Early Investigations on Electrolyte Mixing Issues in Large Flow Battery Tanks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Cycling Procedure
2.3. Fluid Mechanics in the Tanks
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amir, M.; Deshmukh, R.G.; Khalid, H.M.; Said, Z.; Raza, A.; Muyeen, S.; Nizami, A.-S.; Elavarasan, R.M.; Saidur, R.; Sopian, K. Energy storage technologies: An integrated survey of developments, global economical/environmental effects, optimal scheduling model, and sustainable adaption policies. J. Energy Storage 2023, 72, 108694. [Google Scholar] [CrossRef]
- Kueppers, M.; Pineda, S.N.P.; Metzger, M.; Huber, M.; Paulus, S.; Heger, H.J.; Niessen, S. Decarbonization pathways of worldwide energy systems–Definition and modeling of archetypes. Appl. Energy 2021, 285, 116438. [Google Scholar] [CrossRef]
- Bloomfield, H.; Brayshaw, D.; Troccoli, A.; Goodess, C.; De Felice, M.; Dubus, L.; Bett, P.; Saint-Drenan, Y.-M. Quantifying the sensitivity of european power systems to energy scenarios and climate change projections. Renew. Energy 2021, 164, 1062–1075. [Google Scholar] [CrossRef]
- Harsini, A.E. Resilience-oriented district energy system integrated with renewable energy and multi-level seasonal energy storage. J. Energy Storage 2023, 72, 108645. [Google Scholar] [CrossRef]
- Bovo, A.; Poli, N.; Trovò, A.; Marini, G.; Guarnieri, M. Hydrogen energy storage system in a Multi–Technology Microgrid:technical features and performance. Int. J. Hydrogen Energy 2023, 48, 12072–12088. [Google Scholar] [CrossRef]
- Rana, M.; Uddin, M.; Sarkar, R.; Meraj, S.T.; Shafiullah, G.; Muyeen, S.; Islam, A.; Jamal, T. Applications of energy storage systems in power grids with and without renewable energy integration—A comprehensive review. J. Energy Storage 2023, 68, 107811. [Google Scholar] [CrossRef]
- Trovo, A.; Di Noto, V.; Mengou, J.E.; Gamabaro, C.; Guarnieri, M. Fast Response of kW-Class Vanadium Redox Flow Batteries. IEEE Trans. Sustain. Energy 2021, 12, 2413–2422. [Google Scholar] [CrossRef]
- Yamujala, S.; Jain, A.; Bhakar, R.; Mathur, J. Multi-service based economic valuation of grid-connected battery energy storage systems. J. Energy Storage 2022, 52, 104657. [Google Scholar] [CrossRef]
- Jenkins, J.D.; Sepulveda, N.A. Long-duration energy storage: A blueprint for research and innovation. Joule 2021, 5, 2241–2246. [Google Scholar] [CrossRef]
- Horton, L. Sustainable Clean Energy–Foundational Research Challenges and Opportunities. In Proceedings of the Plenary Session at 243rd ECS Meeting, Boston, MA, USA, 29 May 2023; Available online: https://www.youtube.com/watch?v=fp1FwUIGwV4 (accessed on 12 April 2024).
- LDES Council. Available online: https://www.ldescouncil.com/ (accessed on 29 July 2023).
- Zhou, H.; Zhang, R.; Ma, Q.; Li, Z.; Su, H.; Lu, P.; Yang, W.; Xu, Q. Modeling and Simulation of Non-Aqueous Redox Flow Batteries: A Mini-Review. Batteries 2023, 9, 215. [Google Scholar] [CrossRef]
- Viswanathan, V.V.; Crawford, A.J.; Thomsen, E.C.; Shamim, N.; Li, G.; Huang, Q.; Reed, D.M. An Overview of the Design and Optimized Operation of Vanadium Redox Flow Batteries for Durations in the Range of 4–24 Hours. Batteries 2023, 9, 221. [Google Scholar] [CrossRef]
- Gundlapalli, R.; Jayanti, S. Effective splitting of serpentine flow field for applications in large-scale flow batteries. J. Power Sources 2021, 487, 229409. [Google Scholar] [CrossRef]
- Bogdanov, S.; Parsegov, S.; Schubert, C.; Ibanez, F.M.; Pettinger, K.-H.; Pugach, M. Parameter identification algorithm for dynamic modeling of industrial-scale vanadium redox flow batteries. J. Power Sources 2023, 580, 233423. [Google Scholar] [CrossRef]
- Di Noto, V.; Vezzù, K.; Crivellaro, G.; Pagot, G.; Sun, C.; Meda, L.; Rutkowska, I.A.; Kulesza, P.J.; Zawodzinski, T.A. A general electrochemical formalism for vanadium redox flow batteries. Electrochim. Acta 2022, 408, 139937. [Google Scholar] [CrossRef]
- Arenas, L.F.; de León, C.P.; Walsh, F.C. Redox flow batteries for energy storage: Their promise, achievements and challenges. Curr. Opin. Electrochem. 2019, 16, 117–126. [Google Scholar] [CrossRef]
- Aberoumand, S.; Dubal, D.; Woodfield, P.; Parsa, S.M.; Mahale, K.; Pham, H.D.; Tung, T.; Nguyen, H.-Q.; Dao, D.V. Enhancement in vanadium redox flow battery performance using reduced graphene oxide nanofluid electrolyte. J. Energy Storage 2023, 72, 108343. [Google Scholar] [CrossRef]
- Roe, S.; Menictas, C.; Skyllas-Kazacos, M. A High Energy Density Vanadium Redox Flow Battery with 3 M Vanadium Electrolyte. J. Electrochem. Soc. 2015, 163, A5023–A5028. [Google Scholar] [CrossRef]
- Banerjee, R.; Bevilacqua, N.; Eifert, L.; Zeis, R. Characterization of carbon felt electrodes for vanadium redox flow batteries—A pore network modeling approach. J. Energy Storage 2019, 21, 163–171. [Google Scholar] [CrossRef]
- Greco, K.V.; Forner-Cuenca, A.; Mularczyk, A.; Eller, J.J.; Brushett, F.R. Elucidating the nuanced effects of thermal pretreatment on carbon paper electrodes for vanadium redox flow batteries. ACS Appl. Mater. Interfaces 2018, 10, 44430–44442. [Google Scholar] [CrossRef]
- Sun, C.; Zlotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Di Noto, V. [Nafion/(WO3)x] hybrid membranes for vanadium redox flow batteries. Solid State Ionics 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Gandomi, Y.A.; Aaron, D.S.; Mench, M.M. Influence of Membrane Equivalent Weight and Reinforcement on Ionic Species Crossover in All-Vanadium Redox Flow Batteries. Membranes 2017, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Darling, R.M.; Perry, M.L. The influence of electrode and channel configurations on flow battery performance. J. Electrochem. Soc. 2014, 161, A1381–A1387. [Google Scholar] [CrossRef]
- Kumar, S.; Jayanti, S. Effect of flow field on the performance of an all-vanadium redox flow battery. J. Power Sources 2016, 307, 782–787. [Google Scholar] [CrossRef]
- Ke, X.; Prahl, J.M.; Alexander, J.I.D.; Wainright, J.S.; Zawodzinski, T.A.; Savinell, R.F. Rechargeable redox flow batteries: Flow fields, stacks and design considerations. Chem. Soc. Rev. 2018, 47, 8721–8743. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Xing, F.; Li, X.; Ning, G.; Zhang, H. Flow field design and optimization based on the mass transport polarization regulation in a flow-through type vanadium flow battery. J. Power Sources 2016, 324, 402–411. [Google Scholar] [CrossRef]
- Chen, F.; Cheng, X.; Liu, L.; Han, L.; Liu, J.; Chen, H.; Zhang, Q.; Yan, C. Modification of carbon felt electrode by MnO@C from metal-organic framework for vanadium flow battery. J. Power Sources 2023, 580, 233421. [Google Scholar] [CrossRef]
- Kim, J.D.; Park, H. Synergistic effect of nanofluid as catalyst with carbon foam electrode for improved rheological properties of aqueous electrolytes for vanadium redox flow battery. J. Power Sources 2021, 500, 229974. [Google Scholar] [CrossRef]
- Poli, N.; Trovò, A.; Fischer, P.; Noack, J.; Guarnieri, M. Electrochemical rebalancing process for vanadium flow batteries: Sizing and economic assessment. J. Energy Storage 2023, 58, 106404. [Google Scholar] [CrossRef]
- Bhattarai, A.; Ghimire, P.C.; Whitehead, A.; Schweiss, R.; Scherer, G.G.; Wai, N.; Hng, H.H. Novel approaches for solving the capacity fade problem during operation of a vanadium redox flow battery. Batteries 2018, 4, 48. [Google Scholar] [CrossRef]
- Trinidad, P.; Walsh, F.C.; Gilroy, D. Conversion expressions for electrochemical reactors which operate under mass transport controlled reaction conditions, Part I: Batch reactor, PFR and CSTR. Int. J. Engng. 1998, 14, 431–441. [Google Scholar]
- Tang, A.; Ting, S.; Bao, J.; Skyllas-Kazacos, M. Thermal modelling and simulation of the all-vanadium redox flow battery. J. Power Sources 2012, 203, 165–176. [Google Scholar] [CrossRef]
- Wang, Y.; Smith, K.C. Numerical investigation of convective transport in redox flow battery tanks: Using baffles to increase utilization. J. Energy Storage 2019, 25, 100840. [Google Scholar] [CrossRef]
- Prieto-Díaz, P.A.; Ibáñez, S.E.; Vera, M. Fluid dynamics of mixing in the tanks of small vanadium redox flow batteries: Insights from order-of-magnitude estimates and transient two-dimensional simulations. Int. J. Heat Mass Transf. 2023, 216, 124567. [Google Scholar] [CrossRef]
- Trovò, A.; Poli, N.; Guarnieri, M. New strategies for the evaluation of Vanadium Flow Batteries: Testing prototypes. Curr. Opin. Chem. Eng. 2022, 37, 100853. [Google Scholar] [CrossRef]
- McArdle, S.; Marshall, A.T. Why electrode orientation and carbon felt heterogeneity can influence the performance of flow batteries. J. Power Sources 2023, 562, 232755. [Google Scholar] [CrossRef]
- Trovo, A.; Guarnieri, M. Battery management system with testing protocols for kW-class vanadium redox flow batteries. In Proceedings of the 2020 2nd IEEE International Conference on Industrial Electronics for Sustainable Energy Systems (IESES), Cagliari, Italy, 1–3 September 2020; pp. 33–38. [Google Scholar] [CrossRef]
- Mousa, A. Chemical and Electrochemical Studies of V (III) and V (II) Solutions in Sulfuric Acid Solution for Vanadium Battery Ap-plications. Ph.D. Thesis, University of New South Wales, Sydney, Australia, 2003. [Google Scholar]
- Ressel, S.; Bill, F.; Holtz, L.; Janshen, N.; Chica, A.; Flower, T.; Weidlich, C.; Struckmann, T. State of charge monitoring of vanadium redox flow batteries using half cell potentials and electrolyte density. J. Power Sources 2018, 378, 776–783. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery—A review. ChemSusChem 2016, 9, 1521–1543. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiong, J.; Tang, A.; Qin, Y.; Liu, J.; Yan, C. Investigation of the use of electrolyte viscosity for online state-of-charge monitoring design in vanadium redox flow battery. Appl. Energy 2018, 211, 1050–1059. [Google Scholar] [CrossRef]
- Tang, A.; Bao, J.; Skyllas-Kazacos, M. Studies on pressure losses and flow rate optimization in vanadium redox flow battery. J. Power Sources 2014, 248, 154–162. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Gao, H.; Liu, J.; Yan, C.; Tang, A. Numerical modelling and in-depth analysis of multi-stack vanadium flow battery module incorporating transport delay. Appl. Energy 2019, 247, 13–23. [Google Scholar] [CrossRef]
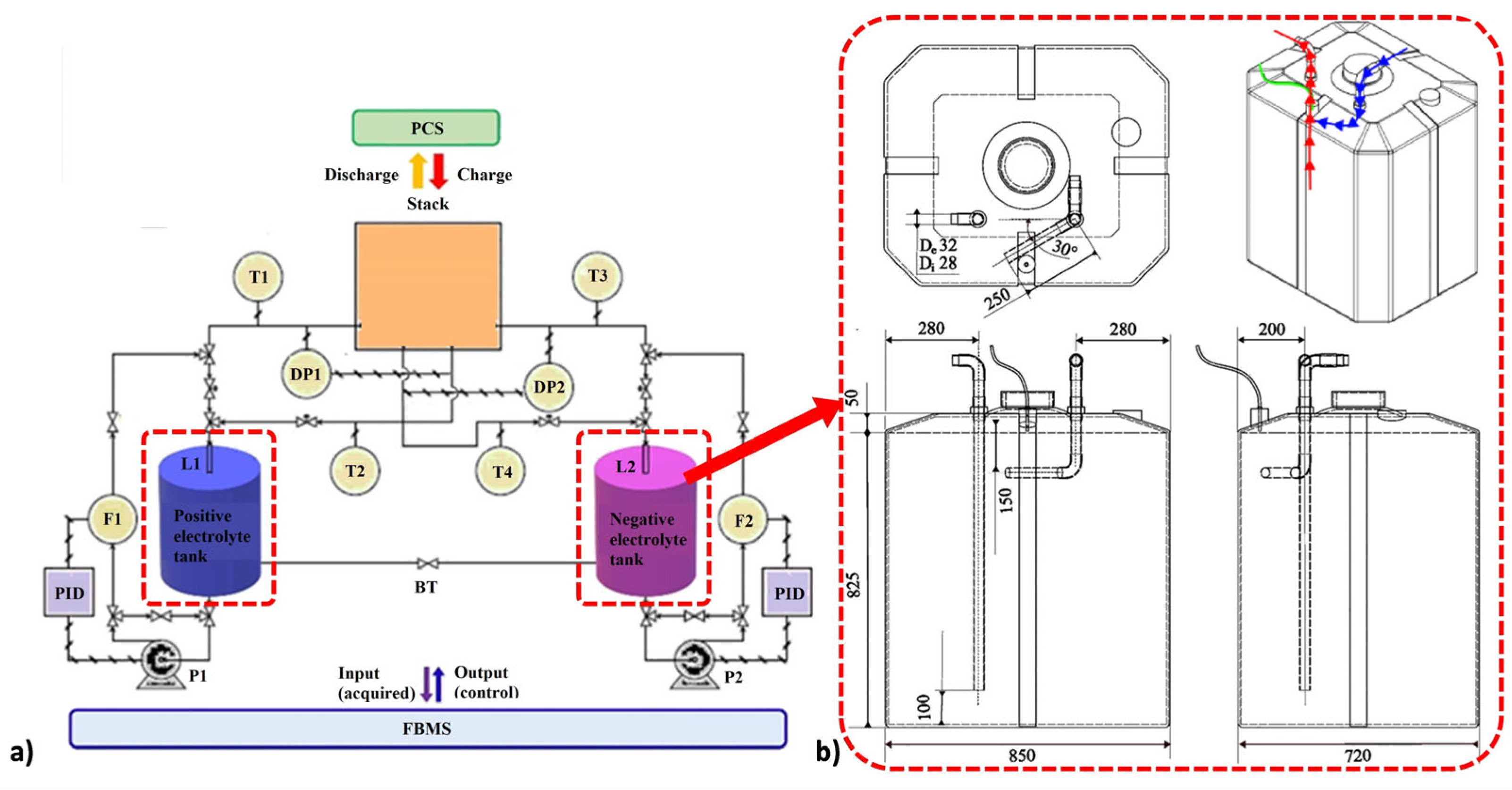
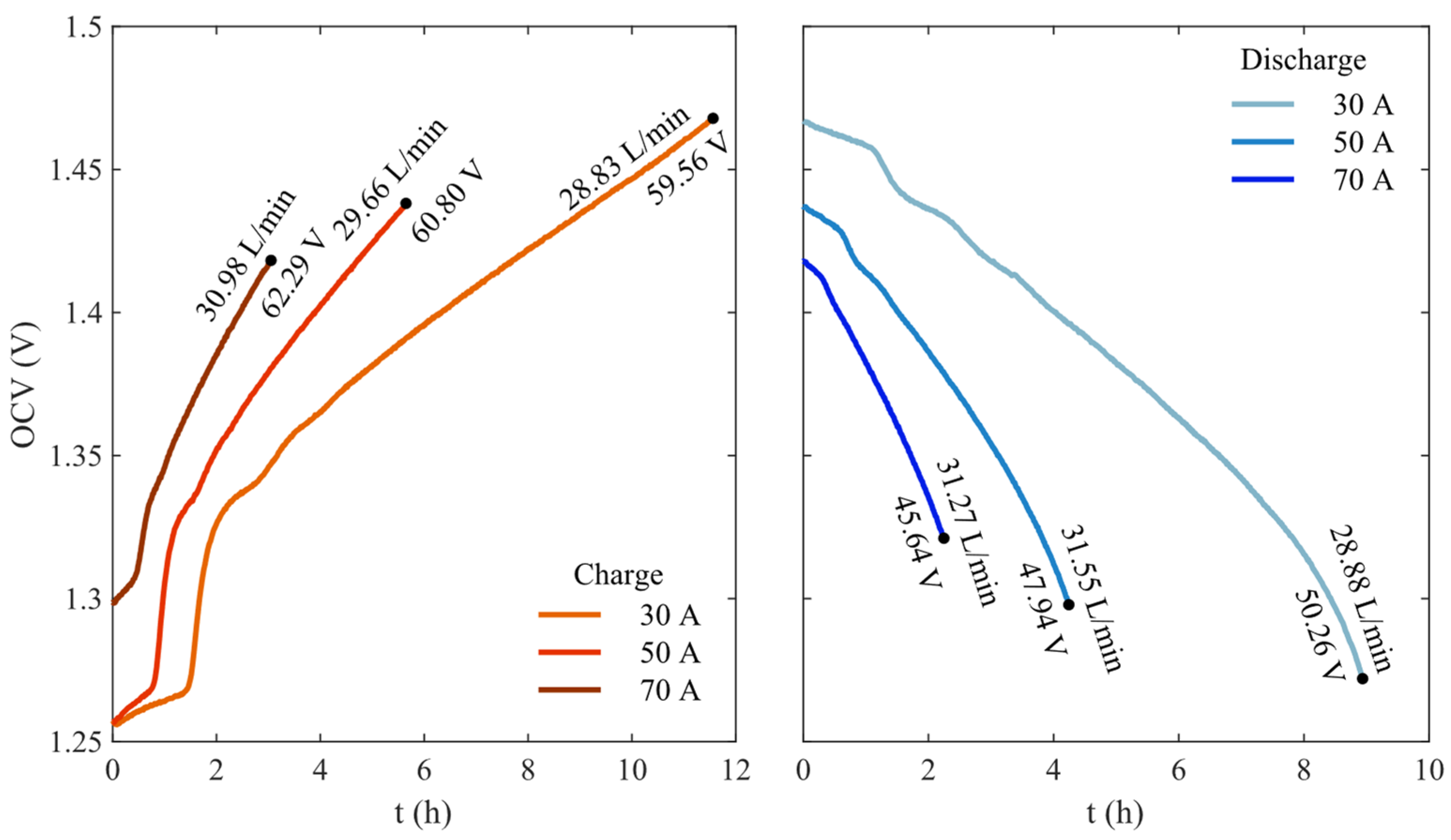

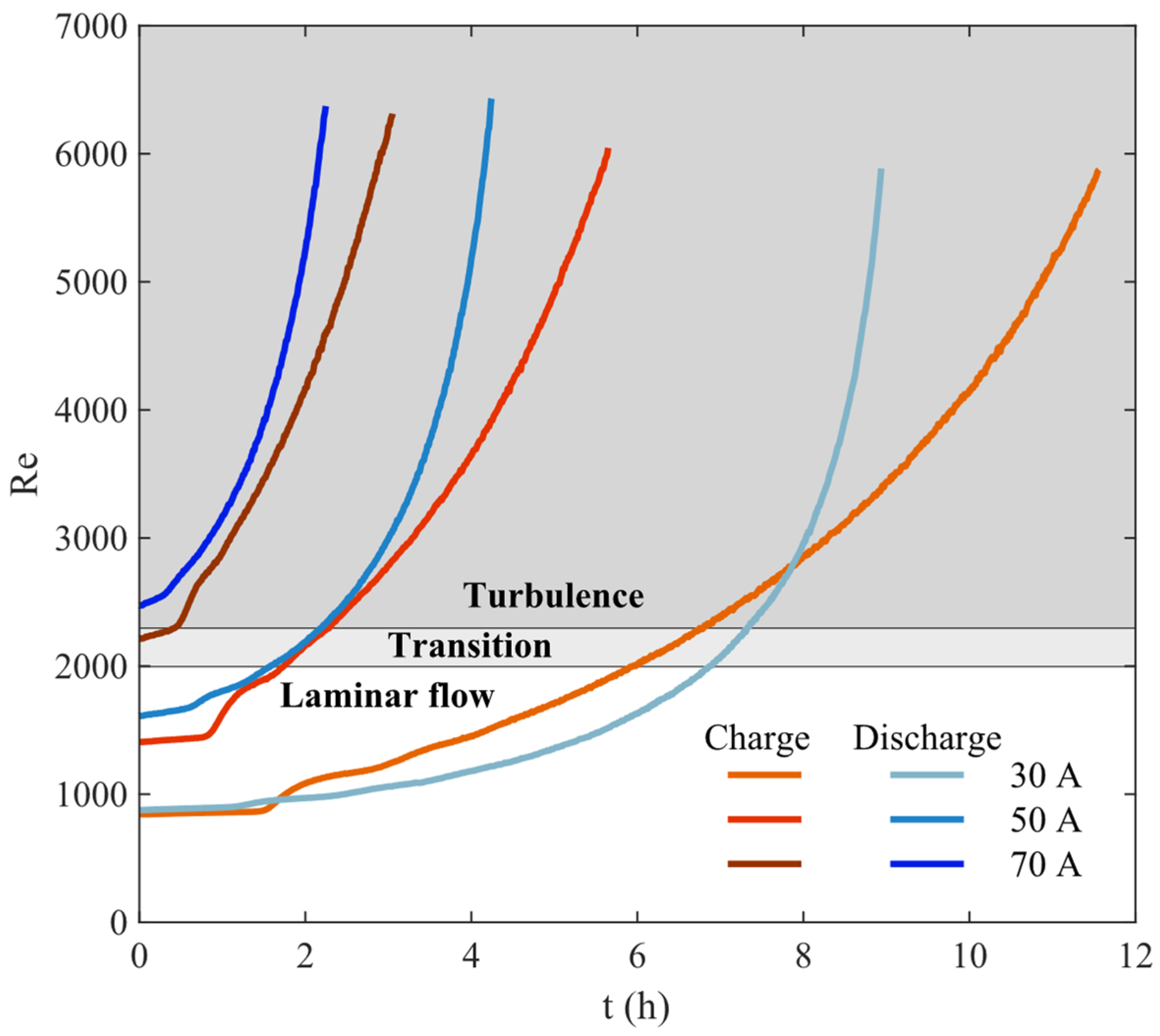
| Parameter | Value | References |
|---|---|---|
| [42] | ||
| [41] | ||
| [36,42] | ||
| [36,41,42] | ||
| - | ||
| [43] |
| I [A] | SoC Range [%] | Q [L min−1] |
|---|---|---|
| 30 | 17–63 | 4.2–30.3 |
| 50 | 23–56 | 7.8–32.6 |
| 70 | 29–52 | 11.8–32.3 |
| Current | Charge | Discharge | ||
|---|---|---|---|---|
| I [A] | tPD [h] | tPF [h] | tPD [h] | tPF [h] |
| 30 | 1.498 | 1.387 | 1.182 | 1.328 |
| 50 | 0.813 | 0.829 | 0.616 | 0.717 |
| 70 | 0.471 | 0.522 | 0.297 | 0.464 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trovò, A.; Prieto-Díaz, P.A.; Zatta, N.; Picano, F.; Guarnieri, M. Early Investigations on Electrolyte Mixing Issues in Large Flow Battery Tanks. Batteries 2024, 10, 133. https://doi.org/10.3390/batteries10040133
Trovò A, Prieto-Díaz PA, Zatta N, Picano F, Guarnieri M. Early Investigations on Electrolyte Mixing Issues in Large Flow Battery Tanks. Batteries. 2024; 10(4):133. https://doi.org/10.3390/batteries10040133
Chicago/Turabian StyleTrovò, Andrea, Pablo A. Prieto-Díaz, Nicolò Zatta, Francesco Picano, and Massimo Guarnieri. 2024. "Early Investigations on Electrolyte Mixing Issues in Large Flow Battery Tanks" Batteries 10, no. 4: 133. https://doi.org/10.3390/batteries10040133
APA StyleTrovò, A., Prieto-Díaz, P. A., Zatta, N., Picano, F., & Guarnieri, M. (2024). Early Investigations on Electrolyte Mixing Issues in Large Flow Battery Tanks. Batteries, 10(4), 133. https://doi.org/10.3390/batteries10040133








