Controlling the Magnetic Properties of La0.9A0.1Mn0.9Cr0.1O3 (A: Li, K, Na) Powders and Ceramics by Alkali Ions Doping
Abstract
1. Introduction
2. Materials and Methods
3. Results
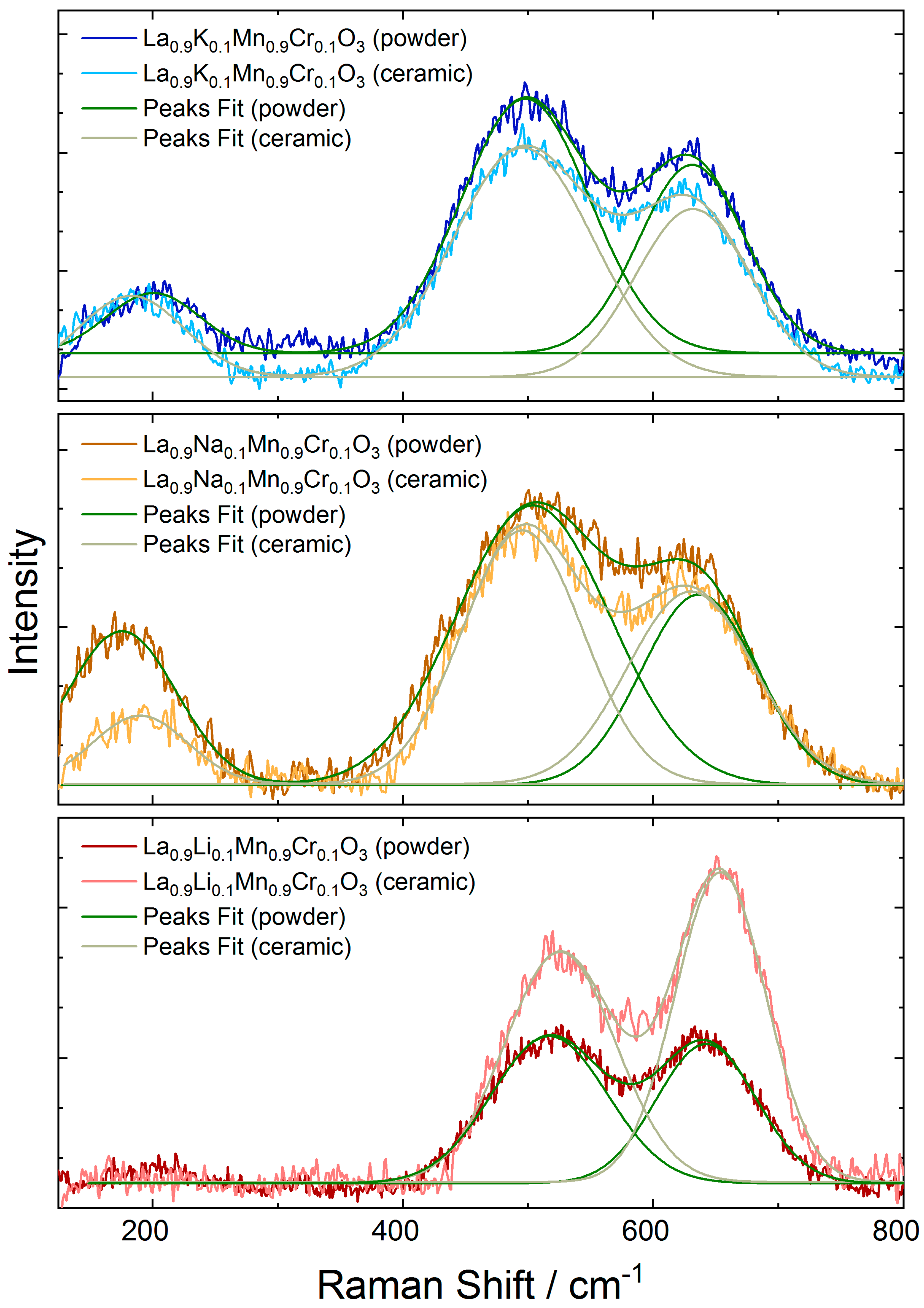
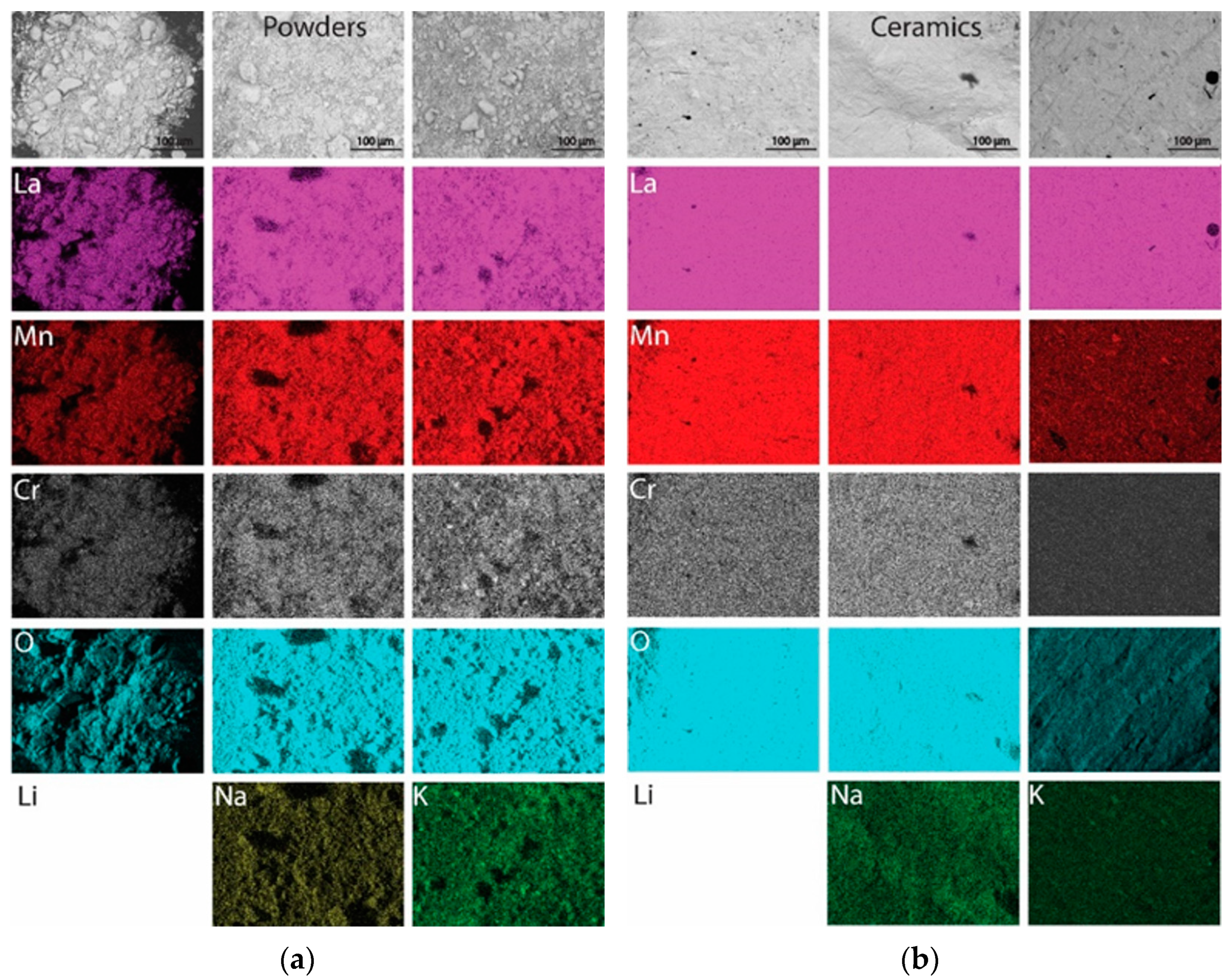
3.1. Structure and Morphology
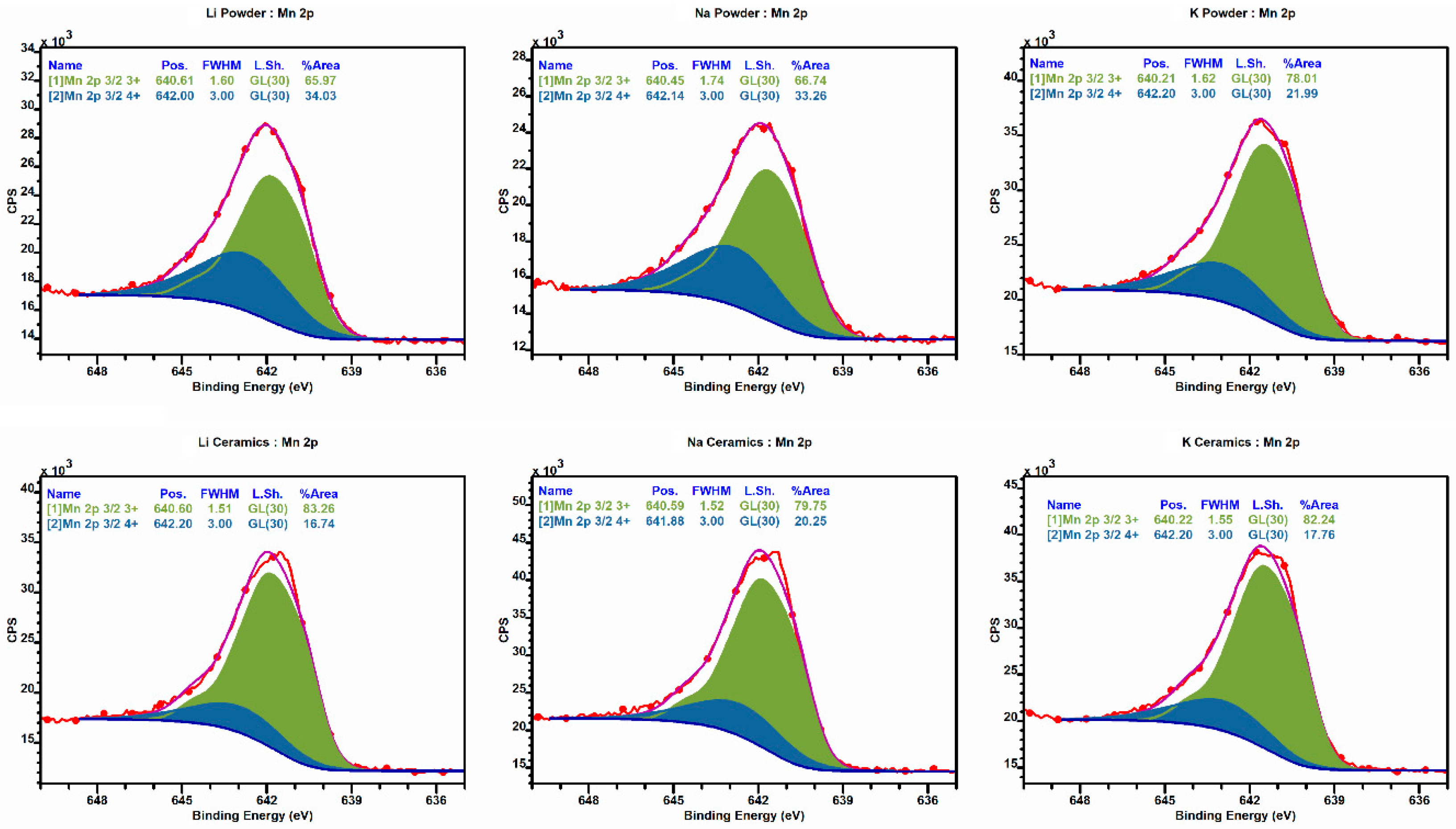
3.2. Oxidation States of Mn and Cr Ions
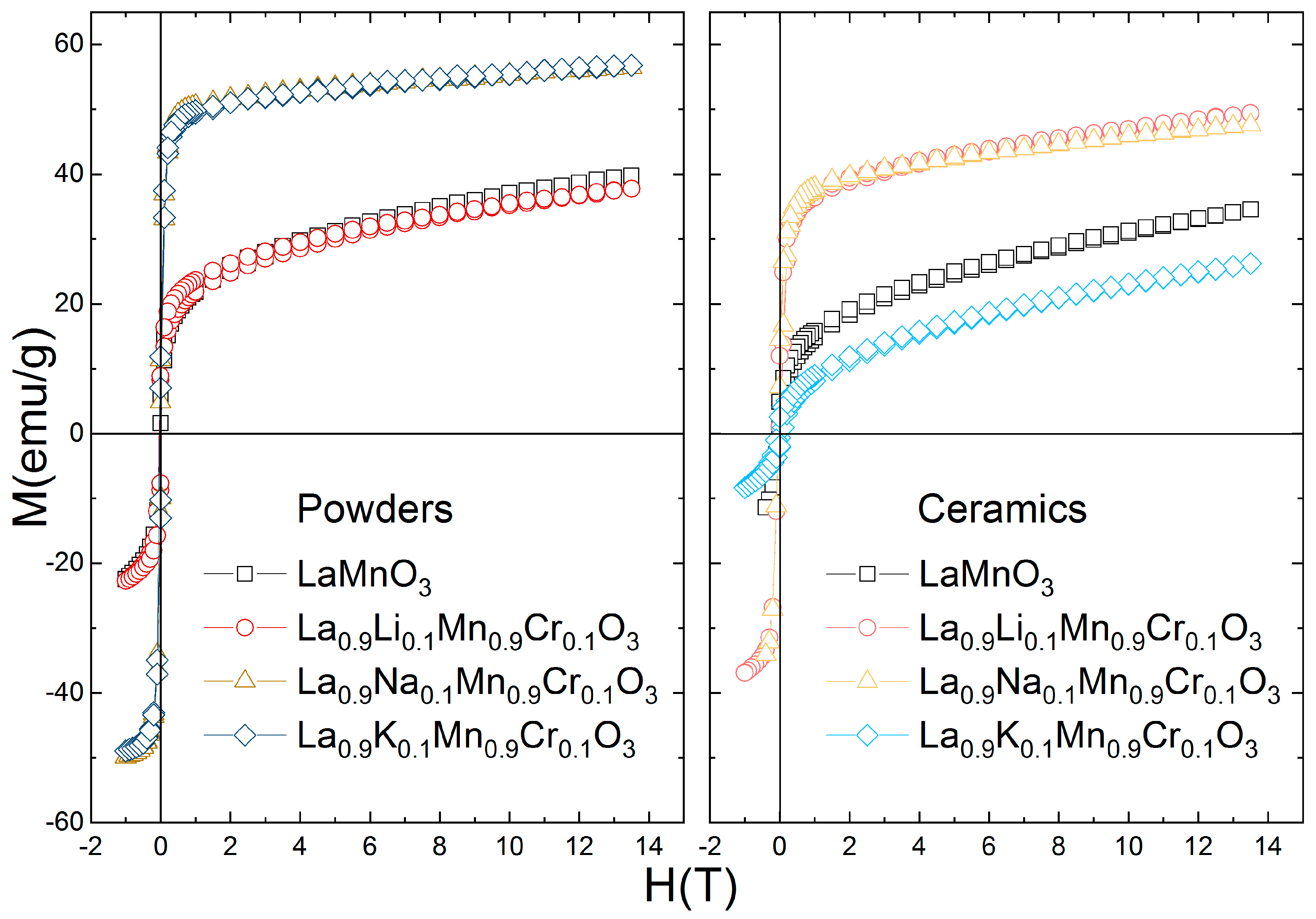
3.3. Magnetic Properties
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmid, H. Multi-ferroic magnetoelectrics. Ferroelectrics 1994, 162, 317–338. [Google Scholar] [CrossRef]
- Schmid, H. On ferrotoroidics and electrotoroidic, magnetotoroidic and piezotoroidic effects. Ferroelectrics 2001, 252, 41–50. [Google Scholar] [CrossRef]
- Kimura, T. Spiral magnets as magnetoelectrics. Annu. Rev. Mater. Res. 2007, 37, 387–413. [Google Scholar] [CrossRef]
- Tokura, Y.; Seki, S.; Nagaosa, N. Multiferroics of spin origin. Rep. Prog. Phys. 2014, 77, 076501. [Google Scholar] [CrossRef] [PubMed]
- Mostovoy, M. Multiferroics: A whirlwind of opportunities. Nat. Mater. 2010, 9, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Coey, J.M.D.; Viret, M.; Von Molnár, S. Mixed-valence manganites. Adv. Phys. 1999, 48, 167–293. [Google Scholar] [CrossRef]
- Hernández, E.; Sagredo, V.; Delgado, G.E. Synthesis and magnetic characterization of LaMnO3 nanoparticles. Rev. Mex. Fis. 2015, 61, 166–169. [Google Scholar]
- Naish, V.E. Models of Crystal Structures of doped Lanthanum Manganites. Fiz. Met. Met. 1998, 85, 589–600. [Google Scholar] [CrossRef]
- Tokura, Y. Critical features of colossal magnetoresistive manganites. Rep. Prog. Phys. 2006, 69, 797–851. [Google Scholar] [CrossRef]
- Zener, C. Interaction between the d-shells in the transition metals. II. Ferromagnetic compounds of manganese with Perovskite structure. Phys. Rev. 1951, 82, 403–405. [Google Scholar] [CrossRef]
- Phan, M.H.; Yu, S.C. Review of the magnetocaloric effect in manganite materials. J. Magn. Magn. Mater. 2007, 308, 325–340. [Google Scholar] [CrossRef]
- Naik, V.B.; Barik, S.K.; Mahendiran, R.; Raveau, B. Magnetic and calorimetric investigations of inverse magnetocaloric effect in Pr0.46 Sr0.54 MnO3. Appl. Phys. Lett. 2011, 98, 2011–2014. [Google Scholar] [CrossRef]
- Bahl, C.R.H.; Velázquez, D.; Nielsen, K.K.; Engelbrecht, K.; Andersen, K.B.; Bulatova, R.; Pryds, N. High performance magnetocaloric perovskites for magnetic refrigeration. Appl. Phys. Lett. 2012, 100, 121905. [Google Scholar] [CrossRef]
- Balli, M.; Jandl, S.; Fournier, P.; Kedous-Lebouc, A. Advanced materials for magnetic cooling: Fundamentals and practical aspects. Appl. Phys. Rev. 2017, 4, 021305. [Google Scholar] [CrossRef]
- Shivakumara, C.; Hegde, M.S.; Srinivasa, T.; Vasanthacharya, N.Y.; Subbanna, G.N.; Lalla, N.P. Synthesis, structure and magnetic properties of Ln1-xAxMnO3 (Ln = Pr, Nd; A = Na, K) from NaCl or KCl flux. J. Mater. Chem. 2001, 11, 2572–2579. [Google Scholar] [CrossRef]
- Sun, Y.; Kamarad, J.; Arnold, Z.; Kou, Z.Q.; Cheng, Z.H. Tuning of magnetocaloric effect in a La0.69Ca0.31MnO3 single crystal by pressure. Appl. Phys. Lett. 2006, 88, 2004–2007. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Palstra, T.T.M.; Cheong, S.W.; Batlogg, B. Pressure effects on the magnetoresistance in doped manganese perovskites. Phys. Rev. B. 1995, 52, 15046–15049. [Google Scholar] [CrossRef]
- Deisenhofer, J.; Paraskevopoulos, M.; Krug von Nidda, H.A.; Loidl, A. Interplay of superexchange and orbital degeneracy in cr-doped (formula presented). Phys. Rev. B Condens. Matter Mater. Phys. 2002, 66, 054414. [Google Scholar] [CrossRef]
- Troyanchuk, I.O.; Bushinsky, M.; Pushkarev, N.; Bespalaya, N.Y. Inhomogeneous magnetic states in the Nd (Mn1-xCrx) O3 system. Phys. Solid State 2004, 46, 1878–1883. [Google Scholar] [CrossRef]
- Sun, Y.; Tong, W.; Xu, X.; Zhang, Y. Tuning colossal magnetoresistance response by Cr substitution in La0.67Sr0.33MnO3. Appl. Phys. Lett. 2001, 78, 643–645. [Google Scholar] [CrossRef]
- Raveau, B.; Maignan, A.; Martin, C. Insulator–Metal Transition Induced by Cr and Co Doping in Pr0.5Ca0.5MnO3. J. Solid State Chem. 1997, 130, 162–166. [Google Scholar] [CrossRef]
- Morales, L.; Allub, R.; Alascio, B.; Butera, A.; Caneiro, A. Structural and magnetotransport properties of LaMn1-xCrxO3.00(0 ≤ x ≤ 0.15): Evidence of Mn3+-O-Cr3+ double-exchange interaction. Phys. Rev. B-Condens. Matter Mater. Phys. 2005, 72, 3–6. [Google Scholar] [CrossRef]
- Troyanchuk, I.O.; Bushinsky, M.V.; Karpinsky, D.V. Magnetic ordering in manganites substituted by chromium ions. J. Exp. Theor. Phys. 2006, 103, 580–588. [Google Scholar] [CrossRef]
- Troyanchuk, I.O.; Samsonenko, N.V.; Kasper, N.V.; Szymczak, H.; Nabialek, A. Magnetic and Transport Properties of EuMnO3+x Substituted by Ca, Sr and Cr Ions. Phys. Status Solidi 1997, 160, 195–203. [Google Scholar] [CrossRef]
- Gao, T.; Cao, S.; Li, W.; Kang, B.; Yu, L.; Yuan, S.; Zhang, J. Spin glass behavior in the half doped Pr0.5Ca0.5Mn1−xCrxO3 system. Phys. B Condens. Matter 2009, 404, 1283–1286. [Google Scholar] [CrossRef]
- Cabeza, O.; Long, M.; Severac, C.; Bari, M.A.; Muirhead, C.M.; Francesconi, M.G.; Greaves, C. Magnetization and resistivity in chromium doped manganites. J. Phys. Condens. Matter 1999, 11, 2569–2578. [Google Scholar] [CrossRef]
- Rivadulla, F.; López-Quintela, M.A.; Hueso, L.E.; Sande, P. Effect of Mn-site doping on the magnetotransport properties of the colossal magnetoresistance compound La2/3Ca1/3Mn1−xAxO3 (A = Co, Cr; x ≤ 0.1). Phys. Rev. B Condens. Matter Mater. Phys. 2000, 62, 5678–5684. [Google Scholar] [CrossRef]
- Paul, D.; Anuradha, K.N.; Bhagyashree, K.S.; Bhat, S.V. Electron Magnetic Resonance Studies of Nanosized Nd0.65Ca0.35 Mn1−xCrxO3 (x = 0, 0.06, 0.1) Manganite. Appl. Magn. Reson. 2015, 46, 1059–1068. [Google Scholar] [CrossRef]
- Kallel, N.; Dhahri, J.; Zemni, S.; Dhahri, E.; Oumezzine, M.; Ghedira, M.; Vincent, H. Effect of Cr doping in La0.7Sr0.3Mn1−xCrxO3 with 0 ≤ x ≤ 0.5. Phys. Status Solidi Appl. Res. 2001, 184, 319–325. [Google Scholar] [CrossRef]
- Yanchevskii, O.Z.; Belous, A.G.; Tovstolytkin, A.I.; V’yunov, O.I.; Durilin, D.A. Structural, electrical, and magnetic properties of La0.7Sr0.3Mn1−yCryO3. Inorg. Mater. 2006, 42, 1121–1125. [Google Scholar] [CrossRef]
- Xiao, X.; Yuan, S.; Miao, J.; Ren, G.; Yu, G.; Wang, Y.; Yin, S. Tuning colossal magnetoresistance response at roomtemperature by La2/3+ySr1/3−yMn1−yCryO3. Mater. Lett. 2007, 61, 2315–2318. [Google Scholar] [CrossRef]
- Głuchowski, P.; Nikonkov, R.; Tomala, R.; Stręk, W.; Shulha, T.; Serdechnova, M.; Zheludkevich, M.; Pakalaniškis, A.; Skaudžius, R.; Kareiva, A.; et al. Magnetic Properties of La0.9A0.1MnO3 (A: Li, Na, K) Nanopowders and Nanoceramics. Materials 2020, 13, 1788. [Google Scholar] [CrossRef]
- Głuchowski, P.; Nikonkov, R.; Tomala, R.; Stręk, W.; Shulh, T.; Serdechnova, M.; Zarkov, A.; Murauskas, T.; Pakalaniškis, A.; Skaudžius, R.; et al. Impact of Alkali Ions Codoping on Magnetic Properties of La0.9A0.1Mn0.9Co0.1O3 (A: Li, K, Na) Powders and Ceramics. Appl. Sci. 2020, 10, 8786. [Google Scholar] [CrossRef]
- Ekambaram, S.; Patil, K.C.; Maaza, M. Synthesis of lamp phosphors: Facile combustion approach. J. Alloys Compd. 2005, 393, 81–92. [Google Scholar] [CrossRef]
- Fedyk, R.; Hreniak, D.; Łojkowski, W.; Strek, W.; Matysiak, H.; Grzanka, E.; Gierlotka, S.; Mazur, P. Method of preparation and structural properties of transparent YAG nanoceramics. Opt. Mater. 2007, 29, 1252–1257. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The high score suite. In Powder Diffraction; Cambridge University Press: Cambridge, UK, 2014; Volume 29, pp. S13–S18. [Google Scholar]
- Gschneidner, K.A.; Bunzli, J.C.G.; Pecharsky, V.K. Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Markovich, V.; Fita, I.; Mogilyansky, D.; Wiśniewski, A.; Puzniak, R.; Titelman, L.; Vradman, L.; Herskowitz, M.; Gorodetsky, G. Effect of particle size on magnetic properties of LaMnO3+δ nanoparticles. Superlattices Microstruct. 2008, 44, 476–482. [Google Scholar] [CrossRef]
- Suzuki, K.; Koushalya, P.R.; Bhat, S.V. Size Dependent Magnetic Properties of Nd0.7Ca0.3MnO3 Nanomanganite. IOP Conf. Ser. Mater. Sci. Eng. 2015, 73, 012007. [Google Scholar]
- Goveas, L.R.; Bhagyashree, K.S.; Anuradha, K.N.; Bhat, S.V. Size dependence of charge order and magnetism in Sm0.35Ca0.65MnO3. AIP Adv. 2021, 11, 25313. [Google Scholar] [CrossRef]
- Markovich, V.; Fita, I.; Mogilyansky, D.; Wisniewski, A.; Puzniak, P.; Titelman, L.; Vradman, L.; Herskowitz, M.; Gorodetsky, G. Magnetic properties of nanocrystalline La1−xMnO3+δ manganites: Size effects. J. Phys. Condens. Matter 2007, 19, 346210. [Google Scholar] [CrossRef]
- Głuchowski, P. Pressure -induced changes in the persistent luminescence of Gd2.994Ce0.006Ga3Al2O12 and Gd2.964Ce0.006Dy0.03Ga3Al2O12 nanoceramics. Dalt. Trans. 2022, 51, 5524–5533. [Google Scholar] [CrossRef]
- Głuchowski, P.; Tomala, R.; Kowalski, R.; Ignatenko, O.; Witkowski, M.E.; Drozdowski, W.; Stręk, W.; Ryba-Romanowski, W.; Solarz, P. “Frozen” pressure effect in GGAG:Ce3+ white light emitting nanoceramics. Ceram. Int. 2019, 45, 21870–21877. [Google Scholar] [CrossRef]
- Iliev, M.; Abrashev, M. Raman spectroscopy of orthorhombic perovskitelike and. Phys. Rev. B Condens. Matter Mater. Phys. 1998, 57, 2872–2877. [Google Scholar] [CrossRef]
- Abrashev, M.V.; Litvinchuk, A.P.; Iliev, M.N.; Meng, R.L.; Popov, V.N.; Ivanov, V.G.; Chakalov, R.A.; Thomsen, C. Comparative study of optical phonons in the rhombohedrally distorted perovskites (formula presented) and (formula presented). Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 4146–4153. [Google Scholar] [CrossRef]
- Gupta, K.M.; Gupta, N. Magnetic Materials: Properties and Behaviour. In Advanced Electrical and Electronics Materials: Processes and Applications; Scrivener Publishing LLC: Beverly, MA, USA, 2015; pp. 379–421. [Google Scholar] [CrossRef]
- Pavlov, V.I.; Bogush, A.K.; Balyko, L.V. Magnetic phase transitions in LaMnO3+λ. Cryst. Res. Technol. 1984, 19, 237–245. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Amano, M.E.; Betancourt, I.; Arellano-Jimenez, M.J.; Sánchez-Llamazares, J.L.; Sánchez-Valdés, C.F. Magnetocaloric response of submicron (LaAg)MnO3 manganite obtained by Pechini method. J. Sol.Gel. Sci. Technol. 2016, 78, 159–165. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Z.; Yu, Y.; Yin, J.; Song, K.; Yang, B.; Yuan, L.; Hu, X. Copper/cobalt-doped LaMnO3 perovskite oxide as a bifunctional catalyst for rechargeable Li-O2 batteries. J. Alloys Compd. 2019, 801, 19–26. [Google Scholar] [CrossRef]
- Lee, Y.N.; Lago, R.M.; Fierro, J.L.G.; Cortés, V.; Sapiña, F.; Martínez, E. Surface properties and catalytic performance for ethane combustion of La1−xKxMnO3+δ perovskites. Appl. Catal. A Gen. 2001, 207, 17–24. [Google Scholar] [CrossRef]
- Wei, Z.-X.; Wei, L.; Gong, L.; Wang, Y.; Hu, C.-W. Combustion synthesis and effect of LaMnO3 and La0.8Sr0.2MnO3 on RDX thermal decomposition. J. Hazard. Mater. 2010, 177, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hu, J.; Tian, G.; Zhu, J.; Song, Q.; Wang, H.; Zhang, C. Efficient Catalysts of K and Ce Co-Doped LaMnO3for NOx-Soot Simultaneous Removal and Reaction Kinetics. ACS Omega 2021, 6, 19836–19845. [Google Scholar] [CrossRef]
- García-Muñoz, J.; Fontcuberta, J.; Martinez, B.; Seffar, A.; Pinol, S.; Obradors, X. Magnetic frustration in mixed valence manganites. Phys. Rev. B-Condens. Matter Mater. Phys 1997, 55, R668–R671. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–766. [Google Scholar] [CrossRef]
- Georgalas, C.; Samartzis, A.; Biniskos, N.; Syskakis, E. Effects of Cr-doping on the Jahn-Teller, the orthorhombic to rhombohedral, and the magnetic transitions in LaMn1-xCrxO3 compounds. Phys. B Condens. Matter 2020, 586, 412101. [Google Scholar] [CrossRef]
- Afify, M.S.; El Faham, M.M.; Eldemerdash, U.; El Rouby, W.M.; El-Dek, S. Room temperature ferromagnetism in Ag doped LaMnO3 nanoparticles. J. Alloys Compd. 2020, 861, 158570. [Google Scholar] [CrossRef]
- Branković, Z.; Đuris, K.; Radojković, A.; Bernik, S.; Jagličić, Z.; Jagodic, M.; Vojisavljević, K.; Branković, G. Magnetic properties of doped LaMnO3 ceramics obtained by a polymerizable complex method. J. Sol-Gel Sci. Technol. 2010, 55, 311–316. [Google Scholar] [CrossRef]
- Das, S.; Poddar, A.; Roy, B.; Giri, S. Studies of transport and magnetic properties of Ce-doped LaMnO3. J. Alloys Compd. 2004, 365, 94–101. [Google Scholar] [CrossRef]
- Supelano, G.I.; Barón-González, A.J.; Santos, A.S.; Ortíz, C.; Gómez, J.A.M.; Vargas, C.A.P. Effect of Mg addition on LaMnO3 ceramic system. J. Mater. Res. Technol. 2018, 7, 77–81. [Google Scholar] [CrossRef]
- Zakhvalinskiǐ, V.S.; Laiho, R.; Lisunov, K.G.; Lähderanta, E.; Petrenko, P.A.; Stepanov, Y.P.; Salminen, J.; Stamov, V.N. Preparation and magnetic properties of LaMnO3+δ (0 ≤ δ ≤ 0.154). Phys. Solid State 2006, 48, 2300–2309. [Google Scholar] [CrossRef]
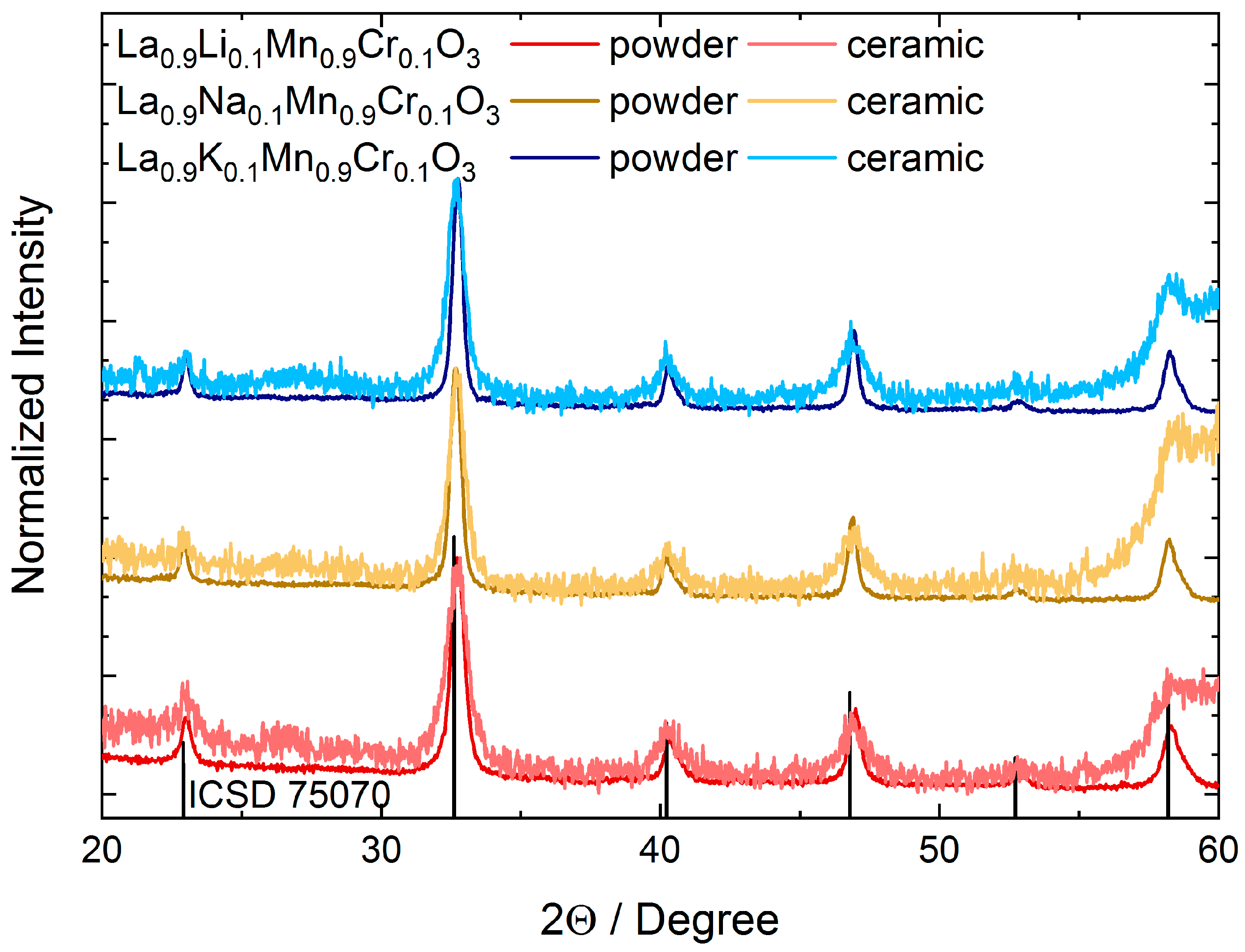

| Size | Strains | a, b | c | V | Atomic Distance | |||
|---|---|---|---|---|---|---|---|---|
| Mn-O(1) | La-O(1) | La-O(2) | ||||||
| nm | % | Å | Å | Å3 | Å | |||
| Powders | ||||||||
| La0.9Li0.1Mn0.9Cr0.1O3 Rexp = 2.0631, GOF = 2.692 | 26 | 0.222 | 5.496(7) | 13.32(3) | 348.6(2) | 1.957(5) | 2.744(1) | 2.463(1) |
| La0.9Na0.1Mn0.9Cr0.1O3 Rexp = 2.0317, GOF = 2.4394 | 34 | 0.18 | 5.496(4) | 13.34(0) | 349.0(1) | 1.958(2) | 2.746(3) | 2.462(9) |
| La0.9K0.1Mn0.9Cr0.1O3 Rexp = 2.0565, GOF = 2.4711 | 37 | 0.177 | 5.503(0) | 13.36(4) | 350.4(8) | 1.961(0) | 2.750(6) | 2.465(9) |
| Ceramics | ||||||||
| La0.9Li0.1Mn0.9Cr0.1O3 Rexp = 4.6257, GOF = 6.5642 | 13 | 0.686 | 5.505(2) | 13.36(3) | 350.7(5) | 1.961(6) | 2.751(9) | 2.467(0) |
| La0.9Na0.1Mn0.9Cr0.1O3 Rexp = 5.8594, GOF = 5.2561 | 16 | 0.672 | 5.482(5) | 13.44(4) | 349.9(6) | 1.959(8) | 2.758(0) | 2.456(7) |
| La0.9K0.1Mn0.9Cr0.1O3 Rexp = 5.3762, GOF = 4.2687 | 18 | 0.892 | 5.488(2) | 13.41(3) | 349.9(0) | 1.942(8) | 2.719(8) | 2.447(7) |
| Raman Modes | Eg2 | Eg3 | Eg4 | Δ |
|---|---|---|---|---|
| La | Bending | Antistretching | ||
| cm−1 | ||||
| Powder | ||||
| LaMnO3 [44] | 198 | 490 | 612 | 122 |
| La0.9Li0.1Mn0.9Cr0.1O3 | - | 517 | 643 | 126 |
| La0.9Na0.1Mn0.9Cr0.1O3 | 177 | 504 | 637 | 133 |
| La0.9K0.1Mn0.9Cr0.1O3 | 185 | 496 | 632 | 136 |
| Ceramic | ||||
| La0.9Li0.1Mn0.9Cr0.1O3 | - | 525 | 653 | 128 |
| La0.9Na0.1Mn0.9Cr0.1O3 | 191 | 495 | 630 | 135 |
| La0.9K0.1Mn0.9Cr0.1O3 | 201 | 497 | 632 | 135 |
| Mn Oxidation State (%) | Cr Oxidation State (%) | |||
|---|---|---|---|---|
| 3+ | 4+ | 3+ | 6+ | |
| Powder | ||||
| La0.9Li0.1Mn0.9Cr0.1O3 | 66.0 | 34.0 | 62.1 | 37.9 |
| La0.9Na0.1Mn0.9Cr0.1O3 | 66.7 | 33.3 | 53.9 | 46.1 |
| La0.9K0.1Mn0.9Cr0.1O3 | 78.0 | 22.0 | 50.4 | 49.6 |
| Ceramic | ||||
| La0.9Li0.1Mn0.9Cr0.1O3 | 83.3 | 16.7 | 40.8 | 59.2 |
| La0.9Na0.1Mn0.9Cr0.1O3 | 79.8 | 20.2 | 85.4 | 14.6 |
| La0.9K0.1Mn0.9Cr0.1O3 | 82.2 | 17.8 | 78.3 | 21.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głuchowski, P.; Nikonkov, R.; Kujawa, D.; Stręk, W.; Murauskas, T.; Pakalniškis, A.; Kareiva, A.; Yaremkevych, A.; Fesenko, O.; Zhaludkevich, A.; et al. Controlling the Magnetic Properties of La0.9A0.1Mn0.9Cr0.1O3 (A: Li, K, Na) Powders and Ceramics by Alkali Ions Doping. Magnetochemistry 2023, 9, 140. https://doi.org/10.3390/magnetochemistry9060140
Głuchowski P, Nikonkov R, Kujawa D, Stręk W, Murauskas T, Pakalniškis A, Kareiva A, Yaremkevych A, Fesenko O, Zhaludkevich A, et al. Controlling the Magnetic Properties of La0.9A0.1Mn0.9Cr0.1O3 (A: Li, K, Na) Powders and Ceramics by Alkali Ions Doping. Magnetochemistry. 2023; 9(6):140. https://doi.org/10.3390/magnetochemistry9060140
Chicago/Turabian StyleGłuchowski, Paweł, Ruslan Nikonkov, Daniela Kujawa, Wiesław Stręk, Tomas Murauskas, Andrius Pakalniškis, Aivaras Kareiva, Andrii Yaremkevych, Olena Fesenko, Aliaksandr Zhaludkevich, and et al. 2023. "Controlling the Magnetic Properties of La0.9A0.1Mn0.9Cr0.1O3 (A: Li, K, Na) Powders and Ceramics by Alkali Ions Doping" Magnetochemistry 9, no. 6: 140. https://doi.org/10.3390/magnetochemistry9060140
APA StyleGłuchowski, P., Nikonkov, R., Kujawa, D., Stręk, W., Murauskas, T., Pakalniškis, A., Kareiva, A., Yaremkevych, A., Fesenko, O., Zhaludkevich, A., & Karpinsky, D. (2023). Controlling the Magnetic Properties of La0.9A0.1Mn0.9Cr0.1O3 (A: Li, K, Na) Powders and Ceramics by Alkali Ions Doping. Magnetochemistry, 9(6), 140. https://doi.org/10.3390/magnetochemistry9060140










