North America’s Potential for an Environmentally Sustainable Nickel, Manganese, and Cobalt Battery Value Chain
Abstract
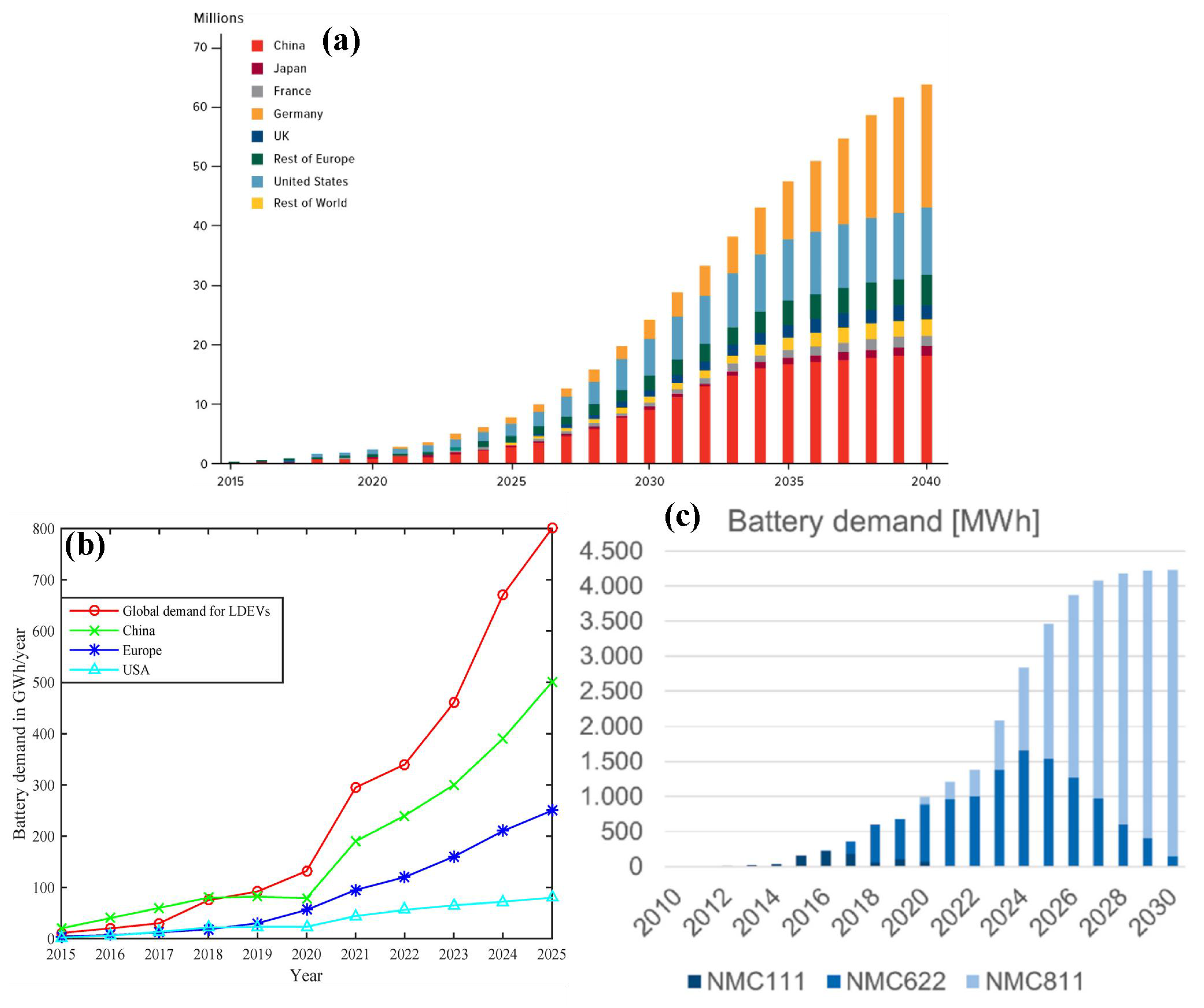
1. Introduction
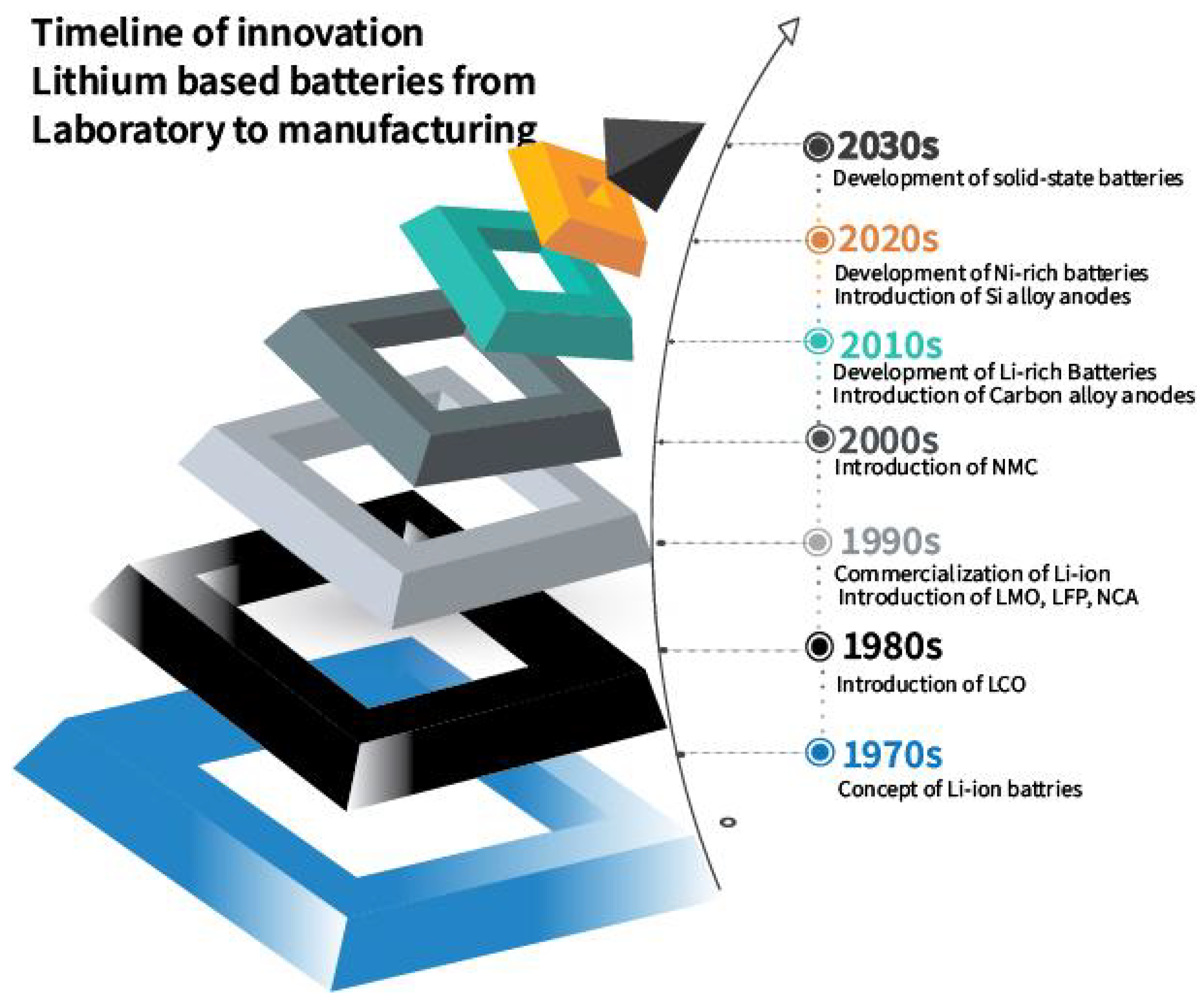
1.1. Changing Battery Chemistries
1.2. Battery Properties
1.2.1. Why NMC?
1.2.2. Cost
1.2.3. Performance
1.2.4. Specific Energy
1.2.5. Safety
2. Mining
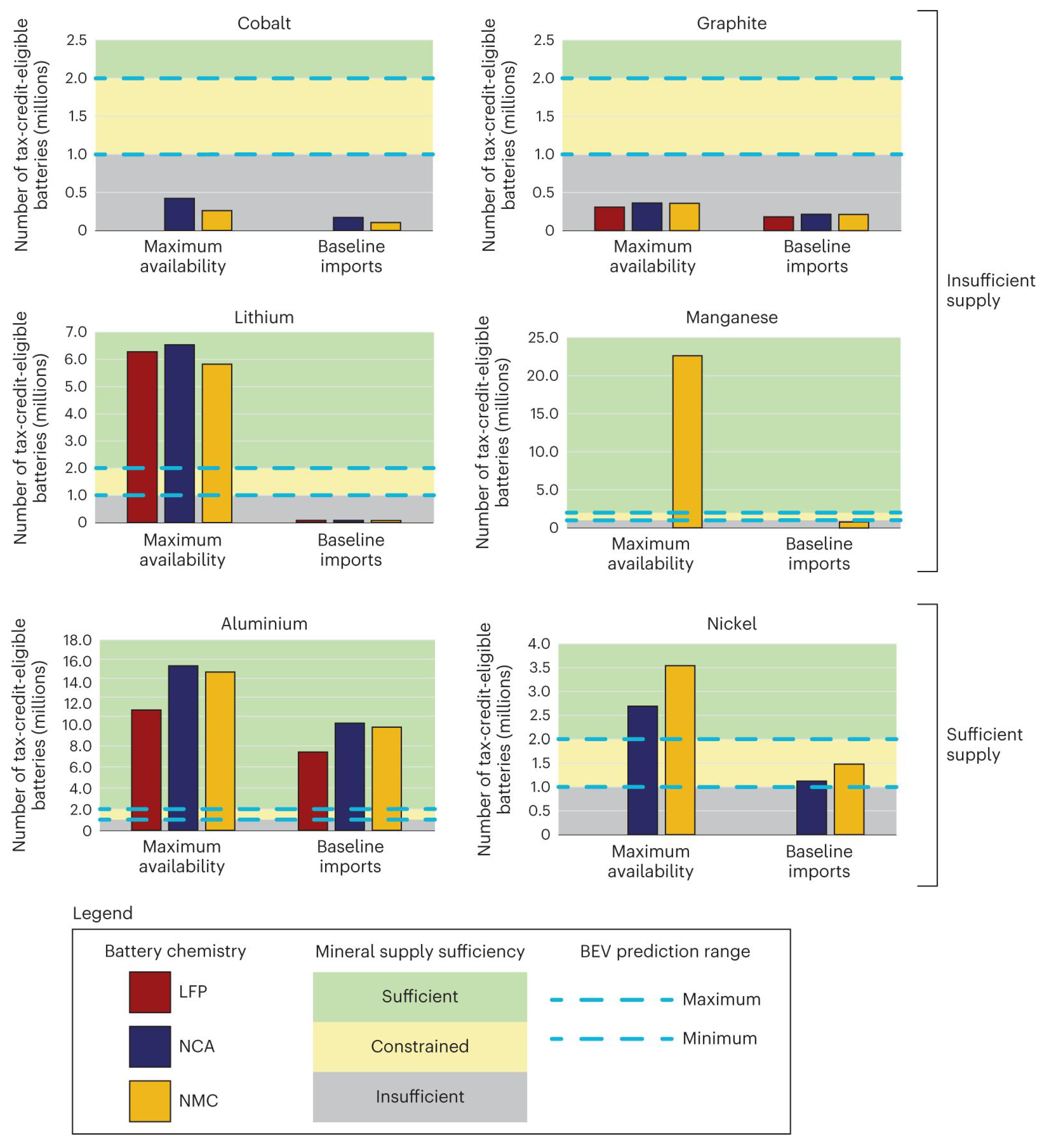
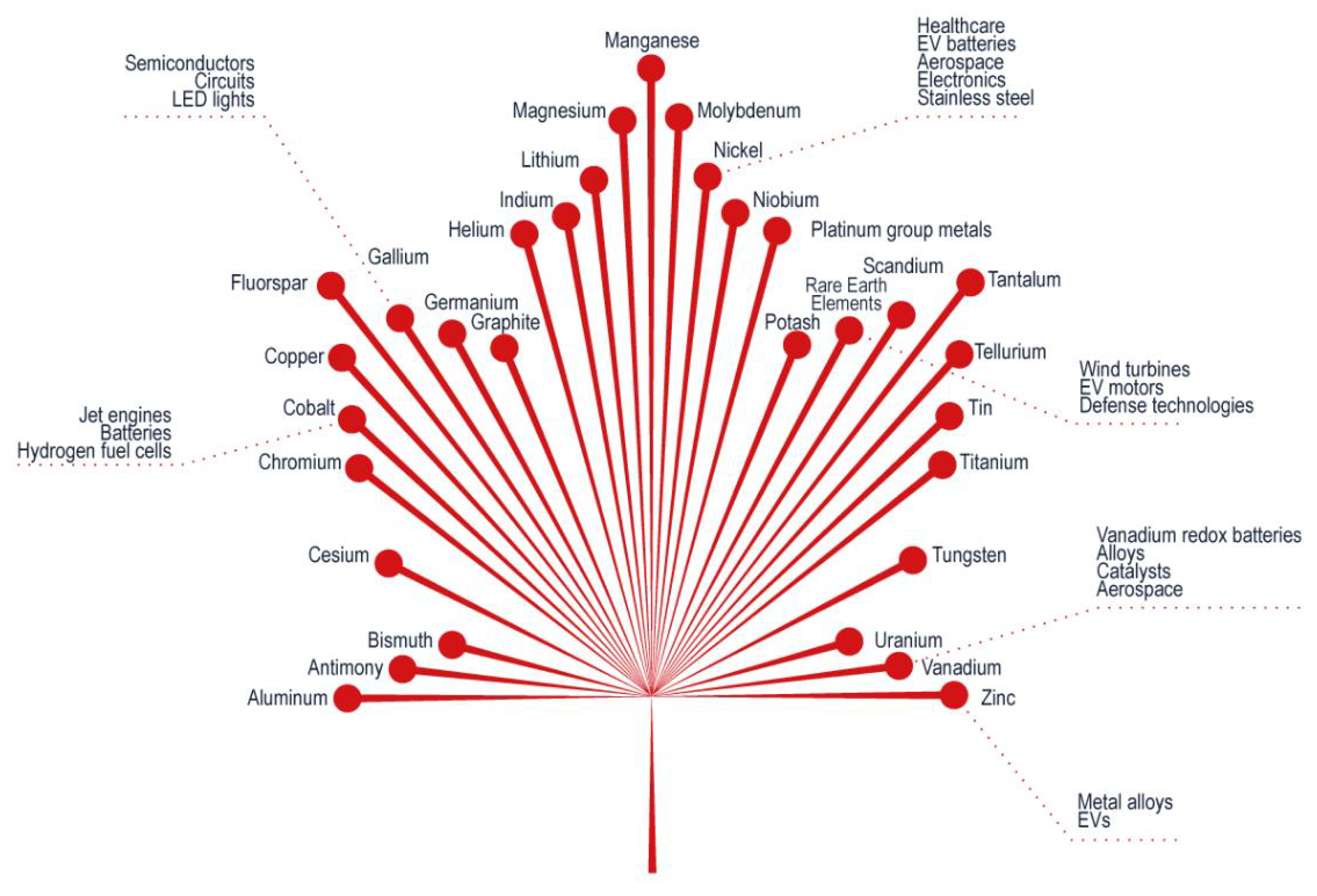
2.1. Availabilities of Critical Minerals
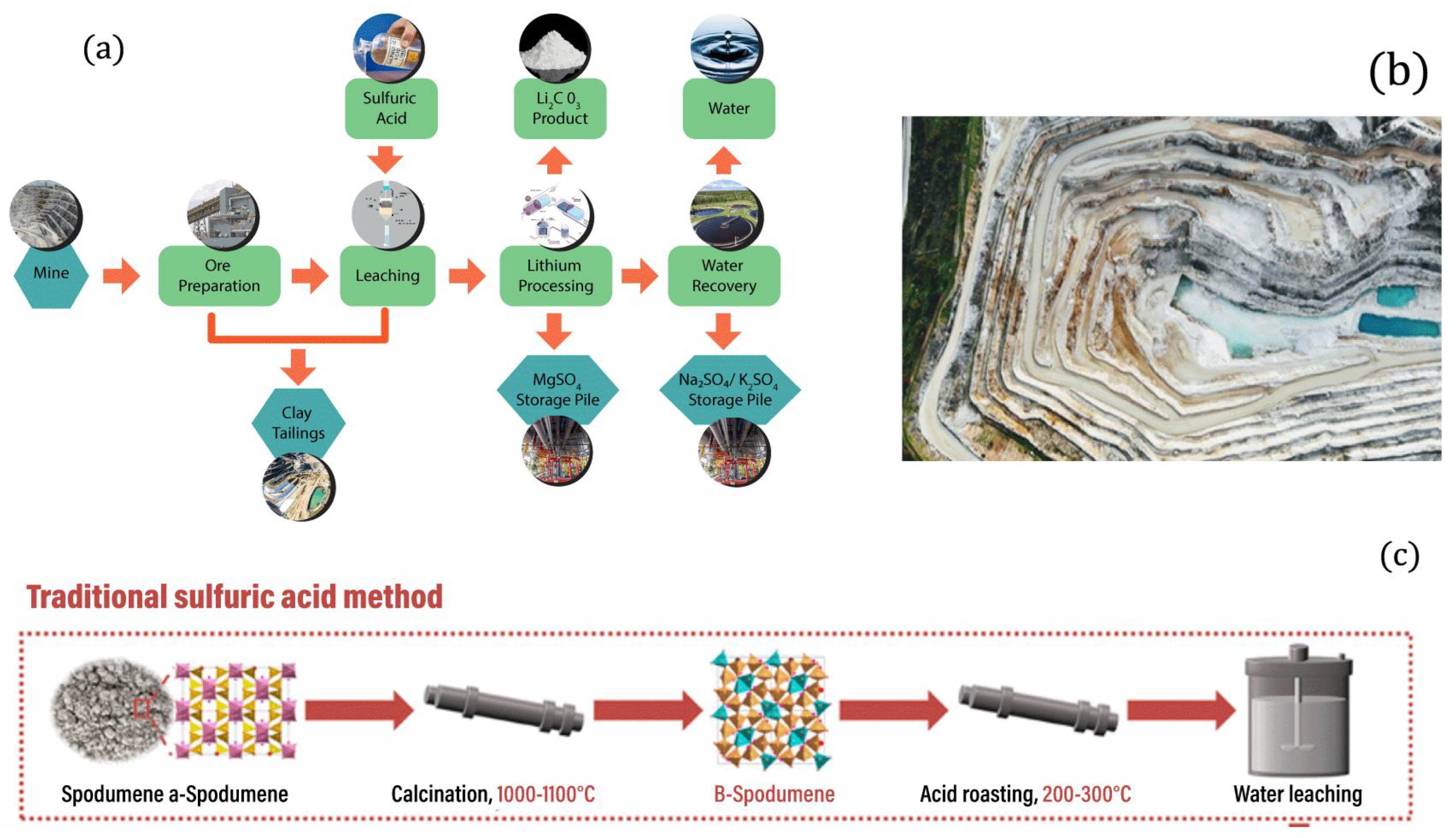
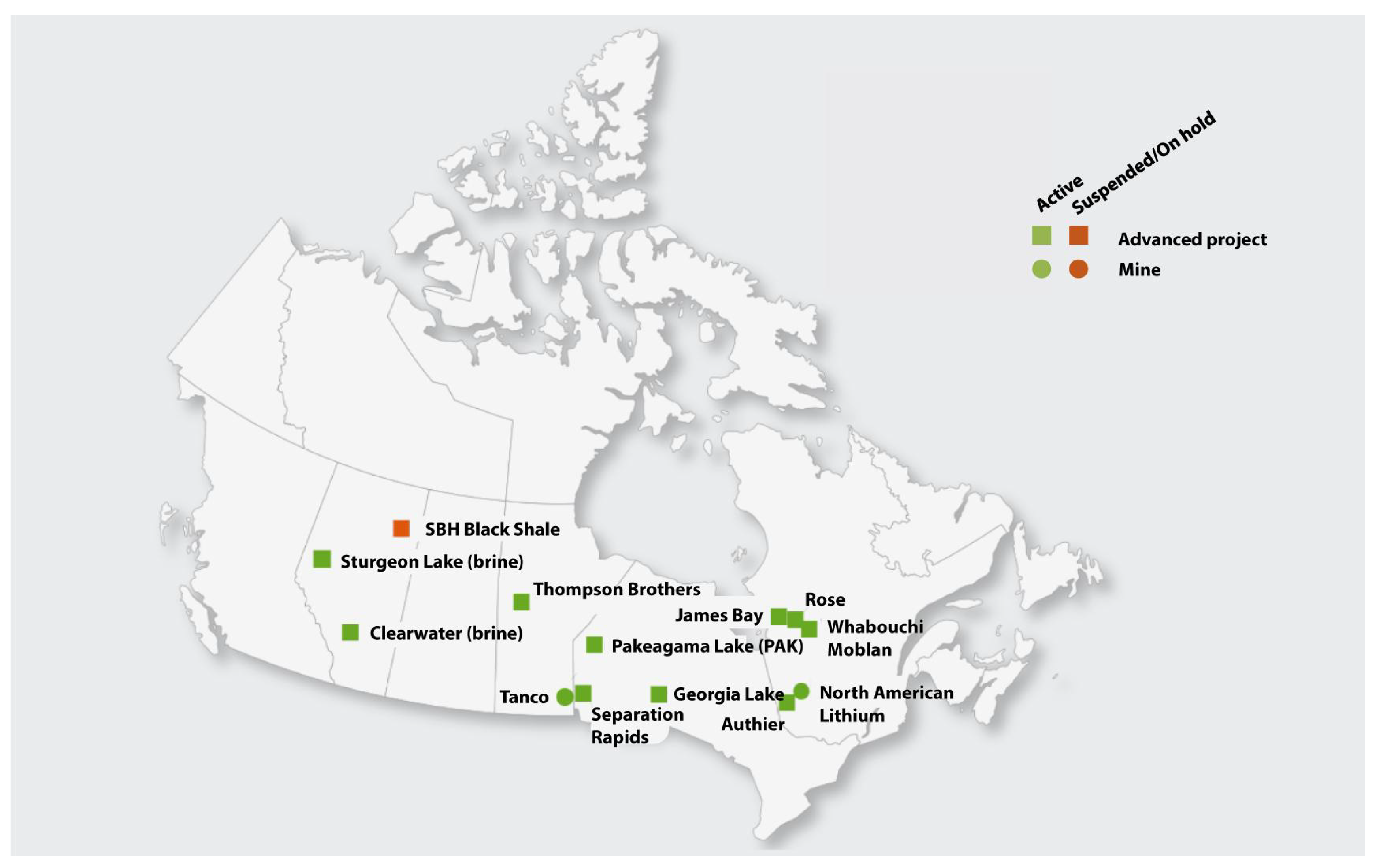
2.2. Lithium (Li) Sources
2.3. Nickel (Ni) Sources
2.4. Manganese (Mn) Sources
2.5. Cobalt (Co) Resources
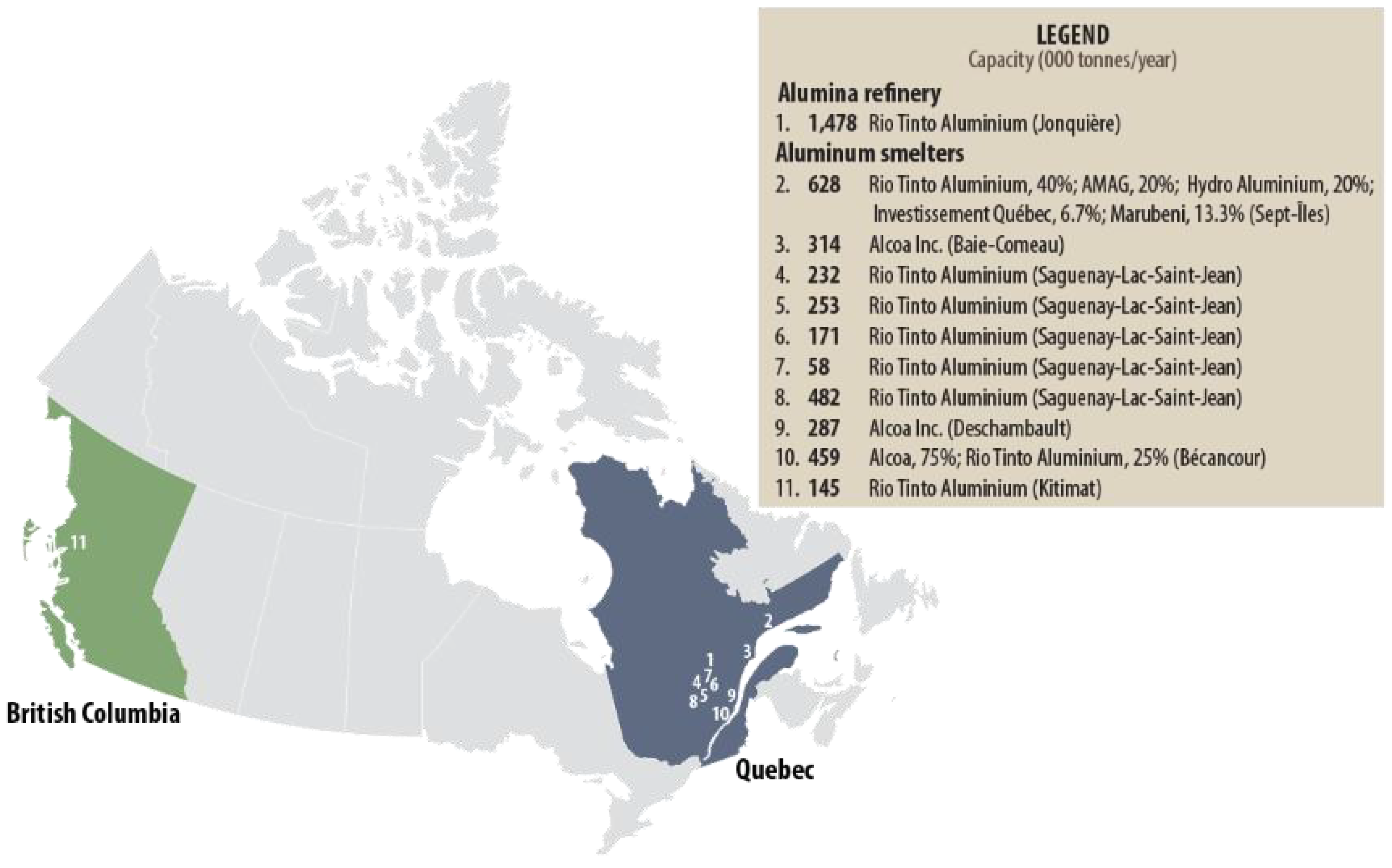
2.6. Aluminum (Al) Sources
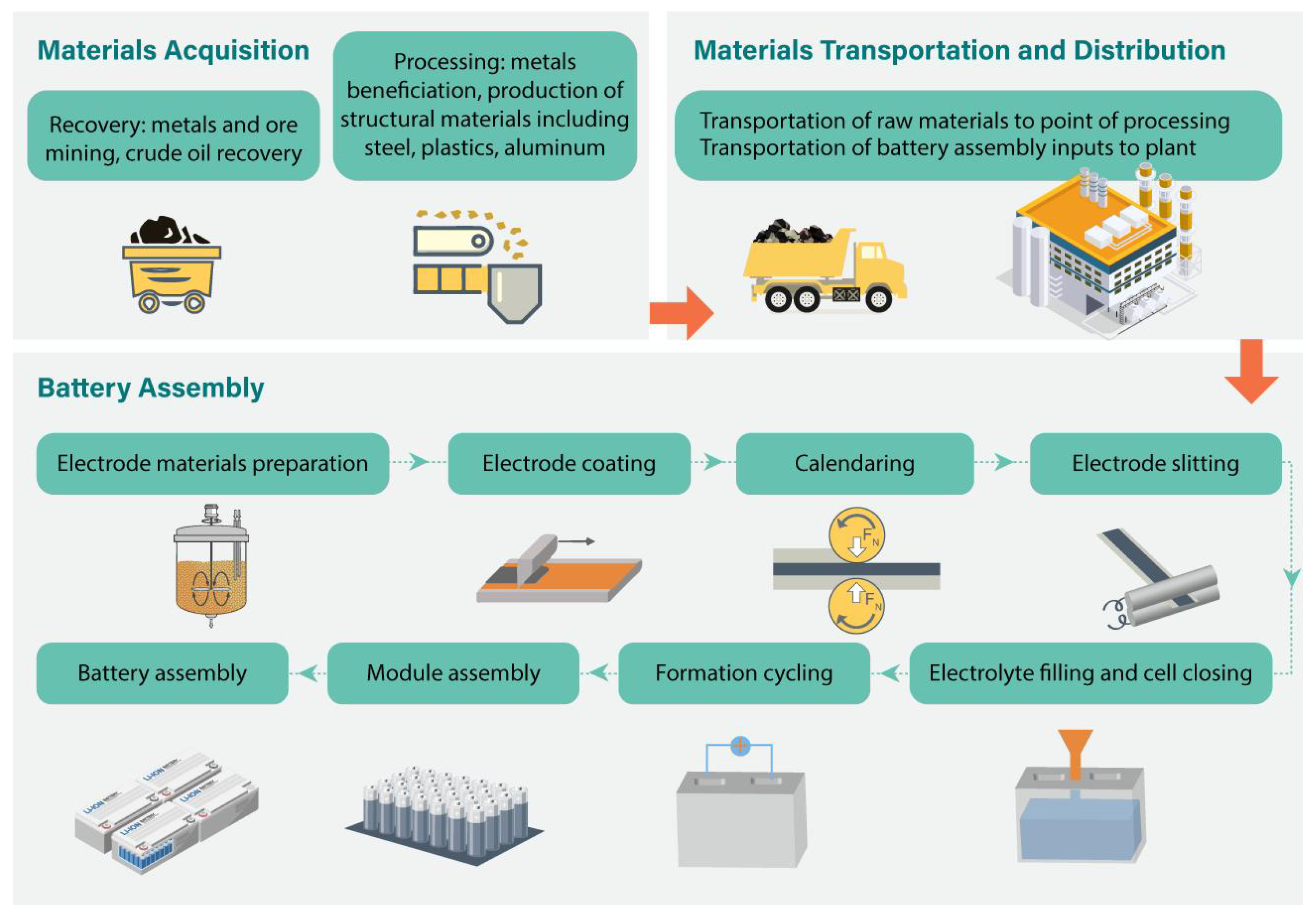
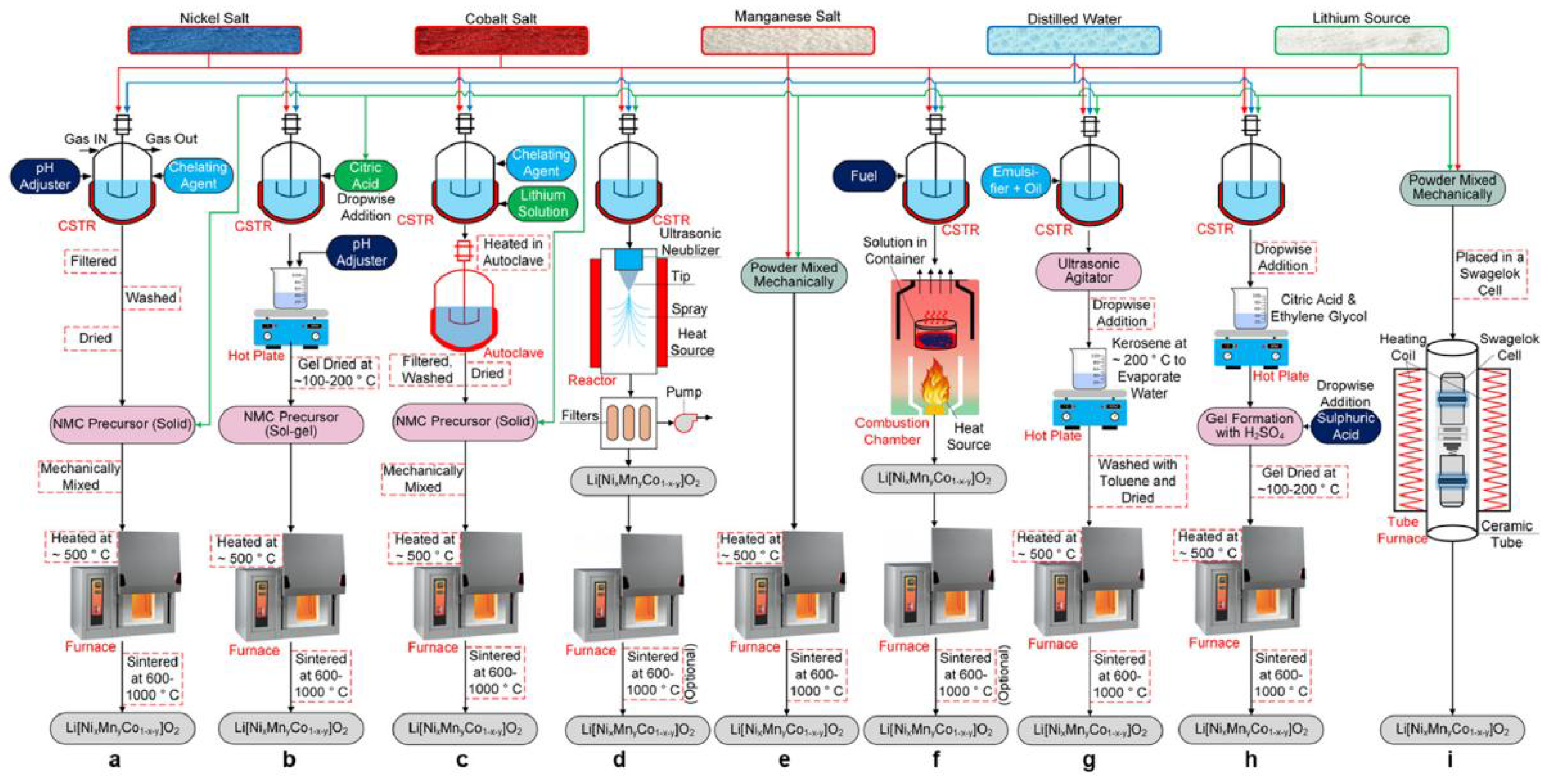
2.7. Manufacturing of NMC Cathodes from Materials to Cells
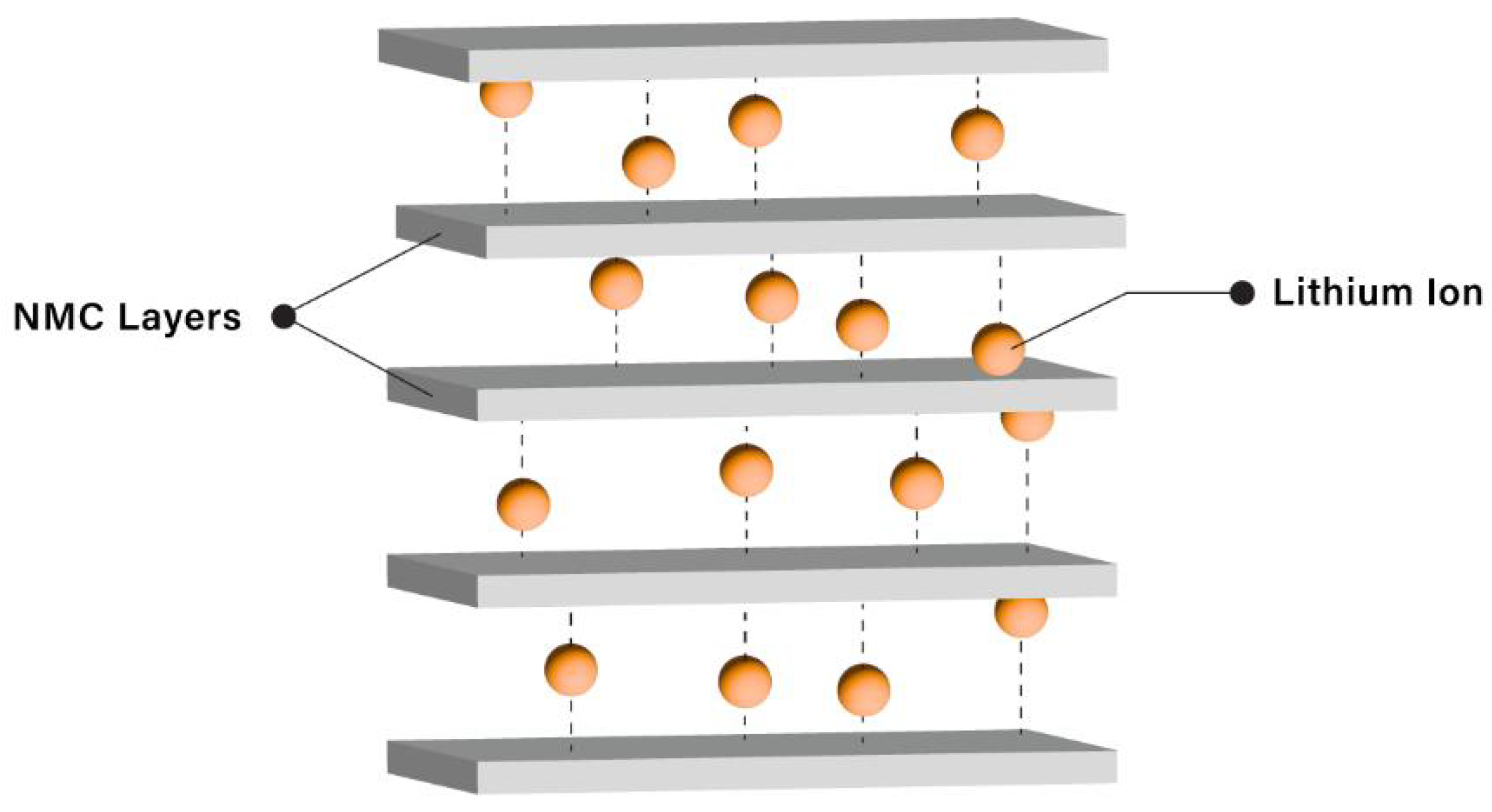
2.8. Lithium-Ion and NMC Cathode Materials
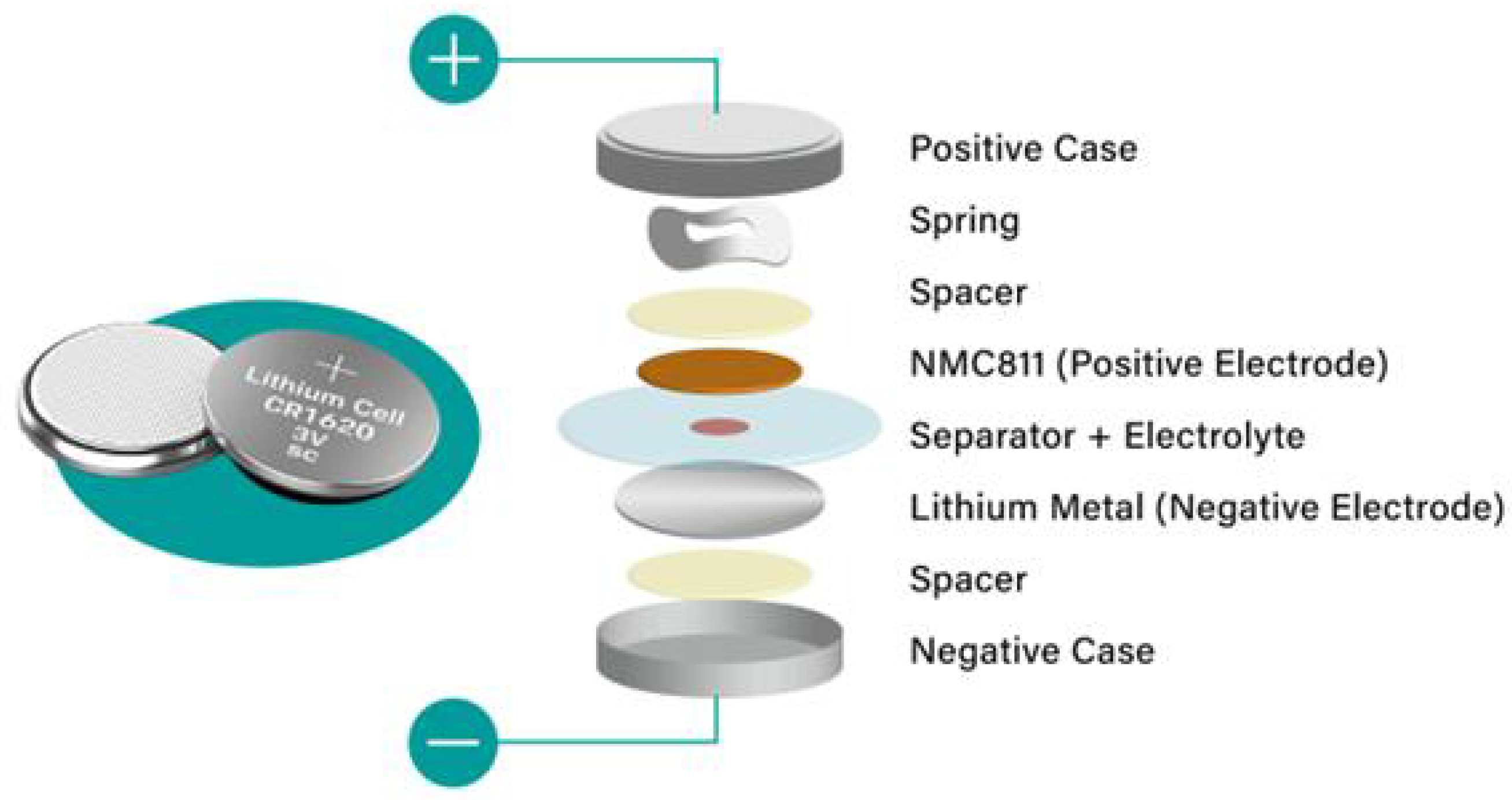
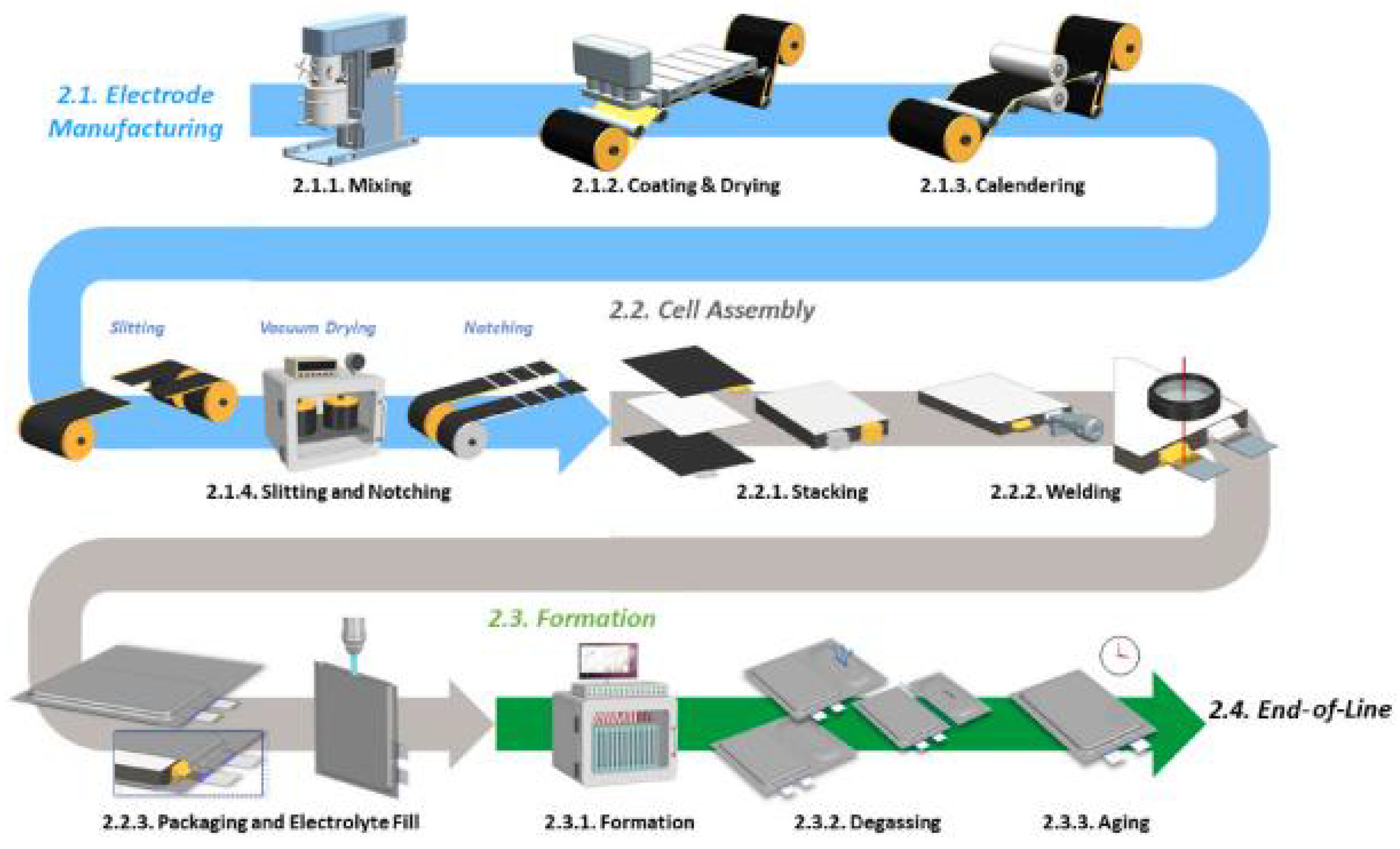
2.9. Cell Production Mechanism
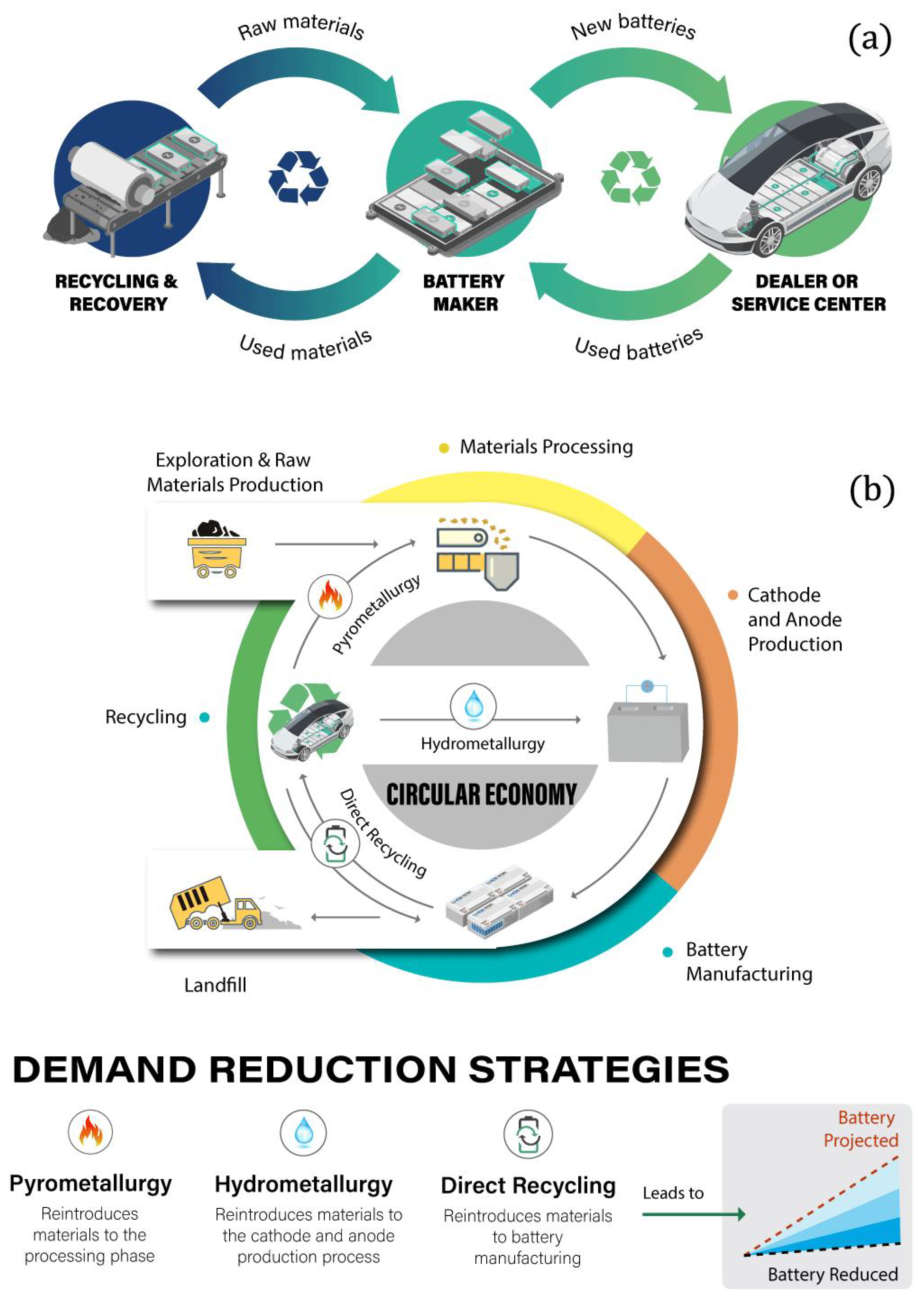
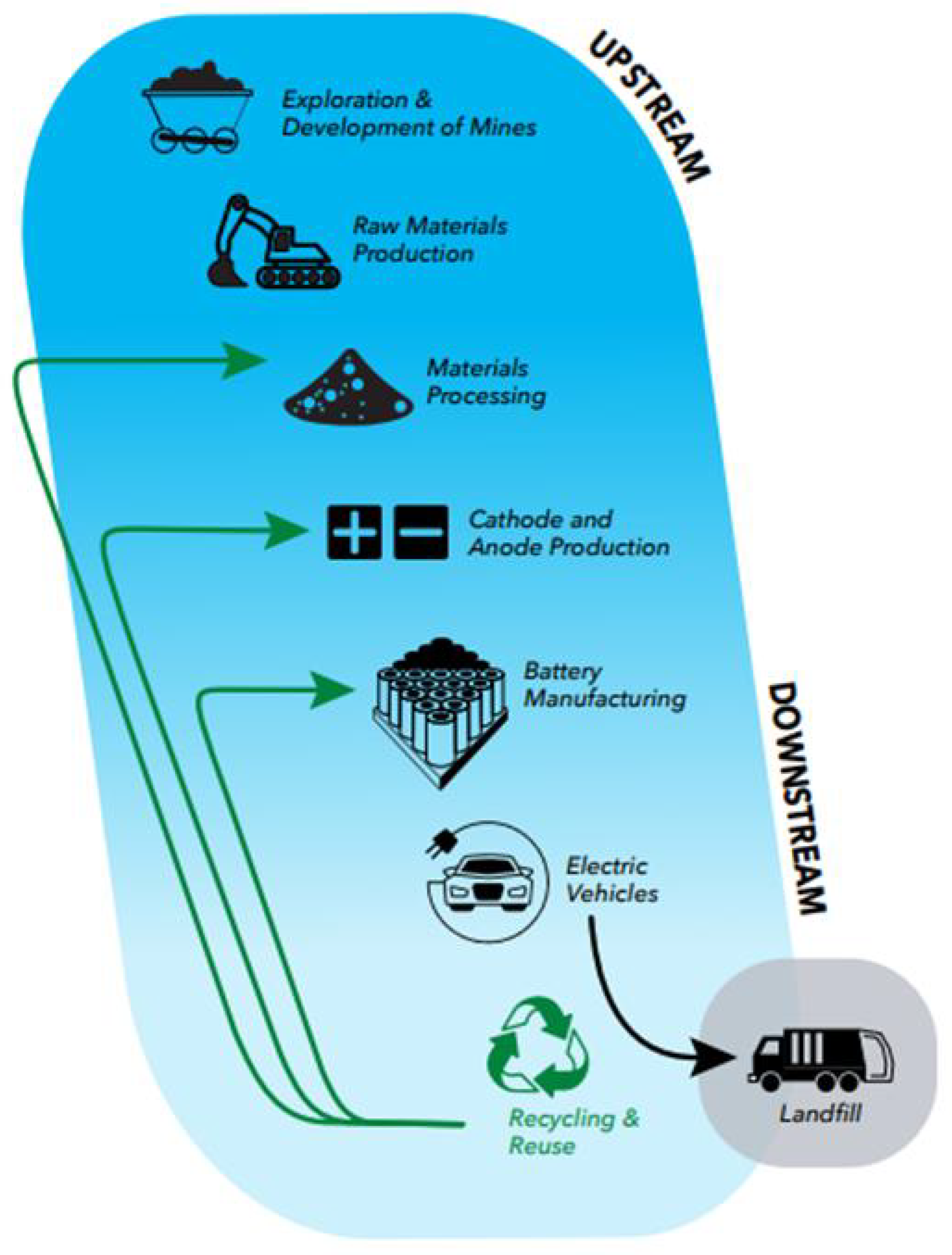
2.10. Zero Waste
2.11. Reusing/Recycling
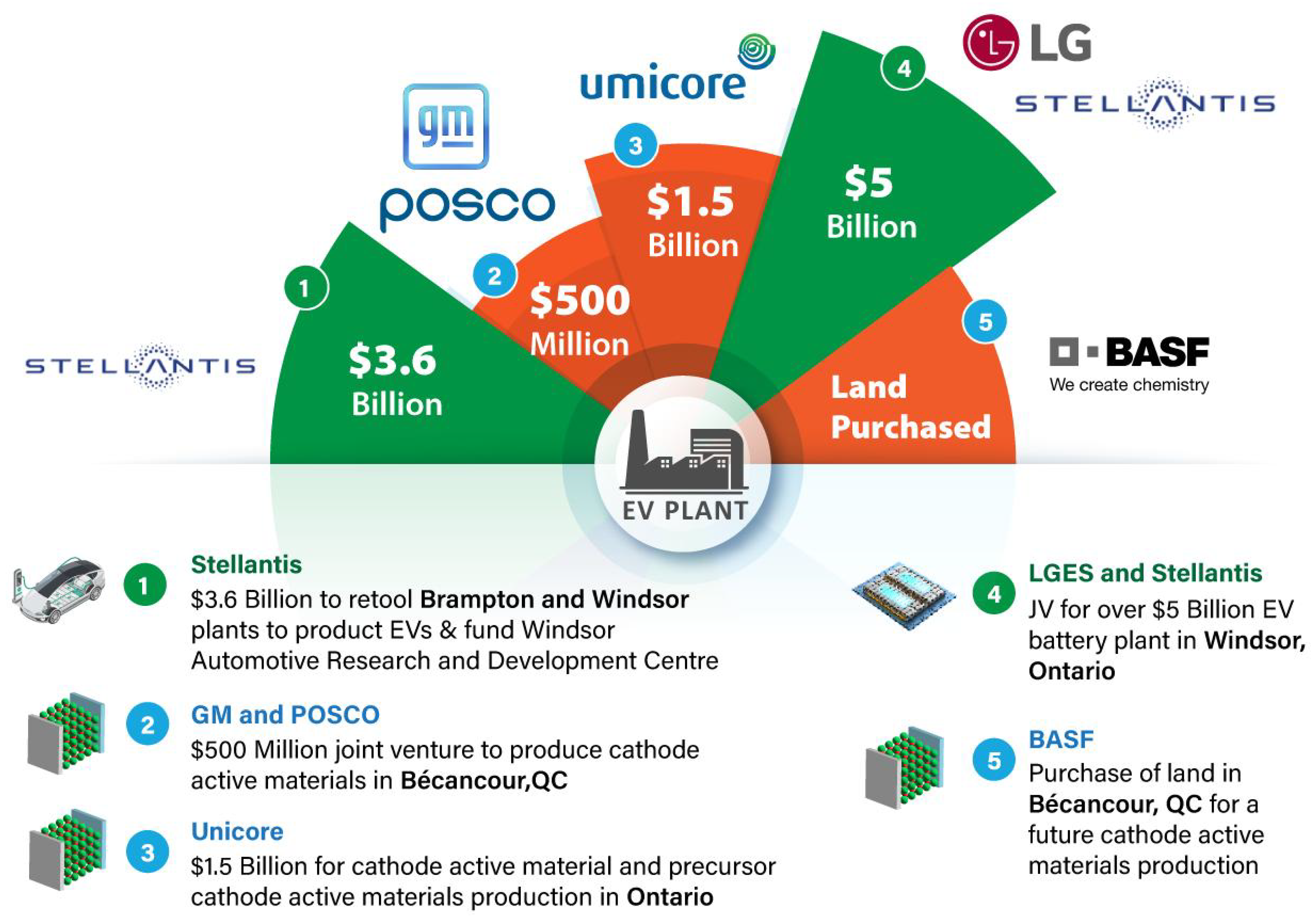
2.12. Creation of New Production Facilities
3. Current Environmental Data on the Production of NMC Cathodes
3.1. Regulatory Changes
3.2. Consumer Demand
3.3. Climate Change and Sustainability
3.4. Environmental Impacts of NMC Cathode Production
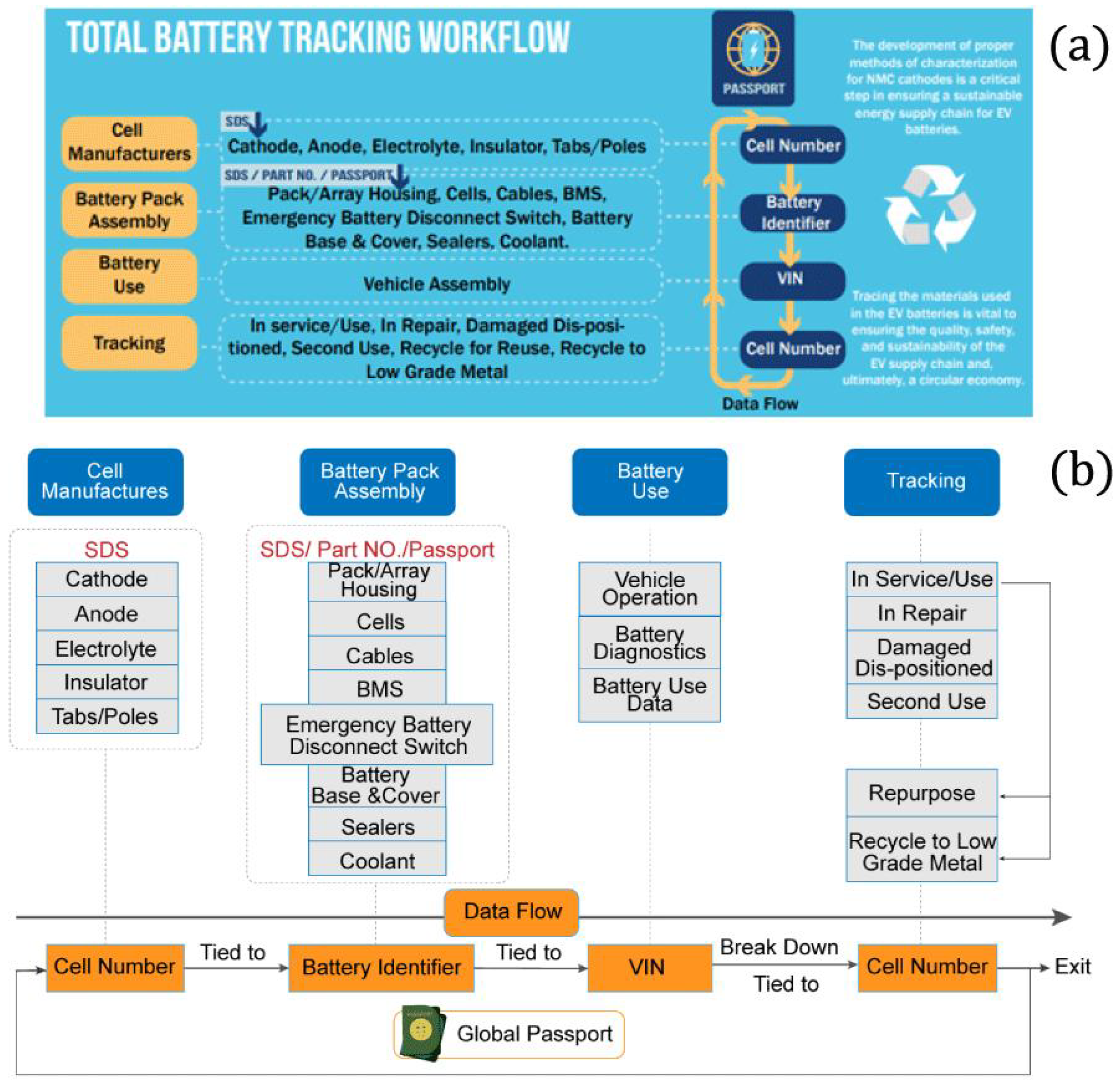
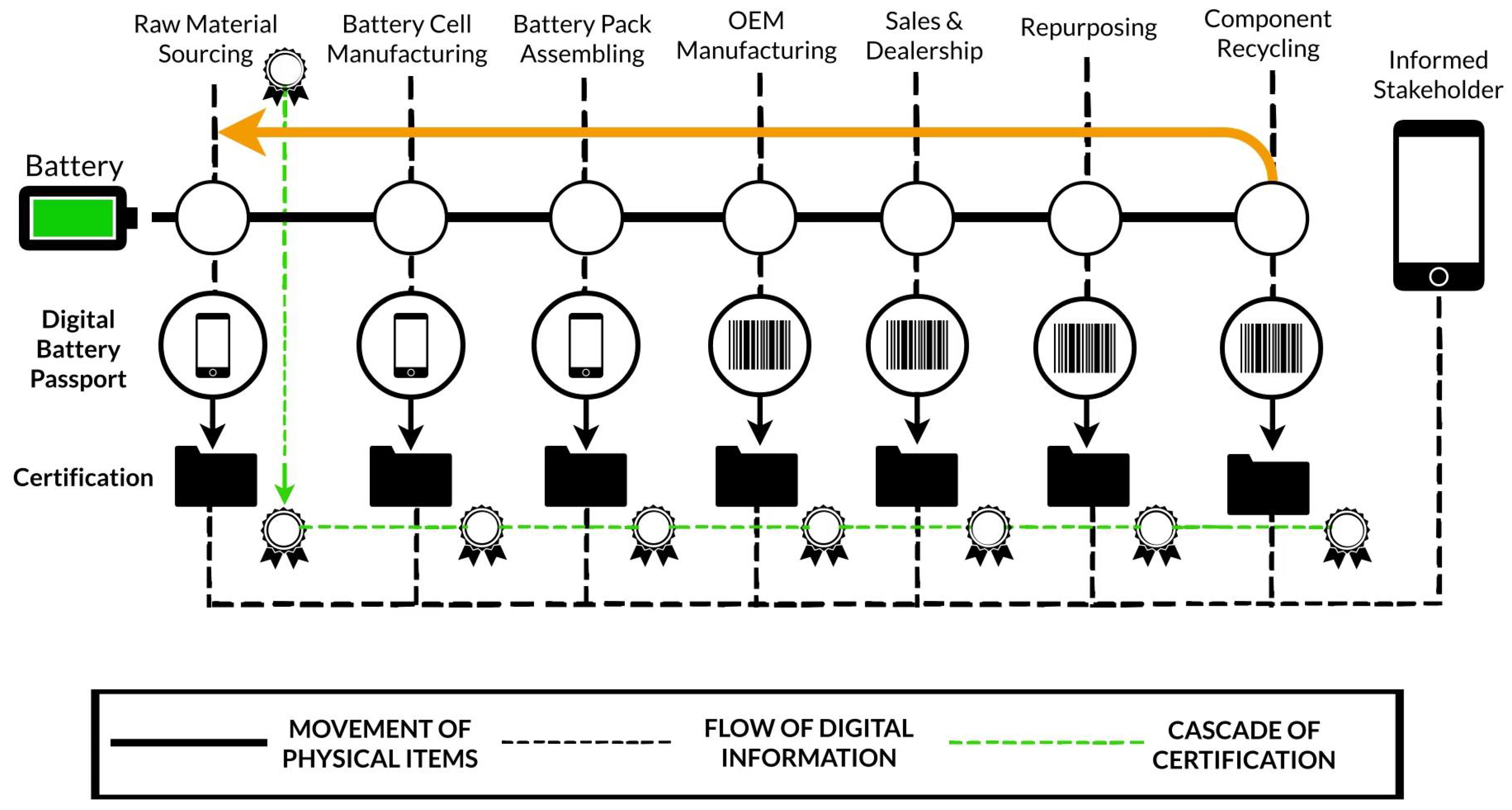
4. Battery Passport (Future Perspectives)
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Workshop on the Characterization and Quantification of Lithium, from the Micro- to the Nano-Scale, from Mining to Energy–Sciencesconf.org. Available online: https://cqlmns.sciencesconf.org/ (accessed on 9 February 2024).
- Global EV Outlook. 2020. Available online: https://www.iea.org/reports/global-ev-outlook-2020 (accessed on 21 October 2024).
- Baars, J. Introduction: Chronological Time and Chronological Age: Problems of Temporal Diversity. In Aging and Time; Routledge: London, UK, 2007. [Google Scholar]
- Fallah, N.; Fitzpatrick, C.; Killian, S.; Johnson, M. End-of-Life Electric Vehicle Battery Stock Estimation in Ireland through Integrated Energy and Circular Economy Modelling. Resour. Conserv. Recycl. 2021, 174, 105753. [Google Scholar] [CrossRef]
- Fallah, N.; Fitzpatrick, C. Is Shifting from Li-Ion NMC to LFP in EVs Beneficial for Second-Life Storages in Electricity Markets? J. Energy Storage 2023, 68, 107740. [Google Scholar] [CrossRef]
- Paris Declaration on Electro-Mobility and Climate Change & Call to Action. Available online: https://unfccc.int/media/521376/paris-electro-mobility-declaration.pdf (accessed on 17 October 2023).
- McGovern, M.E.; Bruder, D.D.; Huemiller, E.D.; Rinker, T.J.; Bracey, J.T.; Sekol, R.C.; Abell, J.A. A Review of Research Needs in Nondestructive Evaluation for Quality Verification in Electric Vehicle Lithium-Ion Battery Cell Manufacturing. J. Power Sources 2023, 561, 232742. [Google Scholar] [CrossRef]
- BloombergNEF. 2017. Available online: https://www.mining.com/web/move-tesla-china-holds-keys-electric-vehicles/ (accessed on 21 October 2024).
- Electric Vehicles and Hybrids Surpass 16% of Total 2023 U.S. Light-Duty Vehicle Sales–U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/todayinenergy/detail.php?id=61344 (accessed on 29 June 2024).
- Driving Electric Vehicles for Good|General Motors. Available online: https://www.gm.com/public/us/en/gm/home/stories/evs-for-good.html (accessed on 17 October 2023).
- U.S. Automakers to Say They Aspire to Up to 50% of EV Sales by 2030-Sources|Reuters. Available online: https://www.reuters.com/business/autos-transportation/us-automakers-say-they-aspire-up-50-ev-sales-by-2030-sources-2021-08-04/ (accessed on 17 October 2023).
- Electric Car Sales, 2016–2023—Charts–Data & Statistics. IEA. Available online: https://www.iea.org/data-and-statistics/charts/electric-car-sales-2016-2023 (accessed on 29 June 2024).
- Cimino, A.; Gnoni, M.G.; Longo, F.; La Rosa, A. Digital Twin (DT) Based Methodology to Support Effective Design of Industrial Production Lines. Procedia Comput. Sci. 2023, 217, 1896–1907. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Zaghib, K.; Groult, H. Comparative Issues of Cathode Materials for Li-Ion Batteries. Inorganics 2014, 2, 132–154. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef]
- Schwich, L.; Küpers, M.; Finsterbusch, M.; Schreiber, A.; Fattakhova-Rohlfing, D.; Guillon, O.; Friedrich, B. Recycling Strategies for Ceramic All-Solid-State Batteries—Part I: Study on Possible Treatments in Contrast to Li-Ion Battery Recycling. Metals 2020, 10, 1523. [Google Scholar] [CrossRef]
- Asare, M.O.; Afriyie, J.O. Ancient Mining and Metallurgy as the Origin of Cu, Ag, Pb, Hg, and Zn Contamination in Soils: A Review. Water Air Soil Pollut. 2021, 232, 240. [Google Scholar] [CrossRef]
- Burkin, A.R. Chemical Hydrometallurgy: Theory and Principles; Imperial College Press: London, UK; World Scientific Publishing Co.: Singapore, 2001. [Google Scholar] [CrossRef]
- Electrochemistry Encyclopedia—Pillars of Modern Electrochemistry: A Brief History. Available online: https://knowledge.electrochem.org/encycl/art-p05-pillars-of-ec.htm (accessed on 17 October 2023).
- Building America’s Clean Energy Future. Energy.gov. Available online: https://www.energy.gov/invest (accessed on 23 February 2024).
- Ballinger, B.; Stringer, M.; Schmeda-Lopez, D.R.; Kefford, B.; Parkinson, B.; Greig, C.; Smart, S. The Vulnerability of Electric Vehicle Deployment to Critical Mineral Supply. Appl. Energy 2019, 255, 113844. [Google Scholar] [CrossRef]
- Roelich, K.; Dawson, D.A.; Purnell, P.; Knoeri, C.; Revell, R.; Busch, J.; Steinberger, J.K. Assessing the Dynamic Material Criticality of Infrastructure Transitions: A Case of Low Carbon Electricity. Appl. Energy 2014, 123, 378–386. [Google Scholar] [CrossRef]
- Church, S.; Laske, N.; Leschiner, D.; Simmons, N.; Verhalen, S.; Willis, M. The Impact of the U.S. Inflation Reduction Act on Global Clean Energy Supply Chains. Available online: https://www.innovationnewsnetwork.com/the-impact-of-the-us-inflation-reduction-act-on-global-clean-energy-supply-chains/45302/ (accessed on 21 October 2024).
- Grimm, V.; Malmendier, U.; Schnitzer, M.; Truger, A.; Werding, M. The Inflation Reduction Act: Is the New U.S. Industrial Policy a Threat to Europe? Cons. Alemão Espec. Econômicos 2023, 1, 1–14. [Google Scholar]
- U.S. Department of the Treasury. Available online: https://home.treasury.gov/news/press-releases (accessed on 11 January 2024).
- Morris, C. Ford Signs Lithium and Nickel Deals to Secure IRA-Compliant Supply. Charged EVs. Available online: https://chargedevs.com/newswire/ford-signs-lithium-and-nickel-deals-to-secure-ira-compliant-supply/ (accessed on 19 September 2023).
- Guzman-Anaya, L. Challenges to Mexico’s Automotive Industry. The USMCA, COVID-19, and Electric Vehicle Production. In Japanese Cooperation and Supporting Industry in Mexico’s Automotive Sector: USMCA, Covid-19 Disruptions, and Electric Vehicle Production; Guzman-Anaya, L., Ed.; New Frontiers in Regional Science: Asian Perspectives; Springer Nature: Singapore, 2023; pp. 1–5. [Google Scholar] [CrossRef]
- Gorachinova, E.; Wolfe, D.A. New Path Development in a Semi-Peripheral Auto Region: The Case of Ontario. Econ. Geogr. 2023, 99, 526–547. [Google Scholar] [CrossRef]
- Praet, N.V. Quebec, Ottawa Offer Financial Aid for GM-Posco Battery Facility; The Globe and Mail Inc.: Toronto, ON, Canada, 2023; p. B1. [Google Scholar]
- Canada.ca. The Canadian Critical Minerals Strategy. Available online: https://www.canada.ca/en/campaign/critical-minerals-in-canada/canadian-critical-minerals-strategy.html (accessed on 17 October 2023).
- Anticipated Demand for Battery Minerals Creates Excitement in Quebec–ProQuest. Available online: https://www.proquest.com/openview/53ba0286ca180a3b15ab4b5b9f03d889/1?pq-origsite=gscholar&cbl=39 (accessed on 10 October 2023).
- What Are the Sources for Lithium? Available online: https://www.ibatterymetals.com/insights/what-are-the-sources-for-lithium (accessed on 3 October 2023).
- BASF and a GM-Posco Joint Venture Select Sites in Canada for Battery Materials. Chemical & Engineering News. Available online: https://cen.acs.org/energy/energy-storage-/BASF-GM-Posco-joint-venture/100/i10 (accessed on 19 September 2023).
- General Motors and POSCO Future M Welcome Québec and Canada Government Support For Bécancour CAM Project to Develop the EV Battery Supply Chain in North America. Available online: https://news.gm.ca/public/ca/en/gm/home/newsroom.detail.html/Pages/news/ca/en/2023/may/0529_posco-announcement.html (accessed on 23 February 2024).
- BASF Acquires Site for North American Battery Materials and Recycling Expansion in Canada. Available online: https://www.basf.com/ca/en/media/News-Releases/2022/basf-acquires-site-for-north-american-battery-materials-and-recy.html (accessed on 23 February 2024).
- Umicore Prepares to Construct Battery Materials Production Plant in Canada. Available online: https://www.umicore.ca/en/news/umicore-prepares-to-construct-battery-materials-production-plant-in-canada/ (accessed on 23 February 2024).
- Chen, Z.; Zhang, W.; Yang, Z. A Review on Cathode Materials for Advanced Lithium Ion Batteries: Microstructure Designs and Performance Regulations. Nanotechnology 2019, 31, 012001. [Google Scholar] [CrossRef] [PubMed]
- Lithium-Ion Battery Market Insight and Growth Projection, 2032. Allied Market Research. Available online: https://www.alliedmarketresearch.com/lithium-ion-battery-market (accessed on 10 October 2023).
- Khaleel, M.; Nassar, Y.; El-Khozondar, H.J.; Elmnifi, M.; Rajab, Z.; Yaghoubi, E.; Yaghoubi, E. Electric Vehicles in China, Europe, and the United States: Current Trend and Market Comparison. Int. J. Electr. Eng. Sustain. 2024, 2, 1–20. [Google Scholar]
- Pereira, P.M.P. Equity Research–Tesla Inc. Master’s Thesis, Universidade de Lisboa, Lisboa, Portugal, 2024. Available online: https://repositorium.sdum.uminho.pt/handle/1822/88250 (accessed on 5 February 2024).
- Barman, P.; Dutta, L.; Azzopardi, B. Electric Vehicle Battery Supply Chain and Critical Materials: A Brief Survey of State of the Art. Energies 2023, 16, 3369. [Google Scholar] [CrossRef]
- Schulz-Mönninghoff, M.; Neidhardt, M.; Niero, M. What Is the Contribution of Different Business Processes to Material Circularity at Company-Level? A Case Study for Electric Vehicle Batteries. J. Clean. Prod. 2023, 382, 135232. [Google Scholar] [CrossRef]
- Kampker, A.; Heimes, H.H.; Offermanns, C.; Vienenkötter, J.; Robben, T. Framework and Classification of Battery System Architectures. World Electr. Veh. J. 2023, 14, 88. [Google Scholar] [CrossRef]
- Diouf, B.; Pode, R. Potential of Lithium-Ion Batteries in Renewable Energy. Renew. Energy 2015, 76, 375–380. [Google Scholar] [CrossRef]
- Xiao, J.; Shi, F.; Glossmann, T.; Burnett, C.; Liu, Z. From Laboratory Innovations to Materials Manufacturing for Lithium-Based Batteries. Nat. Energy 2023, 8, 329–339. [Google Scholar] [CrossRef]
- Naz, B. Proposal of New Solid-State Battery Denser and Safer. 2024. Available online: https://www.onlinescientificresearch.com/articles/proposal-of-new-solidstate-battery-denser-and-safer.html (accessed on 21 October 2024).
- Malik, M.; Chan, K.H.; Azimi, G. Review on the Synthesis of LiNixMnyCo1-x-yO2 (NMC) Cathodes for Lithium-Ion Batteries. Mater. Today Energy 2022, 28, 101066. [Google Scholar] [CrossRef]
- Chu, B.; Guo, Y.-J.; Shi, J.-L.; Yin, Y.-X.; Huang, T.; Su, H.; Yu, A.; Guo, Y.-G.; Li, Y. Cobalt in High-Energy-Density Layered Cathode Materials for Lithium Ion Batteries. J. Power Sources 2022, 544, 231873. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Xiao, B.; Liu, P.; Huang, T.; Li, Y.; Ren, X.; Zhang, Q.; Liu, J.; Ouyang, X.; et al. Challenges and Approaches of Single-Crystal Ni-Rich Layered Cathodes in Lithium Batteries. Natl. Sci. Rev. 2023, 10, nwad252. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, Z.; Norouzi Banis, M.; Deng, S.; Zhao, Y.; Sun, Q.; Wang, C.; Yang, X.; Li, W.; Liang, J.; et al. Unravelling the Chemistry and Microstructure Evolution of a Cathodic Interface in Sulfide-Based All-Solid-State Li-Ion Batteries. ACS Energy Lett. 2019, 4, 2480–2488. [Google Scholar] [CrossRef]
- Kiemel, S.; Glöser-Chahoud, S.; Waltersmann, L.; Schutzbach, M.; Sauer, A.; Miehe, R. Assessing the Application-Specific Substitutability of Lithium-Ion Battery Cathode Chemistries Based on Material Criticality, Performance, and Price. Resources 2021, 10, 87. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-Ion Batteries: Current Status and Future Perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Stephan, A.K. A Pathway to Understand NMC Cathodes. Joule 2020, 4, 1632–1633. [Google Scholar] [CrossRef]
- Cheng, C.; Tan, L.; Liu, H.; Huang, X. High Rate Performances of the Cathode Material LiNi1/3Co1/3Mn1/3O2 Synthesized Using Low Temperature Hydroxide Precipitation. Mater. Res. Bull. 2011, 46, 2032–2035. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, F.; Liu, B.; Zhou, H.; Zhang, Q.; Kong, J.; Wang, Q. Progress in Preparation and Modification of LiNi0.6Mn0.2Co0.2O2 Cathode Material for High Energy Density Li-Ion Batteries. Int. J. Electrochem. 2018, 2018, e6930386. [Google Scholar] [CrossRef]
- Jung, R.; Metzger, M.; Maglia, F.; Stinner, C.; Gasteiger, H.A. Oxygen Release and Its Effect on the Cycling Stability of LiNixMnyCozO2 (NMC) Cathode Materials for Li-Ion Batteries. J. Electrochem. Soc. 2017, 164, A1361. [Google Scholar] [CrossRef]
- Jones, B.; Nguyen-Tien, V.; Elliott, R.J.R. The Electric Vehicle Revolution: Critical Material Supply Chains, Trade and Development. World Econ. 2023, 46, 2–26. [Google Scholar] [CrossRef]
- Nagmani; Pahari, D.; Tyagi, A.; Puravankara, D.S. Lithium-Ion Battery Technologies for Electric Mobility–State-of-the-Art Scenario. ARAI J. Mobil. Technol. 2022, 2, 233–248. [Google Scholar] [CrossRef]
- Wood, D.L.; Li, J.; An, S.J. Formation Challenges of Lithium-Ion Battery Manufacturing. Joule 2019, 3, 2884–2888. [Google Scholar] [CrossRef]
- International Council on Clean Transportation, icct. Available online: https://theicct.org/ (accessed on 11 January 2024).
- Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation Mechanisms and Mitigation Strategies of Nickel-Rich NMC-Based Lithium-Ion Batteries. Electrochem. Energy Rev. 2020, 3, 43–80. [Google Scholar] [CrossRef]
- Nie, K.; Hong, Y.; Qiu, J.; Li, Q.; Yu, X.; Li, H.; Chen, L. Interfaces Between Cathode and Electrolyte in Solid State Lithium Batteries: Challenges and Perspectives. Front. Chem. 2018, 6, 616. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wu, Y.; Wei, G.; Qiu, S.; Qu, J. Enhanced Electrochemical Performance of LiNi0.8Co0.1Mn0.1O2 Cathode via Wet-Chemical Coating of MgO. J. Solid State Electrochem. 2019, 23, 2213–2224. [Google Scholar] [CrossRef]
- Iriyama, Y.; Kurita, H.; Yamada, I.; Abe, T.; Ogumi, Z. Effects of Surface Modification by MgO on Interfacial Reactions of Lithium Cobalt Oxide Thin Film Electrode. J. Power Sources 2004, 137, 111–116. [Google Scholar] [CrossRef]
- Li, S.; Chai, Z.; Wang, Z.; Tai, C.-W.; Zhu, J.; Edström, K.; Ma, Y. A Multiscale, Dynamic Elucidation of Li Solubility in the Alloy and Metallic Plating Process. Adv. Mater. 2023, 35, 2306826. [Google Scholar] [CrossRef]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A Review on the Key Issues for Lithium-Ion Battery Management in Electric Vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Zuo, D.; Tian, G.; Li, X.; Chen, D.; Shu, K. Recent Progress in Surface Coating of Cathode Materials for Lithium Ion Secondary Batteries. J. Alloys Compd. 2017, 706, 24–40. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, Y. Recent Advances in the Research of Polyanion-Type Cathode Materials for Li-Ion Batteries. Energy Environ. Sci. 2011, 4, 3223–3242. [Google Scholar] [CrossRef]
- Hu, M.; Pang, X.; Zhou, Z. Recent Progress in High-Voltage Lithium Ion Batteries. J. Power Sources 2013, 237, 229–242. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.P.; Fu, L.J.; Liu, H.; Wu, Y.P.; Rahm, E.; Holze, R.; Wu, H.Q. Cathode Materials Modified by Surface Coating for Lithium Ion Batteries. Electrochim. Acta 2006, 51, 3872–3883. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0 < x ≤ 1): A new cathode material for batteries of high energy density. Solid State Ion. 1981, 3–4, 171–174. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, X.; Xia, S.; Zhang, K.; Wei, C.; Bak, S.; Shadike, Z.; Liu, X.; Yang, Y.; Xu, R.; et al. High-Voltage Charging-Induced Strain, Heterogeneity, and Micro-Cracks in Secondary Particles of a Nickel-Rich Layered Cathode Material. Adv. Funct. Mater. 2019, 29, 1900247. [Google Scholar] [CrossRef]
- Patry, G.; Romagny, A.; Martinet, S.; Froelich, D. Cost Modeling of Lithium-Ion Battery Cells for Automotive Applications. Energy Sci. Eng. 2015, 3, 71–82. [Google Scholar] [CrossRef]
- Li, M.; Lu, J. Cobalt in Lithium-Ion Batteries. Science 2020, 367, 979–980. [Google Scholar] [CrossRef]
- BatPaC Model Software|Argonne National Laboratory (ANL). 2024. Available online: https://www.anl.gov/cse/batpac-model-software (accessed on 11 January 2024).
- Chae, C.; Park, H.; Kim, D.; Kim, J.; Oh, E.-S.; Lee, J.K. A Li-Ion Battery Using LiMn2O4 Cathode and MnOx/C Anode. J. Power Sources 2013, 244, 214–221. [Google Scholar] [CrossRef]
- Kaur, G.; Gates, B.D. Review—Surface Coatings for Cathodes in Lithium Ion Batteries: From Crystal Structures to Electrochemical Performance. J. Electrochem. Soc. 2022, 169, 043504. [Google Scholar] [CrossRef]
- Ahmed, S.; Nelson, P.A.; Gallagher, K.G.; Susarla, N.; Dees, D.W. Cost and Energy Demand of Producing Nickel Manganese Cobalt Cathode Material for Lithium Ion Batteries. J. Power Sources 2017, 342, 733–740. [Google Scholar] [CrossRef]
- Howell, D. Overview of the DOE Advanced Battery R&D Program; Hybrid Electric Systems Vehicle Technologies Office: Washington, DC, USA, 2014. [Google Scholar]
- Neubauer, J.; Pesaran, A.; Bae, C.; Elder, R.; Cunningham, B. Updating United States Advanced Battery Consortium and Department of Energy Battery Technology Targets for Battery Electric Vehicles. J. Power Sources 2014, 271, 614–621. [Google Scholar] [CrossRef]
- Kraft, L.; Zünd, T.; Schreiner, D.; Wilhelm, R.; Günter, F.J.; Reinhart, G.; Gasteiger, H.A.; Jossen, A. Comparative Evaluation of LMR-NCM and NCA Cathode Active Materials in Multilayer Lithium-Ion Pouch Cells: Part II. Rate Capability, Long-Term Stability, and Thermal Behavior. J. Electrochem. Soc. 2021, 168, 020537. [Google Scholar] [CrossRef]
- Lee, K.K.; Yoon, W.S.; Kim, K.B.; Lee, K.Y.; Hong, S.T. Characterization of LiNi0.85Co0.10M0.05O2 (M = Al, Fe) as a Cathode Material for Lithium Secondary Batteries. J. Power Sources 2001, 97–98, 308–312. [Google Scholar] [CrossRef]
- Gutsch, M.; Leker, J. Costs, Carbon Footprint, and Environmental Impacts of Lithium-Ion Batteries–From Cathode Active Material Synthesis to Cell Manufacturing and Recycling. Appl. Energy 2024, 353, 122132. [Google Scholar] [CrossRef]
- Purwanto, A.; Ikhsanudin, M.N.; Asri, P.P.P.; Giasari, A.S.; Hakam, M.; Yudha, C.S.; Widiyandari, H.; Dyartanti, E.R.; Jumari, A.; Nur, A. The Optimization of Nickel-Rich Cathode-Material Production on a Pilot Plant Scale. Processes 2024, 12, 685. [Google Scholar] [CrossRef]
- Ziegler, M.S.; Song, J.; Trancik, J.E. Determinants of Lithium-Ion Battery Technology Cost Decline. Energy Environ. Sci. 2021, 14, 6074–6098. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, H.; Lin, F. Sustainable Electric Vehicle Batteries for a Sustainable World: Perspectives on Battery Cathodes, Environment, Supply Chain, Manufacturing, Life Cycle, and Policy. Adv. Energy Mater. 2022, 12, 2200383. [Google Scholar] [CrossRef]
- Hawley, W.B.; Li, M.; Li, J. Room-Temperature Eutectic Synthesis for Upcycling of Cathode Materials. Batteries 2023, 9, 498. [Google Scholar] [CrossRef]
- Nisa, S.S.; Rahmawati, M.; Yudha, C.S.; Nilasary, H.; Nursukatmo, H.; Oktaviano, H.S.; Muzayanha, S.U.; Purwanto, A. A Fast Approach to Obtain Layered Transition-Metal Cathode Material for Rechargeable Batteries. Batteries 2022, 8, 4. [Google Scholar] [CrossRef]
- Tahmasebi, M.H.; Zheng, L.; Hatchard, T.D.; Obrovac, M.N. Li[Ni0.6Mn0.2Co0.2]O2 Made From Crystalline Rock Salt Oxide Precursors. J. Electrochem. Soc. 2023, 170, 030531. [Google Scholar] [CrossRef]
- Berry, C. The Paradox of Green Growth: Challenges and Opportunities in Decarbonizing the Lithium-Ion Supply Chain. In Critical Minerals, the Climate Crisis and the Tech Imperium; Kalantzakos, S., Ed.; Archimedes; Springer Nature: Cham, Switzerland, 2023; pp. 107–123. [Google Scholar] [CrossRef]
- Schöberl, J.; Ank, M.; Schreiber, M.; Wassiliadis, N.; Lienkamp, M. Thermal Runaway Propagation in Automotive Lithium-Ion Batteries with NMC-811 and LFP Cathodes: Safety Requirements and Impact on System Integration. eTransportation 2024, 19, 100305. [Google Scholar] [CrossRef]
- Greenwood, M.; Wentker, M.; Leker, J. A Region-Specific Raw Material and Lithium-Ion Battery Criticality Methodology with an Assessment of NMC Cathode Technology. Appl. Energy 2021, 302, 117512. [Google Scholar] [CrossRef]
- Stallard, J.C.; Wheatcroft, L.; Booth, S.G.; Boston, R.; Corr, S.A.; De Volder, M.F.L.; Inkson, B.J.; Fleck, N.A. Mechanical Properties of Cathode Materials for Lithium-Ion Batteries. Joule 2022, 6, 984–1007. [Google Scholar] [CrossRef]
- Celeste, A.; Tuccillo, M.; Menon, A.S.; Brant, W.; Brandell, D.; Pellegrini, V.; Brescia, R.; Silvestri, L.; Brutti, S. On the Elusive Crystallography of Lithium-Rich Layered Oxides: Novel Structural Models. Small Methods 2024, 8, 2301466. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, Z.; Wang, Q.; Zhang, Y.; Ma, X.; Shen, C.; Xu, D.; Liu, J.; Liu, Y.; Gionet, P.; et al. Closed Loop Recycling of Electric Vehicle Batteries to Enable Ultra-High Quality Cathode Powder. Sci. Rep. 2019, 9, 1654. [Google Scholar] [CrossRef]
- Kaboli, S.; Demers, H.; Paolella, A.; Darwiche, A.; Dontigny, M.; Clément, D.; Guerfi, A.; Trudeau, M.L.; Goodenough, J.B.; Zaghib, K. Behavior of Solid Electrolyte in Li-Polymer Battery with NMC Cathode via in-Situ Scanning Electron Microscopy. Nano Lett. 2020, 20, 1607–1613. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.-C.; Zhou, H.; Guo, S. Wide-Temperature-Range Sodium-Metal Batteries: From Fundamentals and Obstacles to Optimization. Energy Environ. Sci. 2023, 16, 4759–4811. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Wade, A.; Tan, C.; Parker, J.E.; Matras, D.; Leach, A.S.; Robinson, J.B.; Llewellyn, A.; Dimitrijevic, A.; Jervis, R.; et al. Identifying the Origins of Microstructural Defects Such as Cracking within Ni-Rich NMC811 Cathode Particles for Lithium-Ion Batteries. Adv. Energy Mater. 2020, 10, 2002655. [Google Scholar] [CrossRef]
- Zhao, E.; Fang, L.; Chen, M.; Chen, D.; Huang, Q.; Hu, Z.; Yan, Q.; Wu, M.; Xiao, X. New Insight into Li/Ni Disorder in Layered Cathode Materials for Lithium Ion Batteries: A Joint Study of Neutron Diffraction, Electrochemical Kinetic Analysis and First-Principles Calculations. J. Mater. Chem. A 2017, 5, 1679–1686. [Google Scholar] [CrossRef]
- McClelland, I.; Booth, S.G.; Anthonisamy, N.N.; Middlemiss, L.A.; Pérez, G.E.; Cussen, E.J.; Baker, P.J.; Cussen, S.A. Direct Observation of Dynamic Lithium Diffusion Behavior in Nickel-Rich, LiNi0.8Mn0.1Co0.1O2 (NMC811) Cathodes Using Operando Muon Spectroscopy. Chem. Mater. 2023, 35, 4149–4158. [Google Scholar] [CrossRef]
- Mayyas, A.; Moawad, K.; Chadly, A.; Alhseinat, E. Can Circular Economy and Cathode Chemistry Evolution Stabilize the Supply Chain of Li-Ion Batteries? Extr. Ind. Soc. 2023, 14, 101253. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Ohzuku, T. Novel Lithium Insertion Material of LiCo1/3Ni1/3Mn1/3O2 for Advanced Lithium-Ion Batteries. J. Power Sources 2003, 119–121, 171–174. [Google Scholar] [CrossRef]
- Tiozzo, A.; Ghaseminezhad, K.; Mazzucco, A.; Giuliano, M.; Rocca, R.; Dotoli, M.; Nicol, G.; Nervi, C.; Baricco, M.; Sgroi, M.F. Investigating the Influence of Three Different Atmospheric Conditions during the Synthesis Process of NMC811 Cathode Material. Crystals 2024, 14, 137. [Google Scholar] [CrossRef]
- White, J.L.; Gittleson, F.S.; Homer, M.; El Gabaly, F. Nickel and Cobalt Oxidation State Evolution at Ni-Rich NMC Cathode Surfaces during Treatment. J. Phys. Chem. C 2020, 124, 16508–16514. [Google Scholar] [CrossRef]
- Aslam, M.K.; Niu, Y.; Hussain, T.; Tabassum, H.; Tang, W.; Xu, M.; Ahuja, R. How to Avoid Dendrite Formation in Metal Batteries: Innovative Strategies for Dendrite Suppression. Nano Energy 2021, 86, 106142. [Google Scholar] [CrossRef]
- Wentker, M.; Greenwood, M.; Leker, J. A Bottom-Up Approach to Lithium-Ion Battery Cost Modeling with a Focus on Cathode Active Materials. Energies 2019, 12, 504. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Q.; Wu, B.; Tan, S.; Shadike, Z.; Bi, Y.; Whittingham, M.S.; Xiao, J.; Yang, X.-Q.; Hu, E. Fundamental Linkage Between Structure, Electrochemical Properties, and Chemical Compositions of LiNi1–x–yMnxCoyO2 Cathode Materials. ACS Appl. Mater. Interfaces 2021, 13, 2622–2629. [Google Scholar] [CrossRef]
- Garcia, J.C.; Bareño, J.; Yan, J.; Chen, G.; Hauser, A.; Croy, J.R.; Iddir, H. Surface Structure, Morphology, and Stability of Li(Ni1/3Mn1/3Co1/3)O2 Cathode Material. J. Phys. Chem. C 2017, 121, 8290–8299. [Google Scholar] [CrossRef]
- Para, M.L.; Querio, A.; Amici, J.; Versaci, D.; Barresi, A.A.; Bodoardo, S.; Marchisio, D. Electrochemical Performance Optimization of NMC811 through the Structure Design of Its Precursor. J. Electroanal. Chem. 2023, 943, 117630. [Google Scholar] [CrossRef]
- Deng, D. Li-Ion Batteries: Basics, Progress, and Challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Zou, P.; Lin, R.; Ma, L.; Li, T.; Hwang, I.; Xu, W.; Sun, C.; Trask, S.; et al. Long-Life Lithium-Ion Batteries Realized by Low-Ni, Co-Free Cathode Chemistry. Nat. Energy 2023, 8, 695–702. [Google Scholar] [CrossRef]
- Moores, S. The Global Battery Arms Race: Lithium-ion Battery Gigafactories and Their Supply Chain. Oxf. Energy Forum 2021, 126, 26–30. [Google Scholar]
- Prina Cerai, A. Geography of Control: A Deep Dive Assessment on Criticality and Lithium Supply Chain. Miner. Econ. 2024, 37, 499–546. [Google Scholar] [CrossRef]
- Trost, J.N.; Dunn, J.B. Assessing the Feasibility of the Inflation Reduction Act’s EV Critical Mineral Targets. Nat. Sustain. 2023, 6, 639–643. [Google Scholar] [CrossRef]
- Gao, T.; Fan, N.; Chen, W.; Dai, T. Lithium Extraction from Hard Rock Lithium Ores (Spodumene, Lepidolite, Zinnwaldite, Petalite): Technology, Resources, Environment and Cost. China Geol. 2023, 6, 137–153. [Google Scholar] [CrossRef]
- Natural Resources Canada. Lithium Facts. Available online: https://natural-resources.canada.ca/our-natural-resources/minerals-mining/minerals-metals-facts/lithium-facts/24009 (accessed on 2 October 2023).
- Song, Y.; Zhao, T.; He, L.; Zhao, Z.; Liu, X. A Promising Approach for Directly Extracting Lithium from α-Spodumene by Alkaline Digestion and Precipitation as Phosphate. Hydrometallurgy 2019, 189, 105141. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2020; U.S. Geological Survey: Reston, VA, USA, 2020. [Google Scholar] [CrossRef]
- Flexer, V.; Baspineiro, C.F.; Galli, C.I. Lithium Recovery from Brines: A Vital Raw Material for Green Energies with a Potential Environmental Impact in Its Mining and Processing. Sci. Total Environ. 2018, 639, 1188–1204. [Google Scholar] [CrossRef]
- Vera, M.L.; Torres, W.R.; Galli, C.I.; Chagnes, A.; Flexer, V. Environmental Impact of Direct Lithium Extraction from Brines. Nat. Rev. Earth Environ. 2023, 4, 149–165. [Google Scholar] [CrossRef]
- South America’s “Lithium Fields” Reveal the Dark Side of Electric Cars. Euronews. Available online: https://www.euronews.com/green/2022/02/01/south-america-s-lithium-fields-reveal-the-dark-side-of-our-electric-future (accessed on 3 October 2023).
- Al-Jawad, J.; Ford, J.; Petavratzi, E.; Hughes, A. Understanding the Spatial Variation in Lithium Concentration of High Andean Salars Using Diagnostic Factors. Sci. Total Environ. 2024, 906, 167647. [Google Scholar] [CrossRef]
- Munk, L.A.; Hynek, S.A.; Bradley, D.C.; Boutt, D.; Labay, K.; Jochens, H. Lithium Brines: A Global Perspective. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2016; Volume 18. [Google Scholar] [CrossRef]
- Kim, T.Y.; Gould, T.; Bennet, S.; Briens, F.; Dasgupta, A.; Gonzales, P. The Role of Critical Minerals in Clean Energy Transitions; International Energy Agency: Washington, DC, USA, 2021. [Google Scholar]
- Liu, C.; Li, Y.; Lin, D.; Hsu, P.-C.; Liu, B.; Yan, G.; Wu, T.; Cui, Y.; Chu, S. Lithium Extraction from Seawater through Pulsed Electrochemical Intercalation. Joule 2020, 4, 1459–1469. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Liu, Y.; Luo, C.; Wang, C. Comparison of Electrochemical Performances of Olivine NaFePO4 in Sodium-Ion Batteries and Olivine LiFePO4 in Lithium-Ion Batteries. Nanoscale 2013, 5, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.; Engel, H.; Eitan, R.; Aurbach, D.; Levi, M.D.; Kosa, M.; Major, D.T. Classical and Quantum Modeling of Li and Na Diffusion in FePO4. J. Phys. Chem. C 2015, 119, 15801–15809. [Google Scholar] [CrossRef]
- Yu, J.; Fang, D.; Zhang, H.; Leong, Z.Y.; Zhang, J.; Li, X.; Yang, H.Y. Ocean Mining: A Fluidic Electrochemical Route for Lithium Extraction from Seawater. ACS Mater. Lett. 2020, 2, 1662–1668. [Google Scholar] [CrossRef]
- Anticipated Demand for Battery Minerals Creates Excitement in Québec|E & MJ. Available online: https://www.e-mj.com/news/australia-and-oceania/anticipated-demand-for-battery-minerals-creates-excitement-in-quebec/ (accessed on 12 October 2023).
- Ibarra-Gutiérrez, S.; Bouchard, J.; Laflamme, M.; Fytas, K. Assessing the Potential of Quebec Lithium Industry: Mineral Reserves, Lithium-Ion Batteries Production and Greenhouse Gas Emissions. Resour. Policy 2021, 74, 102371. [Google Scholar] [CrossRef]
- Campagnol, N.; Hoffman, K.; Lala, A.; Ramsbottom, O. The Future of Nickel: A Class Act; Basic Materials November 2017; McKinsey & Company: Chicago, IL, USA, 20 November 2017. [Google Scholar]
- Nickel Statistics and Information|U.S. Geological Survey. Available online: https://www.usgs.gov/centers/national-minerals-information-center/nickel-statistics-and-information (accessed on 8 February 2024).
- Carmen. The Five Largest Nickel Mines in Operation in Canada. Mining Technology. Available online: https://www.mining-technology.com/marketdata/five-largest-nickel-mines-canada/ (accessed on 8 February 2024).
- Natural Resources Canada. Nickel Facts. Available online: https://natural-resources.canada.ca/our-natural-resources/minerals-mining/minerals-metals-facts/nickel-facts/20519 (accessed on 8 February 2024).
- Ev-Battery-Supply-Chains-Report.Pdf. Available online: https://www.nrdc.org/sites/default/files/2023-07/ev-battery-supply-chains-report.pdf (accessed on 2 October 2023).
- Kelly, J.C.; Dai, Q.; Wang, M. Globally Regional Life Cycle Analysis of Automotive Lithium-Ion Nickel Manganese Cobalt Batteries. Mitig. Adapt. Strateg. Glob. Change 2020, 25, 371–396. [Google Scholar] [CrossRef]
- Clarke, C.; Upson, S. A Global Portrait of the Manganese Industry—A Socioeconomic Perspective. NeuroToxicology 2017, 58, 173–179. [Google Scholar] [CrossRef]
- Winjobi, O.; Kelly, J.C.; Dai, Q. Life-Cycle Analysis, by Global Region, of Automotive Lithium-Ion Nickel Manganese Cobalt Batteries of Varying Nickel Content. Sustain. Mater. Technol. 2022, 32, e00415. [Google Scholar] [CrossRef]
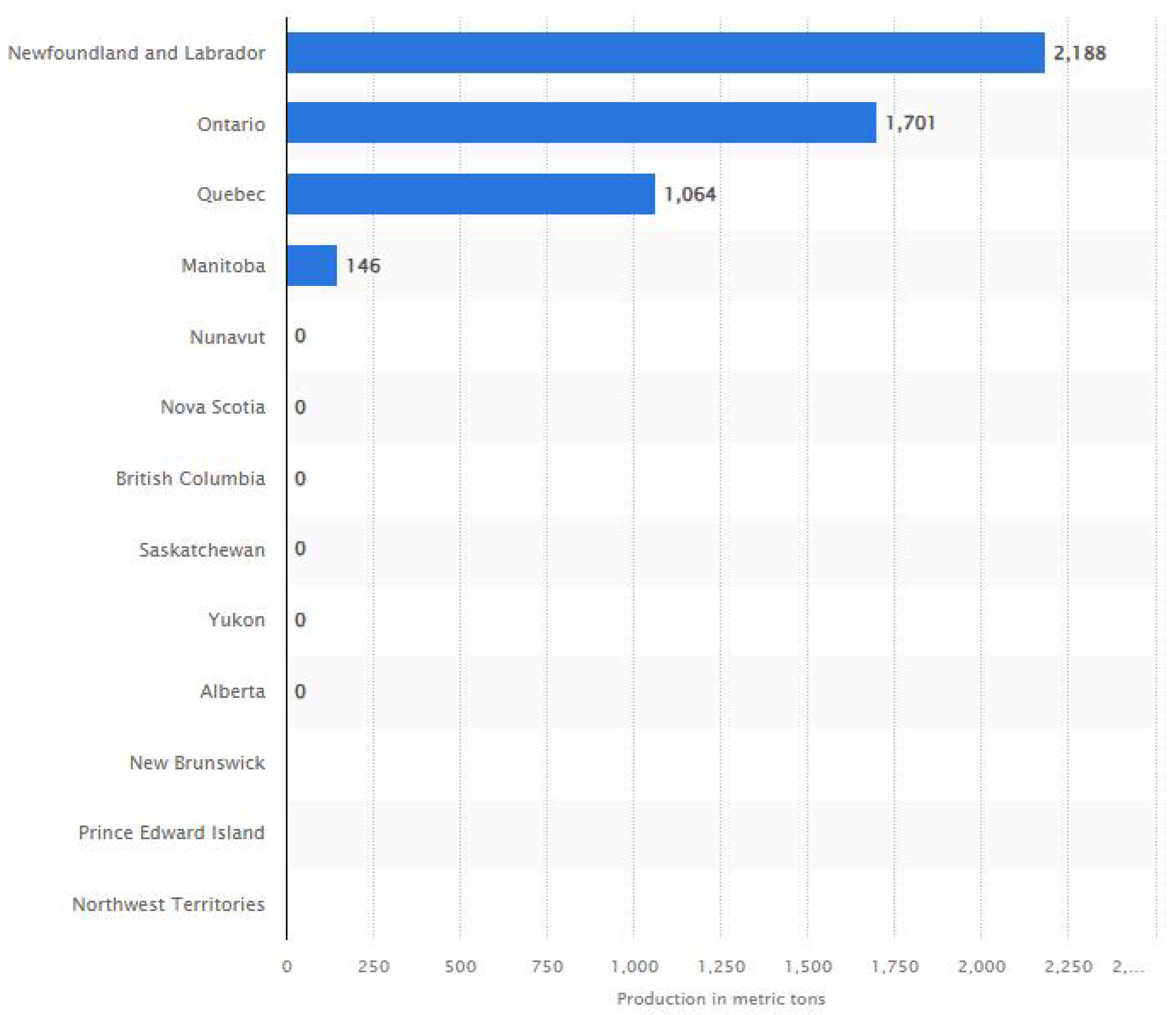
- Natural Resources Canada. Cobalt Facts. Available online: https://natural-resources.canada.ca/our-natural-resources/minerals-mining/mining-data-statistics-and-analysis/minerals-metals-facts/cobalt-facts/24981 (accessed on 8 February 2024).
- Canada: Cobalt Production by Province 2022|Statista. Available online: https://www.statista.com/statistics/434662/estimate-of-cobalt-production-in-canada-by-province/ (accessed on 8 February 2024).
- Sadeghian, A.; Iqbal, N. A Review on Dissimilar Laser Welding of Steel-Copper, Steel-Aluminum, Aluminum-Copper, and Steel-Nickel for Electric Vehicle Battery Manufacturing. Opt. Laser Technol. 2022, 146, 107595. [Google Scholar] [CrossRef]
- Aluminum Statistics and Information|U.S. Geological Survey. Available online: https://www.usgs.gov/centers/national-minerals-information-center/aluminum-statistics-and-information (accessed on 9 February 2024).
- The Industry. Aluminium.ca. Available online: https://aluminium.ca/en/the-industry/ (accessed on 8 February 2024).
- Natural Resources Canada. Aluminum Facts. Available online: https://natural-resources.canada.ca/our-natural-resources/minerals-mining/mining-data-statistics-and-analysis/minerals-metals-facts/aluminum-facts/20510 (accessed on 8 February 2024).
- Material and Energy Flows in the Production of Cathode and Anode Materialsfor Lithium Ion Batteries. Available online: https://publications.anl.gov/anlpubs/2015/10/121442.pdf (accessed on 10 October 2023).
- Dees, D. Enabling High-Energy/Voltage Lithium-Ion Cells: Materials Characterization. Available online: https://www.energy.gov/sites/prod/files/2016/06/f32/es252_dees_2016_o_web.pdf (accessed on 10 October 2023).
- Manufacturing of Lithium-Ion Battery Cell Components. Available online: https://www.pem.rwth-aachen.de/global/show_document.asp?id=aaaaaaaaaffpnvh (accessed on 3 October 2023).
- Chen, S.; Niu, C.; Lee, H.; Li, Q.; Yu, L.; Xu, W.; Zhang, J.-G.; Dufek, E.J.; Whittingham, M.S.; Meng, S.; et al. Critical Parameters for Evaluating Coin Cells and Pouch Cells of Rechargeable Li-Metal Batteries. Joule 2019, 3, 1094–1105. [Google Scholar] [CrossRef]
- Marks, T.; Trussler, S.; Smith, A.J.; Xiong, D.; Dahn, J.R. A Guide to Li-Ion Coin-Cell Electrode Making for Academic Researchers. J. Electrochem. Soc. 2010, 158, A51. [Google Scholar] [CrossRef]
- House Committee on Natural Resources. Available online: https://naturalresources.house.gov/uploadedfiles/testimony_hollomon.pdf (accessed on 26 October 2024).
- Zero Waste & Low Energy Lithium Processing Provisional Patent Filed & Proposed Plant Engineering Study Initiated. Insidexploration. Available online: https://insidexploration.com/zero-waste-low-energy-lithium-processing-provisional-patent-filed-proposed-plant-engineering-study-initiated/ (accessed on 10 February 2024).
- Qian, G.; Li, Z.; Wang, Y.; Xie, X.; He, Y.; Li, J.; Zhu, Y.; Xie, S.; Cheng, Z.; Che, H.; et al. Value-Creating Upcycling of Retired Electric Vehicle Battery Cathodes. Cell Rep. Phys. Sci. 2022, 3, 100741. [Google Scholar] [CrossRef]
- Marchese, D.; Giosuè, C.; Staffolani, A.; Conti, M.; Orcioni, S.; Soavi, F.; Cavalletti, M.; Stipa, P. An Overview of the Sustainable Recycling Processes Used for Lithium-Ion Batteries. Batteries 2024, 10, 27. [Google Scholar] [CrossRef]
- Do, M.P.; Jegan Roy, J.; Cao, B.; Srinivasan, M. Green Closed-Loop Cathode Regeneration from Spent NMC-Based Lithium-Ion Batteries through Bioleaching. ACS Sustain. Chem. Eng. 2022, 10, 2634–2644. [Google Scholar] [CrossRef]
- Gaines, L. Life-Cycle Analysis for Lithium-Ion Battery Production and Recycling; Argonne National Laboratory: Argonne, IL, USA, 2011. [Google Scholar]
- Qiao, D.; Ma, Y.; Bao, Y.; Hong, Y.; Batunacun; Narenmandula; Dai, T. Exploring the Potential Impact of Electric Passenger Vehicle Battery Recycling on China’s Cobalt Supply and Demand under the Goals of Carbon Peaking and Carbon Neutrality during 2010–2060. J. Clean. Prod. 2024, 444, 141139. [Google Scholar] [CrossRef]
- Gonzales-Calienes, G.; Kannangara, M.; Bensebaa, F. Economic and Environmental Viability of Lithium-Ion Battery Recycling—Case Study in Two Canadian Regions with Different Energy Mixes. Batteries 2023, 9, 375. [Google Scholar] [CrossRef]
- Kendall, A.; Slattery, M.; Dunn, J. End of Life EV Battery Policy Simulator: A Dynamic Systems, Mixed-Methods Approach; National Center for Sustainable Transportation: Davis, CA, USA, 2024. [Google Scholar] [CrossRef]
- Gohlke, D.; Zhou, Y.; Wu, X.; Courtney, C. Assessment of Light-Duty Plug-in Electric Vehicles in the United States, 2010–2020; Argonne National Lab (ANL): Argonne, IL, USA, 2021. [Google Scholar]
- Klier, T.; Rubenstein, J.M. North America’s Rapidly Growing Electric Vehicle Market: Implications for the Geography of Automotive Production. Rochester, NY, USA, 1 December 2022. Available online: https://papers.ssrn.com/abstract=4291029 (accessed on 2 October 2023).
- Chandrasekharam, D.; Şener, M.F.; Recepoğlu, Y.K.; Isık, T.; Demir, M.M.; Baba, A. Lithium: An Energy Transition Element, Its Role in the Future Energy Demand and Carbon Emissions Mitigation Strategy. Geothermics 2024, 119, 102959. [Google Scholar] [CrossRef]
- Das, J.; Kleiman, A.; Rehman, A.U.; Verma, R.; Young, M.H. The Cobalt Supply Chain and Environmental Life Cycle Impacts of Lithium-Ion Battery Energy Storage Systems. Sustainability 2024, 16, 1910. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Kelly, J.C.; James, C.; Gallagher, K.G. The Significance of Li-Ion Batteries in Electric Vehicle Life-Cycle Energy and Emissions and Recycling’s Role in Its Reduction. Energy Environ. Sci. 2015, 8, 158–168. [Google Scholar] [CrossRef]
- Kim, J.; Amine, K. A Comparative Study on the Substitution of Divalent, Trivalent and Tetravalent Metal Ions in LiNi1−xMxO2 (M = Cu2+, Al3+ and Ti4+). J. Power Sources 2002, 104, 33–39. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Cha, H.; Yoon, M.; Park, M.; Cho, J. Prospect and Reality of Ni-Rich Cathode for Commercialization. Adv. Energy Mater. 2018, 8, 1702028. [Google Scholar] [CrossRef]
- Bibra, E.M.; Connelly, E.; Gorner, M.; Lowans, C.; Paoli, L.; Tattini, J.; Teter, J. Global EV Outlook 2021: Accelerating Ambitions Despite the Pandemic; TRB: Washington, DC, USA, 2021. [Google Scholar]
- Dai, Q.; Kelly, J.C.; Gaines, L.; Wang, M. Life Cycle Analysis of Lithium-Ion Batteries for Automotive Applications. Batteries 2019, 5, 48. [Google Scholar] [CrossRef]
- Gaines, L. Lithium-Ion Battery Recycling Processes: Research towards a Sustainable Course. Sustain. Mater. Technol. 2018, 17, e00068. [Google Scholar] [CrossRef]
- Emilsson, E.; Dahllöf, L. Lithium-Ion Vehicle Battery Production–Status 2019 on Energy Use, CO2 Emissions, Use of Metals, Products Environmental Footprint, and Recycling; IVL Svenska Miljöinstitutet: Stockholm, Sweden, 2019. [Google Scholar]
- Lewrén, A. Life Cycle Assessment of Nickel-Rich Lithium-Ion Battery for Electric Vehicles A Comparatative LCA Between the Cathode Chemistries NMC 333 and NMC 622; Chalmers ODR: Stockholm, Sweden, 2019. [Google Scholar]
- Lampert, D.J.; Cai, H.; Elgowainy, A. Wells to Wheels: Water Consumption for Transportation Fuels in the United States. Energy Environ. Sci. 2016, 9, 787–802. [Google Scholar] [CrossRef]
- Jacob, M.; Wissel, K.; Clemens, O. Recycling of Solid-State Batteries—Challenge and Opportunity for a Circular Economy? Mater. Futures 2024, 3, 012101. [Google Scholar] [CrossRef]
- Slattery, M.; Dunn, J.; Kendall, A. Charting the Electric Vehicle Battery Reuse and Recycling Network in North America. Waste Manag. 2024, 174, 76–87. [Google Scholar] [CrossRef]
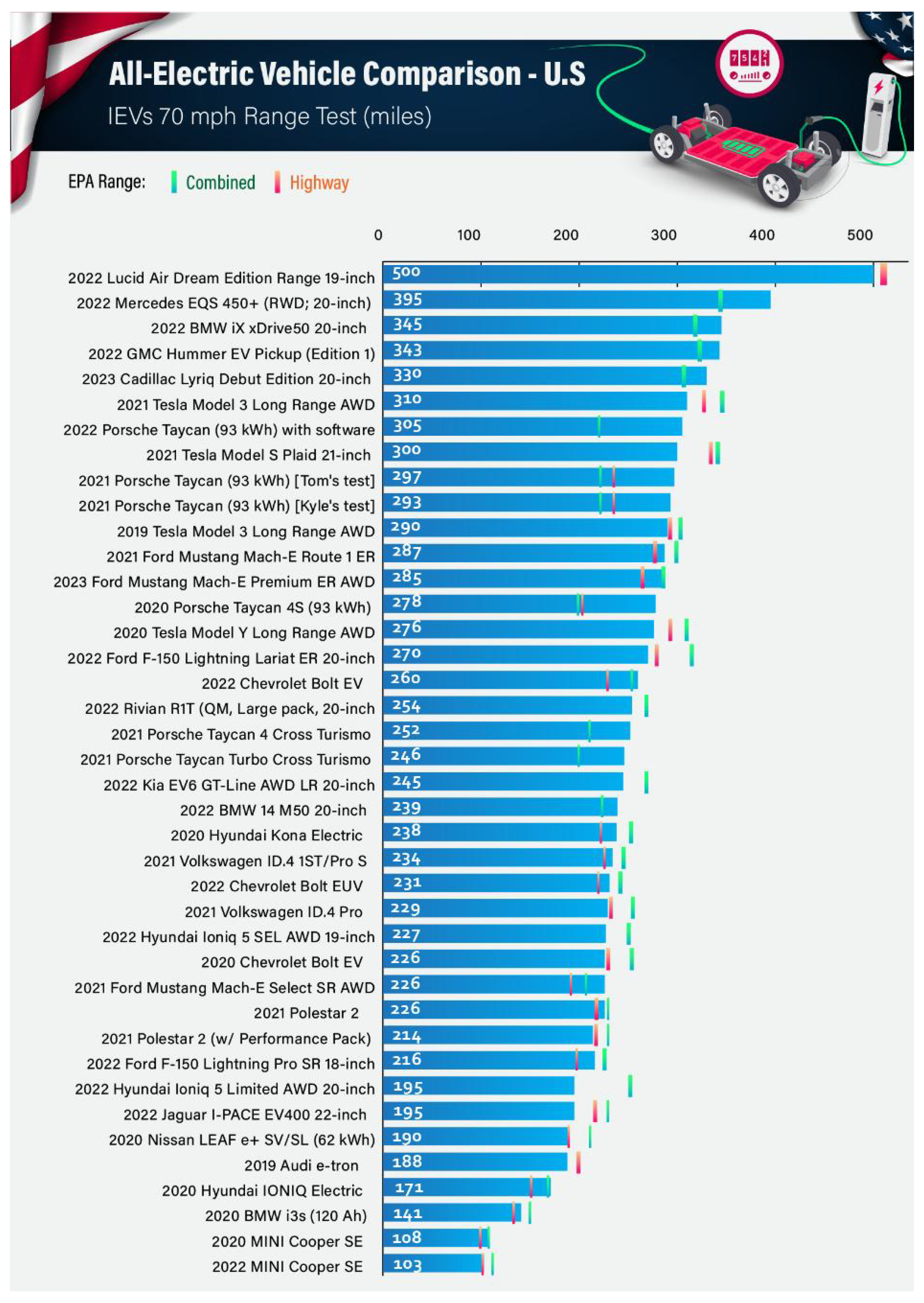
- Osaka, S. Analysis|The Obsession with EV Range Is All Wrong. Washington Post. 19 July 2023. Available online: https://www.washingtonpost.com/climate-solutions/2023/07/07/ev-range-anxiety-battery-myth/ (accessed on 16 October 2023).
- What’s the Real World Highway Range of Today’s Electric Cars? We Test to Find Out. InsideEVs. Available online: https://insideevs.com/reviews/443791/ev-range-test-results/ (accessed on 16 October 2023).
- Yee, M.S. Promoting Electric Vehicles as the Silver Bullet for Tackling Climate Change. In Climate and Energy Governance for a Sustainable Future; Leal-Arcas, R., Ed.; Climate Change Management; Springer Nature: Singapore, 2023; pp. 157–170. [Google Scholar] [CrossRef]
- Neshat, N.; Kaya, M.; Ghaboulian Zare, S. Exploratory Policy Analysis for Electric Vehicle Adoption in European Countries: A Multi-Agent-Based Modelling Approach. J. Clean. Prod. 2023, 414, 137401. [Google Scholar] [CrossRef]
- Rasbash, D.; Dillman, K.J.; Heinonen, J.; Ásgeirsson, E.I. A National and Regional Greenhouse Gas Breakeven Assessment of EVs across North America. Sustainability 2023, 15, 2181. [Google Scholar] [CrossRef]
- Issa, M.; Ilinca, A.; Rousse, D.R.; Boulon, L.; Groleau, P. Renewable Energy and Decarbonization in the Canadian Mining Industry: Opportunities and Challenges. Energies 2023, 16, 6967. [Google Scholar] [CrossRef]
- Shang, H.; Sun, Y.; Huang, D.; Meng, F. Life Cycle Assessment of Atmospheric Environmental Impact on the Large-Scale Promotion of Electric Vehicles in China. Resour. Environ. Sustain. 2024, 15, 100148. [Google Scholar] [CrossRef]
- Sankar, T.K.; Abhilash; Meshram, P. Environmental Impact Assessment in the Entire Life Cycle of Lithium-Ion Batteries. Rev. Environ. Contam. Toxicol. 2024, 262, 5. [Google Scholar] [CrossRef]
- Jannesar Niri, A.; Poelzer, G.A.; Zhang, S.E.; Rosenkranz, J.; Pettersson, M.; Ghorbani, Y. Sustainability Challenges throughout the Electric Vehicle Battery Value Chain. Renew. Sustain. Energy Rev. 2024, 191, 114176. [Google Scholar] [CrossRef]
- Aubertin, A.; Axsen, J.; Gunster, S. Electric Vehicles for Climate, a Green Economy, or Independence? Comparing Policy Discourse in Newspapers across Three Canadian Provinces. Energy Res. Soc. Sci. 2024, 108, 103353. [Google Scholar] [CrossRef]
- Niu, B.; Xu, H.; Mu, Z. To Divide or Not to Divide: Impact of Carbon Market Connections on Firms’ Resilient Profitability and Emission Reduction. Int. J. Prod. Econ. 2024, 270, 109167. [Google Scholar] [CrossRef]
- Bellan, R. Tracking the EV Battery Factory Construction Boom Across North America. TechCrunch. Available online: https://techcrunch.com/2023/08/16/tracking-the-ev-battery-factory-construction-boom-across-north-america/ (accessed on 30 January 2024).
- Gu, X.; Bai, H.; Cui, X.; Zhu, J.; Zhuang, W.; Li, Z.; Hu, X.; Song, Z. Challenges and Opportunities for Second-Life Batteries: Key Technologies and Economy. Renew. Sustain. Energy Rev. 2024, 192, 114191. [Google Scholar] [CrossRef]
- A Vision for a Sustainable Battery Value Chain in 2030 Unlocking the Full Potential to Power Sustainable Development and Climate Change Mitigation. Available online: https://www3.weforum.org/docs/WEF_A_Vision_for_a_Sustainable_Battery_Value_Chain_in_2030_Report.pdf (accessed on 19 September 2023).
- Kellen. SP’s 1st Quarter Meeting of 2020 Held at Honda of Alabama. Suppliers Partnership for the Environment. Available online: https://www.supplierspartnership.org/meetings/sps-1st-quarter-meeting-of-2020-held-at-honda-of-alabama/ (accessed on 8 February 2024).
- Shahzad, K.; Helo, P.; Ranta, M.; Nousiainen, E. Blockchain Technology for Operational Excellence and Supply Chain Resilience: A Framework Based on Use Cases and an Architecture Demonstration. Technol. Anal. Strateg. Manag. 2024, 1–18. [Google Scholar] [CrossRef]
- SP Responsible Battery Work Group. Suppliers Partnership for the Environment. Available online: https://www.supplierspartnership.org/responsible-battery-work-group/ (accessed on 8 February 2024).

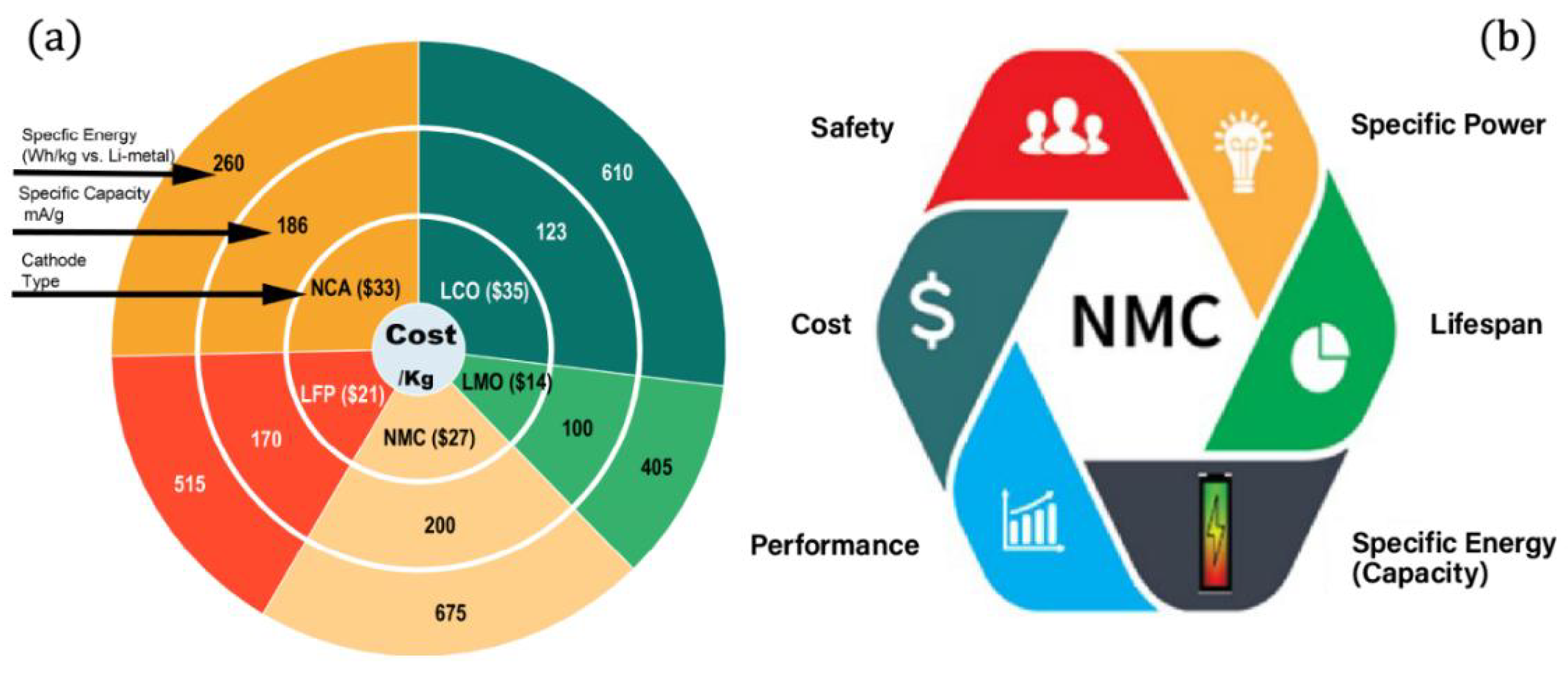
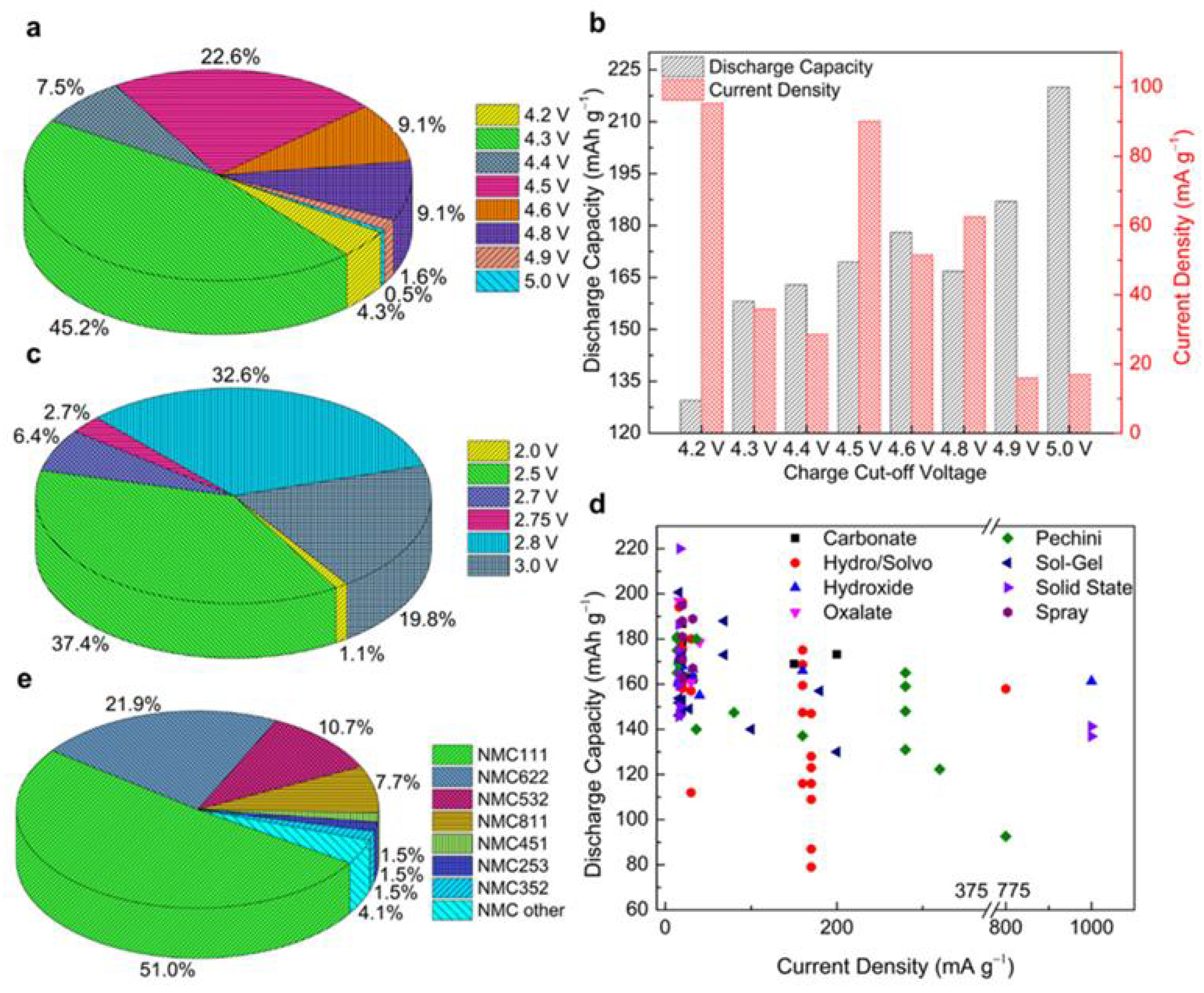
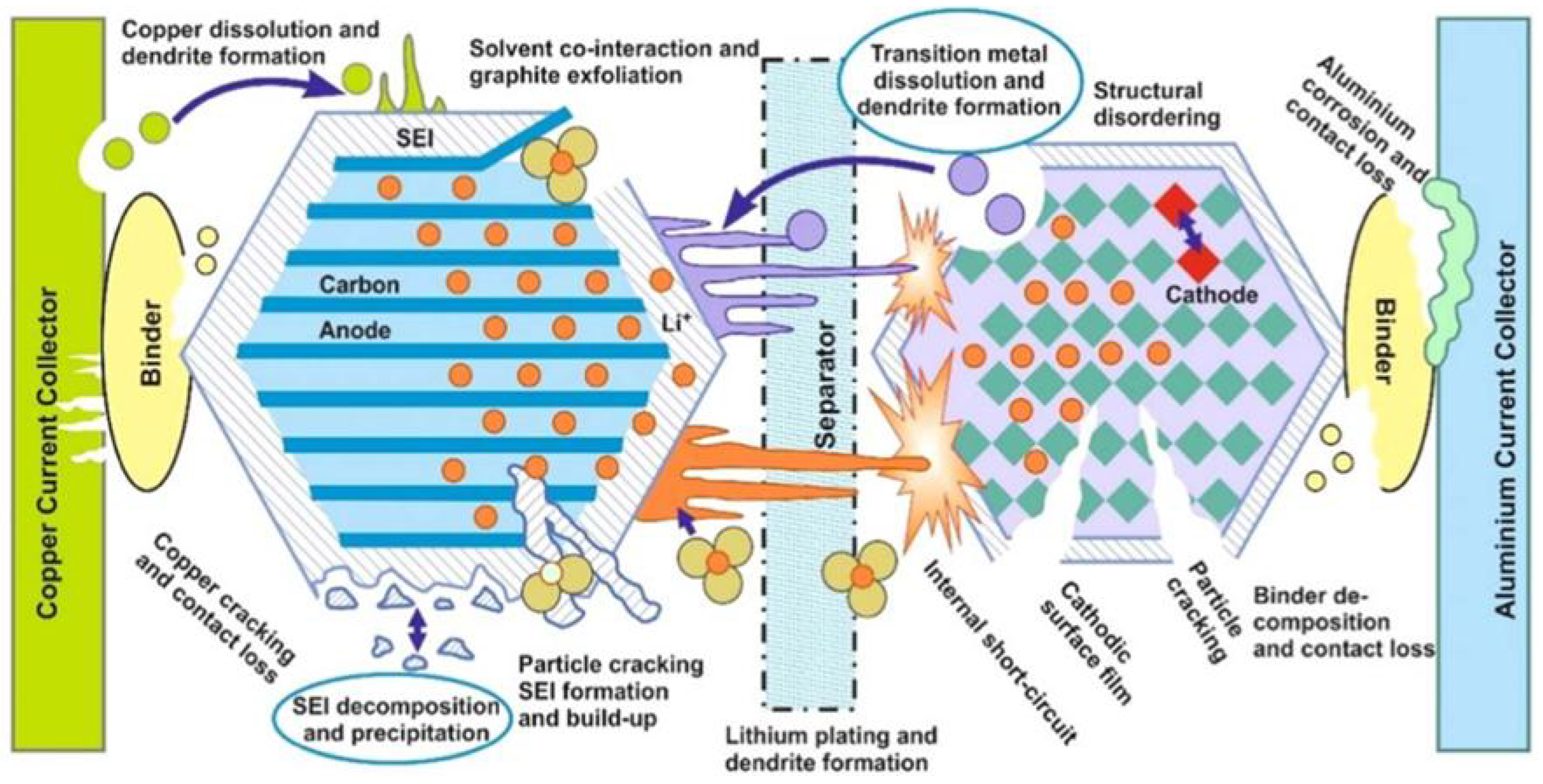
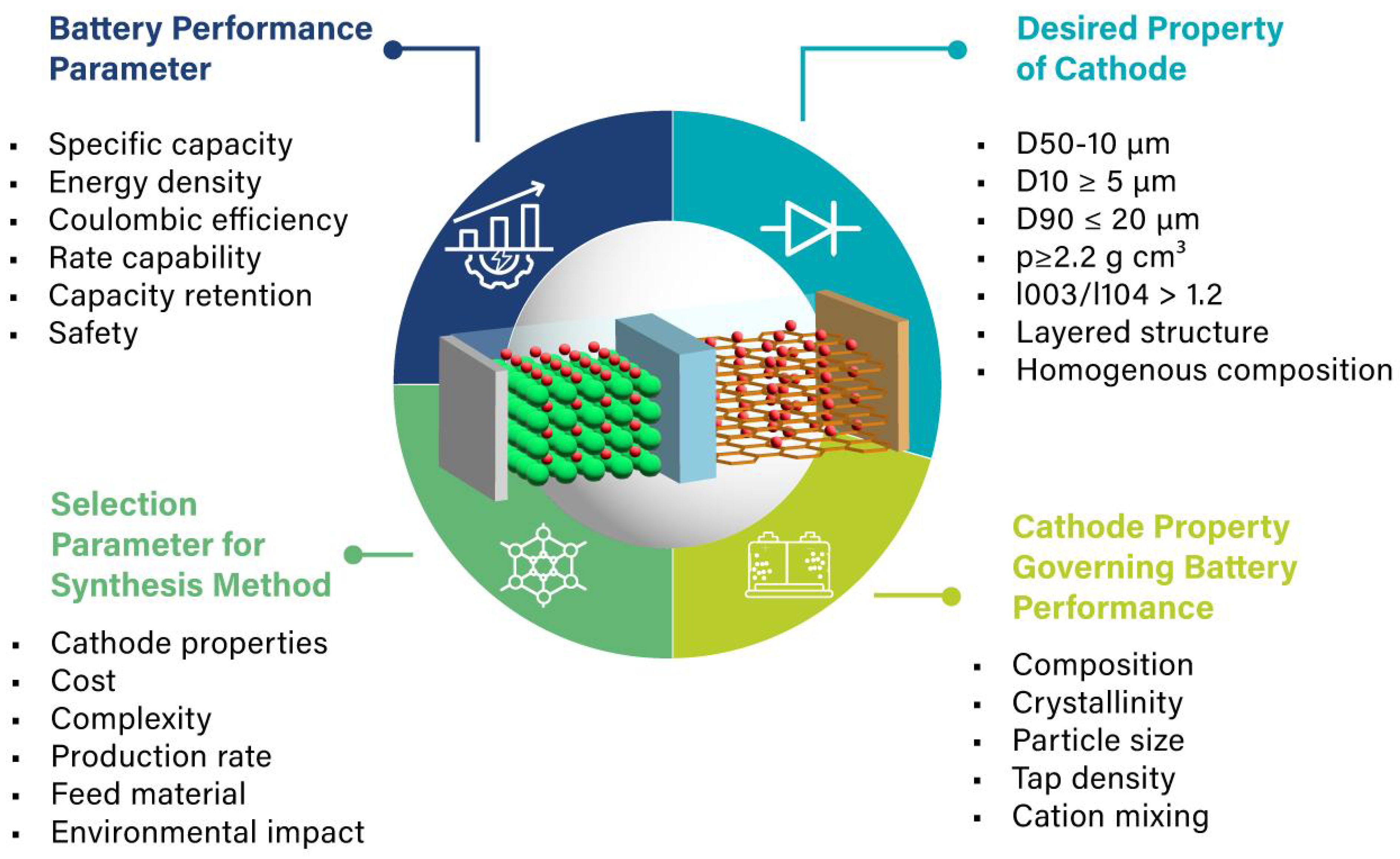
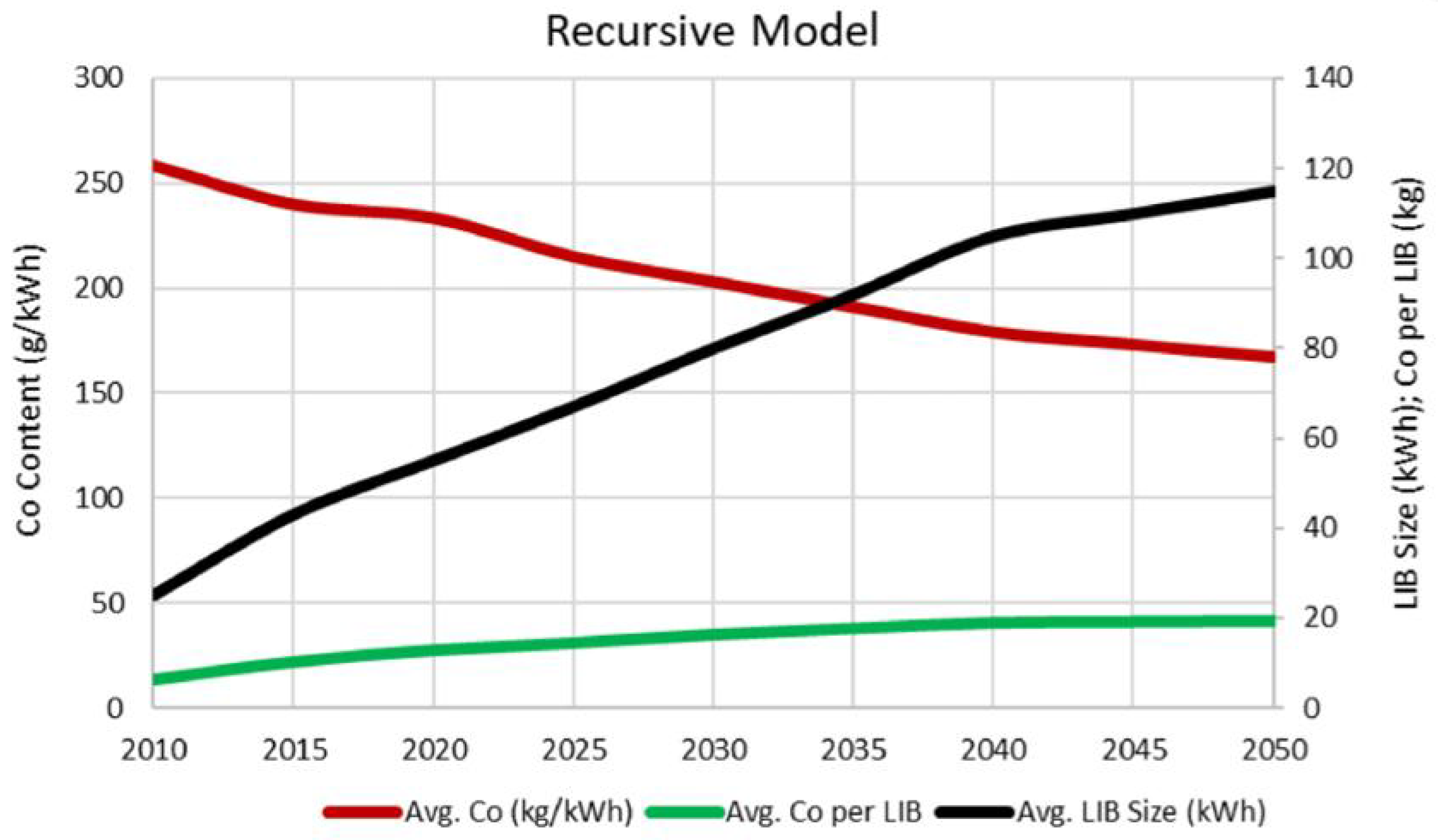








| Reference | Chemical Formula | Abbreviation | Specific Capacity (mA g−1) | Specific Energy (Wh kg−1 vs. Li Metal) | Cost kg−1 | Advantage(s) | Disadvantage(s) |
|---|---|---|---|---|---|---|---|
| [72] | LiCoO2 | LCO | 123 | 610 | 35 | High energy density | High cost and moderate stability |
| [71] | LiMn2O4 | LMO | 100 | 405 | 14 | Low cost and high-power density | Lower energy density and accelerated capacity fade |
| [75,79,80] | LiNixMnyCo1−x−yO2 | NMC | 200 | 675 | 27 | Performs well in all metrics | Moderate cost and moderate stability |
| [72] | LiFePO4 | LFP | 170 | 515 | 21 | High-power density and very stable | Lower energy density |
| [74] | LiNixCoyAl1−x−yO2 | NCA | 186 | 260 | 33 | High energy density | High cost and moderate stability |
| NMC Cathode Operating Parameters | |
|---|---|
| Operation | 2–4 V |
| Charging | 0–45 °C |
| Discharging | −20–55 °C |
| Electrolyte decomposition | 70 °C |
| Solid-phase electrolyte (SEI) decomposes | 90–120 °C |
| Production of flammable gases | >120 °C |
| Separator melts | 130 °C |
| Cathode material decomposes | 150 °C |
| Thermal runaway (self-heating) | 10 C/min * 11 |
| Cathode | 2010 | 2015 | 2020 | 2025 | 2030 | 2035 | 2040 |
|---|---|---|---|---|---|---|---|
| NMC | NMC111 (45%) | NMC111 (35%) | NMC111 (40%) | NMC111 (20%) | NMC111 (10%) | ||
| NMC532 (55%) | NMC532 (50%) | NMC532 (30%) | NMC532 (50%) | NMC532 (35%) | |||
| NMC622 (205) | NMC622 (25%) | NMC622 (30%) | NMC622 (35%) | ||||
| NMC811 (<5%) | NMC811 (<5%) | NMC811 (10%) | NMC811 (20%) | ||||
| kg Co per Battery | 6.34 | 10.8 | 13.39 | 15.17 | 14.68 | ||
| Battery Size kWh (extrapolated) * | 25 | 43 | 55 | 67 | 80 | 92 | 105 |
| NCA ** | 83% Nickel (Ni) (100%) | 83% Ni (100%) | 83% Ni (48%) | 83% Ni (40%) | 83% Ni (25%) | ||
| 87% Ni (52%) | 87% Ni (45%) | 87% Ni (40%) | |||||
| 90% Ni (15%) | 90% Ni (35%) | ||||||
| Recovery Rate | 46.50% | 50% | 70% | 75% | 80% | ||
| Collection Rate *** | 20% | 50% | 60% | 75% | 85% |
| Battery Pack Plants in North America Operating and Announced Since November 2022 | |||
|---|---|---|---|
| Battery Company | Location | Automaker Customer | Production Start Year |
| CATL | Ciudad Juárez, Chihuahua, Mexico | Ford, Tesla | TBD |
| CATL | Big Rapids, Michigan | BMW, Ford | 2026 |
| Envision AESC | Bowling Green, Kentucky | Mercedes | 2025 |
| Envision AESC | Smyrna, Tennessee | Nissan | 2012 |
| Envision AESC | Woodruff, South Carolina | BMW | TBD |
| iM3NY | Endicott, New York | TBD | 2022 |
| LG Chem | Queen Creek, Arizona | TBD | 2024 |
| LG Chem | New Castle, Indiana | GMs | TBD |
| LG Chem | Lansing, Michigan | GMs | 2024 |
| LG Chem | Holland, Michigan | GMs | 2011 |
| LG Chem | Lordstown, Ohio | GMs | 2022 |
| LG Chem | Jeffersonville, Ohio | Honda | 2025 |
| LG Chem | Windsor, Ontario, Canada | Stellantis | 2024 |
| LG Chem | Spring Hill, Tennessee | GMs | 2023 |
| Mercedes | Woodstock, Alabama | Mercedes | TBD |
| Microvast | Clarksville, Tennessee | TBD | 2022 |
| ONE | Van Buren Township, Michigan | TBD | 2024 |
| Panasonic | De Soto, Kansas | Tesla | 2025 |
| Panasonic | Sparks, Nevada | Tesla, others | 2016 |
| Panasonic | TBD, Oklahoma | Tesla | TBD |
| Samsung | Kokomo, Indiana | Stellantis | 2025 |
| SKI | Commerce, Georgia | Ford, VW | 2022 |
| SKI | Glendale, Kentucky | Ford | 2025 |
| SKI | Glendale, Kentucky | Ford | 2026 |
| SKI | Stanton, Tennessee | Ford | 2025 |
| Tesla | Fremont, California | Tesla | 2022 |
| Tesla | Austin, Texas | Telsa | TBD |
| TBMNC | Liberty, North Carolina | Toyota | 2025 |
| VinFast | Sanford, North Carolina | VinFast | 2023 |
| VW | Chattanooga, Tennessee | VW | 2022 |
| Reference | Process Parameter | CO2 Emissions (kg CO2 Eq/kg) |
|---|---|---|
| [94] | NMC emissions (virgin raw material) | 8.9 |
| [129] | CoSO4 (cathode active material; (pCAM)) | 6.73 |
| [129] | NiSO4 (pCAM) | 8.61 |
| [120] | Electricity power (concrentration plant) to produce LiOH monohydrate (raw material) from spodumene | 0.00254 |
| [120] | Electricity power (electrochemical plant) to produce LiOH monohydrate (raw material) from spodumene | 0.01095 |
| [120] | Natural gas (electrochemical plant) to produce LiOH monohydrate (raw material) from spodumene | 1.861 |
| Reference | Process Parameter | CO2 Emissions kg CO2 Eq Kwh−1 | CO2 Emissions kg | SOX Emissions Increase Relative to NMC111 | Water Consumption | Overall Emissions |
|---|---|---|---|---|---|---|
| [120,129] | NMC811 Assembly of single lithium-ion battery | 55.1 | 141.5 | 142% | 12% | |
| [129] | NMC111 | 59.1 | 44% | |||
| [129] | NMC532 | 130% | 28% | |||
| [129] | NMC622 | 130% | 27% | |||
| [120] | (LIB) | 141.5 | ||||
| [94] | NMC cathode (recycling; hydrometallurgy) | >23% | ||||
| [94] | NMC cathode (recycling; pyrometallurgy) | 0% |
| GHG Emissions to Produce 1 Ton Lithium Hydroxide Monohydrate from Spodumene | ||
|---|---|---|
| Process | Amount | Emissions |
| Spodumene concentrate | 5.81 t | |
| Lithium sulfate | 0.054 t | |
| Electricity power (concentration plant) | 7610 MJ | 2.54 kg CO2 |
| Electricity power (electrochemical plant) | 32.85 GJ | 10.95 kg CO2 |
| Natural gas (electrochemical plant) | 36.35 GJ | 1861.14 kg CO2 |
| Total | 69.21 GJ | 1874.63 CO2 |
| Project | Operator/Owner | Fleet Description |
|---|---|---|
| Borden Lake, Ontario | Goldcorp | Canada’s first fully electric underground mine (fully electric fleet) |
| Macassa Mone in Kirkland Lake, Ontario | Agnico Eagle | Twenty-two battery electric scoops with 6 X Z50 trucks (a 50-tonne battery-powered haul truck) |
| Onaping Depth Nickel–Copper Project, Ontario | Glencore Canada | Entire fleet of Epiroc battery–electric mining equipment (scoop tram loader, Minestruck hauler, Boomer fac drilling rig, Cabletec rock bolting rig, and drill rig) |
| Lamaque Gold Mine, Quebec | Eldorado Gold | Two Sandvik TH550B battery–electric trucks |
| NMG open-pit, Quebec | Nouveau Monde Graphite | One X 40-tonne Western Star 6900XD |
| Brucejack Mine, British Columbia | Newcrest Mining | Twelve electric haul trucks |
| Mcllvena Bay Project, Saskatchewan | Foran Mining Corporation | Fleet of 20 BEVs, including trucks, loaders, and drill |
| BHP Jansen Potash Project, Saskatchewan | BHP Group | Ten underground battery–electric loaders and one electric tethered loader |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vegh, G.; Madikere Raghunatha Reddy, A.K.; Li, X.; Deng, S.; Liu, T.; Amine, K.; Zaghib, K. North America’s Potential for an Environmentally Sustainable Nickel, Manganese, and Cobalt Battery Value Chain. Batteries 2024, 10, 377. https://doi.org/10.3390/batteries10110377
Vegh G, Madikere Raghunatha Reddy AK, Li X, Deng S, Liu T, Amine K, Zaghib K. North America’s Potential for an Environmentally Sustainable Nickel, Manganese, and Cobalt Battery Value Chain. Batteries. 2024; 10(11):377. https://doi.org/10.3390/batteries10110377
Chicago/Turabian StyleVegh, Gary, Anil Kumar Madikere Raghunatha Reddy, Xia Li, Sixu Deng, Tongchao Liu, Khalil Amine, and Karim Zaghib. 2024. "North America’s Potential for an Environmentally Sustainable Nickel, Manganese, and Cobalt Battery Value Chain" Batteries 10, no. 11: 377. https://doi.org/10.3390/batteries10110377
APA StyleVegh, G., Madikere Raghunatha Reddy, A. K., Li, X., Deng, S., Liu, T., Amine, K., & Zaghib, K. (2024). North America’s Potential for an Environmentally Sustainable Nickel, Manganese, and Cobalt Battery Value Chain. Batteries, 10(11), 377. https://doi.org/10.3390/batteries10110377












