Behavior of NO3−-Based Electrolyte Additive in Lithium Metal Batteries
Abstract
1. Introduction
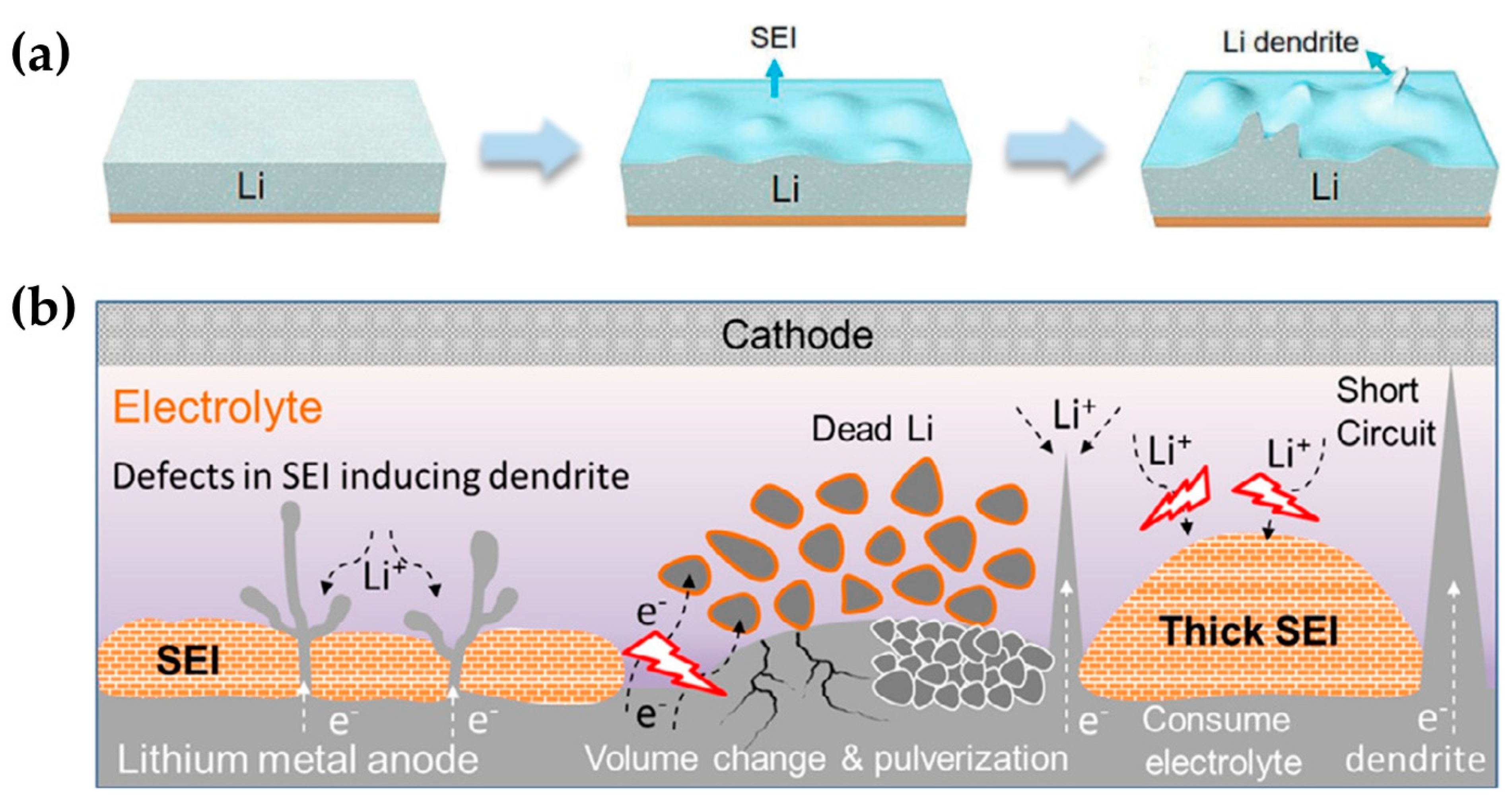
2. Dendrite of Lithium Metal Batteries and Formation of Solid Electrolyte Interface
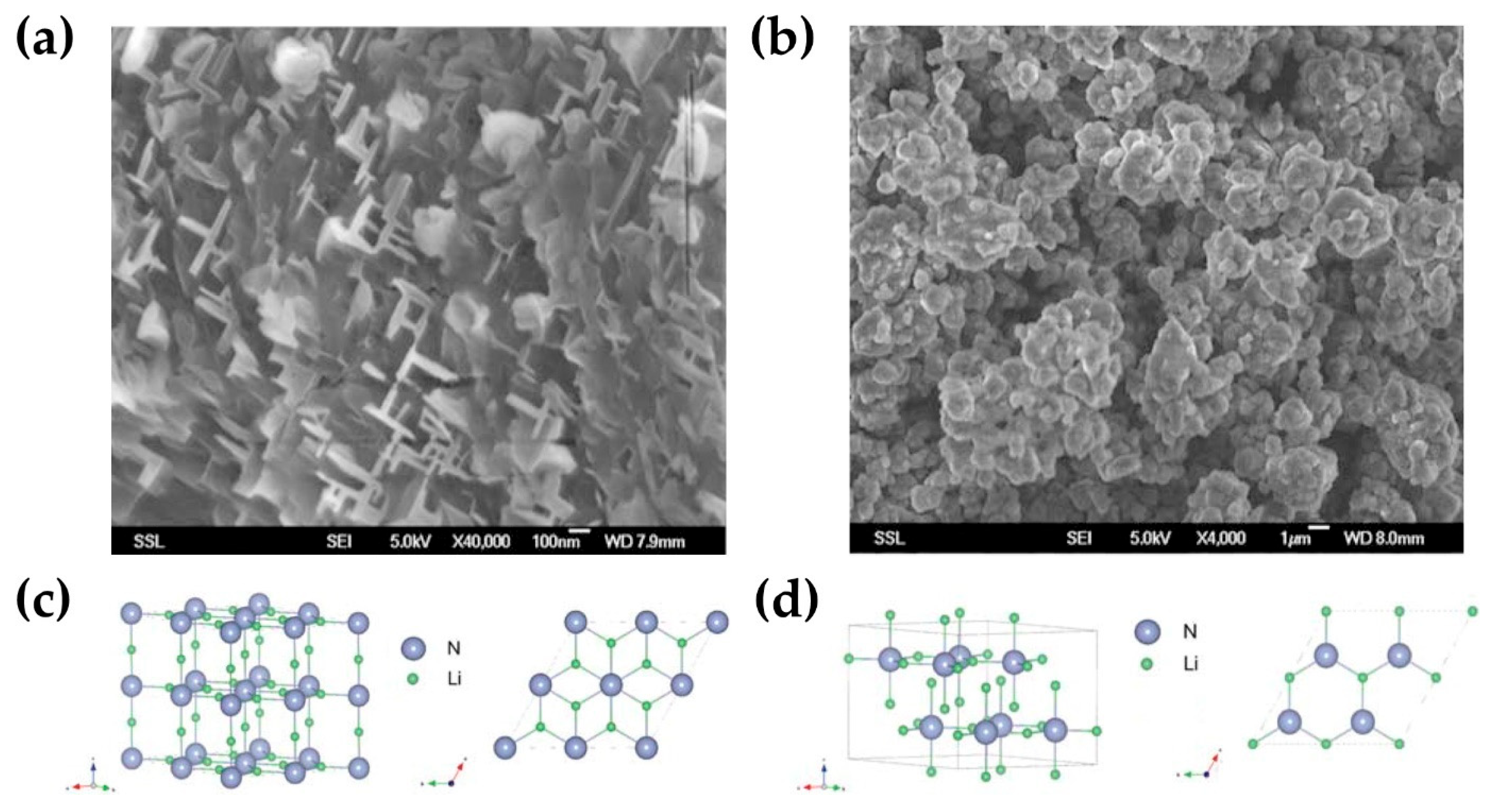
3. Li+ Ion Migration Mechanism of N Compounds in Solid Electrolyte Interfaces
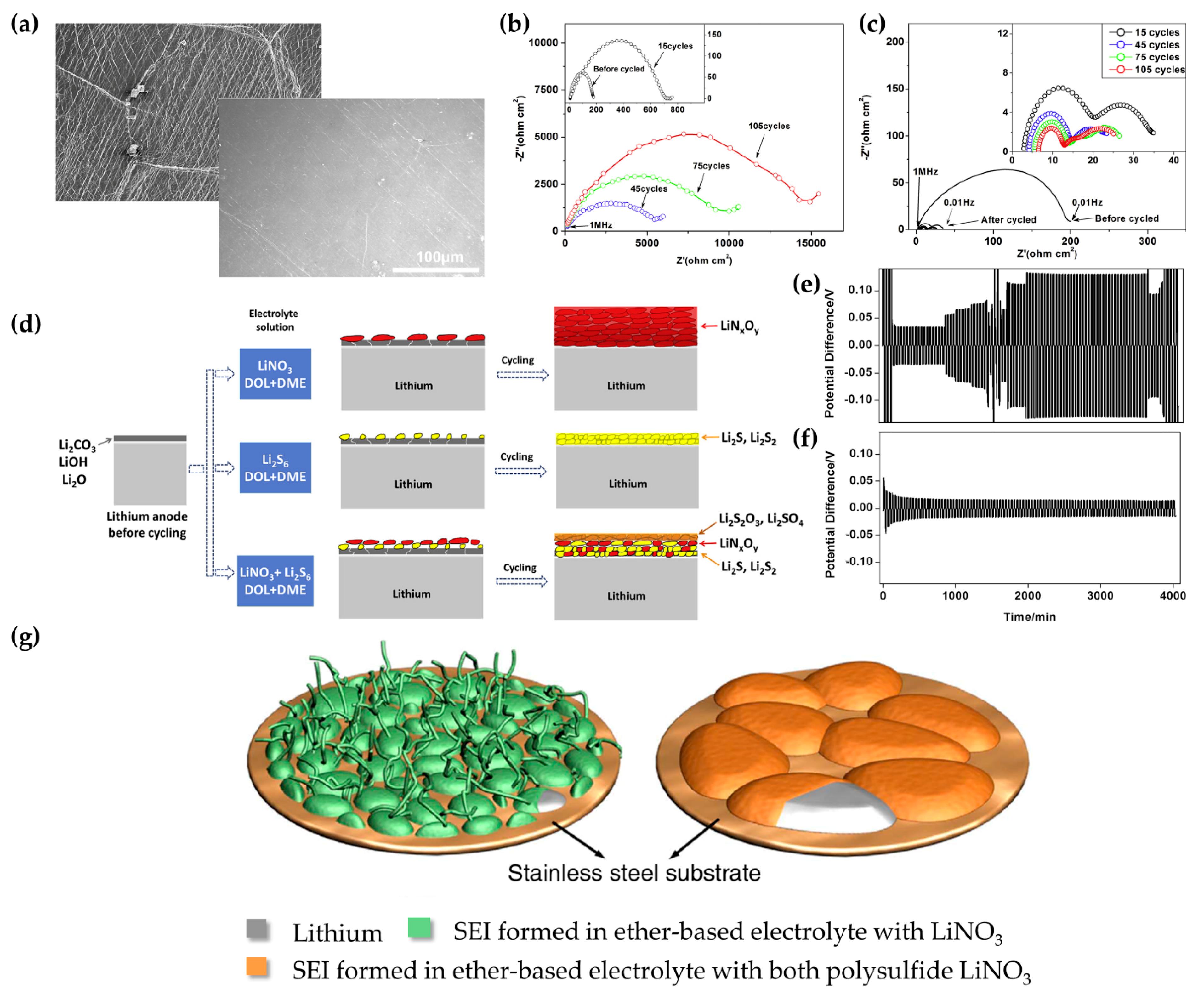
3.1. Ether-Based Electrolytes
3.2. Carbonate-Based Electrolytes
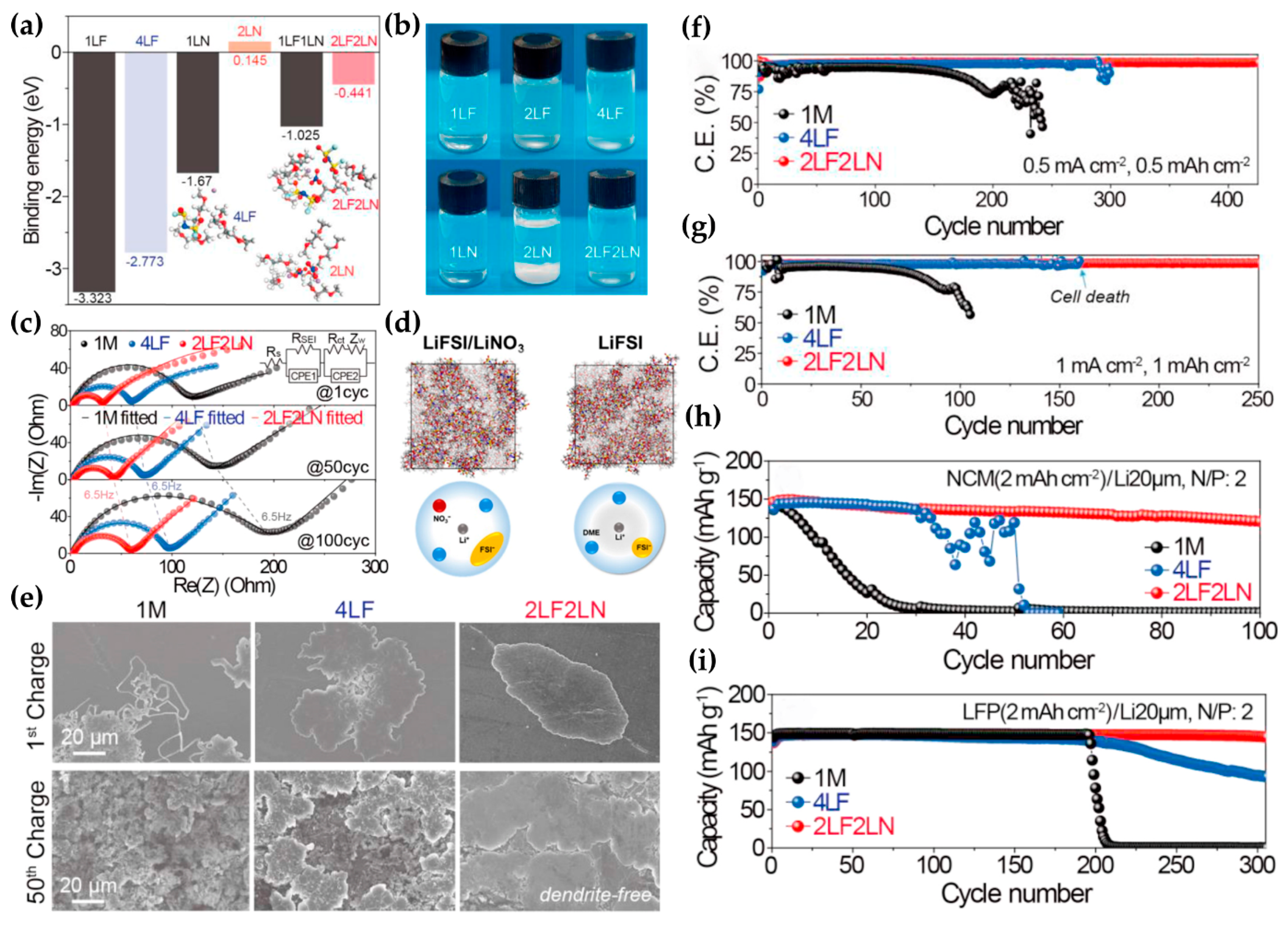
3.3. The New Approach in Carbonate-Based Electrolytes: Solubilizer-Free N-Based Electrolyte Additive
4. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Winter, M.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Seok, J.; Lee, W.; Lee, H.; Park, S.; Chung, C.; Hwang, S.; Yoon, W.-S. Aging Mechanisms of Lithium-ion Batteries. J. Electrochem. Sci. Technol. 2024, 15, 51–66. [Google Scholar] [CrossRef]
- Salunkhe, T.T.; Kadam, A.N.; Hur, J.; Kim, I.T. Green and sustainably designed intercalation-type anodes for emerging lithium dual-ion batteries with high energy density. J. Energy Chem. 2023, 80, 466–478. [Google Scholar] [CrossRef]
- Huy, V.P.H.; So, S.; Kim, I.T.; Hur, J. Self-healing gallium phosphide embedded in a hybrid matrix for high-performance Li-ion batteries. Energy Storage Mater. 2021, 34, 669–681. [Google Scholar] [CrossRef]
- Salunkhe, T.T.; Varma, R.S.; Kadam, A.N.; Lee, S.W.; Lee, Y.C.; Hur, J.; Kim, I.T. Scraps to superior anodes for Li-ion batteries: Sustainable and scalable upgrading of waste rust. J. Hazard. Mater. 2021, 410, 124571. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Bae, J.; Kwon, S.H.; Hur, J.; Lee, S.G.; Kim, I.T. Synergistic effect of antimony-triselenide on addition of conductive hybrid matrix for high-performance lithium-ion batteries. J. Alloys Compd. 2020, 828, 154410. [Google Scholar] [CrossRef]
- Lee, J.H.; Kang, S.G.; Kim, I.T.; Kwon, S.; Lee, I.; Lee, S.G. Adsorption mechanisms of lithium oxides (LixO2) on N-doped graphene: A density functional theory study with implications for lithium-air batteries. Theor. Chem. Acc. 2016, 135, 193–202. [Google Scholar] [CrossRef]
- Lim, Y.E.; Choi, W.S.; Kim, J.H.; Ahn, Y.N.; Kim, I. The Sn-red P-Fe-based alloy materials for efficient Li-ion battery anodes. J. Ind. Eng. Chem. 2023, 121, 299–311. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.I.; Yoon, W.S. Enhancing Electrochemical Performance of Co(OH)2 Anode Materials by Introducing Graphene for Next-Generation Li-ion Batteries. J. Electrochem. Sci. Technol. 2022, 13, 398–406. [Google Scholar] [CrossRef]
- Tran, Q.N.; Park, C.H.; Le, T.H. Nanocrystalline Cellulose-Supported Iron Oxide Composite Materials for High-Performance Lithium-Ion Batteries. Polymers 2024, 16, 691. [Google Scholar] [CrossRef]
- Sin, D.H.; Kim, S.H.; Lee, J.; Lee, H. Modification of Electrode Interface with Fullerene-Based Self-Assembled Monolayer for High-Performance Organic Optoelectronic Devices. Micromachines 2022, 13, 1613. [Google Scholar] [CrossRef] [PubMed]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Ukyo, Y.; Novák, P. Memory effect in a lithium-ion battery. Nat. Mater. 2013, 12, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Chagas, L.G.; Jeong, S.; Hasa, I.; Passerini, S. Ionic Liquid-Based Electrolytes for Sodium-Ion Batteries: Tuning Properties to Enhance the Electrochemical Performance of Manganese-Based Layered Oxide Cathode. ACS Appl. Mater. Interfaces 2019, 11, 22278–22289. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.P.; Tao, J.H.; Yan, P.F.; Henderson, W.A.; Li, Q.Y.; Shao, Y.Y.; Helm, M.L.; Borodin, O.; Graff, G.L.; Polzin, B.; et al. Formation of Reversible Solid Electrolyte Interface on Graphite Surface from Concentrated Electrolytes. Nano Lett. 2017, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.I.; Yoon, W.S. Challenges and Design Strategies for Conversion-Based Anode Materials for Lithium- and Sodium-Ion Batteries. J. Electrochem. Sci. Technol. 2022, 13, 32–53. [Google Scholar] [CrossRef]
- Janek, J.; Zeier, W.G. A solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Yamada, Y.; Usui, K.; Chiang, C.H.; Kikuchi, K.; Furukawa, K.; Yamada, A. General Observation of Lithium Intercalation into Graphite in Ethylene-Carbonate-Free Superconcentrated Electrolytes. ACS Appl. Mater. Interfaces 2014, 6, 10892–10899. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G. Anode-less. Nat. Energy 2019, 4, 637–638. [Google Scholar] [CrossRef]
- Wang, R.H.; Cui, W.S.; Chu, F.L.; Wu, F.X. Lithium metal anodes: Present and future. J. Energy Chem. 2020, 48, 145–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Popovic, J.; Lim, K.; Yin, Y.X.; Maier, J.; Guo, Y.G. Towards better Li metal anodes: Challenges and strategies. Mater. Today 2020, 33, 56–74. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhang, R.; Zhao, C.Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Fu, K.; Zhang, Y.; Hitz, E.; Hu, L.B. Protected Lithium-Metal Anodes in Batteries: From Liquid to Solid. Adv. Mater. 2017, 29, 1701169. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Liu, X.; Han, Q.; Zhang, P.; Song, X.S.; Zhao, Y. A Liquid/Liquid Electrolyte Interface that Inhibits Corrosion and Dendrite Growth of Lithium in Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 6397–6405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Cheng, X.B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene Carbonate Additives to Render Uniform Li Deposits in Lithium Metal Batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Chae, O.B.; Adiraju, V.A.K.; Lucht, B.L. Lithium Cyano Tris(2,2,2-trifluoroethyl) Borate as a Multifunctional Electrolyte Additive for High-Performance Lithium Metal Batteries. ACS Energy Lett. 2021, 6, 3851–3857. [Google Scholar] [CrossRef]
- Wang, S.H.; Yin, Y.X.; Zuo, T.T.; Dong, W.; Li, J.Y.; Shi, J.L.; Zhang, C.H.; Li, N.W.; Li, C.J.; Guo, Y.G. Stable Li Metal Anodes via Regulating Lithium Plating/Stripping in Vertically Aligned Microchannels. Adv. Mater. 2017, 29, 1703729. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Yin, Y.X.; Zhang, S.F.; Li, N.W.; Guo, Y.G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Lin, D.C.; Yuen, P.Y.; Liu, K.; Xie, J.; Dauskardt, R.H.; Cui, Y. An Artificial Solid Electrolyte Interphase with High Li-Ion Conductivity, Mechanical Strength, and Flexibility for Stable Lithium Metal Anodes. Adv. Mater. 2017, 29, 1605531. [Google Scholar] [CrossRef] [PubMed]
- Li, N.W.; Shi, Y.; Yin, Y.X.; Zeng, X.X.; Li, J.Y.; Li, C.J.; Wan, L.J.; Wen, R.; Guo, Y.G. A Flexible Solid Electrolyte Interphase Layer for Long-Life Lithium Metal Anodes. Angew. Chem. Int. Ed. 2018, 57, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Li, P.L.; Dong, X.L.; Li, C.; Liu, J.Y.; Liu, Y.; Feng, W.L.; Wang, C.X.; Wang, Y.G.; Xia, Y.Y. Anchoring an Artificial Solid-Electrolyte Interphase Layer on a 3D Current Collector for High-Performance Lithium Anodes. Angew. Chem. Int. Ed. 2019, 58, 2093–2097. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.Y.; Zhang, X.D.; Zeng, X.X.; Yan, M.; Yin, Y.X.; Xin, S.; Wang, W.P.; Wu, X.W.; Shi, J.L.; Wan, L.J.; et al. Enabling a Durable Electrochemical Interface via an Artificial Amorphous Cathode Electrolyte Interphase for Hybrid Solid/Liquid Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 6585–6589. [Google Scholar] [CrossRef]
- Monroe, C.; Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 2005, 152, A396–A404. [Google Scholar] [CrossRef]
- Kim, Y.; Stepien, D.; Moon, H.; Schönherr, K.; Schumm, B.; Kuenzel, M.; Althues, H.; Bresser, D.; Passerini, S. Artificial Interphase Design Employing Inorganic-Organic Components for High-Energy Lithium-Metal Batteries. ACS Appl. Mater. Interfaces 2023, 15, 20987–20997. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Sun, S.; Park, H.; Kim, J.; Park, K.; Hwang, I.; Jung, Y.; Song, T.; Paik, U. Stable artificial solid electrolyte interphase with lithium selenide and lithium chloride for dendrite-free lithium metal anodes. J. Power Sources 2021, 506, 230158. [Google Scholar] [CrossRef]
- Zuo, T.T.; Wu, X.W.; Yang, C.P.; Yin, Y.X.; Ye, H.; Li, N.W.; Guo, Y.G. Graphitized Carbon Fibers as Multifunctional 3D Current Collectors for High Areal Capacity Li Anodes. Adv. Mater. 2017, 29, 1700389. [Google Scholar] [CrossRef]
- Ma, Q.; Zeng, X.X.; Yue, J.P.; Yin, Y.X.; Zuo, T.T.; Liang, J.Y.; Deng, Q.; Wu, X.W.; Guo, Y.G. Viscoelastic and Nonflammable Interface Design-Enabled Dendrite-Free and Safe Solid Lithium Metal Batteries. Adv. Energy Mater. 2019, 9, 1803854. [Google Scholar] [CrossRef]
- Jaumaux, P.; Liu, Q.; Zhou, D.; Xu, X.F.; Wang, T.Y.; Wang, Y.Z.; Kang, F.Y.; Li, B.H.; Wang, G.X. Deep-Eutectic-Solvent-Based Self-Healing Polymer Electrolyte for Safe and Long-Life Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 9134–9142. [Google Scholar] [CrossRef]
- Lee, J.; Heo, K.; Song, Y.W.; Hwang, D.; Kim, M.Y.; Jeong, H.; Shin, D.C.; Lim, J. Degradation of All-Solid-State Lithium-Sulfur Batteries with PEO-Based Composite Electrolyte. J. Electrochem. Sci. Technol. 2022, 13, 199–207. [Google Scholar] [CrossRef]
- Zhang, S.S. Role of LiNO3 in rechargeable lithium/sulfur battery. Electrochim. Acta 2012, 70, 344–348. [Google Scholar] [CrossRef]
- Shi, Q.W.; Zhong, Y.R.; Wu, M.; Wang, H.Z.; Wang, H.L. High-capacity rechargeable batteries based on deeply cyclable lithium metal anodes. Proc. Natl. Acad. Sci. USA 2018, 115, 5676–5680. [Google Scholar] [CrossRef]
- Wang, H.S.; Liu, Y.Y.; Li, Y.Z.; Cui, Y. Lithium Metal Anode Materials Design: Interphase and Host. Electrochem. Energy Rev. 2019, 2, 509–517. [Google Scholar] [CrossRef]
- Jäckle, M.; Gross, A. Microscopic properties of lithium, sodium, and magnesium battery anode materials related to possible dendrite growth. J. Chem. Phys. 2014, 141, 4701. [Google Scholar] [CrossRef]
- Kim, S.; Park, G.; Lee, S.J.; Seo, S.; Ryu, K.; Kim, C.H.; Choi, J.W. Lithium-Metal Batteries: From Fundamental Research to Industrialization. Adv. Mater. 2023, 35, 2206625. [Google Scholar] [CrossRef]
- Han, Y.Y.; Liu, B.; Xiao, Z.; Zhang, W.K.; Wang, X.L.; Pan, G.X.; Xia, Y.; Xia, X.H.; Tu, J.P. Interface issues of lithium metal anode for high-energy batteries: Challenges, strategies, and perspectives. InfoMat 2021, 3, 155–174. [Google Scholar] [CrossRef]
- Chae, O.B.; Lucht, B.L. Interfacial Issues and Modification of Solid Electrolyte Interphase for Li Metal Anode in Liquid and Solid Electrolytes. Adv. Energy Mater. 2023, 13, 2203791. [Google Scholar] [CrossRef]
- Yuan, W.J.; Chen, J.; Shi, G.Q. Nanoporous graphene materials. Mater. Today 2014, 17, 77–85. [Google Scholar] [CrossRef]
- Amanchukwu, C.V.; Yu, Z.; Kong, X.; Qin, J.; Cui, Y.; Bao, Z.N. A New Class of Ionically Conducting Fluorinated Ether Electrolytes with High Electrochemical Stability. J. Am. Chem. Soc. 2020, 142, 7393–7403. [Google Scholar] [CrossRef]
- Sun, S.; Kim, G.; Lee, D.; Park, E.; Myeong, S.; Son, B.; Lee, K.; Jang, M.; Paik, U.; Song, T. Modulating SEI formation via tuning the solvation sheath for lithium metal batteries. Chem. Commun. 2022, 58, 9834–9837. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.K.; Niu, Y.B.; Hussain, T.; Tabassum, H.; Tang, W.W.; Xu, M.W.; Ahuja, R. How to avoid dendrite formation in metal batteries: Innovative strategies for dendrite suppression. Nano Energy 2021, 86, 106142. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Fu, L.; Wang, X.C.; Chen, Z.H.; Li, Y.J.; Mao, E.Y.; Seh, Z.W.; Sun, Y.M. Insights on “nitrate salt” in lithium anode for stabilized solid electrolyte interphase. Carbon Energy 2022, 4, 12–20. [Google Scholar] [CrossRef]
- Liu, S.F.; Ji, X.; Piao, N.; Chen, J.; Eidson, N.; Xu, J.J.; Wang, P.F.; Chen, L.; Zhang, J.X.; Deng, T.; et al. An Inorganic-Rich Solid Electrolyte Interphase for Advanced Lithium-Metal Batteries in Carbonate Electrolytes. Angew. Chem. Int. Ed. 2021, 60, 3661–3671. [Google Scholar] [CrossRef]
- Huang, S.Z.; Huang, Y.P.; Xia, Y.J.; Ding, J.Y.; Peng, C.Y.; Wang, L.L.; Luo, J.R.; Zhang, X.X.; Zheng, J.R.; Gao, Y.Q.; et al. High Li+ coordinated solvation sheaths enable high-quality Li metal anode. InfoMat 2023, 5, e12411. [Google Scholar] [CrossRef]
- Fu, L.; Wang, X.C.; Wang, L.; Wan, M.T.; Li, Y.J.; Cai, Z.; Tan, Y.C.; Li, G.C.; Zhan, R.M.; Seh, Z.W.; et al. A Salt-in-Metal Anode: Stabilizing the Solid Electrolyte Interphase to Enable Prolonged Battery Cycling. Adv. Funct. Mater. 2021, 31, 2010602. [Google Scholar] [CrossRef]
- Hou, L.P.; Yao, N.; Xie, J.; Shi, P.; Sun, S.Y.; Jin, C.B.; Chen, C.M.; Liu, Q.B.; Li, B.Q.; Zhang, X.Q.; et al. Modification of Nitrate Ion Enables Stable Solid Electrolyte Interphase in Lithium Metal Batteries. Angew. Chem. Int. Ed. 2022, 61, e202201406. [Google Scholar] [CrossRef]
- Wang, Q.D.; Yao, Z.P.; Zhao, C.L.; Verhallen, T.; Tabor, D.P.; Liu, M.; Ooms, F.; Kang, F.Y.; Aspuru-Guzik, A.; Hu, Y.S.; et al. Interface chemistry of an amide electrolyte for highly reversible lithium metal batteries. Nat. Commun. 2020, 11, 4188. [Google Scholar] [CrossRef]
- Aurbach, D.; Pollak, E.; Elazari, R.; Salitra, G.; Kelley, C.S.; Affinito, J. On the Surface Chemical Aspects of Very High Energy Density, Rechargeable Li-Sulfur Batteries. J. Electrochem. Soc. 2009, 156, A694–A702. [Google Scholar] [CrossRef]
- Luo, D.; Li, M.; Zheng, Y.; Ma, Q.; Gao, R.; Zhang, Z.; Dou, H.; Wen, G.; Shui, L.; Yu, A.; et al. Electrolyte Design for Lithium Metal Anode-Based Batteries toward Extreme Temperature Application. Adv. Sci. 2021, 8, 2101051. [Google Scholar] [CrossRef]
- Alpen, U.V. Li3N: A Promising Li Ionic Conductor. J. Solid State Chem. 1979, 29, 379–392. [Google Scholar] [CrossRef]
- Heinz Schulz, K.H.T. Defect Structure of the Ionic Conductor Lithium Nitride. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1979, 35, 309–314. [Google Scholar] [CrossRef]
- LAPP, T.; Skaarup, S.; Hooper, A. Ionic conductivity of pure and doped Li3N. Solid State Ion. 1983, 11, 97–103. [Google Scholar] [CrossRef]
- Kowada, Y.; Tatsumisago, M.; Minami, T. Chemical bonding and lithium ion conductions in Li3N. Solid State Ion. 2009, 180, 462–466. [Google Scholar] [CrossRef]
- Lee, H.S.; Bin Lee, S.; Kim, Y.S.; Kim, H.; Kim, M.J.; Yoon, T.W.; Lee, D.K.; Cho, J.H.; Kim, Y.H.; Kang, B.S. Boosting thermoelectric performance of conjugated polymers via interchain molecular docking: Significance of highly crystalline and percolated morphology. Chem. Eng. J. 2023, 468, 143654. [Google Scholar] [CrossRef]
- Kanwal, S.; Rahman, G. Defects-driven magnetism in bulk α-Li3N. J. Magn. Magn. Mater. 2018, 466, 192–199. [Google Scholar] [CrossRef]
- Li, W.; Wu, G.; Araújo, C.M.; Scheicher, R.H.; Blomqvist, A.; Ahuja, R.; Xiong, Z.; Feng, Y.; Chen, P. Li+ ion conductivity and diffusion mechanism in α-Li3N and β-Li3N. Energy Environ. Sci. 2010, 3, 1524–1530. [Google Scholar] [CrossRef]
- Lee, H.; Li, H.; Kim, Y.S.; Park, S.M.; Lee, D.; Lee, S.; Lee, H.S.; Kim, Y.H.; Kang, B. Novel Dithienopyrrole-Based Conjugated Copolymers: Importance of Backbone Planarity in Achieving High Electrical Conductivity and Thermoelectric Performance. Macromol. Rapid Commun. 2022, 43, 2200277. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Sun, Y.L.; Qian, Z.F.; Wang, R.H. New Insights on the Good Compatibility of Ether-Based Localized High-Concentration Electrolyte with Lithium Metal. ACS Mater. Lett. 2021, 3, 838–844. [Google Scholar] [CrossRef]
- Ren, X.D.; Zou, L.F.; Jiao, S.H.; Mei, D.H.; Engelhard, M.H.; Li, Q.Y.; Lee, H.Y.; Niu, C.J.; Adams, B.D.; Wang, C.M.; et al. High-Concentration Ether Electrolytes for Stable High-Voltage Lithium Metal Batteries. ACS Energy Lett. 2019, 4, 896–902. [Google Scholar] [CrossRef]
- Jozwiuk, A.; Sommer, H.; Janek, J.; Brezesinski, T. Fair performance comparison of different carbon blacks in lithium-sulfur batteries with practical mass loadings-Simple design competes with complex cathode architecture. J. Power Sources 2015, 296, 454–461. [Google Scholar] [CrossRef]
- Jozwiuk, A.; Berkes, B.B.; Weiss, T.; Sommer, H.; Janek, J.; Brezesinski, T. The critical role of lithium nitrate in the gas evolution of lithium-sulfur batteries. Energy Environ. Sci. 2016, 9, 2603–2608. [Google Scholar] [CrossRef]
- Morgan, B.; Madden, P.A. Ion mobilities and microscopic dynamics in liquid (Li,K)Cl. J. Chem. Phys. 2004, 120, 1402–1413. [Google Scholar] [CrossRef]
- Davoodabadi, A.; Li, J.L.; Liang, Y.F.; Wood, D.L.; Singler, T.J.; Jin, C.R. Analysis of electrolyte imbibition through lithium-ion battery electrodes. J. Power Sources 2019, 424, 193–203. [Google Scholar] [CrossRef]
- Warrington, A.; Hasanpoor, M.; Balkis, A.; Howlett, P.C.; Hutt, O.E.; Forsyth, M.; Pringle, J.M. Investigation of properties and performance of three novel highly concentrated ether-functionalised ionic liquid electrolytes for lithium metal batteries. Energy Storage Mater. 2023, 63, 102984. [Google Scholar] [CrossRef]
- Adams, B.D.; Carino, E.V.; Connell, J.G.; Han, K.S.; Cao, R.; Chen, J.; Zheng, J.; Li, Q.; Mueller, K.T.; Henderson, W.A.; et al. Long term stability of Li-S batteries using high concentration lithium nitrate electrolytes. Nano Energy 2017, 40, 607–617. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Lin, X.D.; Xu, P.; Yuan, R.M.; Gupta, D.; Rupp, R.; Barozzino-Consiglio, G.; Xu, H.W.; Dong, Q.F.; Vlad, A. Strong ion pairing at the origin of modified Li-cation solvation and improved performances of dual-salt electrolytes. J. Power Sources 2022, 541, 231644. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Chen, X.; Hou, L.P.; Li, B.Q.; Cheng, X.B.; Huang, J.Q.; Zhang, Q. Regulating Anions in the Solvation Sheath of Lithium Ions for Stable Lithium Metal Batteries. ACS Energy Lett. 2019, 4, 411–416. [Google Scholar] [CrossRef]
- Kang, D.W.; Moon, J.; Choi, H.-Y.; Shin, H.-C.; Kim, B.G. Stable cycling and uniform lithium deposition in anode-free lithium-metal batteries enabled by a high-concentration dual-salt electrolyte with high LiNO3 content. J. Power Sources 2021, 490, 229504. [Google Scholar] [CrossRef]
- Rodriguez, R.; Edison, R.A.; Stephens, R.M.; Sun, H.-H.; Heller, A.; Mullins, C.B. Separator-free and concentrated LiNO3 electrolyte cells enable uniform lithium electrodeposition. J. Mater. Chem. A 2020, 8, 3999–4006. [Google Scholar] [CrossRef]
- Li, W.; Yao, H.; Yan, K.; Zheng, G.; Liang, Z.; Chiang, Y.-M.; Cui, Y. The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 2015, 6, 7436. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Xie, K.; Diao, Y.; Hong, X. Characterization of the solid electrolyte interphase on lithium anode for preventing the shuttle mechanism in lithium–sulfur batteries. J. Power Sources 2014, 246, 840–845. [Google Scholar] [CrossRef]
- Yang, X.F.; Sun, Q.; Zhao, C.T.; Gao, X.J.; Adair, K.; Zhao, Y.; Luo, J.; Lin, X.T.; Liang, J.N.; Huang, H.; et al. Self-healing electrostatic shield enabling uniform lithium deposition in all-solid-state lithium batteries. Energy Storage Mater. 2019, 22, 194–199. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.L.; Shao, Y.Y.; Engelhard, M.H.; Nie, Z.M.; Xiao, J.; et al. Dendrite-Free Lithium Deposition via Self-Healing Electrostatic Shield Mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, J.; Hwang, Y.; Jung, C. Review on Effective Skills to Inhibit Dendrite Growth for Stable Lithium Metal Electrode. J. Korean Electrochem. Soc. 2022, 25, 51–68. [Google Scholar] [CrossRef]
- Lin, H.; Chen, K.H.; Shuai, Y.; He, X.; Ge, K. Influence of CsNO3 as electrolyte additive on electrochemical property of lithium anode in rechargeable battery. J. Cent. South Univ. 2018, 25, 719–728. [Google Scholar] [CrossRef]
- Jung, J.; Cho, H.; Kim, I.; Kim, S.; Jo, W.; Kim, H.T. Dual Functionalities of Rb Cation in Lean Electrolyte Lithium Sulfur Batteries. Energy Storage Mater. 2023, 63, 103040. [Google Scholar] [CrossRef]
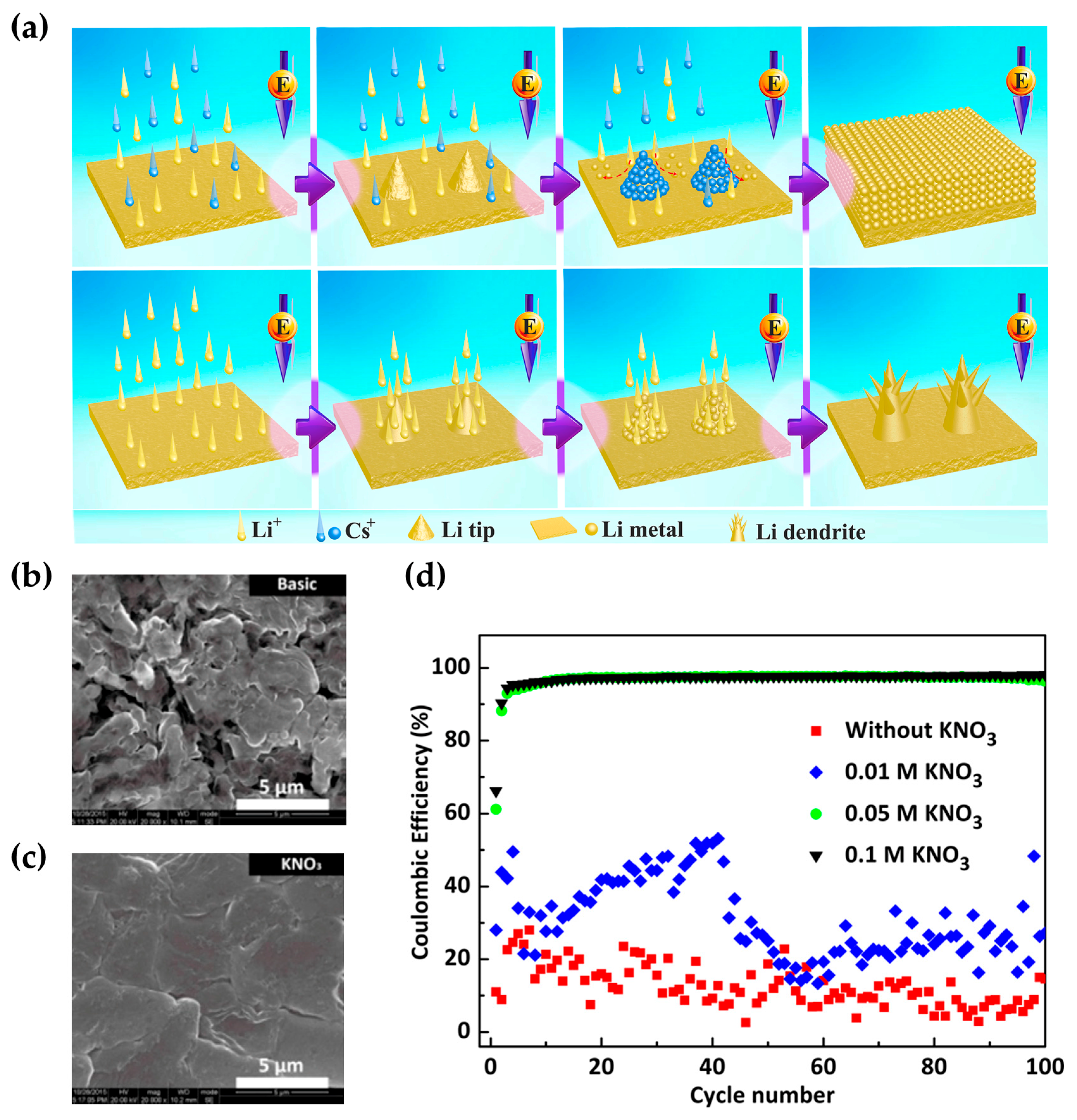
- Jia, W.S.; Fan, C.; Wang, L.P.; Wang, Q.J.; Zhao, M.J.; Zhou, A.J.; Li, J.Z. Extremely Accessible Potassium Nitrate (KNO3) as the Highly Efficient Electrolyte Additive in Lithium Battery. ACS Appl. Mater. Interfaces 2016, 8, 15399–15405. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yang, Y.F.; Shao, H.X. Enhanced cycleability and dendrite-free lithium deposition by adding potassium ion to the electrolyte for lithium metal batteries. Electrochim. Acta 2016, 212, 758–766. [Google Scholar] [CrossRef]
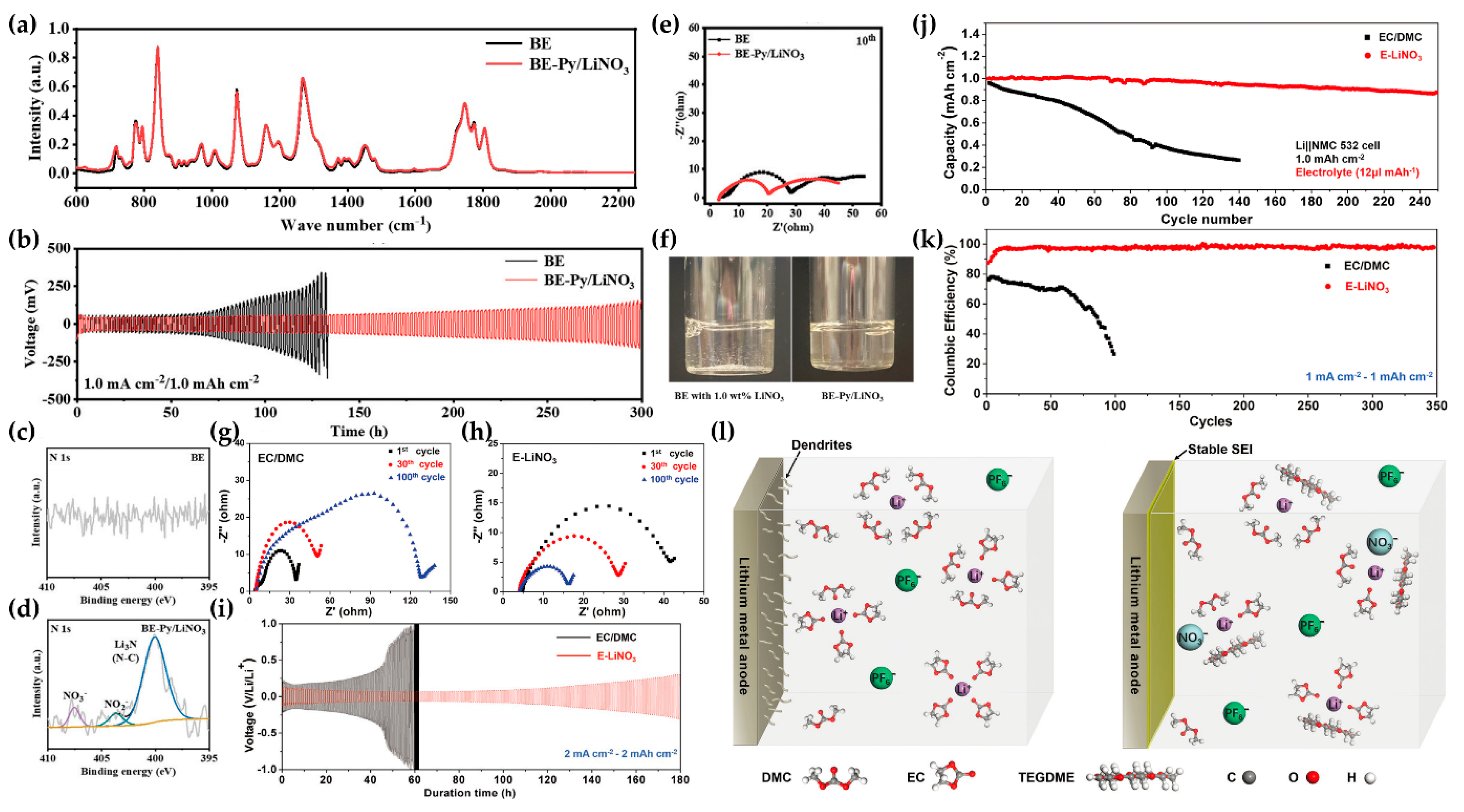
- Shuai, Y.; Zhang, Z.; Chen, K.; Lou, J.; Wang, Y. Highly stable lithium plating by a multifunctional electrolyte additive in a lithium-sulfurized polyacrylonitrile battery. Chem. Commun. 2019, 55, 2376–2379. [Google Scholar] [CrossRef] [PubMed]
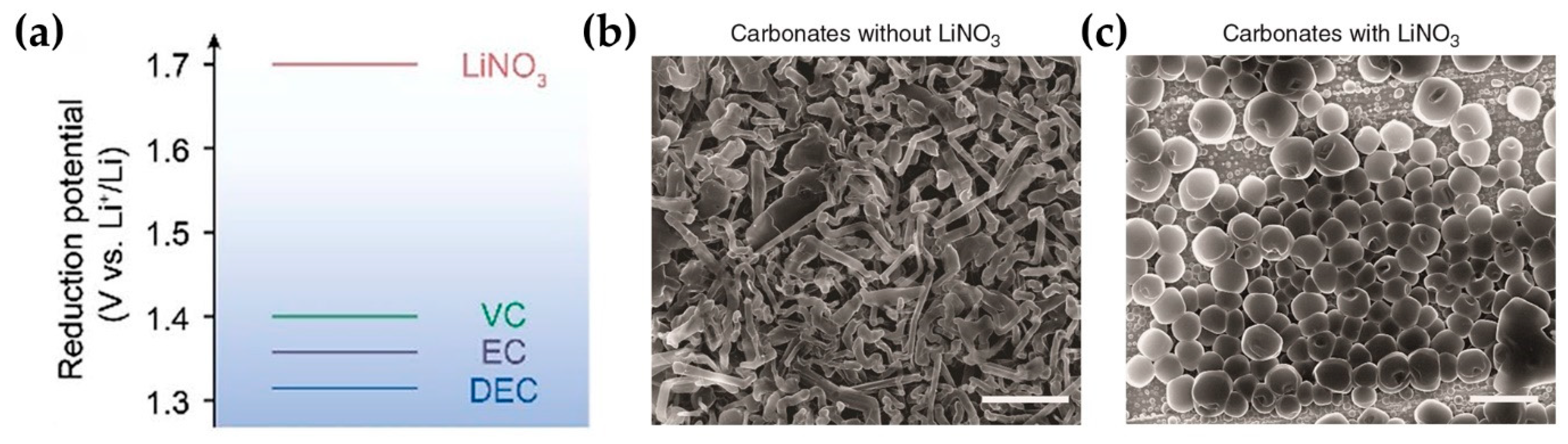
- Hu, L.B.; Zhang, Z.C.; Amine, K. Electrochemical investigation of carbonate-based electrolytes for high voltage lithium-ion cells. J. Power Sources 2013, 236, 175–180. [Google Scholar] [CrossRef]
- Delp, S.A.; Borodin, O.; Olguin, M.; Eisner, C.G.; Allen, J.L.; Jow, T.R. Importance of Reduction and Oxidation Stability of High Voltage Electrolytes and Additives. Electrochim. Acta 2016, 209, 498–510. [Google Scholar] [CrossRef]
- Borodin, O.; Behl, W.; Jow, T.R. Oxidative Stability and Initial Decomposition Reactions of Carbonate, Sulfone, and Alkyl Phosphate-Based Electrolytes. J. Phys. Chem. C 2013, 117, 8661–8682. [Google Scholar] [CrossRef]
- Suo, L.M.; Xue, W.J.; Gobet, M.; Greenbaum, S.G.; Wang, C.; Chen, Y.M.; Yang, W.L.; Li, Y.X.; Li, J. Fluorine-donating electrolytes enable highly reversible 5-V-class Li metal batteries. Proc. Natl. Acad. Sci. USA 2018, 115, 1156–1161. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Li, Y.; Chen, G.; Pei, A.; Nix, O.; Li, Y.; Cui, Y. Solubility-mediated sustained release enabling nitrate additive in carbonate electrolytes for stable lithium metal anode. Nat. Commun. 2018, 9, 3656. [Google Scholar] [CrossRef]
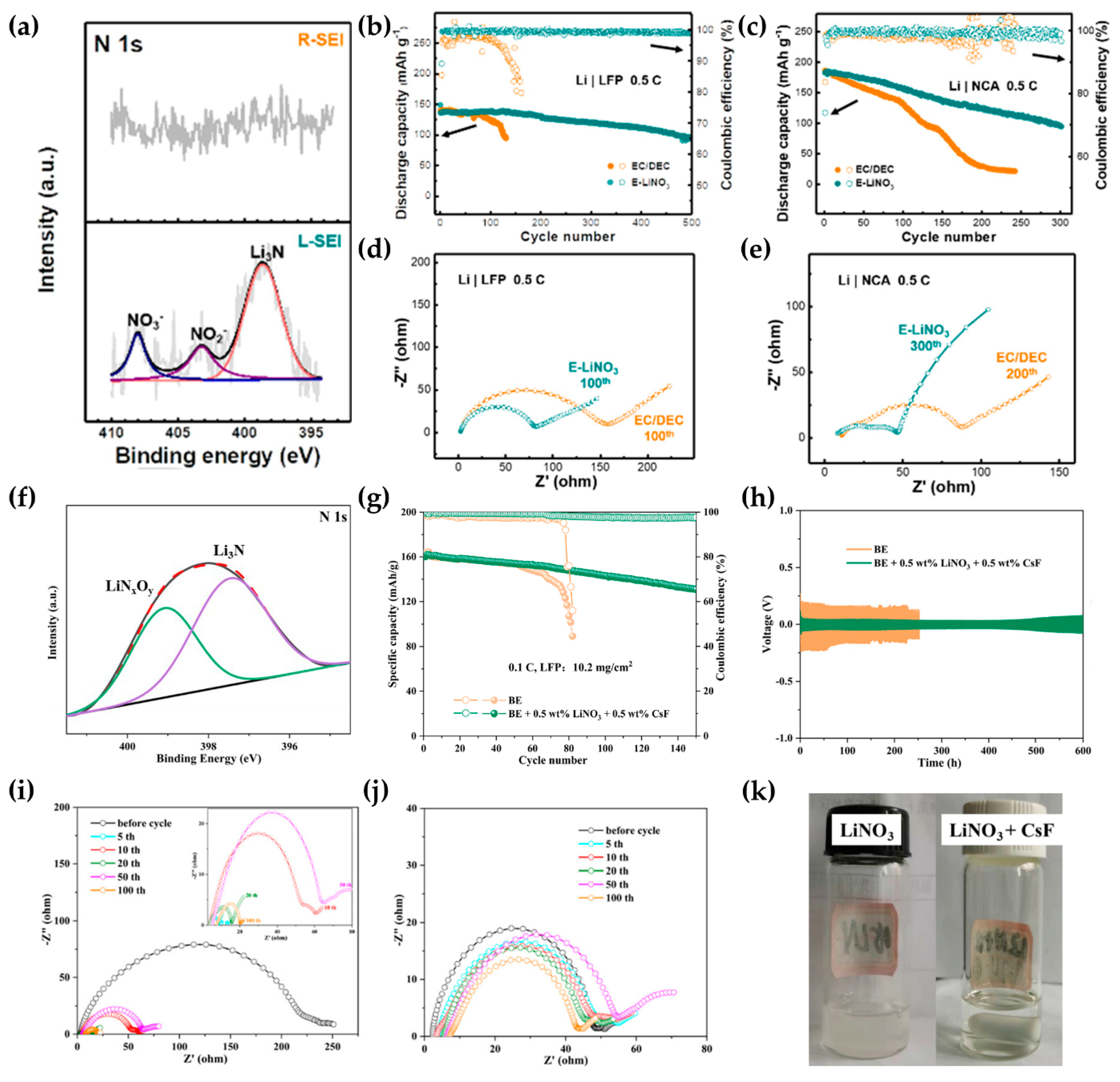
- Yan, C.; Yao, Y.-X.; Chen, X.; Cheng, X.-B.; Zhang, X.-Q.; Huang, J.-Q.; Zhang, Q. Lithium Nitrate Solvation Chemistry in Carbonate Electrolyte Sustains High-Voltage Lithium Metal Batteries. Angew. Chem. Int. Ed. 2018, 57, 14055–14059. [Google Scholar] [CrossRef]
- Zhao, J.; Wan, Z.W.; Zeng, X.M.; Tian, M.M.; Wang, K.; Chen, X.Y.; Ling, M.; Ni, W.B.; Liang, C.D. Solubilized LiNO3 by the cationic size effect of CsF for lithium metal anode protection and dendrite formation prevention in carbonate electrolyte. Inorg. Chem. Front. 2023, 10, 1809–1817. [Google Scholar] [CrossRef]
- Zhang, W.D.; Wu, Q.; Huang, J.X.; Fan, L.; Shen, Z.Y.; He, Y.; Feng, Q.; Zhu, G.N.; Lu, Y.Y. Colossal Granular Lithium Deposits Enabled by the Grain-Coarsening Effect for High-Efficiency Lithium Metal Full Batteries. Adv. Mater. 2020, 32, 2001740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.D.; Shen, Z.Y.; Li, S.Y.; Fan, L.; Wang, X.Y.; Chen, F.; Zang, X.X.; Wu, T.; Ma, F.Y.; Lu, Y.Y. Engineering Wavy-Nanostructured Anode Interphases with Fast Ion Transfer Kinetics: Toward Practical Li-Metal Full Batteries. Adv. Funct. Mater. 2020, 30, 2003800. [Google Scholar] [CrossRef]
- Xia, H.Y.; Wang, Y.K.; Fu, Z.W. Activation of trace LiNO3 additives by BF3 in high-concentration electrolytes towards stable lithium metal batteries. Chem. Commun. 2023, 59, 8680–8683. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wang, Z.X.; Yi, X.L.; Li, X.H.; Guo, H.J.; Peng, W.J.; Wang, J.X.; Yan, G.C. Breaking the Solubility Limit of LiNO3 in Carbonate Electrolyte Assisted by BF3 to Construct a Stable SEI Film for Dendrite-Free Lithium Metal Batteries. Small 2023, 20, 2308678. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Zhang, W.D.; Wu, Q.; Fan, L.; Wang, X.Y.; Wang, X.; Shen, Z.Y.; He, Y.; Lu, Y.Y. Synergistic Dual-Additive Electrolyte Enables Practical Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2020, 59, 14935–14941. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Wu, C.; Pang, W.K.; Mao, J.; Guo, Z. Constructing nitrided interfaces for stabilizing Li metal electrodes in liquid electrolytes. Chem. Sci. 2021, 12, 8945–8966. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.D.; Xiong, X.H.; Liang, Q.W.; Wu, X.W.; Fu, H.K. An inorganic-rich SEI induced by LiNO3 additive for a stable lithium metal anode in carbonate electrolyte. Chem. Commun. 2021, 57, 9232–9235. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.J.; Li, Q.; Luo, D.; Li, G.R.; Liu, H.; Shui, L.L.; Gourley, S.; Zhou, G.F.; Wang, X.; Chen, Z.W. Regulating the Li+-Solvation Structure of Ester Electrolyte for High-Energy-Density Lithium Metal Batteries. Small 2020, 16, 2004688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Yang, G.J.; Liu, Z.P.; Li, X.Y.; Wang, X.F.; Chen, R.J.; Wu, F.; Wang, Z.X.; Chen, L.Q. Competitive Solvation Enhanced Stability of Lithium Metal Anode in Dual-Salt Electrolyte. Nano Lett. 2021, 21, 3310–3317. [Google Scholar] [CrossRef]
- Brown, Z.L.; Heiskanen, S.; Lucht, B.L. Using Triethyl Phosphate to Increase the Solubility of LiNO3 in Carbonate Electrolytes for Improving the Performance of the Lithium Metal Anode. J. Electrochem. Soc. 2019, 166, A2523–A2527. [Google Scholar] [CrossRef]
- Jie, Y.; Liu, X.; Lei, Z.; Wang, S.; Chen, Y.; Huang, F.; Cao, R.; Zhang, G.; Jiao, S. Enabling High-Voltage Lithium Metal Batteries by Manipulating Solvation Structure in Ester Electrolyte. Angew. Chem. Int. Ed. 2020, 59, 3505–3510. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Cheng, X.; Li, B.; Shen, X.; Yan, C.; Huang, J.; Zhang, Q. Highly Stable Lithium Metal Batteries Enabled by Regulating the Solvation of Lithium Ions in Nonaqueous Electrolytes. Angew. Chem. Int. Ed. 2018, 57, 5301–5305. [Google Scholar] [CrossRef] [PubMed]
- Li, P.C.; Chen, L.; Wen, Z.X.; Zhang, D.X.; Zhang, Y.; Zhang, N.; Chen, G.; Liu, X.H. Solvation chemistry of rare earth nitrates in carbonate electrolyte for advanced lithium metal batteries. Chem. Eng. J. 2022, 433, 134468. [Google Scholar] [CrossRef]
- Guo, J.; Wen, Z.Y.; Wu, M.F.; Jin, J.; Liu, Y. Vinylene carbonate-LiNO3: A hybrid additive in carbonic ester electrolytes for SEI modification on Li metal anode. Electrochem. Commun. 2015, 51, 59–63. [Google Scholar] [CrossRef]
- Gu, S.C.; Zhang, S.W.; Han, J.W.; Deng, Y.Q.; Luo, C.; Zhou, G.M.; He, Y.B.; Wei, G.D.; Kang, F.Y.; Lv, W.; et al. Nitrate Additives Coordinated with Crown Ether Stabilize Lithium Metal Anodes in Carbonate Electrolyte. Adv. Funct. Mater. 2021, 31, 2102128. [Google Scholar] [CrossRef]
- Choi, H.; Bae, Y.; Lee, S.M.; Ha, Y.C.; Shin, H.C.; Kim, B.G. A LiPF6-LiFSI Blended-Salt Electrolyte System for Improved Electrochemical Performance of Anode-Free Batteries. J. Electrochem. Sci. Technol. 2022, 13, 78–89. [Google Scholar] [CrossRef]
- Hu, Z.L.; Zhang, S.; Dong, S.M.; Li, W.J.; Li, H.; Cui, G.L.; Chen, L.Q. Poly(ethyl α-cyanoacrylate)-Based Artificial Solid Electrolyte Interphase Layer for Enhanced Interface Stability of Li Metal Anodes. Chem. Mater. 2017, 29, 4682–4689. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Y.; Wang, J.; Zhao, B.; Li, Z.; Wu, H.B. Sustained-Release Nanocapsules Enable Long-Lasting Stabilization of Li Anode for Practical Li-Metal Batteries. Nano-Micro Lett. 2020, 12, 176. [Google Scholar] [CrossRef]
- Adiraju, V.A.K.; Chae, O.B.; Robinson, J.R.; Lucht, B.L. Highly Soluble Lithium Nitrate-Containing Additive for Carbonate-Based Electrolyte in Lithium Metal Batteries. ACS Energy Lett. 2023, 8, 2440–2446. [Google Scholar] [CrossRef]



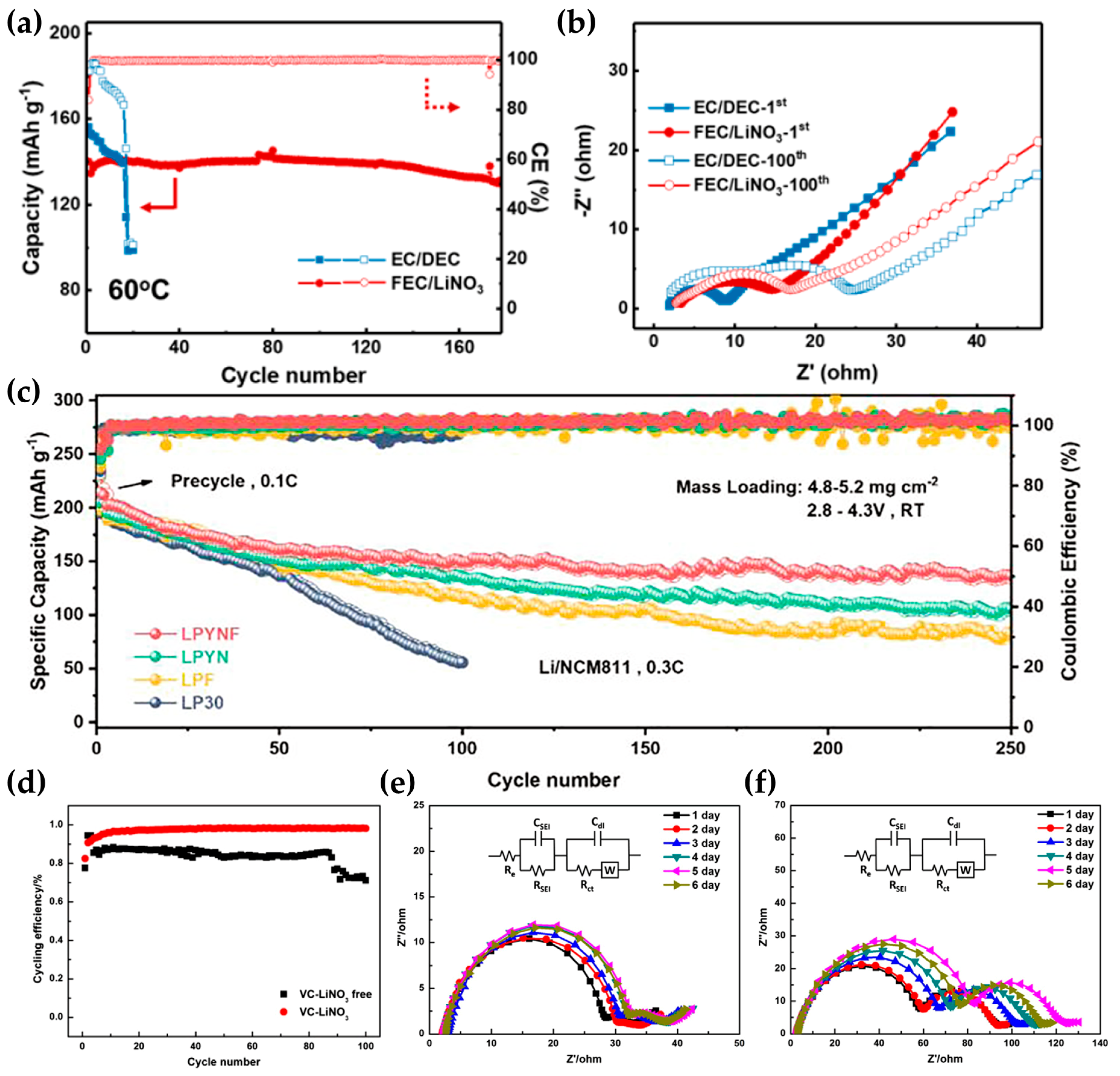
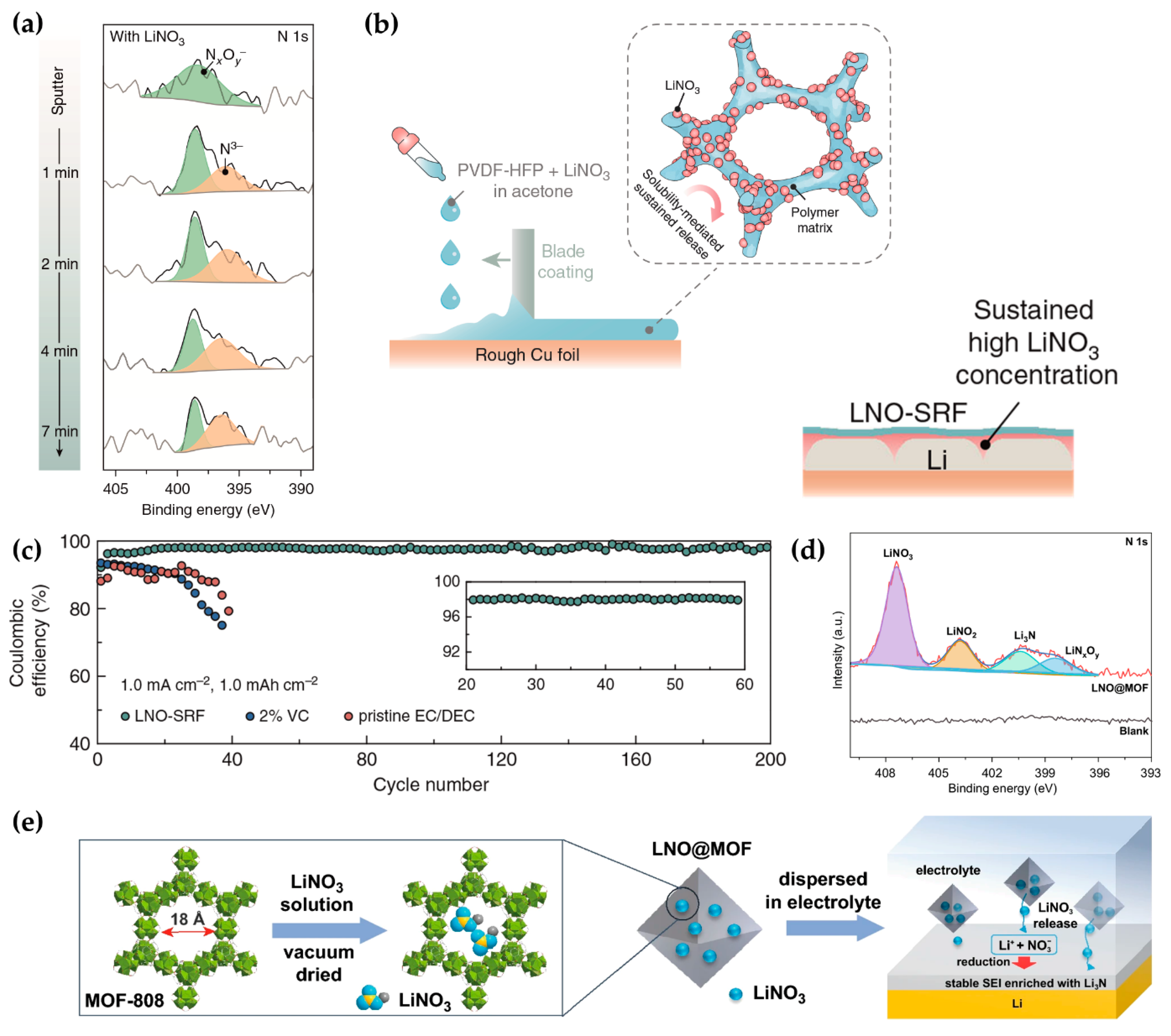
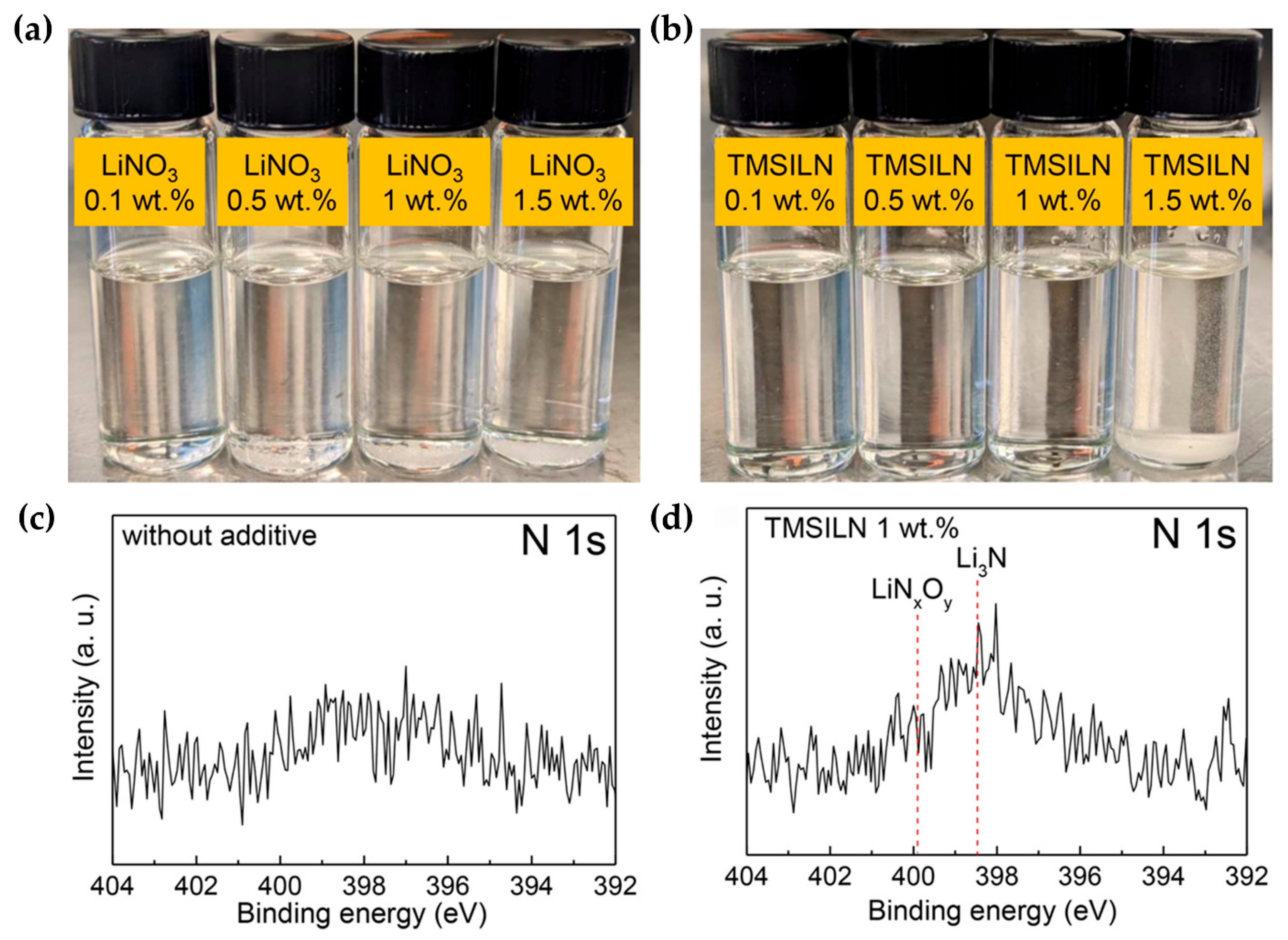
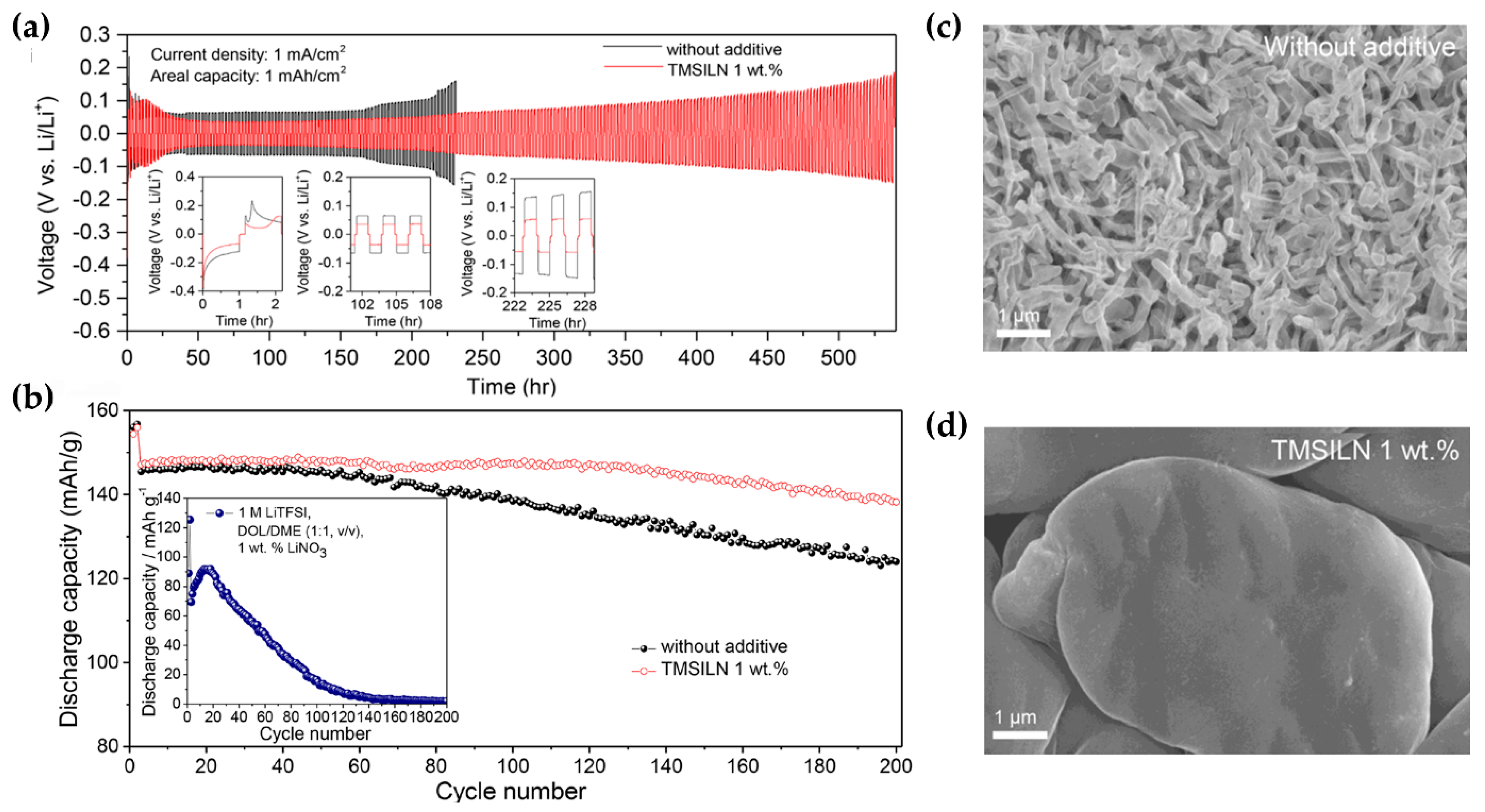
| Additive | Solubility | Capacity Retention | Coulombic Efficiency | Others | Reference |
|---|---|---|---|---|---|
| LiFSI | 2 M | 98.5% (425 cycles) | 47.3–52.7% (100 cycles) | Formation SEI with LiF-Li3N-LiNxOy on lithium metal surface | [80] |
| CuF | 1 wt% | 53% (300 cycles) | 99.5% (300 cycles) | A catalyst not participating in reactions within the battery | [97] |
| Tin(II) | 5 wt% | 89.6% (130 cycles) | 98.4% (300 cycles) | A catalyst not participating in reactions within the battery | [99] |
| CsF | 0.5 wt% | N/A | ~98% (200 cycles) | Synergy effect with SHES effect | [98] |
| Pyridine | Adding 1 wt% to a solvent dissolved in 4.5 M LiNO3 | N/A | ~95.86% (80 h, 1 mAh cm−2, 1 mA cm−2) | High DN | [105] |
| TEGDME | 3 wt% | 87.3% (250 cycles) | 98.2% (350 cycles) | Li+ ion solvation structure modification | [106] |
| GBL | 0.5 M | ~80% (100 cycles) | ~98.8% (200 cycles) | High DN Li+ ion solvation structure modification | [109] |
| TMSILN | 1 wt% | 90% (300 cycles) | N/A | Lithium nitrate-containing salt. No required additional additives | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Yoon, T.; Chae, O.B. Behavior of NO3−-Based Electrolyte Additive in Lithium Metal Batteries. Batteries 2024, 10, 135. https://doi.org/10.3390/batteries10040135
Kim J, Yoon T, Chae OB. Behavior of NO3−-Based Electrolyte Additive in Lithium Metal Batteries. Batteries. 2024; 10(4):135. https://doi.org/10.3390/batteries10040135
Chicago/Turabian StyleKim, Jeongmin, Taeho Yoon, and Oh B. Chae. 2024. "Behavior of NO3−-Based Electrolyte Additive in Lithium Metal Batteries" Batteries 10, no. 4: 135. https://doi.org/10.3390/batteries10040135
APA StyleKim, J., Yoon, T., & Chae, O. B. (2024). Behavior of NO3−-Based Electrolyte Additive in Lithium Metal Batteries. Batteries, 10(4), 135. https://doi.org/10.3390/batteries10040135








