Relativistic Effects from Heavy Main Group p-Elements on the NMR Chemical Shifts of Light Atoms: From Pioneering Studies to Recent Advances
Abstract
1. Introduction
2. Brief Notes on Developing the Relativistic Theory and Computational Methods for NMR Shielding Constants
3. Studies of the Relativistic Effects on NMR Shielding Constants of Light Nuclei from Heavy Main Group p-Elements
3.1. Relativistic Effects on 1H and 13C NMR Chemical Shifts
- (a)
- The overlaps of the 90° rotated occupied frontier MOs with the lowest vacant frontier MOs are significant (see the numerators in Equation (6));
- (b)
- The relevant energy gaps between the frontier occupied and vacant MOs are not “very large” (see the denominators in Equation (6)).
3.2. Relativistic Effects on 19F, 27Al, 29Si, 15N, and 31P NMR Chemical Shifts
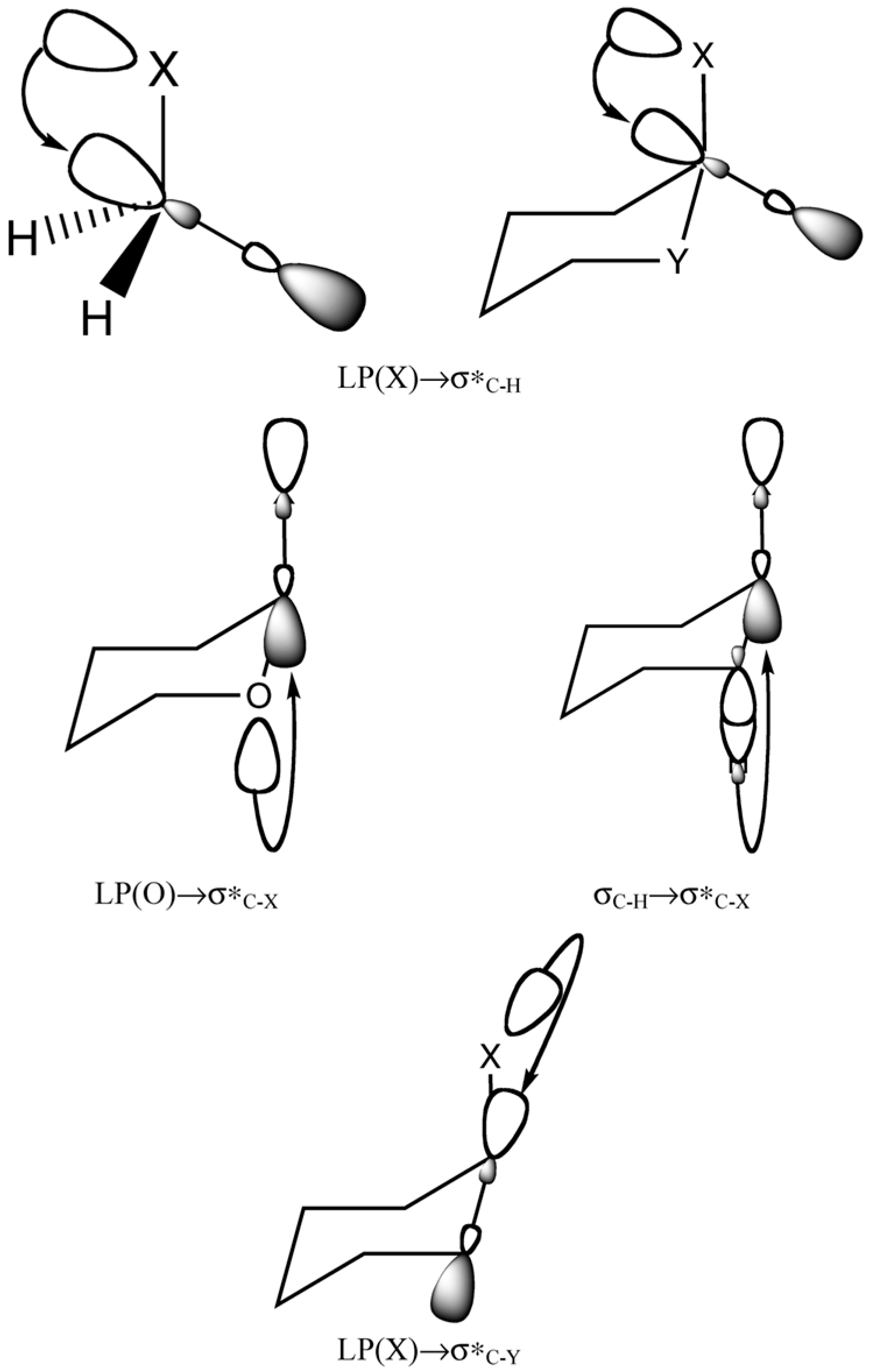
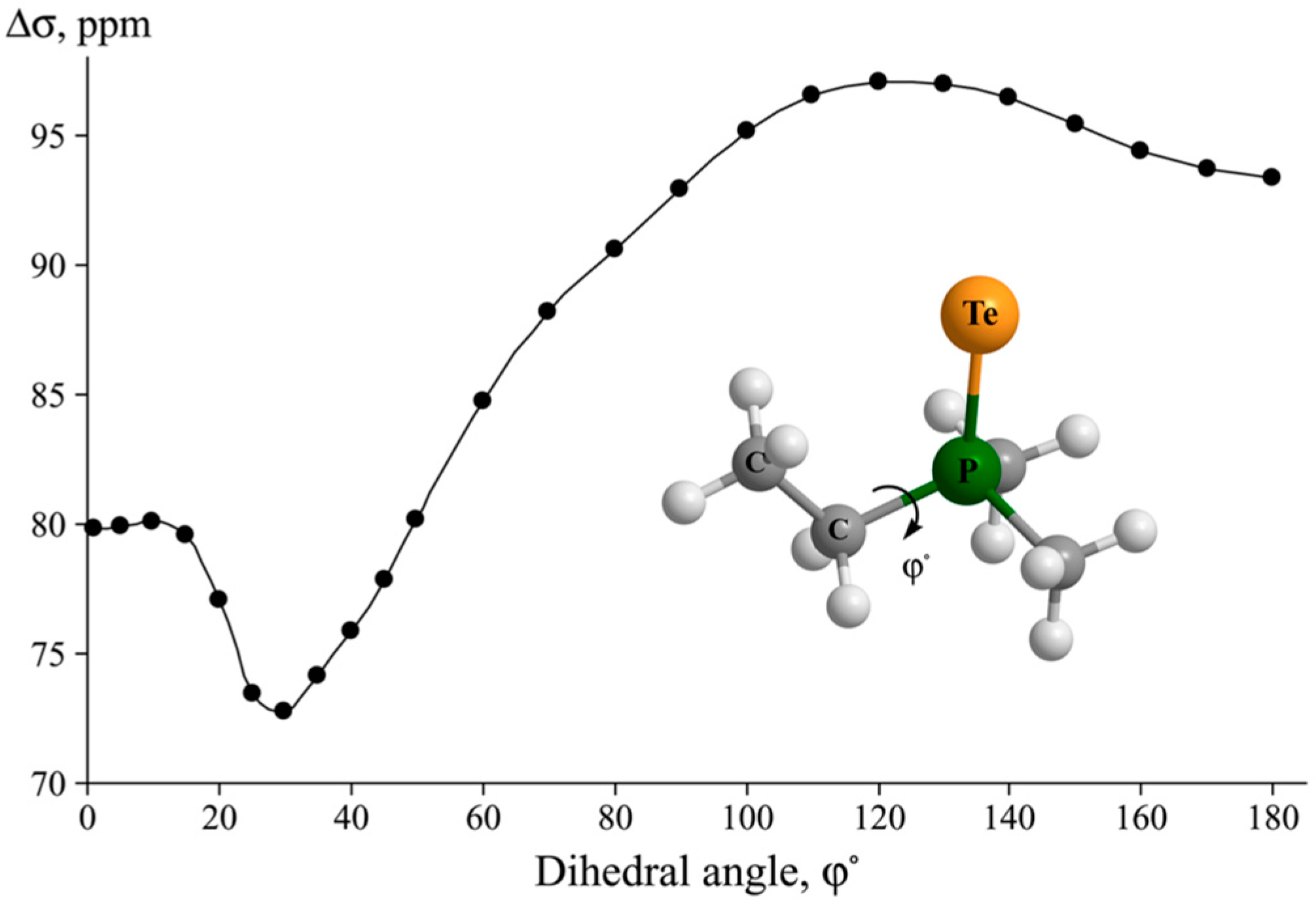
4. Stereochemistry of the Relativistic Effects on the NMR Shielding Constants of Light Nuclei Initiated by Heavy Main Group p-Elements
5. Influence of the Relativistic Effects Initiated by Heavy p-Elements on the Vibrational Contributions to the Shielding Constants of Light Nuclei
6. Conclusions
- The total relativistic HALA effect initiated by the heavy p-elements on the chemical shifts of the light p-block main group nuclei is actually determined by the efficiency of the spin–orbit/Fermi-contact (SO/FC) mechanism that plays a predominant role in the total SO-HALA effect.
- The scalar relativistic HALA corrections to the chemical shifts of light atoms due to the mass-velocity and Darwin relativistic effects are negligible in most cases when the heavy atom belongs to the 16th and 17th groups of PTE. For the 14th group’s heavy atoms, the scalar-HALA can reach significant magnitudes effectively suppressing the SO-HALA mechanism.
- The NHD trends for NMR chemical shifts of light nuclei are due to the relativistic SO-HALA effects.
- The efficiency of the SO/FC (SO-HALA) mechanism was found to depend on the rate of involvement of the valence s orbitals of the light atom in the heavy atom—light atom bond. This is important for the SO/FC mechanism to be efficient, because ns orbitals are responsible for bringing the heavy-atom SOC-induced spin-polarization into contact with the light atom nucleus.
- The most pronounced SO-HALA effect is expected to manifest itself in the 1H NMR chemical shifts of protons directly bound to a heavy atom, because in this case the hydrogen 1s-orbital predominates in bonding.
- The 13C SO-HALA effects increase from sp3 to sp2 to sp hybridization of the carbon atom.
- In the case of p-block main-group central atoms considered as the light NMR spectator atoms, the valence s-orbitals are fully involved in bonding when these atoms are in their highest oxidation states; therefore, large shielding-type SO effects should be expected for them. This explains why the NHD trend is the general behavior for the chemical shifts of the main-group p-elements being in high oxidation states: the bonds have a generally high s-character. In contrast, a low s-character of the bond leads to an inefficient SO/FC mechanism for the p-block main-group elements in low oxidation states, and this may cause the “inverse halogen dependence” (IHD).
- The magnitudes of the energy gaps between the frontier occupied and vacant molecular orbitals (MOs) involved in the SO-HALA mechanism are important for the SO-HALA correction: the less the energy gaps, the larger the magnitude of the SO-HALA effect.
- The magnitude of the spin–orbit coupling (SOC) at heavy atoms is responsible for the efficiency of the SO-HALA mechanism. In particular, the occupation of orbitals with l ≥ 1 and the partial charge on the heavy atom play a decisive role in the magnitude of the spin–orbit splitting. This results in the fact that π-type lone electron pairs (LEPs) on the heavy atom can provide significant contributions to the SO-HALA effect.
- The overlap of the 90°-rotated frontier occupied molecular orbitals (for example, the lone electron pairs of a heavy atom) with lowest unoccupied frontier molecular orbitals (for example, the frontier antibonding molecular orbitals) must be significant to provide considerable MO matrix elements of the spin–orbit and orbital Zeeman (OZ) operators that are included in the main SO/FC–I term.
- Spatial deformation of the chalcogen’s lone electron pairs (LEPs) influences the magnitude of the one-bond SO-HALA effect: the squashing of LEPs at a heavy atom diminishes the magnitude of the SO-HALA effect.
- In the case when inner rotations do not change the SOC on the heavy atom significantly, one can expect a degree of correlation between the stereochemical behaviors of the SO-HALA effect on the shielding constant of a light nuclei and the FC contribution to the corresponding spin–spin coupling constant.
- Like in the case of NHD, the chalcogen and triel dependencies established for the 13C NMR shielding constants were explained by the SO-HALA effect, though, the pnictogen and tetrel dependences appeared to reflect some additional relativistic mechanisms that interfered with the SO-HALA effect.
- The SO-HALA effect on shielding constants of light atoms, such as 1H, 13C or 15N, is cumulative and it increases nonlinearly with the total atomic number of adjacent halogens or chalcogens.
- The deshielding-type SO effect is associated with the occupied σ-type heavy atom—light atom bonding MOs, while the π-type MOs leads to a shielding-type SO effect.
- The α-, β- and γ-HALA effects on 13C NMR chemical shifts alternate in sign, being negative for the α- and γ-carbons (shielding-type effect) and positive for the β-carbons (deshielding-type effect).
- Relativistic SO-HALA effect on the NMR chemical shifts is not restricted to a covalently heavy atom—light atom-bound systems. It manifests itself, in particular, in ion pairs and is known as the “through-space” HALA effect.
- The relativistic rovibrational corrections to the light atom chemical shifts may substantially differ from that calculated at the nonrelativistic level of electronic theory, if there are heavy atoms in the system (especially in the case when the light spectator atom is directly bound to the heavy atom).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauss, J.; Stanton, J.F. Electron-correlated methods for the calculation of NMRchemical shifts. In Calculation of NMR and EPR Parameters, Theory and Applications, 1st ed.; Kaupp, M., Bühl, M., Malkin, V.G., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; Chapter 8; pp. 123–139. [Google Scholar]
- Fukui, H. Theory and calculation of nuclear shielding constants. Prog. Nucl. Magn. Reson. Spectrosc. 1997, 31, 317–342. [Google Scholar] [CrossRef]
- Fukui, H. Theoretical aspects of spin–spin couplings. Nucl. Magn. Reson. 2007, 36, 113–130. [Google Scholar] [CrossRef]
- Webb, G.A.; Fukui, H.; Baba, T. Theoretical Aspects of Spin-spin coupling constants. In Nuclear Magnetic Resonance, 1st ed.; Webb, G.A., Ed.; Royal Society of Chemistry: London, UK, 2003; Volume 32, pp. 126–145. [Google Scholar] [CrossRef]
- Helgaker, T.; Coriani, S.; Jørgensen, P.; Kristensen, K.; Olsen, J.; Ruud, K. Recent advances in wave function-based methods of molecular-property calculations. Chem. Rev. 2012, 112, 543–631. [Google Scholar] [CrossRef]
- Helgaker, T.; Jaszuński, M.; Pecul, M. The quantum-chemical calculation of NMR indirect spin-spin coupling constants. Prog. Nucl. Magn. Reson. Spectrosc. 2008, 53, 249–268. [Google Scholar] [CrossRef]
- Helgaker, T.; Jaszuński, M.; Ruud, K. Ab initio methods for the calculation of NMR shielding and indirect spin-spin coupling constants. Chem. Rev. 1999, 99, 293–352. [Google Scholar] [CrossRef]
- Helgaker, T.; Pecul, M. Spin-Spin Coupling Constants with HF and DFT Methods. In Calculation of NMR and EPR Parameters: Theory and Applications, 1st ed.; Kaupp, M., Bühl, M., Malkin, V.G., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; Chapter 7; pp. 101–121. [Google Scholar]
- Contreras, R.H.; Ferraro, M.B.; de Azúa, M.C.R.; Aucar, G.A. Brief account of nonrelativistic theory of NMR parameters. In High Resolution NMR Spectroscopy. Understanding Molecules and Their Electronic Structures, 1st ed.; Contreras, R.H., Ed.; Elsevier B.V.: London, UK, 2013; Volume 3, Chapter 2; pp. 9–39. [Google Scholar]
- Contreras, R.H.; Tormena, C.F.; Ducati, L.C. Transmission mechanisms of the Fermi-contact term of spin-spin couplings. In High Resolution NMR Spectroscopy. Understanding Molecules and Their Electronic Structures, 1st ed.; Contreras, R.H., Ed.; Elsevier B.V.: London, UK, 2013; Volume 3, Chapter 8; pp. 245–284. [Google Scholar]
- Aucar, G.A.; Romero, R.H.; Maldonado, A.F. Polarization propagators: A powerful theoretical tool for a deeper understanding of NMR spectroscopic parameters. Int. Rev. Phys. Chem. 2010, 29, 1–64. [Google Scholar] [CrossRef]
- Aucar, G.A.; de Azúa, M.C.R.; Giribet, C.G. The polarization propagator approach as a tool to study electronic molecular structures from high-resolution NMR parameters. In High Resolution NMR Spectroscopy. Understanding Molecules and Their Electronic Structures, 1st ed.; Contreras, R.H., Ed.; Elsevier B.V.: London, UK, 2013; Volume 3, Chapter 5; pp. 119–159. [Google Scholar]
- Autschbach, J.; Le Guennic, B. Analyzing and Interpreting NMR Spin–Spin Coupling Constants Using Molecular Orbital Calculations. J. Chem. Educ. 2007, 84, 156–171. [Google Scholar] [CrossRef]
- Autschbach, J. The Calculation of NMR Parameters in Transition Metal Complexes. In Principles and Applications of Density Functional Theory in Inorganic Chemistry I, Structure and Bonding, 1st ed.; Kaltsoyannis, N., McGrady, J.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 112, pp. 1–48. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, W.; Autschbach, J. Relativistic Theories of NMR Shielding. In Handbook of Relativistic Quantum Chemistry, 1st ed.; Liu, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–33. [Google Scholar] [CrossRef]
- Autschbach, J. Calculating NMR Chemical Shifts and J-Couplings for Heavy Element Compounds. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation, Nuclear Magnetic Resonance and Electron Spin Resonance Spectroscopy, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–14. [Google Scholar] [CrossRef]
- Faber, R.; Kaminsky, J.; Sauer, S.P.A. Rovibrational and Temperature Effects in Theoretical Studies of NMR Parameters. In Gas Phase NMR, 1st ed.; Jackowski, K., Jaszuński, M., Eds.; Royal Society of Chemistry: London, UK, 2016; Volume 6, Chapter 7; pp. 218–266. [Google Scholar] [CrossRef]
- Lazzeretti, P. Electronic current densities induced by magnetic fields and nuclear magnetic dipoles: Theory and computation of NMR spectral parameters. In High Resolution NMR Spectroscopy. Understanding Molecules and Their Electronic Structures, 1st ed.; Contreras, R.H., Ed.; Elsevier B. V.: London, UK, 2013; Volume 3, Chapter 7; pp. 209–243. [Google Scholar]
- Cremer, D.; Gräfenstein, J. Calculation and analysis of NMR spin-spin coupling constants. Phys. Chem. Chem. Phys. 2007, 9, 2791–2816. [Google Scholar] [CrossRef] [PubMed]
- De la Vega, J.M.G.; Fabián, J.S. Analysis of Contributions to Spin-Spin Coupling Constants by the Natural J-Coupling Method. In High Resolution NMR Spectroscopy. Understanding Molecules and Their Electronic Structures, 1st ed.; Contreras, R.H., Ed.; Elsevier B. V.: London, UK, 2013; Volume 3, Chapter 6; pp. 161–207. [Google Scholar]
- Rusakova, I.L. Quantum Chemical Approaches to the Calculation of NMR Parameters: From Fundamentals to Recent Advances. Magnetochemistry 2022, 8, 50. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Rusakov, Y.Y. Quantum chemical calculations of 77Se and 125Te nuclear magnetic resonance spectral parameters and their structural applications. Magn. Reson. Chem. 2021, 59, 359–407. [Google Scholar] [CrossRef] [PubMed]
- Rusakova, I.L.; Rusakov, Y.Y.; Krivdin, L.B. Theoretical grounds of relativistic methods for calculation of spin–spin coupling constants in nuclear magnetic resonance spectra. Russ. Chem. Rev. 2016, 85, 356–426. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Krivdin, L.B. Modern quantum chemical methods for calculating spin–spin coupling constants: Theoretical basis and structural applications in chemistry. Russ. Chem. Rev. 2013, 82, 99–130. [Google Scholar] [CrossRef]
- Mulder, F.A.A.; Filatov, M. NMR chemical shift data and ab initio shielding calculations: Emerging tools for protein structure determination. Chem. Soc. Rev. 2010, 39, 578–590. [Google Scholar] [CrossRef]
- Pyykkö, P. Theory of NMR parameters. From Ramsey to Relativity, 1953 to 1983. In Calculation of NMR and EPR Parameters. Theory and Applications, 1st ed.; Kaupp, M., Bühl, M., Malkin, V.G., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; Chapter 2; pp. 7–19. [Google Scholar]
- Facelli, J.C. Chemical shift tensors: Theory and application to molecular structural problems. Prog. Nucl. Magn. Reson. 2011, 58, 176–201. [Google Scholar] [CrossRef]
- Webb, G.A.; Jameson, C.J.; De Dios, A.C. Theoretical and physical aspects of nuclear shielding. In Nuclear Magnetic Resonance, 1st ed.; Webb, G.A., Ed.; Royal Society of Chemistry: London, UK, 2003; Volume 32, pp. 43–74. [Google Scholar] [CrossRef]
- Ebraheem, K.A.K.; Webb, G.A. Semi-empirical calculations of the chemical shifts of nuclei other than protons. Prog. Nucl. Magn. Reson. 1977, 11, 149–181. [Google Scholar] [CrossRef]
- O’Reilly, D.E. Chapter 1 Chemical shift calculations. Prog. Nucl. Magn. Reson. 1967, 2, 1–61. [Google Scholar] [CrossRef]
- Vaara, J.; Jokisaari, J.; Wasylishen, R.E.; Bryce, D.L. Spin–spin coupling tensors as determined by experiment and computational chemistry. Prog. Nucl. Magn. Reson. 2002, 41, 233–304. [Google Scholar] [CrossRef]
- Murrell, J.N. Chapter 1 The theory of nuclear spin-spin coupling in high resolution NMR spectroscopy. Prog. Nucl. Magn. Reson. 1970, 6, 1–60. [Google Scholar] [CrossRef]
- Jameson, C.J. Parameters, Calculation of Nuclear Magnetic Resonance. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation, Nuclear Magnetic Resonance and Electron Spin Resonance Spectroscopy, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–38. [Google Scholar] [CrossRef]
- Pyykko, P. Relativistic effects in structural chemistry. Chem. Rev. 1988, 88, 563–594. [Google Scholar] [CrossRef]
- Nomura, Y.; Takeuchi, Y.; Nakagawa, N. Substituent effects in aromatic proton NMR spectra. III substituent effects caused by halogens. Tetrahedron Lett. 1969, 10, 639–642. [Google Scholar] [CrossRef]
- Vícha, J.; Novotný, J.; Komorovsky, S.; Straka, M.; Kaupp, M.; Marek, R. Relativistic Heavy-Neighbor-Atom Effects on NMR Shifts: Concepts and Trends Across the Periodic Table. Chem. Rev. 2020, 120, 7065–7103. [Google Scholar] [CrossRef] [PubMed]
- Vícha, J.; Komorovsky, S.; Repisky, M.; Marek, R.; Straka, M. Relativistic Spin−Orbit Heavy Atom on the Light Atom NMR Chemical Shifts: General Trends Across the Periodic Table Explained. J. Chem. Theory Comput. 2018, 14, 3025–3039. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.-L.; Cook, T.M.; Lamb, A.C. Trends in NMR chemical shifts of d0 transition metal compounds. J. Organomet. Chem. 2017, 852, 74–93. [Google Scholar] [CrossRef]
- Ehlers, A.W.; Ruiz-Morales, Y.; Baerends, E.J.; Ziegler, T. Dissociation Energies, Vibrational Frequencies, and 13C NMR Chemical Shifts of the 18-Electron Species [M(CO)6]n (M ) Hf-Ir, Mo, Tc, Ru, Cr, Mn, Fe). A Density Functional Study. Inorg. Chem. 1997, 36, 5031–5036. [Google Scholar] [CrossRef]
- Kaupp, M.; Malkin, V.G.; Makina, O.L.; Salahub, D.R. Scalar Relativistic Effects on 17O NMR Chemical Shifts in Transition-Metal Oxo Complexes. An ab Initio ECP/DFT Study. J. Am. Chem. Soc. 1995, 117, 1851–1852. [Google Scholar] [CrossRef]
- Novotny, J.; Vícha, J.; Bora, P.L.; Repisky, M.; Straka, M.; Komorovsky, S.; Marek, R. Linking the Character of Metal-Ligand Bond to the Ligand NMR Shielding in Transition-Metal Complexes: NMR Contributions from Spin-Orbit Coupling. J. Chem. Theory Comput. 2017, 13, 3586–3601. [Google Scholar] [CrossRef] [PubMed]
- Samultsev, D.O.; Semenov, V.A.; Rusakova, I.L.; Krivdin, L.B. Four-Component Relativistic Calculations of NMR Shielding Constants of the Transition Metal Complexes—Part 2: Nitrogen-Coordinated Complexes of Cobalt. Int. J. Mol. Sci. 2022, 23, 13178. [Google Scholar] [CrossRef]
- Bora, P.L.; Novotny, J.; Ruud, K.; Komorovsky, S.; Marek, R. Electron-Spin Structure and Metal-Ligand Bonding in Open-Shell Systems from Relativistic EPR and NMR: A Case Study of Square-Planar Iridium Catalysts. J. Chem. Theory Comput. 2019, 15, 201–214. [Google Scholar] [CrossRef]
- Kaupp, M.; Malkina, O.L. Density functional analysis of 13C and 1H chemical shifts and bonding in mercurimethanes and organomercury hydrides: The role of scalar relativistic, spinorbit, and substituent effects. J. Chem. Phys. 1998, 108, 3648–3659. [Google Scholar] [CrossRef]
- Wodyński, A.; Gryff-Keller, A.; Pecul, M. The Influence of a Presence of a Heavy Atom on 13C Shielding Constants in Organomercury Compounds and Halogen Derivatives. J. Chem. Theory Comput. 2013, 9, 1909–1917. [Google Scholar] [CrossRef]
- Hrobárik, P.; Hrobáriková, V.; Meier, F.; Repiský, M.; Komorovský, S.; Kaupp, M. Relativistic Four-Component DFT Calculations of 1H NMR Chemical Shifts in Transition-Metal Hydride Complexes: Unusual High-Field Shifts Beyond the Buckingham–Stephens Model. Phys. Chem. A 2011, 115, 5654–5659. [Google Scholar] [CrossRef] [PubMed]
- Greif, A.H.; Hrobárik, P.; Hrobáriková, V.; Arbuznikov, A.V.; Autschbach, J.; Kaupp, M. A Relativistic Quantum-Chemical Analysis of the trans Influence on 1H NMR Hydride Shifts in Square-Planar Platinum(II) Complexes. Inorg. Chem. 2015, 54, 15. [Google Scholar] [CrossRef] [PubMed]
- Rocchigiani, L.; Fernandez-Cestau, J.; Chambrier, I.; Hrobárik, P.; Bochmann, M. Unlocking Structural Diversity in Gold(III) Hydrides: Unexpected Interplay of cis/trans-Influence on Stability, Insertion Chemistry, and NMR Chemical Shifts. J. Am. Chem. Soc. 2018, 140, 8287–8302. [Google Scholar] [CrossRef] [PubMed]
- Bagno, A.; Saielli, G. Relativistic DFT Calculations of the NMR Properties and Reactivity of Transition Metal Methane Sigma-Complexes: Insights on C-H Bond Activation. Phys. Chem. Chem. Phys. 2011, 13, 4285–4291. [Google Scholar] [CrossRef]
- Vícha, J.; Novotný, J.; Straka, M.; Repisky, M.; Ruud, K.; Komorovsky, S.; Marek, R. Structure, solvent, and relativistic effects on the NMR chemical shifts in square-planar transitionmetal complexes: Assessment of DFT approaches. Phys. Chem. Chem. Phys. 2015, 17, 24944–24955. [Google Scholar] [CrossRef]
- Vícha, J.; Straka, M.; Munzarová, M.L.; Marek, R. Mechanism of Spin−Orbit Effects on the Ligand NMR Chemical Shift in Transition-Metal Complexes: Linking NMR to EPR. J. Chem. Theory Comput. 2014, 10, 1489–1499. [Google Scholar] [CrossRef]
- Greif, A.H.; Hrobárik, P.; Kaupp, M. Insights into trans-Ligand and Spin-Orbit Effects on Electronic Structure and Ligand NMR Shifts in Transition-Metal Complexes. Chem. Eur. J. 2017, 23, 9790–9803. [Google Scholar] [CrossRef]
- Demissie, T.B.; Kostenko, N.; Komorovsky, S.; Repisky, M.; Isaksson, J.; Bayer, A.; Ruud, K. Experimental and Four-Component Relativistic DFT Studies of Tungsten Carbonyl Complexes. J. Phys. Org. Chem. 2015, 28, 723–731. [Google Scholar] [CrossRef]
- Pawlak, T.; Munzarová, M.; Pazderski, L.; Marek, R. Validation of Relativistic DFT Approaches to the Calculation of NMR Chemical Shifts in Square-Planar Pt2+ and Au3+ Complexes. J. Chem. Theory Comput. 2011, 7, 3909–3923. [Google Scholar] [CrossRef]
- Greif, A.H.; Hrobárik, P.; Autschbach, J.; Kaupp, M. Giant Spin-Orbit Effects on H-1 and C-13 NMR Shifts for Uranium(VI) Complexes Revisited: Role of the Exchange-Correlation Response Kernel, Bonding Analyses, and New Predictions. Phys. Chem. Chem. Phys. 2016, 18, 30462–30474. [Google Scholar] [CrossRef]
- Hrobárik, P.; Hrobáriková, V.; Greif, A.H.; Kaupp, M. Giant Spin-Orbit Effects on NMRShifts in Diamagnetic Actinide Complexes: Guiding the Search of Uranium(VI) Hydride Complexes in the Correct Spectral Range. Angew. Chem. Int. Ed. 2012, 51, 10884–10888. [Google Scholar] [CrossRef] [PubMed]
- Gowda, V.; Laitinen, R.S.; Telkki, V.-V.; Larsson, A.-C.; Antzutkin, O.N.; Lantto, P. DFT Calculations in the Assignment of Solid-State NMR and Crystal Structure Elucidation of a Lanthanum-(III) Complex with Dithiocarbamate and Phenanthroline. Dalton Trans. 2016, 45, 19473–19484. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, M.; Schleyer, P.v.R.; Stoll, H.; Preuss, H. Pseudopotential Approaches to Ca, Sr, and Ba Hydrides. Why Are Some Alkaline Earth MX2 Compounds Bent? J. Chem. Phys. 1991, 94, 1360–1366. [Google Scholar] [CrossRef]
- Nicol, A.T.; Vaughan, R.W. Proton Chemical Shift Tensors of Alkaline Earth Hydrides. J. Chem. Phys. 1978, 69, 5211–5213. [Google Scholar] [CrossRef]
- Morishima, I.; Endo, K.; Yonezawa, T. Effect of the heavy atom on the nuclear shielding constant. I. The proton chemical shifts in hydrogen halides. J. Chem. Phys. 1973, 59, 3356–3364. [Google Scholar] [CrossRef]
- Cheremisin, A.A.; Schastneva, P.V. Effects of spin-orbital interactions on 13 NMR chemical shifts in halogen-substituted methanes. J. Magn. Reson. 1969, 40, 459–468. [Google Scholar] [CrossRef]
- Pyper, N.C. The relativistic theory of the chemical shift. Chem. Phys. Lett. 1983, 96, 204–210. [Google Scholar] [CrossRef]
- Pyper, N.C. Exact relativistic analogues of the non-relativistic hyperfine structure operators. Mol. Phys. 1988, 64, 933–961. [Google Scholar] [CrossRef]
- Pyper, N.C. Relativistic theory of nuclear shielding in one-electron atoms 1. Theoretical foundations and first-order terms. Mol. Phys. 1999, 97, 381–390. [Google Scholar] [CrossRef]
- Pyper, N.C.; Zhang, Z.C. Relativistic theory of nuclear shielding in one-electron atoms 2. Analytical and numerical results. Mol. Phys. 1999, 97, 391–413. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Webb, G.A. On the relativistic molecular orbital theory of diamagnetism and NMR chemical shifts. J. Mol. Struct. Theochem. 1983, 104, 439–444. [Google Scholar] [CrossRef]
- Pyykkö, P. On the relativistic theory of NMR chemical shifts. Chem. Phys. 1983, 74, 1–7. [Google Scholar] [CrossRef]
- Pyykkö, P.; Görling, A.; Rösch, N. A transparent interpretation of the relativistic contribution to the N.M.R. ‘heavy atom chemical shift’. Mol. Phys. 1987, 61, 195–205. [Google Scholar] [CrossRef]
- Nakatsuji, H.; Takashima, H.; Hada, M. Spin-orbit effect on the magnetic shielding constant using the ab initio UHF method. Chem. Phys. Lett. 1995, 233, 95–101. [Google Scholar] [CrossRef]
- Kazunaka, E.; Kyonosuke, Y.; Hiroaki, O. Heavy Atom Effect on 14 Group Nuclear Shielding Constant of SiX4 and CH4−nXn (X = Cl, Br, I; n = 1, 2, 3, 4). Bull. Chem. Soc. Jpn. 1995, 68, 3341–3345. [Google Scholar] [CrossRef]
- Breit, G. The Effect of Retardation on the Interaction of Two Electrons. Phys. Rev. 1929, 34, 553–573. [Google Scholar] [CrossRef]
- Mittleman, M.H. Configuration-Space Hamiltonian for Heavy Atoms and Correction to the Breit Interaction. Phys. Rev. A 1972, 5, 2395–2401. [Google Scholar] [CrossRef]
- Sakurai, J.J. Advanced Quantum Mechanics, 1st ed.; Addison-Wesley Publishing Company, Reading: Chicago, IL, USA, 1967; pp. 108–109. [Google Scholar]
- Ramsey, N.F. Magnetic shielding of nuclei in molecules. Phys. Rev. 1950, 78, 699–703. [Google Scholar] [CrossRef]
- London, F. Théorie quantique des courants interatomiques dans les combinaisons aromatiques. J. Phys. Radium 1937, 8, 397–409. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism I. A gauge-invariant LCAO method for N.M.R. chemical shifts. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- Quiney, H.M.; Skaane, H.; Grant, I.P. Relativistic, quantum electrodynamic and many-body effects in the water molecule. Chem. Phys. Lett. 1998, 290, 473–480. [Google Scholar] [CrossRef]
- Quiney, H.M.; Skaane, H.; Grant, I.P. Ab initio relativistic quantum chemistry: Four-components good, two-components bad! Adv. Quantum Chem. 1998, 32, 1–49. [Google Scholar] [CrossRef]
- Grant, I.P.; Quiney, H.M. Application of relativistic theories and quantum electrodynamics to chemical problems. Int. J. Quantum Chem. 2000, 80, 283–297. [Google Scholar] [CrossRef]
- Aucar, G.A.; Oddershede, J. Relativistic theory for indirect nuclear spin–spin couplings within the polarization propagator approach. Int. J. Quantum Chem. 1993, 47, 425–435. [Google Scholar] [CrossRef]
- Aucar, G.A.; Aucar, I.A. Recent developments in absolute shielding scales for NMR spectroscopy. In Annual Reports on NMR Spectroscopy, 1st ed.; Webb, G., Ed.; Academic Press: London, UK, 2019; Volume 96, Chapter 3; pp. 77–141. [Google Scholar]
- Aucar, G.A.; Maldonado, A.F.; Montero, M.D.A.; Cruz, T.S. Theoretical developments and applications of polarization propagators. Int. J. Quantum Chem. 2019, 119, e25722. [Google Scholar] [CrossRef]
- Melo, J.I.; de Azua, M.C.R.; Giribet, C.G.; Aucar, G.A.; Provasi, P.F. Relativistic effects on nuclear magnetic shielding constants in HX and CH3X (X=Br,I) based on the linear response within the elimination of small component approach. J. Chem. Phys. 2004, 121, 6798–6808. [Google Scholar] [CrossRef]
- Gomez, S.S.; Romero, R.H.; Aucar, G.A. Fully relativistic calculation of nuclear magnetic shieldings and indirect nuclear spin-spin couplings in group-15 and -16 hydrides. J. Chem. Phys. 2002, 117, 7942–7946. [Google Scholar] [CrossRef]
- Maldonado, A.F.; Aucar, G.A.; Melo, J.I. Core-dependent and ligand-dependent relativistic corrections to the nuclear magnetic shieldings in MH4−nYn (n = 0-4; M = Si, Ge, Sn, and Y = H, F, Cl, Br, I) model compounds. J. Mol. Model. 2014, 20, 2417. [Google Scholar] [CrossRef]
- Aucar, G.A.; Saue, T.; Visscher, L.; Jensen, H.J.A. On the origin and contribution of the diamagnetic term in four-component relativistic calculations of magnetic properties. J. Chem. Phys. 1999, 110, 6208–6218. [Google Scholar] [CrossRef]
- Maldonado, A.F.; Aucar, G.A. The UKB prescription and the heavy atom effects on the nuclear magnetic shielding of vicinal heavy atoms. Phys. Chem. Chem. Phys. 2009, 11, 5615–5627. [Google Scholar] [CrossRef]
- Kozioł, K.; Aucar, I.A.; Aucar, G.A. Relativistic and QED effects on NMR magnetic shielding constant of neutral and ionized atoms and diatomic molecules. J. Chem. Phys. 2019, 150, 184301. [Google Scholar] [CrossRef] [PubMed]
- Iliaš, M.; Saue, T.; Enevoldsen, T.; Jensen, H.J.A. Gauge origin independent calculations of nuclear magnetic shieldings in relativistic four-component theory. J. Chem. Phys. 2009, 131, 124119. [Google Scholar] [CrossRef]
- Olejniczak, M.; Bast, R.; Saue, T.; Pecul, M. A simple scheme for magnetic balance in four-component relativistic Kohn-Sham calculations of nuclear magnetic resonance shielding constants in a Gaussian basis. J. Chem. Phys. 2012, 136, 014108. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Xiao, Y.; Liu, W. Four-component relativistic theory for nuclear magnetic shielding: Magnetically balanced gauge-including atomic orbitals. J. Chem. Phys. 2009, 131, 244113. [Google Scholar] [CrossRef]
- Vaara, J.; Pyykkö, P. Relativistic, nearly basis-set-limit nuclear magnetic shielding constants of the rare gases He-Rn: A way to absolute nuclear magnetic resonance shielding scales. J. Chem. Phys. 2003, 118, 2973–2976. [Google Scholar] [CrossRef]
- Antušek, A.; Pecul, M.; Sadlej, J. Relativistic calculation of NMR properties of XeF2, XeF4 and XeF6. Chem. Phys. Lett. 2006, 427, 281–288. [Google Scholar] [CrossRef]
- Schnack-Petersen, A.K.; Simmermacher, M.; Fasshauer, E.; Jensen, H.J.A.; Sauer, S.P.A. The second-order-polarization-propagator-approximation (SOPPA) in a four-component spinor basis. J. Chem. Phys. 2020, 152, 134113. [Google Scholar] [CrossRef]
- Oddershede, J. Response and propagator methods. In Methods in Computational Molecular Physics, 1st ed.; Wilson, S., Diercksen, G.H.F., Eds.; Plenum Press: New York, NY, USA, 1992; Chapter 11; pp. 303–324. [Google Scholar]
- Enevoldsen, T.; Oddershede, J.; Sauer, S.P.A. Correlated calculations of indirect nuclear spin-spin coupling constants using second-order polarization propagator approximations: SOPPA and SOPPA(CCSD). Theor. Chem. Acc. 1998, 100, 275–284. [Google Scholar] [CrossRef]
- Malkin, V.G.; Malkina, O.L.; Salahub, D.R. Spin-orbit correction to NMR shielding constants from density functional theory. Chem. Phys. Lett. 1996, 261, 335–345. [Google Scholar] [CrossRef]
- Kutzelnigg, W.; Fleischer, U.; Schindler, M. The IGLO-Method: Ab-initio Calculation and Interpretation of NMR Chemical Shifts and Magnetic Susceptibilities. In Deuterium and Shift Calculation. NMR Basic Principles and Progress, 1st ed.; Diehl, P., Fluck, E., Gunther, H., Kosfeld, R., Seelig, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; Volume 23, pp. 165–262. [Google Scholar] [CrossRef]
- Komorovsky, S.; Repisky, M.; Malkina, O.L.; Malkin, V.G. Fully relativistic calculations of NMR shielding tensors using restricted magnetically balanced basis and gauge including atomic orbitals. J. Chem. Phys. 2010, 132, 154101. [Google Scholar] [CrossRef]
- Komorovsky, S.; Repisky, M.; Malkina, O.L.; Malkin, V.G.; Ondik, I.M.; Kaupp, M. A fully relativistic method for calculation of nuclear magnetic shielding tensors with a restricted magnetically balanced basis in the framework of the matrix Dirac–Kohn–Sham equation. J. Chem. Phys. 2008, 128, 104101. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Peng, D.; Liu, W. Exact two-component relativistic theory for nuclear magnetic resonance parameters. J. Chem. Phys. 2007, 126, 081101. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, W.; Cheng, L.; Peng, D. Four-component relativistic theory for nuclear magnetic shielding constants: Critical assessments of different approaches. J. Chem. Phys. 2007, 126, 214101. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Reiher, M. Exact decoupling of the relativistic Fock operator. Theor. Chem. Acc. 2012, 131, 1081. [Google Scholar] [CrossRef]
- Kaneko, H.; Hada, M.; Nakajima, T.; Nakatsuji, H. Spin-orbit effect on the magnetic shielding constant using the ab initio UHF method: Tin tetrahalides. Chem. Phys. Lett. 1996, 261, 1–6. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Nakajima, T.; Hada, M.; Nakatsuji, H. Relativistic theory of the magnetic shielding constant: A Dirac–Fock finite perturbation study. Chem. Phys. Lett. 1998, 283, 119–124. [Google Scholar] [CrossRef]
- Fukui, H.; Baba, T.; Inomata, H. Calculation of nuclear magnetic shieldings. X. Relativistic effects. J. Chem. Phys. 1996, 105, 3175–3186. [Google Scholar] [CrossRef]
- Fukui, H.; Baba, T.; Inomata, H. Erratum: Calculation of nuclear magnetic shieldings. X. Relativistic effects. J. Chem. Phys. 1996, 105, 3175, Erratum in J. Chem. Phys. 1997, 106, 2987–2987. [Google Scholar] [CrossRef]
- Fukui, H.; Baba, T. Calculation of nuclear magnetic shieldings. XII. Relativistic no-pair equation. J. Chem. Phys. 1998, 108, 3854–3862. [Google Scholar] [CrossRef]
- Ballard, C.C.; Hada, M.; Kaneko, H.; Nakatsuji, H. Relativistic study of nuclear magnetic shielding constants: Hydrogen halides. Chem. Phys. Lett. 1996, 254, 170–178. [Google Scholar] [CrossRef]
- Sucher, J. Foundations of the relativistic theory of many-electron atoms. Phys. Rev. A 1980, 22, 348–362. [Google Scholar] [CrossRef]
- Hess, B.A. Applicability of the no-pair equation with free-particle projection operators to atomic and molecular structure calculations. Phys. Rev. A 1985, 32, 756–763. [Google Scholar] [CrossRef]
- Hess, B.A. Relativistic electronic-structure calculations employing a two-component no-pair formalism with external-field projection operators. Phys. Rev. 1986, 33, 3742–3748. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, R.; Hada, M.; Nakatsuji, H. Quasirelativistic theory for the magnetic shielding constant. I. Formulation of Douglas–Kroll–Hess transformation for the magnetic field and its application to atomic systems. J. Chem. Phys. 2003, 118, 1015–1026. [Google Scholar] [CrossRef]
- Fukuda, R.; Hada, M.; Nakatsuji, H. Quasirelativistic theory for magnetic shielding constants. II. Gauge-including atomic orbitals and applications to molecules. J. Chem. Phys. 2003, 118, 1027–1035. [Google Scholar] [CrossRef]
- Wan, J.; Fukuda, R.; Hada, M.; Nakatsuji, H. Quasi-Relativistic Study of 199Hg Nuclear Magnetic Shielding Constants of Dimethylmercury, Disilylmercury and Digermylmercury. J. Phys. Chem. A 2001, 105, 128–133. [Google Scholar] [CrossRef]
- Hada, M.; Wan, J.; Fukuda, R.; Nakatsuji, H. Quasirelativistic study of 125Te nuclear magnetic shielding constants and chemical shifts. J. Comput. Chem. 2001, 22, 1502–1508. [Google Scholar] [CrossRef]
- Wolff, S.K.; Ziegler, T. Calculation of DFT-GIAO NMR shifts with the inclusion of spin-orbit coupling. J. Chem. Phys. 1998, 109, 895–905. [Google Scholar] [CrossRef]
- Schreckenbach, G.; Ziegler, T. Calculation of NMR Shielding Tensors Using Gauge-Including Atomic Orbitals and Modern Density Functional Theory. J. Phys. Chem. 1995, 99, 606–611. [Google Scholar] [CrossRef]
- Schreckenbach, G.; Ziegler, T. The calculation of NMR shielding tensors based on density functional theory and the frozen-core approximation. Int. J. Quantum Chem. 1996, 60, 753–766. [Google Scholar] [CrossRef]
- Vaara, J.; Ruud, K.; Vahtras, O.; Ågren, H.; Jokissari, J. Quadratic response calculations of the electronic spin-orbit contribution to nuclear shielding tensors. J. Chem. Phys. 1998, 109, 1212–1222. [Google Scholar] [CrossRef]
- Vaara, J.; Ruud, K.; Vahtras, O. Second- and third-order spin-orbit contributions to nuclear shielding tensors. J. Chem. Phys. 1999, 111, 2900–2909. [Google Scholar] [CrossRef]
- Cramer, C.J. Essentials of Computational Chemistry, Theories and Models, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2004; pp. 1–596. [Google Scholar]
- Jensen, F. Introduction to Computational Chemistry, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2007; pp. 1–599. [Google Scholar]
- Vaara, J.; Malkina, O.L.; Stoll, H.; Malkin, V.G.; Kaupp, M. Study of relativistic effects on nuclear shieldings using density-functional theory and spin–orbit pseudopotentials. J. Chem. Phys. 2001, 114, 61–71. [Google Scholar] [CrossRef]
- Vaara, J.; Manninen, P.; Lantto, P. Perturbational and ECP calculation of relativistic effects in NMR shielding and spin-spin coupling. In Calculation of NMR and EPR Parameters. Theory and Applications, 1st ed.; Kaupp, M., Bühl, M., Malkin, V.G., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; Chapter 13; pp. 209–226. [Google Scholar]
- Manninen, P.; Lantto, P.; Vaara, J.; Ruud, K. Perturbational ab initio calculations of relativistic contributions to nuclear magnetic resonance shielding tensors. J. Chem. Phys. 2003, 119, 2623–2637. [Google Scholar] [CrossRef]
- Manninen, P. Breit-Pauli Hamiltonian and Molecular Magnetic Resonance Properties. Ph.D. Thesis, Department of Physical Sciences, University of Oulu, Oulu, Finland, 2004. [Google Scholar]
- Manninen, P.; Ruud, K.; Lantto, P.; Vaara, J. Leading-order relativistic effects on nuclear magnetic resonance shielding tensors. J. Chem. Phys. 2005, 122, 114107, Erratum in J. Chem. Phys. 2006, 124, 149901. [Google Scholar] [CrossRef]
- Jaszuński, M.; Ruud, K. Nuclear magnetic resonance shielding constants in XH4 group XIV hydrides. Mol. Phys. 2006, 104, 2139–2148. [Google Scholar] [CrossRef]
- Kudo, K.; Fukui, H. Calculation of nuclear magnetic shieldings using an analytically differentiated relativistic shielding formula. J. Chem. Phys. 2005, 123, 114102. [Google Scholar] [CrossRef]
- Jensen, G.; Hess, B.A. Revision of the Douglas-Kroll transformation. Phys. Rev. A 1989, 39, 6016–6017. [Google Scholar] [CrossRef]
- Barysz, M.; Sadlej, A.J. Two-component methods of relativistic quantum chemistry: From the Douglas-Kroll approximation to the exact two-component formalism. J. Mol. Struct. THEOCHEM 2001, 573, 181–200. [Google Scholar] [CrossRef]
- Barysz, M.; Sadlej, A.J. Infinite-order two-component theory for relativistic quantum chemistry. J. Chem. Phys. 2002, 116, 2696–2704. [Google Scholar] [CrossRef]
- Kedziera, D.; Barysz, M. Two-component relativistic methods for the heaviest elements. J. Chem. Phys. 2004, 121, 6719–6727. [Google Scholar] [CrossRef] [PubMed]
- Kedziera, D.; Barysz, M. Non-iterative approach to the infinite-order two-component (IOTC) relativistic theory and the non-symmetric algebraic Riccati equation. Chem. Phys. Lett. 2007, 446, 176–181. [Google Scholar] [CrossRef]
- Sun, Q.; Xiao, Y.; Liu, W. Exact Two-Component Relativistic Theory for NMR Parameters: General Formulation and Pilot Application. J. Chem. Phys. 2012, 137, 174105–174106. [Google Scholar] [CrossRef] [PubMed]
- Autschbach, J. Relativistic calculations of magnetic resonance parameters: Background and some recent developments. Phil. Trans. R. Soc. A 2014, 372, 20120489. [Google Scholar] [CrossRef] [PubMed]
- Dyall, K.G. Interfacing relativistic and nonrelativistic methods. I. Normalized elimination of the small component in the modified Dirac equation. J. Chem. Phys. 1997, 106, 9618–9626. [Google Scholar] [CrossRef]
- Dyall, K.G. Interfacing relativistic and nonrelativistic methods. II. Investigation of a low-order approximation. J. Chem. Phys. 1998, 109, 4201–4208. [Google Scholar] [CrossRef]
- Dyall, K.G.; Enevoldsen, T. Interfacing relativistic and nonrelativistic methods. III. Atomic 4-spinor expansions and integral approximations. J. Chem. Phys. 1999, 111, 10000–10007. [Google Scholar] [CrossRef]
- Dyall, K.G. Interfacing relativistic and nonrelativistic methods. IV. One- and two-electron scalar approximations. J. Chem. Phys. 2001, 115, 9136–9143. [Google Scholar] [CrossRef]
- Dyall, K.G. A systematic sequence of relativistic approximations. J. Comput. Chem. 2002, 23, 786–793. [Google Scholar] [CrossRef]
- Filatov, M.; Cremer, D. Representation of the exact relativistic electronic Hamiltonian within the regular approximation. J. Chem. Phys. 2003, 119, 11526–11540. [Google Scholar] [CrossRef]
- Filatov, M.; Cremer, D. Connection between the regular approximation and the normalized elimination of the small component in relativistic quantum theory. J. Chem. Phys. 2005, 122, 064104. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Filatov, M.; Cremer, D. An improved algorithm for the normalized elimination of the small-component method. Theor. Chem. Acc. 2011, 130, 633–644. [Google Scholar] [CrossRef]
- Seino, J.; Hada, M. Magnetic shielding constants calculated by the infinite-order Douglas-Kroll-Hess method with electron-electron relativistic corrections. J. Chem. Phys. 2010, 132, 174105. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.K.; Ziegler, T.; van Lenthe, E.; Baerends, E.J. Density functional calculations of nuclear magnetic shieldings using the zeroth-order regular approximation (ZORA) for relativistic effects: ZORA nuclear magnetic resonance. J. Chem. Phys. 1999, 110, 7689–7698. [Google Scholar] [CrossRef]
- Chang, C.; Pelissier, M.; Durand, M. Regular Two-Component Pauli-like effective Hamiltonians in Dirac theory. Phys. Scr. 1986, 34, 394–404. [Google Scholar] [CrossRef]
- van Lenthe, E.; Baerends, E.J.; Snijders, J.G. Relativistic regular two-component Hamiltonians. J. Chem. Phys. 1993, 99, 4597–4610. [Google Scholar] [CrossRef]
- Hamaya, S.; Maeda, H.; Funaki, M.; Fukui, H. Relativistic calculation of nuclear magnetic shielding tensor using the regular approximation to the normalized elimination of the small component. III. Introduction of gauge-including atomic orbitals and a finite-size nuclear model. J. Chem. Phys. 2008, 129, 224103. [Google Scholar] [CrossRef]
- Douglas, M.; Kroll, N.M. Quantum electrodynamical corrections to the fine structure of helium. Ann. Phys. 1974, 82, 89–155. [Google Scholar] [CrossRef]
- Reiher, M. Douglas-Kroll-Hess Theory: A relativistic electrons-only theory for chemistry. Theor. Chem. Acc. 2006, 116, 241–252. [Google Scholar] [CrossRef]
- Nakajima, T.; Hirao, K. The higher-order Douglas-Kroll transformation. J. Chem. Phys. 2000, 113, 7786–7789. [Google Scholar] [CrossRef]
- Nakajima, T.; Hirao, K. Numerical illustration of third-order Douglas-Kroll method: Atomic and molecular properties of superheavy element 112. Chem. Phys. Lett. 2000, 329, 511–516. [Google Scholar] [CrossRef]
- Wolf, A.; Reiher, M.; Hess, B.A. The generalized Douglas-Kroll transformation. J. Chem. Phys. 2002, 117, 9215–9226. [Google Scholar] [CrossRef]
- Van Wüllen, C. Relation between different variants of the generalized Douglas-Kroll transformation through sixth order. J. Chem. Phys. 2004, 120, 7307–7313. [Google Scholar] [CrossRef]
- Filatov, M.; Cremer, D. Calculation of indirect nuclear spin-spin coupling constants within the regular approximation for relativistic effects. J. Chem. Phys. 2004, 120, 11407–11422. [Google Scholar] [CrossRef] [PubMed]
- Dyall, K.G.; van Lenthe, E. Relativistic regular approximations revisited: An infinite-order relativistic approximation. J. Chem. Phys. 1999, 111, 1366–1372. [Google Scholar] [CrossRef]
- Wu, W.; Rehe, D.; Hrobárik, P.; Kornienko, A.Y.; Emge, T.J.; Brennan, J.G. Molecular Thorium Compounds with Dichalcogenide Ligands: Synthesis, Structure, 77Se NMR Study, and Thermolysis. Inorg. Chem. 2018, 57, 14821–14833. [Google Scholar] [CrossRef]
- Brownridge, S.; Calhoun, L.; Jenkins, H.D.B.; Laitinen, R.S.; Murchie, M.P.; Passmore, J.; Pietikäinen, J.; Rautiainen, J.M.; Sanders, J.C.P.; Schrobilgen, G.J.; et al. 77Se NMR Spectroscopic, DFT MO, and VBT Investigations of the Reversible Dissociation of Solid (Se6I2)[AsF6]2·2SO2 in Liquid SO2 to Solutions Containing 1,4-Se6I22+ in Equilibrium with Sen2+ (n = 4, 8, 10) and Seven Binary Selenium Iodine Cations: Preliminary Evidence for 1,1,4,4-Se4Br42+ and cyclo-Se7Br+. Inorg. Chem. 2009, 48, 1938–1959. [Google Scholar]
- Ringgold, M.; Rehe, D.; Hrobárik, P.; Kornienko, A.Y.; Emge, T.J.; Brennan, J.G. Thorium Cubanes−Synthesis, Solid-State and Solution Structures, Thermolysis, and Chalcogen Exchange Reactions. Inorg. Chem. 2018, 57, 7129–7141. [Google Scholar] [CrossRef]
- Rautiainen, J.M.; Way, T.; Schatte, G.; Passmore, J.; Laitinen, R.S.; Suontamo, R.J.; Valkonen, J. A Computational and Experimental Study of the Structures and Raman and 77Se NMR Spectra of SeX3+ and SeX2 (X = Cl, Br, I): FT-Raman Spectrum of (SeI3)[AsF6]. Inorg. Chem. 2005, 44, 1904–1913. [Google Scholar] [CrossRef]
- Melo, J.I.; Ruiz de Azua, M.C.; Giribet, C.G.; Aucar, G.A.; Romero, R.H. Relativistic effects on the nuclear magnetic shielding tensor. J. Chem. Phys. 2003, 118, 471–486. [Google Scholar] [CrossRef]
- Aucar, G.A.; Melo, J.I.; Aucar, I.A.; Maldonado, A.F. Foundations of the LRESC model for response properties and some applications. Int. J. Quantum Chem. 2018, 118, e25487. [Google Scholar] [CrossRef]
- Melo, J.I.; Maldonado, A.F.; Aucar, G.A. Performance of the LRESC Model on top of DFT Functionals for Relativistic NMR Shielding Calculations. J. Chem. Inf. Model. 2020, 60, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Zapata−Escobar, A.D.; Maldonado, A.F.; Aucar, G.A. The LRESC-Loc Model to Analyze Magnetic Shieldings with Localized Molecular Orbitals. J. Phys. Chem. A 2022, 126, 9519–9534. [Google Scholar] [CrossRef]
- Visscher, L.; Enevoldsen, T.; Saue, T.; Jensen, H.J.A.; Oddershede, J. Full four-component relativistic calculations of NMR shielding and indirect spin–spin coupling tensors in hydrogen halides. J. Comput. Chem. 1999, 20, 1262–1273. [Google Scholar] [CrossRef]
- Autschbach, J. Calculation of heavy-nucleus chemical shifts. Relativistic all-electron methods. In Calculation of NMR and EPR Parameters. Theory and Applications, 1st ed.; Kaupp, M., Bühl, M., Malkin, V.G., Eds.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004; Chapter 14; pp. 227–248. [Google Scholar]
- Autschbach, J. Perspective: Relativistic effects. J. Chem. Phys. 2012, 136, 150902. [Google Scholar] [CrossRef] [PubMed]
- Autschbach, J. Relativistic Effects on NMR Parameters. In High Resolution NMR Spectroscopy. Understanding Molecules and Their Electronic Structures, 1st ed.; Contreras, R.H., Ed.; Elsevier B. V.: London, UK, 2013; Volume 3, Chapter 4; pp. 69–117. [Google Scholar]
- Autschbach, J.; Ziegler, T. Relativistic computation of NMR shieldings and spin-spin coupling constants. In Encyclopedia of Nuclear Magnetic Resonance: Advances in NMR, 1st ed.; Grant, D.M., Harris, R.K., Eds.; John Wiley and Sons: Chichester, UK, 2002; Volume 9, pp. 306–323. [Google Scholar]
- Oprea, C.I. Theoretical Calculations of Heavy Atom Effects in Magnetic Resonance Spectroscopy. Ph.D. Thesis, Theoretical Chemistry School of Biotechnology Royal Institute of Technology, Stockholm, Sweden, 2006. [Google Scholar]
- Gόmez, S.S.; Romero, R.H.; Aucar, G.A. Relativistic mass-corrections to the heavy atom nuclear magnetic shieldings. Analysis of contributions in terms of localized orbitals. Chem. Phys. Lett. 2003, 367, 265–269. [Google Scholar] [CrossRef]
- Gόmez, S.S.; Melo, J.I.; Romero, R.H.; Aucar, G.A.; Azúa, M.R. Relativistic corrections to the diamagnetic term of the nuclear magnetic shielding: Analysis of contributions from localized orbitals. J. Chem. Phys. 2005, 122, 064103. [Google Scholar] [CrossRef] [PubMed]
- Gόmez, S.S.; Maldonado, A.; Aucar, G.A. Relativistic effects on the nuclear magnetic shieldings of rare-gas atoms and halogen in hydrogen halides within relativistic polarization propagator theory. J. Chem. Phys. 2005, 123, 214108. [Google Scholar] [CrossRef] [PubMed]
- Lantto, P.; Romero, R.H.; Gόmez, S.S.; Aucar, G.A.; Vaara, J. Relativistic heavy-atom effects on heavy-atom nuclear shieldings. J. Chem. Phys. 2006, 125, 184113. [Google Scholar] [CrossRef]
- Melo, J.I.; Maldonado, A.; Aucar, G.A. Relativistic effects on the shielding of SnH2XY and PbH2XY (X, Y = F, Cl, Br and I) heavy atom–containing molecules. Theor. Chem. Acc. 2011, 129, 483–494. [Google Scholar] [CrossRef]
- Melo, J.I.; Maldonado, A.; Aucar, G.A. Relativistic effects on nuclear magnetic shieldings of CHnX4−n and CHXYZ (X, Y, Z = H, F, Cl, Br, I). J. Chem. Phys. 2012, 137, 214319. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, A.; Aucar, G.A. Relativistic and Electron-Correlation Effects on the Nuclear Magnetic Resonance Shieldings of Molecules Containing Tin and Lead Atoms. J. Phys. Chem. A 2014, 118, 7863–7875. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, A.; Melo, J.I.; Aucar, G.A. Theoretical analysis of NMR shieldings of group-11 metal halides on MX (M = Cu, Ag, Au; X = H, F, Cl, Br, I) molecular systems, and the appearance of quasi-instabilities on AuF. Phys. Chem. Chem. Phys. 2015, 17, 25516–25524. [Google Scholar] [CrossRef]
- Gimenez, C.A.; Kozioł, K.; Aucar, G.A. Quantum electrodynamics effects on NMR magnetic shielding constants of He-like and Be-like atomic systems. Phys. Rev. A 2016, 93, 032504. [Google Scholar] [CrossRef]
- Jofré, M.T.; Kozioł, K.; Aucar, I.A.; Gaul, K.; Berger, R.; Aucar, G.A. Relativistic and QED corrections to one-bond indirect nuclear spin–spin couplings in X22+ and X32+ ions (X = Zn, Cd, Hg). J. Chem. Phys. 2022, 157, 064103. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Krivdin, L.B. Relativistic effects in the NMR spectra of compounds containing heavy chalcogens. Mendeleev Commun. 2018, 28, 1–13. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Rusakov, Y.Y. On the heavy atom on light atom relativistic effect in the NMR shielding constants of phosphine tellurides. Magn. Reson. Chem. 2019, 57, 1071–1083. [Google Scholar] [CrossRef]
- Kaupp, M. Relativistic effects on NMR chemical shifts. In Relativistic Electronic Structure Theory, Part 2: Applications. Theoretical and Computational Chemistry, 1st ed.; Schwerdtfeger, P., Ed.; Elsevier B. V.: Amsterdam, The Netherlands, 2004; Volume 14, Chapter 9; pp. 552–597. [Google Scholar]
- Kaupp, M.; Malkina, O.L.; Malkin, V.G.; Pyykkö, P. How Do Spin-Orbit-Induced Heavy-Atom Effects on NMR Chemical Shifts Function? Validation of a Simple Analogy to Spin-Spin Coupling by Density Functional Theory (DFT) Calculations on Some Iodo Compounds. Chem. Eur. J. 1998, 4, 118–126. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. Long-range relativistic heavy atom effect on 1H NMR chemical shifts of selenium- and tellurium-containing compounds. Int. J. Quantum Chem. 2018, 119, e25809. [Google Scholar] [CrossRef]
- Jameson, C.J.; Mason, J. The Parameters of NMR Spectroscopy. In Multinuclear NMR, 1st ed.; Mason, J., Ed.; Plenum Press: New York, NY, USA, 1987; pp. 3–50. [Google Scholar] [CrossRef]
- Jameson, C.J.; Mason, J. The Chemical Shift. In Multinuclear NMR, 1st ed.; Mason, J., Ed.; Plenum Press: New York, NY, USA, 1987; pp. 51–88. [Google Scholar] [CrossRef]
- Schneider, W.G.; Bernstein, H.J.; Pople, J.A. Proton Magnetic Resonance Chemical Shift of Free (Gaseous) and Associated (Liquid) Hydride Molecules. J. Chem. Phys. 1958, 28, 601–607. [Google Scholar] [CrossRef]
- Kidd, R.G. Nuclear Shielding of the Transition Metals. Ann. Rep. NMR Spectrosc. 1980, 10A, 1–79. [Google Scholar] [CrossRef]
- Kidd, R.G. The Oxidation-State Dependence of Transition-Metal Shieldings. Ann. Rep. NMR Spectrosc. 1991, 23, 85–139. [Google Scholar] [CrossRef]
- Takashima, H.; Hada, M.; Nakatsuji, H. Spin-orbit effect on the magnetic shielding constant using the ab initio UHF method: Gallium and indium tetrahalides. Chem. Phys. Lett. 1995, 235, 13–16. [Google Scholar] [CrossRef]
- Nakatsuji, H.; Nakajima, T.; Hada, M.; Takashima, H.; Tanaka, S. Spin-orbit effect on the magnetic shielding constant using the ab initio UHF method: Silicon tetrahalides. Chem. Phys. Lett. 1995, 247, 418–424. [Google Scholar] [CrossRef]
- Nakatsuji, H.; Hada, M.; Tejima, T.; Nakajima, T.; Sugimoto, M. Spin-orbit effect on the magnetic shielding constant using the ab initio UHF method. Electronic mechanism in the aluminum compounds, AlX4− (X = H, F, Cl, Br and I). Chem. Phys. Lett. 1996, 249, 284–289. [Google Scholar] [CrossRef]
- Nakatsuji, H.; Hada, H.; Kaneko, H.; Ballard, C.C. Relativistic study of nuclear magnetic shielding constants: Mercury dihalides. Chem. Phys. Lett. 1996, 255, 195–202. [Google Scholar] [CrossRef]
- Hada, M.; Kaneko, H.; Nakatsuji, H. Relativistic study of nuclear magnetic shielding constants: Tungsten hexahalides and tetraoxide. Chem. Phys. Lett. 1996, 261, 7–12. [Google Scholar] [CrossRef]
- Viesser, R.V.; Ducati, L.C.; Tormena, C.F.; Autschbach, J. The halogen effect on the 13C NMR chemical shift in substituted benzenes. Phys. Chem. Chem. Phys. 2018, 20, 11247–11259. [Google Scholar] [CrossRef]
- Nakagawa, N.; Sinada, S.; Obinata, S. Heavy atom-derived spin polarization shifts. In Proceedings of the “The 6th NMR Symposium”, Japan, Kyoto, 1967; pp. 8–12. [Google Scholar]
- Lohr, L.L., Jr.; Pyykkö, P. Relativistically parameterized extended Hückel theory. Chem. Phys. Lett. 1979, 62, 333–338. [Google Scholar] [CrossRef]
- Hoffmann, R. An Extended Hückel Theory. I. Hydrocarbons. J. Chem. Phys. 1963, 39, 1397–1412. [Google Scholar] [CrossRef]
- Kaupp, M.; Malkina, O.L.; Malkin, V.G. Interpretation of 13C NMR chemical shifts in halomethyl cations. On the importance of spin-orbit coupling and electron correlation. Chem. Phys. Lett. 1997, 265, 55–59. [Google Scholar] [CrossRef]
- Malkin, V.G.; Malkina, O.L.; Casida, M.E.; Salahub, D.R. Nuclear Magnetic Resonance Shielding Tensors Calculated with a Sum-over-States Density Functional Perturbation Theory. J. Am. Chem. Soc. 1994, 116, 5898–5908. [Google Scholar] [CrossRef]
- Malkin, V.G.; Malkina, O.L.; Eriksson, L.A.; Salahub, D.R. The calculation of NMR and ESR spectroscopy parameters using density functional theory. In Theoretical and Computational Chemistry. Modem Density Functional Theory: A Tool for Chemistry, 1st ed.; Seminario, J.M., Politzer, P., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1995; Volume 2, Chapter 9; pp. 273–348. [Google Scholar]
- Pople, J.A.; Mclver, J.W., Jr.; Ostlund, N.S. Self-Consistent Perturbation Theory. II. Nuclear-Spin Coupling Constants. J. Chem. Phys. 1968, 49, 2965–2970. [Google Scholar] [CrossRef]
- Malkina, O.L.; Schimmelpfennig, B.; Kaupp, M.; Hess, B.A.; Chandra, P.; Wahlgren, U.; Malkin, V.G. Spin–orbit corrections to NMR shielding constants from density functional theory. How important are the two-electron terms? Chem. Phys. Lett. 1998, 296, 93–104. [Google Scholar] [CrossRef]
- Kaupp, M.; Aubauer, C.; Engelhardt, G.; Klapötke, T.M.; Malkina, O.L. The PI4+ cation has an extremely large negative 31P nuclear magnetic resonance chemical shift, due to spin–orbit coupling: A quantum-chemical prediction and its confirmation by solid-state nuclear magnetic resonance spectroscopy. J. Chem. Phys. 1999, 110, 3897–3902. [Google Scholar] [CrossRef]
- Kaupp, M.; Malkina, O.L.; Malkin, V.G. The Role of π-Type Nonbonding Orbitals for Spin-Orbit Induced NMR Chemical Shifts: DFT Study of 13C and 19F Shifts in the Series CF3IFn (n = 0, 2, 4, 6). J. Comput. Chem. 1999, 20, 1304–1313. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B 1992, 45, 13244–13249. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Partridge, H. Near Hartree–Fock quality GTO basis sets for the first- and third-row atoms. J. Chem. Phys. 1989, 90, 1043–1047. [Google Scholar] [CrossRef]
- Partridge, H.; Faegri, K. High-quality Gaussian basis sets for fourth-row atoms. Theor. Chim. Acta 1992, 82, 207–212. [Google Scholar] [CrossRef]
- Partridge, H. Near Hartree–Fock quality GTO basis sets for the second-row atoms. J. Chem. Phys. 1987, 87, 6643–6647. [Google Scholar] [CrossRef]
- Kantola, A.M.; Lantto, P.; Vaara, J.; Jokisaari, J. Carbon and proton shielding tensors in methyl halides. Phys. Chem. Chem. Phys. 2010, 12, 2679–2692. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, R.J.; Silver, D.M. Correlation energy in LiH, BH, and HF with many-body perturbation theory using Slater-type atomic orbitals. Int. J. Quantum Chem. 1974, S8, 271–276. [Google Scholar] [CrossRef]
- Binkley, J.S.; Pople, J.A. Møller–Plesset theory for atomic ground state energies. Int. J. Quantum Chem. 1975, 9, 229–236. [Google Scholar] [CrossRef]
- Bartlett, R.J. Many-body perturbation theory and coupled cluster theory for electron correlation in molecules. Annu. Rev. Phys. Chem. 1981, 32, 359–401. [Google Scholar] [CrossRef]
- Gauss, J.; Stanton, J.F. Analytic CCSD(T) second derivatives. Chem. Phys. Lett. 1997, 276, 70–77. [Google Scholar] [CrossRef]
- Knizia, G.; Adler, T.B.; Werner, H.-J. Simplified CCSD(T)-F12 methods: Theory and benchmarks. J. Chem. Phys. 2009, 130, 054104. [Google Scholar] [CrossRef]
- Stanton, J.F.; Gauss, J.; Siehl, H.-U. CCSD(T) calculation of NMR chemical shifts: Consistency of calculated and measured 13C chemical shifts in the 1-cyclopropylcyclopropylidenemethyl cation. Chem. Phys. Lett. 1996, 262, 183–186. [Google Scholar] [CrossRef]
- Rasul, G.; Surya Prakash, G.K.; Olah, G.A. Comparative study of the hypercoordinate ions C7H9+ and C8H9+ by the ab initio/GIAO-CCSD(T) method. J. Phys. Chem. A 2006, 110, 11320–11323. [Google Scholar] [CrossRef]
- Kupka, T.; Stachów, M.; Kaminsky, J.; Sauer, S.P.A. Estimation of isotropic nuclear magnetic shieldings in the CCSD(T) and MP2 complete basis set limit using affordable correlation calculations. Magn. Reson. Chem. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Casella, G.; Bagno, A.; Komorovsky, S.; Repisky, M.; Saielli, G. Four-Component Relativistic DFT Calculations of 13C Chemical Shifts of Halogenated Natural Substances. Chem. Eur. J. 2015, 21, 18834–18840. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.C.; Ducati, L.C.; Rittner, R.; Tormena, C.F.; Contreras, R.H.; Frenking, G. Heavy Halogen Atom Effect on 13C NMR Chemical Shifts in Monohalo Derivatives of Cyclohexane and Pyran. Experimental and Theoretical Study. J. Chem. Theory Comput. 2009, 5, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Hyvärinen, M.; Vaara, J.; Goldammer, A.; Kutzky, B.; Hegetschweiler, K.; Kaupp, M.; Straka, M. Characteristic Spin-Orbit Induced 1H(CH2) Chemical Shifts upon Deprotonation of Group 9 Polyamine Aqua and Alcohol Complexes. J. Am. Chem. Soc. 2009, 131, 11909–11918. [Google Scholar] [CrossRef]
- Hegetschweiler, K.; Kuppert, D.; Huppert, J.; Straka, M.; Kaupp, M. Spin-Orbit-Induced Anomalous pH-Dependence in 1H NMR Spectra of CoIII Amine Complexes: A Diagnostic Tool for Structure Elucidation. J. Am. Chem. Soc. 2004, 126, 6728–6738. [Google Scholar] [CrossRef]
- Viesser, R.V.; Ducati, L.C.; Autschbach, J.; Tormena, C.F. Effects of stereoelectronic interactions on the relativistic spin–orbit and paramagnetic components of the 13C NMR shielding tensors of dihaloethenes. Phys. Chem. Chem. Phys. 2015, 17, 19315–19324. [Google Scholar] [CrossRef]
- Demissie, T.B.; Repisky, M.; Komorovsky, S.; Isaksson, J.; Svendsen, J.S.; Dodziuk, H.; Ruud, K. Four-component relativistic chemical shift calculations of halogenated organic compounds. J. Phys. Org. Chem. 2013, 26, 679–687. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868, Erratum in Phys. Rev. Lett. 1997, 78, 1396–1396. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.C.; Phillips, J.C. Quantum Theory of Molecules and Solids: The Self-Consistent Field for Molecules and Solids, 1st ed.; McGraw-Hill: New York, NY, USA, 1974; Volume 4, pp. 1–583. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calcula-tions: A critical analysis. Can. J. Phys. 1980, 59, 1200–1211. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Burke, K.; Perdew, J.P.; Wang, Y. Derivation of a Generalized Gradient Approximation: The PW91 Density Functional. In Electronic Density Functional Theory, 1st ed.; Dobson, J.F., Vignale, G., Das, M.P., Eds.; Springer: Boston, MA, USA, 1998; pp. 81–111. [Google Scholar] [CrossRef]
- Irwin, J.J.; Shoichet, B.K. ZINC—A free database of commercially available compounds for virtual screening. J. Chem. Inf. Mod. 2004, 45, 177–182. [Google Scholar] [CrossRef]
- Bolton, E.E.; Wang, Y.; Thiessen, P.A.; Bryant, S.H. Chapter 12—PubChem: Integrated Platform of Small Molecules and Biological Activities. Annu. Rep. Comput. Chem. 2008, 4, 217–241. [Google Scholar] [CrossRef]
- Samultsev, D.O.; Rusakov, Y.Y.; Krivdin, L.B. Normal halogen dependence of 13C NMR chemical shifts of halogenomethanes revisited at the four-component relativistic level. Magn. Reson. Chem. 2016, 54, 787–792. [Google Scholar] [CrossRef]
- Handy, N.C.; Cohen, A.J. Left-right correlation energy. Mol. Phys. 2001, 99, 403–412. [Google Scholar] [CrossRef]
- Perdew, J.P. Unified Theory of Exchange and Correlation Beyond the Local Density Approximation. In Electronic Structure of Solids, 91 ed.; Ziesche, P., Eschig, H., Eds.; Akademie Verlag: Berlin, Germany, 1991; pp. 11–20. [Google Scholar]
- Glaser, R.; Chen, N.; Wu, N.; Knotts, N.; Kaupp, M. 13C NMR Study of Halogen Bonding of Haloarenes: Measurements of Solvent Effects and Theoretical Analysis. J. Am. Chem. Soc. 2004, 126, 4412–4419. [Google Scholar] [CrossRef]
- Radula-Janik, K.; Kupka, T.; Ejsmont, K.; Daszkiewicz, Z.; Sauer, S.P.A. Halogen effect on structure and 13C NMR chemical shift of 3,6-disubstituted-N-alkyl carbazoles. Magn. Reson. Chem. 2013, 51, 630–635. [Google Scholar] [CrossRef]
- Zhao, J.; Truhlar, D.G. Improved Description of Nuclear Magnetic Resonance Chemical Shielding Constants Using the M06-L Meta-Generalized-Gradient-Approximation Density Functional. J. Phys. Chem. A 2008, 122, 6794–6799. [Google Scholar] [CrossRef] [PubMed]
- Standara, S.; Maliňáková, K.; Marek, R.; Marek, J.; Hocek, M.; Vaara, J.; Straka, M. Understanding the NMR chemical shifts for 6-halopurines: Role of structure, solvent and relativistic effects. Phys. Chem. Chem. Phys. 2010, 12, 5126–5139. [Google Scholar] [CrossRef] [PubMed]
- Ariai, J.; Saielli, G. “Through-Space” Relativistic Effects on NMR Chemical Shifts of Pyridinium Halide Ionic Liquids. ChemPhysChem 2018, 20, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Vícha, J.; Švec, P.; Růžičková, Z.; Samsonov, M.A.; Bártová, K.; Růžička, A.; Straka, M.; Dračínský, M. Experimental and Theoretical Evidence of Spin-Orbit Heavy Atom on the Light Atom 1H NMR Chemical Shifts Induced through H⋅⋅⋅I− Hydrogen Bond. Chem. Eur. J. 2020, 26, 8698–8702. [Google Scholar] [CrossRef] [PubMed]
- Pickard, C.J.; Mauri, F. All-electron magnetic response with pseudopotentials: NMR chemical shifts. Phys. Rev. B 2001, 63, 245101. [Google Scholar] [CrossRef]
- Bonhomme, C.; Gervais, C.; Babonneau, F.; Coelho, C.; Pourpoint, F.; Azais, T.; Ashbrook, S.E.; Griffin, J.M.; Yates, J.R.; Mauri, F.; et al. First-Principles Calculation of NMR Parameters Using the Gauge Including Projector Augmented Wave Method: A Chemist’s Point of View. Chem. Rev. 2012, 112, 5733–5779. [Google Scholar] [CrossRef]
- Rodriguez, J.I. An efficient method for computing the QTAIM topology of a scalar field: The electron density case. J. Comput. Chem. 2013, 34, 681–686. [Google Scholar] [CrossRef]
- Nakanishi, W.; Hayashi, S.; Narahara, K. Atoms-in-Molecules Dual Parameter Analysis of Weak to Strong Interactions: Behaviors of Electronic Energy Densities versus Laplacian of Electron Densities at Bond Critical Points. J. Phys. Chem. A 2008, 112, 13593–13599. [Google Scholar] [CrossRef]
- Vícha, J.; Foroutan-Nejad, C.; Pawlak, T.; Munzarová, M.L.; Straka, M.; Marek, R. Understanding the electronic factors responsible for ligand spin-orbit NMR shielding in transition-metal complexes. J. Chem. Theory Comput. 2015, 11, 1509–1517. [Google Scholar] [CrossRef]
- Jaszuński, M.; Olejniczak, M. NMR shielding constants in SeH2 and TeH2. Mol. Phys. 2013, 111, 1355–1363. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L.; Krivdin, L.B. On the significant relativistic heavy atom effect on 13C NMR chemical shifts of β- and γ-carbons in seleno- and telluroketones. Mol. Phys. 2017, 115, 3117–3127. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L.; Krivdin, L.B. Relativistic heavy atom effect on the 31P NMR parameters of phosphine chalcogenides. Part 1. Chemical shifts. Magn. Reson. Chem. 2018, 56, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Lantto, P.; Kangasvieri, S.; Vaara, J. Electron correlation and relativistic effects in the secondary NMR isotope shifts of CSe2. Phys. Chem. Chem. Phys. 2013, 15, 17468–17478. [Google Scholar] [CrossRef] [PubMed]
- Kellö, V.; Sadlej, A.J. Medium-size polarized basis sets for high-level-correlated calculations of molecular electric properties. Theor. Chim. Acta 1992, 83, 351–366. [Google Scholar] [CrossRef]
- Berger, R.J.F.; Rettenwander, D.; Spirk, S.; Wolf, C.; Patzschke, M.; Ertl, M.; Monkowius, U.; Mitzel, N.W. Relativistic effects in triphenylbismuth and their influence on molecular structure and spectroscopic properties. Phys. Chem. Chem. Phys. 2012, 14, 15520–15524. [Google Scholar] [CrossRef]
- Bast, R.; Jensen, H.J.A.; Saue, T. Relativistic adiabatic time-dependent density functional theory using hybrid functionals and noncollinear spin magnetization. Int. J. Quantum Chem. 2009, 109, 2091–2112. [Google Scholar] [CrossRef]
- Lévy-Leblond, J.-M. Nonrelativistic particles and wave equations. Commun. Math. Phys. 1967, 6, 286–311. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L. Relativistic heavy atom effect on 13C NMR chemical shifts initiated by adjacent multiple chalcogens. Magn. Reson. Chem. 2018, 56, 716–726. [Google Scholar] [CrossRef]
- Rusakov, Y.Y.; Rusakova, I.L.; Krivdin, L.B. On the HALA effect in the NMR carbon shielding constants of the compounds containing heavy p-elements. Int. J. Quantum Chem. 2016, 116, 1404–1412. [Google Scholar] [CrossRef]
- Demissie, T.B. Relativistic effects on the NMR parameters of Si, Ge, Sn, and Pb alkynyl compounds: Scalar versus spin-orbit effects. J. Chem. Phys. 2017, 147, 174301. [Google Scholar] [CrossRef]
- Vícha, J.; Marek, R.; Straka, M. High-Frequency 1H NMR Chemical Shifts of SnII and PbII Hydrides Induced by Relativistic Effects: Quest for PbII Hydrides. Inorg. Chem. 2016, 55, 10302–10309. [Google Scholar] [CrossRef]
- Vícha, J.; Marek, R.; Straka, M. High-Frequency 13C and 29Si NMR Chemical Shifts in Diamagnetic Low-Valence Compounds of TlI and PbII: Decisive Role of Relativistic Effects. Inorg. Chem. 2016, 55, 1770–1781. [Google Scholar] [CrossRef]
- Gómez, S.S.; Aucar, G.A. Relativistic effects on the nuclear magnetic resonance shielding of FX (X = F, Cl, Br, I, and At) molecular systems. J. Chem. Phys. 2011, 134, 204314. [Google Scholar] [CrossRef]
- Field-Theodore, T.E.; Olejniczak, M.; Jaszuński, M.; Wilson, D.J.D. NMR shielding constants in group 15 trifluorides. Phys. Chem. Chem. Phys. 2018, 20, 23025–23033. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ernzerhof, M.; Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 1996, 105, 9982–9985. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Keal, T.W.; Tozer, D.J. The exchange-correlation potential in Kohn-Sham nuclear magnetic resonance shielding calculations. J. Chem. Phys. 2003, 119, 3015–3024. [Google Scholar] [CrossRef]
- Keal, T.W.; Tozer, D.J. A semiempirical generalized gradient approximation exchange-correlation functional. J. Chem. Phys. 2004, 121, 5654–5660. [Google Scholar] [CrossRef]
- Keal, T.W.; Tozer, D.J.; Helgaker, T. GIAO shielding constants and indirect spin–spin coupling constants: Performance of density functional methods. Chem. Phys. Lett. 2004, 391, 374–379. [Google Scholar] [CrossRef]
- Teale, A.M.; Tozer, D.J. Exchange representations in Kohn–Sham NMR shielding calculations. Chem. Phys. Lett. 2004, 383, 109–114. [Google Scholar] [CrossRef]
- Sugimoto, M.; Kanayama, M.; Nakatsuji, H. Theoretical study on metal NMR chemical shifts. Gallium compounds, GaCl4−nBrn− (n = 0 − 4). J. Phys. Chem. 1993, 97, 5868–5874. [Google Scholar] [CrossRef]
- Fedorov, S.V.; Rusakov, Y.Y.; Krivdin, L.B. Relativistic Environmental Effects in 29Si NMR Chemical Shifts of Halosilanes: Light Nucleus, Heavy Environment. J. Phys. Chem. A 2015, 119, 5778–5789. [Google Scholar] [CrossRef]
- Dyall, K.G. Relativistic and nonrelativistic finite nucleus optimized triple-zeta basis sets for the 4p, 5p and 6p elements. Theor. Chem. Acc. 2002, 108, 335–340. [Google Scholar] [CrossRef]
- Dyall, K.G. Relativistic quadruple-zeta and revised triple-zeta and double-zeta basis Sets for the 4p, 5p, and 6p elements. Theor. Chem. Acc. 2006, 115, 441–447. [Google Scholar] [CrossRef]
- Bühl, M.; Kaupp, M.; Malkina, O.L.; Malkin, V.G. The DFT Route to NMR Chemical Shifts. J. Comput. Chem. 1999, 20, 91–105. [Google Scholar] [CrossRef]
- Chernyshev, K.A.; Krivdin, L.B. Quantum-chemical calculations of NMR chemical shifts of organic molecules: II. Influence of medium, relativistic effects, and vibrational corrections on phosphorus magnetic shielding constants in the simplest phosphines and phosphine chalcogenides. Rus. J. Org. Chem. 2011, 47, 355–362. [Google Scholar] [CrossRef]
- Chernyshev, K.A.; Krivdin, L.B.; Fedorov, S.V.; Arbuzova, S.N.; Ivanova, N.I. Quantum Chemical Calculations of NMR Chemical Shifts of Organic Molecules: XI.* Conformational and Relativistic Effects on the 31P and 77Se Chemical Shifts of Phosphine Selenides. Russ. J. Org. Chem. 2013, 49, 1420–1427. [Google Scholar] [CrossRef]
- Fedorov, S.V.; Rusakov, Y.Y.; Krivdin, L.B. Towards the versatile DFT and MP2 computational schemes for 31P NMR chemical shifts taking into account relativistic corrections. Magn. Reson. Chem. 2014, 52, 699–710. [Google Scholar] [CrossRef]
- Hocek, M.; Masojídková, M.; Holý, A. Synthesis of Acyclic Nucleotide Analogues Derived from 6-Hetarylpurines via Cross-Coupling Reactions of 9-[2-(Diethoxyphosphonylmethoxy)ethyl]-6-iodopurine with Hetaryl Organometallic Reagents. Collect. Czech. Chem. Commun. 1997, 62, 136–146. [Google Scholar] [CrossRef]
- Samultsev, D.O.; Rusakov, Y.Y.; Krivdin, L.B. Relativistic effects of chlorine in 15N NMR chemical shifts of chlorine-containing amines. Russ. J. Org. Chem. 2017, 53, 1738–1739. [Google Scholar] [CrossRef]
- Samultsev, D.O.; Rusakov, Y.Y.; Krivdin, L.B. On the long-range relativistic effects in the 15N NMR chemical shifts of halogenated azines. Magn. Reson. Chem. 2017, 55, 990–995. [Google Scholar] [CrossRef]
- Jensen, F. Basis Set Convergence of Nuclear Magnetic Shielding Constants Calculated by Density Functional Methods. J. Chem. Theory Comput. 2008, 4, 719–727. [Google Scholar] [CrossRef]
- Karplus, M. Contact Electron-Spin Coupling of Nuclear Magnetic Moments. J. Chem. Phys. 1959, 30, 11–15. [Google Scholar] [CrossRef]
- Rusakova, I.L.; Krivdin, L.B. Karplus dependence of spin–spin coupling constants revisited theoretically. Part 1: Second-order double perturbation theory. Phys. Chem. Chem. Phys. 2013, 15, 18195–18203. [Google Scholar] [CrossRef]
- Schraml, J. 13C-NMR spectra of monosubstituted and symmetrically 1,2-disubstituted ethenes. Collect. Czech. Chem. Commun. 1976, 41, 3063–3076. [Google Scholar] [CrossRef]
- Savitsky, G.; Namikawa, K. Carbon-13 Chemical Shifts and Geometric Isomerism About the Ethylenic Double Bond. J. Phys. Chem. 1963, 67, 2754–2756. [Google Scholar] [CrossRef]
- Maciel, G.E.; Ellis, P.D.; Natterstad, J.J.; Savitsky, G.B. The effect of geometric isomerism on the carbon-13 chemical shift of diiodoethylene and related compounds. J. Magn. Reson. 1969, 1, 589–605. [Google Scholar] [CrossRef]
- Minaev, B.; Vaara, J.; Ruud, K.; Vahtras, O.; Ågren, H. Internuclear distance dependence of the spin–orbit coupling contributions to proton NMR chemical shifts. Chem. Phys. Lett. 1998, 295, 455–461. [Google Scholar] [CrossRef]
- Minaev, B.F. Theoretical Analysis and Prognostication of Spin–Orbit Coupling Effects in Molecular Spectroscopy and Chemical Kinetics. Ph.D. Thesis, N.N. Semenov Institute of Chemical Physics, Moscow, Russia, 1981. [Google Scholar]
- Bacskay, G.B. On the calculation of nuclear spin-spin coupling constants. The bond length dependence of the Fermi contact term in H2 and HD. Chem. Phys. Lett. 1995, 242, 507–513. [Google Scholar] [CrossRef]
- Cromp, B.; Carrington, T., Jr.; Salahub, D.R.; Malkina, O.L.; Malkin, V.G. Effect of rotation and vibration on nuclear magnetic resonance chemical shifts: Density functional theory calculations. J. Chem. Phys. 1999, 110, 7153–7159. [Google Scholar] [CrossRef]
- Jameson, C.J.; Jameson, A.K.; Burrell, P.M. 19F nuclear magnetic shielding scale from gas phase studies. J. Chem. Phys. 1980, 73, 6013–6020. [Google Scholar] [CrossRef]
- Lantto, P.; Vaara, J.; Kantola, A.M.; Telkki, V.-V.; Schimmelpfennig, B.; Ruud, K.; Jokisaari, J. Relativistic Spin-Orbit Coupling Effects on Secondary Isotope Shifts of 13C Nuclear Shielding in CX2 (X = O, S, Se, Te). J. Am. Chem. Soc. 2002, 124, 2762–2771. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.A.; Marian, C.M.; Wahlgren, U.; Gropen, O. A mean-field spin-orbit method applicable to correlated wavefunctions. Chem. Phys. Lett. 1996, 251, 365–371. [Google Scholar] [CrossRef]
- Jakubowska, K.; Pecul, M.; Ruud, K. Vibrational Corrections to NMR Spin–Spin Coupling Constants from Relativistic Four-Component DFT Calculations. J. Phys. Chem. A 2022, 126, 7013–7020. [Google Scholar] [CrossRef] [PubMed]






















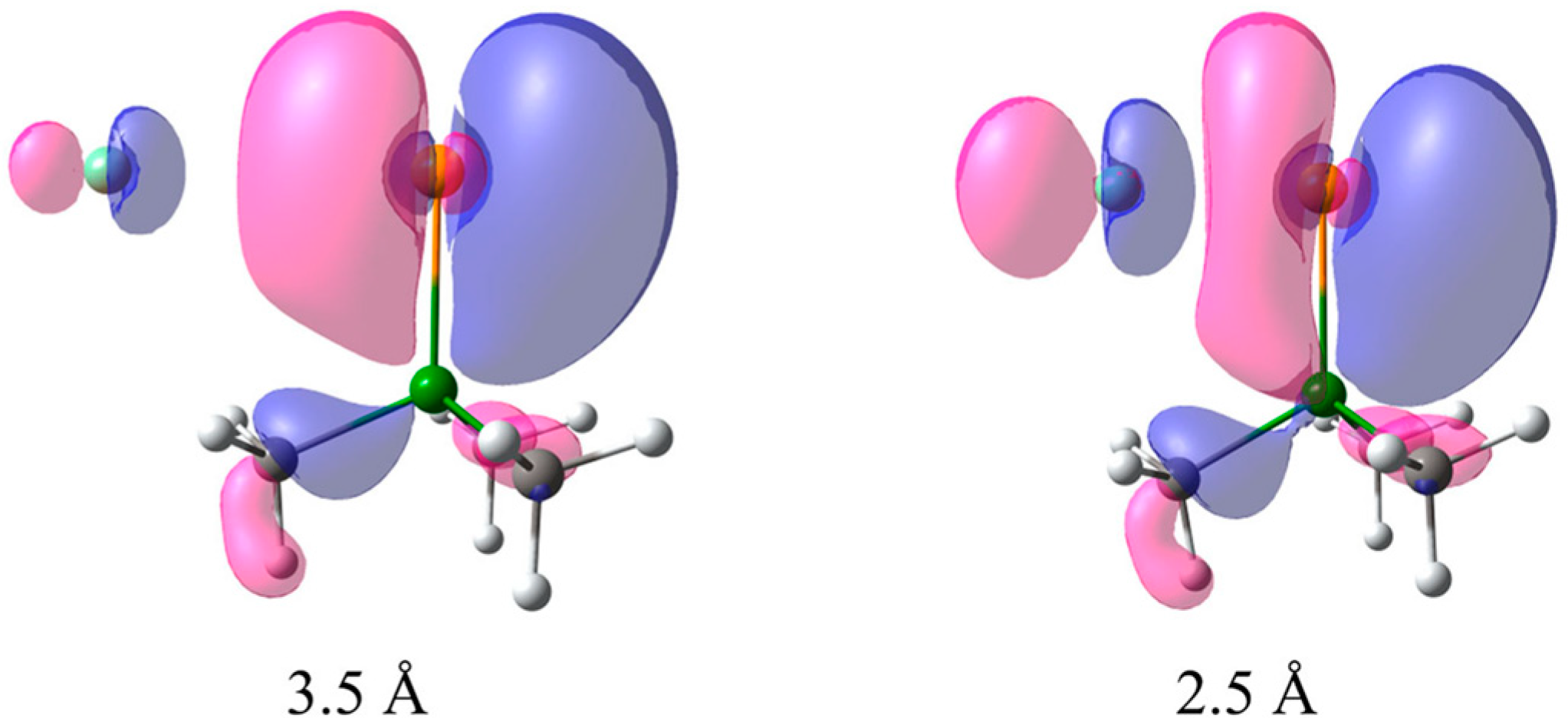






| X | σNR | σSR | σSO/FC–I | σtot | ||
|---|---|---|---|---|---|---|
| AE | ECP | AMFI-AE | SO-ECP | AE | ECP | |
| HX series | ||||||
| F | 30.04 | 29.09 | 0.11 | 0.13 | 30.15 | 29.22 |
| Cl | 31.72 | 30.82 | 0.65 | 0.70 | 32.37 | 31.52 |
| Br | 31.25 | 31.14 | 3.76 | 3.78 | 35.01 | 34.92 |
| I | 31.65 | 31.69 | 10.78 | 11.38 | 42.43 | 43.07 |
| CH3X series | ||||||
| F | 119.98 | 116.59 | 0.48 | 0.47 | 120.46 | 117.06 |
| Cl | 162.78 | 162.77 | 1.82 | 2.07 | 164.60 | 164.84 |
| Br | 171.09 | 168.49 | 10.14 | 10.46 | 181.23 | 178.95 |
| I | 188.76 | 183.00 | 26.99 | 27.81 | 215.75 | 210.81 |
| Molecule | σRPA | σ4RPA | HALA Effect |
|---|---|---|---|
| NH3 | 31.8986 | 30.3269 | −1.5717 |
| PH3 | 29.7512 | 28.6462 | −1.1050 |
| AsH3 | 29.5515 | 28.6451 | −0.9064 |
| SbH3 | 28.9133 | 28.1719 | −0.7414 |
| BiH3 | 27.5737 | 15.4135 | −12.1602 |
| H2O | 31.0213 | 29.1499 | −1.8714 |
| H2S | 31.1767 | 30.7105 | −0.4662 |
| H2Se | 30.4243 | 33.2876 | 2.8633 |
| H2Te | 29.8316 | 39.8276 | 9.9960 |
| H2Po | 28.1430 | 42.4056 | 14.2626 |
| Cmpd. | σnorel | σrel | Δσ | ΔσSO | δnonrel | δrel | δexp |
|---|---|---|---|---|---|---|---|
| (CH3)2Te | 184.6 | 208.7 | 24.1 | 27.2 | 1.8 | −21.4 | −21.5 |
| (CH3)4Te | 157.5 | 163.3 | 5.8 | 7.5 | 28.9 | 24.0 | 20.6 |
| (CH3)6Te | 148.8 | 146.2 | −2.6 | 4.0 | 37.6 | 41.1 | 37.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusakova, I.L.; Rusakov, Y.Y. Relativistic Effects from Heavy Main Group p-Elements on the NMR Chemical Shifts of Light Atoms: From Pioneering Studies to Recent Advances. Magnetochemistry 2023, 9, 24. https://doi.org/10.3390/magnetochemistry9010024
Rusakova IL, Rusakov YY. Relativistic Effects from Heavy Main Group p-Elements on the NMR Chemical Shifts of Light Atoms: From Pioneering Studies to Recent Advances. Magnetochemistry. 2023; 9(1):24. https://doi.org/10.3390/magnetochemistry9010024
Chicago/Turabian StyleRusakova, Irina L., and Yuriy Yu. Rusakov. 2023. "Relativistic Effects from Heavy Main Group p-Elements on the NMR Chemical Shifts of Light Atoms: From Pioneering Studies to Recent Advances" Magnetochemistry 9, no. 1: 24. https://doi.org/10.3390/magnetochemistry9010024
APA StyleRusakova, I. L., & Rusakov, Y. Y. (2023). Relativistic Effects from Heavy Main Group p-Elements on the NMR Chemical Shifts of Light Atoms: From Pioneering Studies to Recent Advances. Magnetochemistry, 9(1), 24. https://doi.org/10.3390/magnetochemistry9010024