Selective Separation of Lithium from Leachate of Spent Lithium-Ion Batteries by Zirconium Phosphate/Polyacrylonitrile Composite: Leaching and Sorption Behavior
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
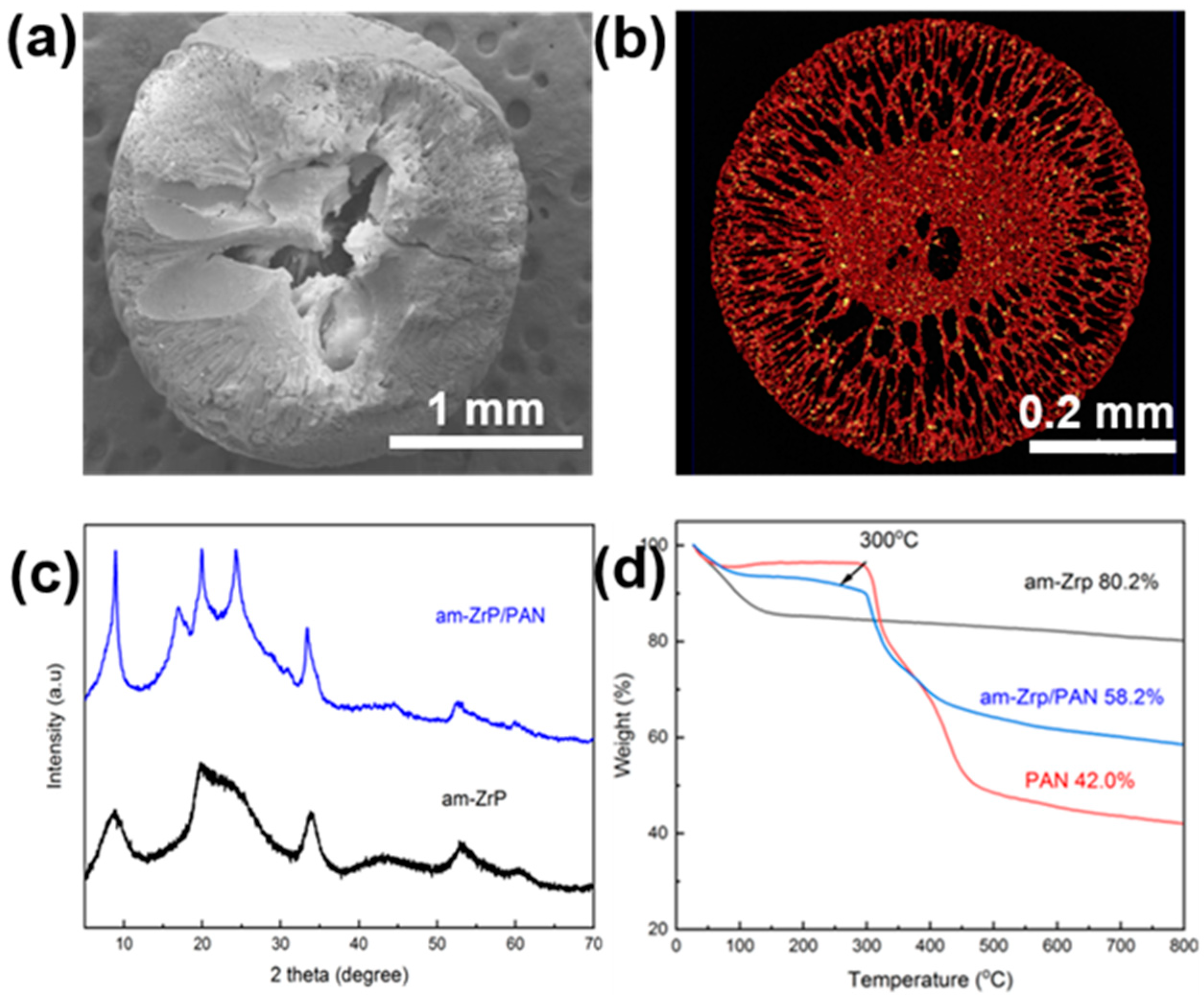
2.2. Characterization
2.3. Synthesis of am-ZrP/PAN
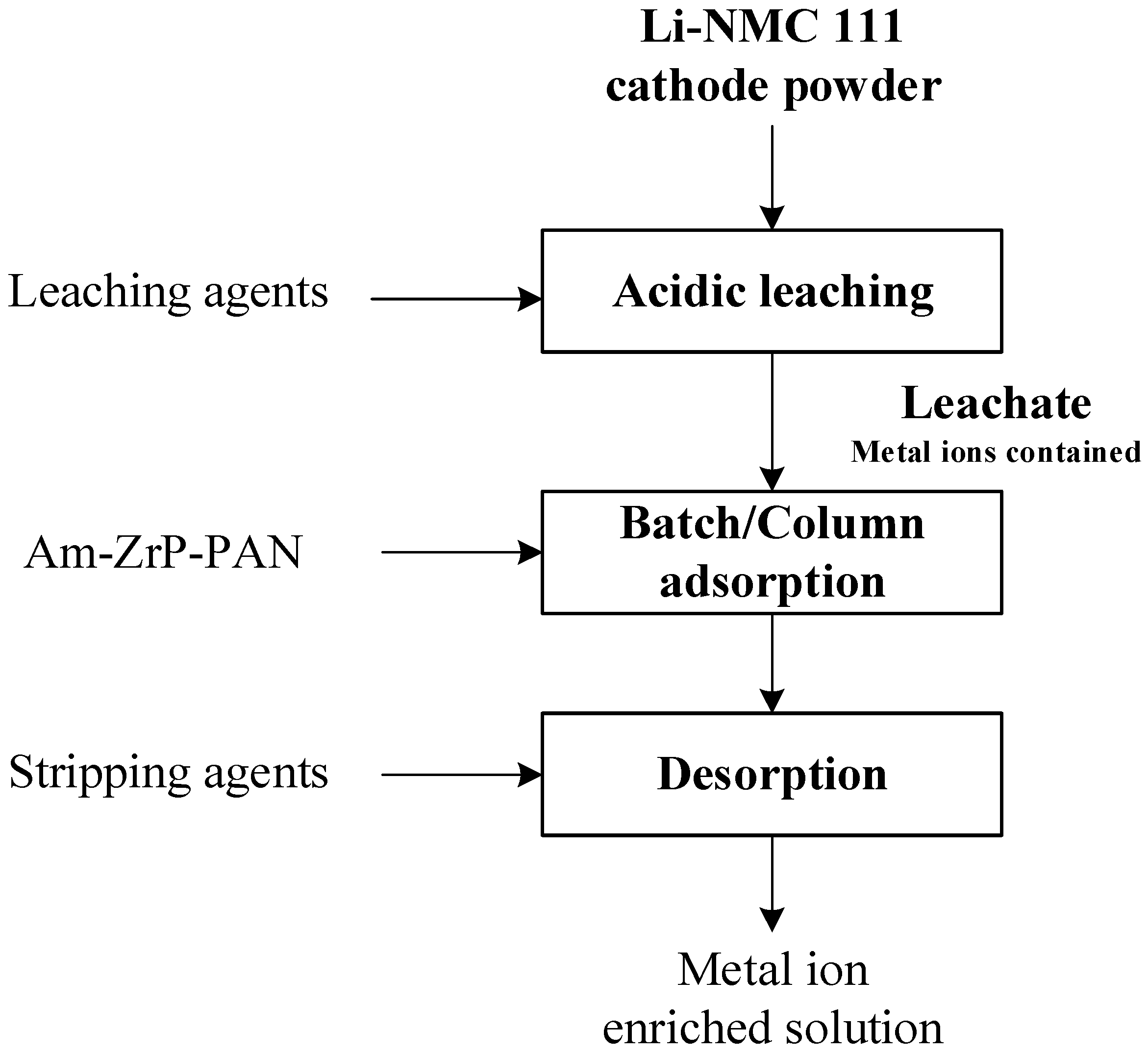
2.4. Leaching Procedure
2.5. Effect of pH on Metal Uptake
2.6. Adsorption Kinetics Studies
2.7. Isotherm Studies
2.8. Column Chromatography Experiments
3. Results and Discussion
3.1. Characterization of am-ZrP/PAN
3.2. Leaching Experiments
3.2.1. Effect of H2SO4 Concentration on Leaching Efficiency
3.2.2. Effect of Temperature on Leaching Efficiency
3.2.3. Effect of H2O2 Concentration on Leaching Efficiency
3.2.4. Effect of Pulp Density on Leaching Efficiency
3.2.5. Effect of Time on Leaching Efficiency
3.3. Sorption Experiments
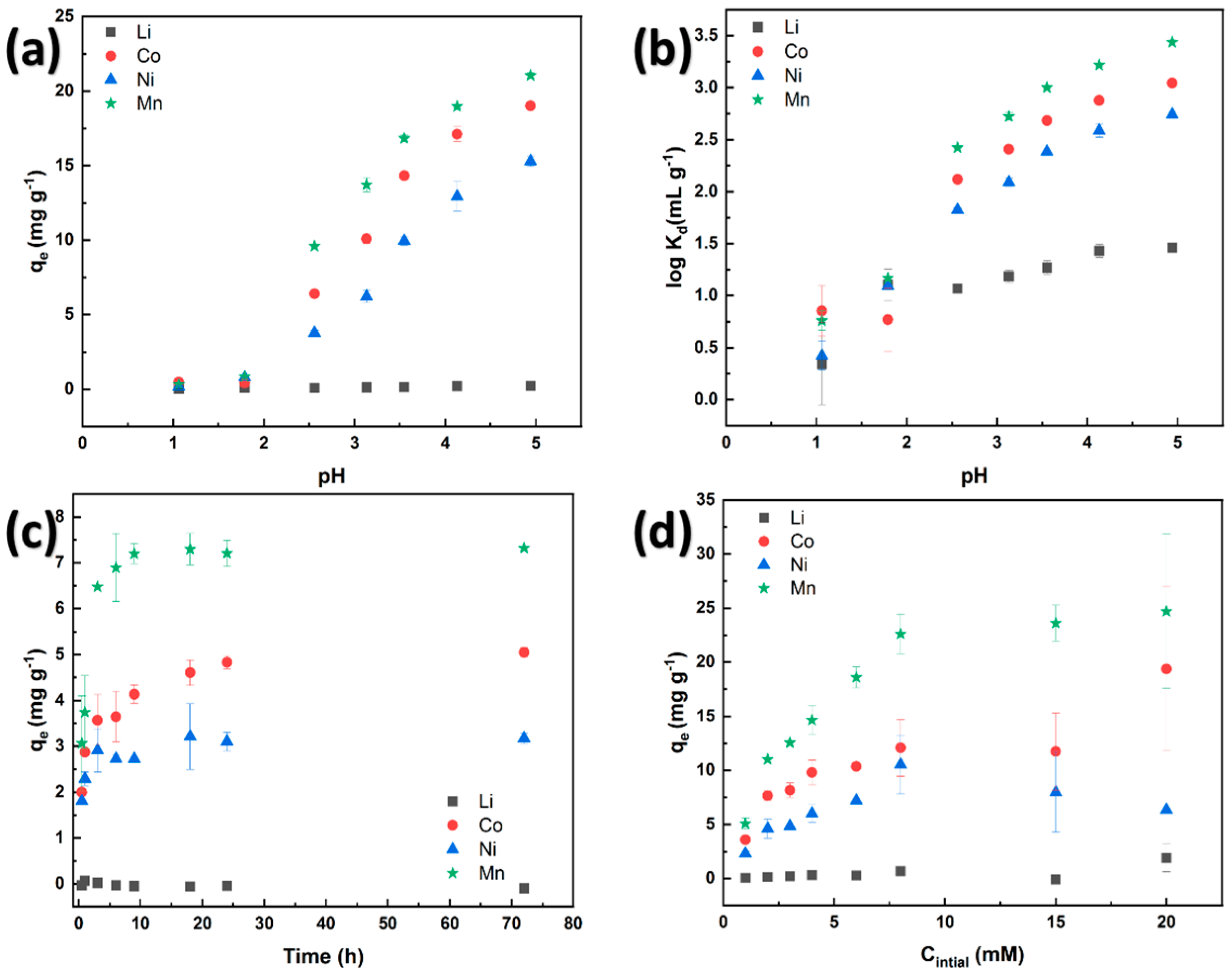
3.3.1. Effect of pH on Adsorption
3.3.2. Kinetics Study
3.3.3. Adsorption Isotherms
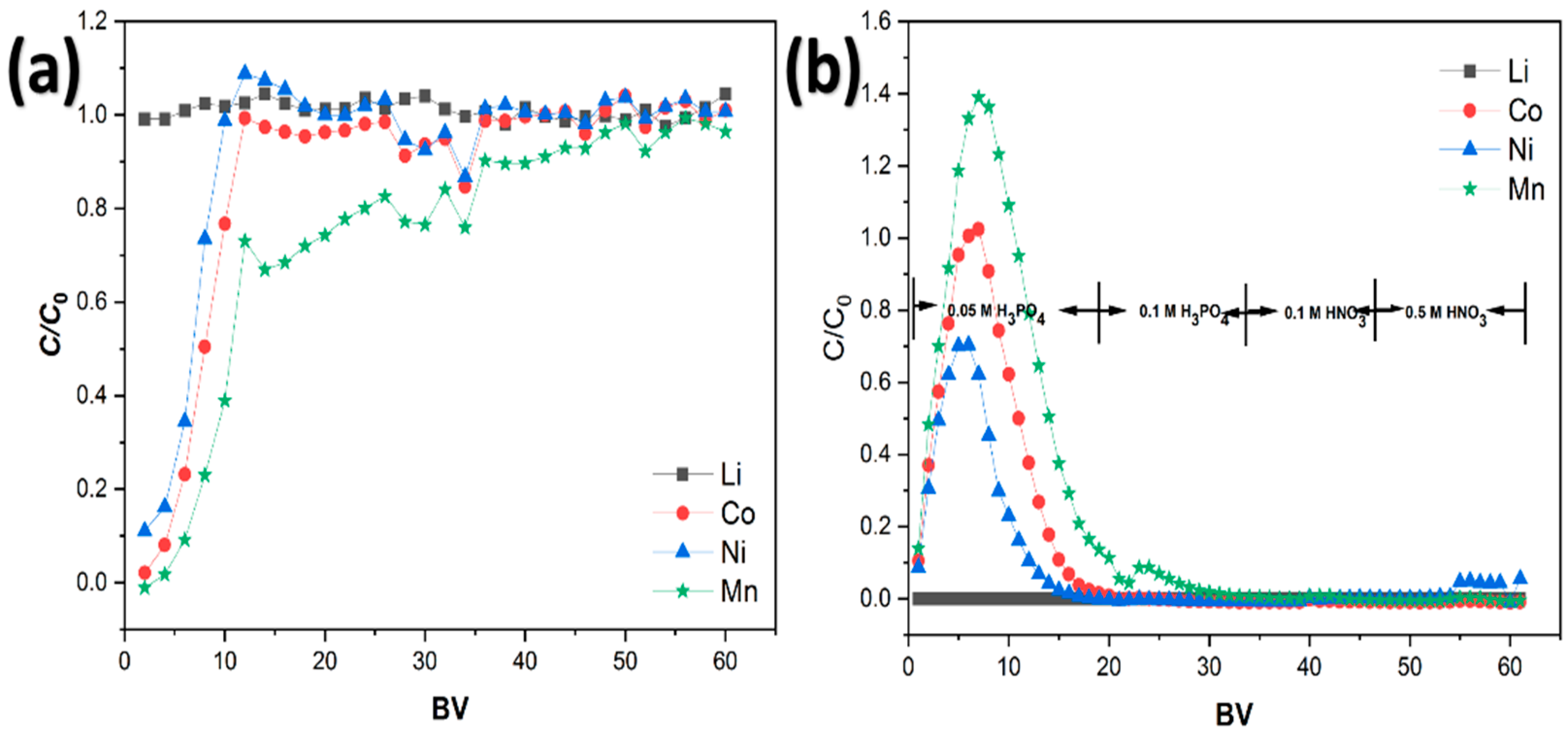
3.4. Column Chromatography Loading and Desorption Studies
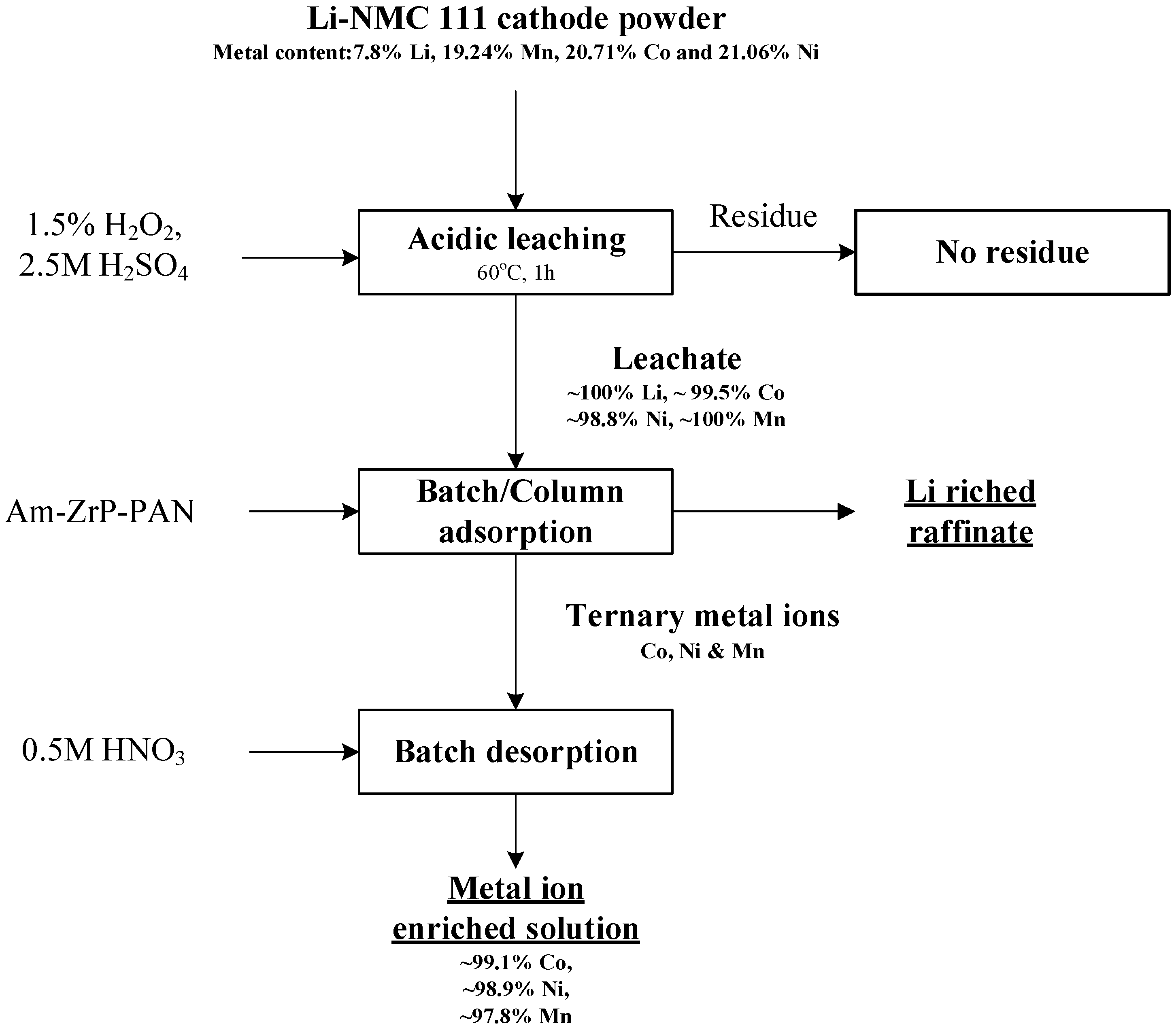
3.5. Preliminary Evaluation of the Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.C.; Bohn, N.; Geßwein, H.; Neumann, M.; Osenberg, M.; Hilger, A.; Manke, I.; Schmidt, V.; Binder, J.R. Hierarchical Structuring of NMC111-Cathode Materials in Lithium-Ion Batteries: An In-Depth Study on the Influence of Primary and Secondary Particle Sizes on Electrochemical Performance. ACS Appl. Energy Mater. 2020, 3, 12565–12574. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Shaju, K.M.; Bruce, P.G. Macroporous Li(Ni1/3Co1/3Mn1/3)O2: A high-rate positive electrode for rechargeable lithium batteries. J. Power Sources 2007, 174, 1201–1205. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Kelly, J.C.; James, C.; Gallagher, K.G. The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction. Energy Environ. Sci. 2015, 8, 158–168. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Kang, D.H.P.; Chen, M.; Ogunseitan, O.A. Potential Environmental and Human Health Impacts of Rechargeable Lithium Batteries in Electronic Waste. Environ. Sci. Technol. 2013, 47, 5495–5503. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229–243. [Google Scholar] [CrossRef]
- Bae, H.; Kim, Y. Technologies of lithium recycling from waste lithium ion batteries: A review. Mater. Adv. 2021, 2, 3234–3250. [Google Scholar] [CrossRef]
- Roy, J.J.; Cao, B.; Madhavi, S. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach. Chemosphere 2021, 282, 130944. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Sahu, S.; Agrawala, M.; Patra, S.R.; Devi, N. Synergistic Approach for Selective Leaching and Separation of Strategic Metals from Spent Lithium-Ion Batteries. ACS Omega 2024, 9, 10556–10565. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; de Souza Braga, A.; Korbel, C.; Chagnes, A. New insights in the leaching kinetics of cathodic materials in acidic chloride media for lithium-ion battery recycling. Hydrometallurgy 2021, 204, 105705. [Google Scholar] [CrossRef]
- Meshram, P.; Virolainen, S.; Abhilash; Sainio, T. Solvent Extraction for Separation of 99.9% Pure Cobalt and Recovery of Li, Ni, Fe, Cu, Al from Spent LIBs. Metals 2022, 12, 1056. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef]
- Provazi, K.; Campos, B.A.; Espinosa, D.C.R.; Tenório, J.A.S. Metal separation from mixed types of batteries using selective precipitation and liquid–liquid extraction techniques. Waste Manag. 2011, 31, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Choubey, P.K.; Dinkar, O.S.; Panda, R.; Kumari, A.; Jha, M.K.; Pathak, D.D. Selective extraction and separation of Li, Co and Mn from leach liquor of discarded lithium ion batteries (LIBs). Waste Manag. 2021, 121, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Yen, C.H.; Lin, J.-L.; Xu, R.-B. Recovery of high-purity metallic cobalt from lithium nickel manganese cobalt oxide (NMC)-type Li-ion battery. J. Mater. Cycles Waste Manag. 2019, 21, 300–307. [Google Scholar] [CrossRef]
- Chen, W.-S.; Ho, H.-J. Recovery of Valuable Metals from Lithium-Ion Batteries NMC Cathode Waste Materials by Hydrometallurgical Methods. Metals 2018, 8, 321. [Google Scholar] [CrossRef]
- Wang, F.; Sun, R.; Xu, J.; Chen, Z.; Kang, M. Recovery of cobalt from spent lithium ion batteries using sulphuric acid leaching followed by solid–liquid separation and solvent extraction. RSC Adv. 2016, 6, 85303–85311. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Davis, K.; Demopoulos, G.P. Hydrometallurgical recycling technologies for NMC Li-ion battery cathodes: Current industrial practice and new R&D trends. RSC Sustain. 2023, 1, 1932–1951. [Google Scholar] [CrossRef]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end of life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; He, Y. Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes. Waste Manag. 2017, 64, 219–227. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, L.; Tang, Y.; Zhu, Y.; Chen, H.; Chen, Y. Resynthesis and electrochemical performance of LiNi0.5Co0.2Mn0.3O2 from spent cathode material of lithium-ion batteries. Vacuum 2018, 156, 317–324. [Google Scholar] [CrossRef]
- Lei, S.; Cao, Y.; Cao, X.; Sun, W.; Weng, Y.; Yang, Y. Separation of lithium and transition metals from leachate of spent lithium-ion batteries by solvent extraction method with Versatic 10. Sep. Purif. Technol. 2020, 250, 117258. [Google Scholar] [CrossRef]
- Xu, J.; Wiikinkoski, W.E.; Koivula, R.; Zhang, W.; Ebin, B.; Harjula, R. HF-free synthesis of a-zirconium phosphate and its use as ion exchanger for separation of Nd(III) and Dy(III) from a ternary Co–Nd–Dy system. J. Sustain. Metall. 2017, 3, 646–658. [Google Scholar] [CrossRef]
- Poole, C.F.; Yu, L.; Sun, Y. Chapter 1—Concepts and milestones in the development of ion-exchange chromatography. In Ion-Exchange Chromatography and Related Techniques; Nesterenko, P.N., Poole, C.F., Sun, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–23. [Google Scholar]
- Wiikinkoski, W.E.; Xu, J.; Zhang, W.; Hietala, S.; Koivula, T.R. Modification of α-zirconium phosphate synthesis—Effects of crystallinity and acidity on Eu(III) and Am(III) ion exchange. ChemistrySelect 2018, 3, 9583–9588. [Google Scholar] [CrossRef]
- Wang, H.; Huang, K.; Zhang, Y.; Chen, X.; Jin, W.; Zheng, S.; Zhang, Y.; Li, P. Recovery of Lithium, Nickel, and Cobalt from Spent Lithium-Ion Battery Powders by Selective Ammonia Leaching and an Adsorption Separation System. ACS Sustain. Chem. Eng. 2017, 5, 11489–11495. [Google Scholar] [CrossRef]
- Strauss, M.L.; Diaz, L.A.; McNally, J.; Klaehn, J.; Lister, T.E. Separation of cobalt, nickel, and manganese in leach solutions of waste lithium-ion batteries using Dowex M4195 ion exchange resin. Hydrometallurgy 2021, 206, 105757. [Google Scholar] [CrossRef]
- Virolainen, S.; Wesselborg, T.; Kaukinen, A.; Sainio, T. Removal of iron, aluminium, manganese and copper from leach solutions of lithium-ion battery waste using ion exchange. Hydrometallurgy 2021, 202, 105602. [Google Scholar] [CrossRef]
- Xu, J.; Virolainen, S.; Zhang, W.; Kuva, J.; Sainio, T.; Koivula, R. Polyacrylonitrile-encapsulated amorphous zirconium phosphate composite adsorbent for Co, Nd and Dy separations. Chem. Eng. J. 2018, 351, 832–840. [Google Scholar] [CrossRef]
- Xu, J.; Koivula, R.; Zhang, W.; Wiikinkoski, E.; Hietala, S.; Harjula, R. Separation of cobalt, neodymium and dysprosium using amorphous zirconium phosphate. Hydrometallurgy 2018, 175, 170–178. [Google Scholar] [CrossRef]
- Ma, F.; Shi, W.; Meng, H.; Li, Z.; Zhou, W.; Zhang, L. Preparation, characterization and ion-exchange behavior of polyantimonic acid-polyacrylonitrile (PAA–PAN) composite beads for strontium(II). J. Radioanal. Nucl. Chem. 2016, 308, 155–163. [Google Scholar] [CrossRef]
- He, L.-P.; Sun, S.-Y.; Mu, Y.-Y.; Song, X.-F.; Yu, J.-G. Recovery of Lithium, Nickel, Cobalt, and Manganese from Spent Lithium-Ion Batteries Using l-Tartaric Acid as a Leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- He, L.-P.; Sun, S.-Y.; Song, X.-F.; Yu, J.-G. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries. Waste Manag. 2017, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]

| Metal Ion | qmax,epo. (mmol g−1) | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | Intra-Particle Diffusion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K1 (h−1) | qeq (mmol g−1) | R2 | K2 (mg g−1 h−1) | qeq (mmol g−1) | R2 | C (mg g−1) | Kini. (mg g−1 h−1/2) | R2 | ||
| Co | 0.045 | 0.101 | 0.047 | 0.976 | 0.095 | 0.045 | 0.983 | 0.107 | 0.065 | 0.882 |
| Ni | 0.037 | 0.006 | 0.017 | 0.997 | 0.015 | 0.037 | 0.989 | 0.121 | 0.055 | 0.814 |
| Mn | 0.095 | 0.060 | 0.176 | 0.976 | 0.015 | 0.095 | 0.993 | 0.027 | 0.013 | 0.933 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haruna, B.; Luo, Z.; Muhammad, M.A.; Tang, J.; Kuva, J.; Koivula, R.; Bao, H.; Xu, J. Selective Separation of Lithium from Leachate of Spent Lithium-Ion Batteries by Zirconium Phosphate/Polyacrylonitrile Composite: Leaching and Sorption Behavior. Batteries 2024, 10, 254. https://doi.org/10.3390/batteries10070254
Haruna B, Luo Z, Muhammad MA, Tang J, Kuva J, Koivula R, Bao H, Xu J. Selective Separation of Lithium from Leachate of Spent Lithium-Ion Batteries by Zirconium Phosphate/Polyacrylonitrile Composite: Leaching and Sorption Behavior. Batteries. 2024; 10(7):254. https://doi.org/10.3390/batteries10070254
Chicago/Turabian StyleHaruna, Baffa, Zhongyan Luo, Mujtaba Aminu Muhammad, Jinfeng Tang, Jukka Kuva, Risto Koivula, Hongli Bao, and Junhua Xu. 2024. "Selective Separation of Lithium from Leachate of Spent Lithium-Ion Batteries by Zirconium Phosphate/Polyacrylonitrile Composite: Leaching and Sorption Behavior" Batteries 10, no. 7: 254. https://doi.org/10.3390/batteries10070254






