Influence of Acetonitrile on the Electrochemical Behavior of Ionic Liquid-Based Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrolyte Preparation
2.2. Preparation of the Carbon Electrodes and Supercapacitor Cell Assembly
2.3. Electrochemical Characterization
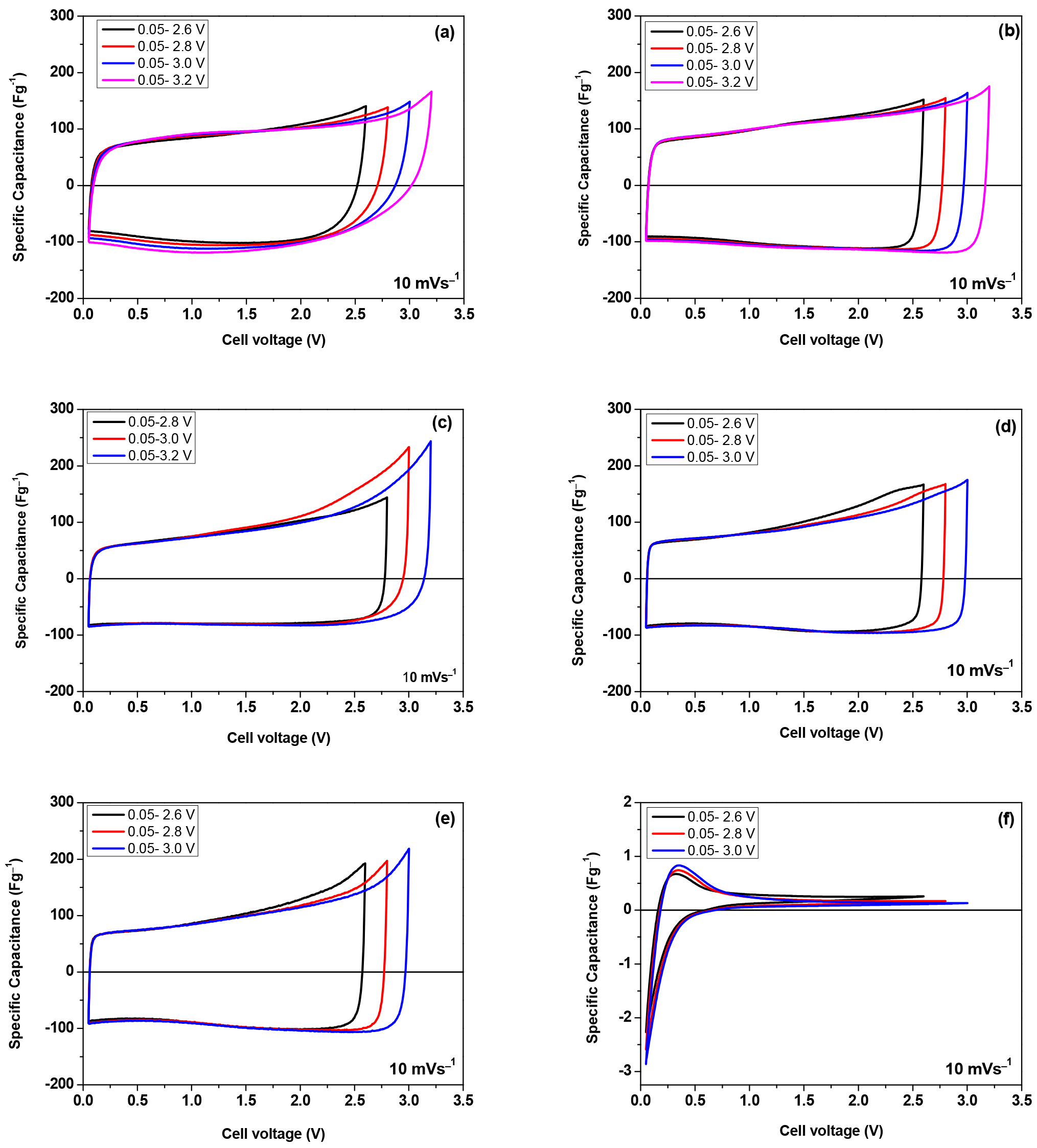
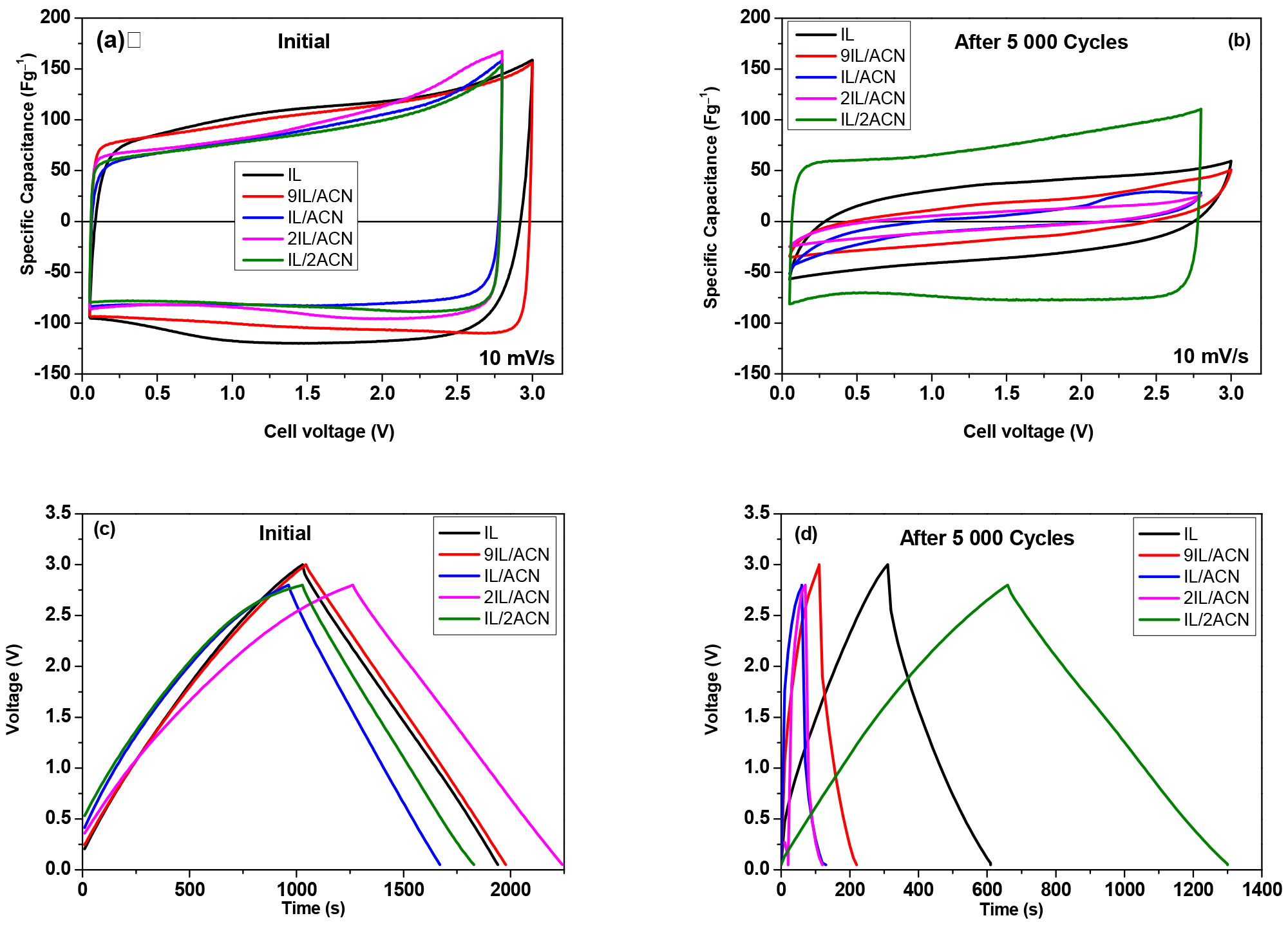
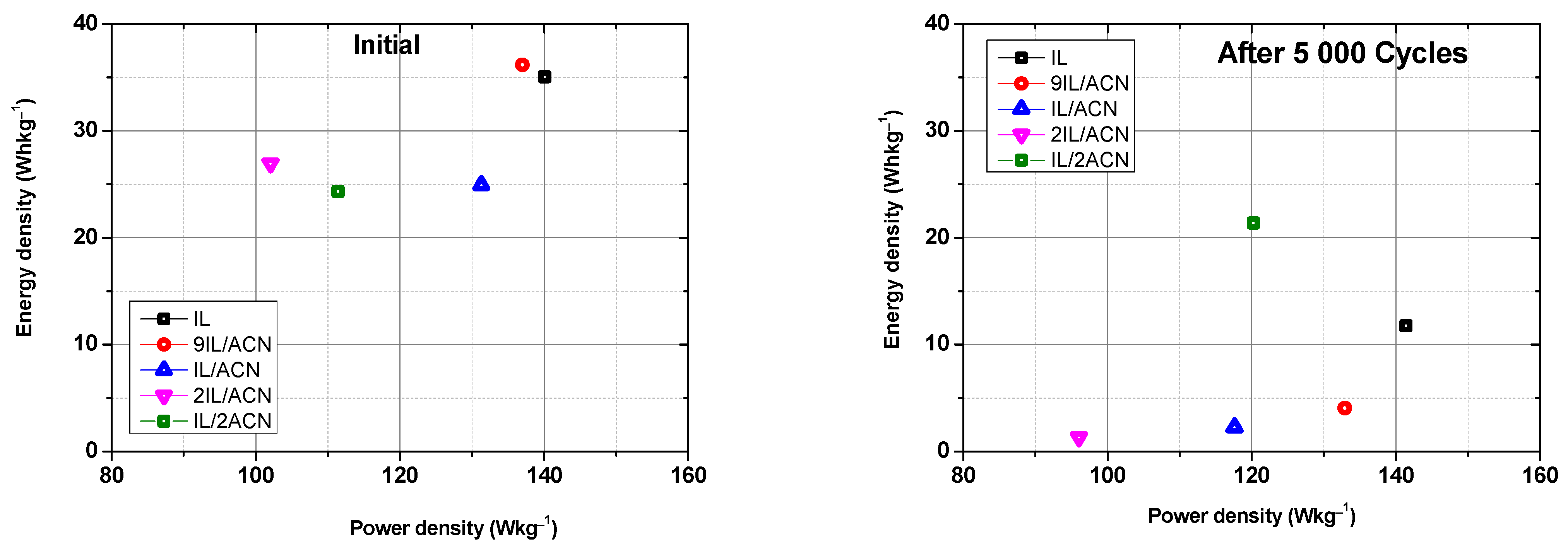
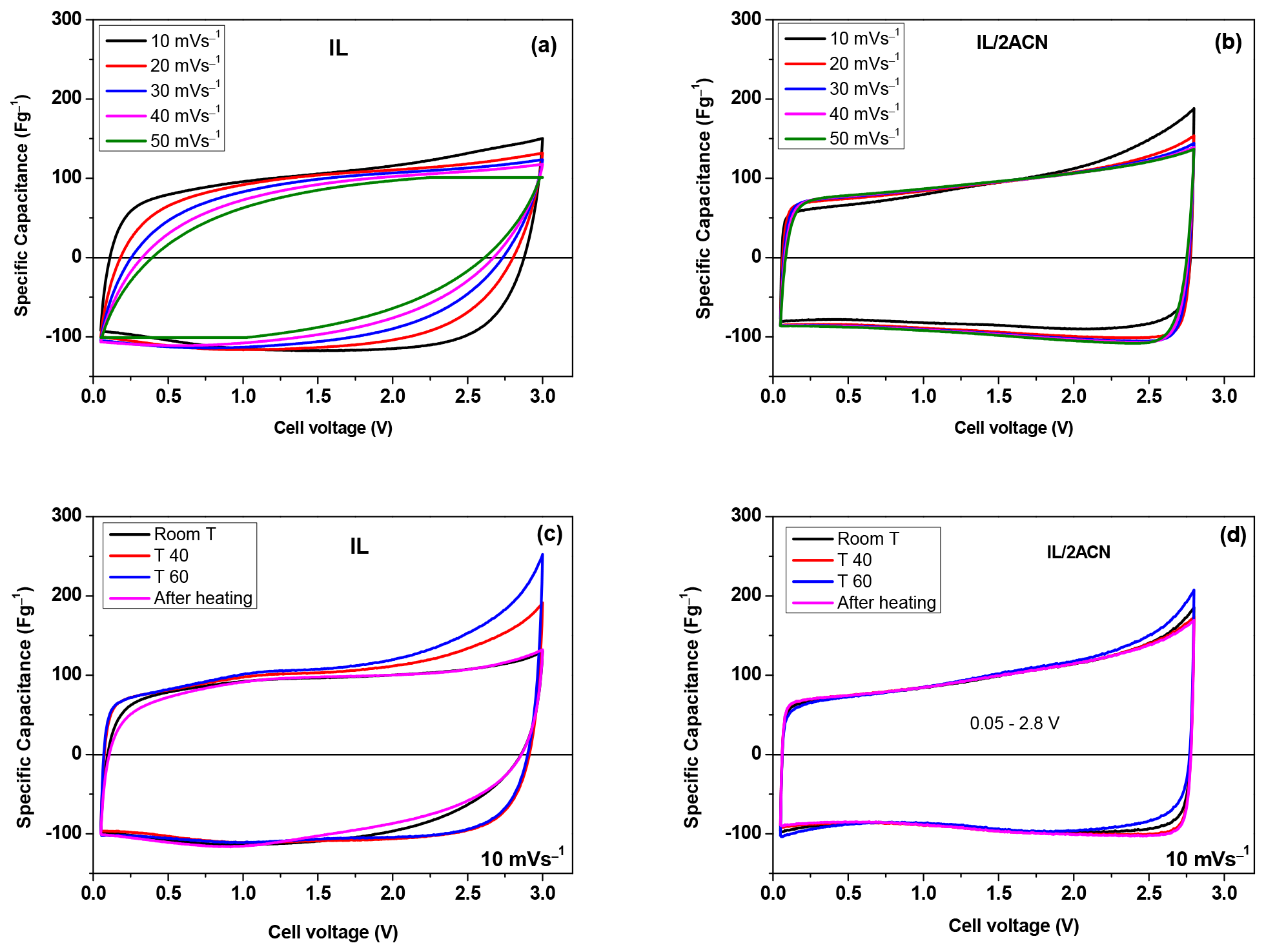
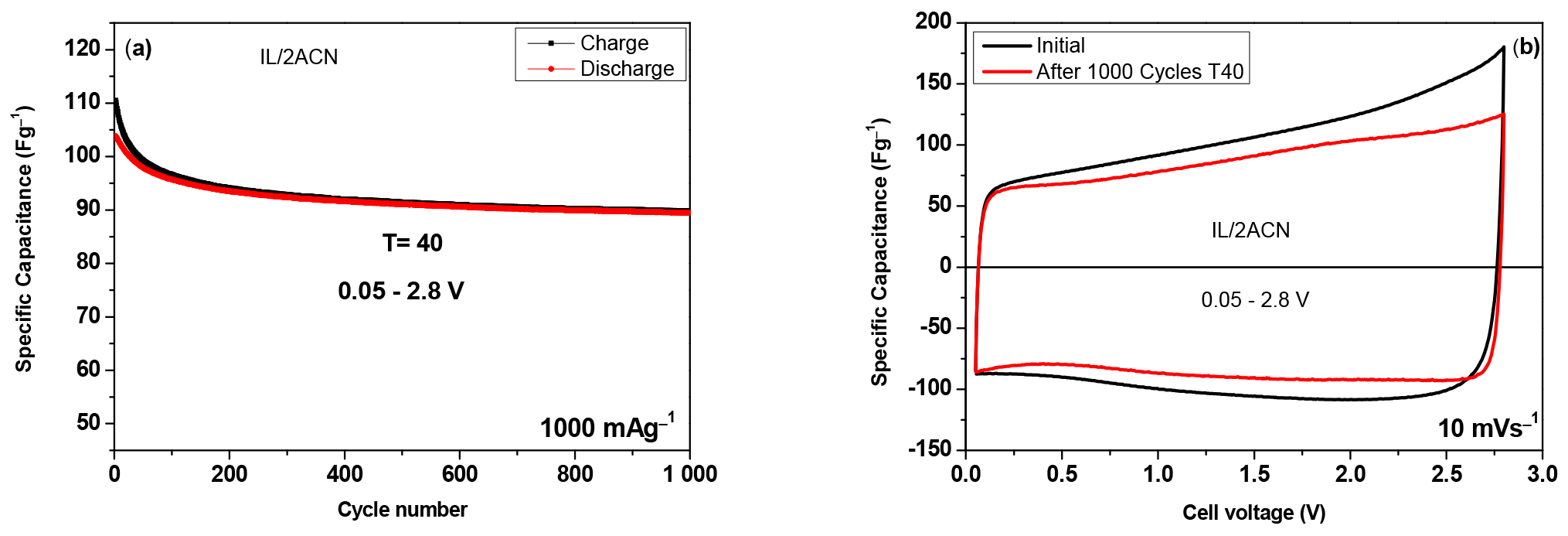
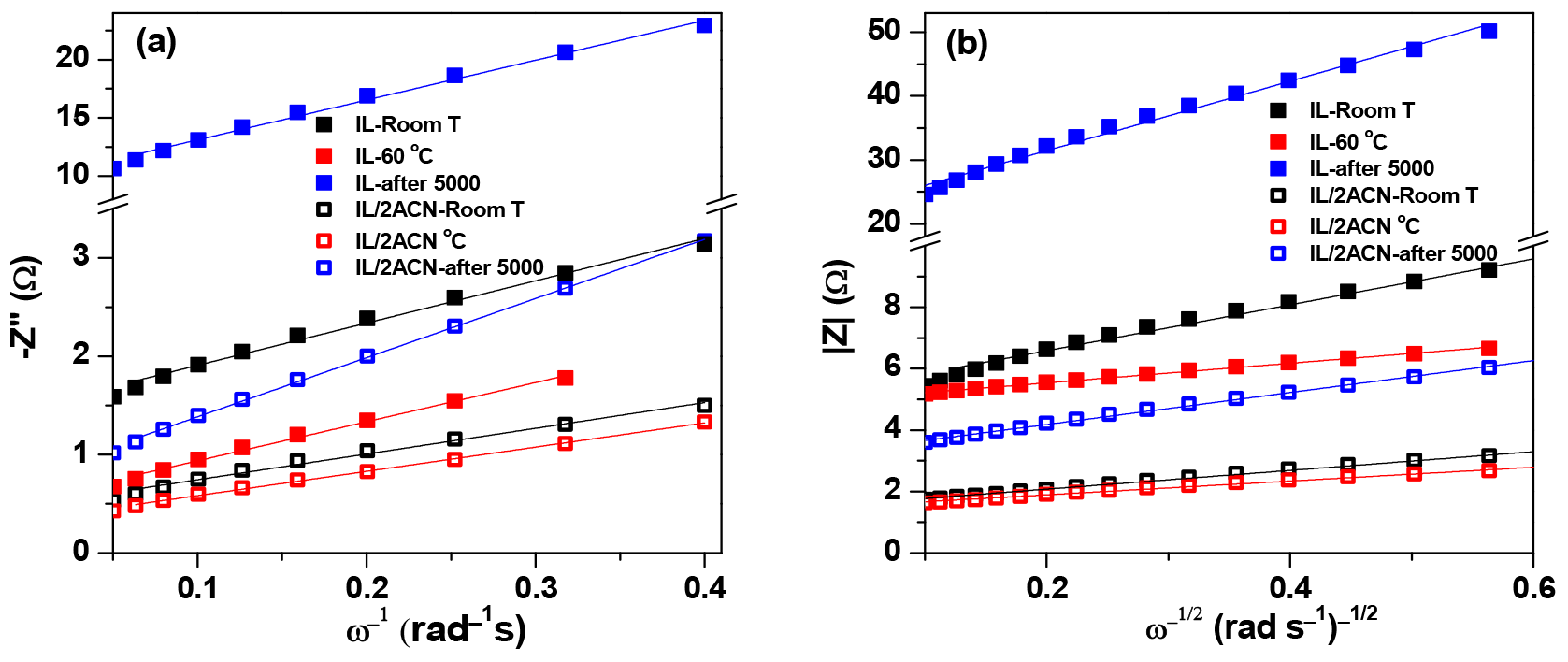
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Czagany, M.; Hompoth, S.; Keshri, A.K.; Pandit, N.; Galambos, I.; Gacsi, Z.; Baumli, P. Supercapacitors: An Efficient Way for Energy Storage Application. Materials 2024, 17, 702. [Google Scholar] [CrossRef]
- Liu, K.; Wu, J. Boosting the Performance of Ionic-Liquid-Based Supercapacitors with Polar Additives. J. Phys. Chem. C 2016, 120, 24041–24047. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, K.; Yan, C.; Li, Y.; Zhang, X.; Su, K.; Ma, P.; Wan, S.; Lang, J. Tuning of Ionic Liquid–Solvent Electrolytes for High-Voltage Electrochemical Double Layer Capacitors: A Review. Batteries 2024, 10, 54. [Google Scholar] [CrossRef]
- Venâncio, R.; Vicentini, R.; Pinzón, C.M.J.; Corrêa, D.A.; Miranda, A.N.; Queiroz, A.C.; Degasperi, F.T.; Siqueira, L.J.; Da Silva, L.M.; Zanin, H. Combining electrochemical, molecular simulation and operando techniques to investigate the stability of electrodes and organic electrolytes used in EDLCs. Energy Storage Mater. 2023, 62, 102943. [Google Scholar] [CrossRef]
- Mendhe, A.; Panda, H.S. A review on electrolytes for supercapacitor device. Discov. Mater. 2023, 3, 29. [Google Scholar] [CrossRef]
- Xiong, W.; Yin, Z.; Zhang, X.; Tu, Z.; Hu, X.; Wu, Y. Ionic Liquids Endowed with Novel Hybrid Anions for Supercapacitors. ACS Omega 2022, 7, 26368–26374. [Google Scholar] [CrossRef]
- Pan, S.; Yao, M.; Zhang, J.; Li, B.; Xing, C.; Song, X.; Su, P.; Zhang, H. Recognition of Ionic Liquids as High-Voltage Electrolytes for Supercapacitors. Front. Chem. 2020, 8, 261. [Google Scholar] [CrossRef]
- Rahmi, V.A.; Zunita, M. Ionic liquid-based electrolyte in supercapacitors. AIP Conf. Proc. 2024, 3073, 060001. [Google Scholar] [CrossRef]
- Eftekhari, A. Supercapacitors utilising ionic liquids. Energy Storage Mater. 2017, 9, 47–69. [Google Scholar] [CrossRef]
- Chaban, V.V.; Voroshylova, I.V.; Kalugin, O.N.; Prezhdo, O.V. Acetonitrile Boosts Conductivity of Imidazolium Ionic Liquids. J. Phys. Chem. B 2012, 116, 7719–7727. [Google Scholar] [CrossRef]
- Yang, H.; Yang, J.; Bo, Z.; Chen, X.; Shuai, X.; Kong, J.; Yan, J.; Cen, K. Kinetic-Dominated Charging Mechanism within Representative Aqueous Electrolyte-based Electric Double-Layer Capacitors. J. Phys. Chem. Lett. 2017, 8, 3703–3710. [Google Scholar] [CrossRef]
- Wu, G.; Tan, P.; Wang, D.; Li, Z.; Peng, L.; Hu, Y.; Wang, C.; Zhu, W.; Chen, S.; Chen, W. High-performance Supercapacitors Based on Electrochemical-induced Vertical-aligned Carbon Nanotubes and Polyaniline Nanocomposite Electrodes. Sci. Rep. 2017, 7, 43676. [Google Scholar] [CrossRef]
- Yu, C.; Masarapu, C.; Rong, J.; Wei, B.; Jiang, H. Stretchable Supercapacitors Based on Buckled Single-Walled Carbon-Nanotube Macrofilms. Adv. Mater. 2009, 21, 4793–4797. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, Z.; Wang, H.; Sun, X.; Lu, L.; Zhao, X.S. A high-energy-density supercapacitor with graphene–CMK-5 as the electrode and ionic liquid as the electrolyte. J. Mater. Chem. A 2013, 1, 2313–2321. [Google Scholar] [CrossRef]
- Wong, S.I.; Lin, H.; Sunarso, J.; Wong, B.T.; Jia, B. Optimization of ionic-liquid based electrolyte concentration for high-energy density graphene supercapacitors. Appl. Mater. Today 2020, 18, 100522. [Google Scholar] [CrossRef]
- Frackowiak, E. Supercapacitors based on carbon materials and ionic liquids. J. Braz. Chem. Soc. 2006, 17, 1074–1082. [Google Scholar] [CrossRef]
- Zhang, S.; Brahim, S.; Maat, S. High-voltage operation of binder-free CNT supercapacitors using ionic liquid electrolytes. J. Mater. Res. 2018, 33, 1179–1188. [Google Scholar] [CrossRef]
- Chaban, V.V.; Voroshylova, I.V.; Kalugin, O.N.; Prezhdo, O.V. Acetonitrile Drastically Boosts Conductivity of Ionic Liquids. arXiv 2012. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Huang, K.; Yin, X.; Liu, Y.; Peng, S. BMIMBF4 Ionic Liquid Mixtures Electrolyte for Li-ion Batteries. Int. J. Electrochem. Sci. 2012, 7, 1688–1698. [Google Scholar] [CrossRef]
- Kwon, H.-N.; Jang, S.-J.; Kang, Y.C.; Roh, K.C. The effect of ILs as co-salts in electrolytes for high voltage supercapacitors. Sci. Rep. 2019, 9, 1180. [Google Scholar] [CrossRef]
- Ruiz, V.; Huynh, T.; Sivakkumar, S.R.; Pandolfo, A.G. Ionic liquid–solvent mixtures as supercapacitor electrolytes for extreme temperature operation. RSC Adv. 2012, 2, 5591–5598. [Google Scholar] [CrossRef]
- Deshpande, A.; Kariyawasam, L.; Dutta, P.; Banerjee, S. Enhancement of Lithium Ion Mobility in Ionic Liquid Electrolytes in Presence of Additives. J. Phys. Chem. C 2013, 117, 25343–25351. [Google Scholar] [CrossRef]
- Liu, W.; Yan, X.; Lang, J.; Xue, Q. Effects of concentration and temperature of EMIMBF4/acetonitrile electrolyte on the supercapacitive behavior of graphene nanosheets. J. Mater. Chem. 2012, 22, 8853–8861. [Google Scholar] [CrossRef]
- Bahaa, A.; Alhammadi, A.; Lethesh, K.C.; Susantyoko, R.A.; Bamgbopa, M.O. Ionic liquid electrolyte selection for high voltage supercapacitors in high-temperature applications. Front. Chem. 2024, 12, 1349864. [Google Scholar] [CrossRef]
- Kötz, R.; Hahn, M.; Gallay, R. Temperature behavior and impedance fundamentals of supercapacitors. J. Power Sources 2006, 154, 550–555. [Google Scholar] [CrossRef]
- Likitchatchawankun, A.; Whang, G.; Lau, J.; Munteshari, O.; Dunn, B.; Pilon, L. Effect of temperature on irreversible and reversible heat generation rates in ionic liquid-based electric double layer capacitors. Electrochim. Acta 2020, 338, 135802. [Google Scholar] [CrossRef]
- Thomas, M.; Veleva, S.; Karamanova, B.; Brigandì, A.; Rey-Raap, N.; Arenillas, A.; Stoyanova, A.; Lufrano, F. Highly stable and reliable asymmetric solid-state supercapacitors with low self-discharge rates. Sustain. Mater. Technol. 2023, 38, e00770. [Google Scholar] [CrossRef]
- Nishida, T.; Tashiro, Y.; Yamamoto, M. Physical and electrochemical properties of 1-alkyl-3-methylimidazolium tetrafluoroborate for electrolyte. J. Fluor. Chem. 2003, 120, 135–141. [Google Scholar] [CrossRef]
- Kazemiabnavi, S.; Zhang, Z.; Thornton, K.; Banerjee, S. Electrochemical Stability Window of Imidazolium-Based Ionic Liquids as Electrolytes for Lithium Batteries. J. Phys. Chem. B 2016, 120, 5691–5702. [Google Scholar] [CrossRef]
- Yamada, Y.; Furukawa, K.; Sodeyama, K.; Kikuchi, K.; Yaegashi, M.; Tateyama, Y.; Yamada, A. Unusual Stability of Acetonitrile-Based Superconcentrated Electrolytes for Fast-Charging Lithium-Ion Batteries. J. Am. Chem. Soc. 2014, 136, 5039–5046. [Google Scholar] [CrossRef]
- Kim, E.; Han, J.; Ryu, S.; Choi, Y.; Yoo, J. Ionic Liquid Electrolytes for Electrochemical Energy Storage Devices. Materials 2021, 14, 4000. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.-J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.-S. Ionic Liquid-Based Electrolytes for Energy Storage Devices: A Brief Review on Their Limits and Applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef]
- Salanne, M. Ionic Liquids for Supercapacitor Applications. Top. Curr. Chem. 2017, 375, 63. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F.; Zeng, Y. Supercapacitive studies on amorphous MnO2 in mild solutions. J. Power Sources 2008, 184, 691–694. [Google Scholar] [CrossRef]
- Sudhan, N.; Subramani, K.; Karnan, M.; Ilayaraja, N.; Sathish, M. Biomass-Derived Activated Porous Carbon from Rice Straw for a High-Energy Symmetric Supercapacitor in Aqueous and Non-aqueous Electrolytes. Energy Fuels 2017, 31, 977–985. [Google Scholar] [CrossRef]
- Karnan, M.; Subramani, K.; Sudhan, N.; Ilayaraja, N.; Sathish, M. Aloe vera Derived Activated High-Surface-Area Carbon for Flexible and High-Energy Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 35191–35202. [Google Scholar] [CrossRef]
- Subramani, K.; Sudhan, N.; Karnan, M.; Sathish, M. Orange Peel Derived Activated Carbon for Fabrication of High-Energy and High-Rate Supercapacitors. ChemistrySelect 2017, 2, 11384–11392. [Google Scholar] [CrossRef]
- Boujibar, O.; Ghamouss, F.; Ghosh, A.; Achak, O.; Chafik, T. Activated carbon with exceptionally high surface area and tailored nanoporosity obtained from natural anthracite and its use in supercapacitors. J. Power Sources 2019, 436, 226882. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, L.; Zeng, Y.; Cao, X.; Huang, H.; Liu, J.; Chen, X.; Wei, S.; Gan, L.; Yang, P.; et al. Three-dimensional hierarchical porous carbon derived from resorcinol formaldehyde-zinc tatrate/poly(styrene-maleic anhydride) for high performance supercapacitor electrode. J. Alloys Compd. 2021, 886, 161176. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Y.; Zhu, J.; Yang, C.; Huang, H.; Chen, X.; Wang, R.; Yan, P.; Wei, S.; Liu, M.; et al. From dual-aerogels with semi-interpenetrating polymer network structure to hierarchical porous carbons for advanced supercapacitor electrodes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 12935. [Google Scholar] [CrossRef]
- Zaccagnini, P.; Serrapede, M.; Armandi, M.; Bianco, S.; Carminati, S.; Zampato, M.; Melis, G.; Pirri, C.F.; Lamberti, A. A high-temperature high-pressure supercapacitor based on ionic liquids for harsh environment applications. Electrochim. Acta 2023, 447, 142124. [Google Scholar] [CrossRef]
- Penner, R.; Martin, C. Electrochemical investigations of electronically conductive polymers. 2. Evaluation of charge-transport rates in polypyrrole using an alternating current impedance method. Phys. Chem. 1989, 93, 984–989. [Google Scholar] [CrossRef]
- Ahuja, P.; Sahu, V.; Ujjain, S.K.; Sharma, R.K.; Singh, G. Performance evaluation of Asymmetric Supercapacitor based on Cobalt manganite modified graphene nanoribbons. Electrochim. Acta 2014, 14, 429–436. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.K.; Kanojia, R. Electrochemical behaviour of manganese & ruthenium mixed oxide@ reduced graphene oxide nanoribbon composite in symmetric and asymmetric supercapacitor. Appl. Surf. Sci. 2018, 427 Pt A, 102–111. [Google Scholar] [CrossRef]
- KVan Aken, K.L.; McDonough, J.K.; Li, S.; Feng, G.; Chathoth, S.M.; Mamontov, E.; Fulvio, P.F.; Cummings, P.T.; Dai, S.; Gogotsi, Y. Effect of cation on diffusion coefficient of ionic liquids at onion-like carbon electrodes. J. Phys. Condens. Matter 2014, 26, 284104. [Google Scholar] [CrossRef]





| Electrolyte Composition, IL:AN | Capacitance Retention after 5000 GDC, % | iR Drops—Initial, V | iR Drops—after 5000 GDC, V |
|---|---|---|---|
| IL | 33 | 0.087 | 0.452 |
| 9Il/ACN | 12 | 0.025 | 1.101 |
| 2IL/ACN | 5 | 0.021 | 1.943 |
| IL/ACN | 9 | 0.019 | 1.975 |
| IL/2ACN | 87 | 0.016 | 0.082 |
| Electrolyte Composition, IL/AN | Temperature | Rel Ω | Rp Ω | Ri Ω |
|---|---|---|---|---|
| IL | room temperature | 2.2 | 2.3 | 11.5 |
| after 5000 cycles | 6.8 | - | 52 | |
| 40 °C | 1.7 | 3.7 | 8.9 | |
| 60 °C | 1.3 | 3.3 | 6.78 | |
| Il/2ACN | room temperature | 1.13 | 0.35 | 3.2 |
| after 5000 cycles | 1.7 | 0.9 | 6.2 | |
| 40 °C | 1.02 | 0.53 | 2.9 | |
| 60 °C | 0.94 | 0.48 | - |
| Electrolyte Composition, IL/AN | Temperature | D m2s−1 |
|---|---|---|
| IL | room temperature | 7.01 × 10−8 |
| after 5000 cycles | 1.05 × 10−7 | |
| 60 °C | 3.85 × 10−7 | |
| Il/2ACN | room temperature | 1.06 × 10−7 |
| after 5000 cycles | 1.64 × 10−7 | |
| 60 °C | 2.38 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamanova, B.; Soserov, L.; Lefterova, E.; Stankulov, T.; Stoyanova, A. Influence of Acetonitrile on the Electrochemical Behavior of Ionic Liquid-Based Supercapacitors. Batteries 2024, 10, 266. https://doi.org/10.3390/batteries10080266
Karamanova B, Soserov L, Lefterova E, Stankulov T, Stoyanova A. Influence of Acetonitrile on the Electrochemical Behavior of Ionic Liquid-Based Supercapacitors. Batteries. 2024; 10(8):266. https://doi.org/10.3390/batteries10080266
Chicago/Turabian StyleKaramanova, Boryana, Luybomir Soserov, Elefteria Lefterova, Toma Stankulov, and Antonia Stoyanova. 2024. "Influence of Acetonitrile on the Electrochemical Behavior of Ionic Liquid-Based Supercapacitors" Batteries 10, no. 8: 266. https://doi.org/10.3390/batteries10080266






