Interactions Between Trivalent Elements Enable Ultrastable LDH Cathode for High-Performance Zinc Battery
Abstract
1. Introduction
2. Experimental Section
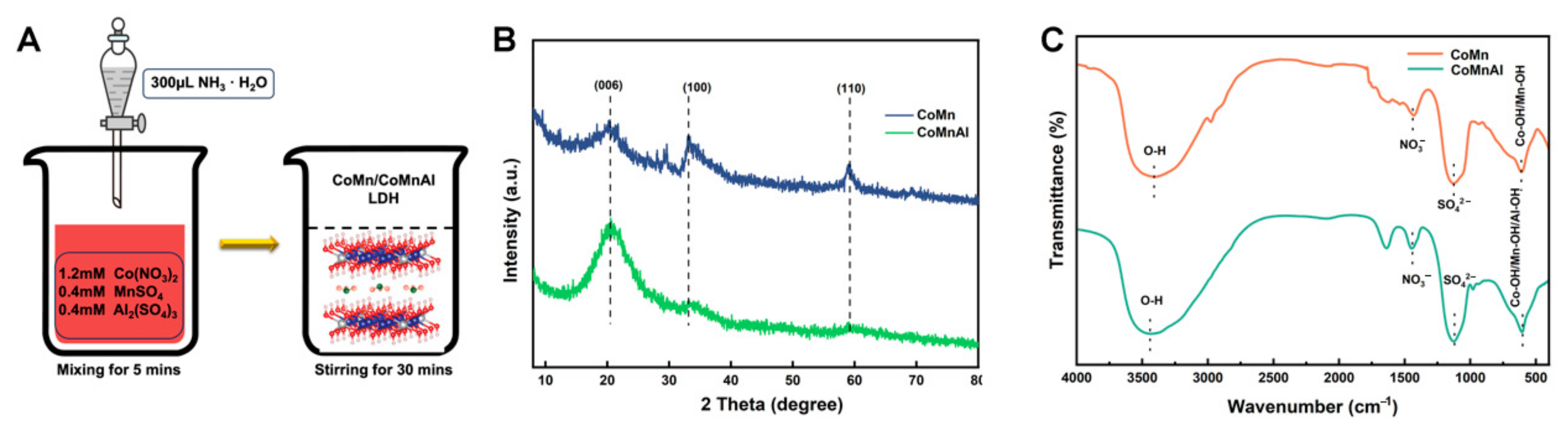
2.1. Synthesis of CoMn, CoAl, and CoMnAl LDHs
2.2. Physical Characterization
2.3. Electrochemical Evaluation
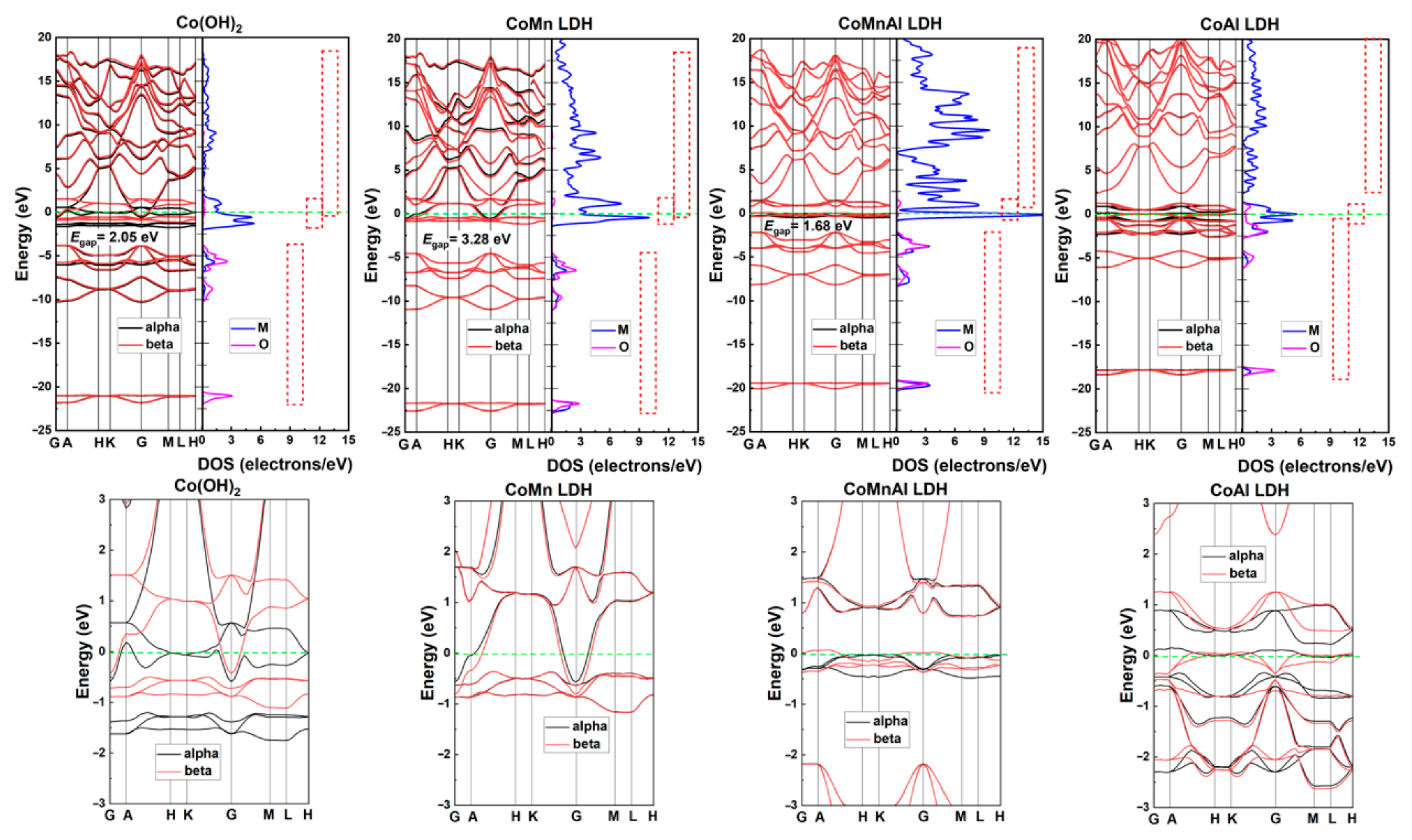
2.4. DFT Calculations
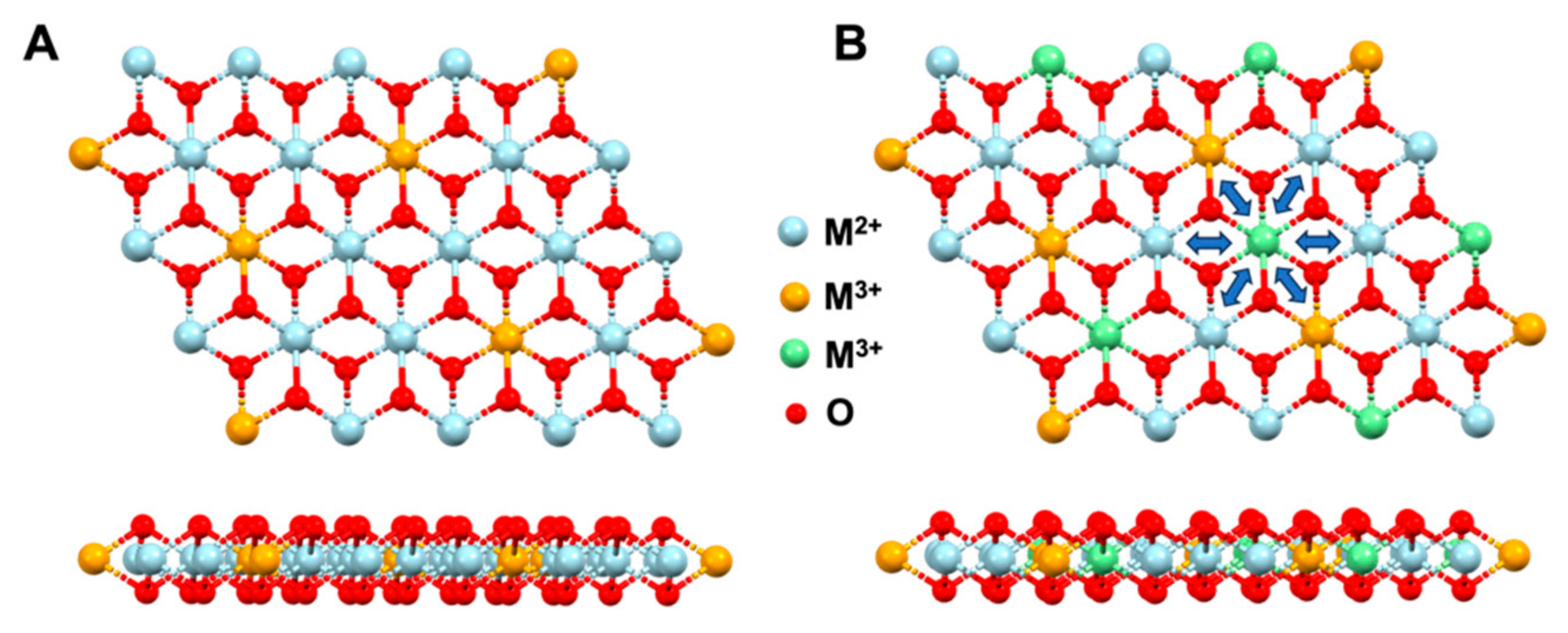
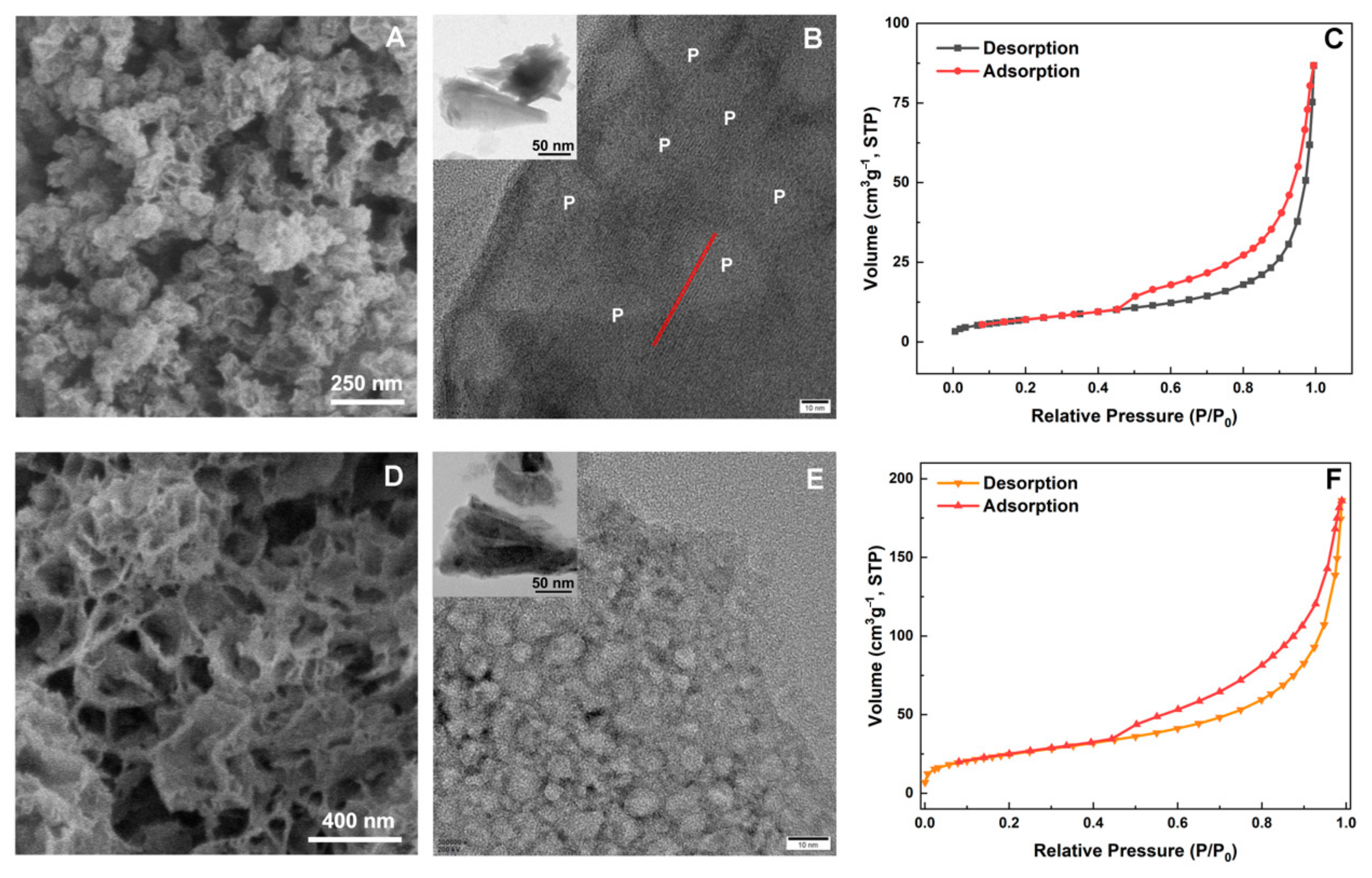
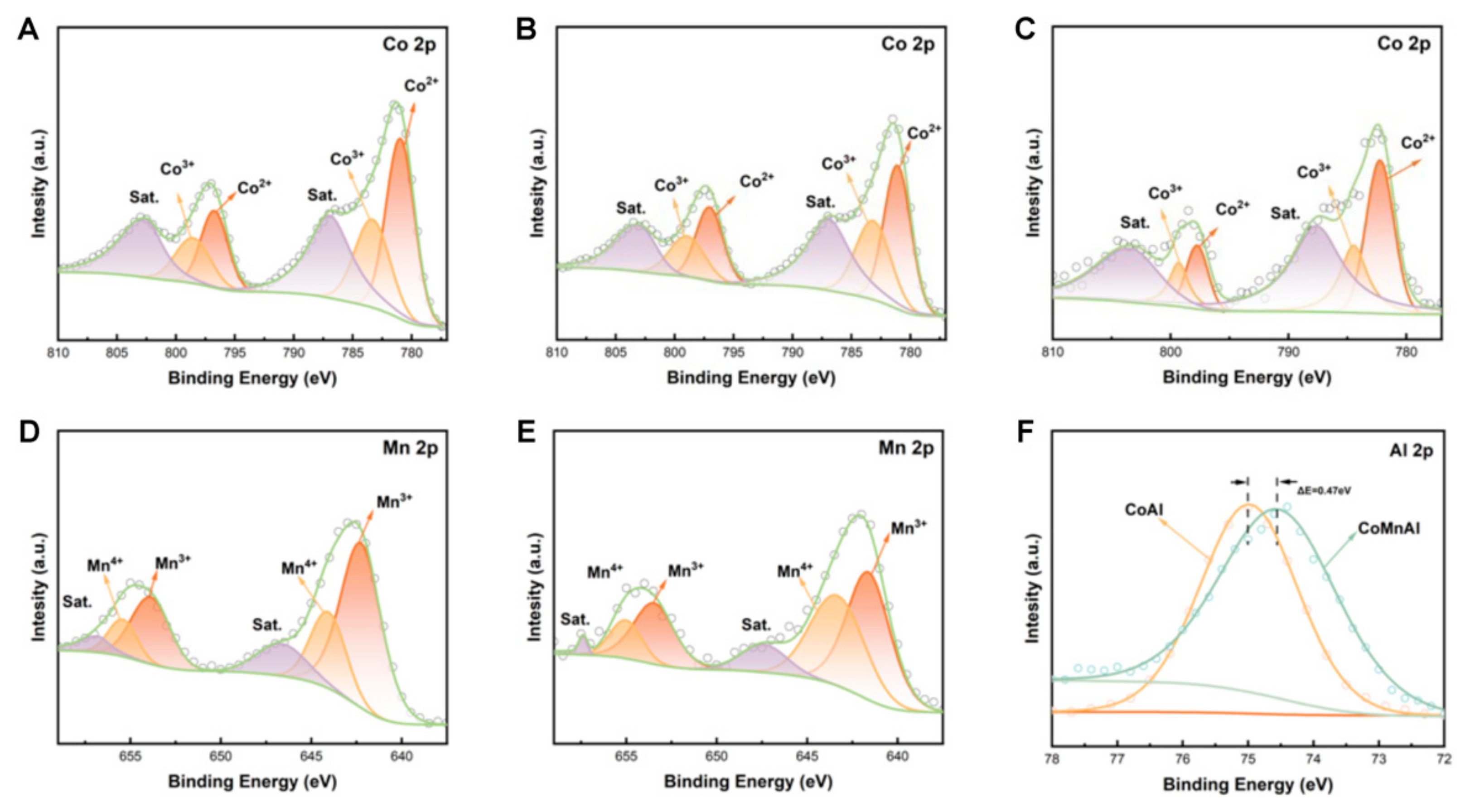
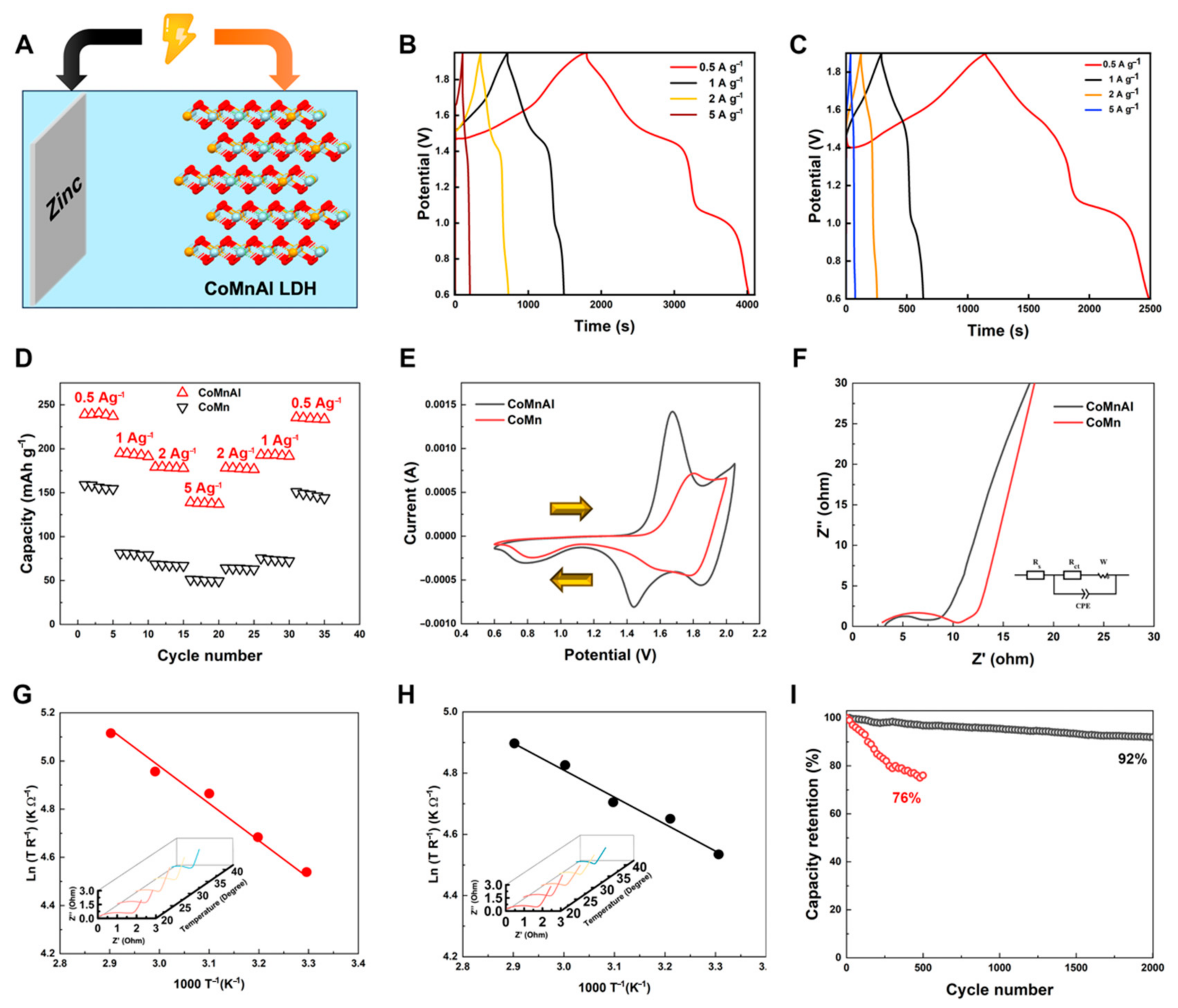
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, W.; Wang, S.; Wu, X.; Liu, W.; Yang, F.; Liu, S.; Jun, S.C.; Dai, L.; He, Z.; Zhang, Q. Tailoring desolvation strategies for aqueous zinc-ion batteries. Energy Environ. Sci. 2024, 17, 4819–4846. [Google Scholar] [CrossRef]
- Zheng, X.; Han, C.; Lee, C.-S.; Yao, W.; Zhi, C.; Tang, Y. Materials challenges for aluminum ion based aqueous energy storage devices: Progress and prospects. Prog. Mater. Sci. 2024, 143, 101253. [Google Scholar] [CrossRef]
- Li, C.; Hu, L.; Ren, X.; Lin, L.; Zhan, C.; Weng, Q.; Sun, X.; Yu, X. Asymmetric Charge Distribution of Active Centers in Small Molecule Quinone Cathode Boosts High-Energy and High-Rate Aqueous Zinc-Organic Batteries. Adv. Funct. Mater. 2024, 34, 2313241. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Hong, H.; Wang, S.; Zhu, J.; Zhi, C. Interface regulation and electrolyte design strategies for zinc anodes in high-performance zinc metal batteries. iScience 2025, 28, 111751. [Google Scholar] [CrossRef]
- Peng, Z.; Shen, X.; Li, B.; Cheng, J.; He, Z.; Sun, Z.; Li, B.; Zhang, Z.; Zhuang, Z.; Wu, X.; et al. Comprehensive crystallographic engineering for high-efficiency and durable zinc metal anodes. Prog. Mater. Sci. 2025, 152, 101453. [Google Scholar] [CrossRef]
- Cao, J.; Wu, H.; Zhang, D.; Luo, D.; Zhang, L.; Yang, X.; Qin, J.; He, G. In-Situ Ultrafast Construction of Zinc Tungstate Interface Layer for Highly Reversible Zinc Anodes. Angew. Chem. Int. Ed. 2024, 63, e202319661. [Google Scholar] [CrossRef]
- Cao, J.; Sun, M.; Zhang, D.; Zhang, Y.; Yang, C.; Luo, D.; Yang, X.; Zhang, X.; Qin, J.; Huang, B.; et al. Tuning Vertical Electrodeposition for Dendrites-Free Zinc-Ion Batteries. ACS Nano 2024, 18, 16610–16621. [Google Scholar] [CrossRef]
- Chen, F.; Li, j.; Shao, Y.; Zhu, Z.; Shen, T.; Chen, K.; Chen, Y.; Chen, Y. ZIF-67 wraps Ni-Mn LDHs nanosheets to enhance the capacitive contribution of supercapacitors. Chem. Eng. J. 2025, 507, 160454. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Gao, S.; Liu, C.; Chen, X. Design and synthesis of nickel-cobalt-aluminum hydroxide battery electrode materials for high-performance hybrid supercapacitors. J. Power Sources 2025, 632, 236370. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Lv, D.; Peng, H.; Song, X.; Yang, J.; Qian, Y. Interface Engineering by Hydrophilic and Zincophilic Aluminum Hydroxide Fluoride for Anode-Free Zinc Metal Batteries at Low Temperature. Adv. Energy Mater. 2023, 13, 2204388. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Liang, J.; Xia, X.; Ren, L.; Song, L.; Liu, W.; Sun, X. Unlocking Layered Double Hydroxide as a High-Performance Cathode Material for Aqueous Zinc-Ion Batteries. Adv. Mater. 2022, 34, e2204320. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, C.; Zhu, Y.; Du, W.; Liu, W.; Yao, M.; Wang, Y.; Qian, Y.; Feng, S. Unlocking the critical role of Mg doping in α-MnO2 cathode for aqueous zinc ion batteries. Chem. Eng. J. 2024, 485, 150077. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, F.; Li, H.; Dong, H.; Yan, C.; Meng, C.; Sang, Y.; Liu, H.; Guo, Y.-G.; Wang, S. Dynamic heterostructure design of MnO2 for high-performance aqueous zinc-ion batteries. Energy Environ. Sci. 2024, 17, 3629–3640. [Google Scholar] [CrossRef]
- Liang, X.; Liu, X.; Wang, P.; Guo, Z.; Chen, X.; Yao, J.; Li, J.; Gan, Y.; Lv, L.; Tao, L.; et al. Ion-exchange induced Ni doping of α-MnO2 cathode with structural modification for aqueous zinc ion batteries. J. Power Sources 2025, 635, 236518. [Google Scholar] [CrossRef]
- Fu, H.; Wang, X.; Ye, L.; Wu, Z.; Yang, J.; Shi, M.; Ang, E.H. Optimizing Fe in Mn-based Prussian blue analogs with dual redox-active sites to enhance operating voltage and durability in Zn-ion batteries. Chem. Eng. J. 2025, 506, 160308. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Li, C.-e.; Yan, X.-h.; Zhang, Y.-y.; Yin, R.-b.; Wei, Y.-f.; Gao, K.-z.; Gao, H.-l. Advanced strategies for enhancing electrochemical performance of NiAl LDH electrodes in supercapacitors. Coord. Chem. Rev. 2025, 531, 216497. [Google Scholar] [CrossRef]
- Bai, S.; Liu, G.; Shen, T.; Wu, Z.; Chen, W.; Song, Y.-F. Controllable synthesis of layered double hydroxides: From macroscopic morphology to microscopic coordination at the atomic level. Coord. Chem. Rev. 2025, 529, 216437. [Google Scholar] [CrossRef]
- Bujdosó, T.; Patzkó, Á.; Galbács, Z.; Dékány, I. Structural characterization of arsenate ion exchanged MgAl-layered double hydroxide. Appl. Clay Sci. 2009, 44, 75–82. [Google Scholar] [CrossRef]
- Wang, H.; Lin, T.; Song, Z.; Huang, M.; Chai, R.; An, S.; Song, Y.-F. Simultaneous mineralization of Cd(II), Pb(II) and As(V) using MgAl-NO3: Performance and mechanism. Sep. Purif. Technol. 2025, 362, 131853. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, L.; Zhang, C.; Shang, D.; Wu, L. Preparation of ZnAl layered double hydroxides supported by silica for the treatment of Cr(VI) and Cu(II) in aqueous solution. Sci. Rep. 2025, 15, 2522. [Google Scholar] [CrossRef]
- Wang, C.; Han, L.; Yang, S.; Liu, Z.; Liu, M.; Li, B. Nanosheet-structured ZnCo-LDH microsphere as active material for rechargeable zinc batteries. J. Colloid Interface Sci. 2024, 659, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Afkhami, A.; Madrakian, T.; Moazami, H.R. Electrosynthesis of Co Mn layered-double-hydroxide as a precursor for Co-Mn-MOFs and subsequent electrochemical sulfurization for supercapacitor application. J. Energy Storage 2023, 71, 108177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Gao, J.; Lu, W.; Feng, J.; Deng, T. Interactions Between Trivalent Elements Enable Ultrastable LDH Cathode for High-Performance Zinc Battery. Batteries 2025, 11, 170. https://doi.org/10.3390/batteries11050170
Zeng J, Gao J, Lu W, Feng J, Deng T. Interactions Between Trivalent Elements Enable Ultrastable LDH Cathode for High-Performance Zinc Battery. Batteries. 2025; 11(5):170. https://doi.org/10.3390/batteries11050170
Chicago/Turabian StyleZeng, Junhua, Jinlei Gao, Wenyao Lu, Jiashuo Feng, and Ting Deng. 2025. "Interactions Between Trivalent Elements Enable Ultrastable LDH Cathode for High-Performance Zinc Battery" Batteries 11, no. 5: 170. https://doi.org/10.3390/batteries11050170
APA StyleZeng, J., Gao, J., Lu, W., Feng, J., & Deng, T. (2025). Interactions Between Trivalent Elements Enable Ultrastable LDH Cathode for High-Performance Zinc Battery. Batteries, 11(5), 170. https://doi.org/10.3390/batteries11050170






