Role of Graphene Oxide and Reduced Graphene Oxide in Electric Double-Layer Capacitors: A Systematic Review
Abstract
1. Introduction
1.1. Advances in EDLCs
1.2. Innovations in Graphene for EDLCs
1.3. Advanced Synthesis and Processing Techniques for Graphene-Based EDLCs
1.4. Enhanced Performance of Graphene-Based Materials in Energy Storage Applications
2. Methodology
2.1. Goal of the Systematic Review
- “What are the effects of using GO and rGO on the performance metrics of EDLCs?”.
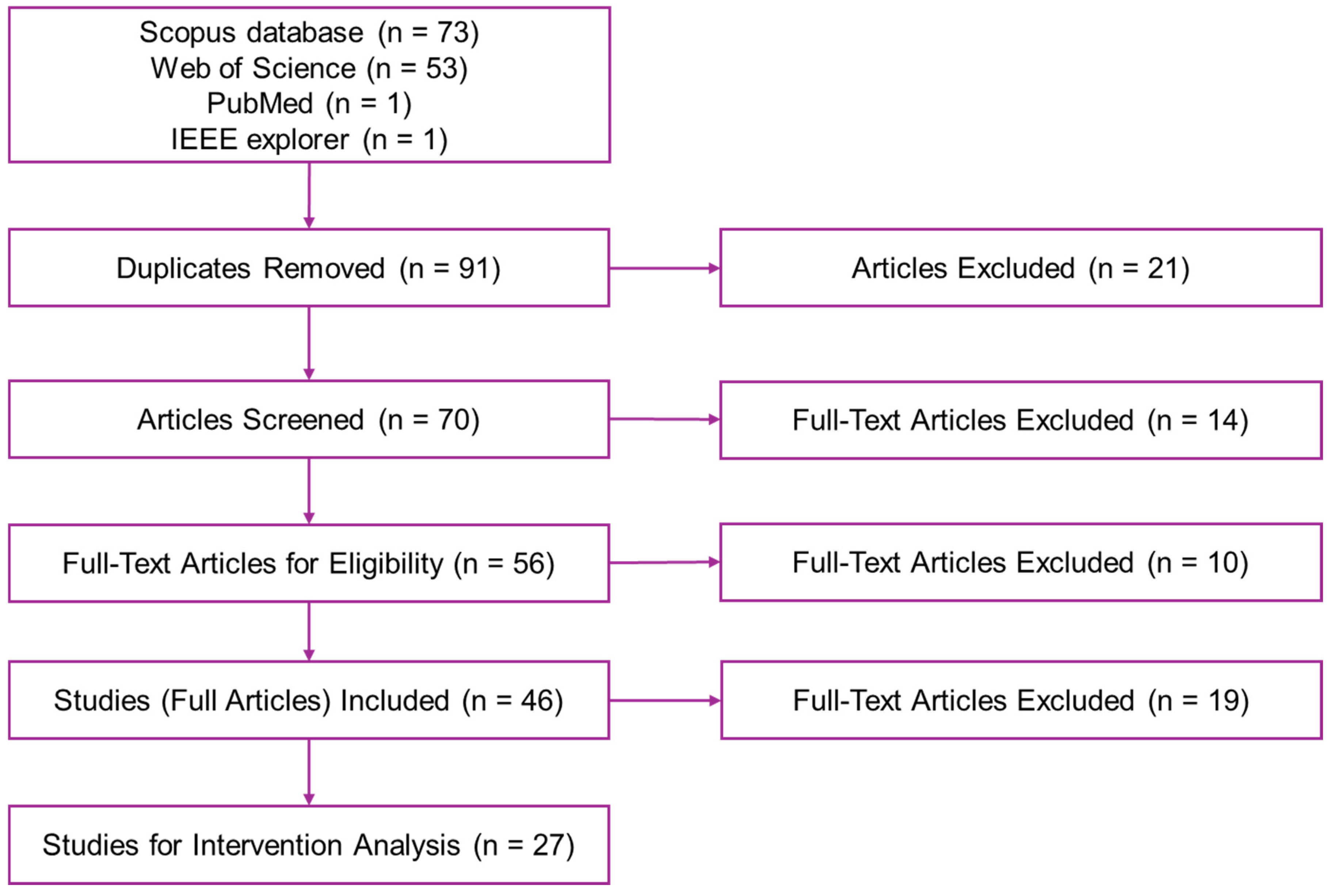
2.2. Identification Stage
2.3. Screening Stage
- Inclusion of only review articles, full articles, and proceedings papers;
- Focus on articles specifically about GO and rGO;
- Relevance to EDLC technology;
- Inclusion of articles in any language.
- Seventeen articles were not related to oxidized graphenes;
- Three articles did not pertain to EDLC technology;
- One article was not available.
2.4. Eligibility Stage
- The full text of the article is available in any language;
- The article focuses on the use of GO or rGO in supercapacitor technology;
- The article is centered on EDLC technology based on carbon materials;
- The article specifically discusses the application of GO or rGO in EDLC technology.
- Eight articles did not focus on EDLC technology and oxidized graphenes.
- Six articles were narrative review papers on the general topic of graphene-based supercapacitors.
2.5. Included Stage
- Energy density;
- Capacitance;
- Power density;
- Cycling stability;
- Efficiency;
- Performance enhancement.
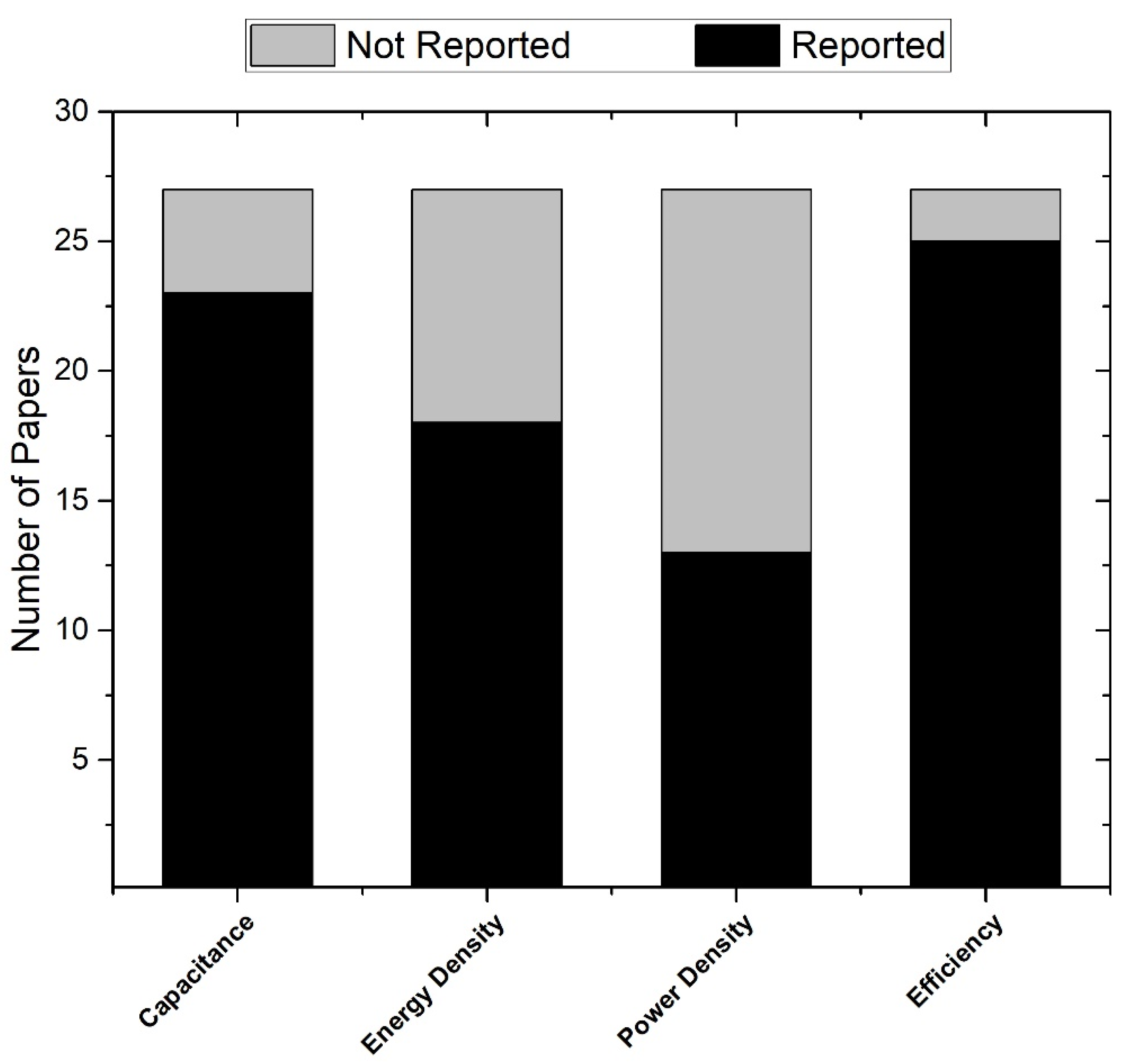
- Nine articles did not report capacitance or energy density;
- One article contained only theoretical predictions about electrode types with no reported metrics.
3. Results
3.1. Summary of Search Results
- Type of intervention describes the specific approach or modification applied;
- Preparation method details the synthesis and preparation techniques used for GO and rGO;
- Electrode fabrication discusses the methods and materials used in the creation of electrodes;
- Electrolyte used specifies the types of electrolytes employed in the studies;
- Comparison details provide a comparison of the methods and materials used across different studies;
- Results and findings summarize key results, including statistical significance and cycling stability.
- Capacitance is the measurement of the ability to store charge;
- Energy density is the amount of energy stored per unit volume or mass;
- Power density is the rate at which energy can be delivered;
- Efficiency is the overall performance efficiency of the EDLCs.
3.2. Interventions Outcomes
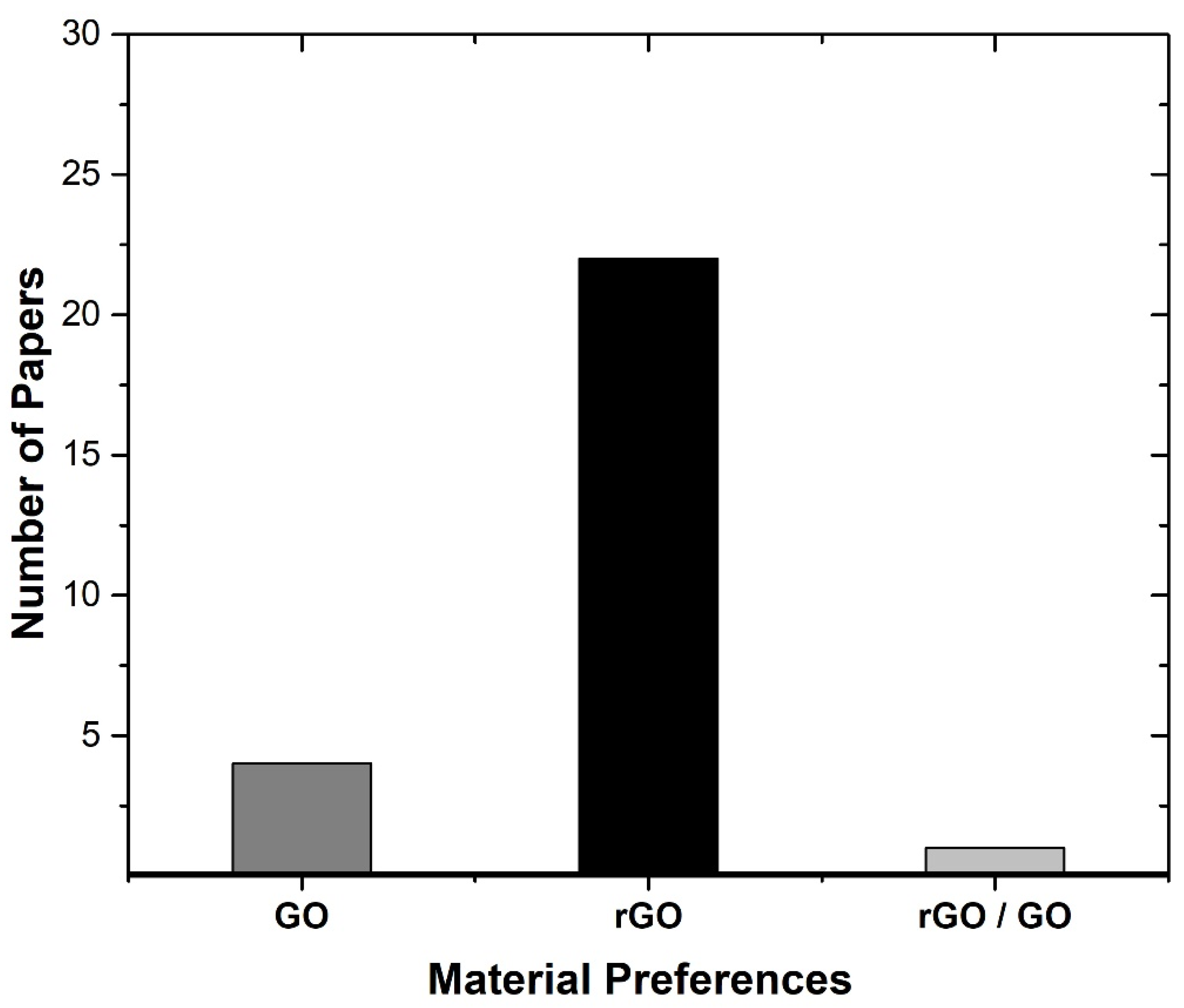
3.3. Data Analysis
- rGO typically offers higher electrical conductivity than GO, making it more suitable for applications requiring efficient charge and discharge cycles, which is critical for supercapacitors;
- rGO tends to have a suitable surface area compared to GO, which enhances its ability to store charge, thus improving the capacitance and overall performance of supercapacitors;
- rGO generally shows better electrochemical stability, making it more reliable for long-term applications in energy storage devices.
3.4. Generated Data for PICOS PRISMA Process
- Identification collects initial articles from databases such as Scopus, Web of Science (WoS), IEEE Explorer, and PubMed;
- Screening review titles and abstracts to filter out irrelevant studies;
- Eligibility assesses full-text articles based on specific inclusion criteria;
- Inclusion selects studies that meet all the criteria for the final review;
- Extraction extracts detailed data from the included studies for thorough analysis.
4. Discussion
4.1. Preparation Methods Suitable in EDLCs
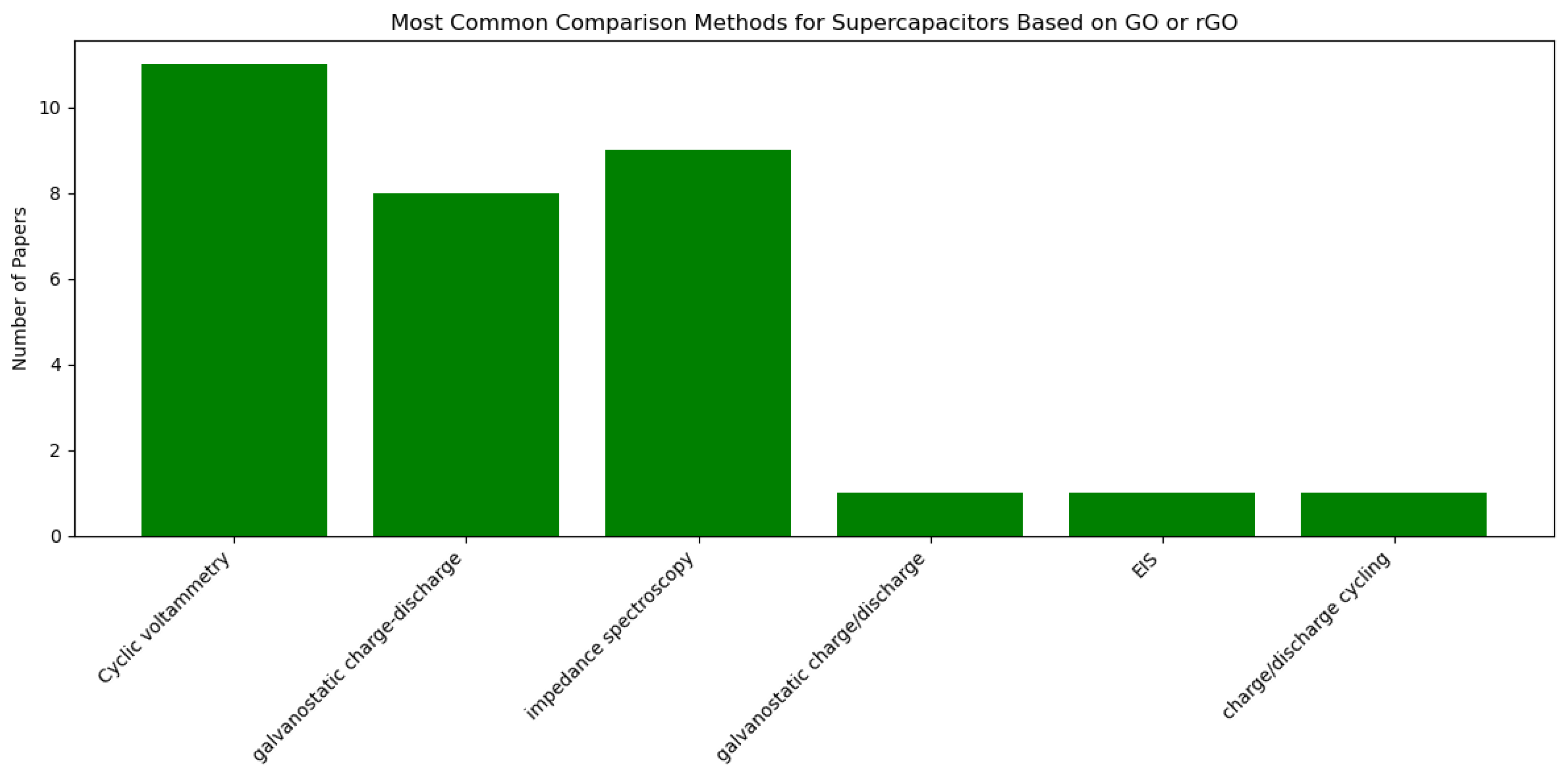
4.2. Comparison of Electrodes and Electrolytes for EDLCs
4.3. Commonly Materials and Methods Used
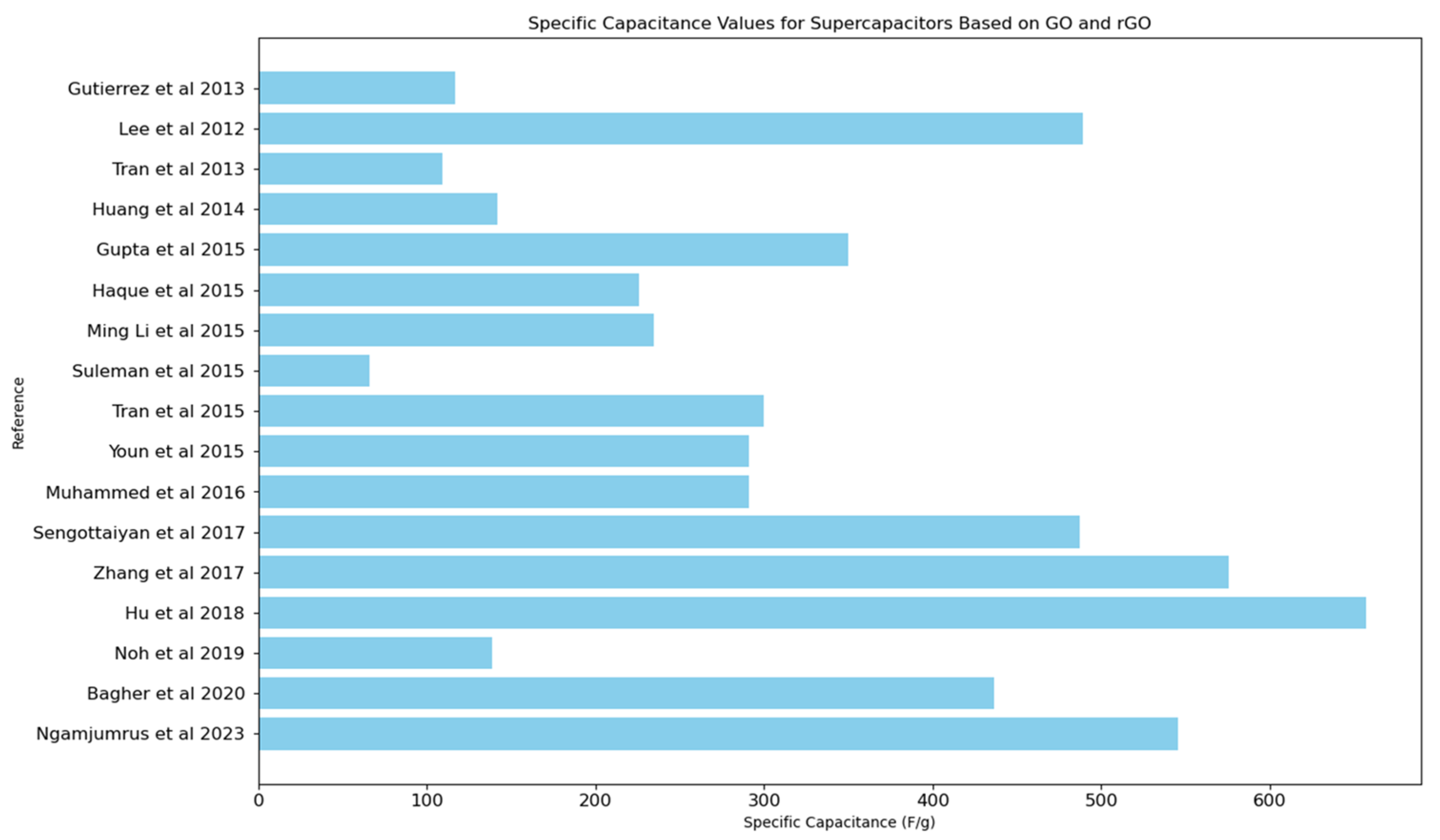
4.4. Performance Metrics: Capacitance
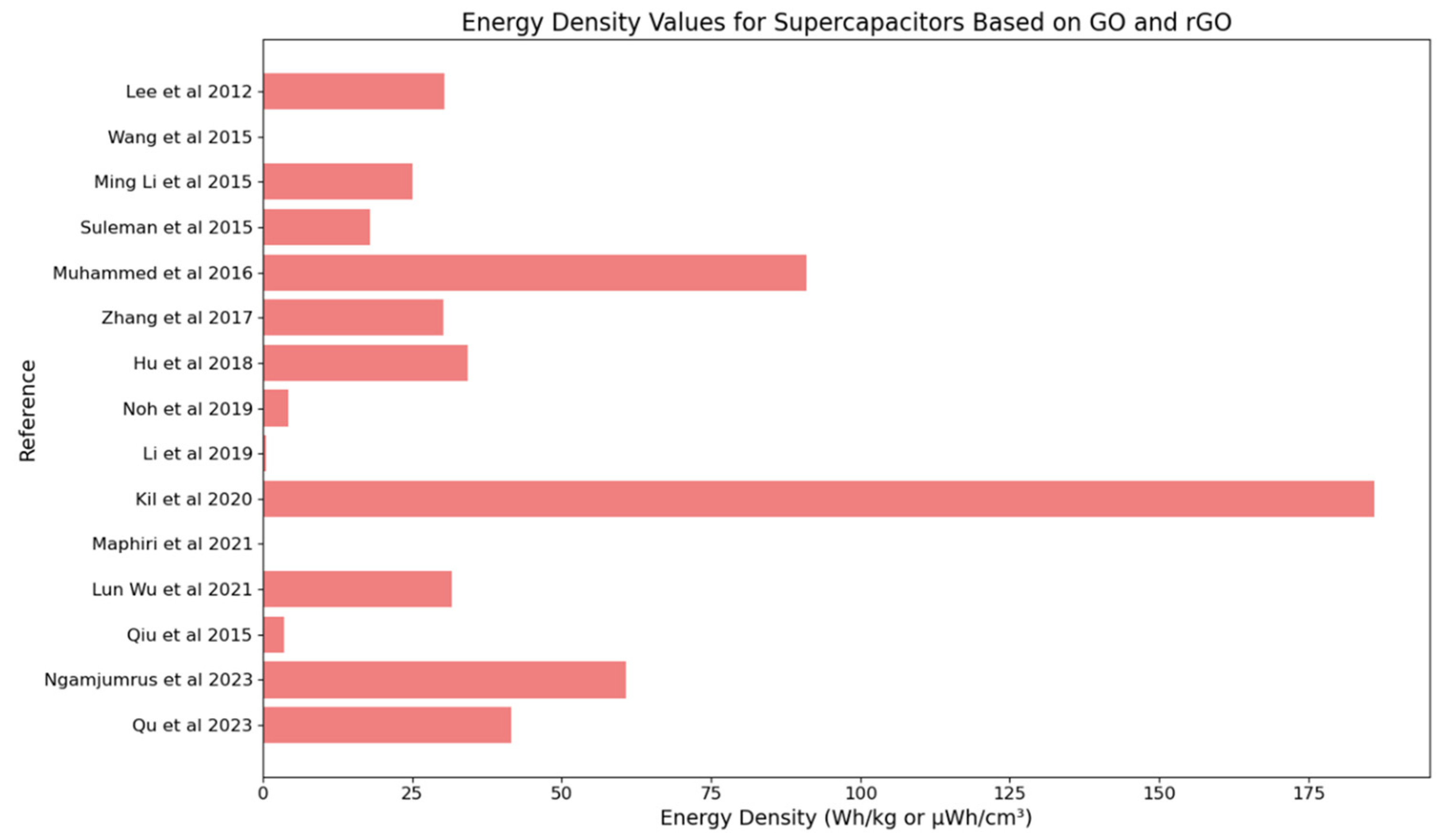
4.5. Performance Metrics: Energy Density
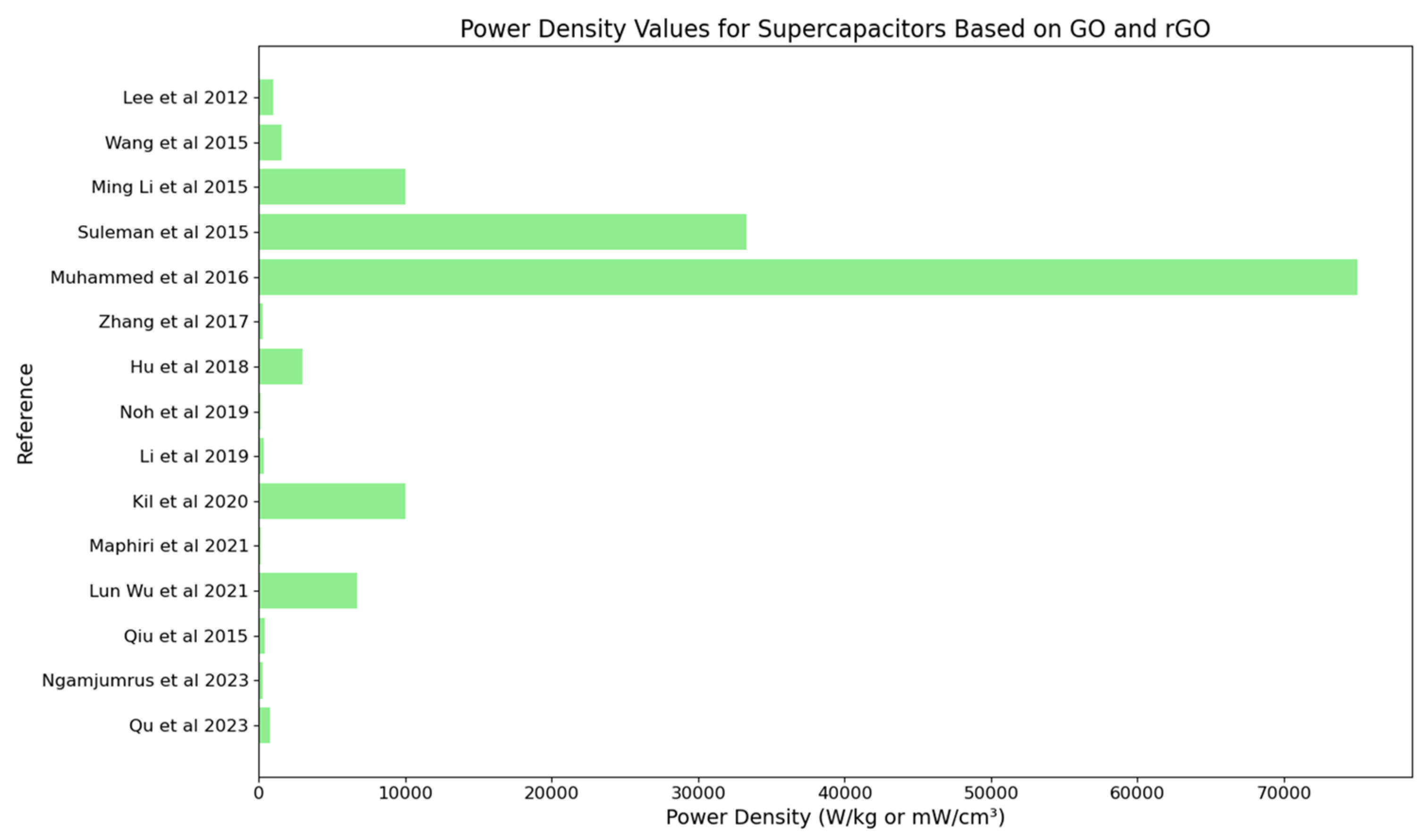
4.6. Performance Metrics: Power Density
4.7. Performance Metrics: Efficiency
5. Limitations and Recommendations
5.1. Limitations of the Current Systematic Review
- Population (P):
- The review focuses solely on studies involving EDLCs incorporating GO and rGO. This narrow focus could exclude other types of capacitors and energy storage devices that might benefit from similar materials.
- Intervention (I):
- There is considerable variability in the synthesis methods and conditions for producing GO and rGO across different studies. This heterogeneity makes it challenging to directly compare results and draw generalized conclusions about the effectiveness of specific interventions.
- The review includes various interventions (e.g., doping, composite formation, etc.) combined with GO and rGO, leading to diverse performance outcomes. The interactions between different interventions and their combined effects on EDLC performance are complex and not fully understood.
- Comparison (C):
- Many studies included in the review lack standardized comparison or control materials, making it difficult to isolate the specific contributions of GO and rGO to performance improvements.
- The performance metrics of EDLCs vary widely depending on the baseline materials and conditions used in the comparative studies, further complicating the comparison of results.
- Outcomes (O):
- There are inconsistencies in how performance metrics such as capacitance, energy density, and cycling stability are measured and reported across studies. This lack of uniformity hampers the ability to perform meta-analyses and aggregate data effectively.
- Many studies focus on short-term performance metrics, while long-term stability and degradation behavior, which are critical for practical applications, are less frequently reported.
- Study Design (S):
- There is a potential for publication bias, as studies with positive results are more likely to be published than those with negative or inconclusive findings. This bias can skew the overall understanding of the effectiveness of GO and rGO in EDLCs.
5.2. Recommendations for Future Research
- Standardization of Synthesis Methods:
- Standardized protocols for the synthesis of GO and rGO should be developed and adopted to ensure consistency in material properties across different studies. This standardization will facilitate more reliable comparisons and meta-analyses of research findings.
- Scalable synthesis methods that can be implemented at an industrial level should be a focus of study. Research should aim to optimize these methods to maintain the high performance of GO and rGO while ensuring economic feasibility and environmental sustainability.
- Comprehensive Performance Metrics:
- Future studies should include long-term performance metrics, such as cycling stability over extended periods and under various operating conditions. Understanding the degradation mechanisms of GO and rGO-based EDLCs is crucial for their practical application.
- Standardized testing conditions and protocols for measuring key performance metrics, such as capacitance, energy density, power density, and efficiency, should be established. Consistent reporting of these metrics will enable more accurate comparisons between studies.
- Environmental and Sustainable Approaches:
- Investment should be made in developing environmentally friendly synthesis processes for GO and rGO that minimize the use of toxic chemicals and reduce the overall environmental impact. Utilizing renewable resources and waste materials for the synthesis of graphene derivatives can also contribute to sustainability.
- Lifecycle analyses of GO and rGO-based EDLCs should be conducted to evaluate their environmental impact from production to disposal. This analysis would help identify areas for improvement in sustainability.
- Material Optimization:
- The effects of various doping elements and functional groups on the electrochemical performance of GO and rGO should be further explored. Tailoring the oxygen content and introducing heteroatoms or other functional groups can optimize the properties of these materials for specific applications.
- The potential of hybrid materials that combine GO and rGO with other high-performance materials, such as metal oxides, conductive polymers, and carbon nanotubes, should be investigated. These hybrid materials can synergistically enhance the performance of EDLCs.
- Interfacial Engineering:
- Research should focus on innovative electrode designs that enhance the interfacial contact between GO/rGO and the electrolyte. Improved electrode architecture can lead to better ion transport, increased surface area, and enhanced overall performance.
- The compatibility of GO and rGO with various electrolytes, including aqueous, organic, and ionic liquid electrolytes, should be explored. Understanding the interactions between the electrode material and the electrolyte will help optimize the performance of EDLCs.
- Application-Specific Research:
- Application-specific research should be conducted to tailor GO and rGO-based EDLCs for targeted applications, such as portable electronics, electric vehicles, and grid energy storage. Each application may have unique requirements for performance metrics and material properties.
- Researchers should move beyond laboratory-scale experiments to develop and test prototypes of GO and rGO-based EDLCs in real-world scenarios. Prototyping will provide practical insights into the challenges and potential of these materials for commercial applications.
6. Potential Challenges and Solutions for Implementing GO and rGO in Realistic Applications
6.1. Challenges and Proposed Solutions
- Scalability and Production Costs
- Challenge: The synthesis of high-quality GO and rGO on a large scale remains a significant challenge. The methods commonly used in laboratories, such as Hummers’ method for GO and chemical reduction for rGO, are often not cost-effective or scalable.
- Solution: The development of scalable production techniques, such as continuous flow processes and green synthesis methods, can help in reducing costs and improving yield. Collaborations between academia and industry could foster innovations in manufacturing technologies, making large-scale production more feasible.
- Material Consistency and Quality Control
- Challenge: Ensuring consistency in the quality of GO and rGO is critical for their performance in applications. Variations in the degree of oxidation, reduction, and functionalization can lead to significant differences in material properties.
- Solution: Implementing standardized protocols for the synthesis and characterization of GO and rGO can help in maintaining consistency. Advanced characterization techniques and real-time monitoring systems can be employed to ensure high-quality production.
- Integration with Existing Technologies
- Challenge: Integrating GO and rGO into existing technologies and systems can be challenging due to compatibility issues with other materials and components.
- Solution: Research on hybrid materials and composites that combine GO and rGO with conventional materials can enhance compatibility. Developing adhesive interfaces and surface treatments can also facilitate better integration.
- Performance Stability and Durability
- Challenge: Maintaining the stability and durability of GO and rGO-based devices under operational conditions is crucial. Issues such as electrode degradation and loss of capacitance over time need to be addressed.
- Solution: Enhancing the structural integrity of GO and rGO through doping, cross-linking, and encapsulation techniques can improve their stability. Conducting long-term performance studies and accelerated aging tests can provide insights into improving durability.
6.2. Environmental Sustainability of GO and rGO
- Chemical Synthesis and Waste Management
- Impact: The chemical processes involved in the synthesis of GO and rGO often generate hazardous waste, including strong acids, oxidizing agents, and reducing agents. Improper disposal of these chemicals can lead to environmental pollution and health risks.
- Solution: Developing greener synthesis methods that minimize the use of hazardous chemicals and generate less waste is essential. Techniques such as electrochemical exfoliation and biomass-derived precursors offer more environmentally friendly alternatives. Implementing strict waste management protocols and recycling processes can also mitigate environmental impact.
- Toxicity and Biocompatibility
- Impact: The potential toxicity of GO and rGO to humans and the environment is a concern, particularly for applications involving direct human contact or disposal in natural ecosystems.
- Solution: Conducting comprehensive toxicity and biocompatibility studies is necessary to understand the potential risks. Functionalizing GO and rGO with biocompatible molecules and coatings can reduce toxicity. Regulations and guidelines for safe handling, usage, and disposal should be established and followed.
- Energy Consumption and Carbon Footprint
- Impact: The energy-intensive nature of GO and rGO production can contribute to a high carbon footprint, which is counterproductive to the goal of sustainable energy storage solutions.
- Solution: Optimizing production processes to reduce energy consumption and adopting renewable energy sources for manufacturing can lower the carbon footprint. Life cycle assessments (LCA) can help identify areas for improvement and track the environmental impact of GO and rGO production.
7. Conclusions
- In terms of capacitance, the studies reviewed demonstrate that both GO and rGO exhibit high specific capacitance values, which are further enhanced through hybridization with conductive polymers and metal oxides. The introduction of nitrogen doping and functionalization with other materials significantly boosts electrochemical performance, making these materials promising for high-capacity energy storage devices.
- In terms of energy density, GO and rGO-based EDLCs show considerable potential in achieving high energy densities, especially when combined with innovative composite structures. The introduction of nitrogen atoms and the development of advanced hybrid materials result in substantial improvements in energy storage capabilities, making these materials suitable for a wide range of applications requiring high energy density.
- In terms of power density, the review highlights the remarkable power densities achieved with GO and rGO-based supercapacitors, particularly those utilizing nitrogen-doped reduced graphene oxide and advanced composites. These materials exhibit excellent power delivery capabilities, essential for applications requiring rapid energy discharge.
- The efficiency of GO and rGO-based EDLCs, measured in terms of capacitance retention after multiple charge–discharge cycles, varies significantly across different studies. High-efficiency materials typically involve advanced composites, doping strategies, and innovative synthesis techniques that enhance structural integrity and electrochemical properties. The durability and long-term stability of these materials are promising for practical applications.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Tene, T.; Usca, G.T.; Guevara, M.; Molina, R.; Veltri, F.; Arias, M.; Caputi, L.S.; Gomez, C.V. Toward Large-Scale Production of Oxidized Graphene. Nanomaterials 2020, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Perrozzi, F.; Prezioso, S.; Ottaviano, L. Graphene oxide: From fundamentals to applications. J. Phys. Condens. Matter 2015, 27, 013002. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; James, D.K.; Tour, J.M. New Routes to Graphene, Graphene Oxide and Their Related Applications. Adv. Mater. 2012, 24, 4924–4955. [Google Scholar] [CrossRef] [PubMed]
- Tene, T.; Arias, F.A.; Guevara, M.; Nuñez, A.; Villamagua, L.; Tapia, C.; Pisarra, M.; Torres, F.J.; Caputi, L.S.; Gomez, C.V. Removal of mercury(II) from aqueous solution by partially reduced graphene oxide. Sci. Rep. 2022, 12, 6326. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Ma, Q.; Yi, S.; Chen, H.; Liu, H.; Chen, Y.; Yang, L. A facile one-pot fabrication of flowerlike graphene-based particles for electric double-layer capacitors. Mater. Chem. Phys. 2014, 148, 631–638. [Google Scholar] [CrossRef]
- Saraf, M.; Dar, R.A.; Natarajan, K.; Srivastava, A.K.; Mobin, S.M. A Binder-Free Hybrid of CuO-Microspheres and rGO Nanosheets as an Alternative Material for Next Generation Energy Storage Application. ChemistrySelect 2016, 1, 2826–2833. [Google Scholar] [CrossRef]
- George, D.; James, G.; Manjally, J.K.; Thomas, J.; Kuriakose, M.S.; Aryamol, K.S.; Benny, A.; George, S.C. Graphene PANI Electrode Material Characterization for Supercapacitor Application. Mater. Today Proc. 2020, 24, 1734–1741. [Google Scholar] [CrossRef]
- Liu, K.-K.; Jin, B.; Meng, L.-Y. Glucose/Graphene-Based Aerogels for Gas Adsorption and Electric Double Layer Capacitors. Polymers 2019, 11, 40. [Google Scholar] [CrossRef]
- Wang, L.; Ye, X.; Zhao, P.; Jiang, H.; Zhu, Y.; Wan, Z.; Rao, G.; You, S.; Zeng, G.; Fu, J.; et al. Toward high capacitance and rate capability supercapacitor: Three dimensional graphene network fabricated by electric field-assisted assembly method. Appl. Surf. Sci. 2018, 467–468, 949–953. [Google Scholar] [CrossRef]
- Allagui, A.; Alnaqbi, H.; Elwakil, A.S.; Said, Z.; Hachicha, A.A.; Wang, C.; Abdelkareem, M.A. Fractional-order electric double-layer capacitors with tunable low-frequency impedance phase angle and energy storage capabilities. Appl. Phys. Lett. 2020, 116, 013902. [Google Scholar] [CrossRef]
- Loganathan, N.N.; Munusamy, K.R.; Perumal, V.; Pandian, B.R. Laser Scribed Graphene from Oil Palm Lignin for Supercapacitor Applications. J. Water Environ. Nanotechnol. 2021, 6, 356–366. [Google Scholar] [CrossRef]
- Mouli, C.L.R. Supercapacitors as Future Energy Storage Devices for Electric Vehicles. In Proceedings of the 2022 IEEE Delhi Section Conference (DELCON), New Delhi, India, 11–13 February 2022. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Chen, Z.-D.; Han, D.-D.; Zhang, Y.-L.; Xia, H.; Sun, H.-B. Laser Fabrication of Graphene-Based Supercapacitors. Photonics Res. 2020, 8, 577–588. [Google Scholar] [CrossRef]
- Khadem, A.H. Recent Advances in Functional Fabric-Based Wearable Supercapacitors. Adv. Mater. Interfaces 2024, 11, 2300724. [Google Scholar] [CrossRef]
- Imae, I. Reduction of Graphene Oxide Using an Environmentally Friendly Method and Its Application to Energy-Related Materials. Coatings 2021, 11, 297. [Google Scholar] [CrossRef]
- Pershaanaa, M.; Bashir, S.; Ramesh, S.; Ramesh, K. Every bite of Supercap: A brief review on construction and enhancement of supercapacitor. J. Energy Storage 2022, 50, 104599. [Google Scholar] [CrossRef]
- Banik, U.; Malik, M.T.U.; Rahat, S.M.S.M.; Mallik, A.K. Performance Enhancement of Ruthenium-Based Supercapacitors: A Review. Energy Storage 2022, 5, e380. [Google Scholar] [CrossRef]
- Gao, D.; Luo, Z.; Liu, C.; Fan, S. A Survey of Hybrid Energy Devices Based on Supercapacitors. Green Energy Environ. 2023, 8, 972–988. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Meng, Q. The Preparation and Characterization of Graphene-Cross-Linked Phenol-Formaldehyde Hybrid Carbon Xerogels. J. Sol-Gel Sci. Technol. 2013, 64, 123–133. [Google Scholar] [CrossRef]
- Morimoto, N.; Kubo, T.; Nishina, Y. Tailoring the Oxygen Content of Graphite and Reduced Graphene Oxide for Specific Applications. Sci. Rep. 2016, 6, 21715. [Google Scholar] [CrossRef] [PubMed]
- Ciszewski, M.; Szatkowska, E.; Koszorek, A.; Majka, M. Carbon Aerogels Modified with Graphene Oxide, Graphene, and CNT as Symmetric Supercapacitor Electrodes. J. Mater. Sci. Mater. Electron. 2017, 28, 4897–4903. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Masikhwaa, T.M.; Lindberg, S.; Matic, A.; Johansson, P.; Manyala, N. Comparison of Ionic Liquid Electrolyte to Aqueous Electrolytes on Carbon Nanofibres Supercapacitor Electrode Derived from Oxygen-Functionalized Graphene. Chem. Eng. J. 2019, 375, 121906. [Google Scholar] [CrossRef]
- Shanmugaraj, P.; Swaminathan, A.; Ravi, R.K.; Dasaiah, M.; Kumar, P.S.; Sakunthala, A. Preparation and characterization of porous PVdF-HFP/graphene oxide composite membranes by solution casting technique. J. Mater. Sci. Mater. Electron. 2019, 30, 20079–20087. [Google Scholar] [CrossRef]
- Tamilselvi, R.; Ramesh, M.; Lekshmi, G.S.; Bazaka, O.; Levchenko, I.; Bazaka, K.; Mandhakini, M. Graphene oxide—Based supercapacitors from agricultural wastes: A step to mass production of highly efficient electrodes for electrical transportation systems. Renew. Energy 2019, 151, 731–739. [Google Scholar] [CrossRef]
- Bulakhe, R.N.; Lee, J.; Tran, C.V.; In, J.B. Mesoporous nanohybrids of 2D layered Cu–Cr phosphate and rGO for high-performance asymmetric hybrid supercapacitors. J. Alloys Compd. 2022, 926, 166864. [Google Scholar] [CrossRef]
- Maphiri, V.M.; Bakhoum, D.T.; Sarr, S.; Sylla, N.F.; Rutavi, G.; Manyala, N. Impact of Thermally Reducing Temperature on Graphene Oxide Thin Films and Microsupercapacitor Performance. Nanomaterials 2022, 12, 2211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, X.; Chen, R.; Wang, X.; Ma, H.; Chai, Y.; Cao, T.; Rao, W.; Chen, J.; Ji, J.; et al. Selective Ion Transport in Assembled Graphene Oxide-Modified Separator and the Novel Intra-Series Architecture for Improved Aqueous Batteries. Chem. Eng. J. 2024, 450 Pt 2, 138061. [Google Scholar] [CrossRef]
- Tien, C.-P.; Teng, H. Polymer/Graphite Oxide Composites as High-Performance Materials for Electric Double Layer Capacitors. J. Power Sources 2010, 195, 2414–2418. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Li, Y.; Wang, T. Superparamagnetic Magnetite Nanocrystals-Graphene Oxide Nanocomposites: Facile Synthesis and Their Enhanced Electric Double-Layer Capacitor Performance. J. Nanosci. Nanotechnol. 2012, 12, 4583–4590. [Google Scholar] [CrossRef]
- Yu, H.; He, J.; Sun, L.; Tanaka, S.; Fugetsu, B. Influence of the Electrochemical Reduction Process on the Performance of Graphene-Based Capacitors. Carbon 2013, 51, 94–101. [Google Scholar] [CrossRef]
- Wang, J.-D.; Peng, T.-J.; Sun, H.-J.; Hou, Y.-D. Effect of the Hydrothermal Reaction Temperature on Three-Dimensional Reduced Graphene Oxide’s Appearance, Structure, and Supercapacitor Performance. Acta Phys. -Chim. Sin. 2014, 30, 2077–2084. [Google Scholar] [CrossRef]
- Qiu, H.; Bechtold, T.; Le, L.; Lee, W.Y. Evaporative Assembly of Graphene Oxide for Electric Double-Layer Capacitor Electrode Application. Powder Technology 2015, 270, 192–196. [Google Scholar] [CrossRef]
- Ma, W.; Chen, S.; Yang, S.; Chen, W.; Weng, W.; Zhu, M. Bottom-Up Fabrication of Activated Carbon Fiber for All-Solid-State Supercapacitor with Excellent Electrochemical Performance. ACS Appl. Mater. Interfaces 2016, 8, 14622–14627. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, A.; Tian, Z.R.; Ang, S.S. MoS2 Reduced Graphene Oxide-Based 2D Nanocomposites for Boosting the Energy Density of Electric Double-Layer Capacitor. MRS Adv. 2016, 1, 1619–1624. [Google Scholar] [CrossRef]
- Park, J.; Kumar, V.; Wang, X.; Lee, P.-S.; Kim, W. Investigation of Charge Transfer Kinetics at Carbon/Hydroquinone Interfaces for Redox-Active-Electrolyte Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 33728–33734. [Google Scholar] [CrossRef] [PubMed]
- Vermisoglou, E.C.; Giannakopoulou, T.; Romanos, G.; Boukos, N.; Psycharis, V.; Lei, C.; Lekakou, C.; Petridis, D.; Yu, J.G.; Trapalis, C. Graphene-Based Materials via Benzidine-Assisted Exfoliation and Reduction of Graphite Oxide and Their Electrochemical Properties. Appl. Surf. Sci. 2016, 392, 244–255. [Google Scholar] [CrossRef]
- Arnaiz, M.; Botas, C.; Carriazo, D.; Mysyk, R.; Mijangos, F.; Rojo, T.; Ajuria, J.; Goikolea, E. Reduced Graphene Oxide Decorated with SnO2 Nanoparticles as Negative Electrode for Lithium Ion Capacitors. Electrochim. Acta 2018, 284, 542–550. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Kumar, S.; Bae, J.; Seo, Y. CVD-Graphene for Low Equivalent Series Resistance in rGO/CVD-Graphene/Ni-Based Supercapacitors. Nanotechnology 2018, 29, 195404. [Google Scholar] [CrossRef]
- Sun, Z.; Du, S.; Li, F.; Yang, L.; Zhang, D.; Song, W. High-performance cellulose based nanocomposite soft actuators with porous high-conductivity electrode doped by graphene-coated carbon nanosheet. Cellulose 2018, 25, 5807–5819. [Google Scholar] [CrossRef]
- Strauss, V.; Muni, M.; Borenstein, A.; Badamdorj, B.; Heil, T.; Kowala, M.D.; Kaner, R. Patching Laser-Reduced Graphene Oxide with Carbon Nanodots. Nanoscale 2019, 11, 12712–12719. [Google Scholar] [CrossRef] [PubMed]
- Ortega, P.F.R.; dos Santos Junior, G.A.; Trigueiro, J.P.C.; Silva, G.G.; Quintanal, N.; Blanco, C.; Lavall, R.L.; Santamaría, R. Insights on the Behavior of Imidazolium Ionic Liquids as Electrolytes in Carbon-Based Supercapacitors: An Applied Electrochemical Approach. J. Phys. Chem. C 2020, 124, 15818–15830. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Cai, Q.; Ma, J.-N.; Zhu, L.; Han, D.-D.; Zhang, Y.-L. Free-standing and flexible graphene supercapacitors of high areal capacitance fabricated by laser holography reduction of graphene oxide. Appl. Phys. Lett. 2021, 118, 071601. [Google Scholar] [CrossRef]
- Syed, A.W.; Mohammad, M.A. Laser Scribed Graphene-Based Flexible Microsupercapacitors With Fractal Design. IEEE Access 2021, 9, 154957–154964. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J. Study on Modification and Electrochemical Performance of Graphene/Nickel Matrix Composite. J. Mater. Sci. Mater. Electron. 2022, 33, 4081–4092. [Google Scholar] [CrossRef]
- Men, L.; Chen, C.; Liu, A.; Zhou, J.; Yu, S.; Wei, Z. Activated carbon fiber–loaded single layer graphene oxide–based capacitive deionization electrode materials for water desalination applications. Ionics 2022, 28, 1903–1913. [Google Scholar] [CrossRef]
- Chang, P.; Li, X.; Zhang, C.; Li, L.; Luo, Y.; Dong, J.; Yang, T. Preparation of Highly Condensed Porous Carbon Used in EDLCs with High Voltage and High Volumetric Energy Density. Diam. Relat. Mater. 2023, 134, 109779. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.; Shamseer, L.; Tetzlaff, J.; Akl, E.; Brennan, S.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Falagas, M.E.; Pitsouni, E.I.; Malietzis, G.A.; Pappas, G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. FASEB J. 2008, 22, 338–342. [Google Scholar] [CrossRef]
- Mongeon, P.; Paul-Hus, A. The journal coverage of Web of Science and Scopus: A comparative analysis. Scientometrics 2016, 106, 213–228. [Google Scholar] [CrossRef]
- Lefebvre, C.; Glanville, J.; Briscoe, S.; Littlewood, A.; Marshall, C.; Metzendorf, M.-I.; Noel-Storr, A.; Rader, T.; Shokraneh, F.; Thomas, J.; et al. Searching for and selecting studies. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley Sons: Chichester, UK, 2019; pp. 67–108. [Google Scholar] [CrossRef]
- Poli, R. An Analysis of Publications on Particle Swarm Optimisation Applications; Technical Report CSM-469; Department of Computer Science, University of Essex: Colchester, UK, May 2007; ISSN 1744-8050. (revised November 2007). [Google Scholar]
- Gutierrez, D.H.; Peaslee, D.; Tanaka, Z.; Londoño, N.J.; Meyyapan, M.; Chen, B. Ionic, Organic and Strong Electrolytes with Graphene on Metal Oxide Composite Electrodes for Supercapacitors. ECS Trans. 2013, 45, 31. [Google Scholar] [CrossRef]
- Lee, T.; Yun, T.; Park, B.; Sharma, B.; Song, H.-K.; Kim, B.-S. Hybrid Multilayer Thin Film Supercapacitor of Graphene Nanosheets with Polyaniline: Importance of Establishing Intimate Electronic Contact through Nanoscale Blending. J. Mater. Chem. 2012, 22, 21092–21099. [Google Scholar] [CrossRef]
- Tran, M.-H.; Yang, C.S.; Jeong, H.K. Enhanced Electric Double Layer Capacitance of New Poly Sodium 4-Styrenesulfonate Intercalated Graphene Oxide Electrodes. Chem. Phys. Lett. 2013, 578, 106–109. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Wu, P.-F.; Zhang, M.-Q.; Ruan, W.-H.; Giannelis, E.P. Boron Cross-Linked Graphene Oxide/Polyvinyl Alcohol Nanocomposite Gel Electrolyte for Flexible Solid-State Electric Double Layer Capacitor with High Performance. Electrochim. Acta 2014, 132, 103–111. [Google Scholar] [CrossRef]
- Gao, S.; Wang, K.; Du, Z.; Wang, Y.; Yuan, A.; Lu, W.; Chen, L. High Power Density Electric Double-Layer Capacitor Based on a Porous Multi-Walled Carbon Nanotube Microsphere as a Local Electrolyte Micro-Reservoir. Carbon 2015, 92, 254–261. [Google Scholar] [CrossRef]
- Gupta, S.; Price, C. Scanning Electrochemical Microscopy of Graphene/Polymer Hybrid Thin Films as Supercapacitors: Physical-Chemical Interfacial Processes. AIP Adv. 2015, 5, 107113. [Google Scholar] [CrossRef]
- Haque, E.; Islam, M.M.; Pourazadi, E.; Hassan, M.; Faisal, S.N.; Roy, A.K.; Konstantinov, K.; Harris, A.T.; Minett, A.I.; Gomes, V.G. Nitrogen Doped Graphene via Thermal Treatment of Composite Solid Precursors as a High Performance Supercapacitor. RSC Adv. 2015, 5, 30679–30686. [Google Scholar] [CrossRef]
- Li, S.-M.; Yang, S.-Y.; Wang, Y.-S.; Tsai, H.-P.; Tien, H.-W.; Hsiao, S.-T.; Liao, W.-H.; Chang, C.-L.; Ma, C.-C.M.; Hu, C.-C. N-doped structures and surface functional groups of reduced graphene oxide and their effect on the electrochemical performance of supercapacitor with organic electrolyte. J. Power Sources 2015, 278, 218–229. [Google Scholar] [CrossRef]
- Suleman, M.; Kumar, Y.; Hashmi, S.A. High-rate Supercapacitive Performance of GO/r-GO Electrodes Interfaced with Plastic-Crystal-Based Flexible Gel Polymer Electrolyte. Electrochim. Acta 2015, 182, 995–1007. [Google Scholar] [CrossRef]
- Tran, M.-H.; Jeong, H.K. One-pot synthesis of graphene/glucose/nickel oxide composite for the supercapacitor application. Electrochim. Acta 2015, 180, 679–686. [Google Scholar] [CrossRef]
- Youn, H.-C.; Bak, S.-M.; Kim, M.-S.; Jaye, C.; Fischer, D.A.; Lee, C.-W.; Yang, X.-Q.; Roh, K.C.; Kim, K.-B. High-surface-area nitrogen-doped reduced graphene oxide for electric double-layer capacitors. ChemSusChem 2015, 8, 1875–1884. [Google Scholar] [CrossRef]
- Muhammed Shafi, P.; Jose, V.K.; Chandra Bose, A. Graphene oxide-MnO2 nanocomposite for supercapacitor application. Proc. SPIE 2016, 9932, 99320I. [Google Scholar] [CrossRef]
- Wang, A.J.; Liu, L.; Lin, X.X.; Yuan, J.; Feng, J.J. One-pot synthesis of 3D freestanding porous PtAg hollow chain-like networks as efficient electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2017, 245, 883–892. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, R.; Liu, X.; Zhang, Y.; Liu, J. Carbon Fiber Electrodes Enhanced with Graphene Oxide for Supercapacitors. J. Power Sources 2019, 430, 15–22. [Google Scholar] [CrossRef]
- Sengottaiyan, C.; Jayavel, R.; Bairi, P.; Shrestha, R.G.; Ariga, K.; Shrestha, L.K. Cobalt Oxide/Reduced Graphene Oxide Composite with Enhanced Electrochemical Supercapacitance Performance. Bull. Chem. Soc. Jpn. 2017, 90, 955–962. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Liu, J.; Wu, J.; Chen, H.; Bi, H. Design and preparation of a ternary composite of graphene oxide/carbon dots/polypyrrole for supercapacitor application: Importance and unique role of carbon dots. Carbon 2017, 115, 134–146. [Google Scholar] [CrossRef]
- Hu, T.; Liu, Y.; Zhang, Y.; Chen, M.; Zheng, J.; Tang, J.; Meng, C. 3D Hierarchical Porous V3O7·H2O Nanobelts/CNT/Reduced Graphene Oxide Integrated Composite with Synergistic Effect for Supercapacitors with High Capacitance and Long Cycling Life. J. Colloid Interface Sci. 2018, 531, 382–393. [Google Scholar] [CrossRef]
- Noh, Y.; Kim, Y.; Han, H.; Jung, W.-G.; Kim, J.G.; Kim, Y.; Kim, H.J.; Kim, B.-J.; Kim, W.B. Improved Ion-Transfer Behavior and Capacitive Energy Storage Characteristics of SnO2 Nanospacer-Incorporated Reduced Graphene Oxide Electrodes. ChemElectroChem 2019, 6, 2503–2509. [Google Scholar] [CrossRef]
- Park, M.-J.; Lee, J.-S. Foldable and Biodegradable Energy-Storage Devices on Copy Papers. Adv. Electron. Mater. 2018, 5, 1800411. [Google Scholar] [CrossRef]
- Bakhshandeh, M.B.; Kowsari, E. Specifying the Effects of Functionalization of Highly Reduced Graphene Oxide by an Ionic Liquid on Supercapacitive Features. J. Electron. Mater. 2020, 49, 3920–3927. [Google Scholar] [CrossRef]
- Kil, H.-J.; Yun, K.; Yoo, M.-E.; Kim, S.; Park, J.-W. Solution-processed graphene oxide electrode for supercapacitors fabricated using low temperature thermal reduction. RSC Adv. 2020, 10, 22102–22111. [Google Scholar] [CrossRef]
- Maphiri, V.M.; Rutavi, G.; Sylla, N.F.; Adewinbi, S.A.; Fasakin, O.; Manyala, N. Novel thermally reduced graphene oxide microsupercapacitor fabricated via mask-free AxiDraw direct writing. Nanomaterials 2021, 11, 1909. [Google Scholar] [CrossRef]
- Wu, C.-L.; Chen, D.-H. Fabrication of rGO/CoSx-rGO/rGO Hybrid Film via Coassembly and Sulfidation of 2D Metal Organic Framework Nanoflakes and Graphene Oxide as Free-Standing Supercapacitor Electrode. J. Alloys Compd. 2021, 872, 159702. [Google Scholar] [CrossRef]
- Zhao, D.; Yuan, X.; Wang, R.; Zhao, Q.; Guo, S. Nanocarved Vanadium Nitride Nanowires Encapsulated in Lamellar Graphene Layers as Supercapacitor Electrodes. J. Mater. Sci. Mater. Electron. 2021, 32, 21197–21205. [Google Scholar] [CrossRef]
- Su, Y.-H.; Shih, C.-Y.; Su, C.-H.; Lee, Y.-L.; Hsieh, C.-T.; Teng, H. Dielectric gel electrolytes for safe charge storage from −20 to 80 °C by double-layer capacitors. J. Taiwan Inst. Chem. Eng. 2022, 134, 104309. [Google Scholar] [CrossRef]
- Ngamjumrus, N.; Silakaew, K.; Thompho, S.; Sriwong, C.; Ruttanapun, C. Two Steps for Improving Reduced Graphene Oxide Activated Durian Shell Carbon Composite by Hydrothermal and 3-D Ball Milling Process for Symmetry Supercapacitor Device. Energies 2023, 16, 6962. [Google Scholar] [CrossRef]
- Qu, X.; Jeon, S.; Jeong, J.; Kang, W.; Xing, B.; Zhang, C.; Hong, S. Optimized synthetic route for reduced graphene oxide-decorated Cu0.33Co0.67Se2 nanorods on Ni foam integrated with N, S co-doped porous carbon to design high-performance hybrid supercapacitor electrodes. J. Alloys Compd. 2023, 966, 171421. [Google Scholar] [CrossRef]


| P | Population | EDLCs |
| I | Intervention | The use of GO and rGO |
| C | Comparison | EDLCs using other materials or no GO/rGO |
| O | Outcomes | Performance metrics including capacitance, energy density, cycling stability, and other relevant electrochemical properties |
| S | Study Design | A systematic review of peer-reviewed articles, research papers, and conference papers |
| Database | Query | Results |
|---|---|---|
| Scopus | (“Graphene Oxide” OR “Reduced Graphene Oxide”) AND (“Electric Double Layer Capacitor” OR “Electric Double-Layer Capacitor”) | 73 |
| Web of Sciences | (“Graphene Oxide” OR “Reduced Graphene Oxide”) AND (“Electric Double Layer Capacitor” OR “Electric Double-Layer Capacitor”) | 53 |
| PubMed | (“Graphene Oxide” OR “Reduced Graphene Oxide”) AND (“Electric Double Layer Capacitor” OR “Electric Double-Layer Capacitor”) | 1 |
| IEEE Xplore | (“Graphene Oxide” OR “Reduced Graphene Oxide”) AND (“Electric Double-Layer Capacitor” OR (“Electric Double Layer Capacitor”) | 1 |
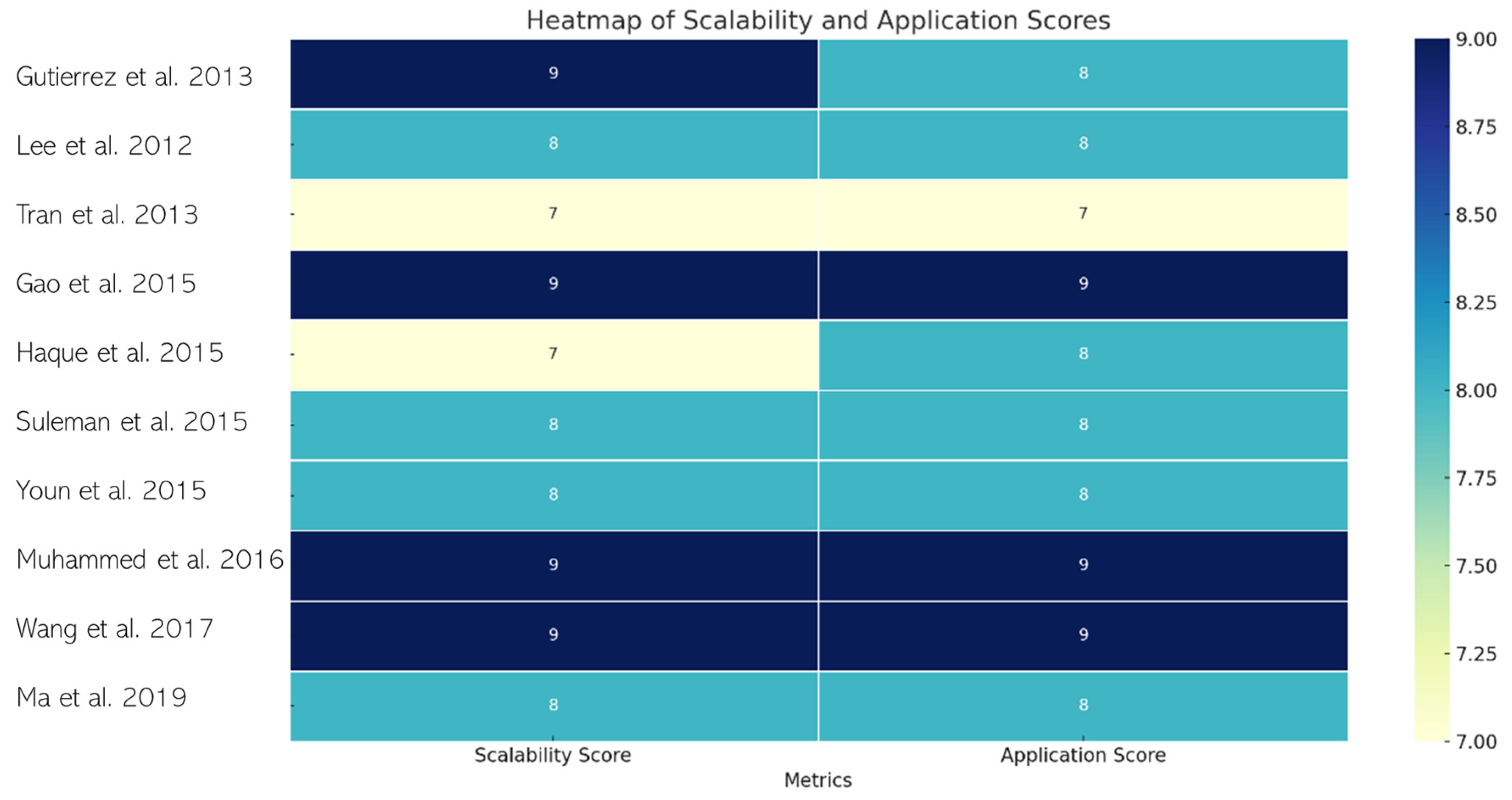
| Reference | Type of Intervention: | Capacitance | Energy Density | Power Density | Efficiency |
|---|---|---|---|---|---|
| Gutierreza et al., 2013 [55] | Development of a scalable prototype supercapacitor using metal oxides and graphene oxide with an ionic liquid electrolyte to combine pseudocapacitance and electric double-layer capacitance. | 0.356 F/g, 117 F/g | Not reported | Not reported | Not reported |
| Lee et al., 2012 [56] | Research on supercapacitors, specifically focusing on the synergy between graphene nanosheets and the conducting polymer polyaniline. | 402.5–219.4 F/g (PG10), 489.0–304.8 F/g (PG10-H), 240.1–103.5 F/g (PG10-HC), 24.6–10.9 F/g (GG10-H) | 18.92 Wh/kg (PG10), 30.34 Wh/kg (PG10-H) | 1.0 kW/kg | 90.7% retention of initial capacitance after 500 cycles (PG10-H) |
| Tran et al., 2013 [57] | Development of new poly sodium 4-styrenesulfonate intercalated graphene oxide electrodes for supercapacitors | 88 F/g (new composite), 109 F/g (PSSGO5) | Not reported | Not reported | 94% retention of initial capacitance after 3000 cycles |
| Huang et al., 2014 [58] | Development of new gel electrolyte for EDLCs | 141.8 F/g | Not reported | Not reported | Not reported |
| Gao et al., 2015 [59] | Development of a high-power density electric double-layer capacitor using porous multi-walled carbon nanotube microspheres as a local electrolyte micro-reservoir | 118 mF/cm2, 136 mF/cm2 | 9 μWh/cm2 | 1540 mW/cm2 | 98% retention after 5000 cycles. |
| Gupta et al., 2015 [60] | Development of graphene/polymer hybrid thin films as supercapacitors, utilizing physical–chemical interfacial processes for enhanced electrochemical performance. | 270–350 F/g (for hybrids with ErGO), 210–300 F/g (for hybrids with GO), 10–20 F/g (for PPy and PAni), 70–80 F/g (for GO and ErGO) | Not reported | Not reported | Not reported |
| Haque et al., 2015 [61] | Development of nitrogen-doped graphene via thermal treatment of graphene oxide and aminoterephthalic acid for use in supercapacitors | 210 F/g (at 1 A/g), 226 F/g (from CV) | Not reported | Not reported | >90% capacity retention after 5000 cycles. |
| Ming Li et al., 2015 [62] | Development of N-doped reduced graphene oxide (N-rGO) using a novel method for enhanced performance in supercapacitors | 234.3 F/g (in 0.5 M H2SO4), 187.8 F/g (in 1 M TEABF4/PC) | 25.1 Wh/kg | 10 kW/kg | 97.9% capacitance retention after 730 cycles |
| Suleman et al., 2015 [63] | Development of high-rate supercapacitive EDLCs using GO and r-GO electrodes interfaced with a plastic-crystal-based gel polymer electrolyte (GPE) | 66 F/g (GO), 60 F/g (r-GO) | 18 Wh/kg (GO), 15.6 Wh/kg (r-GO) | 33.3 kW/kg (GO), 54.9 kW/kg (r-GO) | Stable up to 11,000–13,500 charge–discharge cycles |
| Tran et al., 2015 [64] | Development of a one-pot synthesis method for a graphene/glucose/nickel oxide composite for use in supercapacitors | Up to five times higher than that of reduced GO, about 300 F/g | Not reported | Not reported | Maintains the same specific capacitance up to 5000 cycles |
| Youn et al., 2015 [65] | Development of high-surface-area N-RGO for use in EDLCs | 291 F/g (at 1 A/g), 261 F/g (at 50 A/g) | 91.0 Wh/kg | 75.0 kW/kg | 96% capacitance retention after 100,000 cycles |
| Muhammed et al., 2016 [66] | Development of graphene oxide–MnO2 nanocomposite for use in supercapacitors | 305 F/g for GO, 545 F/g for GO-MnO2 composite | 91.0 Wh/kg | 75.0 kW/kg | 96% capacitance retention after 100,000 cycles |
| Wang et al., 2017 [67] | Development of a graphene-based lithium-ion capacitor (LIC) with enhanced gravimetric energy and power densities | Not reported | Up to 200 Wh/kg. | 10 kW/kg | 70% capacity retention after 5000 cycles. |
| Ma et al., 2019 [68] | Development and electrochemical characterization of graphene-based electrodes with different fabrication parameters for supercapacitors | 2.12 F/g | 244.46 mWh/kg | 54.6 W/kg | Not reported |
| Sengottaiyan et al., 2017 [69] | Development of cobalt oxide/reduced graphene oxide (Co3O4/rGO) composites for supercapacitors using a one-pot hydrothermal synthetic route without surfactants | 487 F/g | Not reported | Not reported | 96.6% capacitance retention after 2000 cycles |
| Zhang et al., 2017 [70] | Synthesis of a ternary composite of graphene oxide/carbon dots/polypyrrole (GO/CDs/PPy) for supercapacitor applications | 576 F/g | 30.1 Wh/kg | 250 W/kg | 92.9% capacitance retention after 5000 cycles |
| Hu et al., 2018 [71] | Development of a 3D hierarchical porous V3O7·H2O nanobelts/CNT/reduced graphene oxide composite for supercapacitors | 657 F/g | 34.3 Wh/kg | 3000 W/kg | 99.7% capacitance retention after 10,000 cycles |
| Noh, et al., 2019 [72] | Development and investigation of SnO2 nanospacer-incorporated reduced graphene oxide electrodes for enhanced supercapacitor performance | 138.4 F/g for RGO–SnO2–NR, 78.4 F/g for RGO–SnO2–NP, 70.0 F/g for bare RGO | 4.17 Wh/kg for RGO–SnO2–NR. | 123.0 W/kg for RGO–SnO2–NR | Not reported |
| Li et al., 2019 [73] | Development of SnO2 nanospacer-incorporated reduced graphene oxide electrodes for improved capacitive energy storage | 6.12 F/g | 0.48 Wh/kg | 371 W/kg | 99% capacitance retention after 2400 cycles |
| Bagher et al., 2020 [74] | Functionalization of highly rGO with butyl methyl imidazolium ionic liquid to enhance supercapacitive features | 436.7 F/g for IFG, 262.5 F/g for RGO | Not reported | Not reported | Not reported |
| Kil et al., 2020 [75] | Development of rGO electrodes for supercapacitors using a solution-processed method with low-temperature thermal reduction | Not reported | 186 Wh/kg at 142 W/kg, 100 Wh/kg at 10 kW/kg. | 10 kW/kg | 70% capacity retention after 5000 cycles |
| Maphiri, et al., 2021 [76] | Development of thermally reduced graphene oxide microsupercapacitors using a mask-free AxiDraw direct writing technique | The highest areal capacitance of 0.5421 mF/cm2 at 10 mV/s for TRGO-200. | Volumetric energy density of 14.61 mWh/cm3 for TRGO-500. | Volumetric power density of 142.67 mW/cm3 for TRGO-500. | Capacitance retention and coulombic efficiency of 95% and 100%, respectively, at a current density of 0.83 µA/cm2 for 4000 cycles. |
| Lun Wu et al., 2021 [77] | Development of rGO/CoSx-rGO/rGO hybrid films using a novel method involving co-assembly and sulfidation of 2D metal organic framework (MOF) nanoflakes and graphene oxide | 375 F/g | 31.68 Wh/kg | 6750 W/kg | 96.6% |
| Zhao et al., 2021 [78] | Development of nanocarved vanadium nitride (VN) nanowires encapsulated in lamellar graphene layers for supercapacitor electrodes | 222 F/g at 0.5 A/g, 65 F/g at 10 A/g | Not reported | Not reported | Not reported |
| Qiu et al., 2015 [79] | Development of gel polymer electrolytes (GPEs) using GO-decorated polymers for enhanced performance in EDLCs under extreme temperature conditions | 40–75 F/g (from CV curves), ~100 F/g at a current density of 0.1 A/g | 3.52 Wh/kg | 0.44 kW/kg | Not reported |
| Ngamjumrus, et al., 2023 [80] | Development of a supercapacitor using hydrothermal and 3-D ball milling processes to improve the properties of reduced graphene oxide activated durian shell carbon composites | 545.78 F/g (highest), 65.585 F/g (coin cell) | 60.834 Wh/kg (highest), 5.123 Wh/kg (coin cell) | 260.834 W/kg (highest), 47.286 W/kg (coin cell) | Not reported |
| Qu et al., 2023 [81] | Development of hybrid supercapacitor electrodes using an optimized synthetic route for Cu0.33Co0.67Se2 nanorods on Ni foam integrated with N, S co-doped porous carbon (NSPC) | ~458 mAh/g at 1 A/g | ~41.5 Wh/kg | ~801.5 W/kg | Not reported |
| Reference | Intervention Type | Preparation Method | Scalability and Application |
|---|---|---|---|
| Gutierrez et al. (2013) [55] | Scalable supercapacitor | Modified Hummers’ method, ammonium evaporation, acid digestion | Mass production |
| Lee et al. (2012) [56] | Synergy in supercapacitors | Layer-by-layer assembly | Industrial scale |
| Tran et al. (2013) [57] | GO electrodes for supercapacitors | Controlled oxidation time | Customizable, practical |
| Gao et al. (2015) [59] | High-power density EDLC | Spray drying of MWCNT microspheres | Mass production |
| Haque et al. (2015) [61] | Nitrogen-doped graphene | Thermal treatment at 750 °C | Improved performance, existing setups |
| Suleman et al. (2015) [63] | High-rate EDLCs | Fabrication of GPE film | Flexible, high-performance |
| Youn et al. (2015) [65] | High-surface-area N-doped rGO | Microwave irradiation, NH3 heat treatment | High-performance |
| Muhammed et al. (2016) [66] | GO-MnO2 nanocomposite | Hummers’ method for GO, soft chemical route for MnO2 | Superior energy storage |
| Wang et al. (2017) [67] | Graphene-based LIC | 3D macroporous foam from rGO, SnO2 nanoparticles | Industrial applications |
| Ma et al. (2019) [68] | VN nanowires in graphene layers | Freeze-casting, NH3 nitridation | Practical supercapacitor |
| Reference | Comparison Materials | Comparison Method |
|---|---|---|
| Lee et al., 2012 [56] | GO, PANi, combinations | Cyclic voltammetry, galvanostatic charge–discharge, impedance spectroscopy |
| Tran et al., 2013 [57] | GO, PSSGO, modified PSSGO | Cyclic voltammetry, galvanostatic charge–discharge, impedance spectroscopy |
| Suleman et al., 2015 [63] | GO, r-GO electrodes | Cyclic voltammetry, galvanostatic charge–discharge, EIS |
| Tran et al., 2015 [64] | RGO, RGO/nickel oxide | Cyclic voltammetry, charge/discharge cycling, impedance spectroscopy |
| Youn et al., 2015 [65] | rGO, other graphene materials | Cyclic voltammetry, galvanostatic charge–discharge, impedance spectroscopy |
| Muhammed et al., 2016 [66] | GO, MnO2 composite | Cyclic voltammetry, galvanostatic charge–discharge |
| Noh et al., 2019 [72] | SnO2 nanorod bundles, SnO2 nanoparticles, rGO | Cyclic voltammetry, galvanostatic charge–discharge, impedance spectroscopy |
| Li et al., 2019 [73] | rGO electrodes without SnO2 | Cyclic voltammetry, galvanostatic charge–discharge, impedance spectroscopy |
| Bagher et al., 2020 [74] | Functionalized rGO, non-functionalized rGO | Cyclic voltammetry, impedance spectroscopy |
| Kil et al., 2020 [75] | rGO preparation methods | Cyclic voltammetry, galvanostatic charge–discharge, impedance spectroscopy |
| Maphiri et al., 2021 [76] | Microsupercapacitors at different levels of thermal reduction | Cyclic voltammetry, galvanostatic charge–discharge, impedance spectroscopy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tene, T.; Bellucci, S.; Guevara, M.; Romero, P.; Guapi, A.; Gahramanli, L.; Straface, S.; Caputi, L.S.; Vacacela Gomez, C. Role of Graphene Oxide and Reduced Graphene Oxide in Electric Double-Layer Capacitors: A Systematic Review. Batteries 2024, 10, 256. https://doi.org/10.3390/batteries10070256
Tene T, Bellucci S, Guevara M, Romero P, Guapi A, Gahramanli L, Straface S, Caputi LS, Vacacela Gomez C. Role of Graphene Oxide and Reduced Graphene Oxide in Electric Double-Layer Capacitors: A Systematic Review. Batteries. 2024; 10(7):256. https://doi.org/10.3390/batteries10070256
Chicago/Turabian StyleTene, Talia, Stefano Bellucci, Marco Guevara, Paul Romero, Alberto Guapi, Lala Gahramanli, Salvatore Straface, Lorenzo S. Caputi, and Cristian Vacacela Gomez. 2024. "Role of Graphene Oxide and Reduced Graphene Oxide in Electric Double-Layer Capacitors: A Systematic Review" Batteries 10, no. 7: 256. https://doi.org/10.3390/batteries10070256
APA StyleTene, T., Bellucci, S., Guevara, M., Romero, P., Guapi, A., Gahramanli, L., Straface, S., Caputi, L. S., & Vacacela Gomez, C. (2024). Role of Graphene Oxide and Reduced Graphene Oxide in Electric Double-Layer Capacitors: A Systematic Review. Batteries, 10(7), 256. https://doi.org/10.3390/batteries10070256













