Recent Progress in Cathode-Free Zinc Electrolytic MnO2 Batteries: Electrolytes and Electrodes
Abstract
:1. Introduction
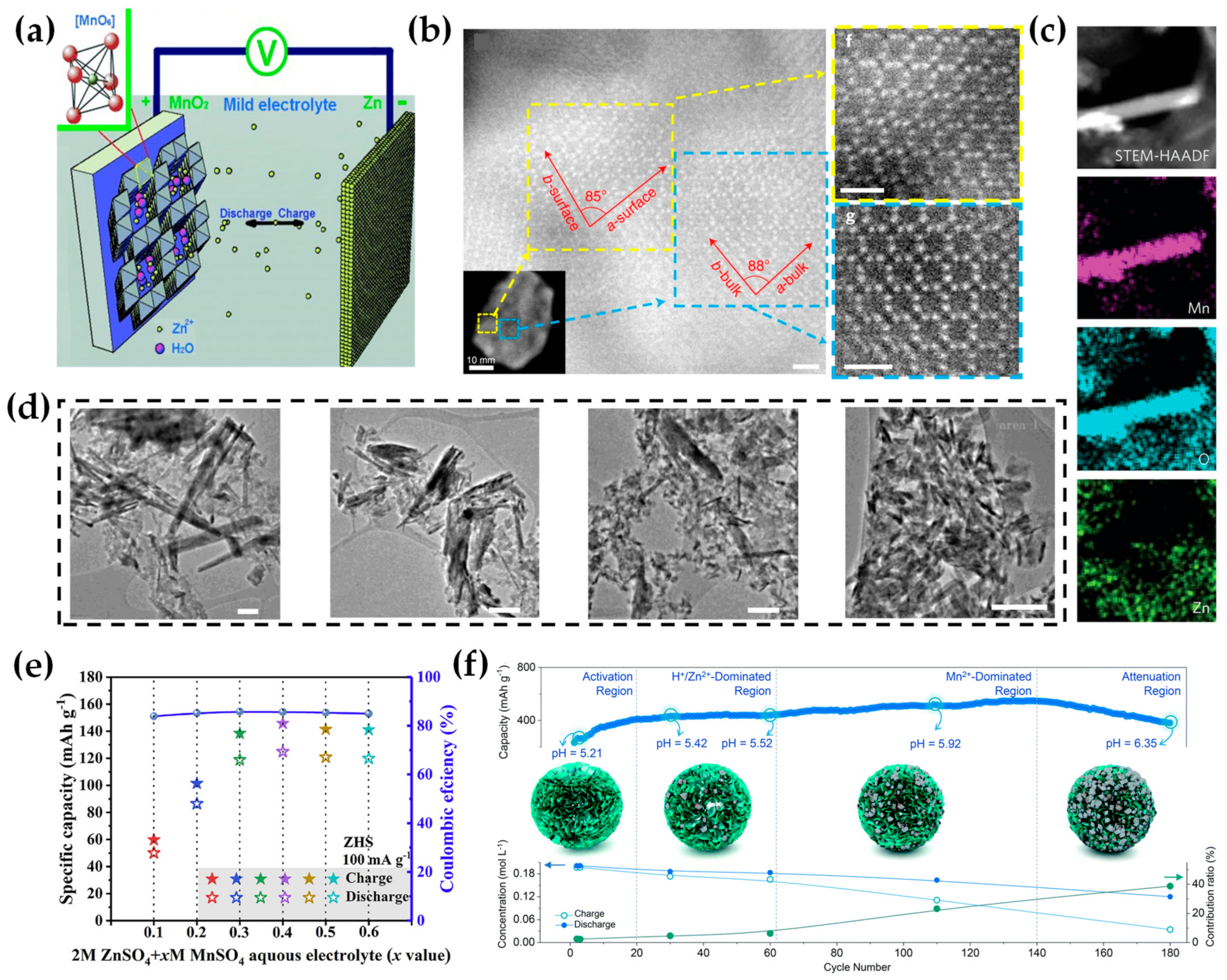
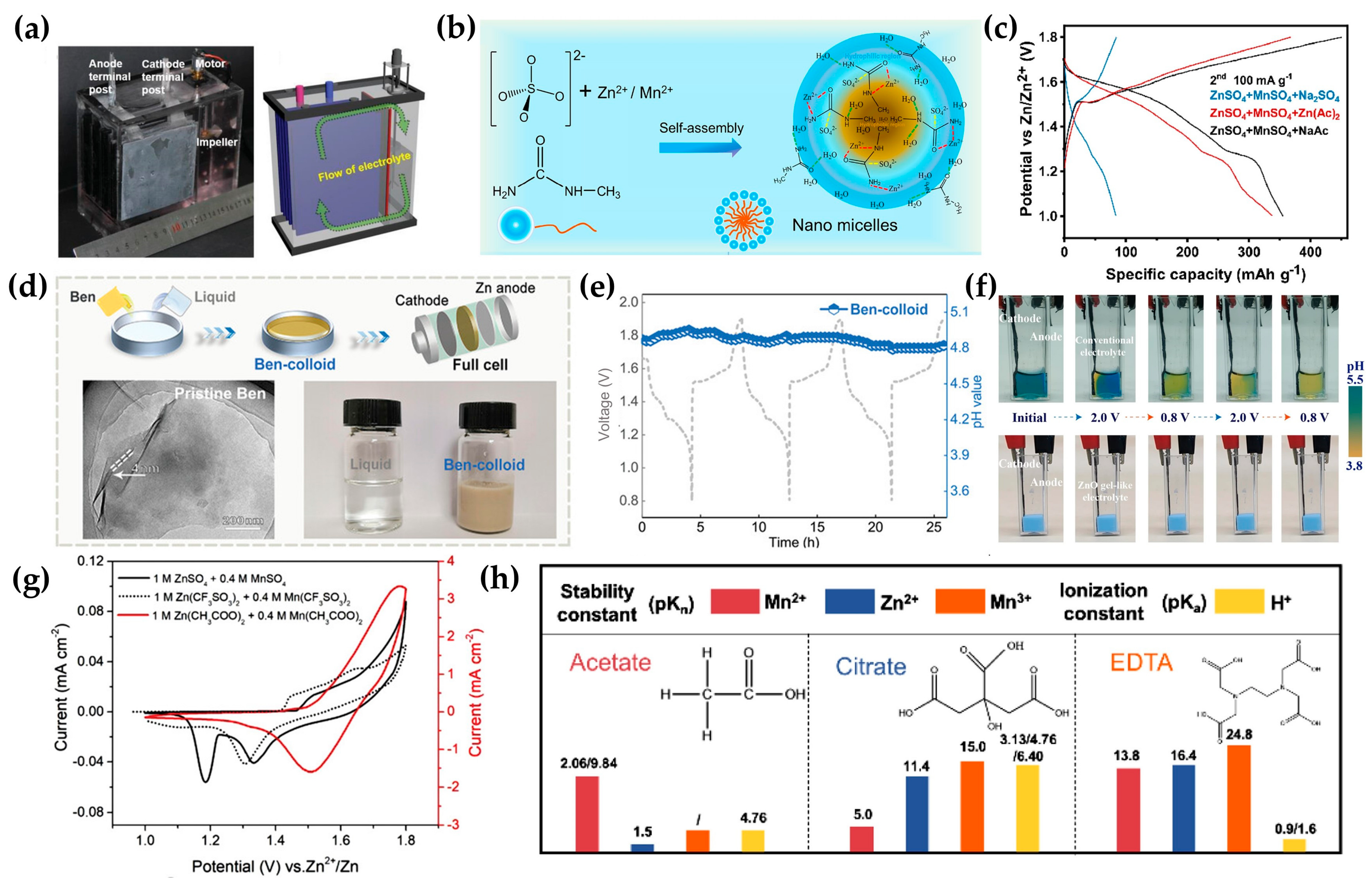
2. Electrolytes Modifications
2.1. Acid Electrolytes

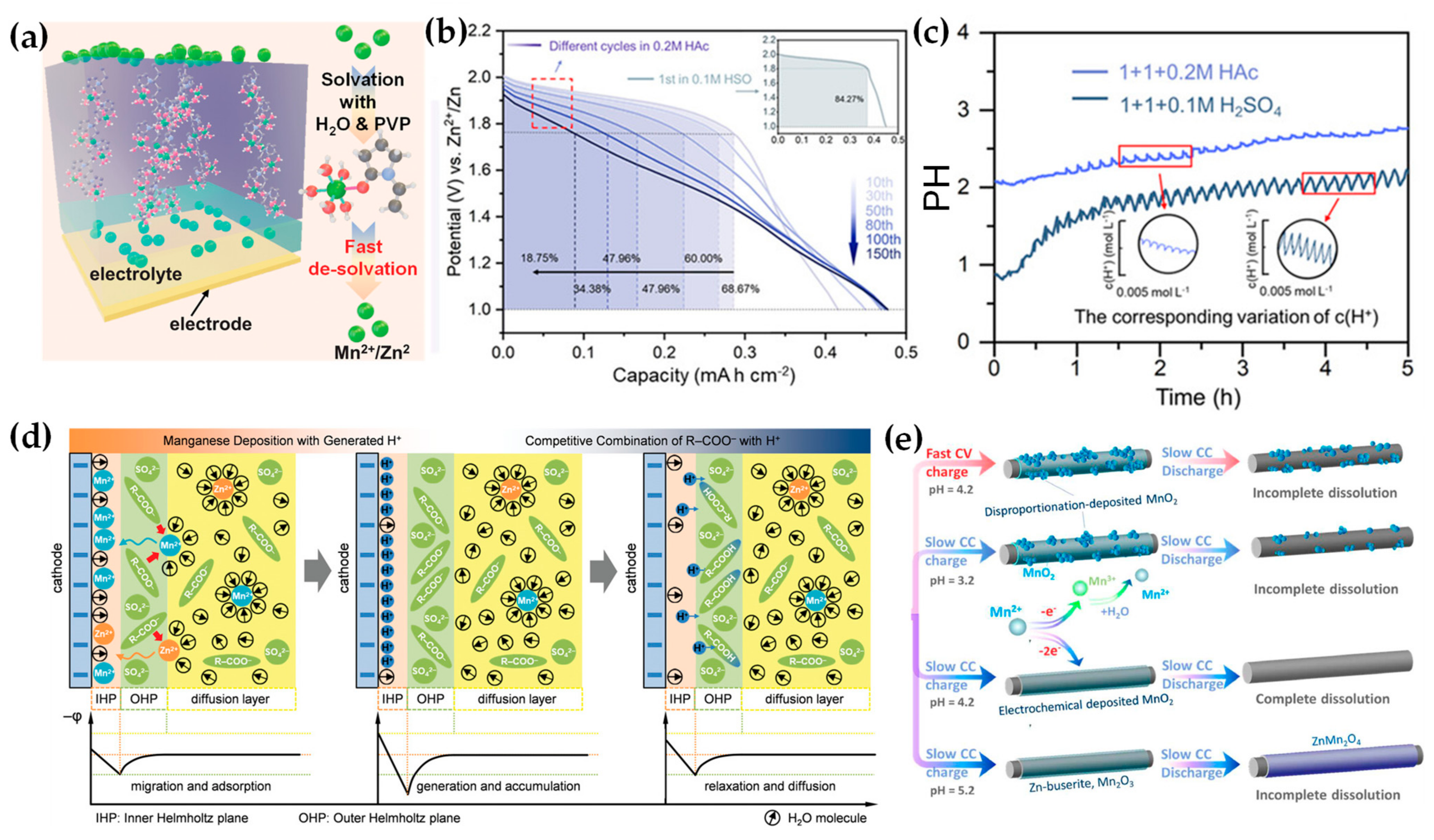
2.2. Dual Electrolytes
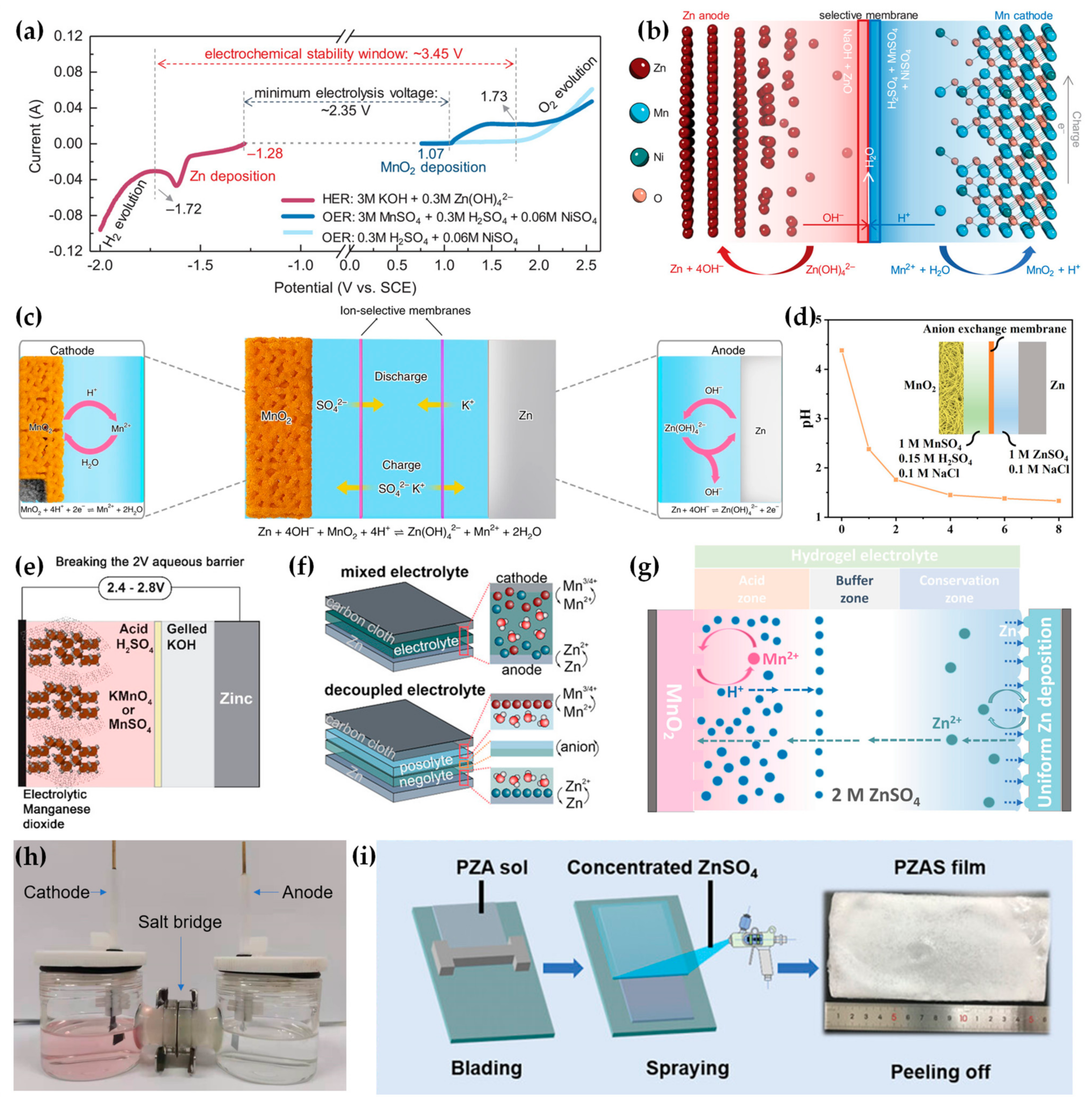
2.3. Near Neutral Electrolytes
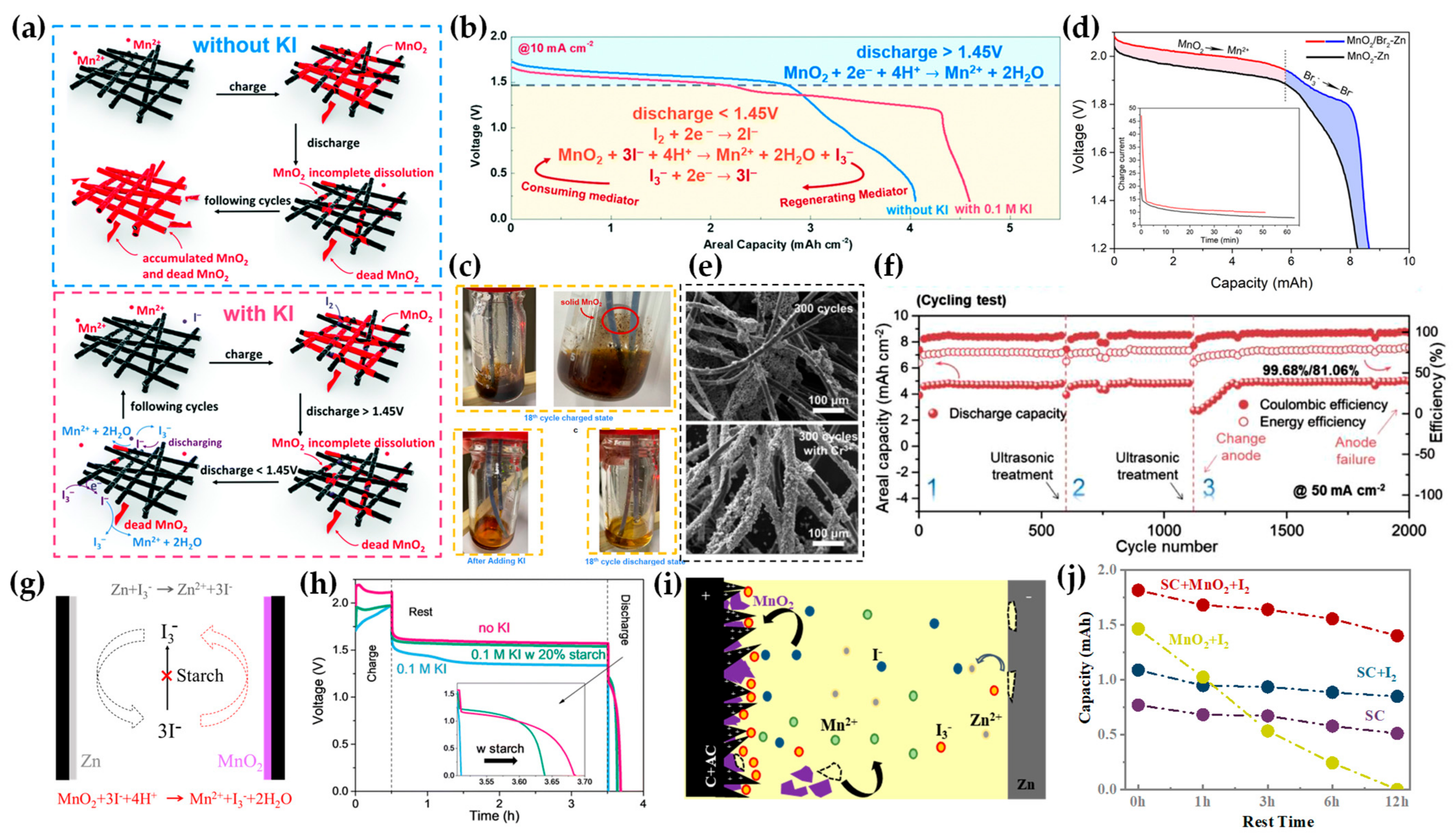
2.4. Mediator Additives
3. Electrode Modifications
3.1. Anode Modifications
3.2. Cathode Modifications
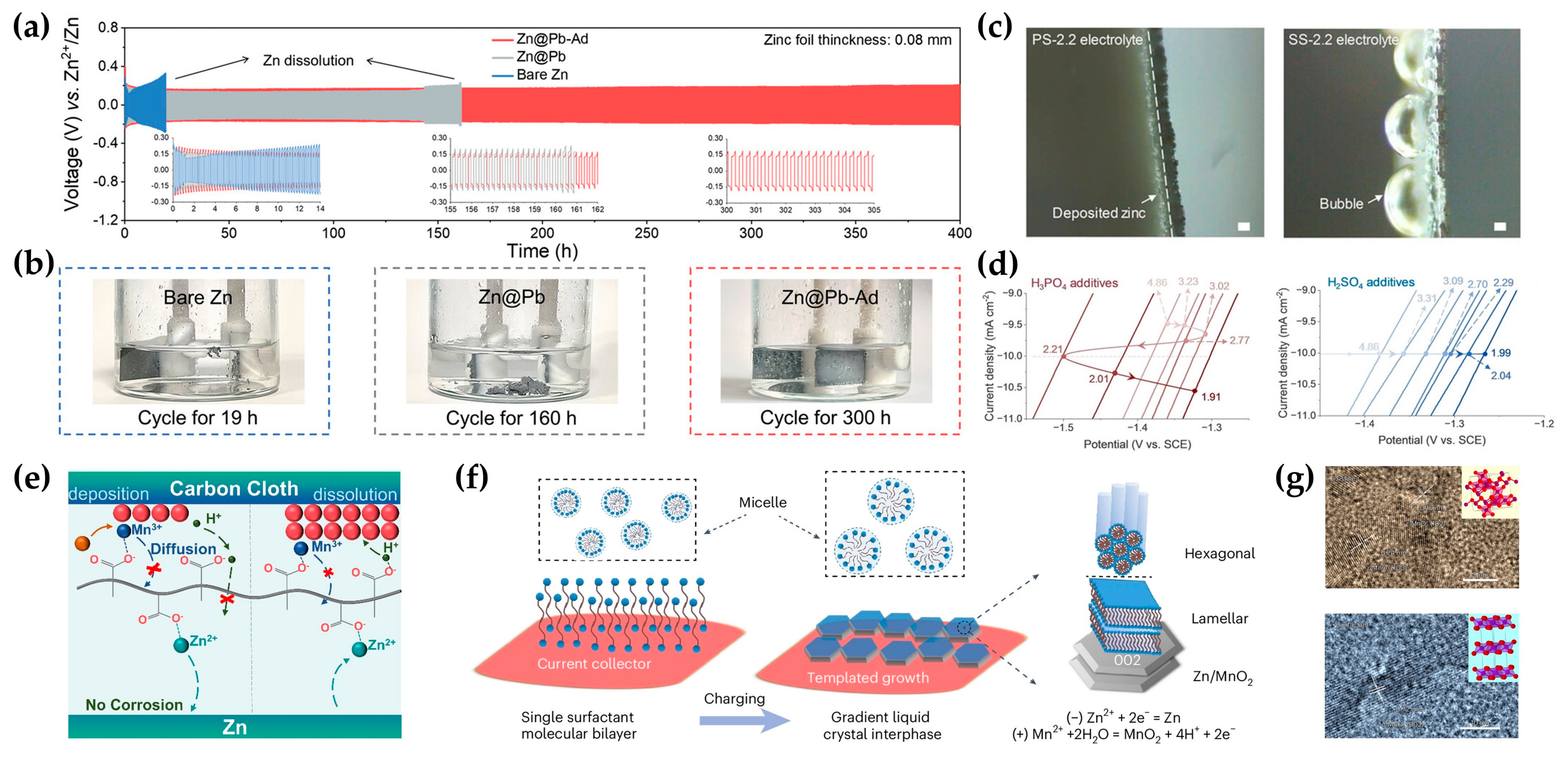
3.3. Current Collectors
4. Outlooks and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luan, J.; Yuan, H.; Liu, J.; Zhong, C. Recent advances on charge storage mechanisms and optimization strategies of Mn-based cathode in zinc-manganese oxides batteries. Energy Storage Mater. 2024, 66, 103206. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Shen, Y.; Wang, R.; Li, H.; Zhou, M.; Wang, W.; Wang, K.; Jiang, K. Issues and opportunities of manganese-based materials for enhanced Zn-ion storage performances. J. Energy Storage 2022, 45, 103729. [Google Scholar] [CrossRef]
- Shoji, T.; Hishinuma, M.; Yamamoto, T. Zinc-manganese dioxide galvanic cell using zinc sulphate as electrolyte. Rechargeability of the cell. J. Appl. Electrochem. 1988, 18, 521–526. [Google Scholar] [CrossRef]
- Xu, C.J.; Li, B.H.; Du, H.D.; Kang, F.Y. Energetic Zinc Ion Chemistry: The Rechargeable Zinc Ion Battery. Angew. Chem. Int. Edit. 2012, 51, 933–935. [Google Scholar] [CrossRef]
- Sambandam, B.; Mathew, V.; Kim, S.; Lee, S.; Kim, S.; Hwang, J.Y.; Fan, H.J.; Kim, J. An analysis of the electrochemical mechanism of manganese oxides in aqueous zinc batteries. Chem 2022, 8, 924–946. [Google Scholar] [CrossRef]
- Zhang, N.; Ji, Y.-R.; Wang, J.-C.; Wang, P.-F.; Zhu, Y.-R.; Yi, T.-F. Understanding of the charge storage mechanism of MnO2-based aqueous zinc-ion batteries: Reaction processes and regulation strategies. J. Energy Chem. 2023, 82, 423–463. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.C.; Guo, Y.F.; Wang, P.F.; Zhu, Y.R.; Yi, T.F. Insights on rational design and energy storage mechanism of Mn-based cathode materials towards high performance aqueous zinc-ion batteries. Coordin. Chem. Rev. 2023, 479, 215009. [Google Scholar] [CrossRef]
- Lee, B.; Lee, H.R.; Kim, H.; Chung, K.Y.; Cho, B.W.; Oh, S.H. Elucidating the intercalation mechanism of zinc ions into alpha-MnO2 for rechargeable zinc batteries. Chem. Commun. 2015, 51, 9265–9268. [Google Scholar] [CrossRef]
- Chamoun, M.; Brant, W.R.; Tai, C.W.; Karlsson, G.; Noreus, D. Rechargeability of aqueous sulfate Zn/MnO2 batteries enhanced by accessible Mn2+ ions. Energy Storage Mater. 2018, 15, 351–360. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Sharpe, R.; He, K.; Li, C.H.; Saray, M.T.; Liu, T.C.; Yao, W.T.; Cheng, M.; Jin, H.L.; Wang, S.; et al. Understanding intercalation chemistry for sustainable aqueous zinc-manganese dioxide batteries. Nat. Sustain. 2022, 5, 890–898. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Kim, S.H.; Oh, S.M. Degradation mechanism of layered MnO2 cathodes in Zn/ZnSO4/MnO2 rechargeable cells. J. Power Sources 1998, 72, 150–158. [Google Scholar] [CrossRef]
- Wu, D.R.; Housel, L.M.; Kim, S.J.; Sadique, N.; Quilty, C.D.; Wu, L.J.; Tappero, R.; Nicholas, S.L.; Ehrlich, S.; Zhu, Y.M.; et al. Quantitative temporally and spatially resolved X-ray fluorescence microprobe characterization of the manganese dissolution-deposition mechanism in aqueous Zn/alpha-MnO2 batteries. Energ. Environ. Sci. 2020, 13, 4322–4333. [Google Scholar] [CrossRef]
- Moon, H.; Ha, K.H.; Park, Y.; Lee, J.; Kwon, M.S.; Lim, J.; Lee, M.H.; Kim, D.H.; Choi, J.H.; Choi, J.H.; et al. Direct Proof of the Reversible Dissolution/Deposition of Mn2+/Mn4+ for Mild-Acid Zn-MnO2 Batteries with Porous Carbon Interlayers. Adv. Sci. 2021, 8, 2003714. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, J.; Bai, C.L.; Li, X.K.; Fang, G.Z.; Liang, S.Q. Zn/MnO2 battery chemistry with dissolution-deposition mechanism. Mater. Today Energy 2020, 16, 100396. [Google Scholar] [CrossRef]
- Chen, H.; Dai, C.L.; Xiao, F.Y.; Yang, Q.J.; Cai, S.N.; Xu, M.W.; Fan, H.J.; Bao, S.J. Reunderstanding the Reaction Mechanism of Aqueous Zn-Mn Batteries with Sulfate Electrolytes: Role of the Zinc Sulfate Hydroxide. Adv. Mater. 2022, 34, 2109092. [Google Scholar] [CrossRef] [PubMed]
- Li, G.D.; Chen, W.; Zhang, H.; Gong, Y.J.; Shi, F.F.; Wang, J.Y.; Zhang, R.F.; Chen, G.X.; Jin, Y.; Wu, T.; et al. Membrane-Free Zn/MnO2 Flow Battery for Large-Scale Energy Storage. Adv. Energy Mater. 2020, 10, 1902085. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, W.H.; Chen, D.; Liu, J.H.; Yuan, Z.Y.; Lu, M.J.; Shen, L.F.; Shulga, V.; Han, W.; Chao, D.L. The origin of capacity fluctuation and rescue of dead Mn-based Zn-ion batteries: A Mn-based competitive capacity evolution protocol. Energ. Environ. Sci. 2021, 15, 1106–1118. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, L.; Lu, B.; Wu, X.; Liang, S.; Zhou, J. Issues and Opportunities Facing Aqueous Mn2+/MnO2-based Batteries. ChemSusChem 2022, 15, e202200348. [Google Scholar] [CrossRef]
- Chao, D.L.; Zhou, W.H.; Ye, C.; Zhang, Q.H.; Chen, Y.G.; Gu, L.; Davey, K.; Qiao, S.Z. An Electrolytic Zn-MnO2 Battery for High-Voltage and Scalable Energy Storage. Angew. Chem. Int. Ed. 2019, 58, 7823–7828. [Google Scholar] [CrossRef]
- Luo, S.; Liu, S.; Yang, G.; Liu, C.; Wu, J.; Hiralal, P.; Nie, Z.; Amaratunga, G.A.J.; Zhou, H. Electrode-free flexible batteries enabled by electro-deposition of both Zn and MnO2 from electrolytes. New J. Chem. 2024, 48, 1462–1466. [Google Scholar] [CrossRef]
- Liang, G.; Mo, F.; Li, H.; Tang, Z.; Liu, Z.; Wang, D.; Yang, Q.; Ma, L.; Zhi, C. A Universal Principle to Design Reversible Aqueous Batteries Based on Deposition–Dissolution Mechanism. Adv. Energy Mater. 2019, 9, 1901838. [Google Scholar] [CrossRef]
- Wang, M.; Chen, N.; Zhu, Z.; Meng, Y.; Shen, C.; Zheng, X.; Liang, D.; Chen, W. Electrode-Less MnO2-Metal Batteries with Deposition and Stripping Chemistry. Small 2021, 17, 2103921. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, G.; Pei, A.; Li, Y.; Liao, L.; Wang, H.; Wan, J.; Liang, Z.; Chen, G.; Zhang, H.; et al. A manganese–hydrogen battery with potential for grid-scale energy storage. Nat. Energy 2018, 3, 428–435. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Z.; Dong, X.; Bin, D.; Wang, Y.; Xia, Y. Low-cost and high safe manganese-based aqueous battery for grid energy storage and conversion. Sci. Bull. 2019, 64, 1780–1787. [Google Scholar] [CrossRef]
- Huang, J.; Yan, L.; Bin, D.; Dong, X.; Wang, Y.; Xia, Y. An aqueous manganese–lead battery for large-scale energy storage. J. Mater. Chem. A 2020, 8, 5959–5967. [Google Scholar] [CrossRef]
- Chuai, M.Y.; Yang, J.L.; Tan, R.; Liu, Z.C.; Yuan, Y.; Xu, Y.; Sun, J.F.; Wang, M.M.; Zheng, X.H.; Chen, N.; et al. Theory-Driven Design of a Cationic Accelerator for High-Performance Electrolytic MnO2-Zn Batteries. Adv. Mater. 2022, 34, 2203249. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Gao, Z.Y.; Xue, Z.Y.; Yang, M.; Zhao, X.Y. Acidity Modulation of Electrolyte Enables High Reversible Mn2+/MnO2 Electrode Reaction of Electrolytic Zn-MnO2 Battery. J. Electron. Mater. 2022, 51, 6041–6046. [Google Scholar] [CrossRef]
- Liu, Z.X.; Yang, Y.Q.; Liang, S.Q.; Lu, B.A.; Zhou, J. pH-Buffer Contained Electrolyte for Self-Adjusted Cathode-Free Zn-MnO2 Batteries with Coexistence of Dual Mechanisms. Small Struct. 2021, 2, 2100119. [Google Scholar] [CrossRef]
- He, Z.F.; Lu, Y.T.; Wei, T.C.; Hu, C.C. Complementary Operando Electrochemical Quartz Crystal Microbalance and UV/Vis Spectroscopic Studies: Acetate Effects on Zinc-Manganese Batteries. ChemSusChem 2023, 16, e202300259. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.N.; Huang, J.Q.; Chi, X.W.; Yang, J.H.; Liu, Y. Bromide-acetate co-mediated high-power density rechargeable aqueous zinc-manganese dioxide batteries. J. Mater. Chem. A 2021, 9, 21888–21896. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Stevenson, G.; Chamoun, M.; Lindström, R.W. MnO2/Mn2+ chemistry: Charging protocol and electrolyte regulation. Energy Storage Mater. 2023, 63, 103008. [Google Scholar] [CrossRef]
- Liu, Z.; Li, L.; Qin, L.; Guo, S.; Fang, G.; Luo, Z.; Liang, S. Balanced Interfacial Ion Concentration and Migration Steric Hindrance Promoting High-Efficiency Deposition/Dissolution Battery Chemistry. Adv. Mater. 2022, 34, 2204681. [Google Scholar] [CrossRef] [PubMed]
- Durena, R.; Zukuls, A. A Short Review: Comparison of Zinc-Manganese Dioxide Batteries with Different pH Aqueous Electrolytes. Batteries 2023, 9, 311. [Google Scholar] [CrossRef]
- Dai, C.; Hu, L.; Jin, X.; Zhao, Y.; Qu, L. The Emerging of Aqueous Zinc-Based Dual Electrolytic Batteries. Small 2021, 17, e2008043. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.; Ye, C.; Xie, F.; Zhou, W.; Zhang, Q.; Gu, Q.; Davey, K.; Gu, L.; Qiao, S.-Z. Atomic Engineering Catalyzed MnO2 Electrolysis Kinetics for a Hybrid Aqueous Battery with High Power and Energy Density. Adv. Mater. 2020, 32, 2001894. [Google Scholar] [CrossRef]
- Liu, C.; Chi, X.; Han, Q.; Liu, Y. A High Energy Density Aqueous Battery Achieved by Dual Dissolution/Deposition Reactions Separated in Acid-Alkaline Electrolyte. Adv. Energy Mater. 2020, 10, 1903589. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, B.; Ding, J.; Liu, X.R.; Zhong, Y.W.; Li, Y.; Sun, C.B.; Han, X.P.; Deng, Y.D.; Zhao, N.Q.; et al. Decoupling electrolytes towards stable and high-energy rechargeable aqueous zinc-manganese dioxide batteries. Nat. Energy 2020, 5, 440–449. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.; Choi, J.; Park, J.; Park, J.W.; Park, M. Reversible metal ionic catalysts for high-voltage aqueous hybrid zinc-manganese redox flow batteries. Energy Storage Mater. 2023, 55, 698–707. [Google Scholar] [CrossRef]
- Sun, J.F.; Liu, Z.C.; Li, K.; Yuan, Y.; Zheng, X.H.; Xu, Y.; Wang, M.M.; Chuai, M.; Hu, H.L.; Chen, W. Proton-Trapping Agent for Mitigating Hydrogen Evolution Corrosion of Zn for an Electrolytic MnO2/Zn Battery. ACS Appl. Mater. Interfaces 2022, 14, 51900–51909. [Google Scholar] [CrossRef]
- Cui, Y.F.; Zhuang, Z.B.; Xie, Z.L.; Cao, R.F.; Hao, Q.; Zhang, N.; Liu, W.Q.; Zhu, Y.H.; Huang, G. High-Energy and Long-Lived Zn-MnO2 Battery Enabled by a Hydrophobic-Ion- Conducting Membrane. ACS Nano 2022, 16, 20730–20738. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.J.; Liu, F.; Liu, Y.; Wu, Z.X.; Wang, L.L.; Zhang, Y.; Huang, Q.H.; Fu, L.J.; Wu, Y.P. A high voltage aqueous zinc-manganese battery using a hybrid alkaline-mild electrolyte. Chem. Commun. 2020, 56, 2039–2042. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.G.; Turney, D.; Huang, J.C.; Wei, X.; Banerjee, S. Breaking the 2 V Barrier in Aqueous Zinc Chemistry: Creating 2.45 and 2.8 V MnO2-Zn Aqueous Batteries. ACS Energy Lett. 2019, 4, 2144–2146. [Google Scholar] [CrossRef]
- Tang, H.; Yin, Y.; Huang, Y.; Wang, J.; Liu, L.; Qu, Z.; Zhang, H.; Li, Y.; Zhu, M.; Schmidt, O.G. Battery-Everywhere Design Based on a Cathodeless Configuration with High Sustainability and Energy Density. ACS Energy Lett. 2021, 6, 1859–1868. [Google Scholar] [CrossRef]
- Shen, Z.X.; Tang, Z.Q.; Li, C.W.; Luo, L.; Pu, J.; Wen, Z.R.; Liu, Y.; Ji, Y.; Xie, J.P.; Wang, L.T.; et al. Precise Proton Redistribution for Two-Electron Redox in Aqueous Zinc/Manganese Dioxide Batteries. Adv. Energy Mater. 2021, 11, 2102055. [Google Scholar] [CrossRef]
- Xu, Q.; Xie, Q.X.; Xue, T.; Cheng, G.; Wu, J.D.; Ning, L.; Yan, X.H.; Lu, Y.J.; Zou, Z.L.; Wang, B.P.; et al. Salt Bridge-intermediated three phase decoupling electrolytes for high voltage electrolytic aqueous Zinc-Manganese dioxides battery. Chem. Eng. J. 2023, 451, 138775. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, J.L.; Liu, Z.C.; Tan, R.; Chuai, M.; Sun, J.F.; Xu, Y.; Zheng, X.H.; Wang, M.M.; Ahmad, T.; et al. A Proton-Barrier Separator Induced via Hofmeister Effect for High-Performance Electrolytic MnO2-Zn Batteries. Adv. Energy Mater. 2022, 12, 2103705. [Google Scholar] [CrossRef]
- Durena, R.; Zukuls, A.; Vanags, M.; Sutka, A. How to increase the potential of aqueous Zn-MnO2 batteries: The effect of pH gradient electrolyte. Electrochim. Acta 2022, 434, 141275. [Google Scholar] [CrossRef]
- Panda, M.R.; El Meragawi, S.; Mirshekarloo, M.S.; Chen, W.; Shaibani, M.; Majumder, M. Acidity-Aided Surface Modification Strategy to Enhance In Situ MnO2 Deposition for High Performance Zn-MnO2 Battery Prototypes. Small 2024, 2311933. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Fan, M.; Zhan, B.; Zuo, L.-J.; Chen, C.; Yan, L. Nanomicellar Electrolyte to Control Release Ions and Reconstruct Hydrogen Bonding Network for Ultrastable High-Energy-Density Zn-Mn Battery. J. Am. Chem. Soc. 2023, 145, 20109–20120. [Google Scholar] [CrossRef]
- Zhong, Z.P.; Li, J.Y.; Li, L.Y.; Xi, X.Y.; Luo, Z.G.; Fang, G.Z.; Liang, S.Q.; Wang, X.Y. Improving performance of zinc-manganese battery via efficient deposition/dissolution chemistry. Energy Storage Mater. 2022, 46, 165–174. [Google Scholar] [CrossRef]
- Xie, X.; Fu, H.; Fang, Y.; Lu, B.; Zhou, J.; Liang, S. Manipulating Ion Concentration to Boost Two-Electron Mn4+/Mn2+ Redox Kinetics through a Colloid Electrolyte for High-Capacity Zinc Batteries. Adv. Energy Mater. 2021, 12, 2102393. [Google Scholar] [CrossRef]
- Wu, J.; Tang, Y.; Xu, H.; Ma, G.; Jiang, J.; Xian, C.; Xu, M.; Bao, S.-J.; Chen, H. ZnO Additive Boosts Charging Speed and Cycling Stability of Electrolytic Zn–Mn Batteries. Nano-Micro Lett. 2024, 16, 74. [Google Scholar] [CrossRef]
- Zeng, X.H.; Liu, J.T.; Mao, J.F.; Hao, J.N.; Wang, Z.J.; Zhou, S.; Ling, C.D.; Guo, Z.P. Toward a Reversible Mn4+/Mn2+ Redox Reaction and Dendrite-Free Zn Anode in Near-Neutral Aqueous Zn/MnO2 Batteries via Salt Anion Chemistry. Adv. Energy Mater. 2020, 10, 1904163. [Google Scholar] [CrossRef]
- Liu, Z.X.; Yang, Y.Q.; Lu, B.A.; Liang, S.Q.; Fan, H.J.; Zhou, J. Insights into complexing effects in acetate-based Zn-MnO2 batteries and performance enhancement by all-round strategies. Energy Storage Mater. 2022, 52, 104–110. [Google Scholar] [CrossRef]
- Kong, M.Z.; Zhang, T.; Bu, H.X.; Li, C.L.; Zhang, X.X.; Zhang, J.; Liu, W.J.; Li, D.Z.; Wang, C.G.; Xu, X.J. Enhancing the efficiency of two-electron zinc-manganese batteries enabled by Glycine complexation of manganese ions and compatibility with zinc anodes. Chem. Eng. J. 2024, 499, 156426. [Google Scholar] [CrossRef]
- Yang, L.; Meng, L.; Ji, X.; Cheng, S. Construction of Zinc-based Anodes for Electrolytic Zinc-MnO2 Batteries with High Discharge Voltage and Good Durability. Batter. Supercaps 2023, 6, e202300158. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Qin, Z.M.; Yang, X.P.; Liu, J.; Liu, X.X.; Sun, X.Q. High-Voltage Manganese Oxide Cathode with Two-Electron Transfer Enabled by a Phosphate Proton Reservoir for Aqueous Zinc Batteries. ACS Energy Lett. 2022, 7, 1814–1819. [Google Scholar] [CrossRef]
- Mateos, M.; Makivic, N.; Kim, Y.-S.; Limoges, B.; Balland, V. Accessing the Two-Electron Charge Storage Capacity of MnO2 in Mild Aqueous Electrolytes. Adv. Energy Mater. 2020, 10, 2000332. [Google Scholar] [CrossRef]
- Lv, H.; Song, Y.; Qin, Z.; Zhang, M.; Yang, D.; Pan, Q.; Wang, Z.; Mu, X.; Meng, J.; Sun, X.; et al. Disproportionation enabling reversible MnO2/Mn2+ transformation in a mild aqueous Zn-MnO2 hybrid battery. Chem. Eng. J. 2022, 430, 133064. [Google Scholar] [CrossRef]
- Xie, C.X.; Li, T.Y.; Deng, C.Z.; Song, Y.; Zhang, H.M.; Li, X.F. A highly reversible neutral zinc/manganese battery for stationary energy storage. Energ. Environ. Sci. 2020, 13, 135–143. [Google Scholar] [CrossRef]
- Ye, X.; Han, D.; Jiang, G.; Cui, C.; Guo, Y.; Wang, Y.; Zhang, Z.; Weng, Z.; Yang, Q.-H. Unraveling the deposition/dissolution chemistry of MnO2 for high-energy aqueous batteries. Energ. Environ. Sci. 2023, 16, 1016–1023. [Google Scholar] [CrossRef]
- Lei, J.F.; Yao, Y.X.; Wang, Z.Y.; Lu, Y.C. Towards high-areal-capacity aqueous zinc-manganese batteries: Promoting MnO2 dissolution by redox mediators. Energ. Environ. Sci. 2021, 14, 4418–4426. [Google Scholar] [CrossRef]
- Zheng, X.; Luo, R.; Ahmad, T.; Sun, J.; Liu, S.; Chen, N.; Wang, M.; Yuan, Y.; Chuai, M.; Xu, Y.; et al. Development of High Areal Capacity Electrolytic MnO2–Zn Battery via an Iodine Mediator. Energy Environ. Mater. 2023, 6, e12433. [Google Scholar] [CrossRef]
- Wu, J.; Huang, J.Q.; Yang, J.H.; Chi, X.W.; Liu, Y. Mn2+/I− Hybrid Cathode with Superior Conversion Efficiency for Ultrahigh-Areal-Capacity Aqueous Zinc Batteries. ACS Appl. Mater. Inter. 2022, 14, 53627–53635. [Google Scholar] [CrossRef]
- Zheng, X.H.; Wang, Y.C.; Xu, Y.; Ahmad, T.; Yuan, Y.; Sun, J.F.; Luo, R.H.; Wang, M.M.; Chuai, M.Y.; Chen, N.; et al. Boosting Electrolytic MnO2-Zn Batteries by a Bromine Mediator. Nano Lett. 2021, 21, 8863–8871. [Google Scholar] [CrossRef]
- Liu, S.; Luo, S.; Amaratunga, G.A.J.; Hiralal, P.; Nie, Z. Thin-film flexible zinc hybrid electrochemical device integrating three chemical pathways. J. Power Sources 2024, 614, 235045. [Google Scholar] [CrossRef]
- Liu, S.; Hiralal, P.; Nie, Z.; Amaratunga, G.A.J.; Luo, S. Thin-film electrolytic MnO2-Zn batteries with limited amount of acetate electrolyte. Mater. Lett. 2024, 357, 135780. [Google Scholar] [CrossRef]
- Ruan, P.; Chen, X.; Qin, L.; Tang, Y.; Lu, B.; Zeng, Z.; Liang, S.; Zhou, J. Achieving Highly Proton-Resistant Zn-Pb Anode through Low Hydrogen Affinity and Strong Bonding for Long-Life Electrolytic Zn//MnO2 Battery. Adv. Mater. 2023, 35, e2300577. [Google Scholar] [CrossRef]
- Yang, H.; Li, L.; Chen, D.; Wang, J.; Tan, Y.; Jiang, Z.; Zhang, Y.; Miao, C.; Zhang, W.; Han, W.; et al. Stimulating the potential of Zn anode to operate in low pH and harsh environments for highly sustainable Zn batteries. Angew. Chem. Int. Ed. 2025, 10, e202419394. [Google Scholar] [CrossRef]
- Li, X.L.; Qi, K.W.; Qin, Z.L.; Ding, X.; Zhu, Y.C.; Hou, Z.G.; Qian, Y.T. Ion-Anchored Strategy for MnO2/Mn2+ Chemistry without “Dead Mn” and Corrosion. ACS Nano 2024, 18, 27016–27025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, G.; Wu, J.; Chi, X.; Liu, Y. An Organic Coordination Manganese Complex as Cathode for High-Voltage Aqueous Zinc-metal Battery. Angew. Chem. Int. Ed. 2023, 62, e202309430. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yuan, H.; Liu, T.; Zhang, R.; Zhu, J.; Cui, H.; Wang, Y.; Cao, D.; Wang, D.; Zhi, C. Distinguish MnO2/Mn2+ Conversion/Zn2+ Intercalation/H+Conversion Chemistries at Different Potentials in Aqueous Zn∥MnO2 Batteries. Angew. Chem. Int. Ed. 2024, 63, e202403504. [Google Scholar] [CrossRef]
- Yao, H.; Dong, W.; Ji, X.; Cheng, S. Construction of self-standing MnO2-based films for reversible and practical electrolytic Zn//MnO2 batteries in acidic environment. Surf. Interfaces 2024, 44, 103669. [Google Scholar] [CrossRef]
- Wang, M.; Meng, Y.; Chen, N.; Chuai, M.; Shen, C.; Zheng, X.; Yuan, Y.; Sun, J.; Xu, Y.; Chen, W. Electrolyte Regulation of Bismuth Ions toward High-Performance Aqueous Manganese-based Batteries. ACS Mater. Lett. 2021, 3, 1558–1565. [Google Scholar] [CrossRef]
- Gou, L.; Li, J.R.; Liang, K.; Zhao, S.P.; Li, D.L.; Fan, X.Y. Bi-MOF Modulating MnO2 Deposition Enables Ultra-Stable Cathode-Free Aqueous Zinc-Ion Batteries. Small 2023, 19, 2208233. [Google Scholar] [CrossRef]
- Qin, Z.M.; Song, Y.; Yang, D.; Zhang, M.Y.; Shi, H.Y.; Li, C.C.; Sun, X.Q.; Liu, X.X. Enabling Reversible MnO2/Mn2+ Transformation by Al3+ Addition for Aqueous Zn-MnO2 Hybrid Batteries. ACS Appl. Mater. Inter. 2022, 14, 10526–10534. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zheng, X.L.; Carlson, E.Z.; Xiao, X.; Chi, X.W.; Greenburg, L.C.; Zhang, G.; Zhang, E.; Liu, C.W.; Yang, Y.F.; et al. In situ formation of liquid crystal interphase in electrolytes with soft templating effects for aqueous dual-electrode-free batteries. Nat. Energy 2024, 9, 1350–1359. [Google Scholar] [CrossRef]
- Dai, C.; Hu, L.; Jin, X.; Wang, Y.; Wang, R.; Xiao, Y.; Li, X.; Zhang, X.; Song, L.; Han, Y.; et al. Fast constructing polarity-switchable zinc-bromine microbatteries with high areal energy density. Sci. Adv. 2022, 8, eabo6688. [Google Scholar] [CrossRef]

| Anode | Cathode | Electrolyte | Device Structure | Capacity (mAh/cm2) | Ref. |
|---|---|---|---|---|---|
| Pt/C | Carbon felt | 1 M MnSO4 + 0.05 M H2SO4 | Swagelok cell | 4 | [24] |
| Cylindrical cell | 2 | ||||
| Cu plate | Carbon cloth | 0.3 M CuSO4 + 0.3 M MnSO4 + 0.5M H2SO4 | Pouch cell | 0.8 | [22] |
| Carbon cloth | 0.1 M Bi(NO3)3 + 0.1 M MnSO4 + 1M HNO3 | ||||
| Zn plate | 0.3 M ZnSO4 + 0.3 M MnSO4 | ||||
| PbSO4/Pb | Carbon felt | 0.5 M H2SO4 + 1 M MnSO4 | Pouch cell | 24.4 | [26] |
| Flow cell | 4.88 | ||||
| Carbon cloth | Carbon cloth | 0.5 M CdSO4 + 0.5 M MnSO4 + 0.5 M H2SO4 | Home-made cell | 10 | [23] |
| 0.5 M CuSO4 + 0.5 M MnSO4 + 0.5 M H2SO4 | 0.5 | ||||
| 1 M ZnSO4 + 1 M MnSO4 + 0.1 M H2SO4 | 0.5 | ||||
| Zn foam | Carbon cloth | 1 M ZnSO4 + 1 M MnSO4 + 0.1 M H2SO4 | Home-made cell | 10 | [20] |
| Zn foil | Carbon felt | 1 M ZnSO4 + 1 M MnSO4 + 0.1 M H2SO4 + 0.07 mM polvinylpyrrolidone (PVP) | Beaker cell | 4 | [27] |
| Zn foil | Carbon felt | 1 M ZnCl2 + 1 M MnCl2 + 0.2 M HCl | Home-made cell | 0.5 | [28] |
| Zn foil | Carbon felt | 1 M ZnSO4 + 1 M MnSO4 + 0.2 M HAc | Pouch cell | 2 | [29] |
| Zn foil | Carbon felt | 1 M ZnAc2 + 0.5 M HAc+0.5 M MnAc2 + 1 M KBr | Beaker cell | 17 | [31] |
| Zn plate | Carbon felt | 1 M MnAc2 + 1 M ZnO + 2.5 M MSA + 1 M KCl | Beaker cell | 2 | [32] |
| 3D porous Zn @carbon-felt | Carbon felt | 3 M NaOH + 0.3 M ZnO/bipolar membrane/ 3 M MnSO4 + 0.3 M H2SO4 + 0.06 M NiSO4 | Home-made cell | 13 | [36] |
| Zn foil | Carbon cloth | 2.4 M KOH + 0.1 M ZnAc2/bipolar membrane/ 1 M MnSO4 + 0.5 M H2SO4 | Home-made cell Flow cell | 15 | [37] |
| Zn foil | MnO2 loaded carbon felt | 6 M KOH + 0.2 M ZnO + 5 mM vanillin //0.1 M K2SO4//3 M H2SO4 + 0.1 M MnSO4 | Pack | 66.3 | [38] |
| Zn metal on carbon felt | Bi2O3 coated carbon felt | 4 M NaOH + 0.1 M ZnO + 5 mM vanillin //3 M Li2SO4//0.1 M MnSO4 + 2.5 M H2SO4 + 0.03 M NiSO4 + 0.01 M MgSO4 | Flow cell | 22.6 | [39] |
| Zn foil | Carbon felt | 0.8 M ZnSO4 + 0.2 M ZnAc2 + 0.1 M NaCl //1 M MnSO4 + 0.15 M H2SO4 + 0.1 M NaCl | Beaker cell | 7.3 | [40] |
| Zn foil | Carbon paper | 0.5 M ZnSO4 + 1 M LiTFSI /PILG/1 M MnSO4 + 1 M HTFSI | Home-made cell | 18 | [41] |
| Zn foil | MnO2 loaded carbon felt | 1 M NaOH + 0.01 M ZnAc2/Na+-form Nafion membrane/2 M ZnSO4 + 0.1 M MnSO4 | Glass cylinders | - | [42] |
| Zn foil | Carbon cloth | 2 M ZnSO4 in PAM /12 M MnSO4 + 1 M H2SO4 in PAM | Simple pouch | 0.7 | [44] |
| Zn foil | Carbon cloth | 3 M KOH + 0.3 M ZnO/NaSO4 agar /3 M MnSO4 + 0.3 M H2SO4 | H-shaped electrolytic cell | 1.35 | [46] |
| Zn foil | Carbon felt | 1 M ZnSO4 + 1 M MnSO4 | Flow cell | 2 | [17] |
| Zn foil | Carbon cloth | 1 M ZnSO4 + 2 M MnSO4 | Beaker cell | 2 | [49] |
| Zn foil | Carbon cloth | 1 M ZnSO4 + 0.5 M MnSO4 + 7 M methylurea | Open thin-film cell | 0.5 | [50] |
| Zn foil | Carbon cloth | 1 M ZnSO4 + 0.1 M MnSO4 + 3 M Glycine | Coin cell | 0.5 | [56] |
| Zn foil | Carbon nanotube film | 1 M ZnSO4 + 0.1 M MnSO4 + 0.2 g/mL ZnO | Coin cell | 2.5 | [53] |
| +0.25 g/mL ZnO | Pouch cell | 0.46 | |||
| ZnO@C | Carbon cloth | ZnSO4+PVA/1 M MnCl2 + 1 M ZnSO4 + H2SO4 | - | 0.5 | [57] |
| Zn foil | Graphite foil | 3 M ZnCl2 + 0.1 M MnCl2 | Home-made cell | 10 | [60] |
| Zn foil | Carbon cloth | 1 M ZnAc2 + 0.4 M MnAc2 | Coin cell | 0.5 | [54] |
| Swagelok cell | 1 | ||||
| Zn on graphite felt | Carbon black on graphite | 1.5 M MnAc2 + 1.5 M ZnCl2 + 3 M KCl | Flow cell | 20 | [61] |
| Zn foil | Carbon felt | 1 M ZnAc2 + 1 M MnAc2 + 2 M KCl + 0.1 M KI | Flow cell | 20 | [63] |
| Zn foil | Carbon felt | 1 M MnSO4 + 1 M ZnSO4 + 0.03 M ZnI2 | Home-made cell | 20 | [64] |
| Zn foil | Carbon felt | 0.5 M HAc + 0.5 M MnAc2 + 1 M ZnAc2 + 1 M KBr + 0.1 M KI | Home-made cell | 20 | [65] |
| Zn foil | Carbon felt | 1 M ZnSO4 + 1 M MnSO4 + 0.2 M H2SO4 + 0.05 M ZnBr2 + 0.2 M Br2 | Home-made cell | 13.3 | [66] |
| Zn foil | Carbon cloth Carbon felt | 0.5 M ZnCl2 + 0.5 M MnAc2 + 2 M KCl + 1.75 M HAc + 0.05 M CrCl3 | Pouch cell | 5 | [55] |
| Printed zinc | Printed graphite | 1 M ZnAc2 + 1 M MnAc2 + 2 M KCl + 0.1 M KI | Thin-film cell | 0.1 | [68] |
| Zn@Pb | Graphite felt | 0.2/0.1 M H2SO4 + 1 M ZnSO4 + 1 M MnSO4 + PbAc2 | Home-made cell | 5 | [69] |
| Phytic acid modified Zn | Carbon cloth | 1 M ZnSO4 + 0.5 M MnSO4 + H3PO4 | Pouch cell | 10 | [70] |
| Zn foil | PMAA@CC | 1 M ZnAc2 + 0.4 M MnAc2 | Pouch cell | 1 | [71] |
| Zn foil | PAAMn@SS | 1 M ZnSO4 + 0.5 M MnSO4 | - | 0.1 | [72] |
| Zn foil | BiMOF@Carbon paper | 1 M ZnSO4 + 0.2 M MnSO4 | - | 0.17 | [76] |
| Zn foil | Graphite foil | 0.25 M Al2(SO4)3 + 2 M ZnSO4 + 0.5 M MnSO4 | Home-made cell | 2 | [77] |
| Cu foil | Carbon felt | 1 M ZnSO4 + 0.2 M MnSO4 + 0.1 mM surfactant | Coin cell | 3 | [78] |
| Printed graphite | Printed graphite | 1 M ZnAc2 + 1 M MnAc2 + 2 M KCl + 0.1 M KI | Thin-film cell | 0.1 | [21] |
| Printed zinc | Printed activated carbon | 1 M ZnAc2 + 1 M MnAc2 + 2 M KCl + 0.1 M KI | Thin-film cell | 0.5 | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Liang, Z.; Zhou, H.; Cai, W.; Wu, J.; Zhang, Q.; Yang, G.; Daoud, W.A.; Nie, Z.; Hiralal, P.; et al. Recent Progress in Cathode-Free Zinc Electrolytic MnO2 Batteries: Electrolytes and Electrodes. Batteries 2025, 11, 171. https://doi.org/10.3390/batteries11050171
Liu S, Liang Z, Zhou H, Cai W, Wu J, Zhang Q, Yang G, Daoud WA, Nie Z, Hiralal P, et al. Recent Progress in Cathode-Free Zinc Electrolytic MnO2 Batteries: Electrolytes and Electrodes. Batteries. 2025; 11(5):171. https://doi.org/10.3390/batteries11050171
Chicago/Turabian StyleLiu, Shiwei, Zhongqi Liang, Hang Zhou, Weizheng Cai, Jiazhen Wu, Qianhui Zhang, Guoshen Yang, Walid A. Daoud, Zanxiang Nie, Pritesh Hiralal, and et al. 2025. "Recent Progress in Cathode-Free Zinc Electrolytic MnO2 Batteries: Electrolytes and Electrodes" Batteries 11, no. 5: 171. https://doi.org/10.3390/batteries11050171
APA StyleLiu, S., Liang, Z., Zhou, H., Cai, W., Wu, J., Zhang, Q., Yang, G., Daoud, W. A., Nie, Z., Hiralal, P., Luo, S., & Amaratunga, G. A. J. (2025). Recent Progress in Cathode-Free Zinc Electrolytic MnO2 Batteries: Electrolytes and Electrodes. Batteries, 11(5), 171. https://doi.org/10.3390/batteries11050171








