Superparamagnetic-like Micrometric Single Crystalline Magnetite for Biomedical Application Synthesis and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and SCMPIO Synthesis
- −
- First, the Fe-EDTA complex was obtained in a 1.05 × 10−1 M solution of FAS, 1.05 × 10−1 M solution of Na4EDTA and a 9.71 × 10−1 M urea solution (all aqueous); the Fe(III)-EDTA complex formation is indicated by the dark red color.
- −
- Second, the hydrothermal method was used for [Fe(III)EDTA]− + Na+ decomposition, for which the Fe-EDTA solution was transferred into a 70 mL Teflonstainless steel line autoclave and was heated up to 230 °C, at a rate of 1.7 °C/min. The degree of filling was 50%. After 30 h of high-temperature treatment, the autoclave was abruptly water-cooled to ensure the freezing of any ongoing processes; inside the autoclave, the pH of the solution was 9.4. After washing in bidistilled water, a fine powder of shining black particles was obtained.
2.2. SCMPIO Characterization
3. Results and Discussion
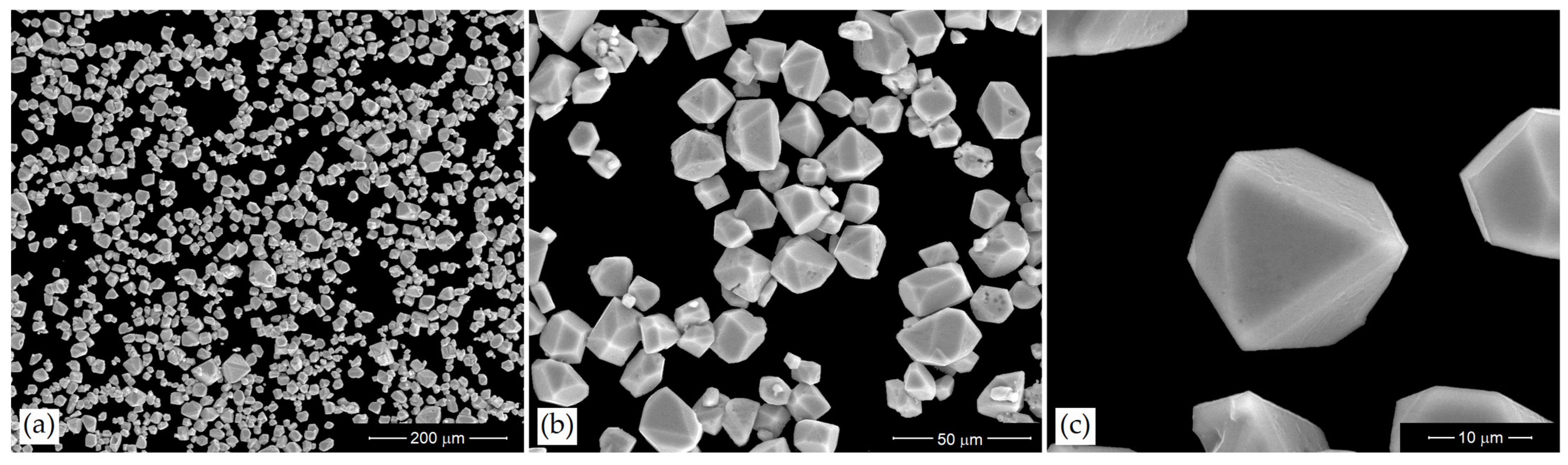
3.1. Morphology
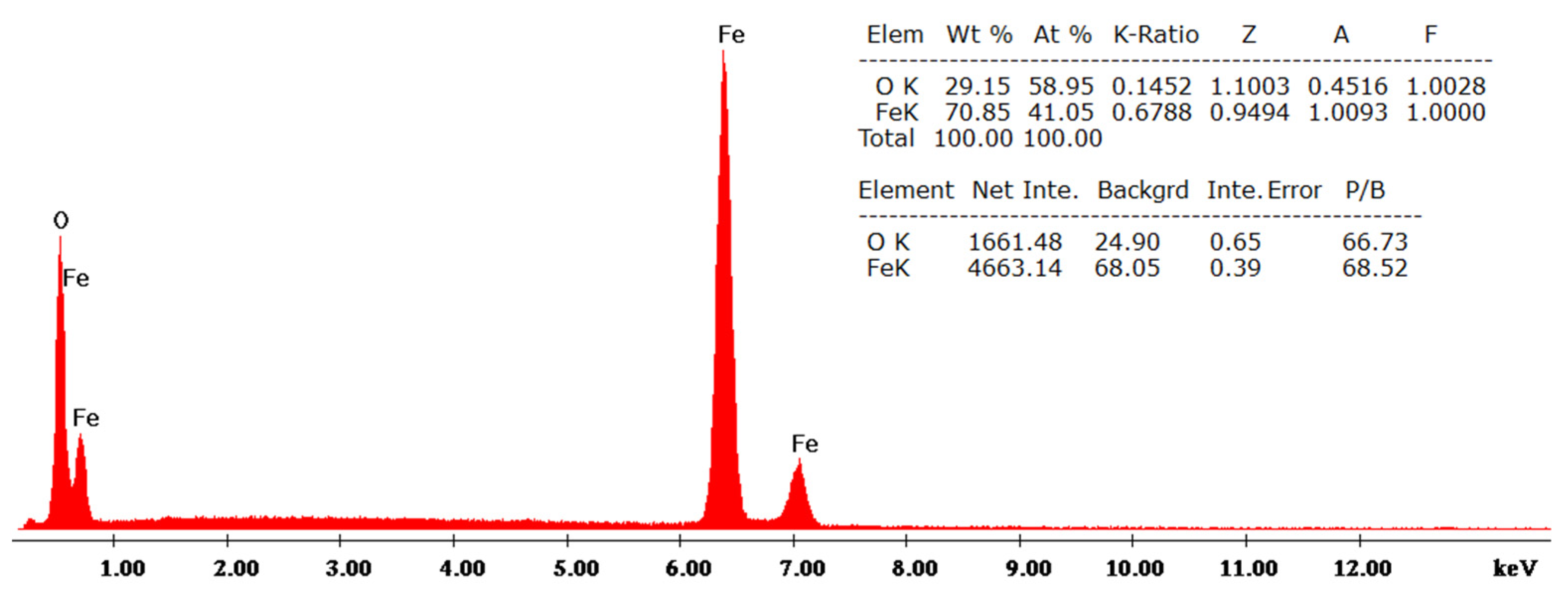
3.2. Elemental Analysis
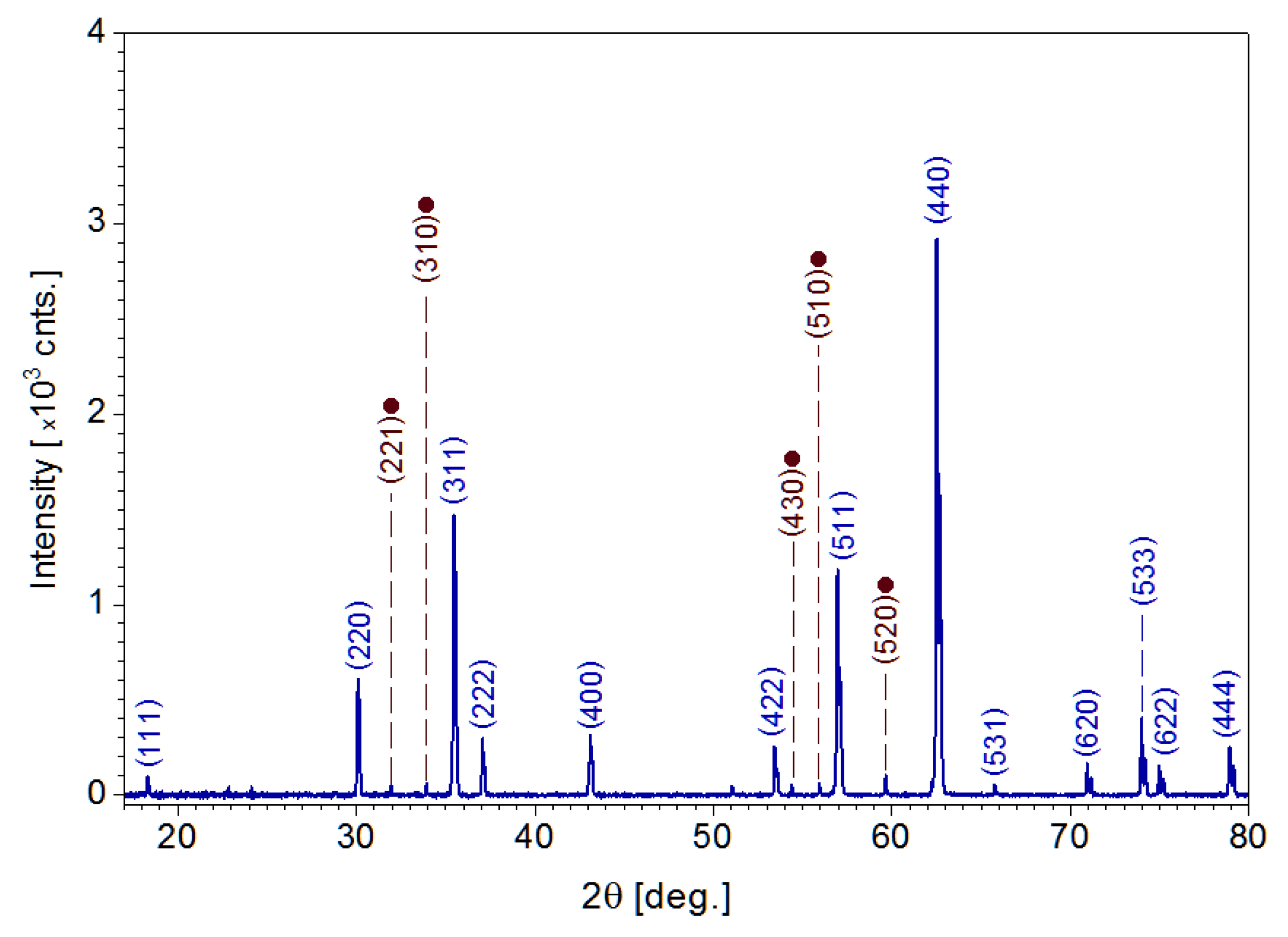
3.3. Crystal Structure
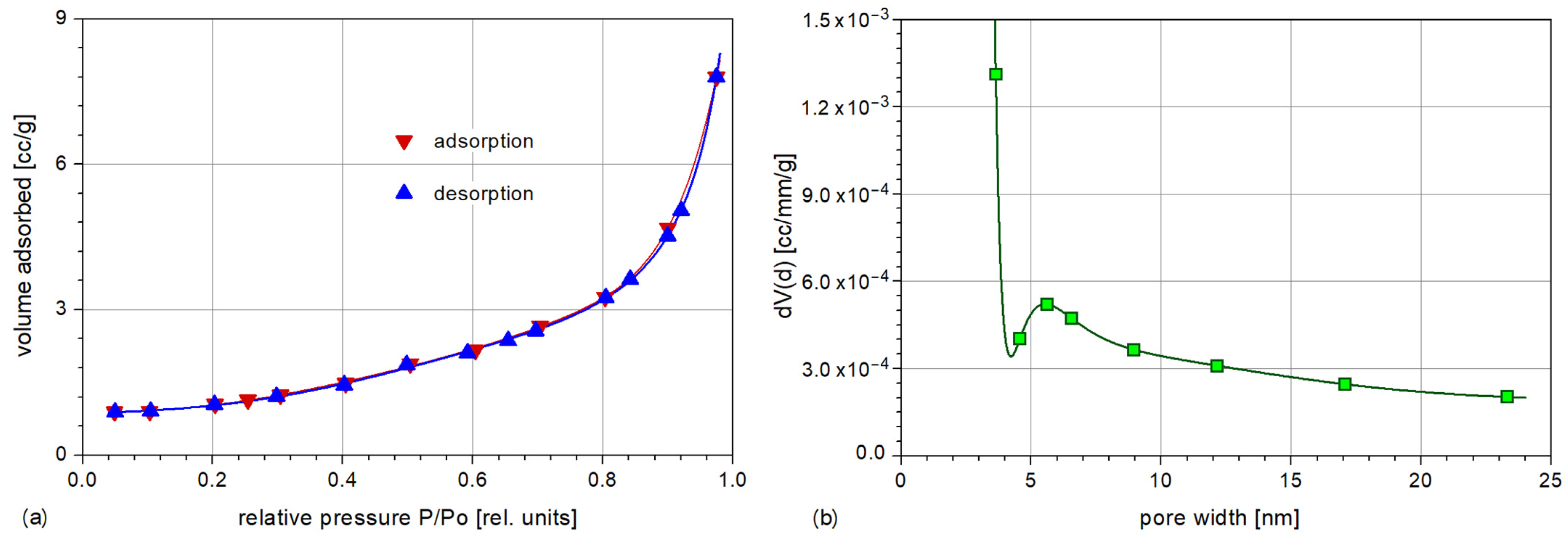
3.4. Specific Surface and Porosity
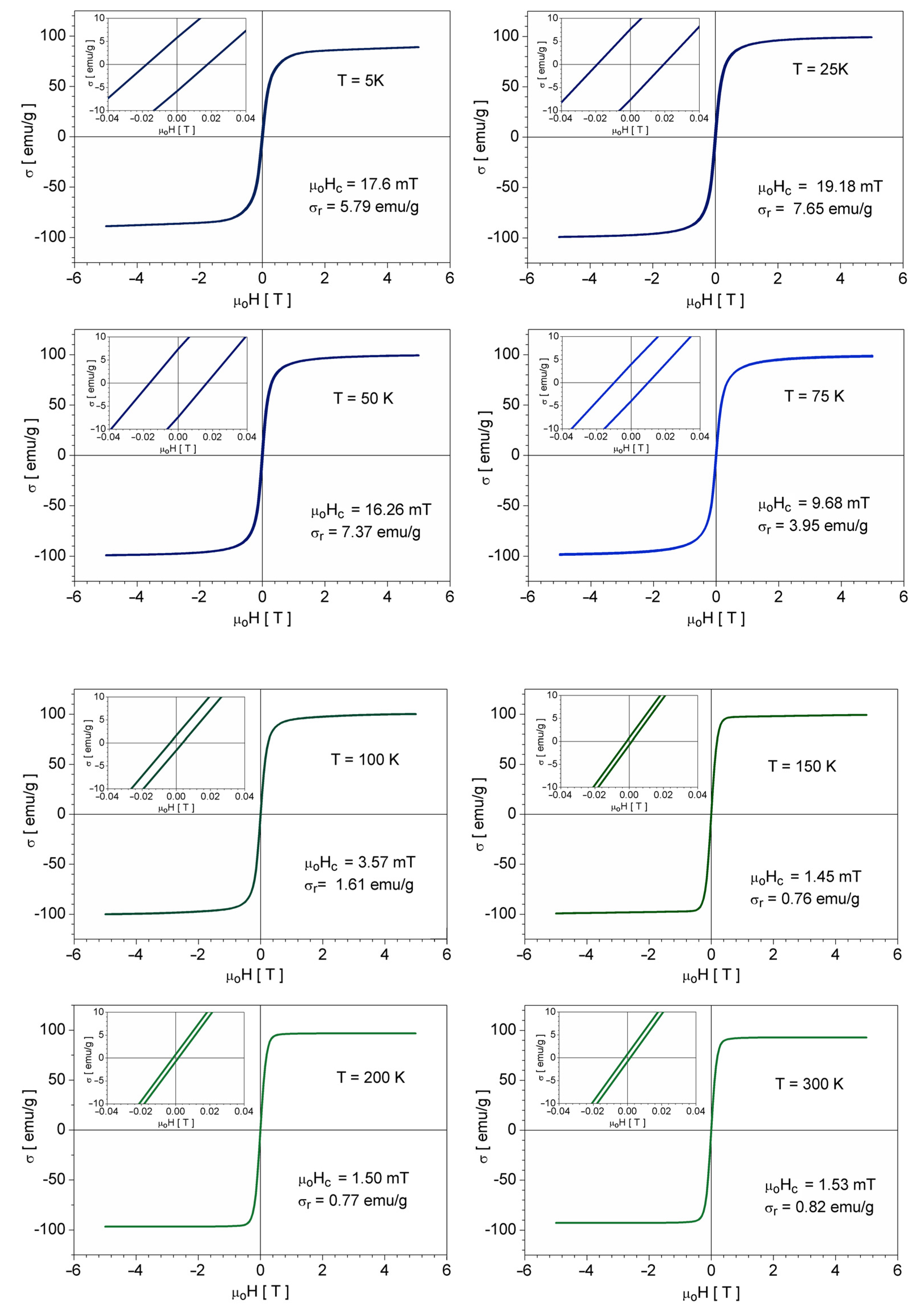
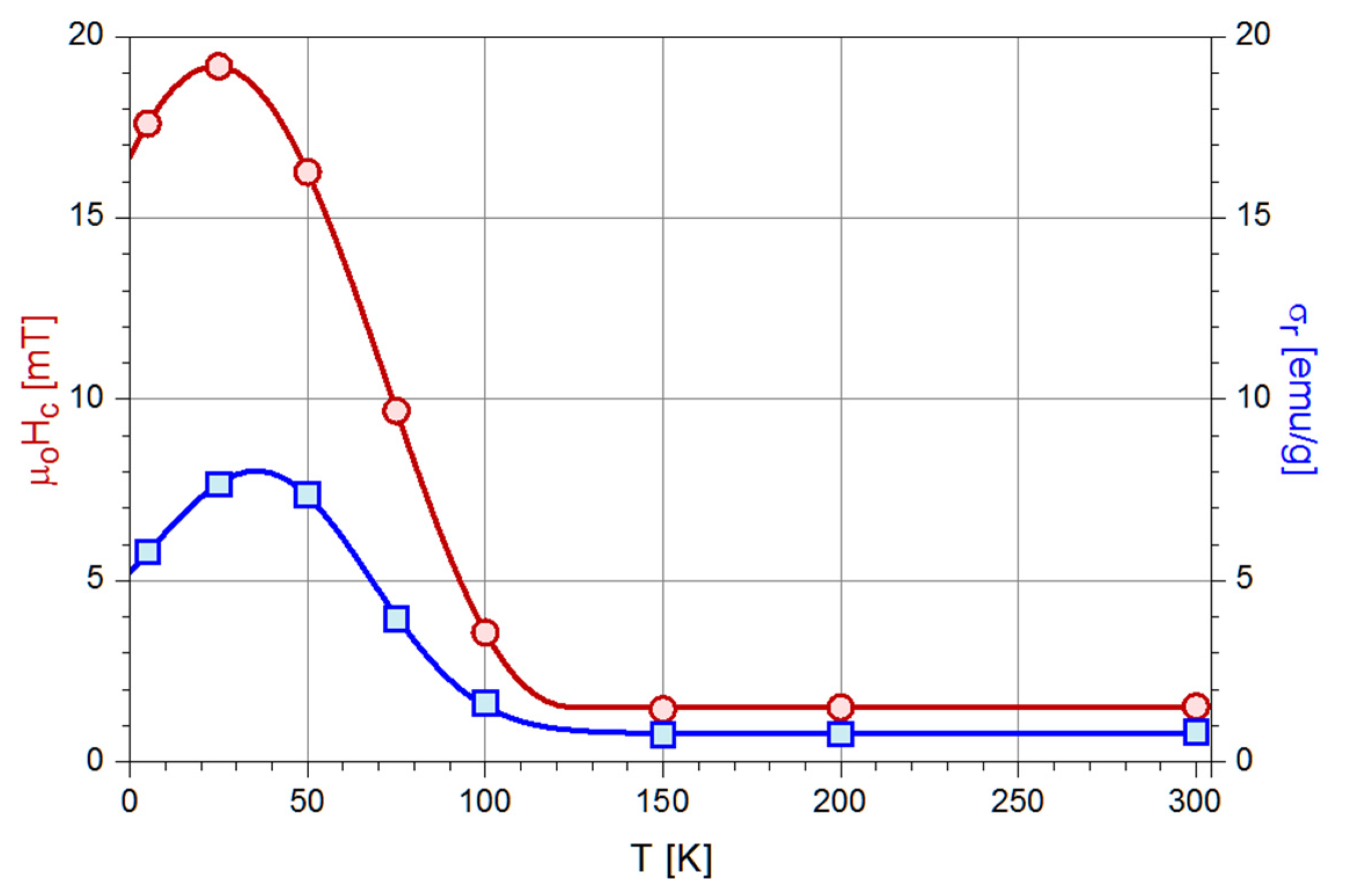
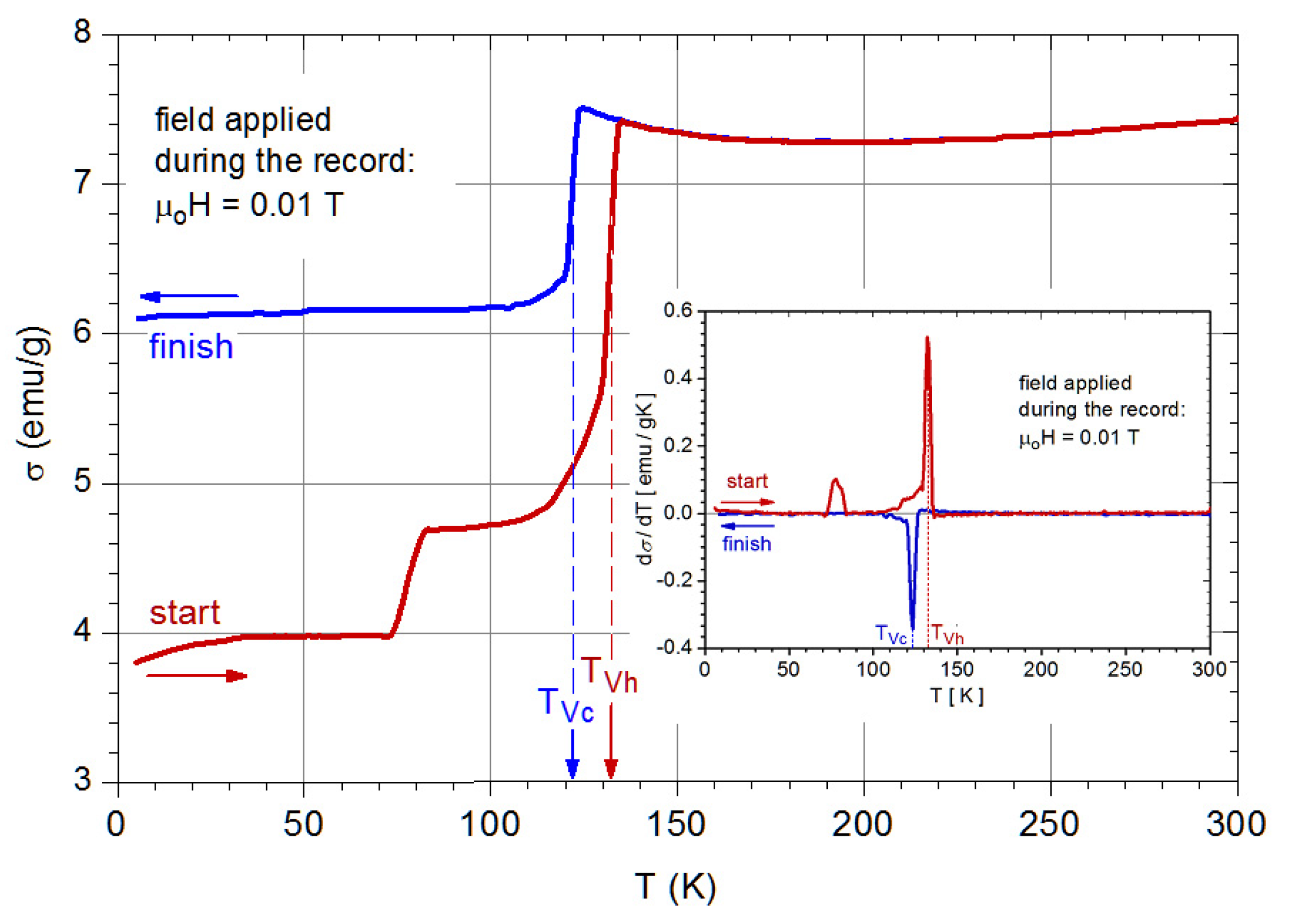
3.5. Magnetic Properties
- −
- The coincidence of the isothermal magnetization curves (or cycles) when plotted against H/T (instead of H), a direct consequence of the fact that the profile of the σ(H) dependence of an assembly of monodisperse superparamagnetic particles, is described by a Langevin function:where μo is the vacuum permeability, kB is Boltzman’s constant, and mp is the magnetic moment of a particle, or by a superposition of such functions in the case of a polydisperse assembly (mp depends on the particle volume).
- −
- The specific profile of the ZFC thermomagnetic record, i.e., the presence of a maximum at the blocking temperature (TB), which for a given compositionis directly related to the nanoparticle size.
3.6. Hydrothermal Synthesis and Chemical Reactions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Néel, L. Théorie du trainage magnétique desferromagnétiques en grains fins avec applications aux terres cuites. Ann. De Géogr. 1949, 5, 99–136. [Google Scholar]
- Hughes, E. Big problems with little particles? Chem. World 2015, 12, 52–55. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Yuan, Y.; Rende, D.; Altan, C.L.; Bucak, S.; Ozisik, R.; Borca-Tasciuc, D.A. Effect of Surface Modification on Magnetization of Iron Oxide Nanoparticle Colloids. Langmuir 2012, 28, 13051–13059. [Google Scholar] [CrossRef] [PubMed]
- Bean, C.; Livingston, J. Superparamagnetism. J. Appl. Phys. 1959, 30, 120–129. [Google Scholar] [CrossRef]
- Silva, L.; Carvalho, J.F.; Pontes, T.R.; Oliveira, E.E.; Francelino, B.L.; Medeiros, A.C.; Egito, E.S.T.D.; Araujo, J.H.; Carriço, A.S. Development of a magnetic system for the treatment of Helicobacter pylori infections. J. Magn. Magn. Mater. 2009, 321, 1566–1570. [Google Scholar] [CrossRef]
- Hinds, K.; Hill, J.; Shapiro, E.; Laukkanen, M.; Silva, A.; Combs, C.; Varney, T.; Balaban, R.; Koretsky, A.; Dunbar, C. Highly efficient endosomal labeling of progenitor and stem cells with large magnetic particles allows magnetic resonance imaging of single cells. Blood 2003, 102, 867–872. [Google Scholar] [CrossRef]
- Mankia, K.S.; McAteer, M.A.; Choudhury, R.P. Microparticle-Based Molecular MRI of Atherosclerosis, Thrombosis, and Tissue Ischemia. Curr. Cardiovasc. Imaging Rep. 2011, 4, 17–23. [Google Scholar] [CrossRef]
- Wu, Y.; Foley, Q.Y.L. In situ labelling of immune cells with iron oxide particles: An approach to detect organ rejection by cellular MRI. Proc. Natl. Acad. Sci. USA 2006, 103, 1852–1857. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Wu, Y.; Foley, L.E.A. Longitudinal tracking of recipient macrophages in a rat chronic cardiac allograft rejection model with non-invasive magnetic resonance imaging using micrometer-sized paramagnetic iron oxide particles. Circulation 2008, 118, 14. [Google Scholar] [CrossRef]
- McAteer, M.; Schneider, J.; Ali, Z.E. A Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide, Atheroscler. Thromb. Vasc. Biol. 2008, 28, 77–83. [Google Scholar] [CrossRef] [Green Version]
- McAteer, M.A.; Sibson, N.R.; von zur Muhlen, C.; Schneider, J.E.; Lowe, A.S.; Warrick, N.; Channon, K.M.; Anthony, D.C.; Choudhury, R.P. In vivo magnetic resonance imaging of acute brain inflammation using microparticles of iron oxide. Nat. Med. 2007, 13, 1253–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieu, J.B.; Martel, S. Magnetic steering of iron oxide microparticles using propulsion gradient coils in MRI. In Proceedings of the 28th IEEE EMBS Annual International Conference, New York, NY, USA, 30 August–3 September 2006. [Google Scholar]
- Chirita, M.; Banica, R.; Ieta, A.; Grozescu, I. Superparamagnetic Unusual Behavior of Micrometric Magnetite Monodisperse Monocrystals Synthesized by Fe-EDTA Thermal Decomposition. Part. Sci. Technol. 2012, 30, 354–363. [Google Scholar] [CrossRef]
- Kiss, M.L.; Chirita, M.; Beljung, C.A.; Polanek, R.; Savii, C.; Ieta, A.; Chirita, A.I.M. Single Crystalline Iron Oxide Micrometric Particles with Superparamagnetic Behaviour for MRI Applications. NSTI-Nanotech 2014, 1, 448–451. [Google Scholar]
- Farcaș, C.; Macasoi, I.; Pinzaru, I.; Chirita, M.; Chirita, M.M.C.; Dehelean, C.; Avram, S.; Loghin, F.; Mocanu, L.; Rotaru, V.; et al. Controlled Synthesis and Characterization of Micrometric Single Crystalline Magnetite With Superparamagnetic Behavior and Cytocompatibility/Cytotoxicity Assessments. Front. Pharm. 2020, 11, 410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedrich, W. The Verwey transition—A topical review. J. Phys. Condens. Matter 2002, 14, 12. [Google Scholar]
- Kosterov, A. Magnetic properties, low temperature. In Encyclopedia of Geomagnetism and Paleomagnetism; Gubbins, D., Herrero-Bervera, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 515–525. [Google Scholar]
- Miller, S. Dancing electrons solve a long standing puzzle in the oldest magnetic material. Space Daily, 21 March 2020; Retrieved 2 September 2021. [Google Scholar]
- Aragón, R.; Buttrey, D.J.; Shepherd, J.P.; Honig, J.M. Influence of non stoichiometry on the Verwey transition. Phys. Rev. B 1985, 31, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, Ö.; Dunlop, D.J.; Moskowitz, B.M. The effect of oxidation on the Verwey transition in magnetite. Geophys. Res. Lett. 1993, 20, 1671–1674. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodroguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef] [Green Version]
- Nicola, R.; Costisor, O.; Ianasi, C.; Lazau, R.; Sacarescu, L.; Niznansky, D.; Ercuta, A.; Putz, A.-M.; Savii, C. Fractal surface maghemite nanoparticles prepared by co-precipitation: The influence of iron concentration and base nature. Stud. Ubb Chem. LXIII 2018, 63, 15–29. [Google Scholar] [CrossRef]
- Ozdemir, O. Coercive force of single crystals of magnetite at low temperatures. Geophys. J. Int. 2000, 141, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Hubert, A.; Schäfer, R. Magnetic Domains. In The Analysis of Magnetic Microstructures; Springer: Berlin/Heidelberg, Germany, 1998; Volume 496. [Google Scholar]
- Bertotti, G. Domain Wall Motion. In Hysteresis in Magnetism for Physicists, Material Scientists, and Engineers; Elsevier Inc.: Amsterdam, The Netherlands, 1998; Volume 285. [Google Scholar]
- Liu, Q.; Yu, Y.; Muxworthy, A.R.; Roberts, A.P. Effects of internal stress on remanence intensity jumps across the Verwey transition for multi-domain magnetite. Phys. Earth Planet. Int. 2008, 169, 100–107. [Google Scholar] [CrossRef] [Green Version]
- O’Handley, R.C.; Allen, S.M. Shape-Memory Alloys, Magnetically Activated Ferromagnetic Shape-Memory Materials. In Encyclopedia of Smart Materials; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Chirita, M.; Ieta, A. FeCO3 Microparticle Synthesis by Fe-EDTA Hydrothermal Decomposition. Cryst. Growth Des. 2012, 12, 883–886. [Google Scholar] [CrossRef]
- Szilagyi, P.A. Study of Iron-Chelates in Solid State and Aqueous Solutions Using Mossbauer Spectroscopy. Ph.D. Thesis, Universite de Tolouse, Toulouse, France, 2007. [Google Scholar]
- Motekaitis, R.J.; Cox, B., III; Taylor, P.; Martell, A.E.; Miles, B.; Tvedt, T.J., Jr. Thermal degradation of EDTA chelates in aqueous solution. Can. J. Chem. 1982, 60, 1207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirita, M.; Bezergheanu, A.; Bazil Cizmas, C.; Ercuta, A. Superparamagnetic-like Micrometric Single Crystalline Magnetite for Biomedical Application Synthesis and Characterization. Magnetochemistry 2023, 9, 5. https://doi.org/10.3390/magnetochemistry9010005
Chirita M, Bezergheanu A, Bazil Cizmas C, Ercuta A. Superparamagnetic-like Micrometric Single Crystalline Magnetite for Biomedical Application Synthesis and Characterization. Magnetochemistry. 2023; 9(1):5. https://doi.org/10.3390/magnetochemistry9010005
Chicago/Turabian StyleChirita, Marius, Adrian Bezergheanu, Corneliu Bazil Cizmas, and Aurel Ercuta. 2023. "Superparamagnetic-like Micrometric Single Crystalline Magnetite for Biomedical Application Synthesis and Characterization" Magnetochemistry 9, no. 1: 5. https://doi.org/10.3390/magnetochemistry9010005
APA StyleChirita, M., Bezergheanu, A., Bazil Cizmas, C., & Ercuta, A. (2023). Superparamagnetic-like Micrometric Single Crystalline Magnetite for Biomedical Application Synthesis and Characterization. Magnetochemistry, 9(1), 5. https://doi.org/10.3390/magnetochemistry9010005





