Emerging Capacitive Materials for On-Chip Electronics Energy Storage Technologies
Abstract
:1. Introduction
2. Materials for Electrostatic (Nano-/Micro-) Capacitors
2.1. Binary Oxide Dielectric Materials
| Dielectric | Thickness (nm) | Preparation Technique | Breakdown Voltage (MVcm−1) | ESD (Jcm−3) | Reference |
|---|---|---|---|---|---|
| Al2O3 | 25 | ALD | 4.8 | - | [67] |
| BT-BMZ | 230 | Sputtering | 7.9 | 87.26 | [73] |
| BSMT | 280 | Sputtering | 3 | 91 | [75] |
| BZT | 130 | Sputtering | 4.56 | 40.6 | [37] |
| Sm-BFO-BTO | 650 | PLD | 3.5 | 152 | [103] |
| BFO25-BTO75-2.5Mn | 200 | PLD | 3 | 80 | [104] |
| BST–BMN | 400 | PLD | 5 | 86 | [105] |
| BZT | 60 | PLD | 6.5 | 65.1 | [106] |
| BZT | 90 | PLD | 7.94 | 89.9 | [74] |
| La:HZO | 10 | ALD | 4 | 50 | [102] |
| Si:HfO2 | 9 | ALD | 3.3 | 40 | [107] |
| Si:HZO | 10 | ALD | 4.5 | 53 | [108] |
| Al:HZO | 10 | ALD | 5 | 52 | [108] |
| HZO/Hf0.25Zr0.75O2 | 1/9 | ALD | 6 | 71.97 | [109] |
| TZT | 8 | ALD | 5.38 | 114.5 | [110] |
| HfO2/ZrO2 | 2.2/6.6 | ALD | 4 | 49.9 | [111] |
2.2. Ternary Oxide Dielectric Materials
3. Materials for Micro-Supercapacitors

- MSC topology
- Capacitance retention of the MSC
- Material/electrolyte conductivity
- Material surface area
- Material/electrolyte ion transport properties.
- Mass-loading of the active materials
- The width of the safe electrochemical window
3.1. Electric Double-Layer Capacitor Materials
3.1.1. Graphene-Based Materials
Exfoliated Graphene
| Electrode Material | Substrate | Synthesis/Fabrication | Thickness | Electrolyte | Cell Voltage/ Pot. Window | Areal Cap. | Vol. Cap. | Energy Density | Power Density | Configuration | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| nm | V | mFcm−2 | Fcm−3 | mWhcm−3 | Wcm−3 | ||||||
| Graphene | PET | CVD/DLW | 17 | PVA-H2SO4 hydrogel | 1 | 0.063 | 36.8 | 5.1 | 1714 | Intedigitated | [170] |
| Graphene | PET | CVD/DLW | 17 | EMITFSI/Fumed silica ionogel | 2.5 | 0.045 | 147 | 23 | 1860 | Intedigitated | [107] |
| Graphene | Si/SiO2 | CVD/Lithography | 5 | PVA-H2SO4 hydrogel | 1 | 0.066 | 131 | NA | NA | Intedigitated | [185] |
| Graphene | Glass/Ni | Drop casting/plasma etching | 1000 | PVA-H2SO4 hydrogel | 0.8 | 0.1 | 20 | 1.5 | 2000 | Intedigitated | [186] |
| Graphene | Si/SiO2 | CVD/Plasma etching | 5 | PVA-H2SO4 hydrogel | 1 | 0.2 | 307 | 42.6 | 2000 | Intedigitated | [187] |
| Graphene | Si/SiO2 and PET | Spin coating/Inkjet printing | 750 | PSSH | 1 | 0.7 | 9.3 | 1.3 | 0.6 | Intedigitated | [187] |
| Graphene (Exfoliated) | PET | Electrochemical exfoliation/Mask-assisted filtration | 600 | PVA-H2SO4 hydrogel | 1.2 | 0.822 | 13.7 | 2.74 | 493 | Intedigitated | [188] |
| Graphene (Exfoliated) | PET | Electrochemical exfoliation/Mask-assisted filtration | 600 | KTFSI-P14TFSI ionogel | 3.4 | 0.54 | 9.03 | 14.5 | 2.6 | Intedigitated | [188] |
| Graphene | Polyimide | Laser scribing | 25,000 | H2SO4 | 1 | 4 | 1.6 | 0.4 | 50 | Intedigitated | [189] |
| rGO | Polyimide | Mask-assisted vacuum filtration | 1020 | PVA-H3PO4 hydrogel | 0.8 | 0.6845 | 6.7 | 0.37 | 5 | Intedigitated | [190] |
| rGO | PET | Spin-coating/lithography | 15 | PVA-H2SO4 hydrogel | 1 | 0.08 | 17.9 | 2.5 | 495 | Intedigitated | [168] |
| rGO | Si/SiO2 | Spin coating/Laser scribing | 7600 | EMITFSI/Fumed silica ionogel | 2.5 | 2.32 | 2.32 | 2.1 | 200 | Intedigitated | [160] |
| rGO-CNTs | Si/SiO2/TiAu | Electrostatic spray deposition (ESD)/photolithography | 6000 | 3 M KCl | 1 | 5.6 | 4.9 | 0.68 | 77 | Intedigitated | [164] |
| Graphene-CNTs | PET | Wet-Jet Milling Exfoliation/screen printing | 27,000 | PVA-H3PO4 hydrogel | 1.8 | 1.32 | 0.49 | 0.22 | <1 | Intedigitated | [191] |
| CNTs | 3D Printing | 27,600 | PVA-H3PO4 hydrogel | 1 | 4.69 | 1.7 | 0.12 | 3.72 | Intedigitated | [152] | |
| CNTs | Si/SiO2 | Injection/Plasma etching | 5000 | PVA-H3PO4 hydrogel | 0.8 | 2.75 | 5.5 | 0.4 | 0.19 | Intedigitated | [153] |
| CNTs | Si/SiO2/Fe | Plasma enhanced CVD | PVA–KCl hydrogel | 0.8 | 5 | 2.5 | - | - | Single electrode | [192] | |
| CNTs-carbon nanosheet (CN) | Si/SiO2 | CVD/photolithography | 100,000 | PVA-H3PO4 hydrogel | 1 | 110 | 11 | 2 | 0.45 | Intedigitated | [193] |
| Carbon nanowires (CNWs) | Si/Si3N4/Cr/Pt | Electrodeposition + CVD/direct laser writing | 12,000 | PVA-H3PO4-SiWA | 0.8 | 5.7 | 4.75 | NA | NA | Single electrode | [194] |
| Activated carbon | Si/SiO2 | Photolithography/etching process/inkjet printing | 20,000 | 1 M Et4NBF4 | 2.5 | 2.1 | - | - | - | Intedigitated | [155] |
| TiC-CDC | Si/SiO2 | DC magnetron sputtering + Chlorintion | 1400 | 1 M H2SO4 | 0.9 | 49 | 350 | 10.1 | 2 | Intedigitated | [54] |
| TiC-CDC | Si/SiO2 | DC magnetron sputtering + Chlorintion | 4100 | 2 M EMI, BF4:CH3CN | 3 | 61.5 | 150 | 35 | 1 | parallel plates | [54] |
| TiC-CDC | Si/SiO2 | DC magnetron sputtering + Chlorintion | 2200 | 2 M EMI, BF4:CH3CN | 3 | 35.2 | 160 | 30 | 10 | parallel plates | [54] |
| TiC-CDC | Si/SiO2 | DC magnetron sputtering + Chlorination | 5000 | 1 M H2SO4 | 0.8 | 205 | 410 | NA | NA | Single electrode | [54] |
| TiC-CDC | Si/SiO2 | DC magnetron sputtering + Chlorination | 3200 | 1 M H2SO4 | 0.9 | 112 | 350 | 39 | 0.94 | parallel plates | [161] |
| TiC-CDC | Si/SiO2 | DC magnetron sputtering + Chlorination | 4600 | 1 M KOH | 1.1 | 71 | 152 | NA | NA | Single electrode | [156] |
| TiC-CDC-Anthraquinone (AQ) | Si/SiO2 | DC magnetron sputtering + Chlorination + Electrochemical grafting | 4600 | 1 M KOH | 1.1 | 44 | 338 | NA | NA | Single electrode | [156] |
Laser-Induced Graphene
3.1.2. Carbon Nanotubes (CNTs)
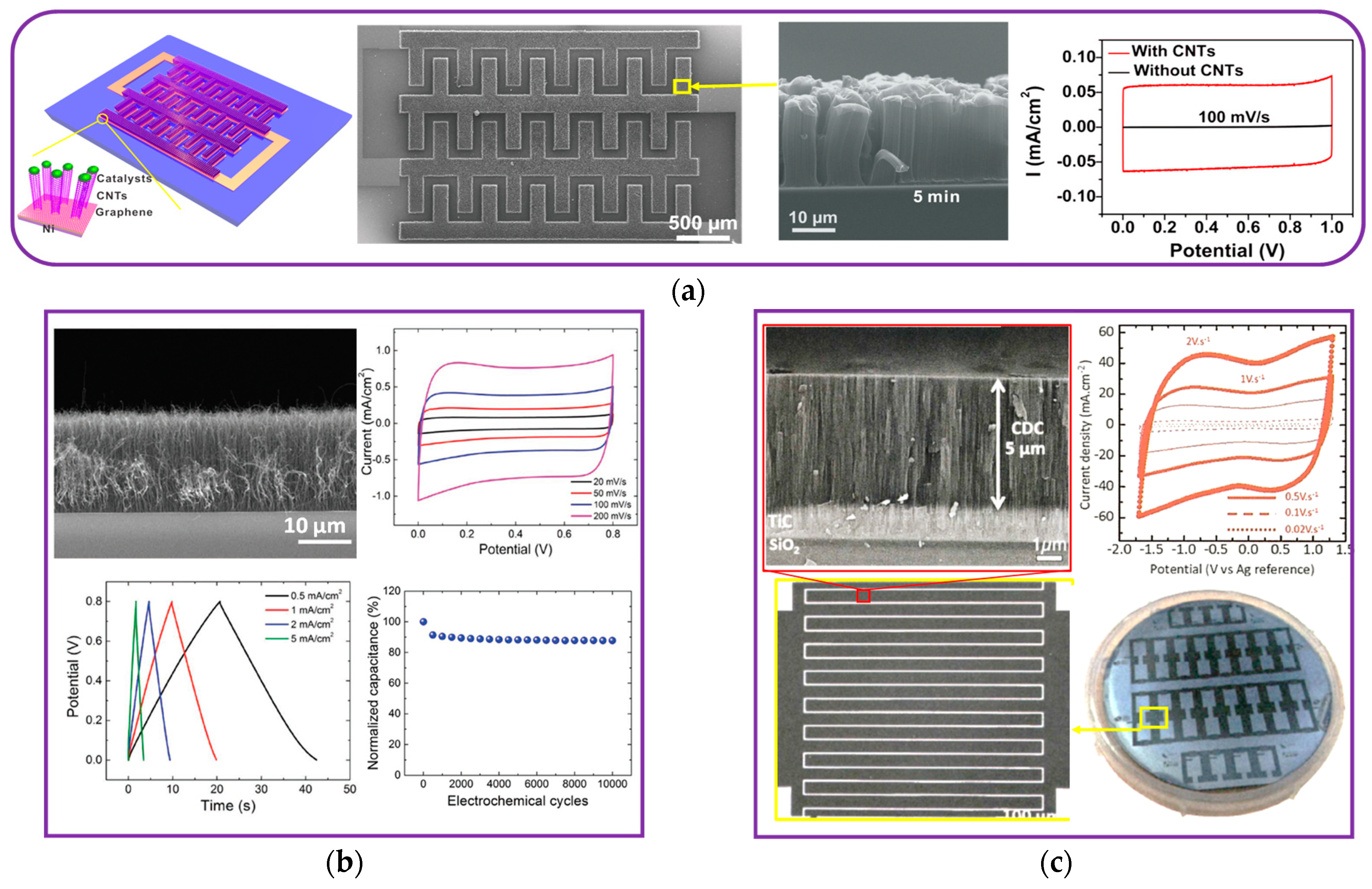
3.1.3. Porous Carbide-Derived Carbons
3.2. Pseudocapacitive Electrodes
- i
- Metal oxides/hydroxides
- ii
- Metalnitrides
- iii
- 2D transition metal carbides/carbonitrides (MXene)
3.2.1. Metal Oxides
- valence changes (electron transfer) of the metallic species located near the oxide surface,
- serving as redox-active centers through cation intercalation/deintercalation, surface adsorption/desorption, or
- surface redox reactions with anions.
Ruthenium Oxides
| Electrode Material | Substrate | Synthesis/Fabrication | Thickness (nm) | Electrolyte | Cell Vol. /Pot. Window, V | Areal Cap. mF cm−2 | Configuration | Reference |
|---|---|---|---|---|---|---|---|---|
| RuO2 | 3D-Pt nanotubes | Electrodeposition | 130 | 0.5 M H2SO4 | 1.35 | 320 | 3D Single electrode | [261] |
| RuO2 | Si/SiO2/Ti/Au | Electrodeposition/photolithography | 0.5 M H2SO4 | 0.9 | 3 | [262] | ||
| hRuO2 | 3D silicon microtubes scaffold + Al2O3/Pt | Electrodeposition/photolithography | 427 | 0.5M H2SO4 | 1 | 4300 | 3D-parallel plates | [251] |
| RuO2-Au | Electrodeposition | NA | PVA-H3PO4-SiWA | 0.9 | 3473 | 3D-parallel plates | [260] | |
| hRuO2-carbon nanowires (CNWs) | Si/Si3N4/Cr/Pt | Electrodeposition + CVD/direct laser writing | 12,000 | 0.5 M H2SO4 | 0.8 | 1094 | Single electrode | [194] |
| RuO2-graphene | Si-SiO2 | PECVD + reactive sputtering | 550 | PVA-H3PO4 hydrogel | 1 | 15.3 | Single electrode | [249] |
| RuOxNySz | SiO2-Ti-Au-3D Porous Au | Electrodeposition/photolithography | 200,000 | 0.5 M H2SO4 | 0.85 | 14,300 | 3D Single electrode | [257] |
| RuOxNySz | SiO2-Ti-Au-3D Porous Au | Electrodeposition/photolithography | 30,000 | PVA-SIWA | 1.1 | 714 | 3D Interdigitated | [257] |
| MnO2 | 3D silicon microtubes scaffold | Electrodeposition | 200 | 5 M LiNO3 | 1 | 185 | Single electrode | [250] |
| MnO2 | Si-SiO2 | Electrodeposition/photolithigraphy | 3000 | 1 M Na2SO4 | 0.8 | 56.3 | Intedigitated | [228] |
| MnO2 | 3D silicon microtubes scaffold + Al2O3/Pt | Electrodeposition/photolithography | 350 | 0.5 M Na2SO4 | 0.8 | 650 | 3D-interdigitated | [263] |
| MnO2 | 3D siliconmicrotubes + Al2O3/Pt | Electrodeposition | 15 | 0.5 M Na2SO4 | 0.8 | 670 | 3D-interdigitated | [162] |
| MnO2nanotubes | PET/3D polycarbonate (PC) membrane | Electrodeposition/PDMS-assisted transfer/photolithigraphy | 8000 | PVA-Na2SO4 hydrogel | 1 | 13.2 | 3D-interdigitated | [264] |
| MnO2-graphene | Graphene (LSG) | Electrodeposition/direct laser writing | 15,000 | 1 M Na2SO4 | 0.9 | 852 | Interdigitated | [201] |
| MnOx-Au | PET | E-beam evaporation/photolithography | 50 | PVA-H2SO4 hydrogel | 0.8 | 0.164 | Interdigitated | [265] |
| Ni(OH)2 | PET-Ni | hydrothermal + spin coating/photolithigraphy | 600 | PVA-KOH hydrogel | 0.7 | 0.528 | Interdigitated | [266] |
| Ni(OH)2 | Polyethylene naphthalate (PEN) sheet | chemical bath deposition process (CBD)/photolithigraphy | 500 | 1 M KOH | 0.6 | 16 | Interdigitated | [239] |
| NiFe2O4 | PET-Ni | electrospinning/photolithography | 300 | PVA-KOH hydrogel | 0.8 | 0.067 | Interdigitated | [267] |
| FeWO4 | Si/Al2O3/Pt | Reactive DC magnetron sputtering | 900 | 5 M LiNO3 | 0.6 | 3.5 | Single electrode | [128] |
| (Mn,Fe)3O4 | Si/Al2O3/Pt | Reactive DC magnetron sputtering | 3870 | 1 M Na2SO4 | 1 | 80 | Single electrode | [236] |
| IrOx | Si/SiC/Ti/Pt | DC magnetron sputtering/lift-off photolithography | 300 | Phosphate-buffered saline (PBS) | 1 | 12.75 | 3D Interdigitated | [245] |
| S-doped graphene | Si/SiO2 | Spin coating/plama etching | 10 | PVA-H2SO4 hydrogel | 1 | 0.582 | Interdigitated | [199] |
| F-doped graphene | PET | Mask-assisted filtration | 1000 | EMIMBF4/PVDF-HFP ionogel | 3.5 | 17.4 | Intedigitated | [197] |
| P-doped graphene | Kevlar fabric | Laser direct writing | 59,400 | PVA-H3PO4 hydrogel | 0.8 | 125.35 | Intedigitated | [198] |
| Cl-doped graphene | PET | Mask-assisted filtration | 500 | EMIMBF4/PVDF-HFP ionogel | 3.5 | 8 | Intedigitated | [268] |
| O-N-S-co-doped graphene | Wood | Slurry coating/Laser direct writing | 60,100 | PVA-H2SO4 hydrogel | 0.8 | 82.1 | Intedigitated | [200] |
| Graphen-Thiophene | PET | Vacuum-filtering/Plasma etching | 105 | PVA-H2SO4 hydrogel | 1 | 3.9 | Intedigitated | [269] |
| Graphene-Phosphorene | PET | Mask-assisted vacuum filtration | 2000 | BMIMPF6 | 3 | 9.8 | Intedigitated | [177] |
| Graphene-PEDOT | PET | Pen lithography | 20,700 | PVA-H2SO4 hydrogel | 1.2 | 16 | Intedigitated | [204] |
| Graphene-CNTs/Ag nanowires | Si/SiO2 and PET | Plasma-jet based 3D printing | 20,000 | PVA-H3PO4 hydrogel | 0.8 | 21.6 | Intedigitated | [270] |
| rGO-RuO2 | Free-standing fiber sheet | modified Hummers + hydrothermal | 42,500 | 1 M H2SO4 | 1 | 4479.5 | Free-standing fiber sheet | [202] |
| rGO-RuO2 | Free-standing fiber sheet | modified Hummers + hydrothermal | 42,500 | PVA-H3PO4 hydrogel | 1 | 833.425 | 3D-interdigitated | [202] |
| rGO-MnO2 | NA (tansfarable to any substrate) | Photomodulation/shaped femtosecond laser (SSFL) | 3000 | 0.5 M Na2SO4 | 2 | 128 | 3D-interdigitated | [271] |
| rGO-WO3 | highly oriented pyrolytic graphite (HOPG) | Electrodeposition | 31,500 | 0.158 M H2SO4 | 1 | 178 | parallel plates | [271] |
| rGO-MnO2 | Polyimide/Au | Doctor blade + electrodeposition/Laser scribing | 15,000 | 0.5 M Na2SO4 | 0.9 | 852 | 3D-interdigitated | [201] |
| rGO-ZnO | PET | hydrothermal reaction/Laser scribing | 11,000 | PVA-H2SO4 hydrogel | 1 | 4.3 | Intedigitated | [272] |
| Graphene-V2O5 | PET | Electrochemical exfoliation + hydrothermal method/Plasma etching | 300 | PVA-LiCl hydrogel | 1 | 3.9 | Intedigitated | [273] |
| rGO-V8C7 | PET | Continuous centrifugal coating and laser scribing | 13,000 | PVA-LiCl hydrogel | 0.8 | 49.5 | Intedigitated | [274] |
| rGO-PPy | Free-standing nanosheets | Polymerization + Mask-assisted filtration | 7000 | PVA-H2SO4 hydrogel | 0.8 | 81 | Intedigitated | [275] |
| CNTs-Ni | Polycarbonate (PC) sheet | Maskless laser-assisted dry transfer/direct laser writing | 50,350 | TMOS:FA:EMI TFSI ionogel | 3 | 0.43 | Intedigitated | [211] |
Manganese Oxides
Molybdenum Oxides
Iridium Oxide (IrO2)
Iron Oxides
Tungsten Oxides
Vanadium Oxides
Other Binary Metal Oxides
Mixed-Metal Oxide Electrodes
3.2.2. Metal Nitrides
Vanadium Nitride
Ruthenium Nitride
| Electrode Material | Substrate | Synthesis/Fabrication | Thickness (nm) | Electrolyte | Cell Volt./Pot. Window, V | Areal Cap. mF cm−2 | Vol. Cap. F cm−3 | Configuration | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| VN | Ta foil | Sputtering/nitridation | 400 | 1 M KOH | 0.6 | 3 | 75 | Single electrode | [401] |
| VN | Si/Si3N4 | Sputtering | 16,000 | 1 M KOH | 0.6 | 1200 | 700 | parallel plates | [399] |
| VN | Si/Si3N4 | Sputtering | 3400 | 1 M KOH | 0.6 | 220 | 650 | Single electrode | [59] |
| VN | Si/Si3N4 | Sputtering | 2000 | 1 M KOH | 0.6 | 40 | Interdigitated | [59] | |
| VN | Si/Si3N4 | Sputtering | 32,200 | 1 M KOH | 0.6 | 1400 | Single electrode | [255] | |
| Cr-doped VN | Si | Sputtering | NA | 1 M KOH | 1 | 190 | NA | Single electrode | [403] |
| VN-carbon nanosheets | Carbon nanosheets | Nitridation | 17,000 | PVA-KOH hydrogel | 0.9 | 2046.12 | 1203.6 | Single electrode | [404] |
| W2N | Si/Si3N4 | Sputtering | 7900 | 1 M KOH | 0.6 | 550 | 700 | Single electrode | [395] |
| Mo2N | Ti foils | Sputtering | 1242 | 0.5 M Li2SO4 | 0.9 | 55 | 722 | Single electrode | [405] |
| RuN | Si | Sputtering | 450 | 1 M KOH | 0.9 | 6 | 133 | Single electrode | [397] |
| RuN | Si/Si3N4 | Sputtering | 10,000 | 1 M KOH | 0.85 | 3200 | 3200 | Single electrode | [53] |
| VWNz | Si/Si3N4 | Sputtering | 150 | 1 M KOH | 0.6 | 11.5 | 700 | Single electrode | [406] |
| TiN | Si | Sputtering | 15,600 | 0.5 M H2SO4 | 0.8 | 27.3 | 42.6 | parallel plates | [407] |
| TiN | Si | Sputtering | 770 | 0.5 M K2SO4 | 0.7 | 12 | 146.4 | Single electrode | [408] |
| TiVN | Si | Sputtering | 270 | 1 M KOH | 1 | 15 | 500 | Single electrode | [409] |
| Nb4 N5 | Nb foil | Electrodeposition | 17,400 | 1 M H2SO4 | 0.6 | 225.8 | 72.3 | Single electrode | [410] |
| Nb4N5-N-doped carbon | Nb foil | Electrodeposition | 17,402 | 1 M H2SO4 | 1 | 232.9 | 133.85 | parallel plates | [410] |
| CrN | Si | Sputtering | 1100 | 0.5 M H2SO4 | 0.8 | 12.8 | 116.36 | parallel plates | [411] |
| CrN | Si | Sputtering | 2100 | 0.5 M H2SO4 | 0.8 | 17.7 | 83.26 | parallel plates | [412] |
| CrN | Si | Sputtering | 1100 | 0.5 M H2SO4 | 0.8 | 31.3 | 284.55 | parallel plates | [413] |
| Mn3N2 | Stainless steel | Sputtering | NA | 1 M KOH | 0.9 | 118 | NA | Single electrode | [414] |
| MoNx-TiN | Ti foils | Electrodeposition + nitridation | 4000 | 1.0 M LiOH | 0.6 | 121.5 | NA | Single electrode | [415] |
| Co3N | Si | Sputtering | 929 | 6 M KOH | 0.6 | 79.1 | 851.4 | Single electrode | [416] |
| CNTs-TiN (3.4/1.2 µm) | Si/amorphous carbon film | CVD + Sputtering | 4600 | K2SO4 | 0.7 | 18.13 | - | Single electrode | [417] |
| CNTs-VN (3.4/1.3 µm) | Si/SiO2 | CVD + Sputtering | 4700 | K2SO4 | 0.5 | 37.5 | - | Single electrode | [214] |
| CNTs-VN | CNT fibers | Solvothermal | 30,000 | 3 M KOH | 1 | 564 | - | Single electrode | [215] |
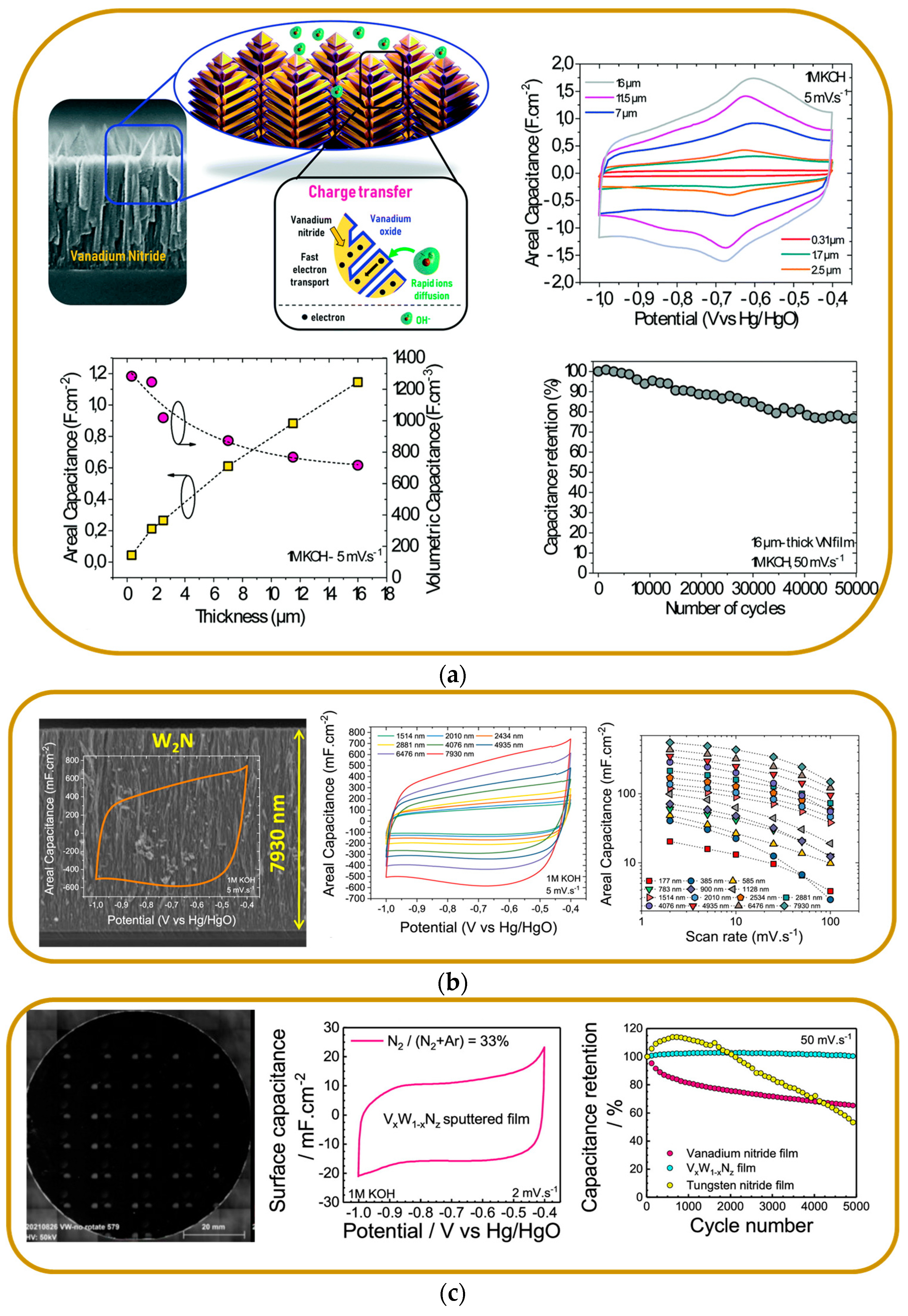
Other Binary Nitride Electrodes
Mixed-Metal Nitride Electrodes
3.2.3. Metal Carbides/Carbonitrides
| Electrode Material | Substrate | Synthesis/Fabrication | Thickness (nm) | Electrolyte | Areal Cap. mF cm−2 | Vol. Cap F cm−3 | Energy Density mWh cm−3 | Power Density W cm−3 | Configuration | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Ti3C2Tx | Free-standing | Etching | 20,000 | 1 M KOH | 680 | 340 | NA | NA | Single electrode | [231] |
| Ti3C2Tx | Free-standing | Etching | 12,000 | PVA-KOH hydrogel | 636 | 530 | NA | NA | Single electrode | [438] |
| Ti3C2Tx | Paper | Screen printing | 20,000 | PVA-H2SO4 hydrogel | 1108 | - | - | - | Intedigitated | [431] |
| Ti3C2Tx | 3D LIG | direct CO2 laser-scribing/lift-off lithography | NA | 2 M H2SO4 | 1348 | - | - | - | Intedigitated | [230] |
| Ti3C2Tx | PET | 3D Printing | NA | PVA-H2SO4 hydrogel | 2337 | - | - | - | Intedigitated | [443] |
| Ti3C2Tx | Glass | Extrusion 3D Printing | NA | PVA-H2SO4 hydrogel | 1035 | - | 1 | 0.005 | Intedigitated | [432] |
| Ti3C2Tx | Glass | Spray coating/laser cutting | 1300 | PVA-H2SO4 hydrogel | 27.3 | 356.8 | 18 | 15 | Intedigitated | [441] |
| Ti3C2Tx | PET | Stamping | 690 | PVA-H2SO4 hydrogel | 61 | 884 | 44.2 | 90 | Intedigitated | [444] |
| Ti3C2Tx | PET | CO2 laser machining + spray coating | 4000 | PVA-H3PO4 hydrogel | 23 | 57.5 | 2.8 | 0.744 | Intedigitated | [3] |
| Ti3C2Tx-Graphene | PET | Spray coating/Mask-assisted filtration | 1000 | PVA-H3PO4 hydrogel | 3.26 | 33 | 3.4 | 0.2 | Intedigitated | [445] |
| Ti3C2Tx | Paper | Vacuum-assisted filtration/laser printing | 2200 | PVA-H2SO4 hydrogel | 27.29 | 124.05 | 6.1 | 0.85 | Intedigitated | [446] |
| Ti3C2Tx-RuO2 | Paper | Screen printing | 270 | PVA–KOH hydrogel | 23.3 | 864.2 | 13.5 | 48.5 | Intedigitated | [433] |
| Ti3C2Tx | PET/Au/PDMS | Vacuum-assisted filtration/Laser marking | 4000 | PVA-H2SO4 hydrogel | 73 | 183 | 12.4 | 4.38 | Intedigitated | [447] |
| Ti3C2Tx | Glass | Dip coating/scalpel engraving | 150 | PVA-H3PO4 hydrogel | 0.283 | 18.9 | 0.67 | 6.67 | Intedigitated | [435] |
| Ti3C2Tx-graphene | Si-SiO2 | Scratch method | 5000 | PVA-H3PO4 hydrogel | 18.2 | 36.4 | 2.3 | 0.16 | Intedigitated | [448] |
| Ti3C2Tx-sodium alginate-Fe2+ | Si | Etching/inkjet-printing | 730 | PVA-H3PO4 hydrogel | 123.8 | 1696 | 85 | 4.6 | Intedigitated | [449] |
3.2.4. Conducting Polymers
4. Electrolytes
4.1. Aqueous Electrolytes (Alkaline, Neutral, and Acidic)
4.2. Organic Electrolytes
4.3. Ionic Liquids
4.4. Solid-State Electrolytes
4.5. Redox-Active Electrolytes
5. Conclusions and Perspectives
- -
- 2D materials like graphene, MXenes, and transition metal dichalcogenides (TMDs) have a high surface area, excellent electrical conductivity, and tunable properties, affording them the leverage to provide higher energy density and faster charge/discharge cycles.
- -
- 3D material developments using similar 3D templates to fabricate MBs, MSCs, and µ capacitors could be particularly valuable and cost-effective with a streamlined fabrication process, enhancing space efficiency and performance synergy. However, from a technological point of view, some issues may arise, including material compatibility, constraints due to the device’s configuration/topology, and fabrication challenges due to the specific requirements.
- -
- Surface area enhancements/topology
- -
- Solid-state electrolytes to offer enhanced safety, ionic conductivity, stability, a wider voltage window to improve energy density, and compatibility with on-chip integration.
- -
- Cycle life improvements by enhancing the active material properties.
- -
- Materials preparation techniques compatible with MEMS technology
Author Contributions
Funding
Conflicts of Interest
References
- Yun, J.; Song, C.; Lee, H.; Park, H.; Jeong, Y.R.; Kim, J.W.; Jin, S.W.; Oh, S.Y.; Sun, L.; Zi, G.; et al. Stretchable Array of High-Performance Micro-Supercapacitors Charged with Solar Cells for Wireless Powering of an Integrated Strain Sensor. Nano Energy 2018, 49, 644–654. [Google Scholar] [CrossRef]
- Guo, R.; Chen, J.; Yang, B.; Liu, L.; Su, L.; Shen, B.; Yan, X.; Guo, R.S.; Chen, J.T.; Yang, B.J.; et al. In-Plane Micro-Supercapacitors for an Integrated Device on One Piece of Paper. Adv. Funct. Mater. 2017, 27, 1702394. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, C.; Wang, Z.; Wang, A.C.; He, J.H.; Wang, Z.L.; Alshareef, H.N. MXene Electrochemical Microsupercapacitor Integrated with Triboelectric Nanogenerator as a Wearable Self-Charging Power Unit. Nano Energy 2018, 45, 266–272. [Google Scholar] [CrossRef]
- Wang, X.; Yin, Y.; Yi, F.; Dai, K.; Niu, S.; Han, Y.; Zhang, Y.; You, Z. Bioinspired Stretchable Triboelectric Nanogenerator as Energy-Harvesting Skin for Self-Powered Electronics. Nano Energy 2017, 39, 429–436. [Google Scholar] [CrossRef]
- Sudevalayam, S.; Kulkarni, P. Energy Harvesting Sensor Nodes: Survey and Implications. IEEE Commun. Surv. Tutor. 2011, 13, 443–461. [Google Scholar] [CrossRef]
- Lethien, C.; Le Bideau, J.; Brousse, T. Challenges and Prospects of 3D Micro-Supercapacitors for Powering the Internet of Things. Energy Environ. Sci. 2019, 12, 96–115. [Google Scholar] [CrossRef]
- Shen, C.; Xu, S.; Xie, Y.; Sanghadasa, M.; Wang, X.; Lin, L. A Review of On-Chip Micro Supercapacitors for Integrated Self-Powering Systems. J. Microelectromechanical Syst. 2017, 26, 949–965. [Google Scholar] [CrossRef]
- Zheng, S.; Shi, X.; Das, P.; Wu, Z.S.; Bao, X. The Road Towards Planar Microbatteries and Micro-Supercapacitors: From 2D to 3D Device Geometries. Adv. Mater. 2019, 31, 1900583. [Google Scholar] [CrossRef] [PubMed]
- Kyeremateng, N.A.; Brousse, T.; Pech, D. Microsupercapacitors as Miniaturized Energy-Storage Components for on-Chip Electronics. Nat. Nanotechnol. 2017, 12, 7–15. [Google Scholar] [CrossRef]
- Spurney, R.G.; Sharma, H.; Pulugurtha, M.R.; Tummala, R.; Lollis, N.; Weaver, M.; Gandhi, S.; Romig, M.; Brumm, H. Ultra-High Density, Thin-Film Tantalum Capacitors with Improved Frequency Characteristics for MHz Switching Power Converters. J. Electron. Mater. 2018, 47, 5632–5639. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, G.; Zheng, X.; Chen, F.; Duan, H. Emerging Miniaturized Energy Storage Devices for Microsystem Applications: From Design to Integration. Int. J. Extrem. Manuf. 2020, 2, 042001. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, S.; Qiu, T.; Lang, X.; Tan, H.; Wang, Y.; Li, Y. Recent Advances on Energy Storage Microdevices: From Materials to Configurations. Energy Storage Mater. 2022, 45, 741–767. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Chen, J. Design and Integration of Flexible Planar Micro-Supercapacitors. Nano Res. 2017, 10, 1524–1544. [Google Scholar] [CrossRef]
- Hu, H.; Pei, Z.; Ye, C. Recent Advances in Designing and Fabrication of Planar Micro-Supercapacitors for on-Chip Energy Storage. Energy Storage Mater. 2015, 1, 82–102. [Google Scholar] [CrossRef]
- Park, S.H.; Goodall, G.; Kim, W.S. Perspective on 3D-Designed Micro-Supercapacitors. Mater. Des. 2020, 193, 108797. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Y.; Liu, Z.; Zhang, L.; Chen, X.; Qi, D.; Liu, Y.; Liu, Z.; Zhang, L.; Chen, X. Design of Architectures and Materials in In-Plane Micro-Supercapacitors: Current Status and Future Challenges. Adv. Mater. 2017, 29, 1602802. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Zhou, T.; Sun, S.; Zhang, J.; Zhou, T.; Zhang, G.; Sun, S. Recent Developments of Planar Micro-Supercapacitors: Fabrication, Properties, and Applications. Adv. Funct. Mater. 2020, 30, 1910000. [Google Scholar] [CrossRef]
- Wu, Z.; Li, L.; Yan, J.M.; Zhang, X.B. Materials Design and System Construction for Conventional and New-Concept Supercapacitors. Adv. Sci. 2017, 4, 1600382. [Google Scholar] [CrossRef]
- Song, W.J.; Yoo, S.; Song, G.; Lee, S.; Kong, M.; Rim, J.; Jeong, U.; Park, S. Recent Progress in Stretchable Batteries for Wearable Electronics. Batter. Supercaps 2019, 2, 181–199. [Google Scholar] [CrossRef]
- Liu, W.; Song, M.S.; Kong, B.; Cui, Y. Flexible and Stretchable Energy Storage: Recent Advances and Future Perspectives. Adv. Mater. 2017, 29, 1603436. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, J. Development of Flexible Li-Ion Batteries for Flexible Electronics. InfoMat 2020, 2, 866–878. [Google Scholar] [CrossRef]
- Chen, D.; Lou, Z.; Jiang, K.; Shen, G. Device Configurations and Future Prospects of Flexible/Stretchable Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1805596. [Google Scholar] [CrossRef]
- Jetybayeva, A.; Umirzakov, A.; Uzakbaiuly, B.; Bakenov, Z.; Mukanova, A. Towards Li–S Microbatteries: A Perspective Review. J. Power Sources 2023, 573, 233158. [Google Scholar] [CrossRef]
- Xia, Q.; Zan, F.; Zhang, Q.; Liu, W.; Li, Q.; He, Y.; Hua, J.; Liu, J.; Xu, J.; Wang, J.; et al. All-Solid-State Thin Film Lithium/Lithium-Ion Microbatteries for Powering the Internet of Things. Adv. Mater. 2023, 35, 2200538. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, X. Advances in Micro Lithium-Ion Batteries for on-Chip and Wearable Applications. J. Micromechanics Microengineering 2021, 31, 114002. [Google Scholar] [CrossRef]
- Selinis, P.; Farmakis, F. Review—A Review on the Anode and Cathode Materials for Lithium-Ion Batteries with Improved Subzero Temperature Performance. J. Electrochem. Soc. 2022, 169, 010526. [Google Scholar] [CrossRef]
- Yue, C.; Li, J.; Lin, L. Fabrication of Si-Based Three-Dimensional Microbatteries: A Review. Front. Mech. Eng. 2017, 12, 459–476. [Google Scholar] [CrossRef]
- Gao, Y.; Tenhaeff, W.E. Synthesis and Characterization of Thin Film Polyelectrolytes for Solid-State Lithium Microbatteries. J. Vac. Sci. Technol. B 2019, 37, 051401. [Google Scholar] [CrossRef]
- Sheil, R.; Chang, J.P. Synthesis and Integration of Thin Film Solid State Electrolytes for 3D Li-Ion Microbatteries. J. Vac. Sci. Technol. 2020, 38, 032411. [Google Scholar] [CrossRef]
- Jetybayeva, A.; Uzakbaiuly, B.; Mukanova, A.; Myung, S.T.; Bakenov, Z. Recent Advancements in Solid Electrolytes Integrated into All-Solid-State 2D and 3D Lithium-Ion Microbatteries. J. Mater. Chem. A. 2021, 9, 15140–15178. [Google Scholar] [CrossRef]
- Ni, J.; Li, L. Self-Supported 3D Array Electrodes for Sodium Microbatteries. Adv. Funct. Mater. 2018, 28, 1704880. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, J.; Wu, H.; Li, Q.; Fan, S.; Wang, J. Progress and Challenges of Flexible Lithium Ion Batteries. J. Power Sources 2020, 454, 227932. [Google Scholar] [CrossRef]
- Radzuan, R.; Salleh, M.K.M.; Hamzah, M.K.; Zulkefle, H.; Md Sin, N.D.; Rusop, M. Development of Thin Film Capacitors for Power System Applications by PVD Technique. In Proceedings of the SHUSER 2012—2012 IEEE Symposium on Humanities, Science and Engineering Research, Kuala Lumpur, Malaysia, 24–27 June 2012; IEEE: New York, NY, USA, 2012; pp. 1097–1100. [Google Scholar] [CrossRef]
- Nguyen, P.; Ng, H.T.; Yamada, Y.; Smith, M.K.; Li, J.; Han, J.; Meyyappan, M.; Lett, N.; Yamada, T.; Chen, Y.P.; et al. High-Dielectric-Constant Silver–Epoxy Composites as Embedded Dielectrics. Adv. Mater. 2005, 17, 1777–1781. [Google Scholar] [CrossRef]
- Özçelik, V.O.; Ciraci, S. High-Performance Planar Nanoscale Dielectric Capacitors. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 91, 195445. [Google Scholar] [CrossRef]
- Yao, S.; Yuan, J.; Mehedi, H.-A.; Gheeraert, E.; Sylvestre, A. Carbon Nanotube Forest Based Electrostatic Capacitor with Excellent Dielectric Performances. Carbon 2017, 116, 648–654. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, M.; Shen, L.; Lu, L.; Ma, C.; Lu, X.; Lou, X.; Jia, C.L. All-Inorganic Flexible Embedded Thin-Film Capacitors for Dielectric Energy Storage with High Performance. ACS Appl. Mater. Interfaces 2019, 11, 5247–5255. [Google Scholar] [CrossRef]
- Tombak, A.; Maria, J.P.; Ayguavives, F.T.; Jin, Z.; Stauf, G.T.; Kingon, A.I.; Mortazawi, A. Voltage-Controlled RF Filters Employing Thin-Film Barium-Strontium-Titanate Tunable Capacitors. IEEE Trans. Microw. Theory Tech. 2003, 51, 462–467. [Google Scholar] [CrossRef]
- Vorobiev, A.; Gevorgian, S. Fabrication of Ferroelectric Components and Devices. In Ferroelectrics in Microwave Devices, Circuits and Systems: Physics, Modeling, Fabrication and Measurements; Springer: Berlin/Heidelberg, Germany, 2009; pp. 61–113. [Google Scholar]
- Han, K.; Wang, Q. 10.40—Polymers for Thin Film Capacitors: Energy Storage—Li Conducting Polymers. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1–10, pp. 811–830. [Google Scholar]
- Butzen, N.; Krishnarnurthy, H.; Ahmed, Z.; Weng, S.; Ravichandran, K.; Zelikson, M.; Tschanz, J.; Douglas, J. 14.4 A Monolithic 26A/Mm2Imax, 88.5% Peak-Efficiency Continuously Scalable Conversion-Ratio Switched-Capacitor DC-DC Converter. In Proceedings of the Digest of Technical Papers—IEEE International Solid-State Circuits Conference, San Francisco, CA, USA, 19–23 February 2023; IEEE: New York, NY, USA, 2023; Volume 2023, pp. 232–234. [Google Scholar]
- Sharma, S.; Chand, P. Supercapacitor and Electrochemical Techniques: A Brief Review. Results Chem. 2023, 5, 100885. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, Related Two-Dimensional Crystals, and Hybrid Systems for Energy Conversion and Storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Chmiola, J.; Celine Largeot, P.L.T.; Simon, P.; Gogotsi, Y. Monolithic Carbide-Derived Carbon Films for Micro-Supercapacitors. Science 2010, 328, 480–483. [Google Scholar] [CrossRef]
- Dinh, K.H.; Roussel, P.; Lethien, C. Advances on Microsupercapacitors: Real Fast Miniaturized Devices toward Technological Dreams for Powering Embedded Electronics? ACS Omega 2022, 8, 8990. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Grant, P.S. One-Step Spray Processing of High Power All-Solid-State Supercapacitors. Sci. Rep. 2013, 3, 2393. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, T.; Ruiz, V.; Sivakkumar, S.; Nerkar, J. General Properties of Electrochemical Capacitors. Supercapacitors Mater. Syst. Appl. 2013, 69–109. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Cesar, R.; Moreira, C.M.R.; Santos, J.H.M.; De Souza, L.G.; Pires, B.M.; Vicentini, R.; Nunes, W.; Zanin, H. Reviewing the Fundamentals of Supercapacitors and the Difficulties Involving the Analysis of the Electrochemical Findings Obtained for Porous Electrode Materials. Energy Storage Mater. 2020, 27, 555–590. [Google Scholar] [CrossRef]
- Ceraolo, M.; Lutzemberger, G.; Poli, D. State-Of-Charge Evaluation Of Supercapacitors. J. Energy Storage 2017, 11, 211–218. [Google Scholar] [CrossRef]
- Saha, P.; Dey, S.; Khanra, M. Accurate Estimation of State-of-Charge of Supercapacitor under Uncertain Leakage and Open Circuit Voltage Map. J. Power Sources 2019, 434, 226696. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Simon, P. True Performance Metrics in Electrochemical Energy Storage. Science 2011, 334, 917–918. [Google Scholar] [CrossRef]
- Burke, A. Ultracapacitors: Why, How, and Where Is the Technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef]
- Dinh Khac, H.; Whang, G.; Iadecola, A.; Makhlouf, H.; Barnabé, A.; Teurtrie, A.; Marinova, M.; Huvé, M.; Roch-Jeune, I.; Douard, C.; et al. Nanofeather Ruthenium Nitride Electrodes for Electrochemical Capacitors. Nat. Mater. 2024, 23, 670–679. [Google Scholar] [CrossRef]
- Huang, P.; Lethien, C.; Pinaud, S.; Brousse, K.; Laloo, R.; Turq, V.; Respaud, M.; Demortière, A.; Daffos, B.; Taberna, P.L.; et al. On-Chip and Freestanding Elastic Carbon Films for Micro-Supercapacitors. Science 2016, 351, 691–695. [Google Scholar] [CrossRef]
- Kim, M.S.; Hsia, B.; Carraro, C.; Maboudian, R. Flexible Micro-Supercapacitors with High Energy Density from Simple Transfer of Photoresist-Derived Porous Carbon Electrodes. Carbon 2014, 74, 163–169. [Google Scholar] [CrossRef]
- Beidaghi, M.; Gogotsi, Y. Capacitive Energy Storage in Micro-Scale Devices: Recent Advances in Design and Fabrication of Micro-Supercapacitors. Energy Environ. Sci. 2014, 7, 867–884. [Google Scholar] [CrossRef]
- Chen, F.; Xu, Z.-L. Design and Manufacture of High-Performance Microbatteries: Lithium and Beyond. Microstructures 2022, 2, 2022012. [Google Scholar] [CrossRef]
- Zhu, Z.; Kan, R.; Hu, S.; He, L.; Hong, X.; Tang, H.; Luo, W. Recent Advances in High-Performance Microbatteries: Construction, Application, and Perspective. Small 2020, 16, 2003251. [Google Scholar] [CrossRef] [PubMed]
- Robert, K.; Douard, C.; Demortière, A.; Blanchard, F.; Roussel, P.; Brousse, T.; Lethien, C. On Chip Interdigitated Micro-Supercapacitors Based on Sputtered Bifunctional Vanadium Nitride Thin Films with Finely Tuned Inter- and Intracolumnar Porosities. Adv. Mater. Technol. 2018, 3, 1800036. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, T.; Qian, F.; Han, T.Y.J.; Duoss, E.B.; Kuntz, J.D.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Supercapacitors Based on Three-Dimensional Hierarchical Graphene Aerogels with Periodic Macropores. Nano Lett. 2016, 16, 3448–3456. [Google Scholar] [CrossRef]
- Manoccio, M.; Esposito, M.; Passaseo, A.; Cuscunà, M.; Tasco, V. Focused Ion Beam Processing for 3d Chiral Photonics Nanostructures. Micromachines 2021, 12, 6. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, F.; Yu, M.; Zhuang, X.; Feng, X. Two-Dimensional Materials for Miniaturized Energy Storage Devices: From Individual Devices to Smart Integrated Systems. Chem. Soc. Rev. 2018, 47, 7426–7451. [Google Scholar] [CrossRef]
- Bao, Z.; Chen, X. Flexible and Stretchable Devices. Adv. Mater. 2016, 28, 4177–4179. [Google Scholar] [CrossRef]
- Strambini, L.; Paghi, A.; Mariani, S.; Sood, A.; Kalliomäki, J.; Järvinen, P.; Toia, F.; Scurati, M.; Morelli, M.; Lamperti, A.; et al. Three-Dimensional Silicon-Integrated Capacitor with Unprecedented Areal Capacitance for on-Chip Energy Storage. Nano Energy 2020, 68, 104281. [Google Scholar] [CrossRef]
- Ulaby, F.T.; Ravaioli, U. Fundamentals of Applied Electromagnetics, 8th ed.; Pearson: London, UK, 2023; ISBN 13: 978-1-292-43676-0. [Google Scholar]
- Wang, M.; Zhang, J.; Wang, Y.; Lu, Y. Material and Structural Design of Microsupercapacitors. J. Solid. State Electrochem. 2022, 26, 313–334. [Google Scholar] [CrossRef]
- Banerjee, P.; Perez, I.; Henn-Lecordier, L.; Lee, S.B.; Rubloff, G.W. Nanotubular Metal-Insulator-Metal Capacitor Arrays for Energy Storage. Nat. Nanotechnol. 2009, 4, 292–296. [Google Scholar] [CrossRef]
- Jayakrishnan, A.R.; Anina Anju, B.; P Nair, S.K.; Dutta, S.; Silva, J.P.B. Recent Development of Lead-Free Relaxor Ferroelectric and Antiferroelectric Thin Films as Energy Storage Dielectric Capacitors. J. Eur. Ceram. Soc. 2024, 44, 4332–4349. [Google Scholar] [CrossRef]
- Jayakrishnan, A.R.; Silva, J.P.B.; Kamakshi, K.; Dastan, D.; Annapureddy, V.; Pereira, M.; Sekhar, K.C. Are Lead-Free Relaxor Ferroelectric Materials the Most Promising Candidates for Energy Storage Capacitors? Prog. Mater. Sci. 2023, 132, 101046. [Google Scholar] [CrossRef]
- Shim, J.H.; Choi, H.J.; Kim, Y.; Torgersen, J.; An, J.; Lee, M.H.; Prinz, F.B. Process-Property Relationship in High-: K ALD SrTiO3 and BaTiO3: A Review. J. Mater. Chem. C Mater. 2017, 5, 8000–8013. [Google Scholar] [CrossRef]
- Go, D.; Shin, J.W.; Lee, S.; Lee, J.; Yang, B.C.; Won, Y.; Motoyama, M.; An, J. Atomic Layer Deposition for Thin Film Solid-State Battery and Capacitor. Int. J. Precis. Eng. Manuf. -Green. Technol. 2023, 10, 851–873. [Google Scholar] [CrossRef]
- Klootwijk, J.; Kemmeren, A.; Wolters, R.; Roozeboom, F.; Verhoeven, J.; Heuvel, E. van Den Extremely High-Density Capacitors with ALD High-K Dielectric Layers. In Defects in High-k Gate Dielectric Stacks; Springer: Heidelberg, Germany, 2006; pp. 17–28. [Google Scholar]
- Pan, H.; Li, F.; Liu, Y.; Zhang, Q.; Wang, M.; Lan, S.; Zheng, Y.; Ma, J.; Gu, L.; Shen, Y.; et al. Ultrahigh—Energy Density Lead-Free Dielectric Films via Polymorphic Nanodomain Design. Science 2019, 365, 578–582. [Google Scholar] [CrossRef]
- Liu, Y.; Han, H.; Pan, H.; Lan, S.; Lin, Y.; Ma, J. Enhancement of Breakdown Strength in Relaxor Ferroelectric Ba(Zr0.3Ti0.7)O3 Thin Film via Manipulating Growth Oxygen Pressure. J. Alloys Compd. 2023, 937, 168452. [Google Scholar] [CrossRef]
- Lv, P.; Yang, C.; Qian, J.; Wu, H.; Huang, S.; Cheng, X.; Cheng, Z. Flexible Lead-Free Perovskite Oxide Multilayer Film Capacitor Based on (Na0.8K0.2)0.5Bi0.5TiO3/Ba0.5Sr0.5(Ti0.97Mn0.03)O3 for High-Performance Dielectric Energy Storage. Adv. Energy Mater. 2020, 10, 1904229. [Google Scholar] [CrossRef]
- Lee, S.W.; Han, J.H.; Kwon, O.S.; Hwang, C.S. Influences of a Crystalline Seed Layer during Atomic Layer Deposition of SrTiO3 Thin Films Using Ti(O-IPr)2(Thd)2, Sr(Thd)2, and H2O. J. Electrochem. Soc. 2008, 155, G253. [Google Scholar] [CrossRef]
- Robertson, J.; Wallace, R.M. High-K Materials and Metal Gates for CMOS Applications. Mater. Sci. Eng. R Rep. 2015, 88, 1–41. [Google Scholar] [CrossRef]
- Jeon, W. Recent Advances in the Understanding of High-k Dielectric Materials Deposited by Atomic Layer Deposition for Dynamic Random-Access Memory Capacitor Applications. J. Mater. Res. 2020, 35, 775–794. [Google Scholar] [CrossRef]
- Smitha, P.S.; Babu, V.S.; Shiny, G. Critical Parameters of High Performance Metal-Insulator-Metal Nanocapacitors: A Review. Mater. Res. Express 2019, 6, 122003. [Google Scholar] [CrossRef]
- Mondal, S.; Pan, T.M. High-Performance Ni/Lu2O3/TaN Metal-Insulator-Metal Capacitors. IEEE Electron. Device Lett. 2011, 32, 1576–1578. [Google Scholar] [CrossRef]
- Austin, D.Z.; Allman, D.; Price, D.; Hose, S.; Conley, J.F. Plasma Enhanced Atomic Layer Deposition of Al2O3/SiO2 MIM Capacitors. IEEE Electron. Device Lett. 2015, 36, 496–498. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kodama, D.; Matsui, Y.; Hiratani, M.; Furusawa, T.; Hisamoto, D. High-Capacitance Cu/Ta2O5/Cu MIM Structure for SoC Applications Featuring a Single-Mask Add-on Process. In Proceedings of the Technical Digest—International Electron Devices Meeting, San Francisco, CA, USA, 8–11 December 2002; IEEE: New York, NY, USA, 2002; pp. 940–942. [Google Scholar]
- Kim, K.D.; Park, M.H.; Kim, H.J.; Kim, Y.J.; Moon, T.; Lee, Y.H.; Hyun, S.D.; Gwon, T.; Hwang, C.S. Ferroelectricity in Undoped-HfO2 Thin Films Induced by Deposition Temperature Control during Atomic Layer Deposition. J. Mater. Chem. C Mater. 2016, 4, 6864–6872. [Google Scholar] [CrossRef]
- Kambale, K.R.; Mahajan, A.; Butee, S.P.; Kulkarni, A.R.; Venkataramani, N. Effect of Sm2O3 Addition on the Dielectric Behaviour of BaTiO3. Mater. Today Proc. 2022, 67, 404–409. [Google Scholar] [CrossRef]
- Khomenkova, L.; Merabet, H.; Chauvat, M.P.; Frilay, C.; Portier, X.; Labbe, C.; Marie, P.; Cardin, J.; Boudin, S.; Rueff, J.M.; et al. Comprehensive Investigation of Er2O3 Thin Films Grown with Different ALD Approaches. Surf. Interfaces 2022, 34, 102377. [Google Scholar] [CrossRef]
- Singh, E.R.; Alam, M.W.; Singh, N.K. Capacitive and RRAM Forming-Free Memory Behavior of Electron-Beam Deposited Ta2O5 Thin Film for Nonvolatile Memory Application. ACS Appl. Electron. Mater. 2023, 5, 3462–3469. [Google Scholar] [CrossRef]
- Nam, M.; Lee, S.; Jeong, H.; Yoon, A.; Park, J.S.; Jeon, W. Optimizing the Crystallinity of a ZrO2 Thin Film Insulator for InGaZnO-Based Metal–Insulator–Semiconductor Capacitors. Adv. Mater. Interfaces 2024, 11, 2300883. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, H.B.; Kwon, S.H.; Ahn, J.H. Enhanced Electrical Properties of ZrO2-TiN Based Capacitors by Introducing Ultrathin Metal Oxides. Mater. Lett. 2020, 279, 128490. [Google Scholar] [CrossRef]
- Jeong, J.; Han, Y.; Sohn, H. Effect of La Doping on Dielectric Constant and Tetragonality of ZrO2 Thin Films Deposited by Atomic Layer Deposition. J. Alloys Compd. 2022, 927, 166961. [Google Scholar] [CrossRef]
- Mehmood, F.; Alcala, R.; Vishnumurthy, P.; Xu, B.; Sachdeva, R.; Mikolajick, T.; Schroeder, U. Reliability Improvement from La2O3 Interfaces in Hf0.5Zr0.5O2-Based Ferroelectric Capacitors. Adv. Mater. Interfaces 2023, 10, 2202151. [Google Scholar] [CrossRef]
- Clark, R.D. Emerging Applications for High K Materials in VLSI Technology. Materials 2014, 7, 2913–2944. [Google Scholar] [CrossRef]
- Ho, C.S.; Chang, S.J.; Chen, S.C.; Liou, J.J.; Li, H. A Reliable Si3N4/Al2O3-HfO2 Stack MIM Capacitor for High-Voltage Analog Applications. IEEE Trans. Electron. Devices 2014, 61, 2944–2949. [Google Scholar] [CrossRef]
- Patil, S.R.; Barhate, V.N.; Patil, V.S.; Agrawal, K.S.; Mahajan, A.M. The Effect of Post-Deposition Annealing on the Chemical, Structural and Electrical Properties of Al/ZrO2/La2O3/ZrO2/Al High-k Nanolaminated MIM Capacitors. J. Mater. Sci. Mater. Electron. 2022, 33, 11227–11235. [Google Scholar] [CrossRef]
- Patil, S.R.; Borokar, V.Y.; Rasadujjaman, M.; Zhang, J.; Ding, S.J.; Mahajan, A.M. Investigation of PEALD ZrO2/La2O3-Based High-k Nanolaminates Sandwiched between Al and Ti Electrodes for MIM Capacitors. J. Mater. Sci. Mater. Electron. 2023, 34, 1284. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.; Li, G.; Yu, H.; Tang, S.; Wu, Y.; Li, X.; Tian, W. Improved Dielectric Properties of La2O3–ZrO2 Bilayer Films for Novel Gate Dielectrics. Vacuum 2020, 178, 109448. [Google Scholar] [CrossRef]
- Ding, S.J.; Hu, H.; Lim, H.F.; Kim, S.J.; Yu, X.F.; Zhu, C.; Li, M.F.; Cho, B.J.; Chan, D.S.H.; Rustagi, S.C.; et al. High-Performance MIM Capacitor Using ALD High-κ HfO 2-Al2O3 Laminate Dielectrics. IEEE Electron. Device Lett. 2003, 24, 730–732. [Google Scholar] [CrossRef]
- Jeannot, S.; Bajolet, A.; Manceau, J.P.; Crémer, S.; Deloffre, E.; Oddou, J.P.; Perrot, C.; Benoit, D.; Richard, C.; Bouillon, P.; et al. Toward next High Performances MIM Generation: Up to 30fF/Μm2 with 3D Architecture and High-k Materials. In Proceedings of the 2007 IEEE Technical Digest—International Electron Devices Meeting International Electron Devices Meeting, Washington, DC, USA, 10–12 December 2007; IEEE: New York, NY, USA, 2007; pp. 997–1000. [Google Scholar] [CrossRef]
- Chang, P.; Xie, Y. Evaluation of HfO-Based Ferroelectric Resonant Tunnel Junction by Band Engineering. IEEE Electron. Device Lett. 2023, 44, 168–171. [Google Scholar] [CrossRef]
- Xiong, L.; Hu, J.; Yang, Z.; Li, X.; Zhang, H.; Zhang, G. Dielectric Properties Investigation of Metal–Insulator–Metal (MIM) Capacitors. Molecules 2022, 27, 3951. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, K.S.; Kim, S.W.; Lee, D.J.; Park, S.J.; Kim, S. Characterizing Voltage Linearity and Leakage Current of High Density Al2O3/HfO2/Al2/O3 MIM Capacitors. IEEE Electron. Device Lett. 2011, 32, 384–386. [Google Scholar] [CrossRef]
- Park, M.H.; Kim, H.J.; Kim, Y.J.; Moon, T.; Kim, K.D.; Hwang, C.S. Thin HfxZr1-XO2 Films: A New Lead-Free System for Electrostatic Supercapacitors with Large Energy Storage Density and Robust Thermal Stability. Adv. Energy Mater. 2014, 4, 1400610. [Google Scholar] [CrossRef]
- Kozodaev, M.G.; Chernikova, A.G.; Khakimov, R.R.; Park, M.H.; Markeev, A.M.; Hwang, C.S. La-Doped Hf0.5Zr0.5O2 Thin Films for High-Efficiency Electrostatic Supercapacitors. Appl. Phys. Lett. 2018, 113, 123902. [Google Scholar] [CrossRef]
- Pan, H.; Lan, S.; Xu, S.; Zhang, Q.; Yao, H.; Liu, Y.; Meng, F.; Guo, E.J.; Gu, L.; Yi, D.; et al. Ultrahigh Energy Storage in Superparaelectric Relaxor Ferroelectrics. Science 2021, 374, 100–104. [Google Scholar] [CrossRef]
- Kursumovic, A.; Li, W.W.; Cho, S.; Curran, P.J.; Tjhe, D.H.L.; MacManus-Driscoll, J.L. Lead-Free Relaxor Thin Films with Huge Energy Density and Low Loss for High Temperature Applications. Nano Energy 2020, 71, 104536. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Z.; Chen, Y.; Huang, W.; Dong, X. A Novel Lead-Free and High-Performance Barium Strontium Titanate-Based Thin Film Capacitor with Ultrahigh Energy Storage Density and Giant Power Density. J. Mater. Chem. C Mater. 2019, 8, 50–57. [Google Scholar] [CrossRef]
- Liang, Z.; Ma, C.; Shen, L.; Lu, L.; Lu, X.; Lou, X.; Liu, M.; Jia, C.L. Flexible Lead-Free Oxide Film Capacitors with Ultrahigh Energy Storage Performances in Extremely Wide Operating Temperature. Nano Energy 2019, 57, 519–527. [Google Scholar] [CrossRef]
- Hoffmann, M.; Schroeder, U.; Künneth, C.; Kersch, A.; Starschich, S.; Böttger, U.; Mikolajick, T. Ferroelectric Phase Transitions in Nanoscale HfO2 Films Enable Giant Pyroelectric Energy Conversion and Highly Efficient Supercapacitors. Nano Energy 2015, 18, 154–164. [Google Scholar] [CrossRef]
- Lomenzo, P.D.; Chung, C.C.; Zhou, C.; Jones, J.L.; Nishida, T. Doped Hf0.5Zr0.5O2 for High Efficiency Integrated Supercapacitors. Appl. Phys. Lett. 2017, 110, 232904. [Google Scholar] [CrossRef]
- He, Y.; Zheng, G.; Wu, X.; Liu, W.J.; Zhang, D.W.; Ding, S.J. Superhigh Energy Storage Density On-Chip Capacitors with Ferroelectric Hf0.5Zr0.5O2/Antiferroelectric Hf0.25Zr0.75O2 Bilayer Nanofilms Fabricated by Plasma-Enhanced Atomic Layer Deposition. Nanoscale Adv. 2022, 4, 4648–4657. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.H.; Chan, Y.C.; Mo, C.L.; Lin, H.C.; Chen, M.J. Enhancement of Energy Storage for Electrostatic Supercapacitors through Built-in Electric Field Engineering. Nano Energy 2022, 99, 107342. [Google Scholar] [CrossRef]
- Yang, K.; Lee, E.B.; Lee, D.H.; Park, J.Y.; Kim, S.H.; Park, G.H.; Yu, G.T.; Lee, J.I.; Kim, G.H.; Park, M.H. Energy Conversion and Storage Using Artificially Induced Antiferroelectricity in HfO2/ZrO2 Nanolaminates. Compos. B Eng. 2022, 236, 109824. [Google Scholar] [CrossRef]
- Haspert, L.C.; Lee, S.B.; Rubloff, G.W. Nanoengineering Strategies for Metal-Insulator-Metal Electrostatic Nanocapacitors. ACS Nano 2012, 6, 3528–3536. [Google Scholar] [CrossRef]
- Hourdakis, E.; Botzakaki, M.A.; Xanthopoulos, N.J. Metal-Insulator-Metal Micro-Capacitors for Integrated Energy Storage up to 105 Hz. J. Phys. D Appl. Phys. 2022, 55, 455502. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Song, J.; Zhang, S.; Wang, J.; Dai, X.; Liu, B.; Dong, G.; Zhao, L. AgNbO3 Antiferroelectric Film with High Energy Storage Performance. J. Mater. 2021, 7, 1294–1300. [Google Scholar] [CrossRef]
- Ko, E.C.; Kang, W.; Han, J.H. Improved Dielectric Constant and Leakage Current Characteristics of BaTiO3 Thin Film on SrRuO3 Seed Layer. J. Alloys Compd. 2022, 895, 162579. [Google Scholar] [CrossRef]
- Chien, C.S.; Tsai, M.H.; Huang, C.L. Electrical Properties and Current Conduction Mechanisms of LaGdO3 Thin Film by RF Sputtering for RRAM Applications. J. Asian Ceram. Soc. 2020, 8, 948–956. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, Y.; Chen, M.; Peng, X.; Wang, B.; Shang, J. Design Strategies of Perovskite Energy-Storage Dielectrics for next-Generation Capacitors. J. Eur. Ceram. Soc. 2023, 43, 5713–5747. [Google Scholar] [CrossRef]
- Pan, M.J.; Randall, C. A Brief Introduction to Ceramic Capacitors. IEEE Electr. Insul. Mag. 2010, 26, 44–50. [Google Scholar] [CrossRef]
- Ulrich, R.; Schaper, L.; Nelms, D.; Leftwich, M. Comparison of Paraelectric and Ferroelectric Materials for Applications as Dielectrics in Thin Film Integrated Capacitors. Int. J. Microcircuits Electron. Packag. 2000, 23, 172–181. [Google Scholar]
- Dugu, S.; Pavunny, S.P.; Scott, J.F.; Katiyar, R.S. Si:SrTiO3-Al2O3-Si:SrTiO3 Multi-Dielectric Architecture for Metal-Insulator-Metal Capacitor Applications. Appl. Phys. Lett. 2016, 109, 212901. [Google Scholar] [CrossRef]
- Nguyen, M.D. Ultrahigh Energy-Storage Performance in Lead-Free BZT Thin-Films by Tuning Relaxor Behavior. Mater. Res. Bull. 2021, 133, 111072. [Google Scholar] [CrossRef]
- Moon, S.; Moon Lee, S.; Lim, H.-K.; Jin, H.-J.; Soo Yun, Y.; Moon, S.; Jin, H.; Lee, S.M.; Lim, H.; Yun, Y.S. Relationship between Multivalent Cation Charge Carriers and Organic Solvents on Nanoporous Carbons in 4 V-Window Magnesium Ion Supercapacitors. Adv. Energy Mater. 2021, 11, 2101054. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting through Conversion Reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef]
- Ni, J.; Dai, A.; Yuan, Y.; Li, L.; Lu, J. Three-Dimensional Microbatteries beyond Lithium Ion. Matter 2020, 2, 1366–1376. [Google Scholar] [CrossRef]
- Nasreldin, M.; de Mulatier, S.; Delattre, R.; Ramuz, M.; Djenizian, T. Flexible and Stretchable Microbatteries for Wearable Technologies. Adv. Mater. Technol. 2020, 5, 2000412. [Google Scholar] [CrossRef]
- Tan, H.; Feng, Y.; Rui, X.; Yu, Y.; Huang, S.; Tan, H.T.; Rui, X.H.; Huang, S.M.; Feng, Y.Z.; Yu, Y. Metal Chalcogenides: Paving the Way for High-Performance Sodium/Potassium-Ion Batteries. Small Methods 2020, 4, 1900563. [Google Scholar] [CrossRef]
- Pan, Q.; Tong, Z.; Su, Y.; Qin, S.; Tang, Y.; Pan, Q.G.; Tong, Z.P.; Su, Y.Q.; Qin, S.; Tang, Y.B. Energy Storage Mechanism, Challenge and Design Strategies of Metal Sulfides for Rechargeable Sodium/Potassium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2103912. [Google Scholar] [CrossRef]
- Buvat, G.; Iadecola, A.; Blanchard, F.; Brousse, T.; Roussel, P.; Lethien, C. A First Outlook of Sputtered FeWO 4 Thin Films for Micro-Supercapacitor Electrodes. J. Electrochem. Soc. 2021, 168, 030524. [Google Scholar] [CrossRef]
- Nunes, W.G.; Freitas, B.G.A.; Beraldo, R.M.; Filho, R.M.; Da Silva, L.M.; Zanin, H. A Rational Experimental Approach to Identify Correctly the Working Voltage Window of Aqueous-Based Supercapacitors. Sci. Rep. 2020, 10, 19195. [Google Scholar] [CrossRef]
- Huang, M.; Li, F.; Dong, F.; Zhang, Y.X.; Zhang, L.L. MnO2-Based Nanostructures for High-Performance Supercapacitors. J. Mater. Chem. A Mater. 2015, 3, 21380–21423. [Google Scholar] [CrossRef]
- Kumar, A.; Sanger, A.; Kumar, A.; Mishra, Y.K.; Chandra, R. Performance of High Energy Density Symmetric Supercapacitor Based on Sputtered MnO2 Nanorods. ChemistrySelect 2016, 1, 3885–3891. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, H.; Lei, Y. Advances on Three-Dimensional Electrodes for Micro-Supercapacitors: A Mini-Review. InfoMat 2019, 1, 74–84. [Google Scholar] [CrossRef]
- Yu, M.; Qiu, W.; Wang, F.; Zhai, T.; Fang, P.; Lu, X.; Tong, Y. Three Dimensional Architectures: Design, Assembly and Application in Electrochemical Capacitors. J. Mater. Chem. A Mater. 2015, 3, 15792–15823. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Huang, H.; Wang, Z.; Xu, Z.; Zhao, H.; Wang, Y.; Chen, N.; Yang, W. High-Voltage Asymmetric MXene-Based on-Chip Micro-Supercapacitors. Nano Energy 2020, 74, 104928. [Google Scholar] [CrossRef]
- Jiang, Q.; Kurra, N.; Alhabeb, M.; Gogotsi, Y.; Alshareef, H.N. All Pseudocapacitive MXene-RuO2 Asymmetric Supercapacitors. Adv. Energy Mater. 2018, 8, 1703043. [Google Scholar] [CrossRef]
- Couly, C.; Alhabeb, M.; Van Aken, K.L.; Kurra, N.; Gomes, L.; Navarro-Suárez, A.M.; Anasori, B.; Alshareef, H.N.; Gogotsi, Y. Asymmetric Flexible MXene-Reduced Graphene Oxide Micro-Supercapacitor. Adv. Electron. Mater. 2018, 4, 1700339. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, Q.; Zhao, C.; Beaujuge, P.M.; Alshareef, H.N. Asymmetric Supercapacitors with Metal-like Ternary Selenides and Porous Graphene Electrodes. Nano Energy 2016, 24, 78–86. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, N.; Szunerits, S.; Addad, A.; Roussel, P.; Boukherroub, R. Fabrication of ZnCoS Nanomaterial for High Energy Flexible Asymmetric Supercapacitors. Chem. Eng. J. 2019, 374, 347–358. [Google Scholar] [CrossRef]
- Adalati, R.; Kumar, A.; Sharma, M.; Chandra, R. Pt Enhanced Capacitive Performance of Cr2N Electrode toward Flexible Asymmetric Supercapacitor. Appl. Phys. Lett. 2021, 118, 183901. [Google Scholar] [CrossRef]
- Priyadarsini, S.S.; Saxena, S.; Ladole, A.H.; Nehru, D.; Dasgupta, S. Inkjet-Printed Transparent Asymmetric Microsupercapacitors with Mesoporous NiCo2O4 and Mn2O3 Electrodes. ACS Appl. Energy Mater. 2024, 7, 715–725. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Mu, J.; Luo, M.; Lei, Z. High-Performance Aqueous Asymmetric Supercapacitor Based on Carbon Nanofibers Network and Tungsten Trioxide Nanorod Bundles Electrodes. Electrochim. Acta 2014, 147, 54–61. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, Y.; Jia, Z.; He, L.; Xu, L.; Meng, J.; Tahir, M.; Zhou, Z.; Wang, X.; Mai, L.; et al. Interwoven Nanowire Based On-Chip Asymmetric Microsupercapacitor with High Integrability, Areal Energy, and Power Density. Adv. Energy Mater. 2020, 10, 2001873. [Google Scholar] [CrossRef]
- Shen, K.; Ding, J.; Yang, S.; Shen, K.; Ding, J.W.; Yang, S.B. 3D Printing Quasi-Solid-State Asymmetric Micro-Supercapacitors with Ultrahigh Areal Energy Density. Adv. Energy Mater. 2018, 8, 1800408. [Google Scholar] [CrossRef]
- Ma, B.; Hao, W.; Ruan, W.; Yuan, C.; Wang, Q.; Teng, F. Unveiling Capacitive Behaviors of MoO2 in Different Electrolytes and Flexible MoO2-Based Asymmetric Micro-Supercapacitor. J. Energy Storage 2022, 52, 104833. [Google Scholar] [CrossRef]
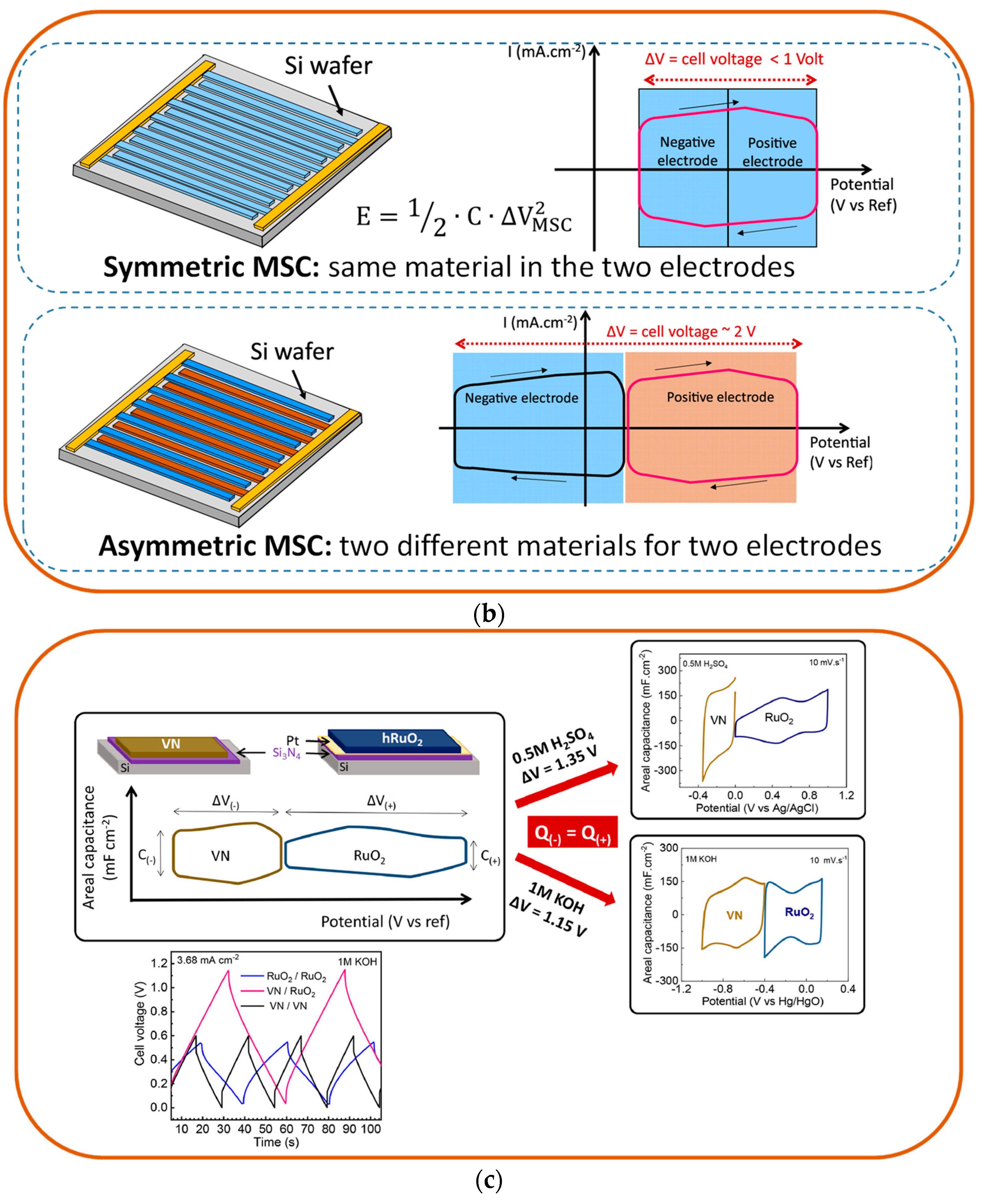
- Asbani, B.; Robert, K.; Roussel, P.; Brousse, T.; Lethien, C. Asymmetric Micro-Supercapacitors Based on Electrodeposited Ruo2 and Sputtered VN Films. Energy Storage Mater. 2021, 37, 207–214. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Ma, J.; Wu, Z.S.; Zhou, F.; He, Y.B.; Kang, F.; Cheng, H.M.; Bao, X. All-Solid-State Flexible Planar Lithium Ion Micro-Capacitors. Energy Environ. Sci. 2018, 11, 2001–2009. [Google Scholar] [CrossRef]
- Bai, C.; Zhang, J.; Chen, R.; Wu, W.; Li, X.; Wang, J.; Lu, Y.; Zhao, Y. A 4 V Planar Li-Ion Micro-Supercapacitor with Ultrahigh Energy Density. ACS Energy Lett. 2024, 9, 410–418. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Z.; Chen, W.; Sun, X.; Luo, M.; Zhang, X.; Li, C.; An, Y.; Song, S.; Wang, K.; et al. A Review on Thermal Behaviors and Thermal Management Systems for Supercapacitors. Batteries 2023, 9, 128. [Google Scholar] [CrossRef]
- Liu, W.; Sun, X.; Yan, X.; Gao, Y.; Zhang, X.; Wang, K.; Ma, Y.; Liu, W.; Sun, X.; Yan, X.; et al. Review of Energy Storage Capacitor Technology. Batteries 2024, 10, 271. [Google Scholar] [CrossRef]
- Li, H.C.; Shen, H.R.; Shi, Y.; Wen, L.; Li, F. Progress and Prospects of Graphene for In-Plane Micro-Supercapacitors. New Carbon Mater. 2022, 37, 781–801. [Google Scholar] [CrossRef]
- Yu, W.; Zhou, H.; Li, B.Q.; Ding, S. 3D Printing of Carbon Nanotubes-Based Microsupercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 4597–4604. [Google Scholar] [CrossRef]
- Kim, S.-K.; Koo, H.-J.; Lee, A.; Braun, P.V.; Kim, S.; Koo, H.; Lee, A.; Braun, P. V Selective Wetting-Induced Micro-Electrode Patterning for Flexible Micro-Supercapacitors. Adv. Mater. 2014, 26, 5108–5112. [Google Scholar] [CrossRef]
- Dousti, B.; Choi, Y., II; Cogan, S.F.; Lee, G.S. A High Energy Density 2D Microsupercapacitor Based on an Interconnected Network of a Horizontally Aligned Carbon Nanotube Sheet. ACS Appl. Mater. Interfaces 2020, 12, 50011–50023. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Taberna, P.L.; Simon, P.; Fabre, N.; Mesnilgrente, F.; Conédéra, V.; Durou, H. Elaboration of a Microstructured Inkjet-Printed Carbon Electrochemical Capacitor. J. Power Sources 2010, 195, 1266–1269. [Google Scholar] [CrossRef]
- Brousse, K.; Martin, C.; Brisse, A.L.; Lethien, C.; Simon, P.; Taberna, P.L.; Brousse, T. Anthraquinone Modification of Microporous Carbide Derived Carbon Films for On-Chip Micro-Supercapacitors Applications. Electrochim. Acta 2017, 246, 391–398. [Google Scholar] [CrossRef]
- Heon, M.; Lofland, S.; Applegate, J.; Nolte, R.; Cortes, E.; Hettinger, J.D.; Taberna, P.L.; Simon, P.; Huang, P.; Brunet, M.; et al. Continuous Carbide-Derived Carbon Films with High Volumetric Capacitance. Energy Environ. Sci. 2010, 4, 135–138. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.L.; Simon, P. Ultrahigh-Power Micrometre-Sized Supercapacitors Based on Onion-like Carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Telpande, S.; Mahapatra, A.D.; Kumar, P.; Misra, A. Role of the Electrode-Edge in Optically Sensitive Three-Dimensional Carbon Foam-MoS2 Based High-Performance Micro-Supercapacitors. J. Mater. Chem. A Mater. 2023, 11, 4963–4976. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Kaner, R.B. Scalable Fabrication of High-Power Graphene Micro-Supercapacitors for Flexible and on-Chip Energy Storage. Nat. Commun. 2013, 4, 1475. [Google Scholar] [CrossRef]
- Létiche, M.; Brousse, K.; Demortière, A.; Huang, P.; Daffos, B.; Pinaud, S.; Respaud, M.; Chaudret, B.; Roussel, P.; Buchaillot, L.; et al. Sputtered Titanium Carbide Thick Film for High Areal Energy on Chip Carbon-Based Micro-Supercapacitors. Adv. Funct. Mater. 2017, 27, 1606813. [Google Scholar] [CrossRef]
- Eustache, E.; Douard, C.; Retoux, R.; Lethien, C.; Brousse, T.; Eustache, E.; Douard, C.; Brousse, T.; Lethien, C. MnO2 Thin Films on 3D Scaffold: Microsupercapacitor Electrodes Competing with “Bulk” Carbon Electrodes. Adv. Energy Mater. 2015, 5, 1500680. [Google Scholar] [CrossRef]
- Mayorov, A.S.; Gorbachev, R.V.; Morozov, S.V.; Britnell, L.; Jalil, R.; Ponomarenko, L.A.; Blake, P.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Micrometer-Scale Ballistic Transport in Encapsulated Graphene at Room Temperature. Nano Lett. 2011, 11, 2396–2399. [Google Scholar] [CrossRef]
- Beidaghi, M.; Wang, C. Micro-Supercapacitors Based on Interdigital Electrodes of Reduced Graphene Oxide and Carbon Nanotube Composites with Ultrahigh Power Handling Performance. Adv. Funct. Mater. 2012, 22, 4501–4510. [Google Scholar] [CrossRef]
- Xia, J.; Chen, F.; Li, J.; Tao, N. Measurement of the Quantum Capacitance of Graphene. Nat. Nanotechnol. 2009, 4, 505–509. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser Scribing of High-Performance and Flexible Graphene-Based Electrochemical Capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef]
- Liang, J.; Mondal, A.K.; Wang, D.W.; Iacopi, F. Graphene-Based Planar Microsupercapacitors: Recent Advances and Future Challenges. Adv. Mater. Technol. 2019, 4, 1800200. [Google Scholar] [CrossRef]
- Wu, Z.S.; Parvez, K.; Feng, X.; Müllen, K. Graphene-Based in-Plane Micro-Supercapacitors with High Power and Energy Densities. Nat. Commun. 2013, 4, 2487. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, L.; Liu, L.; Zhu, B.; Dong, H.; Chen, X. All-Solid-State Flexible Ultrathin Micro-Supercapacitors Based on Graphene. Adv. Mater. 2013, 25, 4035–4042. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Tan, H.; Wu, S.; Ni, K.; Pan, F.; Liu, J.; Tao, Z.; Qu, Y.; Ji, H.; Simon, P.; et al. Direct Laser Writing of Graphene Made from Chemical Vapor Deposition for Flexible, Integratable Micro-Supercapacitors with Ultrahigh Power Output. Adv. Mater. 2018, 30, 1801384. [Google Scholar] [CrossRef]
- Velasco, A.; Ryu, Y.K.; Boscá, A.; Ladrón-De-Guevara, A.; Hunt, E.; Zuo, J.; Pedrós, J.; Calle, F.; Martinez, J. Recent Trends in Graphene Supercapacitors: From Large Area to Microsupercapacitors. Sustain. Energy Fuels 2021, 5, 1235–1254. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The Reduction of Graphene Oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Zhu, Y.; Ni, Z.; Gao, J.; Zhang, D.; Wang, S.; Zhao, J. Fabrication of Flexible Planar Micro-Supercapacitors Using Laser Scribed Graphene Electrodes Incorporated with MXene. J. Phys. Chem. Solids 2023, 183, 111619. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced Graphene Oxide Today. J. Mater. Chem. C Mater. 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Shi, X.; Pei, S.; Zhou, F.; Ren, W.; Cheng, H.M.; Wu, Z.S.; Bao, X. Ultrahigh-Voltage Integrated Micro-Supercapacitors with Designable Shapes and Superior Flexibility. Energy Environ. Sci. 2019, 12, 1534–1541. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Han, Y.; Li, X.; Dai, C.; Zhang, X.; Jin, X.; Shao, C.; Lu, B.; Wang, C.; et al. Fixture-Free Omnidirectional Prestretching Fabrication and Integration of Crumpled in-Plane Micro-Supercapacitors. Sci. Adv. 2022, 8, eabn8338. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, Z.S.; Chen, L.; Zhou, F.; Zheng, S.; Ren, W.; Cheng, H.M.; Bao, X. One-Step Device Fabrication of Phosphorene and Graphene Interdigital Micro-Supercapacitors with High Energy Density. ACS Nano 2017, 11, 7284–7292. [Google Scholar] [CrossRef]
- Chen, M.; Shi, X.; Wang, X.; Liu, H.; Wang, S.; Meng, C.; Liu, Y.; Zhang, L.; Zhu, Y.; Wu, Z.S. Low-Temperature and High-Voltage Planar Micro-Supercapacitors Based on Anti-Freezing Hybrid Gel Electrolyte. J. Energy Chem. 2022, 72, 195–202. [Google Scholar] [CrossRef]
- Li, J.; Sollami Delekta, S.; Zhang, P.; Yang, S.; Lohe, M.R.; Zhuang, X.; Feng, X.; Östling, M. Scalable Fabrication and Integration of Graphene Microsupercapacitors through Full Inkjet Printing. ACS Nano 2017, 11, 8249–8256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Zhang, D.; Sun, X.; Lin, H.; Wang, C.; Ma, Y. One-Step Electrophoretic Deposition of Reduced Graphene Oxide and Ni(OH)2 Composite Films for Controlled Syntheses Supercapacitor Electrodes. J. Phys. Chem. B 2013, 117, 1616–1627. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Z.S.; Qin, J.; Zheng, S.; Wang, S.; Zhou, F.; Sun, C.; Bao, X. Graphene-Based Linear Tandem Micro-Supercapacitors with Metal-Free Current Collectors and High-Voltage Output. Adv. Mater. 2017, 29, 1703034. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, J. Facile Self-Assembly of Exfoliated Graphene/PANI Film for High-Energy Zn-Ion Micro-Supercapacitors. Molecules 2023, 28, 4470. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhu, L.; Liu, H.; Chen, H.; Ju, P.; Li, W. Synthesis of Graphene via Electrochemical Exfoliation in Different Electrolytes for Direct Electrodeposition of a Cu/Graphene Composite Coating. RSC Adv. 2019, 9, 35524–35531. [Google Scholar] [CrossRef]
- Xiang, L.; Shen, Q.; Zhang, Y.; Bai, W.; Nie, C. One-Step Electrodeposited Ni-Graphene Composite Coating with Excellent Tribological Properties. Surf. Coat. Technol. 2019, 373, 38–46. [Google Scholar] [CrossRef]
- Wuttke, M.; Liu, Z.; Lu, H.; Narita, A.; Müllen, K. Direct Metal-Free Chemical Vapor Deposition of Graphene Films on Insulating Substrates for Micro-Supercapacitors with High Volumetric Capacitance. Batter. Supercaps 2019, 2, 929–933. [Google Scholar] [CrossRef]
- Gupta, N.; Mogera, U.; Kulkarni, G.U. Ultrafast Planar Microsupercapacitor Based on Defect-Free Twisted Multilayer Graphene. Mater. Res. Bull. 2022, 152, 111841. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Wang, C.; Wang, H.I.; Wuttke, M.; Wang, X.Y.; Bonn, M.; Chi, L.; Narita, A.; Müllen, K. Bottom-Up, On-Surface-Synthesized Armchair Graphene Nanoribbons for Ultra-High-Power Micro-Supercapacitors. J. Am. Chem. Soc. 2020, 142, 17881–17886. [Google Scholar] [CrossRef]
- Das, P.; Zhang, L.; Zheng, S.; Shi, X.; Li, Y.; Wu, Z.S. Rapid Fabrication of High-Quality Few-Layer Graphene through Gel-Phase Electrochemical Exfoliation of Graphite for High-Energy-Density Ionogel-Based Micro-Supercapacitors. Carbon 2022, 196, 203–212. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-Induced Porous Graphene Films from Commercial Polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Yuan, W.; Zhang, X.; Bai, S.; Chen, Y.; Zhao, B.; Wu, X.; Wang, C.; Huang, H.; et al. Direct Mask-Free Fabrication of Patterned Hierarchical Graphene Electrode for on-Chip Micro-Supercapacitors. J. Mater. Sci. Technol. 2023, 143, 12–19. [Google Scholar] [CrossRef]
- Bellani, S.; Petroni, E.; Del Rio Castillo, A.E.; Curreli, N.; Martín-García, B.; Oropesa-Nuñez, R.; Prato, M.; Bonaccorso, F. Scalable Production of Graphene Inks via Wet-Jet Milling Exfoliation for Screen-Printed Micro-Supercapacitors. Adv. Funct. Mater. 2019, 29, 1807659. [Google Scholar] [CrossRef]
- Cao, C.; Zhou, Y.; Ubnoske, S.; Zang, J.; Cao, Y.; Henry, P.; Parker, C.B.; Glass, J.T. Highly Stretchable Supercapacitors via Crumpled Vertically Aligned Carbon Nanotube Forests. Adv. Energy Mater. 2019, 9, 1900618. [Google Scholar] [CrossRef]
- He, P.; Ding, Z.; Zhao, X.; Liu, J.; Huang, Q.; Peng, J.; Fan, L.Z. Growth of Carbon Nanosheets on Carbon Nanotube Arrays for the Fabrication of Three-Dimensional Micro-Patterned Supercapacitors. Carbon 2019, 155, 453–461. [Google Scholar] [CrossRef]
- Dinh, T.M.; Achour, A.; Vizireanu, S.; Dinescu, G.; Nistor, L.; Armstrong, K.; Guay, D.; Pech, D. Hydrous RuO2/Carbon Nanowalls Hierarchical Structures for All-Solid-State Ultrahigh-Energy-Density Micro-Supercapacitors. Nano Energy 2014, 10, 288–294. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-Induced Graphene: From Discovery to Translation. Adv. Mater. 2019, 31, 1803621. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Z.S.; Zheng, S.; Zhou, F.; Sun, C.; Cheng, H.M.; Bao, X. Scalable Fabrication of Photochemically Reduced Graphene-Based Monolithic Micro-Supercapacitors with Superior Energy and Power Densities. ACS Nano 2017, 11, 4283–4291. [Google Scholar] [CrossRef]
- Zhou, F.; Huang, H.; Xiao, C.; Zheng, S.; Shi, X.; Qin, J.; Fu, Q.; Bao, X.; Feng, X.; Müllen, K.; et al. Electrochemically Scalable Production of Fluorine-Modified Graphene for Flexible and High-Energy Ionogel-Based Microsupercapacitors. J. Am. Chem. Soc. 2018, 140, 8198–8205. [Google Scholar] [CrossRef]
- Rao, Y.; Yuan, M.; Gao, B.; Li, H.; Yu, J.; Chen, X. Laser-Scribed Phosphorus-Doped Graphene Derived from Kevlar Textile for Enhanced Wearable Micro-Supercapacitor. J. Colloid. Interface Sci. 2023, 630, 586–594. [Google Scholar] [CrossRef]
- Wu, Z.S.; Tan, Y.Z.; Zheng, S.; Wang, S.; Parvez, K.; Qin, J.; Shi, X.; Sun, C.; Bao, X.; Feng, X.; et al. Bottom-Up Fabrication of Sulfur-Doped Graphene Films Derived from Sulfur-Annulated Nanographene for Ultrahigh Volumetric Capacitance Micro-Supercapacitors. J. Am. Chem. Soc. 2017, 139, 4506–4512. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wang, Z.; Rao, Y.; Wang, Y.; Gao, B.; Yu, J.; Li, H.; Chen, X. Laser Direct Writing O/N/S Co-Doped Hierarchically Porous Graphene on Carboxymethyl Chitosan/Lignin-Reinforced Wood for Boosted Microsupercapacitor. Carbon 2023, 202, 296–304. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Ihns, M.; Li, M.; Hwang, J.Y.; Mousavi, M.F.; Chaney, L.; Lech, A.T.; Kaner, R.B. Engineering Three-Dimensional Hybrid Supercapacitors and Microsupercapacitors for High-Performance Integrated Energy Storage. Proc. Natl. Acad. Sci. USA 2015, 112, 4233–4238. [Google Scholar] [CrossRef]
- Zhai, S.; Wang, C.; Karahan, H.E.; Wang, Y.; Chen, X.; Sui, X.; Huang, Q.; Liao, X.; Wang, X.; Chen, Y. Nano-RuO2-Decorated Holey Graphene Composite Fibers for Micro-Supercapacitors with Ultrahigh Energy Density. Small 2018, 14, 1800582. [Google Scholar] [CrossRef]
- Gao, M.; Dong, X.; Wang, K.; Duan, W.; Sun, X.; Zhu, C.; Wang, W. Laser Direct Preparation and Processing of Graphene/MnO Nanocomposite Electrodes for Microsupercapacitors. J. Energy Storage 2021, 33, 102162. [Google Scholar] [CrossRef]
- Lee, H.U.; Kim, S.W. Pen Lithography for Flexible Microsupercapacitors with Layer-by-Layer Assembled Graphene Flake/PEDOT Nanocomposite Electrodes. J. Mater. Chem. A Mater. 2017, 5, 13581–13590. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, M.; Xu, L.; Cai, Y.; Tian, X.; Liao, X.; Wang, Z.; Meng, J.; Hong, X.; Mai, L. In-Situ Selective Surface Engineering of Graphene Micro-Supercapacitor Chips. Nano Res. 2022, 15, 1492–1499. [Google Scholar] [CrossRef]
- Yao, B.; Chandrasekaran, S.; Zhang, H.; Ma, A.; Kang, J.; Zhang, L.; Lu, X.; Qian, F.; Zhu, C.; Duoss, E.B.; et al. 3D-Printed Structure Boosts the Kinetics and Intrinsic Capacitance of Pseudocapacitive Graphene Aerogels. Adv. Mater. 2020, 32, 1906652. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Z.; Chen, Z.; Jiang, Z.; Li, H.; Cao, H.; Liu, Y.; Zhu, Y.; Fang, Z.; Yu, X. Rational Design of Novel Ultra-Small Amorphous Fe2O3 Nanodots/Graphene Heterostructures for All-Solid-State Asymmetric Supercapacitors. Nano Res. 2021, 14, 953–960. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Qi, X.; Yu, J.; Cai, J.; Yang, Z. Manganese Monoxide/Biomass-Inherited Porous Carbon Nanostructure Composite Based on the High Water-Absorbent Agaric for Asymmetric Supercapacitor. ACS Sustain. Chem. Eng. 2019, 7, 4284–4294. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, C.; Yan, Z.; Zhu, Y.; Peng, Z.; Hauge, R.H.; Natelson, D.; Tour, J.M. 3-Dimensional Graphene Carbon Nanotube Carpet-Based Microsupercapacitors with High Electrochemical Performance. Nano Lett. 2013, 13, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Le, V.T.; Bae, J.J.; Lee, Y.H. TLM-PSD Model for Optimization of Energy and Power Density of Vertically Aligned Carbon Nanotube Supercapacitor. Sci. Rep. 2013, 3, 2939. [Google Scholar] [CrossRef]
- Hsia, B.; Marschewski, J.; Wang, S.; In, J.B.; Carraro, C.; Poulikakos, D.; Grigoropoulos, C.P.; Maboudian, R. Highly Flexible, All Solid-State Micro-Supercapacitors from Vertically Aligned Carbon Nanotubes. Nanotechnology 2014, 25, 055401. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; De Heer, W.A. Carbon Nanotubes—The Route toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Zhu, S.; Ni, J.; Li, Y. Carbon Nanotube-Based Electrodes for Flexible Supercapacitors. Nano Res. 2020, 13, 1825–1841. [Google Scholar] [CrossRef]
- Ouldhamadouche, N.; Achour, A.; Lucio-Porto, R.; Islam, M.; Solaymani, S.; Arman, A.; Ahmadpourian, A.; Achour, H.; Le Brizoual, L.; Djouadi, M.A.; et al. Electrodes Based on Nano-Tree-like Vanadium Nitride and Carbon Nanotubes for Micro-Supercapacitors. J. Mater. Sci. Technol. 2018, 34, 976–982. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Q.; Sun, J.; Li, C.; Zhao, J.; Zhou, Z.; He, B.; Wang, X.; Man, P.; Li, Q.; et al. Direct Growth of Vanadium Nitride Nanosheets on Carbon Nanotube Fibers as Novel Negative Electrodes for High-Energy-Density Wearable Fiber-Shaped Asymmetric Supercapacitors. J. Power Sources 2018, 382, 122–127. [Google Scholar] [CrossRef]
- Pitkänen, O.; Järvinen, T.; Cheng, H.; Lorite, G.S.; Dombovari, A.; Rieppo, L.; Talapatra, S.; Duong, H.M.; Tóth, G.; Juhász, K.L.; et al. On-Chip Integrated Vertically Aligned Carbon Nanotube Based Super- and Pseudocapacitors. Sci. Rep. 2017, 7, 16594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, B.; Brown, B. Area-Selective Atomic Layer Deposition of Titanium Oxide and Nitride on Vertically Aligned Carbon Nanotubes Patterned by Aerosol Jet Printing for 3D Microsupercapacitors. J. Power Sources 2022, 551, 232154. [Google Scholar] [CrossRef]
- Presser, V.; Heon, M.; Gogotsi, Y. Carbide-Derived Carbons—from Porous Networks to Nanotubes and Graphene. Adv. Funct. Mater. 2011, 21, 810–833. [Google Scholar] [CrossRef]
- Huang, P.; Heon, M.; Pech, D.; Brunet, M.; Taberna, P.L.; Gogotsi, Y.; Lofland, S.; Hettinger, J.D.; Simon, P. Micro-Supercapacitors from Carbide Derived Carbon (CDC) Films on Silicon Chips. J. Power Sources 2013, 225, 240–244. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Bijukumar, D.; Runa, M.; McNallan, M.; Mathew, M. Tribocorrosion Aspects of Implant Coatings: Hip Replacements. In Tribocorrosion: Fundamentals, Methods, and Materials; Academic Press: Cambridge, MA, USA, 2021; pp. 93–126. [Google Scholar]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode Materials for Supercapacitors: A Review of Recent Advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J. Definitions of Pseudocapacitive Materials: A Brief Review. Energy Environ. Mater. 2019, 2, 30–37. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced Energy Storage Devices: Basic Principles, Analytical Methods, and Rational Materials Design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef]
- Conway, B.E. Two-Dimensional and Quasi-Two-Dimensional Isotherms for Li Intercalation and Upd Processes at Surfaces. Electrochim. Acta 1993, 38, 1249–1258. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, W.; Zheng, W. The Debut and Spreading the Landscape for Excellent Vacancies-Promoted Electrochemical Energy Storage of Nano-Architected Molybdenum Oxides. Mater. Today Energy 2022, 30, 101154. [Google Scholar] [CrossRef]
- Diao, Y.; Lu, Y.; Yang, H.; Wang, H.; Chen, H.; D’Arcy, J.M. Direct Conversion of Fe2O3 to 3D Nanofibrillar PEDOT Microsupercapacitors. Adv. Funct. Mater. 2020, 30, 2003394. [Google Scholar] [CrossRef]
- Sung, J.H.; Kim, S.J.; Lee, K.H. Fabrication of Microcapacitors Using Conducting Polymer Microelectrodes. J. Power Sources 2003, 124, 343–350. [Google Scholar] [CrossRef]
- Wang, X.; Myers, B.D.; Yan, J.; Shekhawat, G.; Dravid, V.; Lee, P.S. Manganese Oxide Micro-Supercapacitors with Ultra-High Areal Capacitance. Nanoscale 2013, 5, 4119–4122. [Google Scholar] [CrossRef]
- Choi, C.; Robert, K.; Whang, G.; Roussel, P.; Lethien, C.; Dunn, B. Photopatternable Hydroxide Ion Electrolyte for Solid-State Micro-Supercapacitors. Joule 2021, 5, 2466–2478. [Google Scholar] [CrossRef]
- Lei, Y.; Zhao, W.; Zhu, Y.; Buttner, U.; Dong, X.; Alshareef, H.N. Three-Dimensional Ti3C2Tx MXene-Prussian Blue Hybrid Microsupercapacitors by Water Lift-Off Lithography. ACS Nano 2022, 16, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation Intercalation and High Volumetric Capacitance of Two-Dimensional Titanium Carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Sun, X.; Wu, C.; Peng, L.; Lin, C.; Hu, S.; Yang, J.; Xie, Y. Metallic Few-Layered VS2 Ultrathin Nanosheets: High Two-Dimensional Conductivity for in-Plane Supercapacitors. J. Am. Chem. Soc. 2011, 133, 17832–17838. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zheng, J.; Liu, X.; Meng, C. Hydrothermal Synthesis of VS4/CNTs Composite with Petal-Shape Structures Performing a High Specific Capacity in a Large Potential Range for High-Performance Symmetric Supercapacitors. J. Colloid. Interface Sci. 2019, 554, 191–201. [Google Scholar] [CrossRef]
- Guo, Z.; Yang, L.; Wang, W.; Cao, L.; Dong, B. Ultrathin VS2 Nanoplate with In-Plane and out-of-Plane Defects for an Electrochemical Supercapacitor with Ultrahigh Specific Capacitance. J. Mater. Chem. A Mater. 2018, 6, 14681–14688. [Google Scholar] [CrossRef]
- Karade, S.S.; Banerjee, K.; Majumder, S.; Sankapal, B.R. Novel Application of Non-Aqueous Chemical Bath Deposited Sb2S3 Thin Films as Supercapacitive Electrode. Int. J. Hydrogen Energy 2016, 41, 21278–21285. [Google Scholar] [CrossRef]
- Jolayemi, B.; Buvat, G.; Brousse, T.; Roussel, P.; Lethien, C. Sputtered (Fe,Mn)3O4 Spinel Oxide Thin Films for Micro-Supercapacitor. J. Electrochem. Soc. 2022, 169, 110524. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials—Capacitive, Pseudocapacitive, or Battery-Like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef]
- Nguyen, T.; De Fátima Montemor, M.; Nguyen, T.; Montemor, M.F. Metal Oxide and Hydroxide–Based Aqueous Supercapacitors: From Charge Storage Mechanisms and Functional Electrode Engineering to Need-Tailored Devices. Adv. Sci. 2019, 6, 1801797. [Google Scholar] [CrossRef]
- Huang, T.; Jiang, K.; Chen, D.; Shen, G. Recent Progress and Perspectives of Metal Oxides Based On-Chip Microsupercapacitors. Chin. Chem. Lett. 2018, 29, 553–563. [Google Scholar] [CrossRef]
- Nithya, V.D.; Arul, N.S. Review on α-Fe2O3 Based Negative Electrode for High Performance Supercapacitors. J. Power Sources 2016, 327, 297–318. [Google Scholar] [CrossRef]
- Brousse, K.; Pinaud, S.; Nguyen, S.; Fazzini, P.F.; Makarem, R.; Josse, C.; Thimont, Y.; Chaudret, B.; Taberna, P.L.; Respaud, M.; et al. Facile and Scalable Preparation of Ruthenium Oxide-Based Flexible Micro-Supercapacitors. Adv. Energy Mater. 2020, 10, 1903136. [Google Scholar] [CrossRef]
- Guan, M.; Wang, Q.; Zhang, X.; Bao, J.; Gong, X.; Liu, Y. Two-Dimensional Transition Metal Oxide and Hydroxide-Based Hierarchical Architectures for Advanced Supercapacitor Materials. Front. Chem. 2020, 8, 390. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Zhang, Z. MoOx Thin Films Deposited by Magnetron Sputtering as an Anode for Aqueous Micro-Supercapacitors. Sci. Technol. Adv. Mater. 2013, 14, 065005. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, S.H.; Yan, Y.; Oh, J.; Zhu, K. Controlled Synthesis of Aligned Ni-NiO Core-Shell Nanowire Arrays on Glass Substrates as a New Supercapacitor Electrode. RSC Adv. 2012, 2, 8281–8285. [Google Scholar] [CrossRef]
- Geramifard, N.; Chakraborty, B.; Dousti, B.; Lee, G.S.; Maeng, J. High-Energy-Density Sputtered Iridium Oxide Micro-Supercapacitors Operating in Physiological Electrolytes. J. Electrochem. Soc. 2022, 169, 050508. [Google Scholar] [CrossRef]
- Deng, W.; Ji, X.; Chen, Q.; Banks, C.E. Electrochemical Capacitors Utilising Transition Metal Oxides: An Update of Recent Developments. RSC Adv. 2011, 1, 1171–1178. [Google Scholar] [CrossRef]
- Hota, M.K.; Jiang, Q.; Mashraei, Y.; Salama, K.N.; Alshareef, H.N. Fractal Electrochemical Microsupercapacitors. Adv. Electron. Mater. 2017, 3, 1700185. [Google Scholar] [CrossRef]
- Wang, Y.T.; Lu, A.H.; Zhang, H.L.; Li, W.C. Synthesis of Nanostructured Mesoporous Manganese Oxides with Three-Dimensional Frameworks and Their Application in Supercapacitors. J. Phys. Chem. C 2011, 115, 5413–5421. [Google Scholar] [CrossRef]
- Han, Z.J.; Pineda, S.; Murdock, A.T.; Seo, D.H.; Ostrikov, K.K.; Bendavid, A. RuO2-Coated Vertical Graphene Hybrid Electrodes for High-Performance Solid-State Supercapacitors. J. Mater. Chem. A Mater. 2017, 5, 17293–17301. [Google Scholar] [CrossRef]
- Bounor, B.; Asbani, B.; Douard, C.; Favier, F.; Brousse, T.; Lethien, C. On Chip MnO2-Based 3D Micro-Supercapacitors with Ultra-High Areal Energy Density. Energy Storage Mater. 2021, 38, 520–527. [Google Scholar] [CrossRef]
- Asbani, B.; Buvat, G.; Freixas, J.; Huvé, M.; Troadec, D.; Roussel, P.; Brousse, T.; Lethien, C. Ultra-High Areal Capacitance and High Rate Capability RuO2 Thin Film Electrodes for 3D Micro-Supercapacitors. Energy Storage Mater. 2021, 42, 259–267. [Google Scholar] [CrossRef]
- Bounor, B.; Asbani, B.; Douard, C.; Deresmes, D.; Stiévenard, D.; Roussel, P.; Favier, F.; Lethien, C.; Brousse, T. Nanostructured MnO2 Films for 3D Micro-Supercapacitors: From New Insights of the Growth Mechanism to the Fine Tuning of Areal Capacitance Values. J. Electrochem. Soc. 2023, 170, 030530. [Google Scholar] [CrossRef]
- Wei, W.; Cui, X.; Chen, W.; Ivey, D.G. Manganese Oxide-Based Materials as Electrochemical Supercapacitor Electrodes. Chem. Soc. Rev. 2011, 40, 1697–1721. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-Dimensional, One-Dimensional, Two-Dimensional and Three-Dimensional Nanostructured Materials for Advanced Electrochemical Energy Devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Jrondi, A.; Buvat, G.; Pena, F.D.L.; Marinova, M.; Huvé, M.; Brousse, T.; Roussel, P.; Lethien, C. Major Improvement in the Cycling Ability of Pseudocapacitive Vanadium Nitride Films for Micro-Supercapacitor. Adv. Energy Mater. 2023, 13, 2203462. [Google Scholar] [CrossRef]
- Lim, J.H.; Choi, D.J.; Kim, H.-K.; Cho, W., II; Yoon, Y.S. Thin Film Supercapacitors Using a Sputtered RuO2 Electrode. J. Electrochem. Soc. 2001, 148, A275. [Google Scholar] [CrossRef]
- Patnaik, S.G.; Shamsudeen Seenath, J.; Bourrier, D.; Prabhudev, S.; Guay, D.; Pech, D. Porous RuOxNySzElectrodes for Microsupercapacitors and Microbatteries with Enhanced Areal Performance. ACS Energy Lett. 2021, 6, 131–139. [Google Scholar] [CrossRef]
- Sugimoto, W.; Iwata, H.; Yokoshima, K.; Murakami, Y.; Takasu, Y. Proton and Electron Conductivity in Hydrous Ruthenium Oxides Evaluated by Electrochemical Impedance Spectroscopy: The Origin of Large Capacitance. J. Phys. Chem. B 2005, 109, 7330–7338. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Cho, W.I.; Lim, J.H.; Choi, D.J. Solid-State Thin-Film Supercapacitor with Ruthenium Oxide and Solid Electrolyte Thin Films. J. Power Sources 2001, 101, 126–129. [Google Scholar] [CrossRef]
- Ferris, A.; Garbarino, S.; Guay, D.; Pech, D. 3D RuO2 Microsupercapacitors with Remarkable Areal Energy. Adv. Mater. 2015, 27, 6625–6629. [Google Scholar] [CrossRef] [PubMed]
- Ponrouch, A.; Garbarino, S.; Bertin, E.; Guay, D. Ultra High Capacitance Values of Pt@RuO2 Core-Shell Nanotubular Electrodes for Microsupercapacitor Applications. J. Power Sources 2013, 221, 228–231. [Google Scholar] [CrossRef]
- Dinh, T.M.; Armstrong, K.; Guay, D.; Pech, D. High-Resolution on-Chip Supercapacitors with Ultra-High Scan Rate Ability. J. Mater. Chem. A Mater. 2014, 2, 7170–7174. [Google Scholar] [CrossRef]
- Eustache, E.; Douard, C.; Demortière, A.; De Andrade, V.; Brachet, M.; Le Bideau, J.; Brousse, T.; Lethien, C. High Areal Energy 3D-Interdigitated Micro-Supercapacitors in Aqueous and Ionic Liquid Electrolytes. Adv. Mater. Technol. 2017, 2, 1700126. [Google Scholar] [CrossRef]
- Li, F.; Hu, A.; Zhao, X.; Wu, T.; Chen, W.; Lei, T.; Hu, Y.; Huang, M.; Wang, X. On-Chip High-Energy Interdigital Micro-Supercapacitors with 3D Nanotubular Array Electrodes. J. Mater. Chem. A Mater. 2022, 10, 14051–14059. [Google Scholar] [CrossRef]
- Si, W.; Yan, C.; Chen, Y.; Oswald, S.; Han, L.; Schmidt, O.G. On Chip, All Solid-State and Flexible Micro-Supercapacitors with High Performance Based on MnOx/Au Multilayers. Energy Environ. Sci. 2013, 6, 3218–3223. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, K.; Gu, S.; Yang, H.; Lou, Z.; Chen, D.; Shen, G. Two-Dimensional Ni(OH)2 Nanoplates for Flexible on-Chip Microsupercapacitors. Nano Res. 2015, 8, 3544–3552. [Google Scholar] [CrossRef]
- Li, L.; Lou, Z.; Han, W.; Shen, G. Flexible In-Plane Microsupercapacitors with Electrospun NiFe2O4 Nanofibers for Portable Sensing Applications. Nanoscale 2016, 8, 14986–14991. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Q.; Zhang, L.; Xu, C.; Pan, Z.; Zhou, Q.; Zhou, W.; Wang, J.; Gu, L.; Liu, H.; et al. Electrochemically Exfoliated Chlorine-Doped Graphene for Flexible All-Solid-State Micro-Supercapacitors with High Volumetric Energy Density. Adv. Mater. 2022, 34, 2106309. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Zheng, Y.; Zheng, S.; Wang, S.; Sun, C.; Parvez, K.; Ikeda, T.; Bao, X.; Müllen, K.; Feng, X.; et al. Stacked-Layer Heterostructure Films of 2D Thiophene Nanosheets and Graphene for High-Rate All-Solid-State Pseudocapacitors with Enhanced Volumetric Capacitance. Adv. Mater. 2017, 29, 1602960. [Google Scholar] [CrossRef]
- Liu, L.; Lu, J.Y.; Long, X.L.; Zhou, R.; Liu, Y.Q.; Wu, Y.T.; Yan, K.W. 3D Printing of High-Performance Micro-Supercapacitors with Patterned Exfoliated Graphene/Carbon Nanotube/Silver Nanowire Electrodes. Sci. China Technol. Sci. 2021, 64, 1065–1073. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, L.; Li, X.; Zuo, P.; Xu, C.; Tian, M.; Zhang, X.; Wang, S.; Lu, B.; Shao, C.; et al. Laser Photonic-Reduction Stamping for Graphene-Based Micro-Supercapacitors Ultrafast Fabrication. Nat. Commun. 2020, 11, 6185. [Google Scholar] [CrossRef]
- Jung, J.; Jeong, J.R.; Lee, J.; Lee, S.H.; Kim, S.Y.; Kim, M.J.; Nah, J.; Lee, M.H. In Situ Formation of Graphene/Metal Oxide Composites for High-Energy Microsupercapacitors. NPG Asia Mater. 2020, 12, 50. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, F.; Wang, F.; Wang, J.; Dong, R.; Zhuang, X.; Schmidt, O.G.; Feng, X.; Zhang, P.; Wang, F.; et al. Stimulus-Responsive Micro-Supercapacitors with Ultrahigh Energy Density and Reversible Electrochromic Window. Adv. Mater. 2017, 29, 1604491. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tang, P.; Shen, H.; Hu, T.; Chen, J.; Chen, K.; Qi, F.; Yang, H.; Wen, L.; Li, F. Scalable Fabrication of Vanadium Carbide/Graphene Electrodes for High-Energy and Flexible Microsupercapacitors. Carbon 2021, 183, 840–849. [Google Scholar] [CrossRef]
- Tian, H.; Qin, J.; Hou, D.; Li, Q.; Li, C.; Wu, Z.-S.; Mai, Y.; Tian, H.; Hou, D.; Li, Q.; et al. General Interfacial Self-Assembly Engineering for Patterning Two-Dimensional Polymers with Cylindrical Mesopores on Graphene. Angew. Chem. 2019, 131, 10279–10284. [Google Scholar] [CrossRef]
- Lee, H.Y.; Goodenough, J.B. Supercapacitor Behavior with KCl Electrolyte. J. Solid. State Chem. 1999, 144, 220–223. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Influence of Microstucture on the Charge Storage Properties of Chemically Synthesized Manganese Dioxide. Chem. Mater. 2002, 14, 3946–3952. [Google Scholar] [CrossRef]
- Li, S.H.; Liu, Q.H.; Qi, L.; Lu, L.H.; Wang, H.Y. Progress in Research on Manganese Dioxide Electrode Aterials for Electrochemical Capacitors. Fenxi Huaxue/Chin. J. Anal. Chem. 2012, 40, 339–346. [Google Scholar] [CrossRef]
- Nam, K.W.; Lee, C.W.; Yang, X.Q.; Cho, B.W.; Yoon, W.S.; Kim, K.B. Electrodeposited Manganese Oxides on Three-Dimensional Carbon Nanotube Substrate: Supercapacitive Behaviour in Aqueous and Organic Electrolytes. J. Power Sources 2009, 188, 323–331. [Google Scholar] [CrossRef]
- Yugang, S.; Yuzi, L.; Tu, T.T.; Yang, R. Thermal Transformation of 7sigma;-MnO2 Nanoflowers Studied by in-Situ TEM. Sci. China Chem. 2012, 55, 2346–2352. [Google Scholar] [CrossRef]
- Fischer, A.E.; Pettigrew, K.A.; Rolison, D.R.; Stroud, R.M.; Long, J.W. Incorporation of Homogeneous, Nanoscale MnO2 within Ultraporous Carbon Structures via Self-Limiting Electroless Deposition: Implications for Electrochemical Capacitors. Nano Lett. 2007, 7, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, S.; Zhou, F.; Das, P.; Zheng, S.; Sun, C.; Bao, X.; Wu, Z.S. 2D Mesoporous MnO2 Nanosheets for High-Energy Asymmetric Micro-Supercapacitors in Water-in-Salt Gel Electrolyte. Energy Storage Mater. 2019, 18, 397–404. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Nanostructured Materials for Advanced Energy Conversion and Storage Devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Liu, W.; Lu, C.; Wang, X.; Tay, R.Y.; Tay, B.K. High-Performance Microsupercapacitors Based on Two-Dimensional Graphene/Manganese Dioxide/Silver Nanowire Ternary Hybrid Film. ACS Nano 2015, 9, 1528–1542. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Bélanger, D. Charge Storage Mechanism of MnO2 Electrode Used in Aqueous Electrochemical Capacitor. Chem. Mater. 2004, 16, 3184–3190. [Google Scholar] [CrossRef]
- Pang, S.-C.; Anderson, M.A.; Chapman, T.W. Novel Electrode Materials for Thin-Film Ultracapacitors: Comparison of Electrochemical Properties of Sol-Gel-Derived and Electrodeposited Manganese Dioxide. J. Electrochem. Soc. 2000, 147, 444. [Google Scholar] [CrossRef]
- Hung, C.J.; Hung, J.H.; Lin, P.; Tseng, T.Y. Electrophoretic Fabrication and Characterizations of Manganese Oxide/Carbon Nanotube Nanocomposite Pseudocapacitors. J. Electrochem. Soc. 2011, 158, A942. [Google Scholar] [CrossRef]
- Wang, Y.; Zhitomirsky, I. Electrophoretic Deposition of Manganese Dioxide—Multiwalled Carbon Nanotube Composites for Electrochemical Supercapacitors. Langmuir 2009, 25, 9684–9689. [Google Scholar] [CrossRef]
- Broughton, J.N.; Brett, M.J. Investigation of Thin Sputtered Mn Films for Electrochemical Capacitors. Electrochim. Acta 2004, 49, 4439–4446. [Google Scholar] [CrossRef]
- Agrawal, R.; Wang, C. On-Chip Asymmetric Microsupercapacitors Combining Reduced Graphene Oxide and Manganese Oxide for High Energy-Power Tradeoff. Micromachines 2018, 9, 399. [Google Scholar] [CrossRef]
- Byun, S.; Shim, Y.; Min Yuk, J.; Lee, C.W.; Yoo, J. Reduced Graphene Oxide as a Charge Reservoir of Manganese Oxide: Interfacial Interaction Promotes Charge Storage Property of MnOx-Based Micro-Supercapacitors. Chem. Eng. J. 2022, 439, 135569. [Google Scholar] [CrossRef]
- Dubal, D.P.; Dhawale, D.S.; Salunkhe, R.R.; Pawar, S.M.; Lokhande, C.D. A Novel Chemical Synthesis and Characterization of Mn3O4 Thin Films for Supercapacitor Application. Appl. Surf. Sci. 2010, 256, 4411–4416. [Google Scholar] [CrossRef]
- Yadav, A.A.; Jadhav, S.N.; Chougule, D.M.; Patil, P.D.; Chavan, U.J.; Kolekar, Y.D. Spray Deposited Hausmannite Mn3O4 Thin Films Using Aqueous/Organic Solvent Mixture for Supercapacitor Applications. Electrochim. Acta 2016, 206, 134–142. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S.; Wang, H.; Wang, X.; Zhang, X.; Jin, G. A Novel Solvothermal Synthesis of Mn3O4/Graphene Composites for Supercapacitors. Electrochim. Acta 2013, 90, 210–218. [Google Scholar] [CrossRef]
- Shi, X.; Zeng, Z.; Liao, C.; Tao, S.; Guo, E.; Long, X.; Wang, X.; Deng, D.; Dai, Y. Flexible, Planar Integratable and All-Solid-State Micro-Supercapacitors Based on Nanoporous Gold/ Manganese Oxide Hybrid Electrodes via Template Plasma Etching Method. J. Alloys Compd. 2018, 739, 979–986. [Google Scholar] [CrossRef]
- Li, X.J.; Song, Z.-W.; Zhao, Y.; Wang, Y.; Zhao, X.C.; Liang, M.; Chu, W.G.; Jiang, P.; Liu, Y. Vertically Porous Nickel Thin Film Supported Mn3O4 for Enhanced Energy Storage Performance. J. Colloid. Interface Sci. 2016, 483, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Dong, X.; Mei, X.; Wang, K.; Wang, W.; Zhu, C.; Duan, W.; Sun, X. Laser Direct Writing of Graphene/MnO-Mn3O4 Doped with Sulfur for High-Performance Microsupercapacitors. J. Energy Storage 2022, 49, 104118. [Google Scholar] [CrossRef]
- Li, Y.Q.; Shi, X.M.; Lang, X.Y.; Wen, Z.; Li, J.C.; Jiang, Q. Remarkable Improvements in Volumetric Energy and Power of 3D MnO2 Microsupercapacitors by Tuning Crystallographic Structures. Adv. Funct. Mater. 2016, 26, 1830–1839. [Google Scholar] [CrossRef]
- Bounor, B.; Seenath, J.S.; Patnaik, S.G.; Bourrier, D.; Tran, C.C.H.; Esvan, J.; Weingarten, L.; Descamps-Mandine, A.; Rochefort, D.; Guay, D.; et al. Low-Cost Micro-Supercapacitors Using Porous Ni/MnO2 Entangled Pillars and Na-Based Ionic Liquids. Energy Storage Mater. 2023, 63, 102986. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Zhang, Z. Molybdenum Oxide Film with Stable Pseudocapacitive Property for Aqueous Micro-Scale Electrochemical Capacitor. Electrochim. Acta 2014, 134, 84–91. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, X.; Cheng, S.; Chern, Z.Y.; Jia, J.; Yang, L.; Luo, H.; Yu, J.; Peng, X.; Wang, J.; et al. Fast Energy Storage in Two-Dimensional MoO2 Enabled by Uniform Oriented Tunnels. ACS Nano 2019, 13, 9091–9099. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yong, H.; Yao, J.; Ma, J.; Liu, B.; Hu, J.; Zhang, Y. Influence of the Phase Evolution and Hydrogen Storage Behaviors of Mg-RE Alloy by a Multi-Valence Mo-Based Catalyst. J. Energy Storage 2023, 58, 106397. [Google Scholar] [CrossRef]
- Vincent, R.C.; Luo, Y.; Andrews, J.L.; Zohar, A.; Zhou, Y.; Yan, Q.; Mozur, E.M.; Preefer, M.B.; Weker, J.N.; Cheetham, A.K.; et al. High-Rate Lithium Cycling and Structure Evolution in Mo4O11. Chem. Mater. 2022, 34, 4122–4133. [Google Scholar] [CrossRef]
- Ge, H.; Kuwahara, Y.; Yamashita, H. Development of Defective Molybdenum Oxides for Photocatalysis, Thermal Catalysis, and Photothermal Catalysis. Chem. Commun. 2022, 58, 8466–8479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Qu, G. Molybdenum-Based Electrode Materials Applied in High-Performance Supercapacitors. Batteries 2023, 9, 479. [Google Scholar] [CrossRef]
- Maheswari, N.; Muralidharan, G. Controlled Synthesis of Nanostructured Molybdenum Oxide Electrodes for High Performance Supercapacitor Devices. Appl. Surf. Sci. 2017, 416, 461–469. [Google Scholar] [CrossRef]
- Tang, W.; Liu, L.; Tian, S.; Li, L.; Yue, Y.; Wu, Y.; Zhu, K. Aqueous Supercapacitors of High Energy Density Based on MoO3 Nanoplates as Anode Material. Chem. Commun. 2011, 47, 10058–10060. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Cao, H.; Qian, D. MoO3 Nanowires as Electrochemical Pseudocapacitor Materials. Chem. Commun. 2011, 47, 10305–10307. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Gong, L.G.; Wang, C.X.; Wang, C.M.; Yu, K.; Zhou, B.B. Silver-Doped Molybdenum Oxide Ternary Nanoparticles with Excellent Supercapacitor and Hydrogen Peroxide-Sensing Performance. Mater. Today Energy 2021, 20, 100774. [Google Scholar] [CrossRef]
- Xu, Y.; Deng, P.; Chen, R.; Xie, W.; Xu, Z.; Yang, Y.; Liu, D.; Huang, F.; Zhuang, Z.; Zhitomirsky, I.; et al. Electric Discharge Direct Writing of 3D Mo-MoOx Pseudocapacitive Micro-Supercapacitors with Designable Patterns. Ceram. Int. 2023, 49, 22586–22594. [Google Scholar] [CrossRef]
- Liu, C.; Li, Z.; Zhang, Z. Growth of [010] Oriented α-MoO3 Nanorods by Pulsed Electron Beam Deposition. Appl. Phys. Lett. 2011, 99, 223104. [Google Scholar] [CrossRef]
- Prakash, N.G.; Dhananjaya, M.; Narayana, A.L.; Shaik, D.P.; Rosaiah, P.; Hussain, O.M. High Performance One Dimensional α-MoO3 Nanorods for Supercapacitor Applications. Ceram. Int. 2018, 44, 9967–9975. [Google Scholar] [CrossRef]
- Shaheen, I.; Ahmad, K.S.; Zequine, C.; Gupta, R.K.; Thomas, A.G.; Malik, M.A. Electrochemical Energy Storage by Nanosized MoO3/PdO Material: Investigation of Its Structural, Optical and Electrochemical Properties for Supercapacitor. J. Energy Storage 2021, 36, 102447. [Google Scholar] [CrossRef]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered Mesoporous α-MoO3 with Iso-Oriented Nanocrystalline Walls for Thin-Film Pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Kim, H.S.; Cook, J.B.; Lin, H.; Ko, J.S.; Tolbert, S.H.; Ozolins, V.; Dunn, B. Oxygen Vacancies Enhance Pseudocapacitive Charge Storage Properties of MoO3-x. Nat. Mater. 2017, 16, 454–460. [Google Scholar] [CrossRef]
- Gurusamy, L.; Karuppasamy, L.; Anandan, S.; Liu, N.; Lee, G.J.; Liu, C.H.; Wu, J.J. Enhanced Performance of Charge Storage Supercapattery by Dominant Oxygen Deficiency in Crystal Defects of 2-D MoO3-x Nanoplates. Appl. Surf. Sci. 2021, 541, 148676. [Google Scholar] [CrossRef]
- Xiao, X.; Ding, T.; Yuan, L.; Shen, Y.; Zhong, Q.; Zhang, X.; Cao, Y.; Hu, B.; Zhai, T.; Gong, L.; et al. WO3-x/MoO3-x Core/Shell Nanowires on Carbon Fabric as an Anode for All-Solid-State Asymmetric Supercapacitors. Adv. Energy Mater. 2012, 2, 1328–1332. [Google Scholar] [CrossRef]
- Huang, L.; Yao, B.; Sun, J.; Gao, X.; Wu, J.; Wan, J.; Li, T.; Hu, Z.; Zhou, J. Highly Conductive and Flexible Molybdenum Oxide Nanopaper for High Volumetric Supercapacitor Electrode. J. Mater. Chem. A Mater. 2017, 5, 2897–2903. [Google Scholar] [CrossRef]
- Guru Prakash, N.; Dhananjaya, M.; Lakshmi Narayana, A.; Hussain, O.M. One-Dimensional MoO3/Pd Nanocomposite Electrodes for High Performance Supercapacitors. Mater. Res. Express 2019, 6, 085543. [Google Scholar] [CrossRef]
- Cheng, A.; Shen, Y.; Hong, T.; Zhan, R.; Chen, E.; Chen, Z.; Chen, G.; Liang, M.; Sun, X.; Wang, D.; et al. Self-Assembly Vertical Graphene-Based MoO3 Nanosheets for High Performance Supercapacitors. Nanomaterials 2022, 12, 2057. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Wei, L.; Jin, Y.; Hou, J.; Wang, X.; Guo, X. In-Plane Flexible Solid-State Microsupercapacitors for on-Chip Electronics. Energy 2019, 170, 338–348. [Google Scholar] [CrossRef]
- Chen, J.; Han, S.; Zhao, H.; Bai, J.; Wang, L.; Sun, G.; Zhang, Z.; Pan, X.; Zhou, J.; Xie, E. Robust Wire-Based Supercapacitors Based on Hierarchical A-MoO3 Nanosheet Arrays with Well-Aligned Laminated Structure. Chem. Eng. J. 2017, 320, 34–42. [Google Scholar] [CrossRef]
- Singh, B.K.; Shaikh, A.; Badrayyana, S.; Mohapatra, D.; Dusane, R.O.; Parida, S. Nanoporous Gold-Copper Oxide Based All-Solid-State Micro-Supercapacitors. RSC Adv. 2016, 6, 100467–100475. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Zheng, S.; Qin, S.; Wang, J.; Chen, C.; Liu, D.; Wang, L.; Yang, G.; Su, Y.; et al. Shape-Tailorable High-Energy Asymmetric Micro-Supercapacitors Based on Plasma Reduced and Nitrogen-Doped Graphene Oxide and MoO2 Nanoparticles. J. Mater. Chem. A Mater. 2019, 7, 14328–14336. [Google Scholar] [CrossRef]
- Lin, N.; Chen, H.; Wang, W.; Lu, L. Laser-Induced Graphene/MoO2 Core-Shell Electrodes on Carbon Cloth for Integrated, High-Voltage, and In-Planar Microsupercapacitors. Adv. Mater. Technol. 2021, 6, 2000991. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Chen, Z.; Liu, D.; Wang, J.; Qian, Y.; Chen, C.; Liu, Y.; Wang, L.; Razal, J.; et al. MXene Coupled with Molybdenum Dioxide Nanoparticles as 2D-0D Pseudocapacitive Electrode for High Performance Flexible Asymmetric Micro-Supercapacitors. J. Mater. 2020, 6, 138–144. [Google Scholar] [CrossRef]
- Liu, D.-Q.; Yu, S.-H.; Son, S.-W.; Joo, S.-K. Electrochemical Performance of Iridium Oxide Thin Film for Supercapacitor Prepared by Radio Frequency Magnetron Sputtering Method. ECS Trans. 2008, 16, 103. [Google Scholar] [CrossRef]
- Liao, P.C.; Ho, W.S.; Huang, Y.S.; Tiong, K.K. Characterization of Sputtered Iridium Dioxide Thin Films. J. Mater. Res. 1998, 13, 1318–1326. [Google Scholar] [CrossRef]
- Beknalkar, S.A.; Teli, A.M.; Harale, N.S.; Shin, J.C.; Patil, P.S. Construction of IrO2@Mn3O4 Core-Shell Heterostructured Nanocomposites for High Performance Symmetric Supercapacitor Device. J. Alloys Compd. 2021, 887, 161328. [Google Scholar] [CrossRef]
- Sethuraman, B.; Purushothaman, K.K.; Muralidharan, G. Synthesis of Mesh-like Fe2O3/C Nanocomposite via Greener Route for High Performance Supercapacitors. RSC Adv. 2014, 4, 4631–4637. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, W.; Wang, G. The Perfect Matching between the Low-Cost Fe2O3 Nanowire Anode and the NiO Nanoflake Cathode Significantly Enhances the Energy Density of Asymmetric Supercapacitors. J. Mater. Chem. A Mater. 2015, 3, 6662–6670. [Google Scholar] [CrossRef]
- Kulal, P.M.; Dubal, D.P.; Lokhande, C.D.; Fulari, V.J. Chemical Synthesis of Fe2O3 Thin Films for Supercapacitor Application. J. Alloys Compd. 2011, 509, 2567–2571. [Google Scholar] [CrossRef]
- Xie, K.; Li, J.; Lai, Y.; Lu, W.; Zhang, Z.; Liu, Y.; Zhou, L.; Huang, H. Highly Ordered Iron Oxide Nanotube Arrays as Electrodes for Electrochemical Energy Storage. Electrochem. Commun. 2011, 13, 657–660. [Google Scholar] [CrossRef]
- Gund, G.S.; Dubal, D.P.; Chodankar, N.R.; Cho, J.Y.; Gomez-Romero, P.; Park, C.; Lokhande, C.D. Low-Cost Flexible Supercapacitors with High-Energy Density Based on Nanostructured MnO2 and Fe2O3 Thin Films Directly Fabricated onto Stainless Steel. Sci. Rep. 2015, 5, 12454. [Google Scholar] [CrossRef] [PubMed]
- Shivakumara, S.; Penki, T.R.; Munichandraiah, N. High Specific Surface Area α-Fe2O3 Nanostructures as High Performance Electrode Material for Supercapacitors. Mater. Lett. 2014, 131, 100–103. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Wang, T. Controlled Synthesis of Mesoporous Hematite Nanostructures and Their Application as Electrochemical Capacitor Electrodes. Nanotechnology 2011, 22, 135604. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, M.; Meng, Y.; Fang, P.; Lu, X.; Tong, Y. Iron-Based Supercapacitor Electrodes: Advances and Challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Xia, Q.; Xu, M.; Xia, H.; Xie, J. Nanostructured Iron Oxide/Hydroxide-Based Electrode Materials for Supercapacitors. ChemNanoMat 2016, 2, 588–600. [Google Scholar] [CrossRef]
- Ma, J.; Guo, X.; Yan, Y.; Xue, H.; Pang, H. FeOx-Based Materials for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700986. [Google Scholar] [CrossRef]
- Yu, S.; Hong Ng, V.M.; Wang, F.; Xiao, Z.; Li, C.; Kong, L.B.; Que, W.; Zhou, K. Synthesis and Application of Iron-Based Nanomaterials as Anodes of Lithium-Ion Batteries and Supercapacitors. J. Mater. Chem. A Mater. 2018, 6, 9332–9367. [Google Scholar] [CrossRef]
- El Issmaeli, Y.; Lahrichi, A.; Kalanur, S.S.; Natarajan, S.K.; Pollet, B.G. Recent Advances and Prospects of FeOOH-Based Electrode Materials for Supercapacitors. Batteries 2023, 9, 259. [Google Scholar] [CrossRef]
- Sakurai, S.; Namai, A.; Hashimoto, K.; Ohkoshi, S.I. First Observation of Phase Transformation of All Four Fe2O3 Phases (γ → ε → β → α-Phase). J. Am. Chem. Soc. 2009, 131, 18299–18303. [Google Scholar] [CrossRef]
- Danno, T.; Nakatsuka, D.; Kusano, Y.; Asaoka, H.; Nakanishi, M.; Fujii, T.; Ikeda, Y.; Takada, J. Crystal Structure of β-Fe2O3 and Topotactic Phase Transformation to α-Fe2O3. Cryst. Growth Des. 2013, 13, 770–774. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal Oxide-Based Supercapacitors: Progress and Prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.S.; Wang, H. Boosting the Cycling Stability of Transition Metal Compounds-Based Supercapacitors. Energy Storage Mater. 2019, 16, 545–573. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Chee, M.O.L.; Dong, P.; Ye, M.; Shen, J. Recent Advances in Micro-Supercapacitors. Nanoscale 2019, 11, 5807–5821. [Google Scholar] [CrossRef]
- Sheng, S.; Liu, W.; Zhu, K.; Cheng, K.; Ye, K.; Wang, G.; Cao, D.; Yan, J. Fe3O4 Nanospheres in Situ Decorated Graphene as High-Performance Anode for Asymmetric Supercapacitor with Impressive Energy Density. J. Colloid. Interface Sci. 2019, 536, 235–244. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, W.; Huang, Y.; Huang, Y.; Zhi, C. Proton-Insertion-Enhanced Pseudocapacitance Based on the Assembly Structure of Tungsten Oxide. ACS Appl. Mater. Interfaces 2014, 6, 18901–18910. [Google Scholar] [CrossRef]
- Zheng, F.; Gong, H.; Li, Z.; Yang, W.; Xu, J.; Hu, P.; Li, Y.; Gong, Y.; Zhen, Q. Tertiary Structure of Cactus-like WO3 Spheres Self-Assembled on Cu Foil for Supercapacitive Electrode Materials. J. Alloys Compd. 2017, 712, 345–354. [Google Scholar] [CrossRef]
- Yoon, S.; Kang, E.; Kon Kim, J.; Wee Lee, C.; Lee, J. Development of High-Performance Supercapacitor Electrodes Using Novel Ordered Mesoporous Tungsten Oxide Materials with High Electrical Conductivity. Chem. Commun. 2011, 47, 1021–1023. [Google Scholar] [CrossRef]
- Yang, P.; Sun, P.; Du, L.; Liang, Z.; Xie, W.; Cai, X.; Huang, L.; Tan, S.; Mai, W. Quantitative Analysis of Charge Storage Process of Tungsten Oxide That Combines Pseudocapacitive and Electrochromic Properties. J. Phys. Chem. C 2015, 119, 16483–16489. [Google Scholar] [CrossRef]
- Liu, X.; Sheng, G.; Zhong, M.; Zhou, X. Dispersed and Size-Selected WO3 Nanoparticles in Carbon Aerogel for Supercapacitor Applications. Mater. Des. 2018, 141, 220–229. [Google Scholar] [CrossRef]
- Jia, J.; Liu, X.; Mi, R.; Liu, N.; Xiong, Z.; Yuan, L.; Wang, C.; Sheng, G.; Cao, L.; Zhou, X.; et al. Self-Assembled Pancake-like Hexagonal Tungsten Oxide with Ordered Mesopores for Supercapacitors. J. Mater. Chem. A Mater. 2018, 6, 15330–15339. [Google Scholar] [CrossRef]
- Huang, X.; Liu, H.; Zhang, X.; Jiang, H. High Performance All-Solid-State Flexible Micro-Pseudocapacitor Based on Hierarchically Nanostructured Tungsten Trioxide Composite. ACS Appl. Mater. Interfaces 2015, 7, 27845–27852. [Google Scholar] [CrossRef]
- Shinde, P.A.; Jun, S.C. Review on Recent Progress in the Development of Tungsten Oxide Based Electrodes for Electrochemical Energy Storage. ChemSusChem 2020, 13, 11–38. [Google Scholar] [CrossRef]
- Cong, S.; Geng, F.; Zhao, Z. Tungsten Oxide Materials for Optoelectronic Applications. Adv. Mater. 2016, 28, 10518–10528. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive Oxide Materials for High-Rate Electrochemical Energy Storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Lin, J.; Du, X. High Performance Asymmetric Supercapacitor Based on Hierarchical Carbon Cloth in Situ Deposited with H-Wo3 Nanobelts as Negative Electrode and Carbon Nanotubes as Positive Electrode. Micromachines 2021, 12, 1195. [Google Scholar] [CrossRef]
- Shi, F.; Li, J.; Xiao, J.; Zhao, X.; Li, H.; An, Q.; Zhai, S.; Wang, K.; Wei, L.; Tong, Y. Three-Dimensional Hierarchical Porous Lignin-Derived Carbon/WO3 for High-Performance Solid-State Planar Micro-Supercapacitor. Int. J. Biol. Macromol. 2021, 190, 11–18. [Google Scholar] [CrossRef]
- Hepel, M.; Petrukhina, M.A.; Samuilov, V. High Power-Density WO3-x–Grafted Corannulene-Modified Graphene Nanostructures for Micro-Supercapacitors. J. Electroanal. Chem. 2023, 928, 116990. [Google Scholar] [CrossRef]
- Lillard, R.S.; Kanner, G.S.; Butt, D.P. The Nature of Oxide Films on Tungsten in Acidic and Alkaline Solutions. J. Electrochem. Soc. 1998, 145, 2718. [Google Scholar] [CrossRef]
- Anik, M.; Osseo-Asare, K. Effect of PH on the Anodic Behavior of Tungsten. J. Electrochem. Soc. 2002, 149, B224. [Google Scholar] [CrossRef]
- Di Paola, A.; Di Quarto, F.; Sunseri, C. Electrochromism in Anodically Formed Tungsten Oxide Films. J. Electrochem. Soc. 1978, 125, 1344. [Google Scholar] [CrossRef]
- El-Basiouny, M.S.; Hassan, S.A.; Hefny, M.M. On the Electrochemical Behaviour of Tungsten: The Formation and Dissolution of Tungsten Oxide in Sulphuric Acid Solutions. Corros. Sci. 1980, 20, 909–917. [Google Scholar] [CrossRef]
- Qu, Q.T.; Liu, L.L.; Wu, Y.P.; Holze, R. Electrochemical Behavior of V2O5·0.6H2O Nanoribbons in Neutral Aqueous Electrolyte Solution. Electrochim. Acta 2013, 96, 8–12. [Google Scholar] [CrossRef]
- Yan, Y.; Li, B.; Guo, W.; Pang, H.; Xue, H. Vanadium Based Materials as Electrode Materials for High Performance Supercapacitors. J. Power Sources 2016, 329, 148–169. [Google Scholar] [CrossRef]
- Lai, W.-Y.; Wei Huang, P.; Kang, J.; Zhang, Q.; Wang, Y.; Wen-Yong Lai, S.; Zhang, Y.-Z.; Wang, Y.; Cheng, T.; Yao, L.-Q.; et al. Printed Supercapacitors: Materials, Printing and Applications. Chem. Soc. Rev. 2019, 48, 3229–3264. [Google Scholar] [CrossRef]
- Huang, Z.H.; Song, Y.; Liu, X.X. Boosting Operating Voltage of Vanadium Oxide-Based Symmetric Aqueous Supercapacitor to 2 V. Chem. Eng. J. 2019, 358, 1529–1538. [Google Scholar] [CrossRef]
- Arico, C.; Ouendi, S.; Taberna, P.L.; Roussel, P.; Simon, P.; Lethien, C. Fast Electrochemical Storage Process in Sputtered Nb2O5 Porous Thin Films. ACS Nano 2019, 13, 5826–5832. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Dey, B.; Syed, A.; Bahkali, A.H.; Verma, M.; Yang, D.J.; Choudhury, A. MOFs-Derived Niobium Oxide Nanoparticles/Carbon Nanofiber Hybrid Paper as Flexible Binder-Free Electrode for Solid-State Asymmetric Supercapacitors. J. Alloys Compd. 2023, 957, 170269. [Google Scholar] [CrossRef]
- Brousse, K.; Taberna, P.L.; Simon, P. Current Collector-Free Interdigitated Nb2O5//LiFePO4 Micro-Batteries Prepared by a Simple Laser-Writing Process. J. Electrochem. Soc. 2022, 169, 070534. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, X.; Jiang, L.; Liang, M.; Zhang, X.; Wu, S.; Wu, J.; Tian, M.; Zhao, Y.; Qu, L. Laser Maskless Fast Patterning for Multitype Microsupercapacitors. Nat. Commun. 2023, 14, 3967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, B.; Brown, B. Atomic Layer Deposition of Titanium Oxide and Nitride on Vertically Aligned Carbon Nanotubes for Energy Dense 3D Microsupercapacitors. Appl. Surf. Sci. 2020, 521, 146349. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Das, S.; Rani, S.; Afshan, M.; Pahuja, M.; Jain, A.; Rani, D.; Chaudhary, N.; Jyoti; Ghosh, R.; et al. Phosphorus-Doped Nickel Oxide Micro-Supercapacitor: Unleashing the Power of Energy Storage for Miniaturized Electronic Devices. Small 2023, 20, 2306756. [Google Scholar] [CrossRef]
- Zhang, K.; Ying, G.; Liu, L.; Ma, F.; Su, L.; Zhang, C.; Wu, D.; Wang, X.; Zhou, Y. Three-Dimensional Porous Ti3C2 Tx-NiO Composite Electrodes with Enhanced Electrochemical Performance for Supercapacitors. Materials 2019, 12, 188. [Google Scholar] [CrossRef]
- Rao, Y.; Yuan, M.; Luo, F.; Li, H.; Yu, J.; Chen, X. Laser In-Situ Synthesis of Metallic Cobalt Decorated Porous Graphene for Flexible In-Plane Microsupercapacitors. J. Colloid. Interface Sci. 2022, 610, 775–784. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Wang, Y.; Shi, Y.; Wong, J.I.; Yang, H.Y. CoO Nanoflowers Woven by CNT Network for High Energy Density Flexible Micro-Supercapacitor. Nano Energy 2014, 3, 46–54. [Google Scholar] [CrossRef]
- Li, M.; Jia, C.; Zhang, D.; Luo, Y.; Ma, Y.; Luo, G.; Zhao, L.; Wang, L.; Li, Z.; Lin, Q.; et al. An Efficient Cobalt-Nickel Phosphate Positive Electrode for High-Performance Hybrid Microsupercapacitors. J. Energy Storage 2023, 64, 107144. [Google Scholar] [CrossRef]
- Mahendra, G.; Malathi, R.; Kedhareswara, S.P.; Lakshmi-Narayana, A.; Dhananjaya, M.; Guruprakash, N.; Hussain, O.M.; Mauger, A.; Julien, C.M. RF Sputter-Deposited Nanostructured CuO Films for Micro-Supercapacitors. Appl. Nano 2021, 2, 46–66. [Google Scholar] [CrossRef]
- Sayed, D.M.; Salem, K.E.; Allam, N.K. Optimized Lithography-Free Fabrication of Sub-100 Nm Nb2O5 Nanotube Films as Negative Supercapacitor Electrodes: Tuned Oxygen Vacancies and Cationic Intercalation. ACS Appl. Mater. Interfaces 2022, 14, 25545–25555. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Liu, J.; Zhao, Y.; Chen, C.; Zhao, X.; Li, J.; Jin, H. Oxygen Vacancy Boosted the Electrochemistry Performance of Ti4+ Doped Nb2O5 toward Lithium Ion Battery. Appl. Surf. Sci. 2020, 499, 143905. [Google Scholar] [CrossRef]
- Taffa, D.H.; Dillert, R.; Ulpe, A.C.; Bauerfeind, K.C.L.; Bredow, T.; Bahnemann, D.W.; Wark, M. Photoelectrochemical and Theoretical Investigations of Spinel Type Ferrites (MxFe3−xO4) for Water Splitting: A Mini-Review. J. Photonics Energy 2016, 7, 012009. [Google Scholar] [CrossRef]
- Szotek, Z.; Temmerman, W.M.; Ködderitzsch, D.; Svane, A.; Petit, L.; Winter, H. Electronic Structures of Normal and Inverse Spinel Ferrites from First Principles. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 74, 174431. [Google Scholar] [CrossRef]
- Lin, Y.P.; Wu, N.L. Characterization of MnFe2O4/LiMn2O4 Aqueous Asymmetric Supercapacitor. J. Power Sources 2011, 196, 851–854. [Google Scholar] [CrossRef]
- Zhu, B.; Tang, S.; Vongehr, S.; Xie, H.; Zhu, J.; Meng, X. FeCo2O4 Submicron-Tube Arrays Grown on Ni Foam as High Rate-Capability and Cycling-Stability Electrodes Allowing Superior Energy and Power Densities with Symmetric Supercapacitors. Chem. Commun. 2016, 52, 2624–2627. [Google Scholar] [CrossRef]
- Lannelongue, P.; Le Vot, S.; Fontaine, O.; Brousse, T.; Favier, F. Electrochemical Study of Asymmetric Aqueous Supercapacitors Based on High Density Oxides: C/Ba0.5Sr0.5Co0.8Fe0.2O3-δ and FeWO4/Ba0.5Sr0.5Co0.8Fe0.2O3-δ. Electrochim. Acta 2019, 326, 134886. [Google Scholar] [CrossRef]
- Goubard-Bretesché, N.; Crosnier, O.; Payen, C.; Favier, F.; Brousse, T. Nanocrystalline FeWO4 as a Pseudocapacitive Electrode Material for High Volumetric Energy Density Supercapacitors Operated in an Aqueous Electrolyte. Electrochem. Commun. 2015, 57, 61–64. [Google Scholar] [CrossRef]
- Goubard-Bretesché, N.; Crosnier, O.; Douard, C.; Iadecola, A.; Retoux, R.; Payen, C.; Doublet, M.L.; Kisu, K.; Iwama, E.; Naoi, K.; et al. Unveiling Pseudocapacitive Charge Storage Behavior in FeWO4 Electrode Material by Operando X-Ray Absorption Spectroscopy. Small 2020, 16, 2002855. [Google Scholar] [CrossRef]
- Xing, X.; Gui, Y.; Zhang, G.; Song, C. CoWO4 Nanoparticles Prepared by Two Methods Displaying Different Structures and Supercapacitive Performances. Electrochim. Acta 2015, 157, 15–22. [Google Scholar] [CrossRef]
- Xu, X.; Gao, J.; Huang, G.; Qiu, H.; Wang, Z.; Wu, J.; Pan, Z.; Xing, F. Fabrication of CoWO4@NiWO4 Nanocomposites with Good Supercapacitve Performances. Electrochim. Acta 2015, 174, 837–845. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Shi, W.; Zhou, J.; Gong, D.; Gu, S.; Wang, L.; Xu, Z.; Lu, B. 3D Nanoporous ZnWO4 Nanoparticles with Excellent Electrochemical Performances for Supercapacitors. Mater. Lett. 2016, 177, 34–38. [Google Scholar] [CrossRef]
- Jadhav, S.; Donolikar, P.D.; Chodankar, N.R.; Dongale, T.D.; Dubal, D.P.; Patil, D.R. Nano-Dimensional Iron Tungstate for Super High Energy Density Symmetric Supercapacitor with Redox Electrolyte. J. Solid. State Electrochem. 2019, 23, 3459–3465. [Google Scholar] [CrossRef]
- Kuo, S.-L.; Lee, J.-F.; Wu, N.-L. Study on Pseudocapacitance Mechanism of Aqueous MnFe2O4 Supercapacitor. J. Electrochem. Soc. 2007, 154, A34. [Google Scholar] [CrossRef]
- Kuo, S.L.; Wu, N.L. Electrochemical Capacitor of MnFe2O4 with NaCl Electrolyte. Electrochem. Solid-State Lett. 2005, 8, A495. [Google Scholar] [CrossRef]
- Ouendi, S.; Robert, K.; Stievenard, D.; Brousse, T.; Roussel, P.; Lethien, C. Sputtered Tungsten Nitride Films as Pseudocapacitive Electrode for on Chip Micro-Supercapacitors. Energy Storage Mater. 2019, 20, 243–252. [Google Scholar] [CrossRef]
- Arif, M.; Sanger, A.; Singh, A. Sputter Deposited Chromium Nitride Thin Electrodes for Supercapacitor Applications. Mater. Lett. 2018, 220, 213–217. [Google Scholar] [CrossRef]
- Bouhtiyya, S.; Lucio Porto, R.; Laïk, B.; Boulet, P.; Capon, F.; Pereira-Ramos, J.P.; Brousse, T.; Pierson, J.F. Application of Sputtered Ruthenium Nitride Thin Films as Electrode Material for Energy-Storage Devices. Scr. Mater. 2013, 68, 659–662. [Google Scholar] [CrossRef]
- Adalati, R.; Sharma, M.; Sharma, S.; Kumar, A.; Malik, G.; Boukherroub, R.; Chandra, R. Metal Nitrides as Efficient Electrode Material for Supercapacitors: A Review. J. Energy Storage 2022, 56, 105912. [Google Scholar] [CrossRef]
- Robert, K.; Stiévenard, D.; Deresmes, D.; Douard, C.; Iadecola, A.; Troadec, D.; Simon, P.; Nuns, N.; Marinova, M.; Huvé, M.; et al. Novel Insights into the Charge Storage Mechanism in Pseudocapacitive Vanadium Nitride Thick Films for High-Performance on-Chip Micro-Supercapacitors. Energy Environ. Sci. 2020, 13, 949–957. [Google Scholar] [CrossRef]
- Lucio-Porto, R.; Bouhtiyya, S.; Pierson, J.F.; Morel, A.; Capon, F.; Boulet, P.; Brousse, T. VN Thin Films as Electrode Materials for Electrochemical Capacitors. Electrochim. Acta 2014, 141, 203–211. [Google Scholar] [CrossRef]
- Bondarchuk, O.; Morel, A.; Bélanger, D.; Goikolea, E.; Brousse, T.; Mysyk, R. Thin Films of Pure Vanadium Nitride: Evidence for Anomalous Non-Faradaic Capacitance. J. Power Sources 2016, 324, 439–446. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, W.; Li, T. A Review on Transition Metal Nitrides as Electrode Materials for Supercapacitors. Ceram. Int. 2019, 45, 21062–21076. [Google Scholar] [CrossRef]
- Durai, G.; Kuppusami, P.; Maiyalagan, T.; Theerthagiri, J.; Vinoth Kumar, P.; Kim, H.S. Influence of Chromium Content on Microstructural and Electrochemical Supercapacitive Properties of Vanadium Nitride Thin Films Developed by Reactive Magnetron Co-Sputtering Process. Ceram. Int. 2019, 45, 12643–12653. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Zhang, J.; Tian, W.; Wang, L.; Ren, Z.; Ren, X.; Li, X.; Gao, B.; Peng, X.; et al. Spatially Confined Synthesis of Vanadium Nitride Nanodots Intercalated Carbon Nanosheets with Ultrahigh Volumetric Capacitance and Long Life for Flexible Supercapacitors. Nano Energy 2018, 51, 128–136. [Google Scholar] [CrossRef]
- Chen, L.; Liu, C.; Zhang, Z. Novel [111] Oriented γ-Mo2N Thin Films Deposited by Magnetron Sputtering as an Anode for Aqueous Micro-Supercapacitors. Electrochim. Acta 2017, 245, 237–248. [Google Scholar] [CrossRef]
- Dinh, K.H.; Robert, K.; Thuriot-Roukos, J.; Huvé, M.; Simon, P.; Troadec, D.; Lethien, C.; Roussel, P. Wafer-Scale Performance Mapping of Magnetron-Sputtered Ternary Vanadium Tungsten Nitride for Microsupercapacitors. Chem. Mater. 2023, 35, 8654–8663. [Google Scholar] [CrossRef]
- Wei, B.; Liang, H.; Zhang, D.; Qi, Z.; Shen, H.; Wang, Z. Magnetron Sputtered TiN Thin Films toward Enhanced Performance Supercapacitor Electrodes. Mater. Renew. Sustain. Energy 2018, 7, 11. [Google Scholar] [CrossRef]
- Achour, A.; Porto, R.L.; Soussou, M.A.; Islam, M.; Boujtita, M.; Aissa, K.A.; Le Brizoual, L.; Djouadi, A.; Brousse, T. Titanium Nitride Films for Micro-Supercapacitors: Effect of Surface Chemistry and Film Morphology on the Capacitance. J. Power Sources 2015, 300, 525–532. [Google Scholar] [CrossRef]
- Achour, A.; Lucio-Porto, R.; Chaker, M.; Arman, A.; Ahmadpourian, A.; Soussou, M.A.; Boujtita, M.; Le Brizoual, L.; Djouadi, M.A.; Brousse, T. Titanium Vanadium Nitride Electrode for Micro-Supercapacitors. Electrochem. Commun. 2017, 77, 40–43. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, G.; Liu, X.; Liu, F.; Xie, Y.; Yang, C.; Lin, T.; Gu, H.; Huang, F. Niobium Nitride Nb4N5 as a New High-Performance Electrode Material for Supercapacitors. Adv. Sci. 2015, 2, 1500126. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Liang, H.; Zhang, D.; Wu, Z.; Qi, Z.; Wang, Z. CrN Thin Films Prepared by Reactive DC Magnetron Sputtering for Symmetric Supercapacitors. J. Mater. Chem. A Mater. 2017, 5, 2844–2851. [Google Scholar] [CrossRef]
- Qi, Z.; Wei, B.; Wang, J.; Yang, Y.; Wang, Z. Nanostructured Porous CrN Thin Films by Oblique Angle Magnetron Sputtering for Symmetric Supercapacitors. J. Alloys Compd. 2019, 806, 953–959. [Google Scholar] [CrossRef]
- Wei, B.; Mei, G.; Liang, H.; Qi, Z.; Zhang, D.; Shen, H.; Wang, Z. Porous CrN Thin Films by Selectively Etching CrCuN for Symmetric Supercapacitors. J. Power Sources 2018, 385, 39–44. [Google Scholar] [CrossRef]
- Durai, G.; Kuppusami, P.; Maiyalagan, T.; Ahila, M.; Vinoth kumar, P. Supercapacitive Properties of Manganese Nitride Thin Film Electrodes Prepared by Reactive Magnetron Sputtering: Effect of Different Electrolytes. Ceram. Int. 2019, 45, 17120–17127. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, F. Capacitive Performance of Molybdenum Nitride/Titanium Nitride Nanotube Array for Supercapacitor. Mater. Sci. Eng. B 2017, 215, 64–70. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, W.; Wei, B.; Zheng, J.; Qi, Z.; Wang, Z. Freestanding Co3N Thin Film for High Performance Supercapacitors. Ceram. Int. 2021, 47, 3267–3271. [Google Scholar] [CrossRef]
- Achour, A.; Ducros, J.B.; Porto, R.L.; Boujtita, M.; Gautron, E.; Le Brizoual, L.; Djouadi, M.A.; Brousse, T. Hierarchical Nanocomposite Electrodes Based on Titanium Nitride and Carbon Nanotubes for Micro-Supercapacitors. Nano Energy 2014, 7, 104–113. [Google Scholar] [CrossRef]
- Salman, A.; Padmajan Sasikala, S.; Kim, I.H.; Kim, J.T.; Lee, G.S.; Kim, J.G.; Kim, S.O. Tungsten Nitride-Coated Graphene Fibers for High-Performance Wearable Supercapacitors. Nanoscale 2020, 12, 20239–20249. [Google Scholar] [CrossRef]
- Zhang, W.-B.; Ma, X.-J.; Kong, L.-B.; Luo, Y.-C.; Kang, L. Capacitive Intermetallic Manganese Nitride with High Volumetric Energy Densities. J. Electrochem. Soc. 2016, 163, A2830. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, D.; Liu, W.; Shi, S.; Tian, Z.; Liu, F.; Li, S.; Wang, J.; Ali, F. Tailoring Surface Chemistry and Morphology of Titanium Nitride Electrode for On-Chip Supercapacitors. ACS Sustain. Chem. Eng. 2020, 8, 7869–7878. [Google Scholar] [CrossRef]
- Lv, F.; Ma, H.; Shen, L.; Jiang, Y.; Sun, T.; Ma, J.; Geng, X.; Kiran, A.; Zhu, N. Wearable Helical Molybdenum Nitride Supercapacitors for Self-Powered Healthcare Smartsensors. ACS Appl. Mater. Interfaces 2021, 13, 29780–29787. [Google Scholar] [CrossRef]
- Shen, H.; Wei, B.; Zhang, D.; Qi, Z.; Wang, Z. Magnetron Sputtered NbN Thin Film Electrodes for Supercapacitors. Mater. Lett. 2018, 229, 17–20. [Google Scholar] [CrossRef]
- Gao, Z.; Wu, Z.; Zhao, S.; Zhang, T.; Wang, Q. Enhanced Capacitive Property of HfN Film Electrode by Plasma Etching for Supercapacitors. Mater. Lett. 2019, 235, 148–152. [Google Scholar] [CrossRef]
- Anusha Thampi, V.V.; Nithiyanantham, U.; Nanda Kumar, A.K.; Martin, P.; Bendavid, A.; Subramanian, B. Fabrication of Sputtered Titanium Vanadium Nitride (TiVN) Thin Films for Micro-Supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 12457–12465. [Google Scholar] [CrossRef]
- Yi, C.-Q.; Zou, J.-P.; Yang, H.-Z.; Leng, X. Recent Advances in Pseudocapacitor Electrode Materials: Transition Metal Oxides and Nitrides. Trans. Nonferrous Met. Soc. China (Engl. Ed.) 2018, 28, 1980–2001. [Google Scholar] [CrossRef]
- Zhao, J.; Li, C.; Zhang, Q.; Zhang, J.; Wang, X.; Lin, Z.; Wang, J.; Lv, W.; Lu, C.; Wong, C.P.; et al. An All-Solid-State, Lightweight, and Flexible Asymmetric Supercapacitor Based on Cabbage-like ZnCo2O4 and Porous VN Nanowires Electrode Materials. J. Mater. Chem. A Mater. 2017, 5, 6928–6936. [Google Scholar] [CrossRef]
- Wei, B.; Shang, C.; Shui, L.; Wang, X.; Zhou, G. TiVN Composite Hollow Mesospheres for High-Performance Supercapacitors. Mater. Res. Express 2019, 6, 025801. [Google Scholar] [CrossRef]
- Su, Y.; Zhitomirsky, I. Hybrid MnO2/Carbon Nanotube-VN/Carbon Nanotube Supercapacitors. J. Power Sources 2014, 267, 235–242. [Google Scholar] [CrossRef]
- Wei, B.; Ming, F.; Liang, H.; Qi, Z.; Hu, W.; Wang, Z. All Nitride Asymmetric Supercapacitors of Niobium Titanium Nitride-Vanadium Nitride. J. Power Sources 2021, 481, 228842. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, S.; Cao, Y.; Zhu, Y.; Das, P.; Wang, H.; Liu, Y.; Wang, J.; Chi, L.; Liu, S.; et al. Aqueous MXene/PH1000 Hybrid Inks for Inkjet-Printing Micro-Supercapacitors with Unprecedented Volumetric Capacitance and Modular Self-Powered Microelectronics. Adv. Energy Mater. 2021, 11, 2100746. [Google Scholar] [CrossRef]
- Zheng, S.; Wang, H.; Das, P.; Zhang, Y.; Cao, Y.; Ma, J.; Liu, S.; Wu, Z.S. Multitasking MXene Inks Enable High-Performance Printable Microelectrochemical Energy Storage Devices for All-Flexible Self-Powered Integrated Systems. Adv. Mater. 2021, 33, 2005449. [Google Scholar] [CrossRef] [PubMed]
- Orangi, J.; Hamade, F.; Davis, V.A.; Beidaghi, M. 3D Printing of Additive-Free 2D Ti3C2Tx (MXene) Ink for Fabrication of Micro-Supercapacitors with Ultra-High Energy Densities. ACS Nano 2020, 14, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Liang, J.; Chen, Y. Hydrous RuO2-Decorated MXene Coordinating with Silver Nanowire Inks Enabling Fully Printed Micro-Supercapacitors with Extraordinary Volumetric Performance. Adv. Energy Mater. 2019, 9, 1803987. [Google Scholar] [CrossRef]
- Jiang, Q.; Lei, Y.; Liang, H.; Xi, K.; Xia, C.; Alshareef, H.N. Review of MXene Electrochemical Microsupercapacitors. Energy Storage Mater. 2020, 27, 78–95. [Google Scholar] [CrossRef]
- Salles, P.; Quain, E.; Kurra, N.; Sarycheva, A.; Gogotsi, Y. Automated Scalpel Patterning of Solution Processed Thin Films for Fabrication of Transparent MXene Microsupercapacitors. Small 2018, 14, e1802864. [Google Scholar] [CrossRef]
- Hu, H.; Bai, Z.; Niu, B.; Wu, M.; Hua, T. Binder-Free Bonding of Modularized MXene Thin Films into Thick Film Electrodes for on-Chip Micro-Supercapacitors with Enhanced Areal Performance Metrics. J. Mater. Chem. A Mater. 2018, 6, 14876–14884. [Google Scholar] [CrossRef]
- Biswas, A.; Natu, V.; Puthirath, A.B. Thin-Film Growth of MAX Phases as Functional Materials. Oxf. Open Mater. Sci. 2021, 1, itab020. [Google Scholar] [CrossRef]
- Ling, Z.; Ren, C.E.; Zhao, M.Q.; Yang, J.; Giammarco, J.M.; Qiu, J.; Barsoum, M.W.; Gogotsi, Y. Flexible and Conductive MXene Films and Nanocomposites with High Capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.L.; Barsoum, M.W.; Simon, P.; et al. Ultra-High-Rate Pseudocapacitive Energy Storage in Two-Dimensional Transition Metal Carbides. Nat. Energy 2017, 6, 17105. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Bak, S.M.; Yu, X.; Yang, X.Q.; Barsoum, M.W.; Gogotsi, Y. Probing the Mechanism of High Capacitance in 2D Titanium Carbide Using in Situ X-Ray Absorption Spectroscopy. Adv. Energy Mater. 2015, 5, 1500589. [Google Scholar] [CrossRef]
- Peng, Y.Y.; Akuzum, B.; Kurra, N.; Zhao, M.Q.; Alhabeb, M.; Anasori, B.; Kumbur, E.C.; Alshareef, H.N.; Ger, M.-D.; Gogotsi, Y. All-MXene (2D Titanium Carbide) Solid-State Microsupercapacitors for on-Chip Energy Storage. Energy Environ. Sci. 2016, 9, 2847–2854. [Google Scholar] [CrossRef]
- Jiao, S.; Zhou, A.; Wu, M.; Hu, H. Kirigami Patterning of MXene/Bacterial Cellulose Composite Paper for All-Solid-State Stretchable Micro-Supercapacitor Arrays. Adv. Sci. 2019, 6, 1900529. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Wang, L.; Liu, X.; Du, X.; Zhang, G.; Chang, Y.; Xia, Q.; Hu, Q.; Zhou, A. 3D Printing Quasi-Solid-State Micro-Supercapacitors with Ultrahigh Areal Energy Density Based on High Concentration MXene Sediment. Chem. Eng. J. 2023, 451, 138686. [Google Scholar] [CrossRef]
- Zhang, C.J.; Kremer, M.P.; Seral-Ascaso, A.; Park, S.H.; McEvoy, N.; Anasori, B.; Gogotsi, Y.; Nicolosi, V. Stamping of Flexible, Coplanar Micro-Supercapacitors Using MXene Inks. Adv. Funct. Mater. 2018, 28, 1705506. [Google Scholar] [CrossRef]
- Li, H.; Hou, Y.; Wang, F.; Lohe, M.R.; Zhuang, X.; Niu, L.; Feng, X.; Li, H.; Hou, Y.; Wang, F.; et al. Flexible All-Solid-State Supercapacitors with High Volumetric Capacitances Boosted by Solution Processable MXene and Electrochemically Exfoliated Graphene. Adv. Energy Mater. 2017, 7, 1601847. [Google Scholar] [CrossRef]
- Hu, H.; Hua, T. An Easily Manipulated Protocol for Patterning of MXenes on Paper for Planar Micro-Supercapacitors. J. Mater. Chem. A Mater. 2017, 5, 19639–19648. [Google Scholar] [CrossRef]
- Huang, H.; Su, H.; Zhang, H.; Xu, L.; Chu, X.; Hu, C.; Liu, H.; Chen, N.; Liu, F.; Deng, W.; et al. Extraordinary Areal and Volumetric Performance of Flexible Solid-State Micro-Supercapacitors Based on Highly Conductive Freestanding Ti3C2Tx Films. Adv. Electron. Mater. 2018, 4, 1800179. [Google Scholar] [CrossRef]
- Li, P.; Shi, W.; Liu, W.; Chen, Y.; Xu, X.; Ye, S.; Yin, R.; Zhang, L.; Xu, L.; Cao, X. Fabrication of High-Performance MXene-Based All-Solid-State Flexible Microsupercapacitor Based on a Facile Scratch Method. Nanotechnology 2018, 29, 445401. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, R.; Zhang, H.; Cheng, K. Aqueous MXene Inks for Inkjet-Printing Microsupercapacitors with Ultrahigh Energy Densities. J. Colloid. Interface Sci. 2023, 645, 359–370. [Google Scholar] [CrossRef]
- Jiang, Q.; Kurra, N.; Maleski, K.; Lei, Y.; Liang, H.; Zhang, Y.; Gogotsi, Y.; Alshareef, H.N. On-Chip MXene Microsupercapacitors for AC-Line Filtering Applications. Adv. Energy Mater. 2019, 9, 1901061. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Q.; Ma, J.; Das, P.; Zhang, L.; Liu, H.; Wang, S.; Li, H.; Wu, Z.S. Three-Dimensional (3D)-Printed MXene High-Voltage Aqueous Micro-Supercapacitors with Ultrahigh Areal Energy Density and Low-Temperature Tolerance. Carbon Energy 2024, 6, e481. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Xie, Z.; Wang, D.; Yuan, Y.; Kang, H.; Su, B.; Cheng, Z.; Liu, Y. Modified MXene/Holey Graphene Films for Advanced Supercapacitor Electrodes with Superior Energy Storage. Adv. Sci. 2018, 5, 1800750. [Google Scholar] [CrossRef]
- Kim, E.; Lee, B.J.; Maleski, K.; Chae, Y.; Lee, Y.; Gogotsi, Y.; Ahn, C.W. Microsupercapacitor with a 500 Nm Gap between MXene/CNT Electrodes. Nano Energy 2021, 81, 105616. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Su, Y.; Li, Q.; Zhao, Z.; Geng, F. Molecularly Stacking Manganese Dioxide/Titanium Carbide Sheets to Produce Highly Flexible and Conductive Film Electrodes with Improved Pseudocapacitive Performances. Adv. Energy Mater. 2017, 7, 1602834. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, C.; Que, W.; Liu, X.; Yin, X.; Kong, L.B. Flexible and Free-Standing 2D Titanium Carbide Film Decorated with Manganese Oxide Nanoparticles as a High Volumetric Capacity Electrode for Supercapacitor. J. Power Sources 2017, 359, 332–339. [Google Scholar] [CrossRef]
- Zhao, M.-Q.; Ren, C.E.; Ling, Z.; Lukatskaya, M.R.; Zhang, C.; Van Aken, K.L.; Barsoum, M.W.; Gogotsi, Y.; Zhao, M.; Ren, C.E.; et al. Flexible MXene/Carbon Nanotube Composite Paper with High Volumetric Capacitance. Adv. Mater. 2015, 27, 339–345. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/Graphene Films for Ultrafast Supercapacitors with Outstanding Volumetric Capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Qin, L.; Tao, Q.; Liu, X.; Fahlman, M.; Halim, J.; Persson, P.O.Å.; Rosen, J.; Zhang, F. Polymer-MXene Composite Films Formed by MXene-Facilitated Electrochemical Polymerization for Flexible Solid-State Microsupercapacitors. Nano Energy 2019, 60, 734–742. [Google Scholar] [CrossRef]
- Zhang, C.J.; McKeon, L.; Kremer, M.P.; Park, S.H.; Ronan, O.; Seral-Ascaso, A.; Barwich, S.; Coileáin, C.; McEvoy, N.; Nerl, H.C.; et al. Additive-Free MXene Inks and Direct Printing of Micro-Supercapacitors. Nat. Commun. 2019, 10, 1795. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.Z.; Dubbink, D.; ten Elshof, J.E. Inkjet Printing of δ-MnO2 Nanosheets for Flexible Solid-State Micro-Supercapacitor. Nano Energy 2018, 49, 481–488. [Google Scholar] [CrossRef]
- Li, L.; Secor, E.B.; Chen, K.S.; Zhu, J.; Liu, X.; Gao, T.Z.; Seo, J.W.T.; Zhao, Y.; Hersam, M.C. High-Performance Solid-State Supercapacitors and Microsupercapacitors Derived from Printable Graphene Inks. Adv. Energy Mater. 2016, 6, 1600909. [Google Scholar] [CrossRef]
- Van Toan, N.; Kim Tuoi, T.T.; Li, J.; Inomata, N.; Ono, T. Liquid and Solid States On-Chip Micro-Supercapacitors Using Silicon Nanowire-Graphene Nanowall-Pani Electrode Based on Microfabrication Technology. Mater. Res. Bull. 2020, 131, 110977. [Google Scholar] [CrossRef]
- Wang, K.; Zou, W.; Quan, B.; Yu, A.; Wu, H.; Jiang, P.; Wei, Z. An All-Solid-State Flexible Micro-Supercapacitor on a Chip. Adv. Energy Mater. 2011, 1, 1068–1072. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Pal, B.; Yang, S.; Ramesh, S.; Thangadurai, V.; Jose, R. Electrolyte Selection for Supercapacitive Devices: A Critical Review. Nanoscale Adv. 2019, 1, 3807–3835. [Google Scholar] [CrossRef]
- Mendhe, A.; Panda, H.S. Discover Materials A Review on Electrolytes for Supercapacitor Device. Discov Mater 2023, 3, 29. [Google Scholar] [CrossRef]
- Yu, L.; Chen, G.Z. Ionic Liquid-Based Electrolytes for Supercapacitor and Supercapattery. Front. Chem. 2019, 7, 272. [Google Scholar] [CrossRef]
- Bhat, M.Y.; Hashmi, S.A.; Khan, M.; Choi, D.; Qurashi, A. Frontiers and Recent Developments on Supercapacitor’s Materials, Design, and Applications: Transport and Power System Applications. J. Energy Storage 2023, 58, 106104. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; Cheng, L.; Li, H.; Wang, B.; Zhao, X.S.; Guo, P. Electrochemical Properties of Manganese Ferrite-Based Supercapacitors in Aqueous Electrolyte: The Effect of Ionic Radius. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 94–99. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Wang, J.; Lin, J.; Sun, X.; Mao, S. The Effect of Various Electrolyte Cations on Electrochemical Performance of Polypyrrole/RGO Based Supercapacitors. Phys. Chem. Chem. Phys. 2015, 17, 28666–28673. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, X.; Jiang, L.; Wu, C.; Zhao, Q.; Liu, X.; Hu, B.; Yi, L. The Effects of Electrolyte on the Supercapacitive Performance of Activated Calcium Carbide-Derived Carbon. J. Power Sources 2013, 226, 202–209. [Google Scholar] [CrossRef]
- Lim, Y.; Yoon, J.; Yun, J.; Kim, D.; Hong, S.Y.; Lee, S.J.; Zi, G.; Ha, J.S. Erratum: Biaxially Stretchable, Integrated Array of High Performance Microsupercapacitors. ACS Nano 2014, 8, 11639–11650, Erratum in ACS Nano 2015, 9, 6634. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-Liquid Materials for the Electrochemical Challenges of the Future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Liew, C.W.; Arifin, K.H.; Kawamura, J.; Iwai, Y.; Ramesh, S.; Arof, A.K. Effect of Halide Anions in Ionic Liquid Added Poly(Vinyl Alcohol)-Based Ion Conductors for Electrical Double Layer Capacitors. J. Non Cryst. Solids 2017, 458, 97–106. [Google Scholar] [CrossRef]
- Rennie, A.J.R.; Sanchez-Ramirez, N.; Torresi, R.M.; Hall, P.J. Ether-Bond-Containing Ionic Liquids as Supercapacitor Electrolytes. J. Phys. Chem. Lett. 2013, 4, 2970–2974. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, S.; Olyschläger, T.; Goodrich, P.; Alvarez Vicente, J.; Jacquemin, J.; Balducci, A. Azepanium-Based Ionic Liquids as Green Electrolytes for High Voltage Supercapacitors. J. Power Sources 2015, 273, 931–936. [Google Scholar] [CrossRef]
- Timperman, L.; Skowron, P.; Boisset, A.; Galiano, H.; Lemordant, D.; Frackowiak, E.; Béguin, F.; Anouti, M. Triethylammonium Bis(Tetrafluoromethylsulfonyl)Amide Protic Ionic Liquid as an Electrolyte for Electrical Double-Layer Capacitors. Phys. Chem. Chem. Phys. 2012, 14, 8199–8207. [Google Scholar] [CrossRef]
- Kurig, H.; Vestli, M.; Tõnurist, K.; Jänes, A.; Lust, E. Influence of Room Temperature Ionic Liquid Anion Chemical Composition and Electrical Charge Delocalization on the Supercapacitor Properties. J. Electrochem. Soc. 2012, 159, A944. [Google Scholar] [CrossRef]
- Łatoszyńska, A.A.; Zukowska, G.Z.; Rutkowska, I.A.; Taberna, P.L.; Simon, P.; Kulesza, P.J.; Wieczorek, W. Non-Aqueous Gel Polymer Electrolyte with Phosphoric Acid Ester and Its Application for Quasi Solid-State Supercapacitors. J. Power Sources 2015, 274, 1147–1154. [Google Scholar] [CrossRef]
- Francisco, B.E.; Jones, C.M.; Lee, S.H.; Stoldt, C.R. Nanostructured All-Solid-State Supercapacitor Based on Li2S-P2S5 Glass-Ceramic Electrolyte. Appl. Phys. Lett. 2012, 100, 103902. [Google Scholar] [CrossRef]
- Chong, M.Y.; Numan, A.; Liew, C.W.; Ng, H.M.; Ramesh, K.; Ramesh, S. Enhancing the Performance of Green Solid-State Electric Double-Layer Capacitor Incorporated with Fumed Silica Nanoparticles. J. Phys. Chem. Solids 2018, 117, 194–203. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem. -Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Minakshi, M.; Singh, N.K. Synthesis and Characterization of Solid Polymer Electrolyte Based on Activated Carbon for Solid State Capacitor. Electrochim. Acta 2014, 137, 497–503. [Google Scholar] [CrossRef]
- Pal, B.; Yasin, A.; Kunwar, R.; Yang, S.; Yusoff, M.M.; Jose, R. Polymer versus Cation of Gel Polymer Electrolytes in the Charge Storage of Asymmetric Supercapacitors. Ind. Eng. Chem. Res. 2019, 58, 654–664. [Google Scholar] [CrossRef]

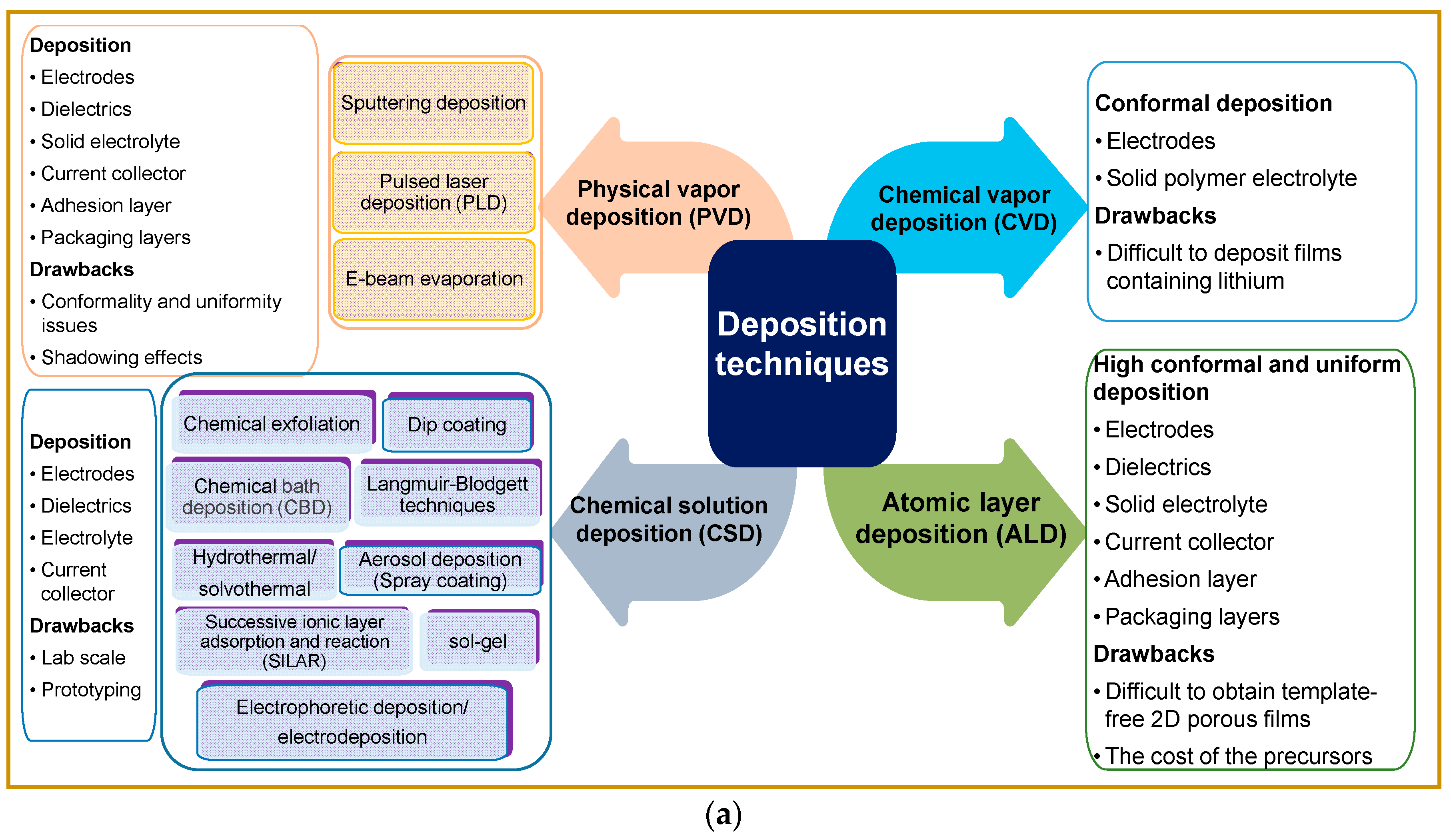

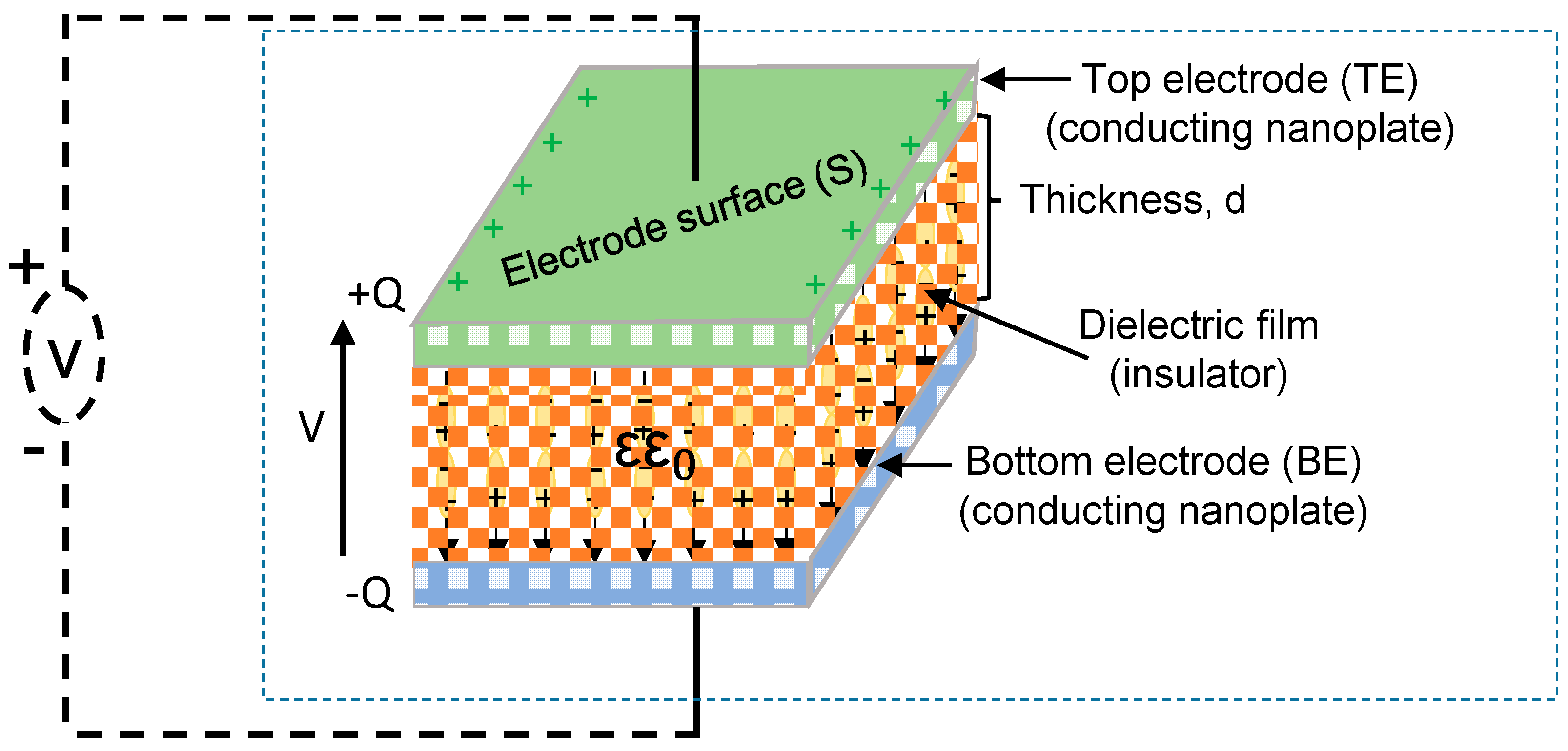
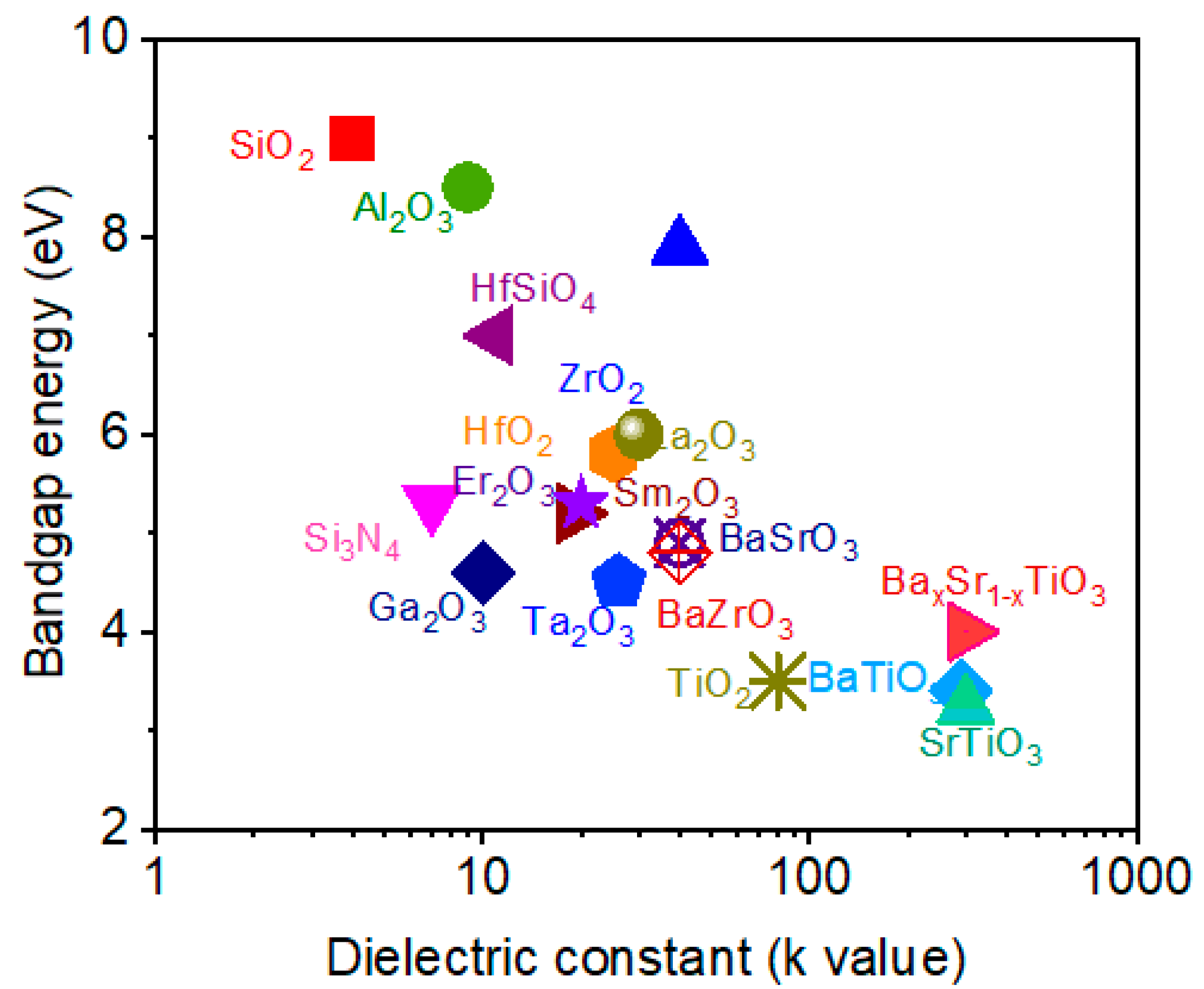

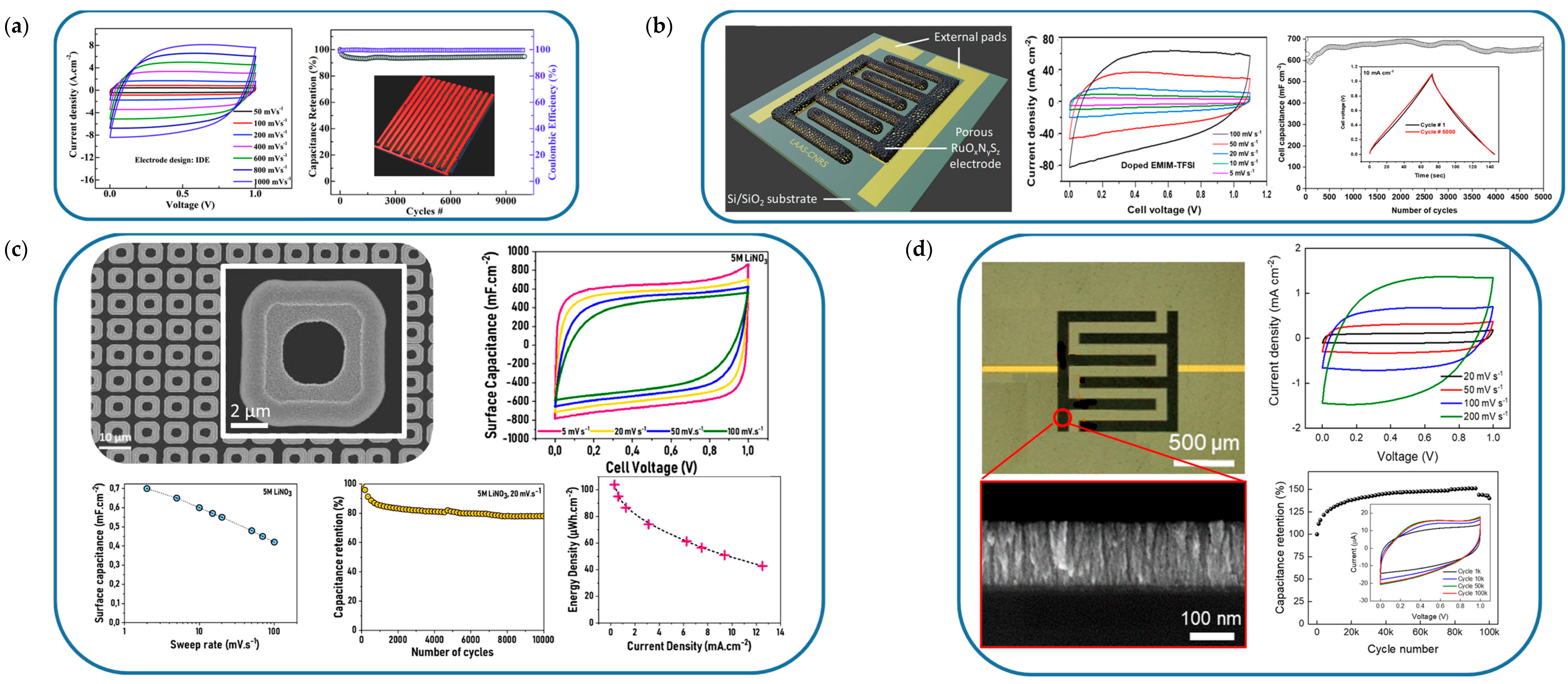
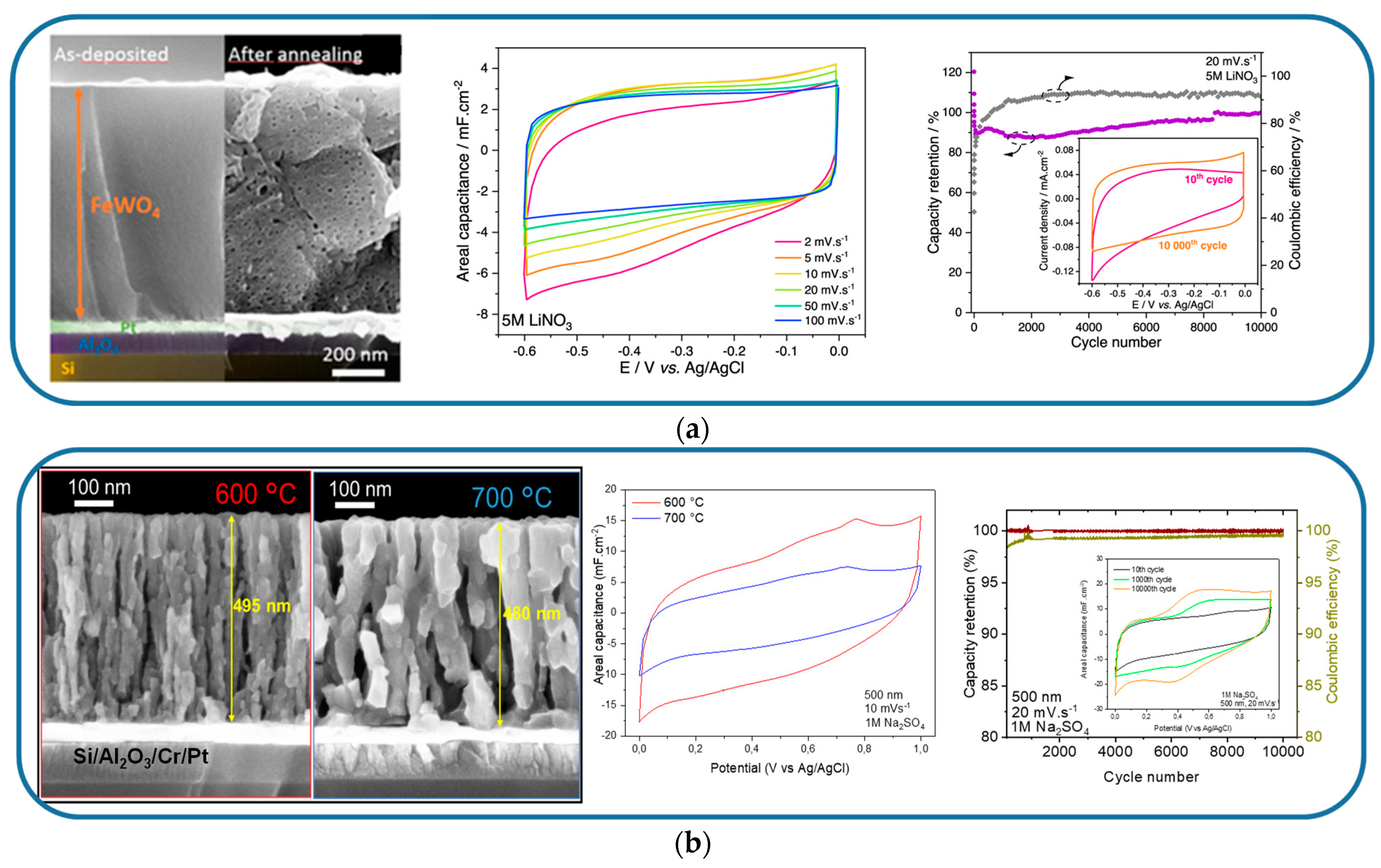

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jolayemi, B.; Buvat, G.; Roussel, P.; Lethien, C. Emerging Capacitive Materials for On-Chip Electronics Energy Storage Technologies. Batteries 2024, 10, 317. https://doi.org/10.3390/batteries10090317
Jolayemi B, Buvat G, Roussel P, Lethien C. Emerging Capacitive Materials for On-Chip Electronics Energy Storage Technologies. Batteries. 2024; 10(9):317. https://doi.org/10.3390/batteries10090317
Chicago/Turabian StyleJolayemi, Bukola, Gaetan Buvat, Pascal Roussel, and Christophe Lethien. 2024. "Emerging Capacitive Materials for On-Chip Electronics Energy Storage Technologies" Batteries 10, no. 9: 317. https://doi.org/10.3390/batteries10090317
APA StyleJolayemi, B., Buvat, G., Roussel, P., & Lethien, C. (2024). Emerging Capacitive Materials for On-Chip Electronics Energy Storage Technologies. Batteries, 10(9), 317. https://doi.org/10.3390/batteries10090317







