Recycling of Lithium Iron Phosphate (LiFePO4) Batteries from the End Product Quality Perspective
Abstract
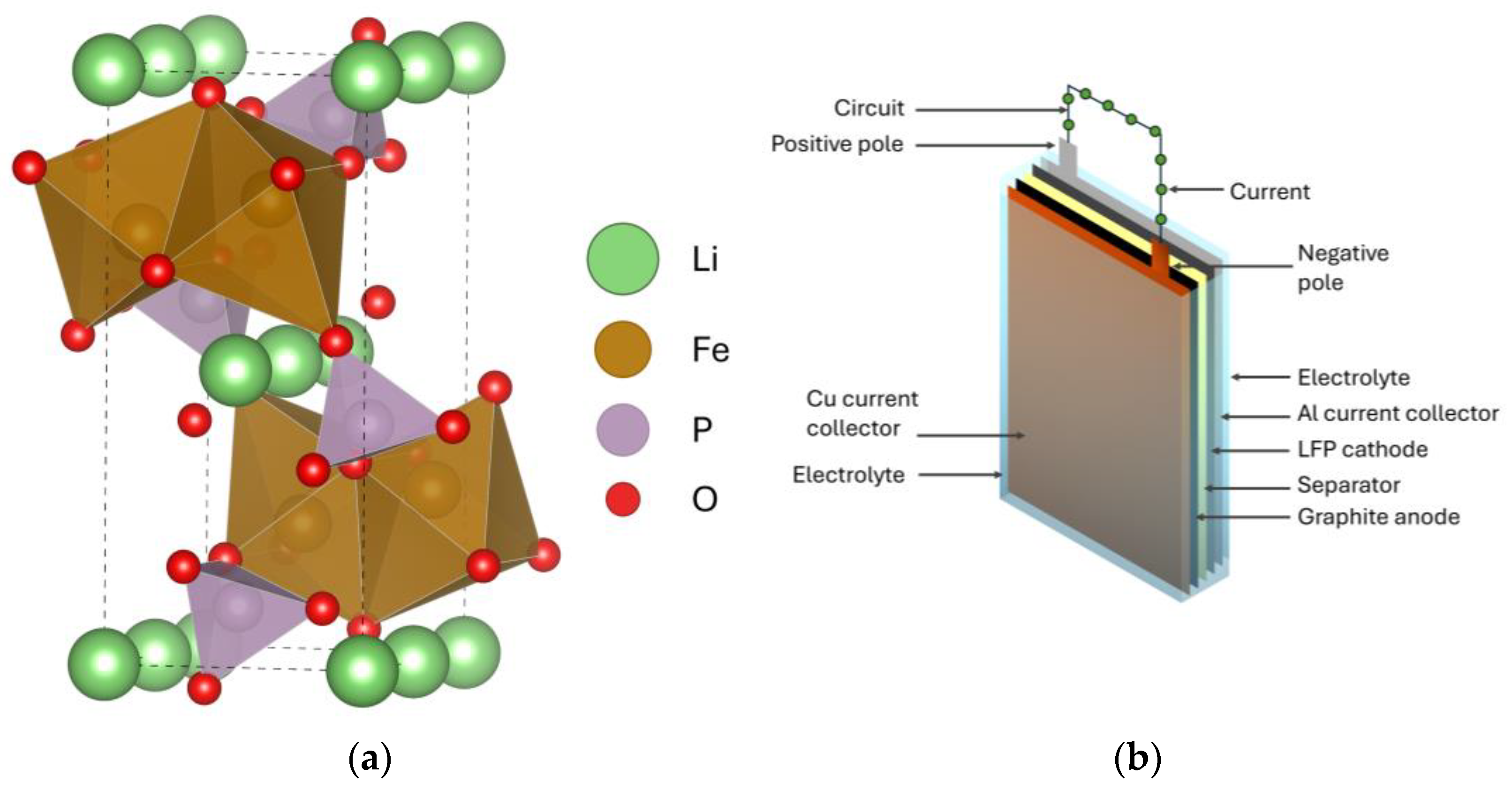
:1. Introduction
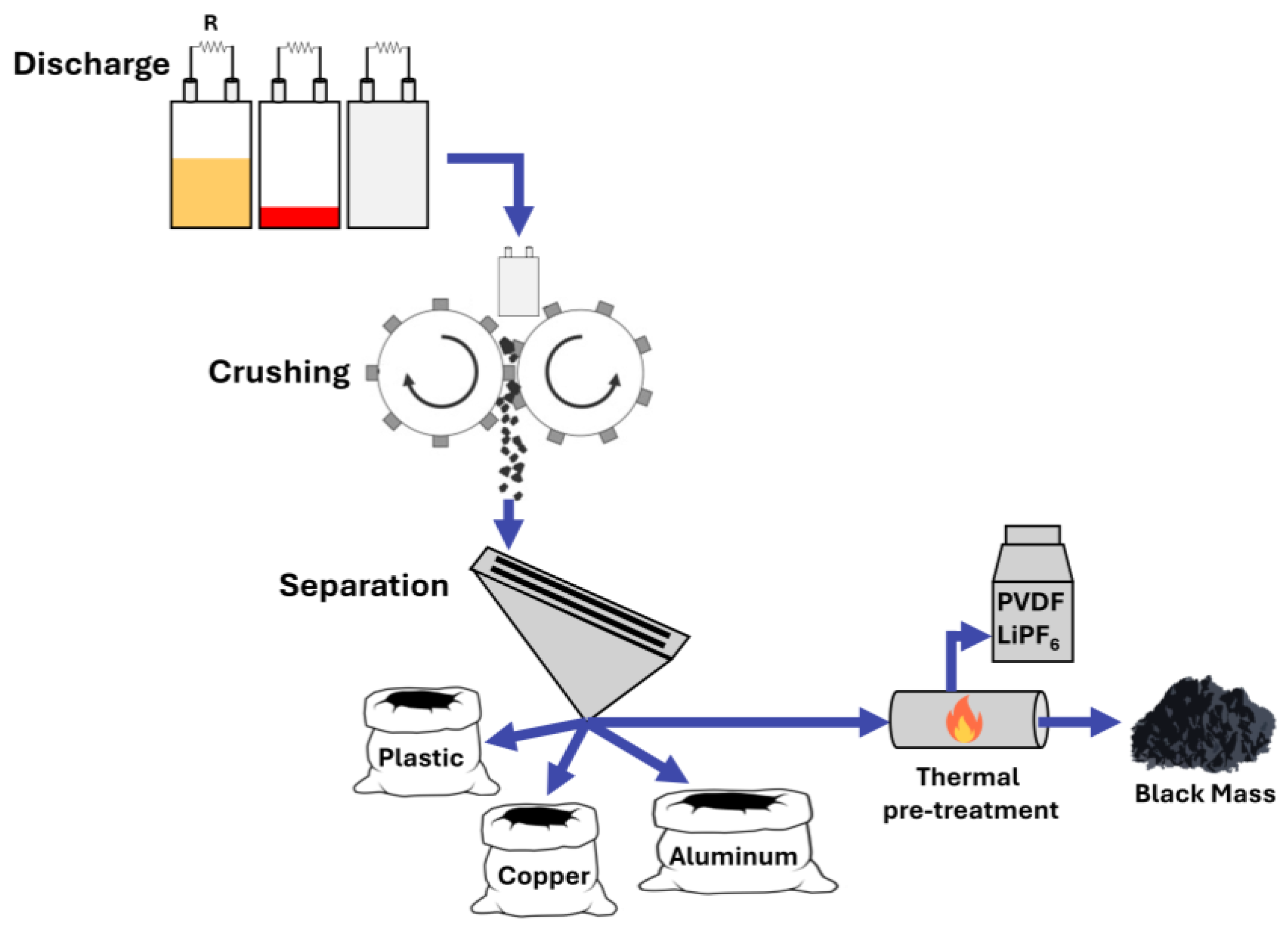
2. Handling and Mechanical Pre-Treatment of Spent Batteries
2.1. Battery Discharge and Dismantling
2.2. Mechanical Processing and Separation
2.3. Black Mass Thermal Pre-Treatment
2.4. Removal of Aluminum and Copper Current Collectors
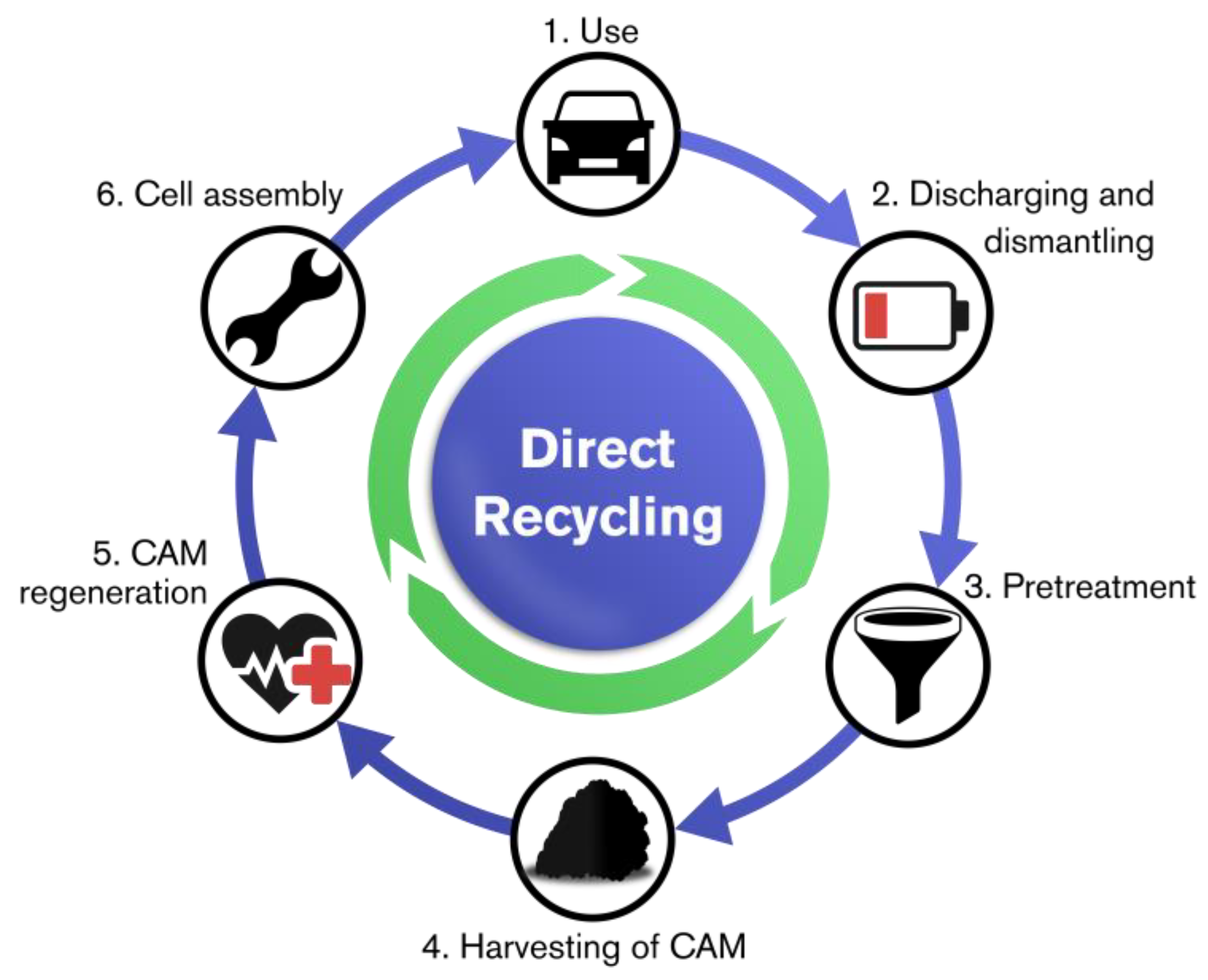
2.5. Methods Applied in the Direct Recycling Approach
2.6. Removal of Graphite
3. Recycling Technologies
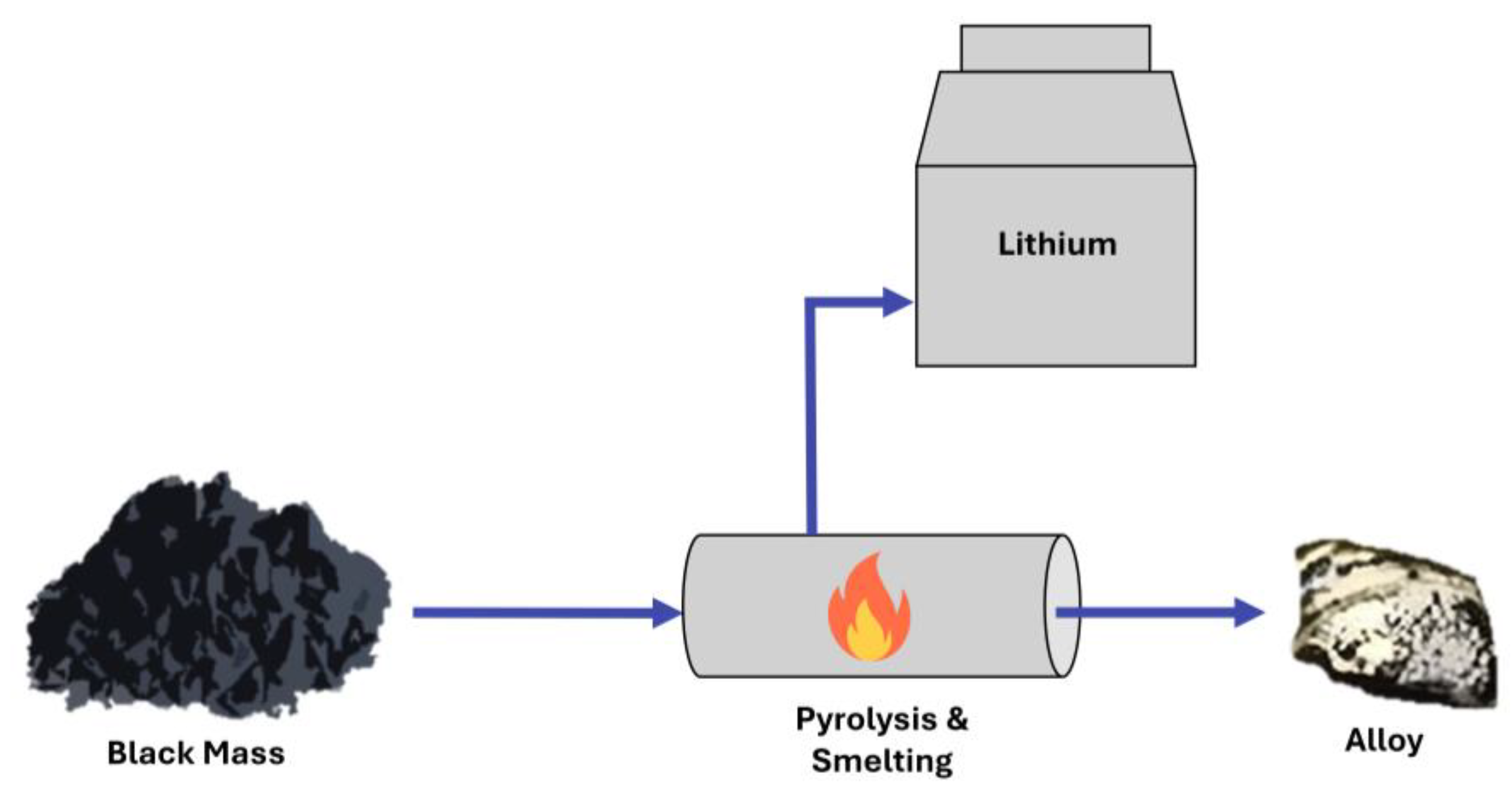
3.1. Pyrometallurgy
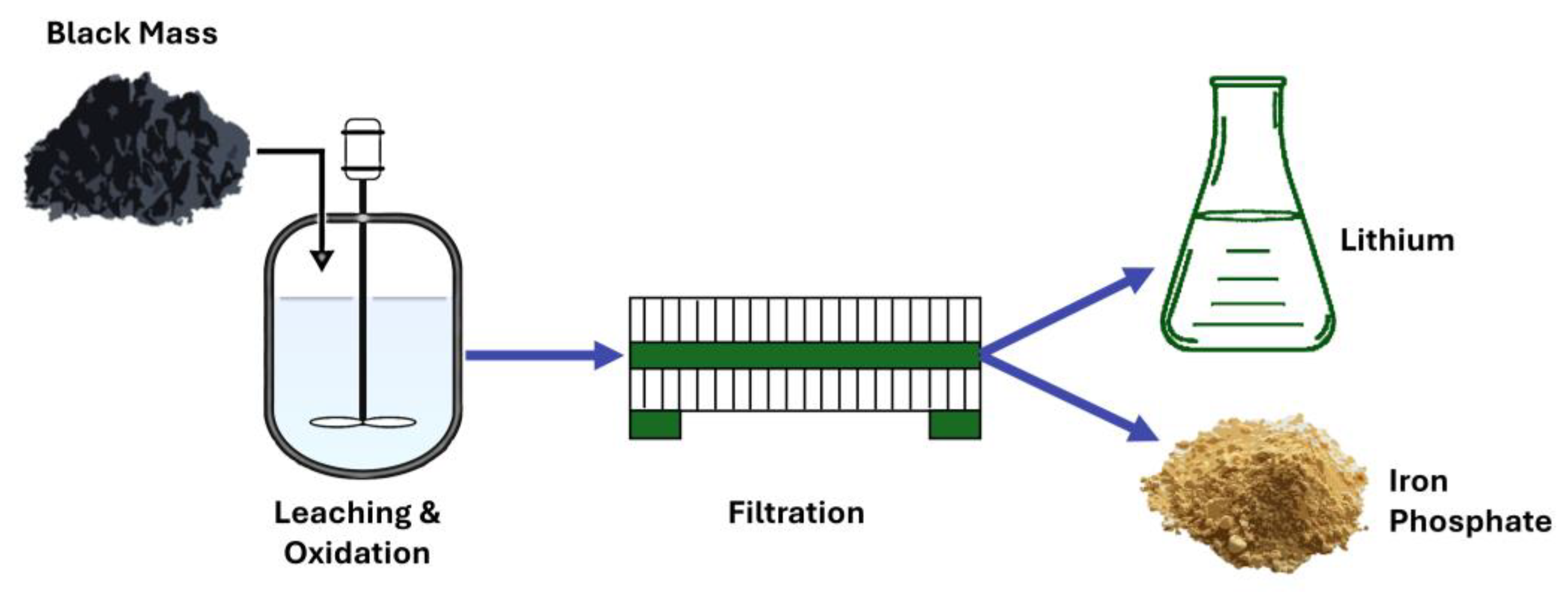
3.2. Hydrometallurgy
3.3. Direct Recycling
3.3.1. Solid-State Thermal Regeneration
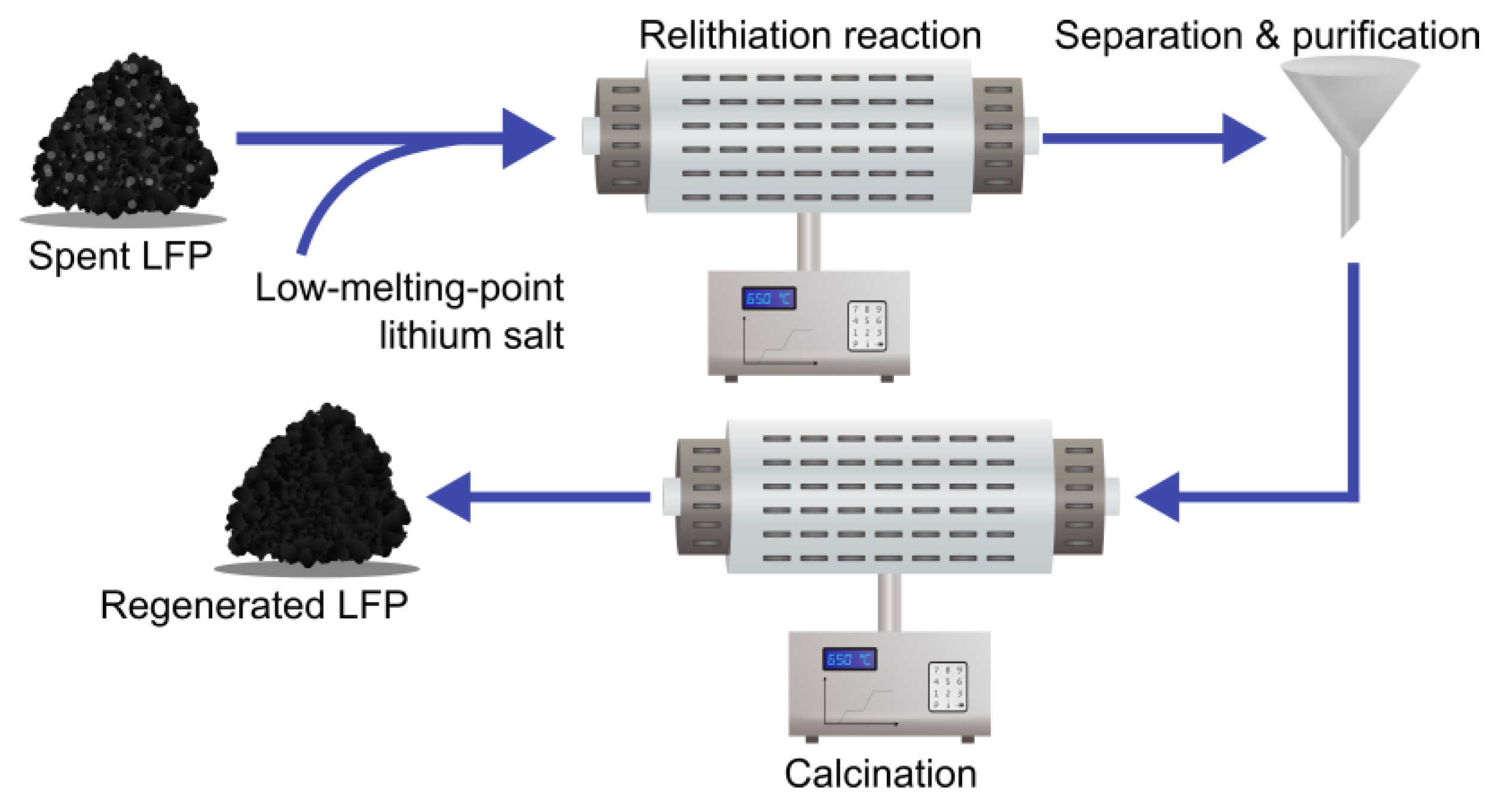
3.3.2. The Molten Salt Approach
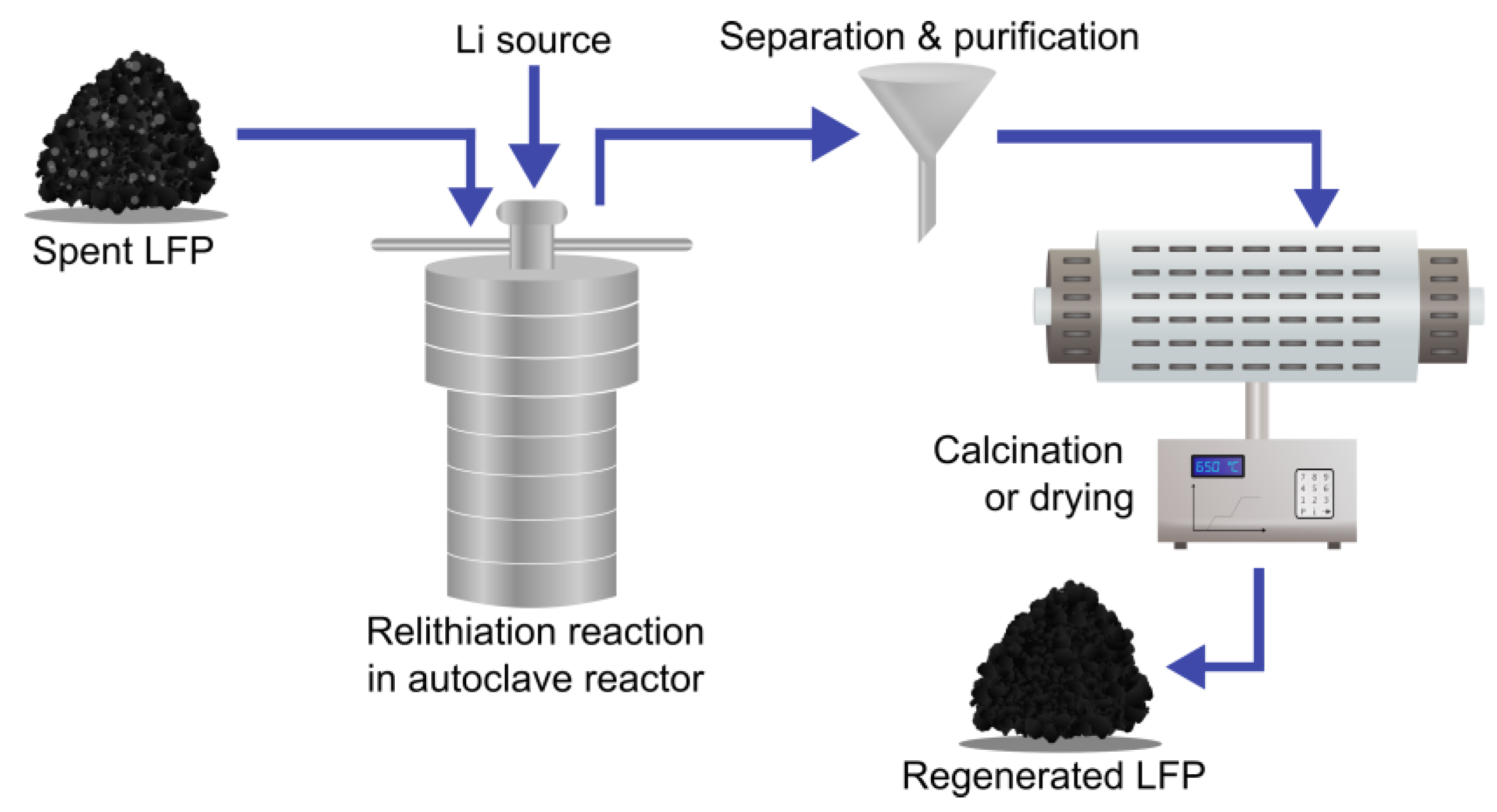
3.3.3. Hydrothermal Regeneration
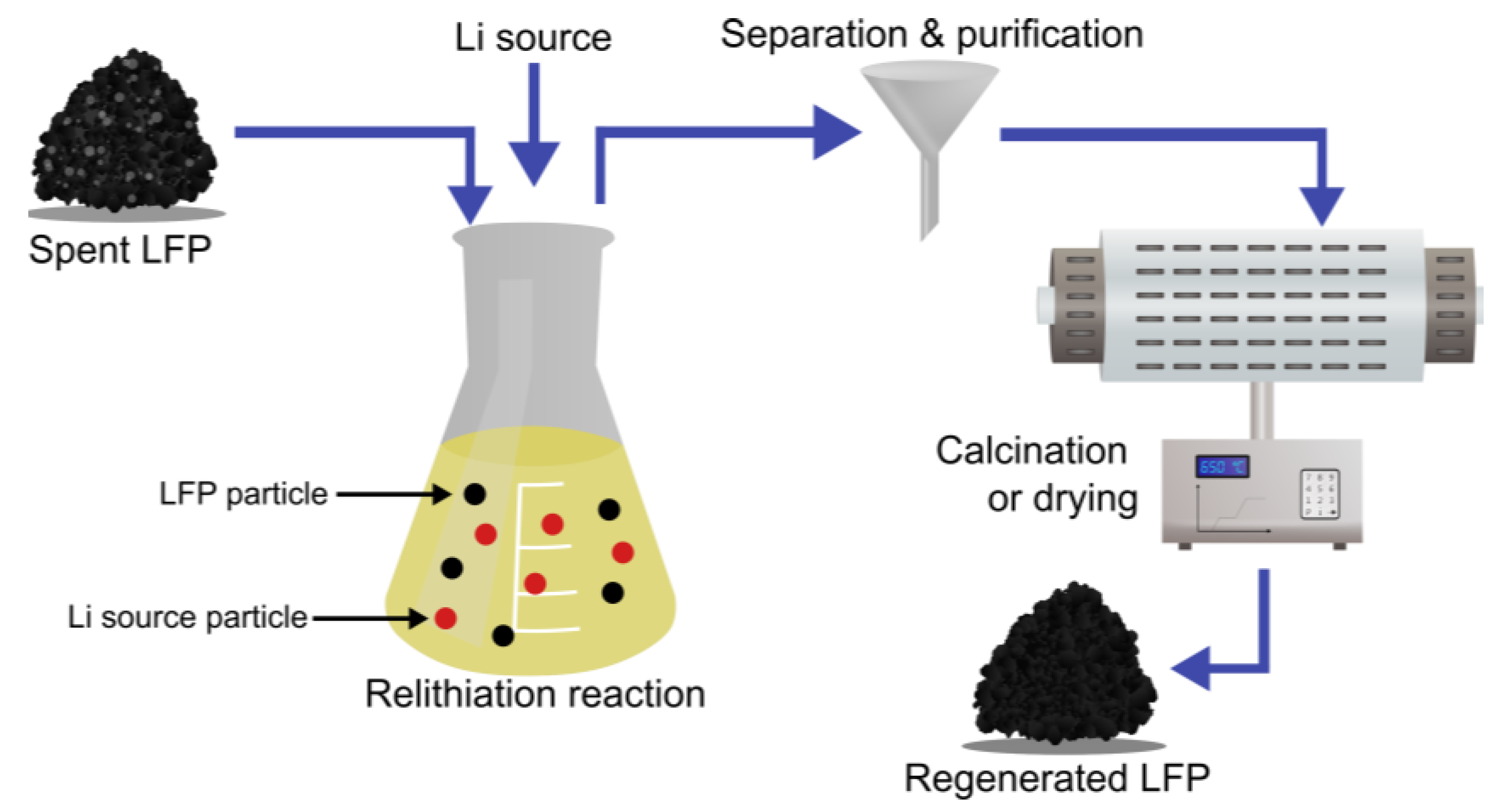
3.3.4. Chemical Regeneration
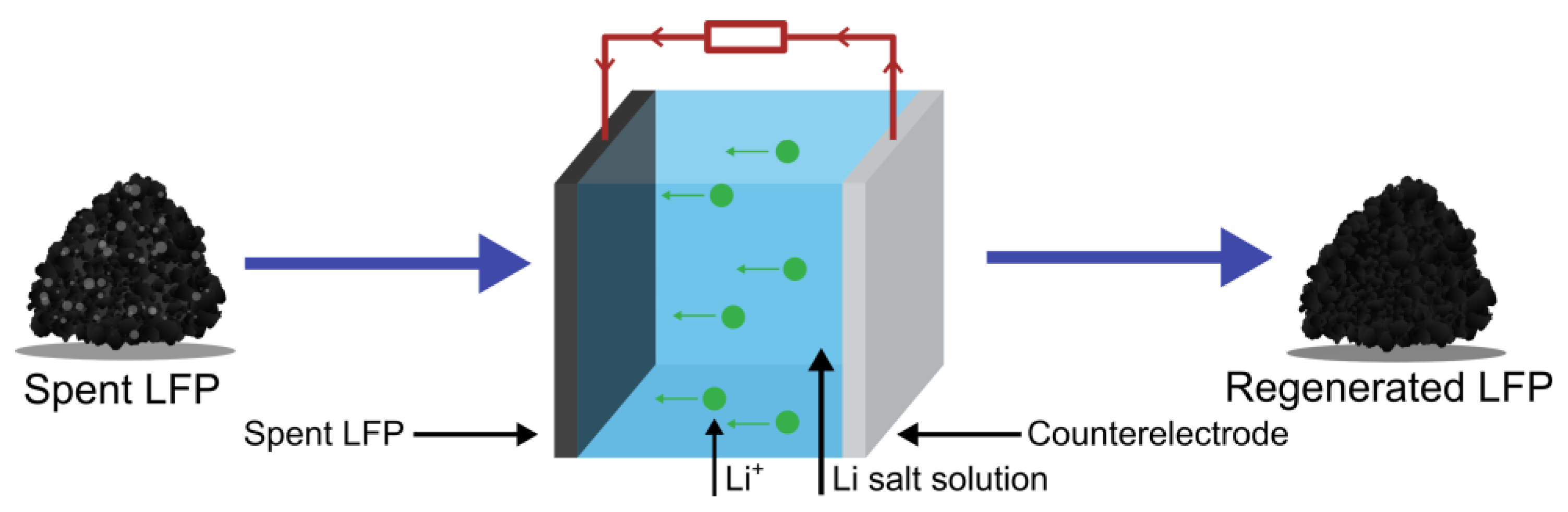
3.3.5. Electrochemical Regeneration
3.4. The Indirect Approach
| Method | Conditions | Electrochemical Performance | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Discharge Capacity [mAh g−1] | At Current Density [C] | Capacity Retention [%] | After Cycles | At Current Density [C] | |||
| Thermal (solid-state) | Thermal annealing under Ar/H2 at 600 °C for 1 h | 132 | 1 | n/r | n/r | n/r | [151] |
| Pristine LFP doping at different ratios, sintering at 700 °C for 8 h under N2 | 144 | 0.1 | 93.75 | 100 | 0.2 | [112] | |
| Thermal annealing under Ar/H2 at 650 °C for 1 h w/Li2CO3 | 147.3 | 0.2 | 95 | 100 | 0.2 | [152] | |
| NaOH wash, annealing at 450 °C for 2 h under air, added Li/Fe/P salts, ball mill, spray drying, annealing at 650 °C for 10 h under N2 | 139 | 0.2 | 95 | 100 | 0.2 | [153] | |
| Li2CO3 + glucose, annealing at 350 °C for 4 h, then 650 °C for 9 h | 161.1 | 0.1 | 91 | 200 | 1 | [107] | |
| Li2CO3 + glucose in DI, thermal annealing at 350–900 °C, ball mill, grinding, calcination | 148 | 0.05 | 92.9 | 100 | 0.1 | [99] | |
| Calcination at 550 °C for 2 h under air, sieving, Li2CO3 + glucose, ball mill, drying at 60 °C for 12 h, annealing at 700 °C for 8/10/12 h under N2 w/TiO2 doping | 147.3 | 0.1 | 94.1 | 100 | 0.5 | [154] | |
| Li2DHBN, grinding, sintering at 800 °C for 6 h under Ar/H2 | 127 | 2 | 88 | 400 | 5 | [155] | |
| Li2CO3 + glucose + CNT, ball mill in EtOH:H2O 1:1, drying at 80 °C, annealing at 350 °C for 2 h, then 650 °C for 12 h under Ar | 155.15 | 0.05 | 93.8 | 100 | 0.1 | [109] | |
| Li2CO3 + glucose + Cu(NO3)2·2.5H2O, ball mill in EtOH, drying, annealing at 350 °C for 2 h, then 650 °C for 12 h under Ar | 160.15 | 0.05 | 81.19 | 1000 | 1 | [108] | |
| Molten salt | LiNO3:LiOH 3:2 + citric acid, heating at 350/450/550 °C for 4 h, DI wash, centrifuge, drying at 80 °C for 12 h | 151.2 | 0.2 | 97.73 | 150 | 1 | [156] |
| LiNO3 + FeC2O4 + sucrose, heating at 300 °C for 1–6 h under Ar, room temp. quench, DI wash, centrifugation, drying at 80 °C for 12 h, annealing at 650 °C for 6 h under Ar | 145 | 0.5 | 90 | 100 | 0.5 | [104] | |
| LiNO3 + anhydrous glucose, ball mill, annealing at different temps., room temp. quench (DI), DI and EtOH wash, centrifuge in ZnCl2(aq), DI wash | 162 | 0.1 | 90 | 500 | 0.1 | [149] | |
| Hydrothermal | Li2SO4·H2O + hydrazine, autoclave, magnetic stirring for 10 min, blast oven for 3 h, filtering and DI wash, drying at 80 °C for 10 h | 141.9 | 1 | 98.6 | 200 | 1 | [150] |
| LiOH + Na2SO3 in autoclave, heating at 150 °C for 24 h, cooling, doping w/Cu(NO3)2, calcination at 600 °C for 4 h under N2 | 144.02 | 0.1 | 92.36 | 100 | 0.2 | [159] | |
| LiOH + ascorbic acid in DI, microwave hydrothermal reduction, graphene doping, MWHT reduction, wash and filter | 161.4 | 0.2 | 94.9 | 100 | 0.2 | [96] | |
| PVP + S-LFP in autoclave w/EtOH, added CH3COOLi, heating at 180 °C for 5 h, centrifuge, vacuum drying at 120 °C for 12 h, sintering at 700 °C for 5 h under Ar | 139.1 | 1 | 80 | 1000 | 10 | [97] | |
| LiOH + ascorbic acid + SDBS in DI, hydrothermal heating, regenerated GO added (5%) to 160 °C for 6 h regeneration | 163.3 | 0.2 | 99.63 | 100 | 0.2 | [158] | |
| Chemical | LiI in EtOH mixed w/SLFP, filtration, washing w/EtOH, drying at 100 °C for 1 h under vacuum, grinding | 160 | 1 | n/r | n/r | n/r | [162] |
| LiOH + citric acid, range of T and time, DI rinse, thermal annealing w/Li2CO3 for 2 h under N2 | 159 | 0.5 | 94.34 | 1000 | 0.5 | [111] | |
| Pristine LFP, chemical oxidation, degraded LFP, + polycyclic aryl-Li compounds, stirring, centrifugation, drying at 80 °C in air overnight | 155 * | 0.5 | 97 * | 100 | 0.5 | [160] | |
| LiI in acetonitrile, stirring for 20 h, vacuum filtration, rinse w/acetonitrile, drying at 100 °C for 1 h under vacuum, grinding | 153 * | 0.1 | n/r | n/r | n/r | [161] | |
| LiOH + glucose + NaCl into DI, stirring at 60 °C for 2 h, freeze drying (vacuum) overnight w/urea, heating at 650 °C for 3 h under Ar, cooling, DI rinse, vacuum drying | 169.74 | 0.1 | 95.7 | 200 | 0.1 | [102] | |
| LiCl + N2H4xH2O in 50% ethylene glycol/water solution, ultrasonication for 25 min—10 min rest—25 min again, centrifuge, DI and EtOH wash | 135.1 | 1 | 97.44 | 100 | 1 | [109] | |
| LiOH + H2O2 in solution, DI rinse to remove LiOH residues, vacuum drying, thermal annealing w/Li2CO3 under Ar at 400–800 °C for 2–10 h | 146.3 | 1 | 84.9 | 1000 | 5 | [163] | |
| Li3PO4 from wastewater + DI, mixed w/Fe(NO3)3 + phosphoric + citric acid in DI and TEG, spray pyrolysis, room T cooling, sintering at 800 °C for 2 h under N2 | 161.3 | 0.1 | 99.87 | 50 | 0.1 | [164] | |
| Electrochemical | Lithiated graphite, cell assembly w/S-LFP wrapped in Al foil, time-controlled regeneration, cleaning w/DMC => drying for 4 h under vacuum | 125.4 | 0.5 | 98.8 | 100 | 0.5 | [166] |
| Cycling w/Li(s), discharge to lithiate positive electrode, coin cell assembly, pairing w/fresh negative electrode | 140 * | 0.1 | 81 * | 50 | 0.1 | [161] | |
| Cycling, washing w/DMC, positive electrode separation, pouch cell assembly w/prelithiated graphite as negative electrode, cycling | 126.6 | 0.5 | 62.5 | 200 | 0.5 | [165] | |
| Cycling, LFP coupled w/fresh graphite + functionalized prelithiation separator, 1 cycle at <0.05 C | 158.4 | 0.5 | 90.7 | 292 | 0.05 | [167] | |
| Leaching Agent | Conditions | Leaching Efficiency [%] | Electrochemical Performance of Resynthesized LFP | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Li | Fe | P | Others | Discharge Capacity [mAh g−1] | Retention [%] (#Cycles) | |||
| H2SO4 | 2.5 M H2SO4, L/S = 10 mL/g, 60 °C, 4 h. Less than 0.005% of impurities. | 97 | 98 | S < 0.018 | 137.8 | n/r | [138] | |
| 9 M H2SO4, L/S = 10 mL/g, air oxygen as oxidant, air flow rate = 60 mL/min, 25 °C, 5 h. No impurities. | 99.3 | 0.02 | 0.02 | 131.7 | 99 (100) | [55] | ||
| H2SO4 = 1.5 times the theoretical amount, L/S = 4 mL/g, ascorbic acid (3%wt) as reducing agent to prevent the formation of Fe3+, 60 °C, 4 h. Purity > 98%. | 98 | 98 | 98 | 133 | ~100 (100) | [175] | ||
| 2 M H2SO4, L/S = 20:1, 70 °C, 2 h. No impurities. Filtrate Li+, added FeSO4, H3PO4, LiOH. | 96.67 | 93.25 | n/r | 105 | 98.6 (300) | [176] | ||
| 2 M H2SO4 and H2O2, S/L = 30 g/L, H2SO4/H2O2 = 4 v/v, 60 °C, 80 min. No impurities. Fe2O3 added Li2CO3. | n/r | n/r | n/r | 141 | 95 (100) | [177] | ||
| 2.5 M H2SO4 and H2O2 (30%mass), L/S = 25 mL/g, 60 °C, 1 h. No impurities. | 98.79 | 94.97 | 98.71 | 160.1 (0.1 C) | 99.7 (100) | [169] | ||
| HCl | LFP powder pre-treated at 700 °C, 10 h, to decompose carbon and oxidize Fe2+ to Fe3+. Leaching: 6 M HCl, 120 °C, 6 h. Recovered: LiOH.H2O. | n/r | n/r | n/r | 139.03 | 98.95 (25) | [168] | |
| LFP powder pre-treated at 600 °C, to oxidize iron. Leaching: 4 M HCl. Recovered: Li3PO4. | n/r | n/r | n/r | 144.3 | 96.7 (200) | [103] | ||
| H3PO4 | 0.5 M H3PO4, 25 °C, 1 h. FePO4·2H2O was precipitated with reflux for 9 h at 85 °C. Recovered: FePO4·2H2O. | n/r | n/r | n/r | 110 (5 C) | 95.4 (100) | [178] | |
| 2.3 M H3PO4 and 0.58 M citric acid, L/S = 5:1, 50 °C, 3 h. | 95.1 | 96.2 | n/r | Al | 145.4 | 97.3 (600) | [173] | |
| Citric acid | Lemon juice and H2O2 (6%vol), S/L = 67 g/L, 25 °C, 90 min. | 94.83 | 4.05 | 0.84 | Cu = 96.9 Al = 47.2 | 135.3 | 98.3 (100) | [52] |
| C6H8O7 and 2 mL H2O2 (30%) in ball mill with zirconia beads, 200 rpm, 30 min. Recovered: LiOH, Li3Cit. | 98.21 | <1.5 | n/r | 102.5 (5 C) | 90 (1000) | [174] | ||
| 0.25 M C6H8O7, 40 °C, 2 h, (i) without and (ii) with H2O2 (6%vol). (iii) Comparing the leaching with 1 M H2SO4, 25 °C, 1 h. | (i) = 90 (ii) = 87 (iii) = 95 | (i) = 99 (iii) = 98 | (i) = 69 (iii) = 96 | S, Al, Cl, Co, V | n/r | n/r | [98] | |
| Acetic acid | 0.8 M CH3COOH and H2O2 (6%vol), S/L = 120 g/L, 50 °C, 30 min. | 95.13 | n/r | n/r | 130 * (0.1 C) | n/r | [143] | |
| Pyrophosphoric acid | 9 M H4P2O7, oxygen as oxidant, S/L = 100 g/L, oxygen flow = 20 L/min, 25 °C, 5 h. | 97.98 | ~100 | n/r | 150.2 | 91.31 (100) | [179] | |
| Sodium hypo- chlorite | NaClO (12% available Cl), S/L = 50 g/L, 30 °C, 2 h. | 98.3 | 0.11 | n/r | Al ~0 | 154.3 | 92.7 (300) | [117] |
| Iron salt | 80 mM FeSO4·7H2O and 400 mM H2O2 (30%vol), slurry density = 20 g/L, 40 °C, 30 min. | 99.9 | n/r | n/r | 138.9 (0.5 C) | 93.6 (50) | [180] | |
| Organic solvent | 4 M methanesulfonic acid (MSA), S/L = 80 g/L; 4 M p-toluenesulfonic acid (TsOH), S/L = 60 g/L. Both with H2O2 (18%vol), 25 °C, 90 min. | MSA = 96 TsOH = 97 | 99 | n/r | MSA: Cu, Al TsOH: Cu | 80 | n/r | [181] |
| H2O2 | H2O2 (20%vol), S/L = 15 g/L, 40 °C, 20 min. | 96.3 | n/r | n/r | 137.1 | 97.9 * (250) | [172] | |
| Ammonium persulfate | (NH4)2S2O8 = 1.4 times the theoretical amount, S/L = 50 g/L, 30 °C, 30 min. | 98.1 | 0.02 | n/r | Al = 0.06 | 130.2 | 98 (400) | [170] |
| LFP and (NH4)2S2O8 in water for 30 min. | 34.3 | 1.31 | 2.03 | 145.2 | 97.9 (200) | [182] | ||
| (NH4)2S2O8 = 1.3 times the theoretical amount, S/L = 150 g/L, 25 °C, 1 h. | 52.4 | n/r | n/r | 156.1 (0.2 C) | 98.4 (100) | [171] | ||
4. Perspectives and Challenges with Direct Recycling
Future Outlooks
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Assembly, U.G. Transforming our World: The 2030 Agenda for Sustainable Development; United Nations: New York, NY, USA, 2020; A/RES/70/1. [Google Scholar]
- Whittingham, M.S. History, evolution, and future status of energy storage. Proc. IEEE 2012, 100, 1518–1534. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Chagnes, A.; Swiatowska, J. Lithium Process Chemistry: Resources, Extraction, Batteries, and Recycling; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Ramström, O. Scientific Background on the Nobel Prize in Chemistry 2019; The Royal Academy of Sciences: Stockholm, Sweden, 2019. [Google Scholar]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Gong, M.; Yu, R.; Zhou, C.; Yu, Y.; Pan, Q.; Dong, C.; Shen, C.; Guan, Y.; Sun, C.; Mai, L.; et al. Mechanically robust current collector with gradient lithiophilicity induced by spontaneous lithium ion diffusion for stable lean-lithium metal batteries. ACS Nano 2024, 18, 20648–20658. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, W.; Yang, L.; Pan, F. Structure and performance of the LiFePO4 cathode material: From the bulk to the surface. Nanoscale 2020, 12, 15036–15044. [Google Scholar] [CrossRef]
- Andersson, A.S.; Thomas, J.O.; Kalska, B.; Häggström, L. Thermal stability of LiFePO4-based cathodes. Electrochem. Solid-State Lett. 1999, 3, 66. [Google Scholar] [CrossRef]
- Padhi, A.; Nanjundaswamy, K.; Masquelier, C.; Okada, S.; Goodenough, J. Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates. J. Electrochem. Soc. 1997, 144, 1609. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 1997, 144, 1188. [Google Scholar] [CrossRef]
- Battery University. BU-216: Summary Table of Lithium-Based Batteries; Battery University: Durant, OK, USA, 2021. [Google Scholar]
- Boston Consulting Group. Batteries for Electric Cars. Challenges, Opportunities, and the Outlook to 2020; BCG Report; Boston Consulting Group: Boston, MA, USA, 2010. [Google Scholar]
- Yang, S.; Song, Y.; Zavalij, P.Y.; Whittingham, M.S. Reactivity, stability and electrochemical behavior of lithium iron phosphates. Electrochem. Commun. 2002, 4, 239–244. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X. Olivine LiFePO4: The remaining challenges for future energy storage. Energy Environ. Sci. 2015, 8, 1110–1138. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Yamada, A.; Chung, S.-C.; Hinokuma, K. Optimized LiFePO4 for lithium battery cathodes. J. Electrochem. Soc. 2001, 148, A224. [Google Scholar] [CrossRef]
- Jugović, D.; Uskoković, D. A review of recent developments in the synthesis procedures of lithium iron phosphate powders. J. Power Sources 2009, 190, 538–544. [Google Scholar] [CrossRef]
- Yoshino, A. The birth of the lithium-ion battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef] [PubMed]
- Gaberscek, M.; Bele, M.; Drofenik, J.; Dominko, R.; Pejovnik, S. Improved carbon anode for lithium batteries pretreatment of carbon particles in a polyelectrolyte solution. Electrochem. Solid-State Lett. 2000, 3, 171. [Google Scholar] [CrossRef]
- Asenbauer, J.; Eisenmann, T.; Kuenzel, M.; Kazzazi, A.; Chen, Z.; Bresser, D. The success story of graphite as a lithium-ion anode material–fundamentals, remaining challenges, and recent developments including silicon (oxide) composites. Sustain. Energy Fuels 2020, 4, 5387–5416. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Drofenik, J.; Gaberscek, M.; Dominko, R.; Poulsen, F.W.; Mogensen, M.; Pejovnik, S.; Jamnik, J. Cellulose as a binding material in graphitic anodes for Li ion batteries: A performance and degradation study. Electrochim. Acta 2003, 48, 883–889. [Google Scholar] [CrossRef]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of lithium-ion batteries—Current state of the art, circular economy, and next generation recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Vasconcelos, D.d.S.; Tenório, J.A.S.; Junior, A.B.B.; Espinosa, D.C.R. Circular recycling strategies for LFP batteries: A review focusing on hydrometallurgy sustainable processing. Metals 2023, 13, 543. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, K.; Jow, T. Low temperature performance of graphite electrode in Li-ion cells. Electrochim. Acta 2002, 48, 241–246. [Google Scholar] [CrossRef]
- Wang, F.; Chen, J.; Tan, Z.; Wu, M.; Yi, B.; Su, W.; Wei, Z.; Liu, S. Low-temperature electrochemical performances of LiFePO4 cathode materials for lithium ion batteries. J. Taiwan Inst. Chem. Eng. 2014, 45, 1321–1330. [Google Scholar] [CrossRef]
- Chen, X.; Gong, Y.; Li, X.; Zhan, F.; Liu, X.; Ma, J. Perspective on low-temperature electrolytes for LiFePO4-based lithium-ion batteries. Int. J. Miner. Metall. Mater. 2023, 30, 1–13. [Google Scholar] [CrossRef]
- Walvekar, H.; Beltran, H.; Sripad, S.; Pecht, M. Implications of the electric vehicle manufacturers’ decision to mass adopt lithium-iron phosphate batteries. IEEE Access 2022, 10, 63834–63843. [Google Scholar] [CrossRef]
- Fernholm, A. They Developed the World’s Most Powerful Battery; The Royal Swedsh Academy of Sciences: Stockholm, Sweden, 2019. [Google Scholar]
- Latini, D.; Vaccari, M.; Lagnoni, M.; Orefice, M.; Mathieux, F.; Huisman, J.; Tognotti, L.; Bertei, A. A comprehensive review and classification of unit operations with assessment of outputs quality in lithium-ion battery recycling. J. Power Sources 2022, 546, 231979. [Google Scholar] [CrossRef]
- Zhang, P.; Yokoyama, T.; Itabashi, O.; Suzuki, T.M.; Inoue, K. Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 1998, 47, 259–271. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, E.J. Re-synthesis and electrochemical characteristics of LiFePO4 cathode materials recycled from scrap electrodes. Bull. Korean Chem. Soc. 2013, 34, 851–855. [Google Scholar] [CrossRef]
- Bergfald, B.; Kristensen, K.; Lystad, H. Recycling of Critical Raw Materials in the Nordics; Nordisk Ministerråd: Copenhagen, Denmark, 2024. [Google Scholar]
- European Union. Regulation (EU) 2023/1542 of the European Parliament and of the Council of 12 July 2023 Concerning Batteries and Waste Batteries, Amending Directive 2008/98/EC and Regulation (EU) 2019/1020 and Repealing Directive 2006/66/EC: Regulation (EU) 2023/1542; European Union: Brussels, Belgium, 2023. [Google Scholar]
- Neubauer, J.; Pesaran, A. The ability of battery second use strategies to impact plug-in electric vehicle prices and serve utility energy storage applications. J. Power Sources 2011, 196, 10351–10358. [Google Scholar] [CrossRef]
- Enache, B.-A.; Seritan, G.-C.; Cepisca, C.; Grigorescu, S.-D.; Argatu, F.-C.; Adochiei, F.-C.; Voicila, T.I. Comparative study of screening methods for second life LiFePO4 batteries. Screening 2020, 5, 14. [Google Scholar]
- Wang, Y.; Tang, B.; Shen, M.; Wu, Y.; Qu, S.; Hu, Y.; Feng, Y. Environmental impact assessment of second life and recycling for LiFePO4 power batteries in China. J. Environ. Manag. 2022, 314, 115083. [Google Scholar] [CrossRef] [PubMed]
- Koch-Ciobotaru, C.; Saez-de-Ibarra, A.; Martinez-Laserna, E.; Stroe, D.-I.; Swierczynski, M.; Rodriguez, P. Second life battery energy storage system for enhancing renewable energy grid integration. In Proceedings of the 2015 IEEE Energy Conversion Congress and Exposition (ECCE), Montreal, QC, Canada, 20–24 September 2015; IEEE: Piscataway, NJ, USA, 2015. [Google Scholar]
- Illa Font, C.H.; Siqueira, H.V.; Neto, J.E.M.; Santos, J.L.F.D.; Stevan, S.L., Jr.; Converti, A.; Corrêa, F.C. Second life of lithium-ion batteries of electric vehicles: A short review and perspectives. Energies 2023, 16, 953. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sheldon, B.W. Deformation and stress in electrode materials for Li-ion batteries. Prog. Mater. Sci. 2014, 63, 58–116. [Google Scholar] [CrossRef]
- Nagpure, S.C.; Bhushan, B.; Babu, S.; Rizzoni, G. Scanning spreading resistance characterization of aged Li-ion batteries using atomic force microscopy. Scr. Mater. 2009, 60, 933–936. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Zhang, X.; Xia, M.; Zhang, X.; Zhang, L.; Shui, M.; Cui, Y.; Shu, J. Surface chemistry of LiFePO4 cathode material as unraveled by HRTEM and XPS. Ionics 2021, 27, 31–37. [Google Scholar] [CrossRef]
- Agubra, V.A.; Fergus, J.W. The formation and stability of the solid electrolyte interface on the graphite anode. J. Power Sources 2014, 268, 153–162. [Google Scholar] [CrossRef]
- Meda, U.S.; Lal, L.; Sushantha, M.; Garg, P. Solid Electrolyte Interphase (SEI), a boon or a bane for lithium batteries: A review on the recent advances. J. Energy Storage 2022, 47, 103564. [Google Scholar] [CrossRef]
- Köbbing, L.; Latz, A.; Horstmann, B. Growth of the solid-electrolyte interphase: Electron diffusion versus solvent diffusion. J. Power Sources 2023, 561, 232651. [Google Scholar] [CrossRef]
- Gachot, G.; Grugeon, S.; Armand, M.; Pilard, S.; Guenot, P.; Tarascon, J.-M.; Laruelle, S. Deciphering the multi-step degradation mechanisms of carbonate-based electrolyte in Li batteries. J. Power Sources 2008, 178, 409–421. [Google Scholar] [CrossRef]
- Demirocak, D.E.; Bhushan, B. Probing the aging effects on nanomechanical properties of a LiFePO4 cathode in a large format prismatic cell. J. Power Sources 2015, 280, 256–262. [Google Scholar] [CrossRef]
- Sharifi-Asl, S.; Lu, J.; Amine, K.; Shahbazian-Yassar, R. Oxygen release degradation in Li-ion battery cathode materials: Mechanisms and mitigating approaches. Adv. Energy Mater. 2019, 9, 1900551. [Google Scholar] [CrossRef]
- Hu, L.-H.; Wu, F.-Y.; Lin, C.-T.; Khlobystov, A.N.; Li, L.-J. Graphene-modified LiFePO4 cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Shen, X.; Li, B.; Liu, H.; Zhao, J. Selective recovery of Li and FePO4 from spent LiFePO4 cathode scraps by organic acids and the properties of the regenerated LiFePO4. Waste Manag. 2020, 113, 32–40. [Google Scholar] [CrossRef]
- Yan, K.; Chen, Q.; Zhang, Z.; Nie, H.; Wang, R.; Xu, Z. A closed-loop process for high-value regeneration of spent LiFePO4 cathodes after selective aluminium precipitation. Green Chem. 2023, 25, 9156–9166. [Google Scholar] [CrossRef]
- Liu, K.; Wang, J.; Wang, M.; Zhang, Q.; Cao, Y.; Huang, L.; Valix, M.; Tsang, D.C. Low-carbon recycling of spent lithium iron phosphate batteries via a hydro-oxygen repair route. Green Chem. 2023, 25, 6642–6651. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, J.; Wang, D.; Jing, Q.; Chen, Y.; Wang, C. Facile and efficient recovery of lithium from spent LiFePO4 batteries via air oxidation–water leaching at room temperature. Green Chem. 2022, 24, 152–162. [Google Scholar] [CrossRef]
- Wang, P.; Lou, X.; Chen, Q.; Liu, Y.; Sun, X.; Guo, Y.; Zhang, X.; Wang, R.; Wang, Z.; Chen, S. Spent LiFePO4: An old but vigorous peroxymonosulfate activator for degradation of organic pollutants in water. Environ. Res. 2022, 214, 113780. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Ali, G.; Kim, H.J.; Yoo, S.H.; Cho, S.O. LiFePO4 microcrystals as an efficient heterogeneous Fenton-like catalyst in degradation of rhodamine 6G. Nanoscale Res. Lett. 2014, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jia, Q.; Wang, L.; Zhao, Y.; Ma, X.; Gong, L.; Zhang, H.; Zuo, T. Highly efficient and selective photocatalytic CO2 reduction using MIL-125 (Ti) and based on LiFePO4 and CuO QDs surface–interface regulation. Catal. Sci. Technol. 2022, 12, 5152–5161. [Google Scholar] [CrossRef]
- Yue, X.-H.; Zhang, C.-C.; Zhang, W.-B.; Wang, Y.; Zhang, F.-S. Recycling phosphorus from spent LiFePO4 battery for multifunctional slow-release fertilizer preparation and simultaneous recovery of Lithium. Chem. Eng. J. 2021, 426, 131311. [Google Scholar] [CrossRef]
- Nasser, O.A.; Petranikova, M. Review of achieved purities after li-ion batteries hydrometallurgical treatment and impurities effects on the cathode performance. Batteries 2021, 7, 60. [Google Scholar] [CrossRef]
- Kaas, A.; Wilke, C.; Vanderbruggen, A.; Peuker, U.A. Influence of different discharge levels on the mechanical recycling efficiency of lithium-ion batteries. Waste Manag. 2023, 172, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, C.; Petranikova, M. Lithium batteries recycling. In Lithium Process Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–267. [Google Scholar]
- Bae, H.; Kim, Y. Technologies of lithium recycling from waste lithium ion batteries: A review. Mater. Adv. 2021, 2, 3234–3250. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, F.-S.; He, K.; Zhang, Z.-Y.; Zhang, C.-C. Avoiding thermal runaway during spent lithium-ion battery recycling: A comprehensive assessment and a new approach for battery discharge. J. Clean. Prod. 2022, 380, 135045. [Google Scholar] [CrossRef]
- Ojanen, S.; Lundström, M.; Santasalo-Aarnio, A.; Serna-Guerrero, R. Challenging the concept of electrochemical discharge using salt solutions for lithium-ion batteries recycling. Waste Manag. 2018, 76, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Punt, T.; Bradshaw, S.M.; van Wyk, P.; Akdogan, G. The efficiency of black mass preparation by discharge and alkaline leaching for LIB recycling. Minerals 2022, 12, 753. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Jeong, S.; Park, J.; Kim, W.; Ko, G.; Park, K.; Kim, H.-I.; Kwon, K. Resynthesis of Ni-rich Li[Ni0.9Co0.05Mn0.05]O2 in simulated Li-ion battery leachate after saline discharge. J. Alloys Compd. 2023, 960, 170910. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, F.-S.; Zhang, Z.-Y.; Zhang, C.-C. Corrosion behavior and corrosion inhibition performance of spent lithium-ion battery during discharge. Sep. Purif. Technol. 2023, 306, 122640. [Google Scholar] [CrossRef]
- Fang, Z.; Duan, Q.; Peng, Q.; Wei, Z.; Cao, H.; Sun, J.; Wang, Q. Comparative study of chemical discharge strategy to pretreat spent lithium-ion batteries for safe, efficient, and environmentally friendly recycling. J. Clean. Prod. 2022, 359, 132116. [Google Scholar] [CrossRef]
- Sonoc, A.; Jeswiet, J.; Soo, V.K. Opportunities to improve recycling of automotive lithium ion batteries. Procedia Cirp 2015, 29, 752–757. [Google Scholar] [CrossRef]
- Zhao, T.; Marthi, R.; Mahandra, H.; Chae, S.; Traversy, M.; Sadri, F.; Choi, Y.; Ghahreman, A. Direct selective leaching of lithium from industrial-grade black mass of waste lithium-ion batteries containing LiFePO4 cathodes. Waste Manag. 2023, 171, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.N.; Lim, G.J.; Cai, Y.; Chua, R.; Guo, Y.; Yan, Y.; Srinivasan, M. Electrolyte designs for safer lithium-ion and lithium-metal batteries. J. Mater. Chem. A 2023, 11, 22688–22717. [Google Scholar] [CrossRef]
- Zachmann, N.; Fox, R.V.; Petranikova, M.; Ebin, B. Implementation of a sub-and supercritical carbon dioxide process for the selective recycling of the electrolyte from spent Li-ion battery. J. CO2 Util. 2024, 81, 102703. [Google Scholar] [CrossRef]
- Botte, G.G.; White, R.E.; Zhang, Z. Thermal stability of LiPF6–EC: EMC electrolyte for lithium ion batteries. J. Power Sources 2001, 97, 570–575. [Google Scholar] [CrossRef]
- Gnanaraj, J.; Zinigrad, E.; Asraf, L.; Gottlieb, H.; Sprecher, M.; Schmidt, M.; Geissler, W.; Aurbach, D. A detailed investigation of the thermal reactions of LiPF6 solution in organic carbonates using ARC and DSC. J. Electrochem. Soc. 2003, 150, A1533. [Google Scholar] [CrossRef]
- Röder, P.; Baba, N.; Friedrich, K.A.; Wiemhöfer, H.-D. Impact of delithiated Li0FePO4 on the decomposition of LiPF6-based electrolyte studied by accelerating rate calorimetry. J. Power Sources 2013, 236, 151–157. [Google Scholar] [CrossRef]
- Shi, G.; Wang, J.; Zhang, S.; Cheng, J.; Shao, X.; Xu, Z.; Chen, X.; Xin, B. Green regeneration and high-value utilization technology of the electrolyte from spent lithium-ion batteries. Sep. Purif. Technol. 2024, 335, 126144. [Google Scholar] [CrossRef]
- Huang, H.; Liu, C.; Sun, Z. In-situ pyrolysis based on alkaline medium removes fluorine-containing contaminants from spent lithium-ion batteries. J. Hazard. Mater. 2023, 457, 131782. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Ebin, B.; Foreman, M.R.S.J.; Steenari, B.-M.; Petranikova, M. Incineration of EV lithium-ion batteries as a pretreatment for recycling–determination of the potential formation of hazardous by-products and effects on metal compounds. J. Hazard. Mater. 2020, 393, 122372. [Google Scholar] [CrossRef]
- Ji, Y.; Jafvert, C.T.; Zyaykina, N.N.; Zhao, F. Decomposition of PVDF to delaminate cathode materials from end-of-life lithium-ion battery cathodes. J. Clean. Prod. 2022, 367, 133112. [Google Scholar] [CrossRef]
- Jie, Y.; Yang, S.; Li, Y.; Hu, F.; Zhao, D.; Chang, D.; Lai, Y.; Chen, Y. Waste organic compounds thermal treatment and valuable cathode materials recovery from spent LiFePO4 batteries by vacuum pyrolysis. ACS Sustain. Chem. Eng. 2020, 8, 19084–19095. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Qu, L.; Feng, Y.; Li, J.; Liu, J.; Zhang, G.; Xie, W. Enhancement in leaching process of lithium and cobalt from spent lithium-ion batteries using benzenesulfonic acid system. Waste Manag. 2019, 88, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Golmohammadzadeh, R.; Dimachki, Z.; Bryant, W.; Zhang, J.; Biniaz, P.; Holl, M.M.B.; Pozo-Gonzalo, C.; Banerjee, P.C. Removal of polyvinylidene fluoride binder and other organics for enhancing the leaching efficiency of lithium and cobalt from black mass. J. Environ. Manag. 2023, 343, 118205. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; May, R.; Ramesh, S.; Chang, W.; Marbella, L.E. Recovery and reuse of composite cathode binder in lithium ion batteries. ChemistryOpen 2021, 10, 545–552. [Google Scholar] [CrossRef]
- He, K.; Zhang, Z.-Y.; Zhang, F.-S. Selectively peeling of spent LiFePO4 cathode by destruction of crystal structure and binder matrix for efficient recycling of spent battery materials. J. Hazard. Mater. 2020, 386, 121633. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mu, D.; Li, R.; Ma, Q.; Zheng, R.; Dai, C. Purification and characterization of reclaimed electrolytes from spent lithium-ion batteries. J. Phys. Chem. C 2017, 121, 4181–4187. [Google Scholar] [CrossRef]
- Fu, Y.; Schuster, J.; Petranikova, M.; Ebin, B. Innovative recycling of organic binders from electric vehicle lithium-ion batteries by supercritical carbon dioxide extraction. Resour. Conserv. Recycl. 2021, 172, 105666. [Google Scholar] [CrossRef]
- Chen, X.; Li, S.; Wu, X.; Zhou, T.; Ma, H. In-situ recycling of coating materials and Al foils from spent lithium ion batteries by ultrasonic-assisted acid scrubbing. J. Clean. Prod. 2020, 258, 120943. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Li, J.; Tao, S.; Gan, Q.; Tang, X. Effect of Cu impurity on the electrochemical performance of regenerated LiFePO4/C electrode materials. J. Mater. Sci. Mater. Electron. 2020, 31, 10460–10469. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, D. Aluminium behaviour in preparation process of lithium iron phosphate and its effects on material electrochemical performance. J. Mater. Res. Technol. 2021, 15, 3575–3584. [Google Scholar] [CrossRef]
- Bi, H.; Zhu, H.; Zu, L.; He, S.; Gao, Y.; Peng, J. Combined mechanical process recycling technology for recovering copper and aluminium components of spent lithium-iron phosphate batteries. Waste Manag. Res. 2019, 37, 767–780. [Google Scholar] [CrossRef]
- Zhu, H.; Bai, Y.; Zu, L.; Bi, H.; Wen, J. Separation of metal and cathode materials from waste lithium iron phosphate battery by electrostatic process. Separations 2023, 10, 220. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, W.; Han, J.; Jiao, F.; Qin, W.; Liu, T.; Zhao, C. Pyrolysis and physical separation for the recovery of spent LiFePO4 batteries. Waste Manag. 2019, 89, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhang, T.; Xie, W.; Zhu, X. Enhancement in liberation of electrode materials derived from spent lithium-ion battery by pyrolysis. J. Clean. Prod. 2018, 199, 62–68. [Google Scholar] [CrossRef]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of lithium-ion batteries: A novel method to separate coating and foil of electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Jiang, Z.; Sun, J.; Jia, P.; Wang, W.; Song, Z.; Zhao, X.; Mao, Y. A sustainable strategy for spent Li-ion battery regeneration: Microwave-hydrothermal relithiation complemented with anode-revived graphene to construct a LiFePO4/MWrGO cathode material. Sustain. Energy Fuels 2022, 6, 2207–2222. [Google Scholar] [CrossRef]
- Jia, K.; Ma, J.; Wang, J.; Liang, Z.; Ji, G.; Piao, Z.; Gao, R.; Zhu, Y.; Zhuang, Z.; Zhou, G. Long-life regenerated LiFePO4 from spent cathode by elevating the d-band center of Fe. Adv. Mater. 2023, 35, 2208034. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.; Francia, C.; Fiore, S. Closed-loop recycling of lithium iron phosphate cathodic powders via citric acid leaching. Environ. Sci. Pollut. Res. 2024, 1–16. [Google Scholar] [CrossRef]
- Qi, C.; Wang, S.; Zhu, X.; Zhang, T.; Gou, Y.; Xie, Z.; Jin, Y.; Wang, Y.; Song, L.; Zhang, M. Environmental-friendly low-cost direct regeneration of cathode material from spent LiFePO4. J. Alloys Compd. 2022, 924, 166612. [Google Scholar] [CrossRef]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A closed loop process for recycling spent lithium ion batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, K.; Zhang, X.; Peng, C.; Jiang, Y.; Chen, W. Aluminum separation by sulfuric acid leaching-solvent extraction from Al-bearing LiFePO4/C powder for recycling of Fe/P. Waste Manag. 2022, 144, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, Z.; Jia, P.; Li, S.; Wang, W.; Song, Z.; Mao, Y.; Zhao, X.; Zhou, B. A sustainable revival process for defective LiFePO4 cathodes through the synergy of defect-targeted healing and in-situ construction of 3D-interconnected porous carbon networks. Waste Manag. 2023, 158, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Zhang, R.; Wang, Y.; Shu, H. Hydrothermal preparation and performance of LiFePO4 by using Li3PO4 recovered from spent cathode scraps as Li source. Waste Manag. 2018, 78, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, M.; Deng, L.; Cheng, Y.-J.; Gao, J.; Xia, Y. Direct regeneration of spent lithium iron phosphate via a low-temperature molten salt process coupled with a reductive environment. Ind. Eng. Chem. Res. 2022, 61, 3831–3839. [Google Scholar] [CrossRef]
- Bai, Y.; Zhu, H.; Zu, L.; Zhang, Y.; Bi, H. Environment-friendly, efficient process for mechanical recovery of waste lithium iron phosphate batteries. Waste Manag. Res. 2023, 41, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, J.; Bai, X.; Wang, S.; Yang, D.; Fu, Y.; He, Y. Separation of the cathode materials from the Al foil in spent lithium-ion batteries by cryogenic grinding. Waste Manag. 2019, 91, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, S.; Wang, Y.; Jiang, Y.; Tan, X.; Han, W.; Wang, S. Recycling of LiFePO4 cathode materials from spent lithium-ion batteries through ultrasound-assisted Fenton reaction and lithium compensation. Waste Manag. 2021, 136, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Zhang, H.; Qi, C.; Ma, J.; Zhou, Z.; Sun, Q.; Song, L.; Jin, Y.; Zhang, M. Effective regeneration of waste LiFePO4 cathode material by Cu doping modification. Appl. Surf. Sci. 2024, 659, 159920. [Google Scholar] [CrossRef]
- Song, L.; Qi, C.; Wang, S.; Zhu, X.; Zhang, T.; Jin, Y.; Zhang, M. Direct regeneration of waste LiFePO4 cathode materials with a solid-phase method promoted by activated CNTs. Waste Manag. 2023, 157, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fang, L.-Z.; Kang, Y.-Q.; Wang, L.; Zhou, Y.-N.; Liu, X.-Y.; Li, T.; Li, Y.-X.; Liang, Z.; Zhang, Z.-X.; et al. A novel three-step approach to separate cathode components for lithium-ion battery recycling. Rare Met. 2021, 40, 1431–1436. [Google Scholar] [CrossRef]
- Xu, P.; Dai, Q.; Gao, H.; Liu, H.; Zhang, M.; Li, M.; Chen, Y.; An, K.; Meng, Y.; Liu, P. Efficient direct recycling of lithium-ion battery cathodes by targeted healing. Joule 2020, 4, 2609–2626. [Google Scholar] [CrossRef]
- Song, X.; Hu, T.; Liang, C.; Long, H.; Zhou, L.; Song, W.; You, L.; Wu, Z.; Liu, J. Direct regeneration of cathode materials from spent lithium iron phosphate batteries using a solid phase sintering method. RSC Adv. 2017, 7, 4783–4790. [Google Scholar] [CrossRef]
- Wang, M.; Tan, Q.; Liu, L.; Li, J. Revealing the dissolution mechanism of polyvinylidene fluoride of spent lithium-ion batteries in waste oil-based methyl ester solvent. ACS Sustain. Chem. Eng. 2020, 8, 7489–7496. [Google Scholar] [CrossRef]
- Fan, X.; Song, C.; Lu, X.; Shi, Y.; Yang, S.; Zheng, F.; Huang, Y.; Liu, K.; Wang, H.; Li, Q. Separation and recovery of valuable metals from spent lithium-ion batteries via concentrated sulfuric acid leaching and regeneration of LiNi1/3Co1/3Mn1/3O2. J. Alloys Compd. 2021, 863, 158775. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, R.; Wang, W.; Tu, S.; Hu, Y.; Wang, X.; Zhan, R.; Wang, J.; Zhao, J.; Liu, S. Reaction-passivation mechanism driven materials separation for recycling of spent lithium-ion batteries. Nat. Commun. 2023, 14, 4648. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Muralidharan, N.; Li, J.; Essehli, R.; Belharouak, I. Sustainable direct recycling of lithium-ion batteries via solvent recovery of electrode materials. ChemSusChem 2020, 13, 5664–5670. [Google Scholar] [CrossRef]
- Kong, Y.; Yuan, L.; Liao, Y.; Shao, Y.; Hao, S.; Huang, Y. Efficient separation and selective Li recycling of spent LiFePO4 cathode. Energy Mater. 2023, 3, 300053. [Google Scholar] [CrossRef]
- Cuesta, N.; Ramos, A.; Cameán, I.; Antuña, C.; García, A.B. Hydrocolloids as binders for graphite anodes of lithium-ion batteries. Electrochim. Acta 2015, 155, 140–147. [Google Scholar] [CrossRef]
- Baboo, J.P.; Yatoo, M.A.; Dent, M.; Najafabadi, E.H.; Lekakou, C.; Slade, R.; Hinder, S.J.; Watts, J.F. Exploring different binders for a LiFePO4 battery, battery testing, modeling and simulations. Energies 2022, 15, 2332. [Google Scholar] [CrossRef]
- Thompson, D.L.; Hartley, J.M.; Lambert, S.M.; Shiref, M.; Harper, G.D.; Kendrick, E.; Anderson, P.; Ryder, K.S.; Gaines, L.; Abbott, A.P. The importance of design in lithium ion battery recycling–a critical review. Green Chem. 2020, 22, 7585–7603. [Google Scholar] [CrossRef]
- Bi, H.; Zhu, H.; Zu, L.; Gao, Y.; Gao, S.; Bai, Y. Environment-friendly technology for recovering cathode materials from spent lithium iron phosphate batteries. Waste Manag. Res. 2020, 38, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Verdugo, L.; Zhang, L.; Etschmann, B.; Bruckard, W.; Menacho, J.; Hoadley, A. Effect of lithium ion on the separation of electrode materials in spent lithium ion batteries using froth flotation. Sep. Purif. Technol. 2023, 311, 123241. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhao, W.; Lu, D.; Ren, G.; Tu, Y. Application of roasting flotation technology to enrich valuable metals from spent LiFePO4 batteries. ACS Omega 2022, 7, 25590–25599. [Google Scholar] [CrossRef]
- Wang, C.; Ding, E.; Zhang, X.; Zeng, Y.; Sun, W.; Wei, Z.; Yang, Y.; Tang, H. Selective flotation separation mechanism of LFPs and graphite electrode materials using CMC as inhibitor. J. Environ. Chem. Eng. 2024, 12, 112297. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, J.; Gan, T.; Lu, D.; Wang, Y.; Zheng, X. High-intensity magnetic separation for recovery of LiFePO4 and graphite from spent lithium-ion batteries. Sep. Purif. Technol. 2022, 297, 121486. [Google Scholar] [CrossRef]
- Cornelio, A.; Zanoletti, A.; Bontempi, E. Recent progress in pyrometallurgy for the recovery of spent lithium-ion batteries: A review of state-of-the-art developments. Curr. Opin. Green Sustain. Chem. 2024, 46, 100881. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical options for recycling spent lithium-ion batteries: A comprehensive review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Narzari, R.; Gogoi, B.; Geed, S.R. Pyrometallurgy: Urban mining and its future implications. In Global E-Waste Management Strategies and Future Implications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 125–142. [Google Scholar]
- Xu, Y.; Zhang, B.; Ge, Z.; Zhang, S.; Song, B.; Tian, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Advances and perspectives towards spent LiFePO4 battery recycling. J. Clean. Prod. 2023, 434, 140077. [Google Scholar] [CrossRef]
- Zhang, J. Pyrometallurgy-based applications in spent lithium-ion battery recycling. In Nano Technology for Battery Recycling, Remanufacturing, and Reusing; Elsevier: Amsterdam, The Netherlands, 2022; pp. 171–182. [Google Scholar]
- Holzer, A.; Windisch-Kern, S.; Ponak, C.; Raupenstrauch, H. A novel pyrometallurgical recycling process for lithium-ion batteries and its application to the recycling of LCO and LFP. Metals 2021, 11, 149. [Google Scholar] [CrossRef]
- Qu, G.; Yang, J.; Wang, H.; Ran, Y.; Li, B.; Wei, Y. Applicability of the reduction smelting recycling process to different types of spent lithium-ion batteries cathode materials. Waste Manag. 2023, 166, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, F.; Gao, S.; Zhao, J.; Wang, D.; Yin, H. NaOH-assisted low-temperature roasting to recover spent LiFePO4 batteries. Waste Manag. 2022, 153, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Qu, X.; Chen, X.; Liu, D.; Zhao, Z.; Xie, H.; Wang, D.; Yin, H. A sodium salt-assisted roasting approach followed by leaching for recovering spent LiFePO4 batteries. J. Hazard. Mater. 2022, 424, 127586. [Google Scholar] [CrossRef] [PubMed]
- Kotaich, K.; Sloop, S.E. Cobalt-free batteries, a new frontier for advanced battery recycling. In Proceedings of the 2009 IEEE International Symposium on Sustainable Systems and Technology, Tempe, AZ, USA, 18–20 May 2009; IEEE: Piscataway, NJ, USA, 2009. [Google Scholar]
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles—Critical issues. J. Clean. Prod. 2010, 18, 1519–1529. [Google Scholar] [CrossRef]
- Holzer, A.; Wiszniewski, L.; Windisch-Kern, S.; Raupenstrauch, H. Optimization of a pyrometallurgical process to efficiently recover valuable metals from commercially used lithium-ion battery cathode materials LCO, NCA, NMC622, and LFP. Metals 2022, 12, 1642. [Google Scholar] [CrossRef]
- Zheng, R.; Zhao, L.; Wang, W.; Liu, Y.; Ma, Q.; Mu, D.; Li, R.; Dai, C. Optimized Li and Fe recovery from spent lithium-ion batteries via a solution-precipitation method. RSC Adv. 2016, 6, 43613–43625. [Google Scholar] [CrossRef]
- Li, H.; Xing, S.; Liu, Y.; Li, F.; Guo, H.; Kuang, G. Recovery of lithium, iron, and phosphorus from spent LiFePO4 batteries using stoichiometric sulfuric acid leaching system. ACS Sustain. Chem. Eng. 2017, 5, 8017–8024. [Google Scholar] [CrossRef]
- Kumar, J.; Neiber, R.R.; Park, J.; Soomro, R.A.; Greene, G.W.; Mazari, S.A.; Seo, H.Y.; Lee, J.H.; Shon, M.; Chang, D.W. Recent progress in sustainable recycling of LiFePO4-type lithium-ion batteries: Strategies for highly selective lithium recovery. Chem. Eng. J. 2022, 431, 133993. [Google Scholar] [CrossRef]
- Zhao, T.; Li, W.; Traversy, M.; Choi, Y.; Ghahreman, A.; Zhao, Z.; Zhang, C.; Zhao, W.; Song, Y. A review on the recycling of spent lithium iron phosphate batteries. J. Environ. Manag. 2024, 351, 119670. [Google Scholar] [CrossRef]
- Shentu, H.; Xiang, B.; Cheng, Y.-J.; Dong, T.; Gao, J.; Xia, Y. A fast and efficient method for selective extraction of lithium from spent lithium iron phosphate battery. Environ. Technol. Innov. 2021, 23, 101569. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, X.; Cao, H.; Lin, X.; Liu, C.; Sun, Y.; Zhang, Y.; Sun, Z. Selective recovery of lithium from spent lithium iron phosphate batteries: A sustainable process. Green Chem. 2018, 20, 3121–3133. [Google Scholar] [CrossRef]
- Jing, Q.; Zhang, J.; Liu, Y.; Yang, C.; Ma, B.; Chen, Y.; Wang, C. E-pH diagrams for the Li-Fe-P-H2O system from 298 to 473 K: Thermodynamic analysis and application to the wet chemical processes of the LiFePO4 cathode material. J. Phys. Chem. C 2019, 123, 14207–14215. [Google Scholar] [CrossRef]
- Qin, Z.; Li, X.; Shen, X.; Cheng, Y.; Wu, F.; Li, Y.; He, Z. Electrochemical selective lithium extraction and regeneration of spent lithium iron phosphate. Waste Manag. 2024, 174, 106–113. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Silvester, D.S.; Banks, C.E.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Direct regeneration of cathode materials in spent lithium-ion batteries toward closed-loop recycling and sustainability. J. Power Sources 2024, 589, 233728. [Google Scholar] [CrossRef]
- Lan, Y.; Li, X.; Zhou, G.; Yao, W.; Cheng, H.M.; Tang, Y. Direct regenerating cathode materials from spent lithium-ion batteries. Adv. Sci. 2024, 11, 2304425. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Tran, D.; Chen, Z. Seeking direct cathode regeneration for more efficient lithium-ion battery recycling. Curr. Opin. Electrochem. 2022, 31, 100875. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, H.; Liu, Z.; Jin, M.; Deng, L.; Li, S.; Huang, Y. A recrystallization approach to repairing spent LiFePO4 black mass. J. Mater. Chem. A 2023, 11, 9057–9065. [Google Scholar] [CrossRef]
- Jing, Q.; Zhang, J.; Liu, Y.; Zhang, W.; Chen, Y.; Wang, C. Direct regeneration of spent LiFePO4 cathode material by a green and efficient one-step hydrothermal method. ACS Sustain. Chem. Eng. 2020, 8, 17622–17628. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Song, J.; Song, D.; Zhang, L.; Shi, X. Environmentally friendly recycling and effective repairing of cathode powders from spent LiFePO4 batteries. Green Chem. 2016, 18, 2500–2506. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Song, D.; Song, J.; Zhang, L. Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries. J. Power Sources 2017, 345, 78–84. [Google Scholar] [CrossRef]
- Liang, Q.; Yue, H.; Wang, S.; Yang, S.; Lam, K.-H.; Hou, X. Recycling and crystal regeneration of commercial used LiFePO4 cathode materials. Electrochim. Acta 2020, 330, 135323. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Zhang, X.; Ge, M.; Zhang, H.; Yin, Y.; Yang, S.-T. High electrochemical performance recycling spent LiFePO4 materials through the preoxidation regeneration strategy. ACS Sustain. Chem. Eng. 2023, 11, 14457–14466. [Google Scholar] [CrossRef]
- Ji, G.; Wang, J.; Liang, Z.; Jia, K.; Ma, J.; Zhuang, Z.; Zhou, G.; Cheng, H.-M. Direct regeneration of degraded lithium-ion battery cathodes with a multifunctional organic lithium salt. Nat. Commun. 2023, 14, 584. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jiao, X.; Bian, H.; Lu, X.-Y.; Zhang, Z. Direct relithiation and efficient regeneration of spent LiFePO4 materials through thermochemical healing. Ionics 2023, 29, 4569–4576. [Google Scholar] [CrossRef]
- Kim, D.-S.; Sohn, J.-S.; Lee, C.-K.; Lee, J.-H.; Han, K.-S.; Lee, Y.-I. Simultaneous separation and renovation of lithium cobalt oxide from the cathode of spent lithium ion rechargeable batteries. J. Power Sources 2004, 132, 145–149. [Google Scholar] [CrossRef]
- Song, W.; Liu, J.; You, L.; Wang, S.; Zhou, Q.; Gao, Y.; Yin, R.; Xu, W.; Guo, Z. Re-synthesis of nano-structured LiFePO4/graphene composite derived from spent lithium-ion battery for booming electric vehicle application. J. Power Sources 2019, 419, 192–202. [Google Scholar] [CrossRef]
- Tang, X.; Wang, R.; Ren, Y.; Duan, J.; Li, J.; Li, P. Effective regeneration of scrapped LiFePO4 material from spent lithium-ion batteries. J. Mater. Sci. 2020, 55, 13036–13048. [Google Scholar] [CrossRef]
- Wu, C.; Hu, J.; Ye, L.; Su, Z.; Fang, X.; Zhu, X.; Zhuang, L.; Ai, X.; Yang, H.; Qian, J. Direct regeneration of spent Li-ion battery cathodes via chemical relithiation reaction. ACS Sustain. Chem. Eng. 2021, 9, 16384–16393. [Google Scholar] [CrossRef]
- Ganter, M.J.; Landi, B.J.; Babbitt, C.W.; Anctil, A.; Gaustad, G. Cathode refunctionalization as a lithium ion battery recycling alternative. J. Power Sources 2014, 256, 274–280. [Google Scholar] [CrossRef]
- Ouaneche, T.; Courty, M.; Stievano, L.; Monconduit, L.; Guéry, C.; Sougrati, M.T.; Recham, N. Room temperature efficient regeneration of spent LiFePO4 by direct chemical lithiation. J. Power Sources 2023, 579, 233248. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B.; Ge, Z.; Wang, H.; Hong, N.; Xiao, X.; Song, B.; Zhang, Y.; Tian, Y.; Deng, W. Direct recovery of degraded LiFePO4 cathode via mild chemical relithiation strategy. Chem. Eng. J. 2023, 477, 147201. [Google Scholar] [CrossRef]
- Im, J.; Heo, K.; Kang, S.-W.; Jeong, H.; Kim, J.; Lim, J. LiFePO4 synthesis using refined Li3PO4 from wastewater in Li-ion battery recycling process. J. Electrochem. Soc. 2019, 166, A3861. [Google Scholar] [CrossRef]
- Wang, T.; Yu, X.; Fan, M.; Meng, Q.; Xiao, Y.; Yin, Y.-X.; Li, H.; Guo, Y.-G. Direct regeneration of spent LiFePO4 via a graphite prelithiation strategy. Chem. Commun. 2020, 56, 245–248. [Google Scholar] [CrossRef]
- Li, C.; Du, H.; Kang, Y.; Zhao, Y.; Tian, Y.; Wozny, J.; Lu, J.; Li, T.; Tavajohi, N.; Huang, M. Room-temperature direct regeneration of spent LiFePO4 cathode using the external short circuit strategy. Next Sustain. 2023, 1, 100008. [Google Scholar] [CrossRef]
- Fan, M.; Meng, Q.; Chang, X.; Gu, C.F.; Meng, X.H.; Yin, Y.X.; Li, H.; Wan, L.J.; Guo, Y.G. In situ electrochemical regeneration of degraded LiFePO4 electrode with functionalized prelithiation separator. Adv. Energy Mater. 2022, 12, 2103630. [Google Scholar] [CrossRef]
- Shin, E.J.; Kim, S.; Noh, J.-K.; Byun, D.; Chung, K.Y.; Kim, H.-S.; Cho, B.-W. A green recycling process designed for LiFePO4 cathode materials for Li-ion batteries. J. Mater. Chem. A 2015, 3, 11493–11502. [Google Scholar] [CrossRef]
- Fu, D.; Zhou, W.; Liu, J.; Zeng, S.-Z.; Wang, L.; Liu, W.; Yu, X.; Liu, X. A facile route for the efficient leaching, recovery, and regeneration of lithium and iron from waste lithium iron phosphate cathode materials. Sep. Purif. Technol. 2024, 342, 127069. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, J.; Zou, J.; Ji, G.; Ye, L.; Li, D.; Zhang, B.; Ou, X. Closed-loop regeneration of LiFePO4 from spent lithium-ion batteries: A “feed three birds with one scone” strategy toward advanced cathode materials. J. Clean. Prod. 2021, 316, 128098. [Google Scholar] [CrossRef]
- Sun, F.; Gao, M.; Jiao, W.; Qi, L.; He, Z.; Zhang, H.; Cao, Y.; Song, D.; Zhang, L. A novel acid-free leaching route of recovering Li2CO3 and FePO4 from spent LiFePO4 black powder. J. Alloys Compd. 2023, 965, 171429. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, X.; Zhang, B.; Di, A.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Start from the source: Direct treatment of a degraded LiFePO4 cathode for efficient recycling of spent lithium-ion batteries. Green Chem. 2022, 24, 7448–7457. [Google Scholar] [CrossRef]
- Hu, G.; Gong, Y.; Peng, Z.; Du, K.; Huang, M.; Wu, J.; Guan, D.; Zeng, J.; Zhang, B.; Cao, Y. Direct recycling strategy for spent lithium iron phosphate powder: An efficient and wastewater-free process. ACS Sustain. Chem. Eng. 2022, 10, 11606–11616. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, X.; Shen, G.; Chen, X.; Wang, J.; Xie, L.; Han, Q.; Zhu, L.; Li, J.; Cao, X. Driving the rapid regeneration of LiFePO4 from spent lithium-ion batteries through one-pot mechanochemical activation. Green Chem. 2024, 26, 1501–1510. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, J.-L.; Jing, Q.-K.; Liu, Y.-B.; Chen, Y.-Q.; Wang, C.-Y. Recovery and regeneration of LiFePO4 from spent lithium-ion batteries via a novel pretreatment process. Int. J. Miner. Metall. Mater. 2021, 28, 1478–1487. [Google Scholar] [CrossRef]
- Song, Y.; Xie, B.; Song, S.; Lei, S.; Sun, W.; Xu, R.; Yang, Y. Regeneration of LiFePO4 from spent lithium-ion batteries via a facile process featuring acid leaching and hydrothermal synthesis. Green Chem. 2021, 23, 3963–3971. [Google Scholar] [CrossRef]
- Qin, X.; Yang, G.; Cai, F.; Jiang, B.; Chen, H.; Tan, C.; Kandasamy, S.; Kandasamy, K.; Sulaiman, M.; Su, N. Recovery and reuse of spent LiFePO4 batteries. J. New Mater. Electrochem. Syst 2019, 22, 119–124. [Google Scholar] [CrossRef]
- Bian, D.; Sun, Y.; Li, S.; Tian, Y.; Yang, Z.; Fan, X.; Zhang, W. A novel process to recycle spent LiFePO4 for synthesizing LiFePO4/C hierarchical microflowers. Electrochim. Acta 2016, 190, 134–140. [Google Scholar] [CrossRef]
- Liu, K.; Yang, S.; Lai, F.; Li, Q.; Wang, H.; Tao, T.; Xiang, D.; Zhang, X. Application of H4P2O7 as leaching acid in one-step selective recovery for metals from spent LiFePO4 batteries. Ionics 2021, 27, 5127–5135. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, L.; Yan, S.; Ma, X. Self-activation of Ferro-chemistry based advanced oxidation process towards in-situ recycling of spent LiFePO4 batteries. Chem. Eng. J. 2023, 471, 144343. [Google Scholar] [CrossRef]
- Yadav, P.; Jie, C.J.; Tan, S.; Srinivasan, M. Recycling of cathode from spent lithium iron phosphate batteries. J. Hazard. Mater. 2020, 399, 123068. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, M.; Cao, S.; Chen, G.; Guo, X.; Wang, X. Regeneration and performance of LiFePO4 with Li2CO3 and FePO4 as raw materials recovered from spent LiFePO4 batteries. Mater. Chem. Phys. 2022, 279, 125750. [Google Scholar] [CrossRef]
- Andersson, A.S.; Kalska, B.; Häggström, L.; Thomas, J.O. Lithium extraction/insertion in LiFePO4: An X-ray diffraction and Mössbauer spectroscopy study. Solid State Ion. 2000, 130, 41–52. [Google Scholar] [CrossRef]
- Sun, C.; Rajasekhara, S.; Goodenough, J.B.; Zhou, F. Monodisperse porous LiFePO4 microspheres for a high power Li-ion battery cathode. J. Am. Chem. Soc. 2011, 133, 2132–2135. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Tobishima, S.; Takei, K.; Sakurai, Y. Characterization of LiFePO4 as the cathode material for rechargeable lithium batteries. J. Power Sources 2001, 97, 508–511. [Google Scholar] [CrossRef]
- Doeff, M.M.; Visco, S.J.; Yanping, M.; Peng, M.; Lei, D.; De Jonghe, L.C. Thin film solid state sodium batteries for electric vehicles. Electrochim. Acta 1995, 40, 2205–2210. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, G.; Duan, Y.; Wu, Y.; Zhang, T.; Zhao, X.; Luo, M.; Liu, Y. The research and industrialization progress and prospects of sodium ion battery. J. Alloys Compd. 2023, 958, 170486. [Google Scholar] [CrossRef]
- Wan, G.; Dou, W.; Zhu, H.; Zhang, W.; Liu, T.; Wang, L.; Lu, J. Empowering higher energy sodium-ion battery cathode by oxygen chemistry. Interdiscip. Mater. 2023, 2, 416–422. [Google Scholar] [CrossRef]


| Leaching | H2SO4 Concentration [M] | L/S [mL/g] | pH | T [°C] | Leaching Efficiency [%] | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Li | Fe | P | ||||||
| Complete | 2.5 | 10.0 | 1.5 | 60 | 97.0 | 98.0 | - | [138] |
| Selective | 0.3 | 10.5 | 3.7 | 60 | 95.7 | 0.017 | 1.97 | [139] |
| Selective | 9.0 | 10.0 | 3.5 | 25 | 99.3 | 0.020 | 0.020 | [55] |
| Method | Advantages | Disadvantages |
|---|---|---|
| Thermal (solid-state) |
|
|
| Molten salt |
|
|
| Hydrothermal |
|
|
| Chemical |
|
|
| Electrochemical |
|
|
| Indirect |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa de Mattos, D.F.; Duda, S.; Petranikova, M. Recycling of Lithium Iron Phosphate (LiFePO4) Batteries from the End Product Quality Perspective. Batteries 2025, 11, 33. https://doi.org/10.3390/batteries11010033
Barbosa de Mattos DF, Duda S, Petranikova M. Recycling of Lithium Iron Phosphate (LiFePO4) Batteries from the End Product Quality Perspective. Batteries. 2025; 11(1):33. https://doi.org/10.3390/batteries11010033
Chicago/Turabian StyleBarbosa de Mattos, Deise F., Simon Duda, and Martina Petranikova. 2025. "Recycling of Lithium Iron Phosphate (LiFePO4) Batteries from the End Product Quality Perspective" Batteries 11, no. 1: 33. https://doi.org/10.3390/batteries11010033
APA StyleBarbosa de Mattos, D. F., Duda, S., & Petranikova, M. (2025). Recycling of Lithium Iron Phosphate (LiFePO4) Batteries from the End Product Quality Perspective. Batteries, 11(1), 33. https://doi.org/10.3390/batteries11010033







