Quantifying the Aging of Lithium-Ion Pouch Cells Using Pressure Sensors
Abstract
:1. Introduction
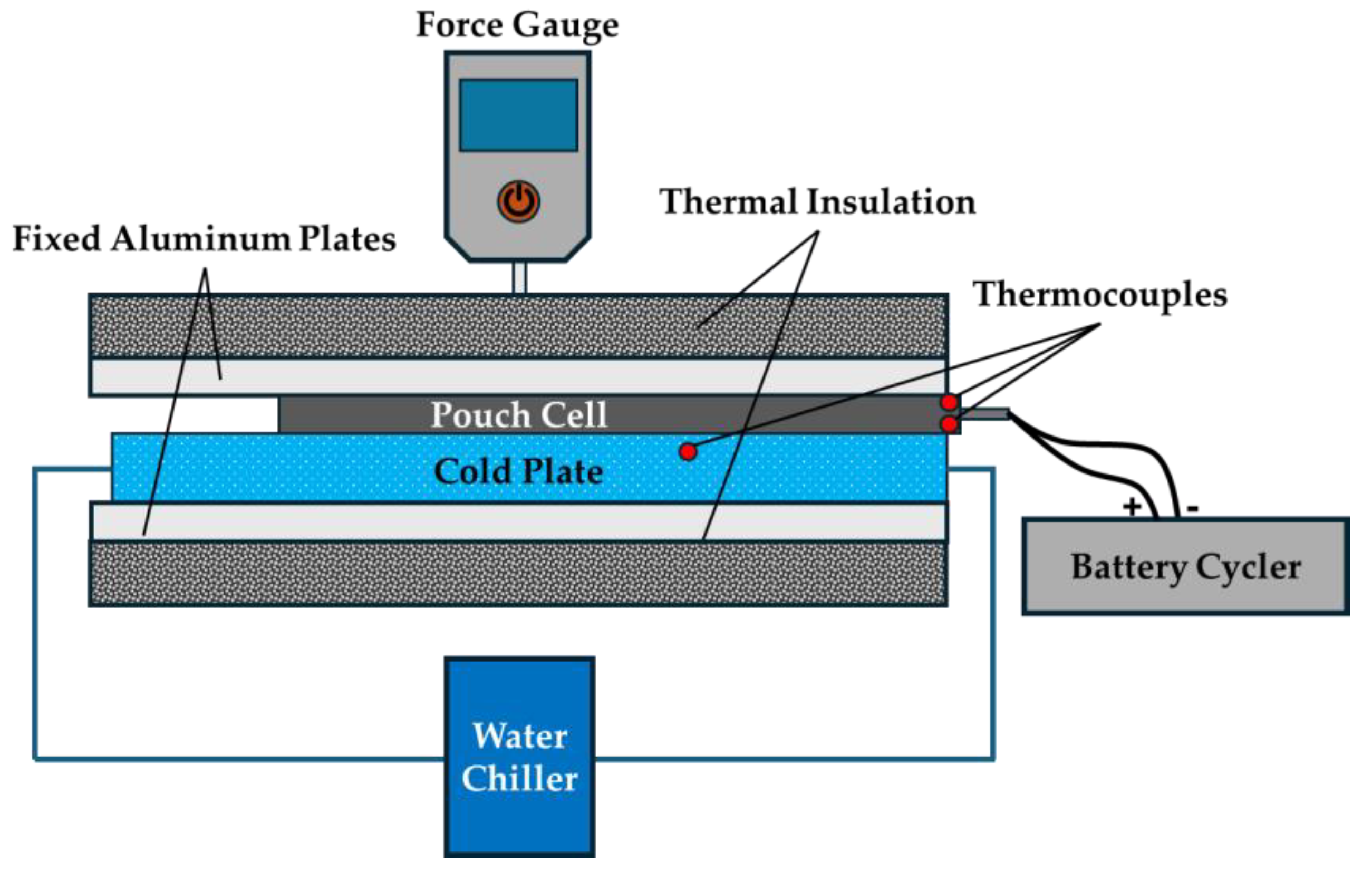
2. Materials and Methods
3. Results and Discussion
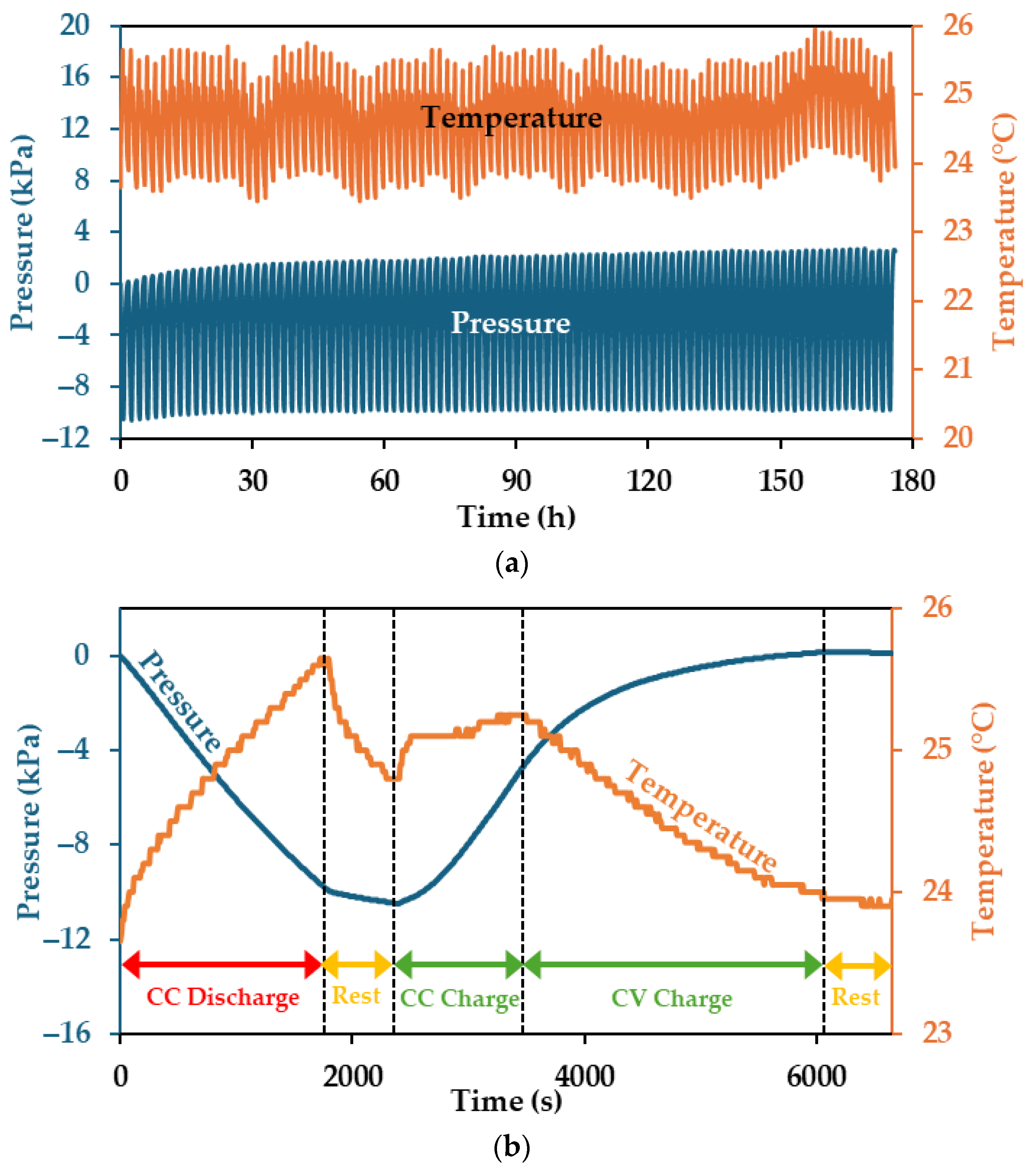
3.1. Pressure and Temperature Trends
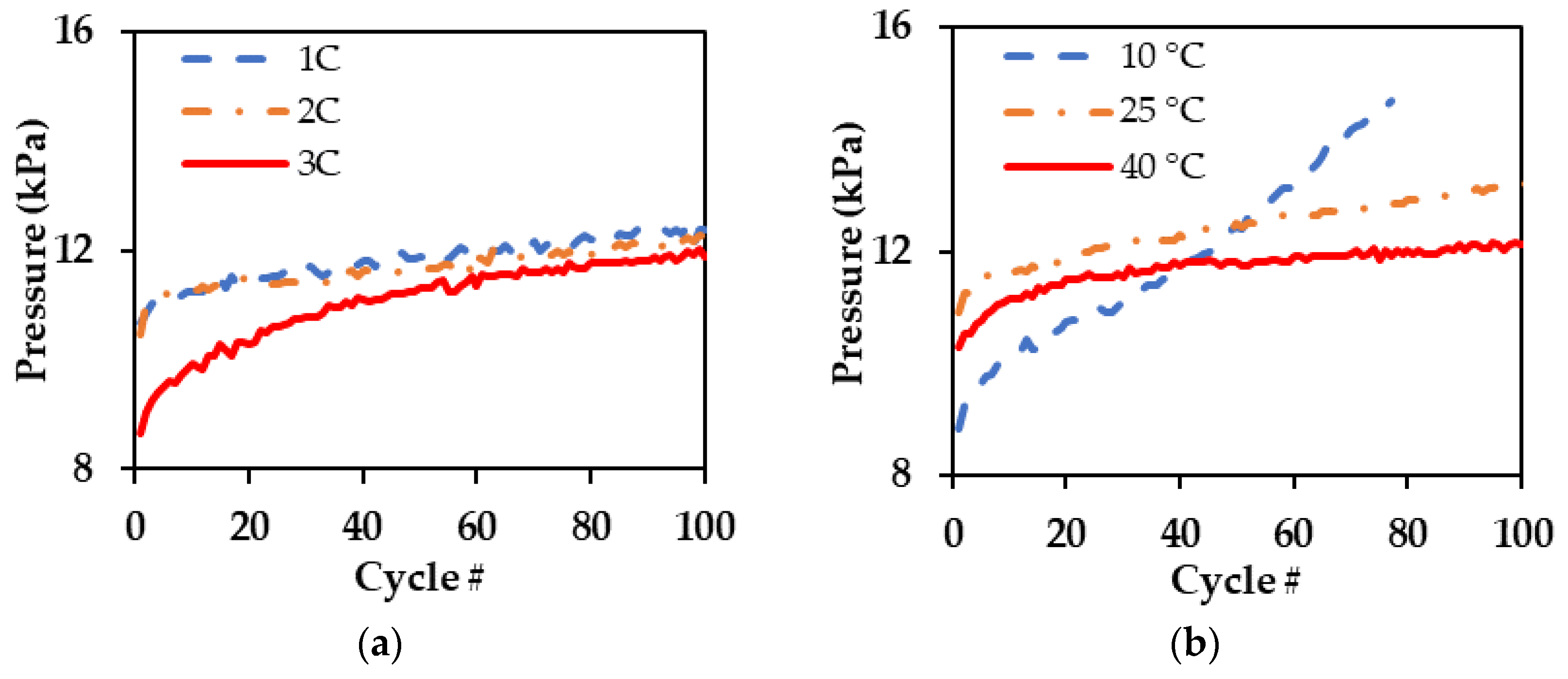
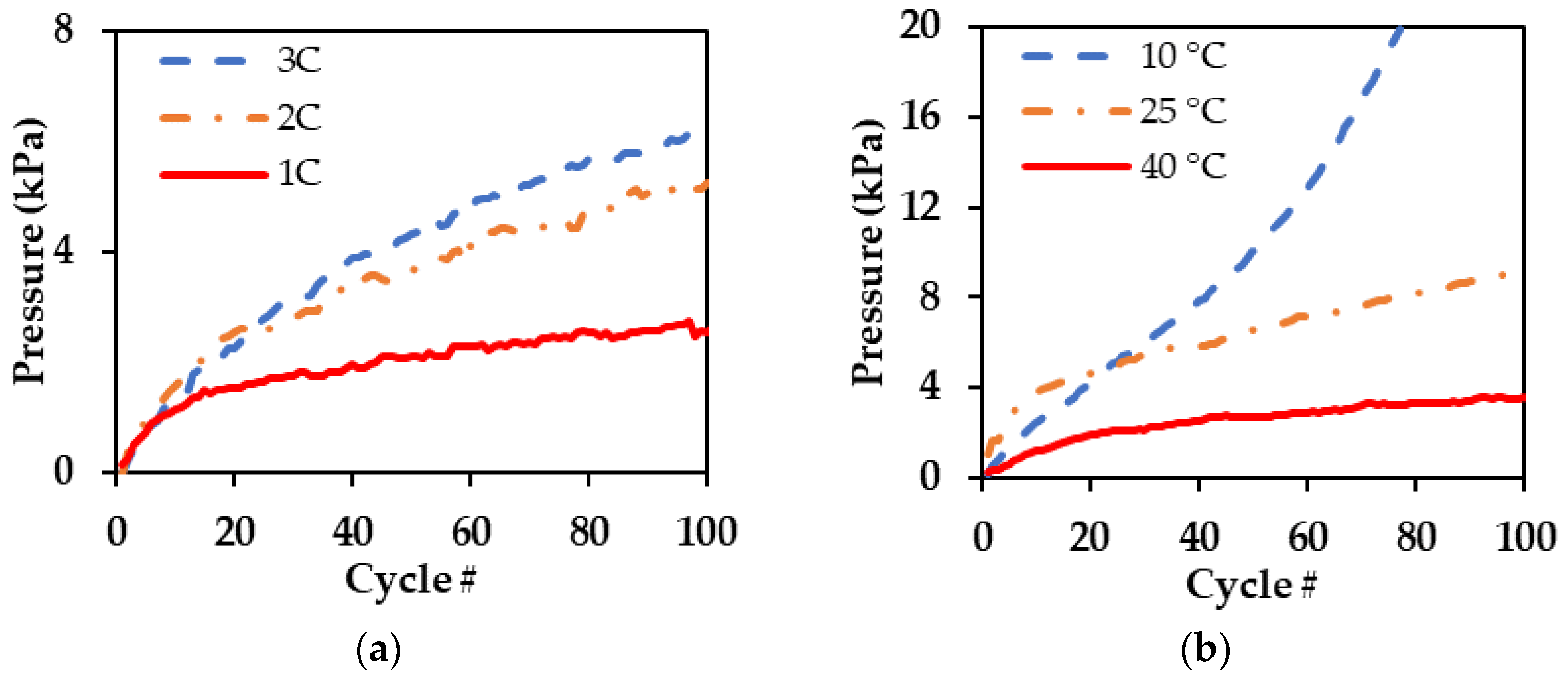
3.2. Pressure Components Analysis
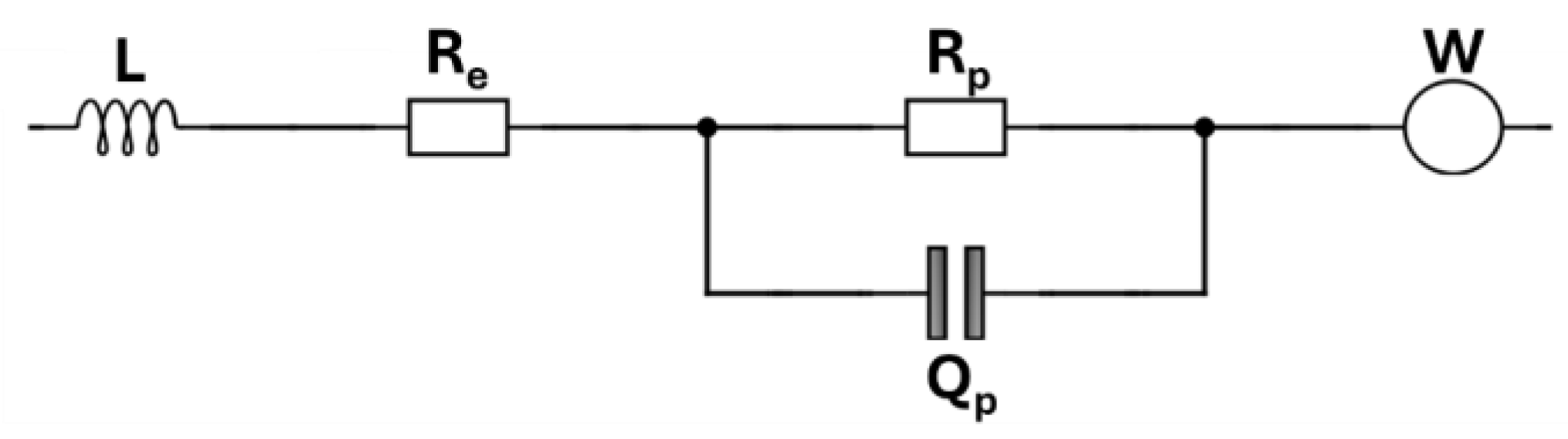
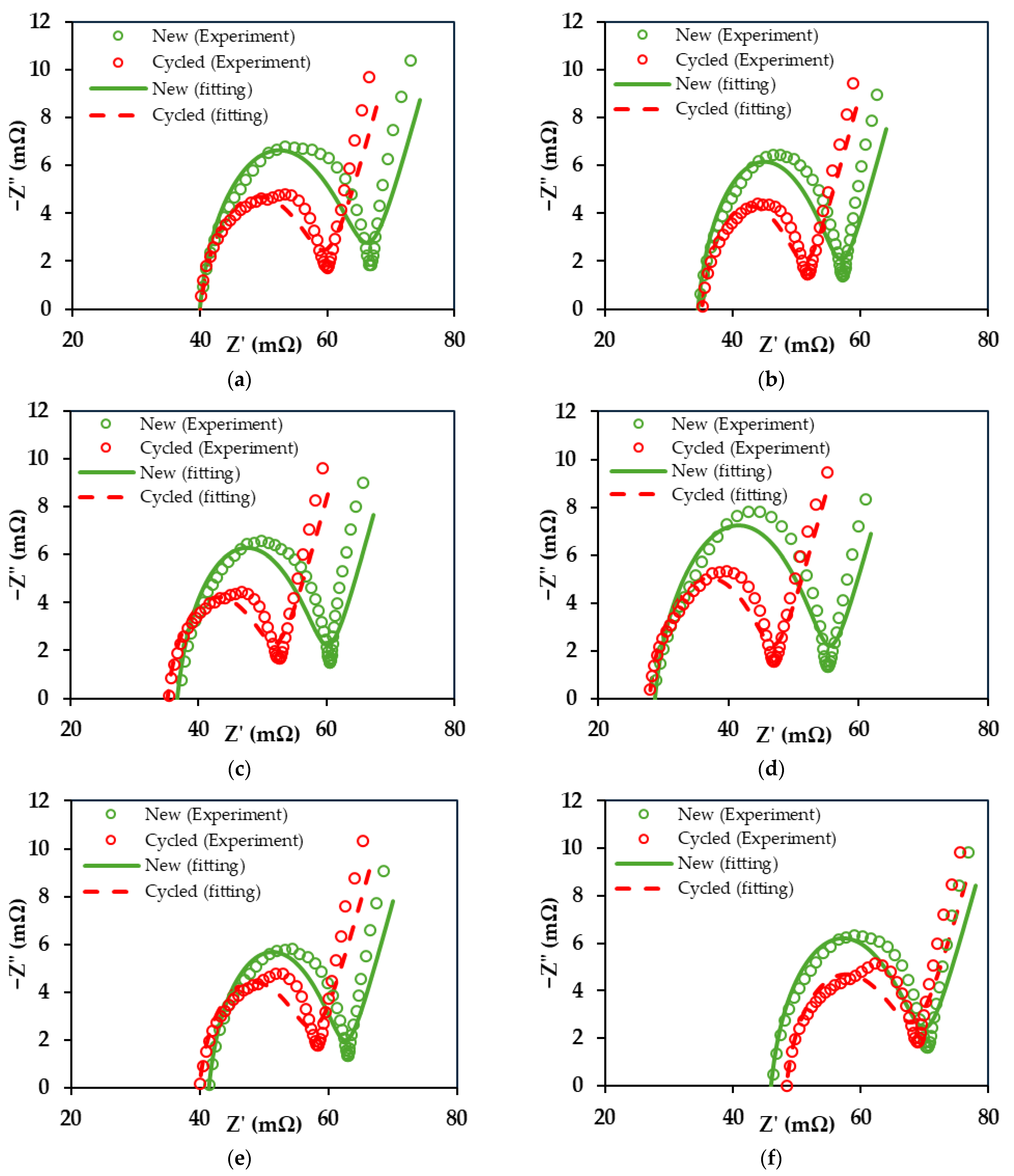
3.3. EIS Analysis
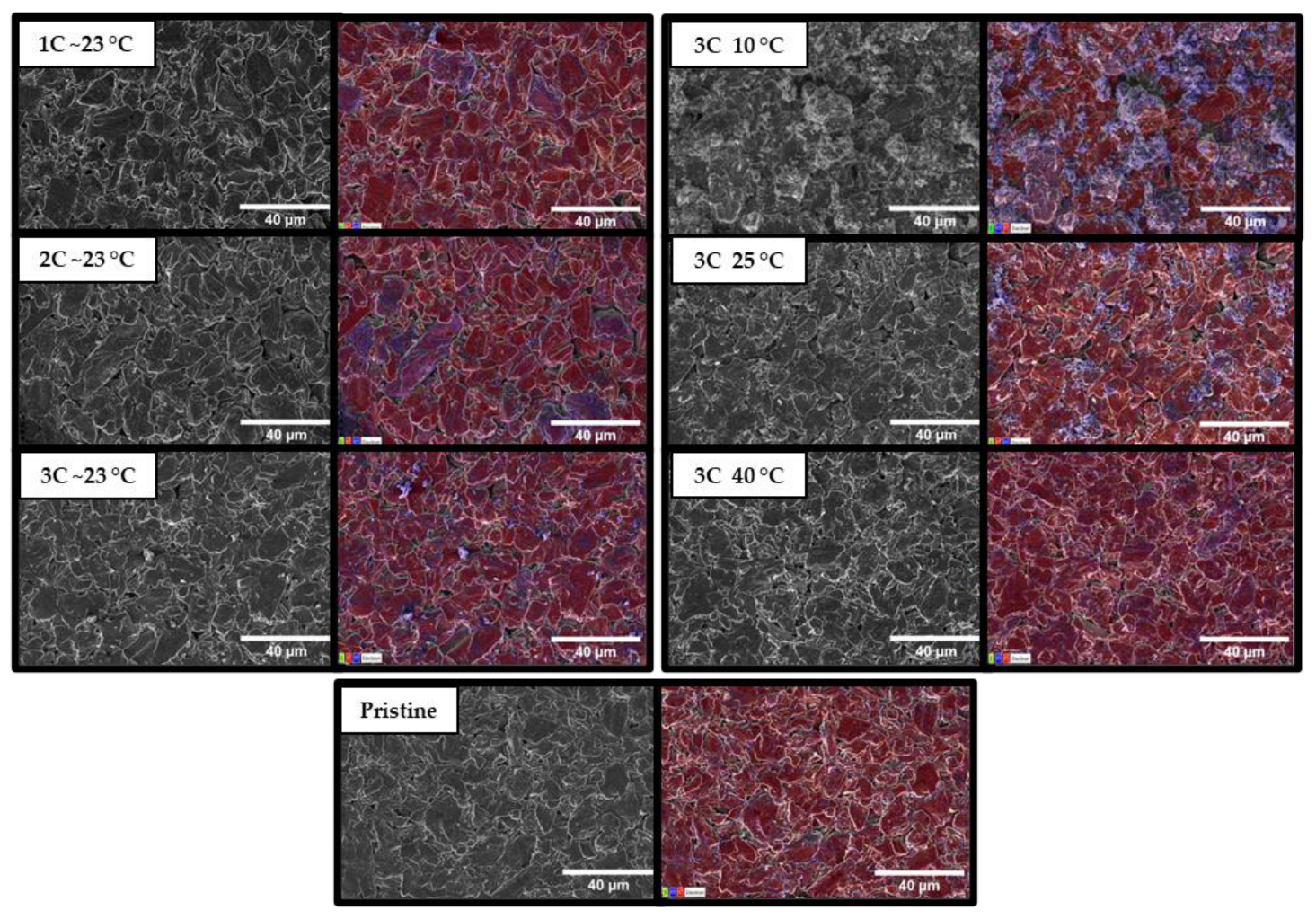
3.4. Postmortem Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arshad, F.; Lin, J.; Manurkar, N.; Fan, E.; Ahmad, A.; Tariq, M.-N.; Wu, F.; Chen, R.; Li, L. Life Cycle Assessment of Lithium-Ion Batteries: A Critical Review. Resour. Conserv. Recycl. 2022, 180, 106164. [Google Scholar] [CrossRef]
- Laresgoiti, I.; Käbitz, S.; Ecker, M.; Sauer, D.U. Modeling Mechanical Degradation in Lithium Ion Batteries during Cycling: Solid Electrolyte Interphase Fracture. J. Power Sources 2015, 300, 112–122. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, H.; Yang, Y. A Comparison of Solid Electrolyte Interphase (SEI) on the Artificial Graphite Anode of the Aged and Cycled Commercial Lithium Ion Cells. Electrochim. Acta 2008, 53, 3539–3546. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing Mechanisms in Lithium-Ion Batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Lin, X.; Khosravinia, K.; Hu, X.; Li, J.; Lu, W. Lithium Plating Mechanism, Detection, and Mitigation in Lithium-Ion Batteries. Prog. Energy Combust. Sci. 2021, 87, 100953. [Google Scholar] [CrossRef]
- Arora, P.; Doyle, M.; White, R.E. Mathematical Modeling of the Lithium Deposition Overcharge Reaction in Lithium-Ion Batteries Using Carbon-Based Negative Electrodes. J. Electrochem. Soc. 1999, 146, 3543. [Google Scholar] [CrossRef]
- Iurilli, P.; Brivio, C.; Wood, V. On the Use of Electrochemical Impedance Spectroscopy to Characterize and Model the Aging Phenomena of Lithium-Ion Batteries: A Critical Review. J. Power Sources 2021, 505, 229860. [Google Scholar] [CrossRef]
- Maheshwari, A.; Heck, M.; Santarelli, M. Cycle Aging Studies of Lithium Nickel Manganese Cobalt Oxide-Based Batteries Using Electrochemical Impedance Spectroscopy. Electrochim. Acta 2018, 273, 335–348. [Google Scholar] [CrossRef]
- Krause, T.; Nusko, D.; Pitta Bauermann, L.; Vetter, M.; Schäfer, M.; Holly, C. Methods for Quantifying Expansion in Lithium-Ion Battery Cells Resulting from Cycling: A Review. Energies 2024, 17, 1566. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, H.; Jin, Y. Experimental and Numerical Study on Mechanical Deformation Characteristics of Lithium Iron Phosphate Pouch Battery Modules under Overcharge Conditions. Energy Fuels 2021, 35, 15172–15184. [Google Scholar] [CrossRef]
- Arora, A.; Lele, S.A.; Medora, N.; Souri, S. Lithium-Ion Battery Failures in Consumer Electronics; Artech House Power Engineering Library; Artech House: Boston, MA, USA, 2019; ISBN 978-1-63081-603-2. [Google Scholar]
- Siegel, J.B.; Stefanopoulou, A.G.; Hagans, P.; Ding, Y.; Gorsich, D. Expansion of Lithium Ion Pouch Cell Batteries: Observations from Neutron Imaging. J. Electrochem. Soc. 2013, 160, A1031. [Google Scholar] [CrossRef]
- Ren, D.; Xie, L.; Wang, L.; He, X. A Practical Approach to Predict Volume Deformation of Lithium-Ion Batteries from Crystal Structure Changes of Electrode Materials. Int. J. Energy Res. 2021, 45, 7732–7740. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Birkenmaier, C.; Bitzer, B.; Harzheim, M.; Hintennach, A.; Schleid, T. Lithium Plating on Graphite Negative Electrodes: Innovative Qualitative and Quantitative Investigation Methods. J. Electrochem. Soc. 2015, 162, A2646–A2650. [Google Scholar] [CrossRef]
- Rieger, B.; Schlueter, S.; Erhard, S.V.; Jossen, A. Strain Propagation in Lithium-Ion Batteries from the Crystal Structure to the Electrode Level. J. Electrochem. Soc. 2016, 163, A1595. [Google Scholar] [CrossRef]
- Hemmerling, J.; Guhathakurta, J.; Dettinger, F.; Fill, A.; Birke, K.P. Non-Uniform Circumferential Expansion of Cylindrical Li-Ion Cells—The Potato Effect. Batteries 2021, 7, 61. [Google Scholar] [CrossRef]
- Zhang, X.; He, J.; Zhou, J.; Chen, H.; Song, W.; Fang, D. Thickness Evolution of Commercial Li-Ion Pouch Cells with Silicon-Based Composite Anodes and NCA Cathodes. Sci. China Technol. Sci. 2021, 64, 83–90. [Google Scholar] [CrossRef]
- Mei, W.; Duan, Q.; Lu, W.; Sun, J.; Wang, Q. An Investigation on Expansion Behavior of Lithium Ion Battery Based on the Thermal-Mechanical Coupling Model. J. Clean. Prod. 2020, 274, 122643. [Google Scholar] [CrossRef]
- Liu, G.; Ouyang, M.; Lu, L.; Li, J.; Han, X. Analysis of the Heat Generation of Lithium-Ion Battery during Charging and Discharging Considering Different Influencing Factors. J. Therm. Anal. Calorim. 2014, 116, 1001–1010. [Google Scholar] [CrossRef]
- Louli, A.J.; Ellis, L.D.; Dahn, J.R. Operando Pressure Measurements Reveal Solid Electrolyte Interphase Growth to Rank Li-Ion Cell Performance. Joule 2019, 3, 745–761. [Google Scholar] [CrossRef]
- Ciorba, S.; Marracci, M.; Antonetti, C.; Martinelli, M.; Caposciutti, G.; Tellini, B.; Raspolli Galletti, A.M. Experimental Analysis of Overcharged Li-Polymer Batteries. Case Stud. Chem. Environ. Eng. 2020, 2, 100012. [Google Scholar] [CrossRef]
- Grimsmann, F.; Brauchle, F.; Gerbert, T.; Gruhle, A.; Parisi, J.; Knipper, M. Impact of Different Aging Mechanisms on the Thickness Change and the Quick-Charge Capability of Lithium-Ion Cells. J. Energy Storage 2017, 14, 158–162. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Y.; Feng, Y.-H.; Fan, X.-Y.; Han, X.; Wang, P.-F. Lithium-Ion Batteries under Low-Temperature Environment: Challenges and Prospects. Materials 2022, 15, 8166. [Google Scholar] [CrossRef]
- Juarez-Robles, D.; Vyas, A.A.; Fear, C.; Jeevarajan, J.A.; Mukherjee, P.P. Overcharge and Aging Analytics of Li-Ion Cells. J. Electrochem. Soc. 2020, 167, 090547. [Google Scholar] [CrossRef]
- Wang, X.; Sone, Y.; Kuwajima, S. In Situ Investigation of the Volume Change in Li-Ion Cell with Charging and Discharging. J. Electrochem. Soc. 2004, 151, A273. [Google Scholar] [CrossRef]
- Wang, X.; Sone, Y.; Segami, G.; Naito, H.; Yamada, C.; Kibe, K. Understanding Volume Change in Lithium-Ion Cells during Charging and Discharging Using In Situ Measurements. J. Electrochem. Soc. 2007, 154, A14. [Google Scholar] [CrossRef]
- Peabody, C.; Arnold, C.B. The Role of Mechanically Induced Separator Creep in Lithium-Ion Battery Capacity Fade. J. Power Sources 2011, 196, 8147–8153. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. Ion Transport Restriction in Mechanically Strained Separator Membranes. J. Power Sources 2013, 226, 149–155. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. Stress Evolution and Capacity Fade in Constrained Lithium-Ion Pouch Cells. J. Power Sources 2014, 245, 745–751. [Google Scholar] [CrossRef]
- Müller, V.; Scurtu, R.-G.; Memm, M.; Danzer, M.A.; Wohlfahrt-Mehrens, M. Study of the Influence of Mechanical Pressure on the Performance and Aging of Lithium-Ion Battery Cells. J. Power Sources 2019, 440, 227148. [Google Scholar] [CrossRef]
- Liu, X.M.; Arnold, C.B. Effects of Cycling Ranges on Stress and Capacity Fade in Lithium-Ion Pouch Cells. J. Electrochem. Soc. 2016, 163, A2501–A2507. [Google Scholar] [CrossRef]
- Bauer, M.; Wachtler, M.; Stöwe, H.; Persson, J.V.; Danzer, M.A. Understanding the Dilation and Dilation Relaxation Behavior of Graphite-Based Lithium-Ion Cells. J. Power Sources 2016, 317, 93–102. [Google Scholar] [CrossRef]
- Li, W.; Xia, Y.; Zhu, J.; Luo, H. State-of-Charge Dependence of Mechanical Response of Lithium-Ion Batteries: A Result of Internal Stress. J. Electrochem. Soc. 2018, 165, A1537–A1546. [Google Scholar] [CrossRef]
- Petzl, M.; Danzer, M.A. Nondestructive Detection, Characterization, and Quantification of Lithium Plating in Commercial Lithium-Ion Batteries. J. Power Sources 2014, 254, 80–87. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. State of Health and Charge Measurements in Lithium-Ion Batteries Using Mechanical Stress. J. Power Sources 2014, 269, 7–14. [Google Scholar] [CrossRef]
- Barai, A.; Tangirala, R.; Uddin, K.; Chevalier, J.; Guo, Y.; McGordon, A.; Jennings, P. The Effect of External Compressive Loads on the Cycle Lifetime of Lithium-Ion Pouch Cells. J. Energy Storage 2017, 13, 211–219. [Google Scholar] [CrossRef]
- Louli, A.J.; Li, J.; Trussler, S.; Fell, C.R.; Dahn, J.R. Volume, Pressure and Thickness Evolution of Li-Ion Pouch Cells with Silicon-Composite Negative Electrodes. J. Electrochem. Soc. 2017, 164, A2689. [Google Scholar] [CrossRef]
- Dai, H.; Yu, C.; Wei, X.; Sun, Z. State of Charge Estimation for Lithium-Ion Pouch Batteries Based on Stress Measurement. Energy 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Samad, N.A.; Kim, Y.; Siegel, J.B.; Stefanopoulou, A.G. Battery Capacity Fading Estimation Using a Force-Based Incremental Capacity Analysis. J. Electrochem. Soc. 2016, 163, A1584. [Google Scholar] [CrossRef]
- Bitzer, B.; Gruhle, A. A New Method for Detecting Lithium Plating by Measuring the Cell Thickness. J. Power Sources 2014, 262, 297–302. [Google Scholar] [CrossRef]
- Burns, J.C.; Stevens, D.A.; Dahn, J.R. In-Situ Detection of Lithium Plating Using High Precision Coulometry. J. Electrochem. Soc. 2015, 162, A959–A964. [Google Scholar] [CrossRef]
- Tippmann, S.; Walper, D.; Balboa, L.; Spier, B.; Bessler, W.G. Low-Temperature Charging of Lithium-Ion Cells Part I: Electrochemical Modeling and Experimental Investigation of Degradation Behavior. J. Power Sources 2014, 252, 305–316. [Google Scholar] [CrossRef]
- Waldmann, T.; Kasper, M.; Wohlfahrt-Mehrens, M. Optimization of Charging Strategy by Prevention of Lithium Deposition on Anodes in High-Energy Lithium-Ion Batteries—Electrochemical Experiments. Electrochim. Acta 2015, 178, 525–532. [Google Scholar] [CrossRef]
- Smart, M.C.; Ratnakumar, B.V. Effects of Electrolyte Composition on Lithium Plating in Lithium-Ion Cells. J. Electrochem. Soc. 2011, 158, A379. [Google Scholar] [CrossRef]
- Agubra, V.; Fergus, J. Lithium Ion Battery Anode Aging Mechanisms. Materials 2013, 6, 1310–1325. [Google Scholar] [CrossRef]
- Zhao, X.; Yin, Y.; Hu, Y.; Choe, S.-Y. Electrochemical-Thermal Modeling of Lithium Plating/Stripping of Li(Ni0.6Mn0.2Co0.2)O2/Carbon Lithium-Ion Batteries at Subzero Ambient Temperatures. J. Power Sources 2019, 418, 61–73. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogg, B.-I.; Kasper, M.; Grolleau, S.; Couceiro, C.G.; Trad, K.; Matadi, B.P.; Wohlfahrt-Mehrens, M. Interplay of Operational Parameters on Lithium Deposition in Lithium-Ion Cells: Systematic Measurements with Reconstructed 3-Electrode Pouch Full Cells. J. Electrochem. Soc. 2016, 163, A1232–A1238. [Google Scholar] [CrossRef]
- Ren, D.; Smith, K.; Guo, D.; Han, X.; Feng, X.; Lu, L.; Ouyang, M.; Li, J. Investigation of Lithium Plating-Stripping Process in Li-Ion Batteries at Low Temperature Using an Electrochemical Model. J. Electrochem. Soc. 2018, 165, A2167–A2178. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium Plating in a Commercial Lithium-Ion Battery—A Low-Temperature Aging Study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Zinth, V.; Von Lüders, C.; Hofmann, M.; Hattendorff, J.; Buchberger, I.; Erhard, S.; Rebelo-Kornmeier, J.; Jossen, A.; Gilles, R. Lithium Plating in Lithium-Ion Batteries at Sub-Ambient Temperatures Investigated by in Situ Neutron Diffraction. J. Power Sources 2014, 271, 152–159. [Google Scholar] [CrossRef]
- Waldmann, T.; Wilka, M.; Kasper, M.; Fleischhammer, M.; Wohlfahrt-Mehrens, M. Temperature Dependent Ageing Mechanisms in Lithium-Ion Batteries—A Post-Mortem Study. J. Power Sources 2014, 262, 129–135. [Google Scholar] [CrossRef]
- Gargh, P.; Sarkar, A.; Lui, Y.H.; Shen, S.; Hu, C.; Hu, S.; Nlebedim, I.C.; Shrotriya, P. Correlating Capacity Fade with Film Resistance Loss in Fast Charging of Lithium-Ion Battery. J. Power Sources 2021, 485, 229360. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature Effect and Thermal Impact in Lithium-Ion Batteries: A Review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Ouyang, D.; He, Y.; Weng, J.; Liu, J.; Chen, M.; Wang, J. Influence of Low Temperature Conditions on Lithium-Ion Batteries and the Application of an Insulation Material. RSC Adv. 2019, 9, 9053–9066. [Google Scholar] [CrossRef]
- Purewal, J.; Wang, J.; Graetz, J.; Soukiazian, S.; Tataria, H.; Verbrugge, M.W. Degradation of Lithium Ion Batteries Employing Graphite Negatives and Nickel–Cobalt–Manganese Oxide + Spinel Manganese Oxide Positives: Part 2, Chemical–Mechanical Degradation Model. J. Power Sources 2014, 272, 1154–1161. [Google Scholar] [CrossRef]
- Chen, Y.; Torres-Castro, L.; Chen, K.-H.; Penley, D.; Lamb, J.; Karulkar, M.; Dasgupta, N.P. Operando Detection of Li Plating during Fast Charging of Li-Ion Batteries Using Incremental Capacity Analysis. J. Power Sources 2022, 539, 231601. [Google Scholar] [CrossRef]
- Schmitt, J.; Maheshwari, A.; Heck, M.; Lux, S.; Vetter, M. Impedance Change and Capacity Fade of Lithium Nickel Manganese Cobalt Oxide-Based Batteries during Calendar Aging. J. Power Sources 2017, 353, 183–194. [Google Scholar] [CrossRef]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Schmidt, J.P.; Ivers-Tiffée, E. Electrochemical Characterization and Post-Mortem Analysis of Aged LiMn2O4–Li(Ni0.5Mn0.3Co0.2)O2/Graphite Lithium Ion Batteries. Part I: Cycle Aging. J. Power Sources 2014, 251, 439–450. [Google Scholar] [CrossRef]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Zhang, M.; Schmidt, J.P.; Ivers-Tiffée, E. Electrochemical Characterization and Post-Mortem Analysis of Aged LiMn2O4–NMC/Graphite Lithium Ion Batteries Part II: Calendar Aging. J. Power Sources 2014, 258, 61–75. [Google Scholar] [CrossRef]
- Schweikert, N.; Hahn, H.; Indris, S. Cycling Behaviour of Li/Li4Ti5O12 Cells Studied by Electrochemical Impedance Spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 6234. [Google Scholar] [CrossRef]
- Waldmann, T.; Hogrefe, C.; Flügel, M.; Pivarníková, I.; Weisenberger, C.; Delz, E.; Bolsinger, M.; Boveleth, L.; Paul, N.; Kasper, M.; et al. Efficient Workflows for Detecting Li Depositions in Lithium-Ion Batteries. J. Electrochem. Soc. 2024, 171, 070526. [Google Scholar] [CrossRef]
- Hogrefe, C.; Waldmann, T.; Hölzle, M.; Wohlfahrt-Mehrens, M. Direct Observation of Internal Short Circuits by Lithium Dendrites in Cross-Sectional Lithium-Ion in Situ Full Cells. J. Power Sources 2023, 556, 232391. [Google Scholar] [CrossRef]
- Grimsmann, F.; Gerbert, T.; Brauchle, F.; Gruhle, A.; Parisi, J.; Knipper, M. Hysteresis and Current Dependence of the Graphite Anode Color in a Lithium-Ion Cell and Analysis of Lithium Plating at the Cell Edge. J. Energy Storage 2018, 15, 17–22. [Google Scholar] [CrossRef]
- Ghanbari, N.; Waldmann, T.; Kasper, M.; Axmann, P.; Wohlfahrt-Mehrens, M. Inhomogeneous Degradation of Graphite Anodes in Li-Ion Cells: A Postmortem Study Using Glow Discharge Optical Emission Spectroscopy (GD-OES). J. Phys. Chem. C 2016, 120, 22225–22234. [Google Scholar] [CrossRef]
- Pivarníková, I.; Flügel, M.; Paul, N.; Cannavo, A.; Ceccio, G.; Vacík, J.; Müller-Buschbaum, P.; Wohlfahrt-Mehrens, M.; Gilles, R.; Waldmann, T. Observation of Preferential Sputtering of Si/Graphite Anodes from Li-Ion Cells by GD-OES and Its Validation by Neutron Depth Profiling. J. Power Sources 2024, 594, 233972. [Google Scholar] [CrossRef]


| C-Rate | Circulating Water Temperature | Average Residual Pressure (Pa/Cycle) | Average Increase in Maximum Pressure (Pa/Cycle) |
|---|---|---|---|
| 1C | - | 2.3 | 13.6 |
| 2C | - | 24.8 | 35.0 |
| 3C | - | 28.6 | 46.1 |
| 3C | 10 °C | 191.6 | 264.6 |
| 3C | 25 °C | 40.1 | 55.6 |
| 3C | 40 °C | 13.1 | 20.0 |
| C-Rate | Discharging Temperature (°C) | Charging Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| Min | Max | Avg | Min | Max | Avg | |
| 1C | 23.7 | 25.6 | 24.8 | 23.8 | 25.1 | 24.4 |
| 2C | 24.5 | 28.6 | 26.9 | 24.9 | 27.4 | 26.2 |
| 3C | 24.2 | 32.2 | 29.1 | 24.6 | 28.9 | 26.3 |
| C-Rate | Circulating Water Temperature | Battery State | Re (mΩ) | Rp (mΩ) | Qp (mF) |
|---|---|---|---|---|---|
| 1C | - | New | 37.5 | 28.6 | 29.4 |
| Cycled | 37.5 | 22.1 | 32.1 | ||
| 2C | - | New | 31.8 | 24.9 | 31.5 |
| Cycled | 33.0 | 18.2 | 41.3 | ||
| 3C | - | New | 33.8 | 26.1 | 29.7 |
| Cycled | 32.8 | 19.2 | 40.8 | ||
| 3C | 10 °C | New | 27.1 | 28.1 | 35.2 |
| Cycled | 26.6 | 20.1 | 48.3 | ||
| 3C | 25 °C | New | 39.0 | 23.4 | 28.4 |
| Cycled | 37.3 | 20.3 | 38.3 | ||
| 3C | 40 °C | New | 43.5 | 26.3 | 29.8 |
| Cycled | 45.7 | 22.6 | 33.9 |
| Battery Cycling Condition | C | O | F |
|---|---|---|---|
| Pristine | 82.8% | 13.0% | 4.2% |
| 1C~23 °C | 74.9% | 19.6% | 5.5% |
| 2C~23 °C | 67.0% | 25.5% | 7.1% |
| 3C~23 °C | 72.4% | 21.8% | 5.8% |
| 3C 10 °C | 59.8% | 36.8% | 3.4% |
| 3C 25 °C | 72.9% | 21.8% | 5.3% |
| 3C 40 °C | 78.7% | 16.9% | 4.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nayfeh, Y.; Vittitoe, J.C.; Li, X. Quantifying the Aging of Lithium-Ion Pouch Cells Using Pressure Sensors. Batteries 2024, 10, 333. https://doi.org/10.3390/batteries10090333
Nayfeh Y, Vittitoe JC, Li X. Quantifying the Aging of Lithium-Ion Pouch Cells Using Pressure Sensors. Batteries. 2024; 10(9):333. https://doi.org/10.3390/batteries10090333
Chicago/Turabian StyleNayfeh, Yousof, Jon C. Vittitoe, and Xianglin Li. 2024. "Quantifying the Aging of Lithium-Ion Pouch Cells Using Pressure Sensors" Batteries 10, no. 9: 333. https://doi.org/10.3390/batteries10090333
APA StyleNayfeh, Y., Vittitoe, J. C., & Li, X. (2024). Quantifying the Aging of Lithium-Ion Pouch Cells Using Pressure Sensors. Batteries, 10(9), 333. https://doi.org/10.3390/batteries10090333






