 56, 297–304. [Google Scholar] [CrossRef]
- Tschulik, K.; Cierpka, C.; Muschke, G.; Gebert, A.; Schultz, L.; Uhlemann, M. Clarifying the mechanism of reverse structuring during electrodeposition in magnetic gradient fields. Anal. Chem. 2012, 84, 2328–2334. [Google Scholar] [CrossRef]
- Aaboubi, O.; Lòs, P.; Amblard, J.; Chopart, J.P.; Olivier, A. Electrochemical investigations of the magnetic field influence on mass transport toward an ultramicrodisk. J. Electrochem. Soc. 2003, 150, E125–E130. [Google Scholar] [CrossRef]
- Devos, O.; Aaboubi, O.; Chopart, J.P.; Merienne, E.; Olivier, A.; Gabrielli, C.; Tribollet, B. A new experimental device for magnetoelectrochemical (M.E.C.) transfer function measurements. Polish J. Chem. 1997, 71, 1160–1170. [Google Scholar]
- Ragsdale, S.R.; Lee, J.; Gao, X.; White, H.S. Magnetic field effects in electrochemistry. Voltammetric reduction of acetophenone at microdisk electrodes. J. Phys. Chem. 1996, 100, 5913–5922. [Google Scholar] [CrossRef]
- Ragsdale, S.R.; Lee, J.; White, H.S. Analysis of the magnetic force generated at a hemispherical microelectrode. Anal. Chem. 1997, 69, 2070–2076. [Google Scholar] [CrossRef]
- Ragsdale, S.R.; Grant, K.M.; White, H.S. Electrochemically generated magnetic forces. Enhanced transport of a paramagnetic redox species in large, nonuniform magetic fields. J. Am. Chem. Soc. 1998, 120, 13461–13468. [Google Scholar] [CrossRef]
- Huang, M.; Marinaro, G.; Yang, X.; Fritzsche, B.; Lei, Z.; Uhlemann, M.; Eckert, K.; Mutschke, G. Mass transfer and electrolyte flow during electrodeposition on a conically shaped electrode under the influence of a magnetic field. J. Electroanal. Chem. 2019, 842, 203–213. [Google Scholar] [CrossRef]
- Dunne, P.; Soucaille, R.; Ackland, K.; Coey, J.M.D. Magnetic structuring of linear copper electrodeposits. J. Appl. Phys. 2012, 111, 07B915. [Google Scholar] [CrossRef] [Green Version]
- Miura, M.; Sugiyama, A.; Oshikiri, Y.; Morimoto, R.; Mogi, I.; Miura, M.; Takagi, S.; Kim, J.; Yamauchi, Y.; Aogaki, R. Excess heat production by the pair annihilation of ionic vacancies in copper redox reactions. Sci. Rep. 2019, 9, 13695. [Google Scholar] [CrossRef]
- Sugiyama, A.; Miura, M.; Oshikiri, Y.; Kim, Y.; Morimoto, R.; Miura, M.; Osaka, T.; Mogi, I.; Yamauchi, Y.; Aogaki, R. Excess heat production in the redox couple reaction of ferricyanide and ferrocyanide. Sci. Rep. 2020, 10, 20072. [Google Scholar] [CrossRef]
- Miura, M.; Oshikiri, Y.; Sugiyama, A.; Morimoto, R.; Mogi, I.; Miura, M.; Takagi, S.; Yamauchi, Y.; Aogaki, R. Magneto-dendrite effect: Copper electrodeposition under high magnetic field. Sci. Rep. 2017, 7, 45511. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, S. Hydrodynamic and Hydromagnetic Stability; Oxford University Press: London, UK, 1961; pp. 87–148. [Google Scholar]
- Aogaki, R. Instability of nonequilibrium fluctuation in electrochemical nucleation. I. Occurrence of instability. J. Chem. Phys. 1996, 103, 8602–8615. [Google Scholar] [CrossRef]
- Reeves, R. The double layer in the absence of specific adsorption. In Comprehensive Treatise of Electrochemistry-1. The Double Layer; Bockris, J.M., Conway, B.E., Yeager, E., Eds.; Plenum Press: New York, NY, USA, 1980; p. 129. [Google Scholar]
- Aogaki, R. Instability of nonequilibrium fluctuation in electrochemical nucleation II. Determination of the critical condition. J. Chem. Phys. 1996, 103, 8616–8625. [Google Scholar] [CrossRef]
- Asanuma, M.; Yamada, A.; Aogaki, R. Self-Organization Process of Secondary Crystal Nodules from Individual Nuclei in Electrodeposition. Jpn. J. Appl. Phys. 2005, 44, 5137–5149. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimoto, R.; Miura, M.; Sugiyama, A.; Miura, M.; Oshikiri, Y.; Mogi, I.; Yamauchi, Y.; Takagi, S.; Aogaki, R. Theory of Chiral Electrodeposition by Chiral Micro-Nano-Vortices under a Vertical Magnetic Field -1: 2D Nucleation by Micro-Vortices. Magnetochemistry 2022, 8, 71. https://doi.org/10.3390/magnetochemistry8070071
Morimoto R, Miura M, Sugiyama A, Miura M, Oshikiri Y, Mogi I, Yamauchi Y, Takagi S, Aogaki R. Theory of Chiral Electrodeposition by Chiral Micro-Nano-Vortices under a Vertical Magnetic Field -1: 2D Nucleation by Micro-Vortices. Magnetochemistry. 2022; 8(7):71. https://doi.org/10.3390/magnetochemistry8070071
Chicago/Turabian StyleMorimoto, Ryoichi, Miki Miura, Atsushi Sugiyama, Makoto Miura, Yoshinobu Oshikiri, Iwao Mogi, Yusuke Yamauchi, Satoshi Takagi, and Ryoichi Aogaki. 2022. "Theory of Chiral Electrodeposition by Chiral Micro-Nano-Vortices under a Vertical Magnetic Field -1: 2D Nucleation by Micro-Vortices" Magnetochemistry 8, no. 7: 71. https://doi.org/10.3390/magnetochemistry8070071
APA StyleMorimoto, R., Miura, M., Sugiyama, A., Miura, M., Oshikiri, Y., Mogi, I., Yamauchi, Y., Takagi, S., & Aogaki, R. (2022). Theory of Chiral Electrodeposition by Chiral Micro-Nano-Vortices under a Vertical Magnetic Field -1: 2D Nucleation by Micro-Vortices. Magnetochemistry, 8(7), 71. https://doi.org/10.3390/magnetochemistry8070071





