Teaching Aid Regarding the Application of Advanced Organic Petrography in Recycling End-of-Life Lithium-Ion Batteries
Abstract
1. Preamble
2. Introduction
2.1. LIB Composition
- The cathode is the positive electrode and consists of active materials, binders, and conductive additives. It is made of lithium metal oxides bound to an Al foil. There are many variations in these oxides, but some common types include LiCoO2 (LCO), LiNixMnyCozO2 (NMC), LiNixCoyAlzO2 (NCA), LiMn2O4 (LMO), LiFePO4 (LFP), and LiNiCoAlO2 (NCA);
- The anode is the negative electrode, usually made of an anode of graphite bound to a Cu foil. However, other materials can be found in the anode, such as carbon-based materials, porous carbon, carbon nanotubes, graphene, silica, lithium titanate (Li4Ti5O12), Si, Ge, Sn, Al, Bi or SnO2, transition metal oxides (MnxOy, NiO, FexOy, CuO, Cu2O, MoO2, etc.), mesoporous heterostructures, nanocomposites, metal sulfides, metal phosphides, and metal nitride, among others [18];
- The electrolyte is typically composed of lithium salts (LiPF6, LiBF4, or LiClO4) dissolved in organic solvents such as ethylene carbonate and dimethyl carbonate/ethyl methyl carbonate;
- The separator is a porous membrane soaked with an ionically conductive liquid electrolyte (typically in LiPF6) and placed between the electrodes. A variety of separators have been used in batteries over the years.
2.2. LIB Recycling
3. Application of Advanced Organic Petrography to LIB Recycling (Examples from Project NEXT-LIB, the ERA-MIN2 Program)
- The separation of the electrolyte from the shreds and solvents;
- The separation of coarse fragments of copper and aluminum foil current collectors, which are sent for copper recyclers;
- The separation of the active materials from the current collectors;
- The structure of the cathode for further reuse;
- The fine particles that may lead to impurities and the presence of current collectors and other materials in the black mass;
- The binder and the solid electrolyte interphase (SEI) from the electrodes;
- The anode graphite transformations caused by the mechanical activation due to shredding and milling.
- X-ray diffraction (XRD) to determine phase composition and lithium metal oxides and graphite amounts;
- SEM-EDS for detailed imaging, phase identification, and semiquantitative chemical analysis including chemical mappings;
- Transmitted electron microscopy (TEM) for crystalline structure assessment and chemical mappings;
- Scanning probe microscopies, including QEMSCAN, for phase identification and characterization;
- Total carbon and carbons forms for organic polymers, inorganic C and graphitic C determination;
3.1. Methods and Analytical Techniques
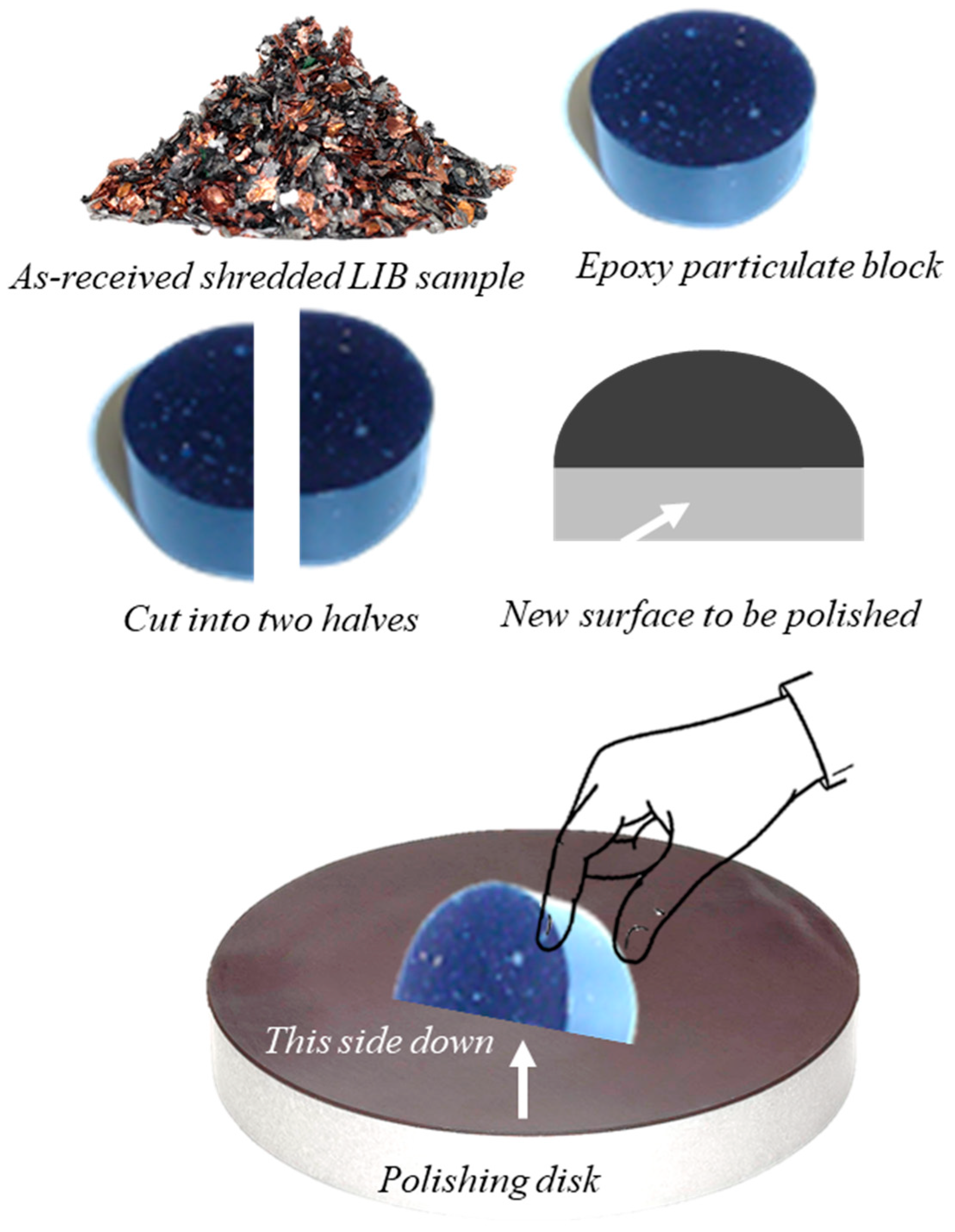
3.1.1. Stereo Microscopy
3.1.2. Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM/EDS)
3.1.3. Incident Light Optical Microscopy with Oil Immersion Objectives
3.1.4. Micro-Raman Spectroscopy
4. Identification of LIB Components and Raw Materials Through Advanced Organic Petrography
4.1. Stereo Microscopic Analysis
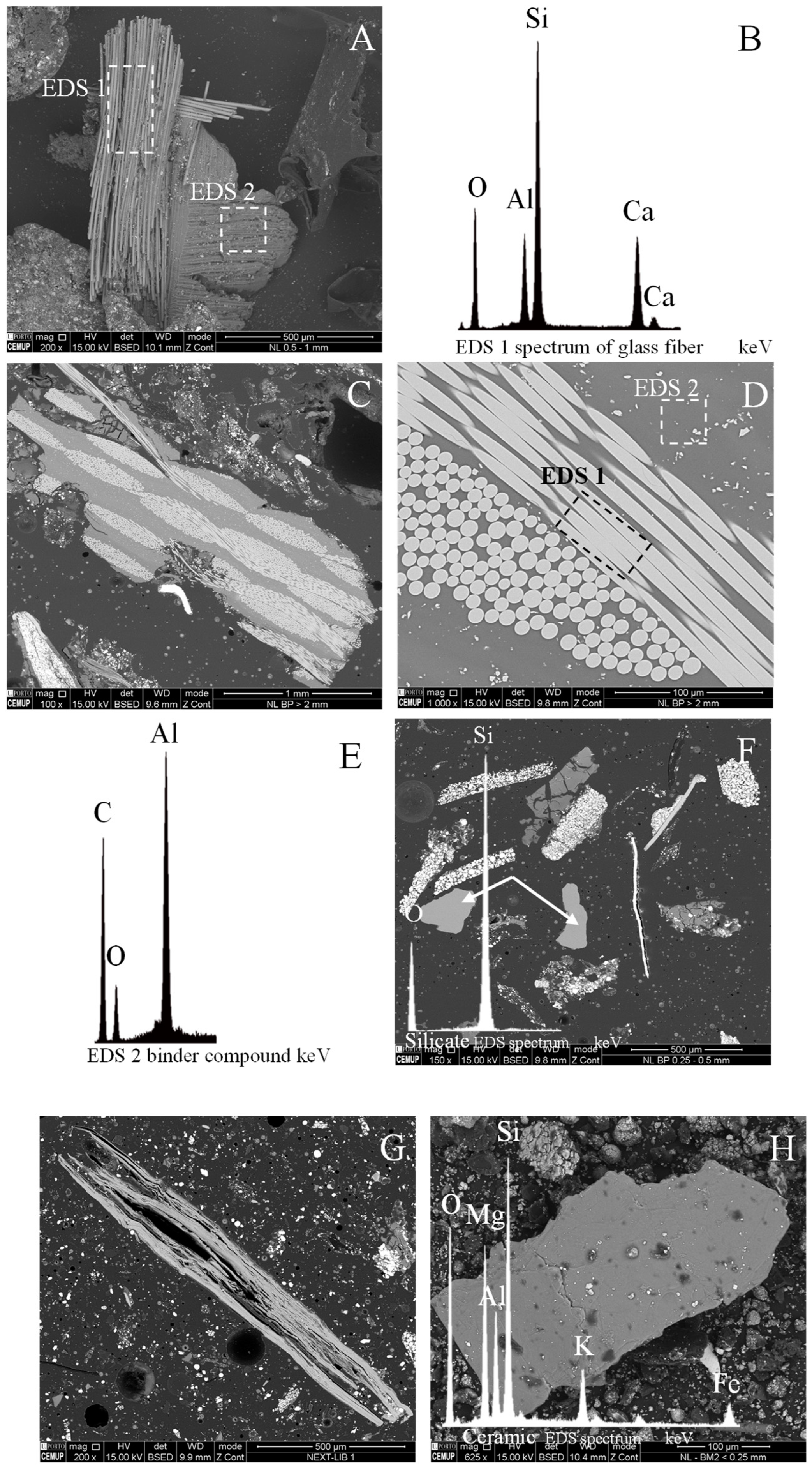
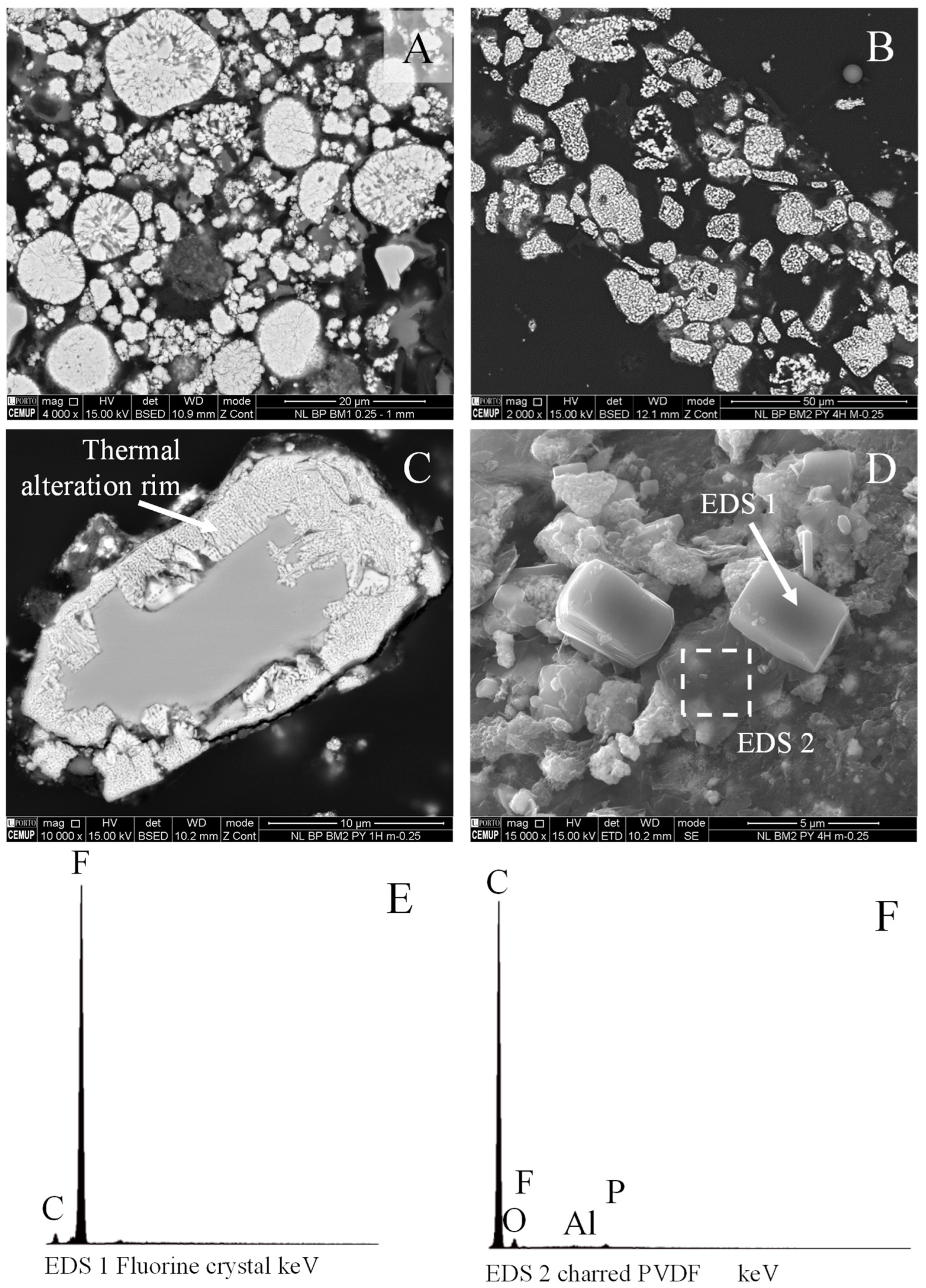
4.2. SEM-EDS Analysis
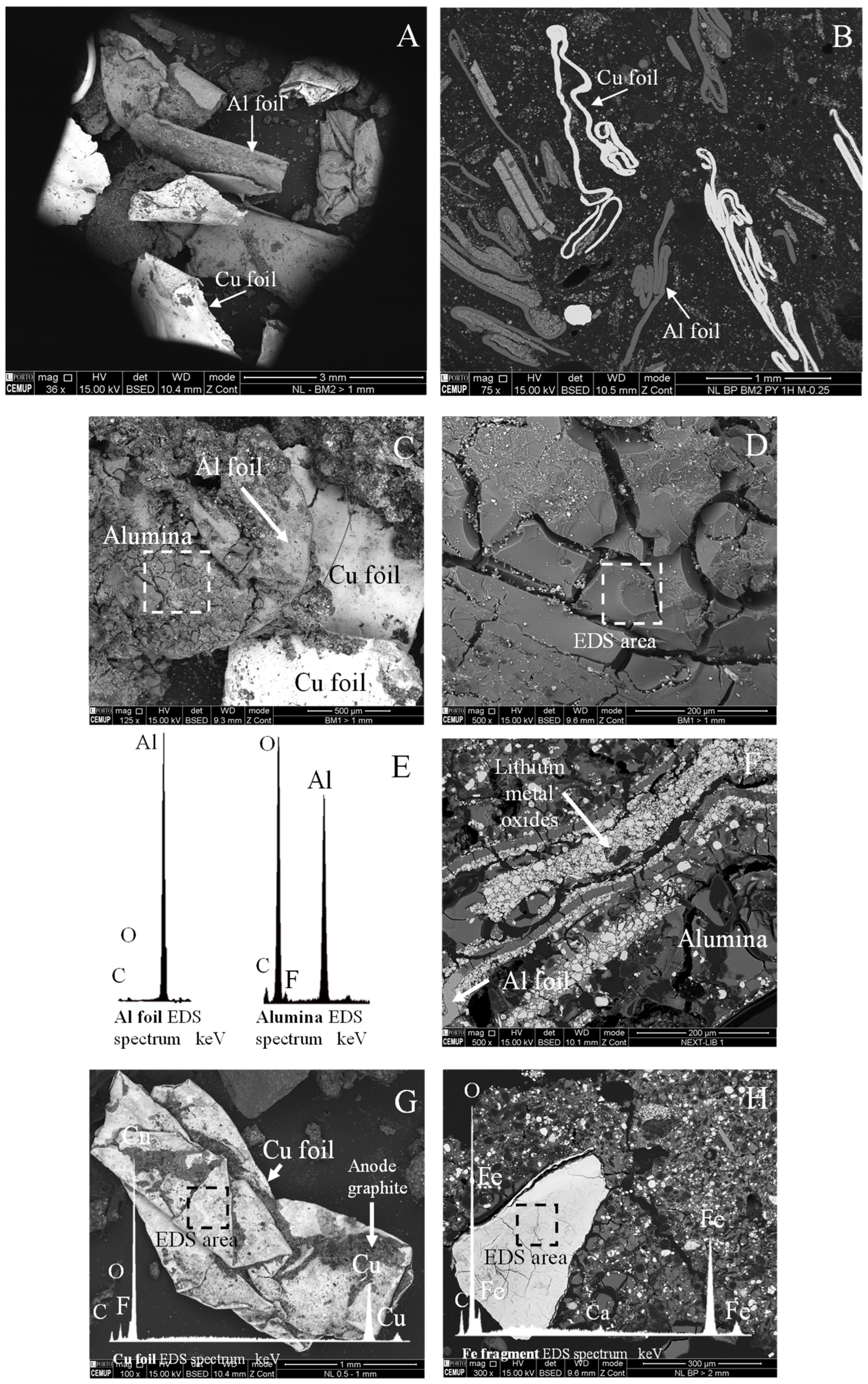
4.2.1. Current Collectors: Al and Cu Foils
4.2.2. Anode Graphite and Organic Polymers
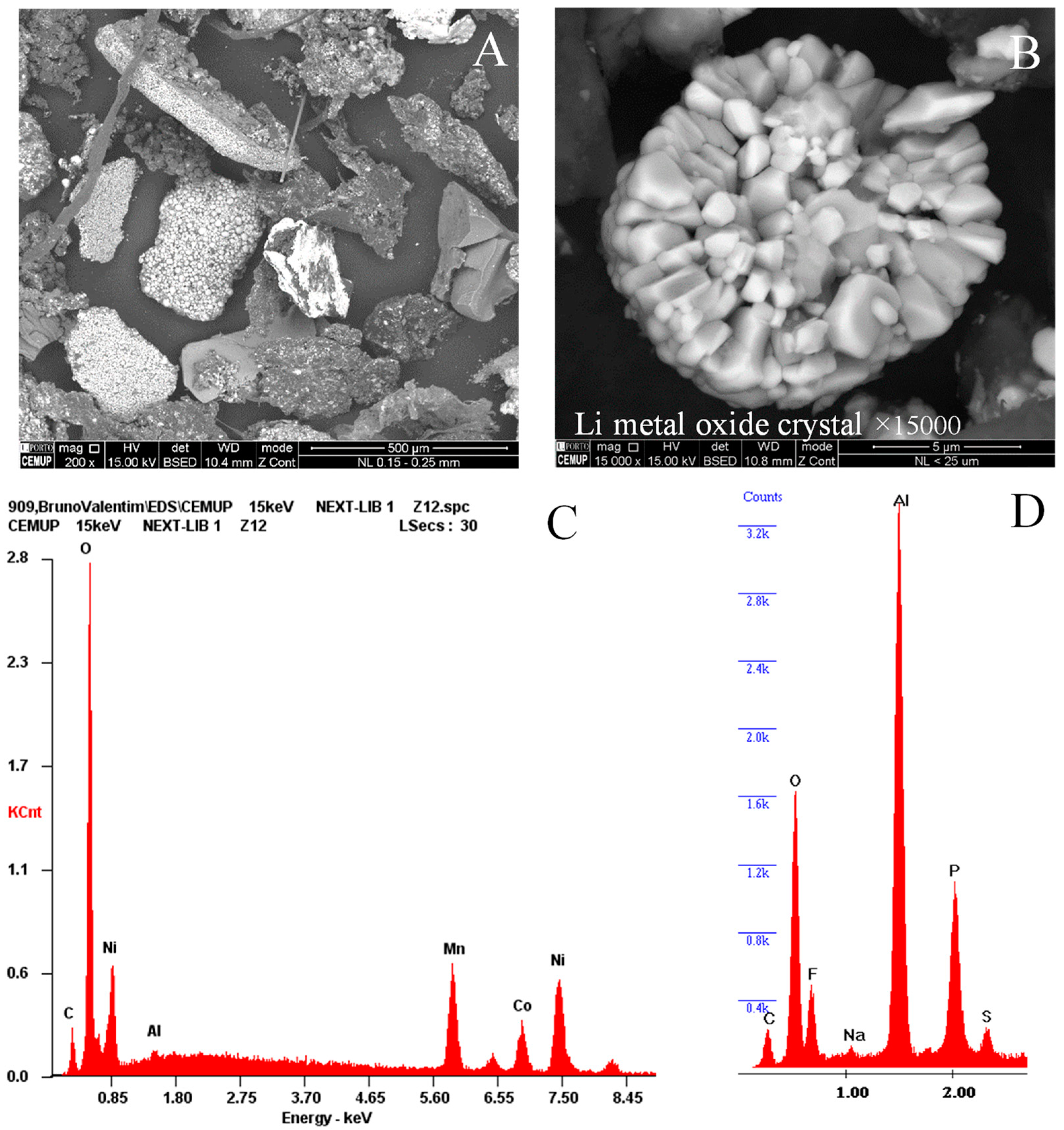
4.2.3. Lithium Metal Oxides
4.2.4. Others
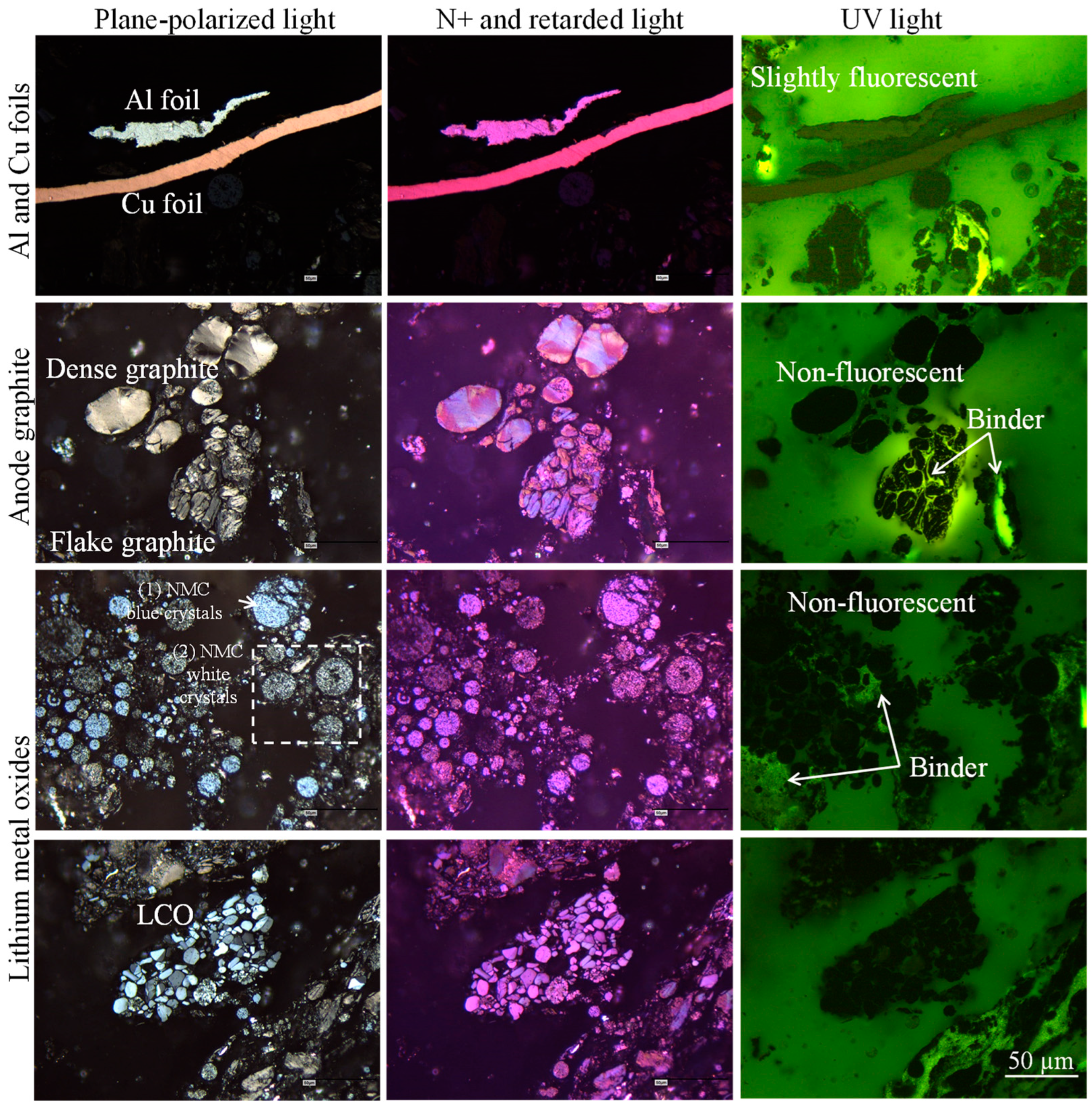
4.3. Analysis Through Incident Light Optical Microscopy with Oil Immersion Objectives
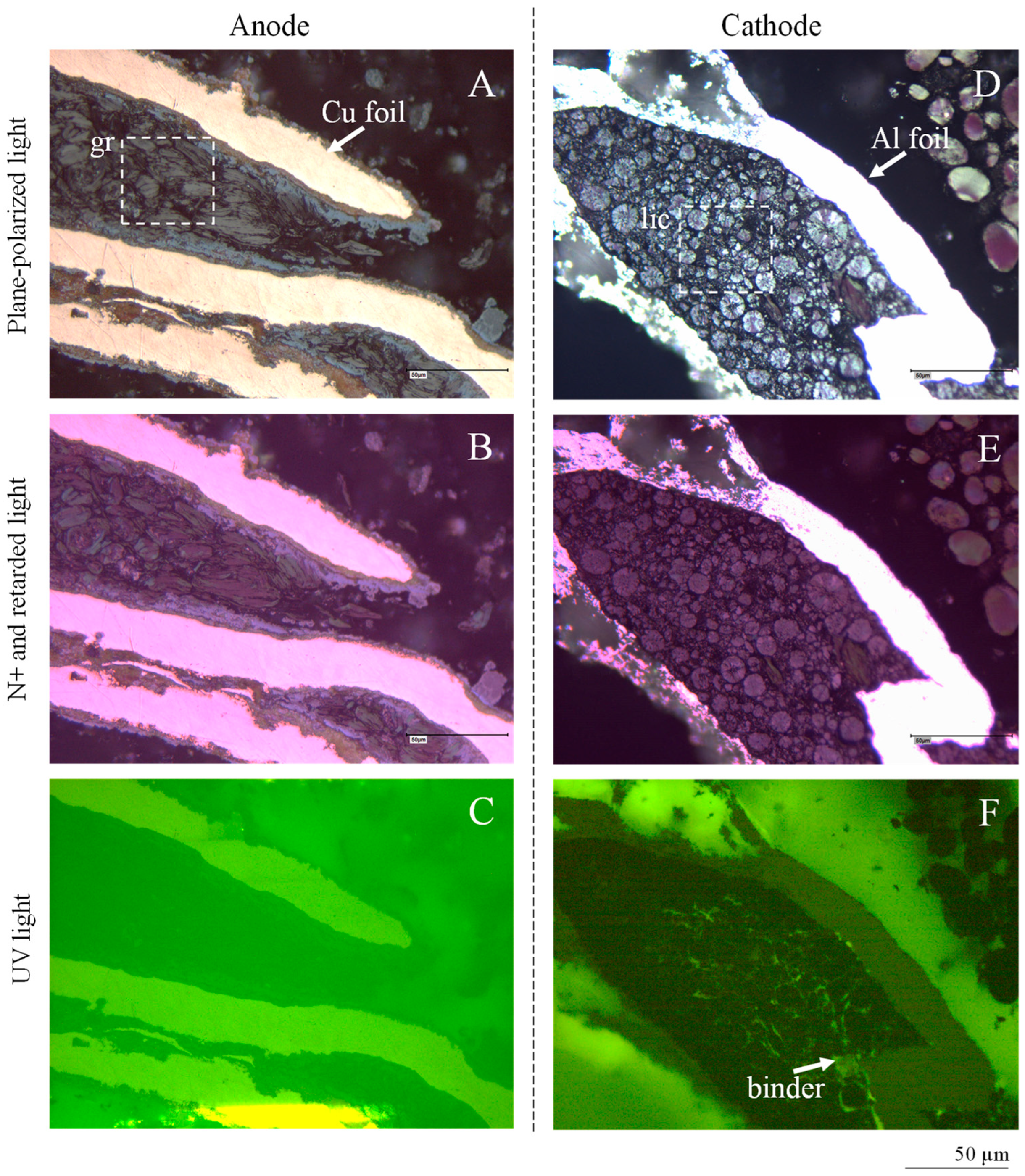
4.3.1. Electrode: Al Foils, Cu Foils, and Alumina
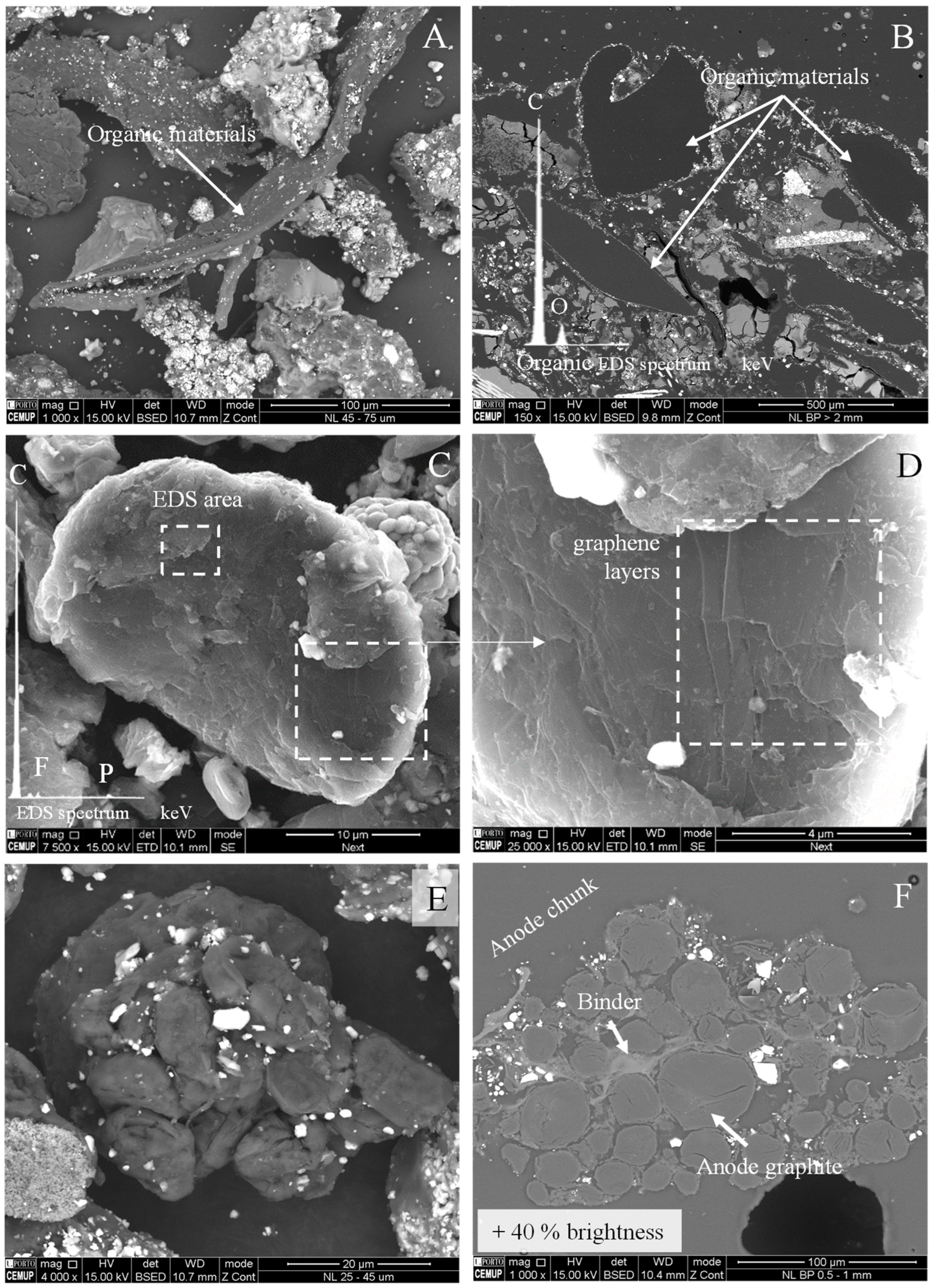
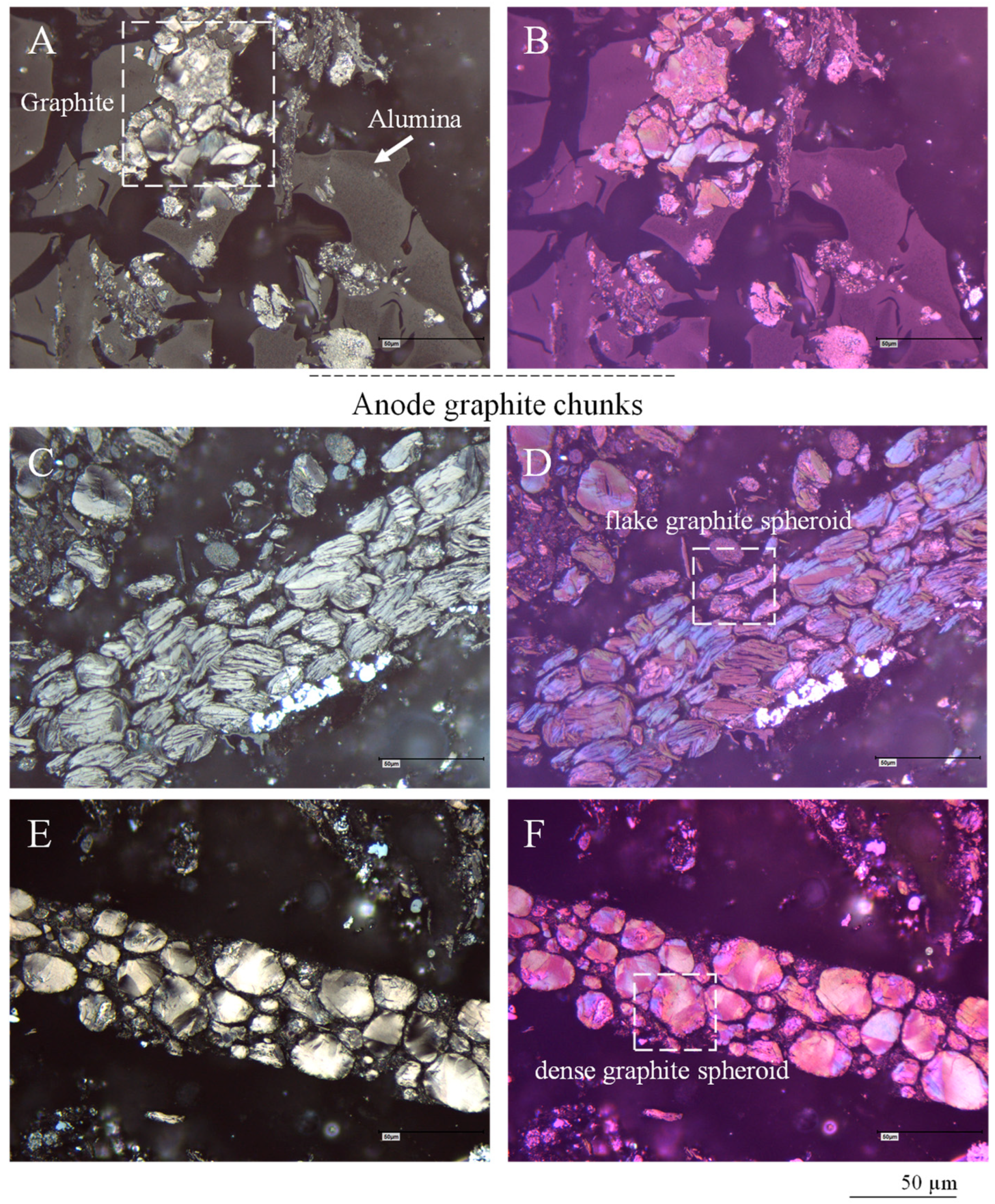
4.3.2. Anode Graphite
- Detached as discrete particles of graphite;
- As anode graphite chunks comprising dozen to hundred particles;
- As residues in the Cu foil surfaces;
- As layer fragments trapped inside Cu folds;
- As complex chunks of anode graphite and lithium metal oxides bound together by alumina (Figure 10A–F).
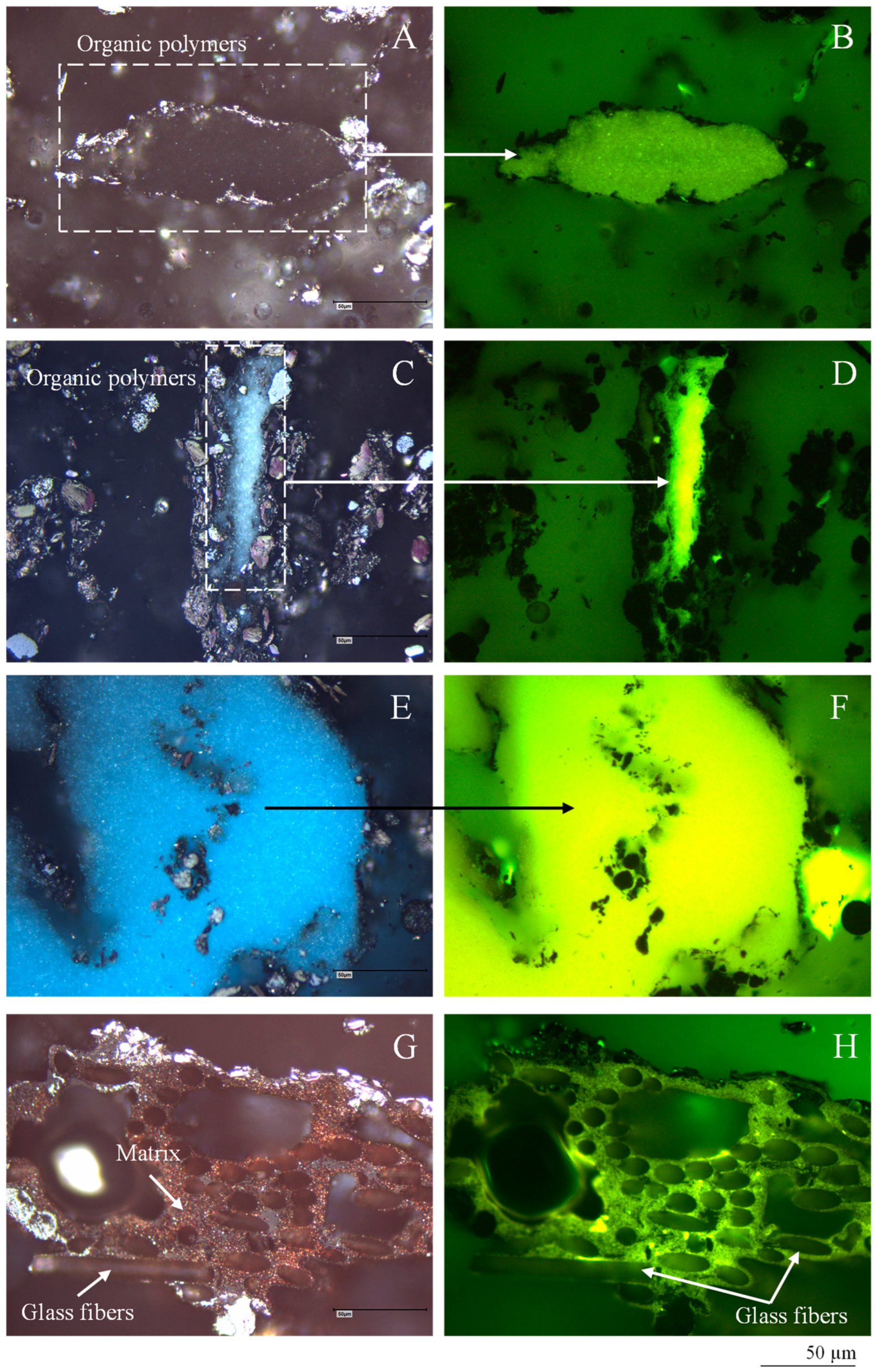
4.3.3. Organic Polymers
4.3.4. Lithium Metal Oxides Under Incident Light Microscopy
- As discrete particles;
- Detached from the Al foils forming chunks;
- As residues in the Al foil surfaces;
- -
- Blue spherical particles (Figure 12C), which are purple under polarized and retarded light, were described as Li-Mn-O after EDS analysis;
- -
- White spherical particles (Figure 12C,H), which are dark purple under polarized and retarded light, were described as Li-Ni-Co-O and Li-Ni-Mn-Co-O after EDS analysis;
- -
- Angular particles composed of twinned crystals and lamellae (Figure 12B,D), with colors varying between white, gray, dark gray, and pale blue, which are purple and pale purple under polarized and retarded light, were described as Li-Co-O after EDS analysis;
- -
- Blue angular crystals with dense or fractured optical structures occurring are associated with lithium crystal spheres (Figure 12E–H), which are intense purple (isotropic) under polarized and retarded light (their EDS analysis is missing).
- -
- None of the lithium crystals observed were fluorescent under UV light (Figure 8).
5. Applications to LIB Recycling
5.1. Advanced Petrography as a Pyrometallurgical Aid (Pyrolysis and Roasting)
5.2. Advanced Organic Petrography as a Hydrometallurgy Aid
6. Final Remarks
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahn, J.; Ehrlich, G.M. Lithium Secondary Cells, 5th ed.; Beard, K.W., Reddy, T.B., Eds.; McGraw-Hill: New York, NY, USA, 2019; Chapter 17; p. 757. ISBN 978-1-26-011593-2. [Google Scholar]
- Contestabile, M.; Panero, S.; Scrosati, B. A laboratory-scale lithium-ion battery recycling process. J. Power Sources 2001, 92, 65–69. [Google Scholar] [CrossRef]
- Zeng, X.L.; Li, J.H.; Ren, Y.S. Prediction of Various Discarded Lithium Batteries in China. In Proceedings of the 2012 IEEE International Symposium on Sustainable Systems and Technology, Boston, MA, USA, 16–18 May 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 1–4. [Google Scholar] [CrossRef]
- Pavel, C.C.; Blagoeva, D.T. Materials Impact on the EU’s Competitiveness of the Renewable Energy, Storage and E-Mobility Sectors—Wind Power, Solar Photovoltaic and Battery Technologies; EUR 28774 EN; JRC108356; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-79-73506-6. [Google Scholar] [CrossRef]
- Richa, K. Sustainable Management of Lithium-Ion Batteries After Use in Electric Vehicles. Ph.D. Thesis, Rochester Institute of Technology, Rochester, NY, USA, 2016. Available online: https://repository.rit.edu/cgi/viewcontent.cgi?params=/context/theses/article/10463/&path_info=KRichaDissertation12_2016.pdf (accessed on 31 October 2024).
- Melin, H.E. State of the Art in Reuse and Recycling of Lithium-ion Batteries—A Research Review. 2019. Available online: https://www.energimyndigheten.se/globalassets/forskning--innovation/overgripande/state-of-the-art-in-reuse-and-recycling-of-lithium-ion-batteries-2019.pdf (accessed on 13 May 2023).
- Available online: https://www.statista.com/topics/2049/lithium-ion-battery-industry/#topicOverview (accessed on 31 October 2024).
- Annex II of the Regulation Proposal COM/2023/160 Final. Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020. Available online: https://single-market-economy.ec.europa.eu/sectors/raw-materials/areas-specific-interest/critical-raw-materials_en#fifth-list-2023-of-critical-raw-materials-for-the-eu (accessed on 31 October 2024).
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
- Gaines, L.; Nelson, P. Lithium-Ion Batteries: Possible Materials Issues. In Proceedings of the 13th International Battery Materials Recycling Seminar and Exhibit, 2010, Broward County Convention Center, Fort Lauderdale, FL, USA, 16–18 March 2009; p. 16. [Google Scholar]
- Dunn, J.B.; Gaines, L.; Barnes, M.; Sullivan, J.; Wang, M. Material and Energy Flows in the Materials Production, Assemble, and End-of-Life Stages of the Automotive Lithium-Ion Battery Life Cycle; Report ANL/ESD/12-3; Argonne National Laboratory (ANL): Argonne, IL, USA, 2012.
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- Diekmann, J.; Hanisch, C.; Froböse, L.; Schälicke, G.; Loellhoeffel, T.; Fölster, A.-S.; Kwadea, A. Ecological Recycling of Lithium-Ion Batteries from Electric Vehicles with Focus on Mechanical Processes. J. Electrochem. Soc. 2017, 164, A6184–A6191. [Google Scholar] [CrossRef]
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling end-of-life electric vehicle lithium-ion batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Gaines, L. Profitable recycling of low-cobalt lithium-ion batteries will depend on new process developments. One Earth 2019, 1, 413–415. [Google Scholar] [CrossRef]
- Li, J.; Shi, P.; Wang, Z.; Chen, Y.; Chang, C.-C. A combined recovery process of metals in spent lithium-ion batteries. Chemosphere 2009, 77, 1132–1136. [Google Scholar] [CrossRef]
- Buchert, M.; Sutter, J. Aktualisierte Ökobilanzen zum Recyclingverfahren LithoRec II für Lithium-Ionen-Batterien (Stand 09/2016). 2016. Available online: https://www.erneuerbar-mobil.de/sites/default/files/2017-01/LithoRec%20II-LCA-Update%202016.pdf (accessed on 25 May 2024).
- Costa, C.M.; Lee, Y.-H.; Kim, J.-H.; Lee, S.-Y.; Lanceros-Méndez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Saunier, J.; Alloin, F.; Sanchez, J.Y.; Barriere, B. Plasticized microporous poly(vinylidene fluoride) separators for lithium-ion batteries. I. Swelling behavior of dense membranes with respect to a liquid electrolyte—Characterization of the swelling equilibrium. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 532–543. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A review of recent developments in membrane separators for rechargeable lithium-ion batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Reddy, T.; Linden, D. Thoroughly Revised, Comprehensive Coverage of Battery Technology, Characteristics, and Applications. In Linden’s Handbook of Batteries, 5th ed.; McGraw-Hill: New York, NY, USA, 2019; ISBN 9781260115925. [Google Scholar]
- Liu, Z.; Fu, W.; Liang, C. Part IV: New Emerging Technologies. Lithium–Sulfur Batteries. In Handbook of Battery Materials, 2nd ed.; Daniel, C., Besenhard, J.O., Eds.; Wiley-VCH Verlag & Co.: Weinheim, Germany, 2011; p. 811. ISBN 978-3-527-32695-2. [Google Scholar]
- Zhang, J.-G.; Bruce, P.G.; Zhang, X.G. Part IV: New Emerging Technologies. Metal–Air Batteries. In Handbook of Battery Materials, 2nd ed.; Daniel, C., Besenhard, J.O., Eds.; Wiley-VCH Verlag & Co.: Weinheim, Germany, 2011; p. 759. ISBN 978-3-527-32695-2. [Google Scholar]
- Zhang, X.; Chung, M.; Kim, H.; Wang, C.-W.; Sastry, A.M. Part V: Performance and Technology Development for Batteries. Mechanics of Battery Cells and Materials. In Handbook of Battery Materials, 2nd ed.; Daniel, C., Besenhard, J.O., Eds.; Wiley-VCH Verlag & Co.: Weinheim, Germany, 2011; p. 877. ISBN 978-3-527-32695-2. [Google Scholar]
- Melin, H.E. The Lithium-Ion Battery End-of-Life Market 2018–2025; Analysis of Volumes, Players, Technologies and Trends. Circular Energy Storage, United Kingdom, 2018. Available online: https://static1.squarespace.com/static/587657ddbe659497fb46664c/t/5b511b990e2e7239c2bc7b0b/1532042145266/Table+of+content+The+lithium-ion+battery+end-of-life+market.pdf (accessed on 31 October 2024).
- FCAB, 2021. Federal Consortium for Advanced Batteries. Executive Summary. National Blueprint for Lithium Batteries. 2021–2030. DOE/EE-2348. Available online: https://www.energy.gov/eere/vehicles/vehicle-technologies-office (accessed on 31 October 2024).
- Gies, E. Recycling: Lazarus batteries. Nature 2015, 526, S100–S101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; He, Y.; Wang, F.; Li, H.; Duan, C.; Wu, C. Surface analysis of cobalt-enriched crushed products of spent lithium-ion batteries by X-ray photoelectron spectroscopy. Sep. Purif. Technol. 2014, 138, 21–27. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.J.; Rarotra, S.; Krikstolaityte, V.; Zhuoran, K.W.; Cindy, Y.D.-I.; Tan, X.Y.; Carboni, M.; Meyer, D.; Yan, Q.; Srinivasan, M. Green recycling methods to treat lithium-ion batteries E-Waste: A circular approach to sustainability. Adv. Mater. 2022, 34, 2103346. [Google Scholar] [CrossRef]
- Sakultung, S.; Pruksathorn, K.; Hunsom, M. Simultaneous recovery of valuable metals from spent mobile phone battery by an acid leaching process. Korean J. Chem. Eng. 2007, 24, 272–277. [Google Scholar] [CrossRef]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A closed loop process for recycling spent lithium ion batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Nayl, A.A.; Elkhashab, R.A.; Badawy, S.M.; El-Khateeb, M.A. Acid leaching of mixed spent Li-ion batteries. Arab. J. Chem. 2017, 10, S3632–S3639. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger. Waste Manag. 2017, 60, 666–679. [Google Scholar] [CrossRef]
- Das, D.; Manna, S.; Puravankara, S. Electrolytes, Additives and Binders for NMC Cathodes in Li-Ion Batteries—A Review. Batteries 2023, 9, 193. [Google Scholar] [CrossRef]
- Krekeler, M.P.S. Transmission electron microscopy (TEM) investigations of Mn-oxide rich cathodic material from spent disposable alkaline batteries. Waste Manag. 2008, 28, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Ruiz, I.; Crelling, J.C. Applied Coal Petrology: The Role of Petrology in Coal Utilization; Elsevier Science: Amsterdam, The Netherlands, 2008; ISBN 978-0-08-045051-3. [Google Scholar]
- ISO 7404-2; Methods for the Petrographic Analysis of Coals—Part 2: Methods of Preparing Coal Samples. International Organization for Standardization: Geneva, Switzerland, 2009; p. 12.
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis, 4th ed.; Springer Nature: Berlin, Germany, 2018; ISBN 978-1-4939-6674-5. ISBN 978-1-4939-6676-9. [Google Scholar] [CrossRef]
- Craig, J.R.; Vaughan, D.J. Ore Microscopy and Ore Petrography, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA; Chichester, UK; Brisbane, Australia; Toronto, ON, Canada; Singapore, 1994; p. 434. ISBN 0-471-55175-9. [Google Scholar]
- Alpern, B.; Cheymol, D. Réflectance et fluorescence des organoclastes du Toarcien du Bassin de Paris en fonction de la profondeur et de la température. Rev. Inst. Francais Pétrole 1978, 33, 515–535. [Google Scholar] [CrossRef]
- Pickel, W.; Kus, J.; Flores, D.; Kalaitzidis, S.; Christanis, K.; Cardott, B.J.; Misz-Kennan, M.; Rodrigues, S.; Hentschel, A.; Hamor-Vido, M.; et al. ICCP. Classification of liptinite—ICCP System 1994. Int. J. Coal Geol. 2017, 169, 40–61. [Google Scholar] [CrossRef]
- ISO 7404-3; Methods for the Petrographic Analysis of Coals—Part 3: Method of Determining Maceral Group Composition. International Organization for Standardization: Geneva, Switzerland, 2009; p. 7.
- Badenhorst, C.; Kuzniarska-Biernacka, I.; Guedes, A.; Mousa, E.; Ramos, V.; Rollinson, G.; Ye, G.; Valentim, B. Recovery of Graphite from Spent Lithium-Ion Batteries. Recycling 2023, 8, 79. [Google Scholar] [CrossRef]
- ISO 7404-5; Methods for the Petrographic Analysis of Coals—Part 5: Method of Determining Microscopically the Reflectance of Vitrinite. International Organization for Standardization: Geneva, Switzerland, 2009; p. 14.
- ASTM D2798-11; Standard Test Method for Microscopical Determination of the Vitrinite Reflectance of Coal. American Society for Testing and Materials: West Conshohocken, PA, USA, 2011; p. 5.
- Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A.A. Comprehensive Review on Raman Spectroscopy Applications. Chemosensors 2021, 9, 262. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Guedes, A.; Valentim, B.; Prieto, A.C.; Noronha, F. Raman spectroscopy of coal macerals and fluidized bed char morphotypes. Fuel 2012, 97, 443–449. [Google Scholar] [CrossRef]
- Gokturk, P.A.; Kakenov, N.; Kocabas, C.; Suzer, S. Raman and X-ray photoelectron spectroscopic studies of graphene devices for identification of doping. Appl. Surf. Sci. 2017, 425, 1130–1137. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Tanimura, M.; Tachibana, M. Temperature dependence of the Raman spectra of multilayer graphene nanoribbons fabricated by unzipping method. Diam. Relat. Mater. 2020, 109, 108047. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zhou, W.; Shi, W.; Dang, W.; Li, X.; Liang, B. The split-up of G band and 2D band in temperature-dependent Raman spectra of suspended graphene. Opt. Laser Technol. 2021, 139, 106960. [Google Scholar] [CrossRef]
- Knight, D.S.; White, W.B. Characterization of diamond films by Raman spectroscopy. J. Mater. Res. 1989, 4, 385–393. [Google Scholar] [CrossRef]
- Adams, R.A.; Li, B.; Kazmi, J.; Adams, T.E.; Tomar, V.; Pol, V.G. Dynamic impact of LiCoO2 electrodes for Li-ion battery aging evaluation. Electrochim. Acta 2018, 292, 586–593. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. In situ Raman analyses of electrode materials for Li-ion batteries. AIMS Mater. Sci. 2018, 5, 650–698. [Google Scholar] [CrossRef]
- Beccard, B.; Karavadra, S.N. Lithium-Ion Battery Manufacturing and Quality Control: Raman Spectroscopy, an Analytical Technique of Choice. Spectrosc. Suppl. Raman Technol. Todays Spectrosc. 2022, 37, 46–53. [Google Scholar] [CrossRef]
- Severin, K.P. Energy Dispersive Spectrometry of Common Rock Forming Minerals; Springer Nature: Berlin, Germany, 2004; ISBN 1-4020-2840-7. ISBN 1-4020-2841-5. [Google Scholar]
- Fischer, S.; Doose, S.; Müller, J.; Höfels, C.; Kwade, A. Impact of Spheroidization of Natural Graphite on Fast-Charging Capability of Anodes for LIB. Batteries 2023, 9, 305. [Google Scholar] [CrossRef]
- Lampe-Onnerud, C.; Shi, J.; Onnerud, P.; Chamberlain, R.; Barnett, B. Benchmark study on high performing carbon anode materials. J. Power Sources 2001, 97, 133–136. [Google Scholar] [CrossRef]
- Glazier, S.L.; Li, J.; Louli, A.J.; Allen, J.P.; Dahn, J.R. An Analysis of Artificial and Natural Graphite in Lithium Ion Pouch Cells Using Ultra-High Precision Coulometry, Isothermal Microcalorimetry, Gas Evolution, Long Term Cycling and Pressure Measurements. J. Electrochem. Soc. 2017, 164, A3545. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material—Fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387. [Google Scholar] [CrossRef]
- Kwiecinska, B.; Petersen, H.I. Graphite, semi-graphite, natural coke, and natural char classification—ICCP system. Int. J. Coal Geol. 2004, 57, 99–116. [Google Scholar] [CrossRef]
- Crelling, J.C.; Rimmer, S.M. Crelling’s Petrographic Atlas of Coals and Carbons. Southern Illinois University Carbondale. 2015. Available online: https://coalandcarbonatlas.siu.edu/ (accessed on 20 July 2024).
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Zhan, R.; Yang, Z.; Bloom, I.; Pan, L. Significance of a Solid Electrolyte Interphase on Separation of Anode and Cathode Materials from Spent Li-Ion Batteries by Froth Flotation. ACS Sustain. Chem. Eng. 2021, 9, 531–540. [Google Scholar] [CrossRef]
- Mousa, E.; Hu, X.; Ånnhagen, L.; Ye, G.; Cornelio, A.; Fahimi, A.; Bontempi, E.; Frontera, P.; Badenhorst, C.; Santos, A.C.; et al. Thermal Treatment and Characterisation of the Black Mass from Spent Lithium-ion Batteries. Sustainability 2023, 15, 15. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentim, B. Teaching Aid Regarding the Application of Advanced Organic Petrography in Recycling End-of-Life Lithium-Ion Batteries. Batteries 2024, 10, 391. https://doi.org/10.3390/batteries10110391
Valentim B. Teaching Aid Regarding the Application of Advanced Organic Petrography in Recycling End-of-Life Lithium-Ion Batteries. Batteries. 2024; 10(11):391. https://doi.org/10.3390/batteries10110391
Chicago/Turabian StyleValentim, Bruno. 2024. "Teaching Aid Regarding the Application of Advanced Organic Petrography in Recycling End-of-Life Lithium-Ion Batteries" Batteries 10, no. 11: 391. https://doi.org/10.3390/batteries10110391
APA StyleValentim, B. (2024). Teaching Aid Regarding the Application of Advanced Organic Petrography in Recycling End-of-Life Lithium-Ion Batteries. Batteries, 10(11), 391. https://doi.org/10.3390/batteries10110391





