Electrospun Interconnected Bead-Like P2-NaxCoyMn1−yO2 (x = 0.66, y = 0.1) Cathode Material for Stable Sodium-Ion Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
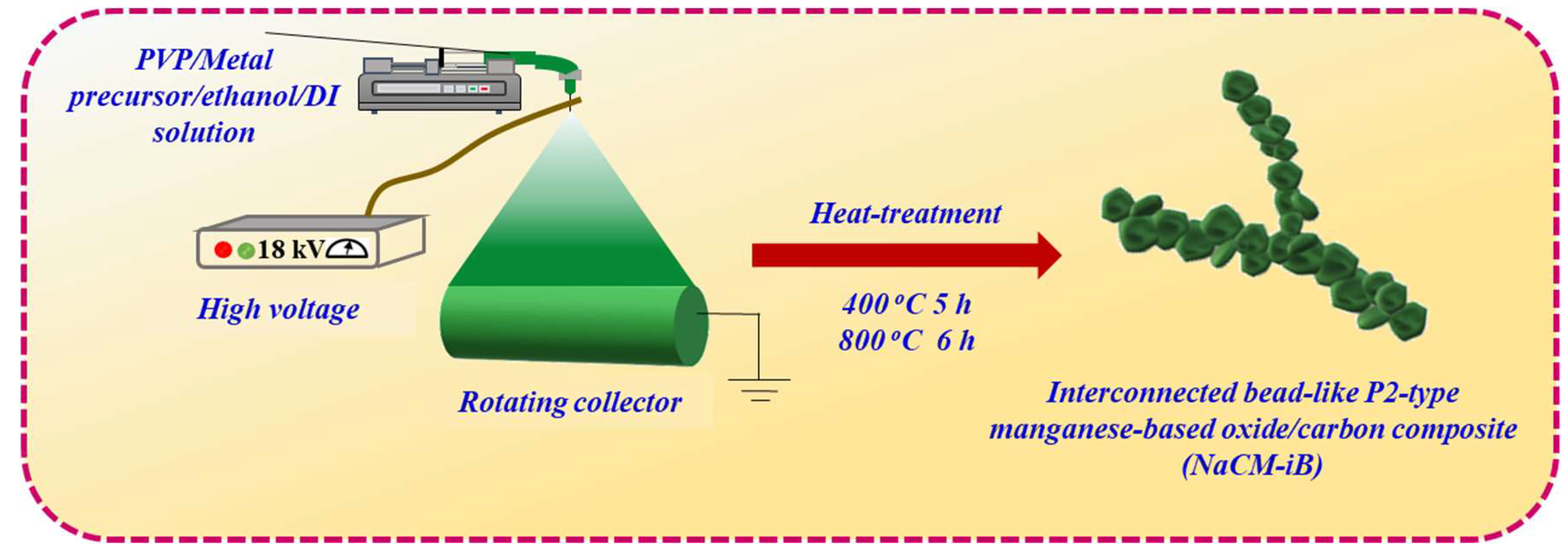
2.2. Synthesis of an Interconnected Bead-Like P2-Type Manganese-Based Oxide/C
2.3. Material Characterization
2.4. Electrochemical Characterizations
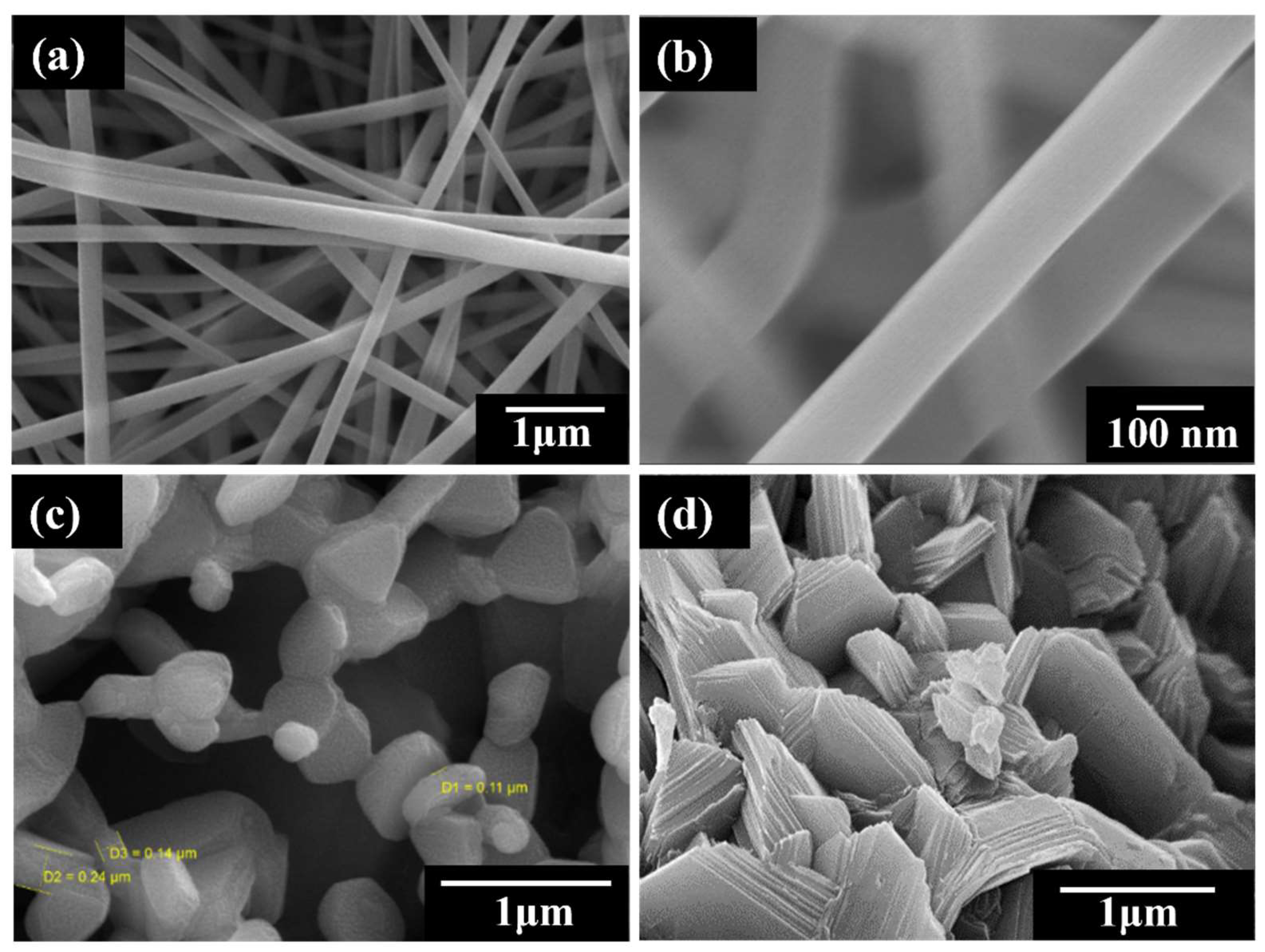
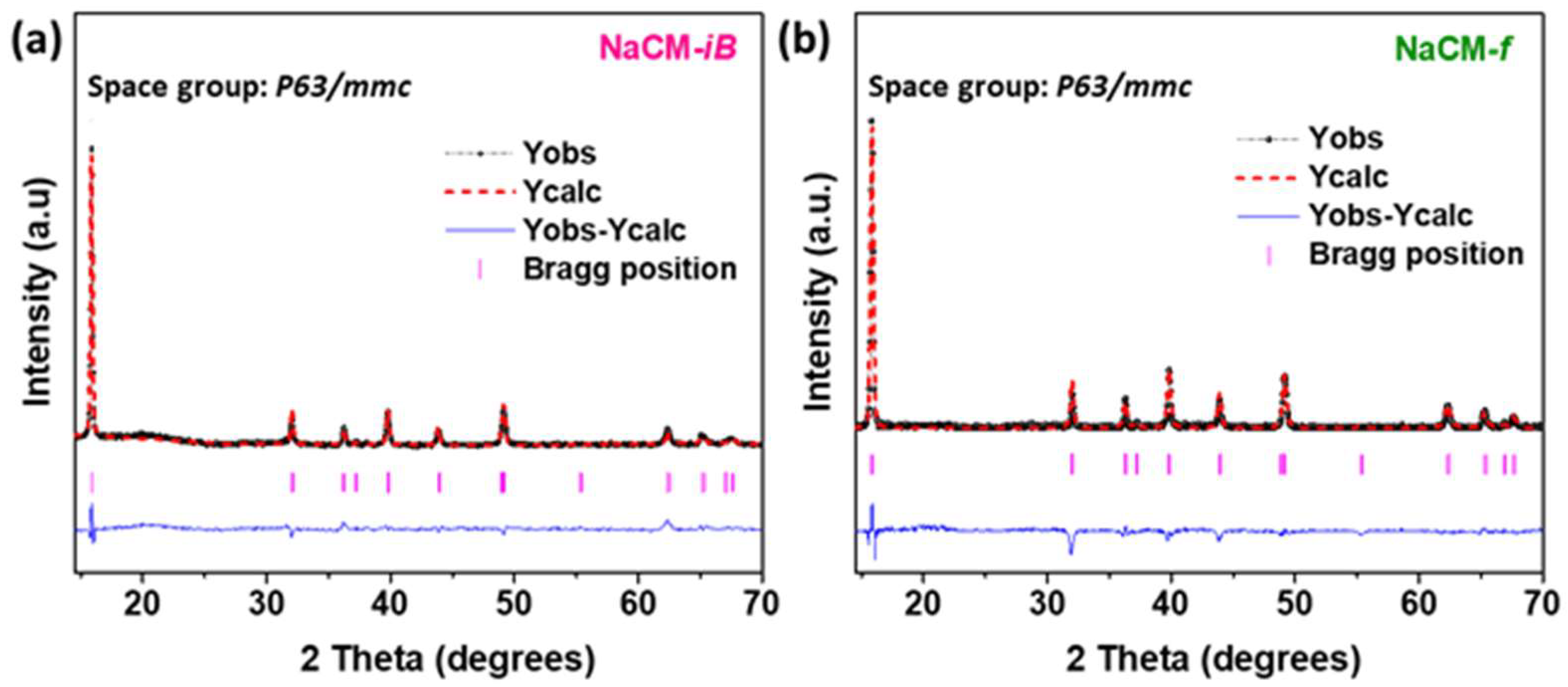
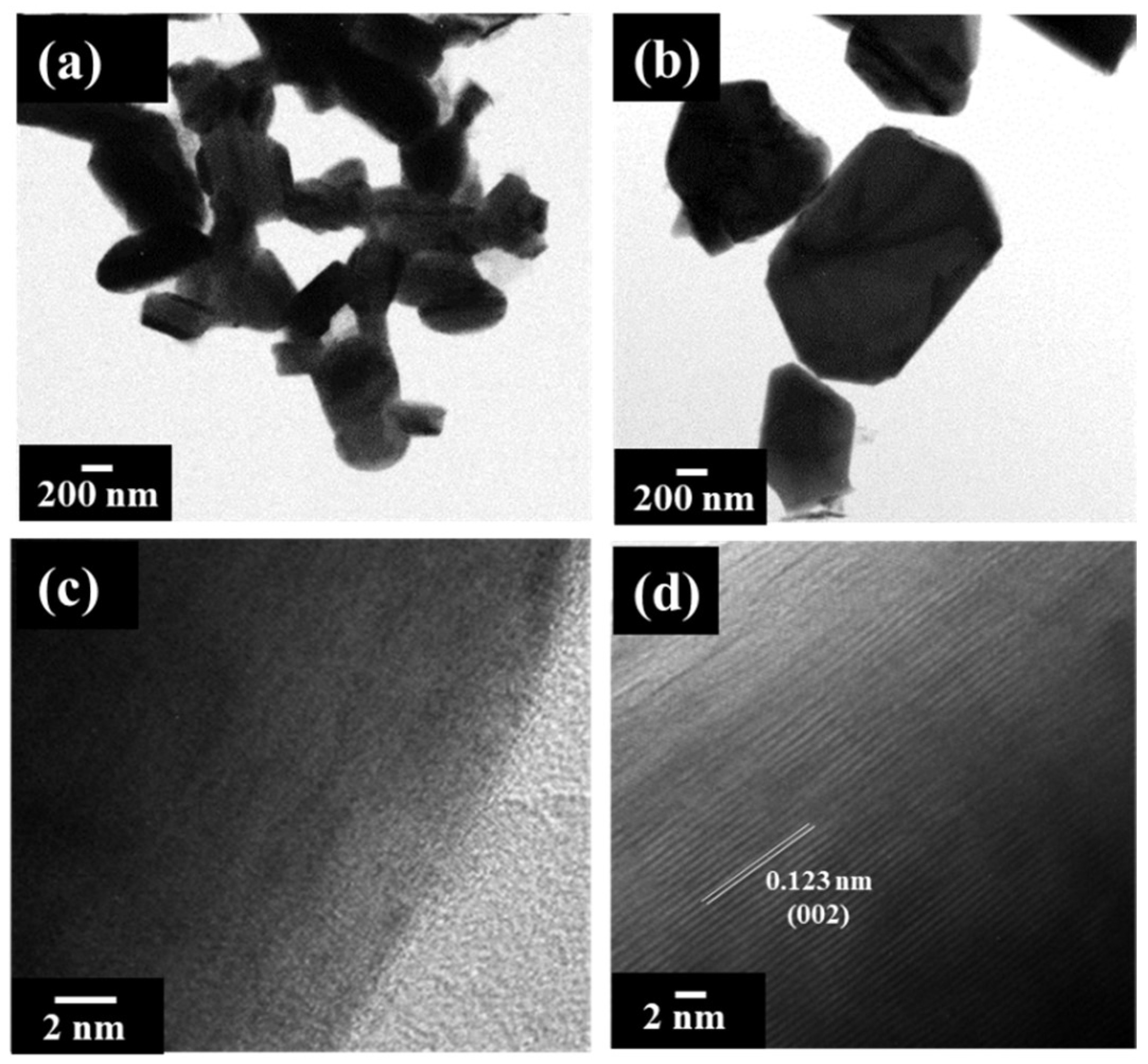
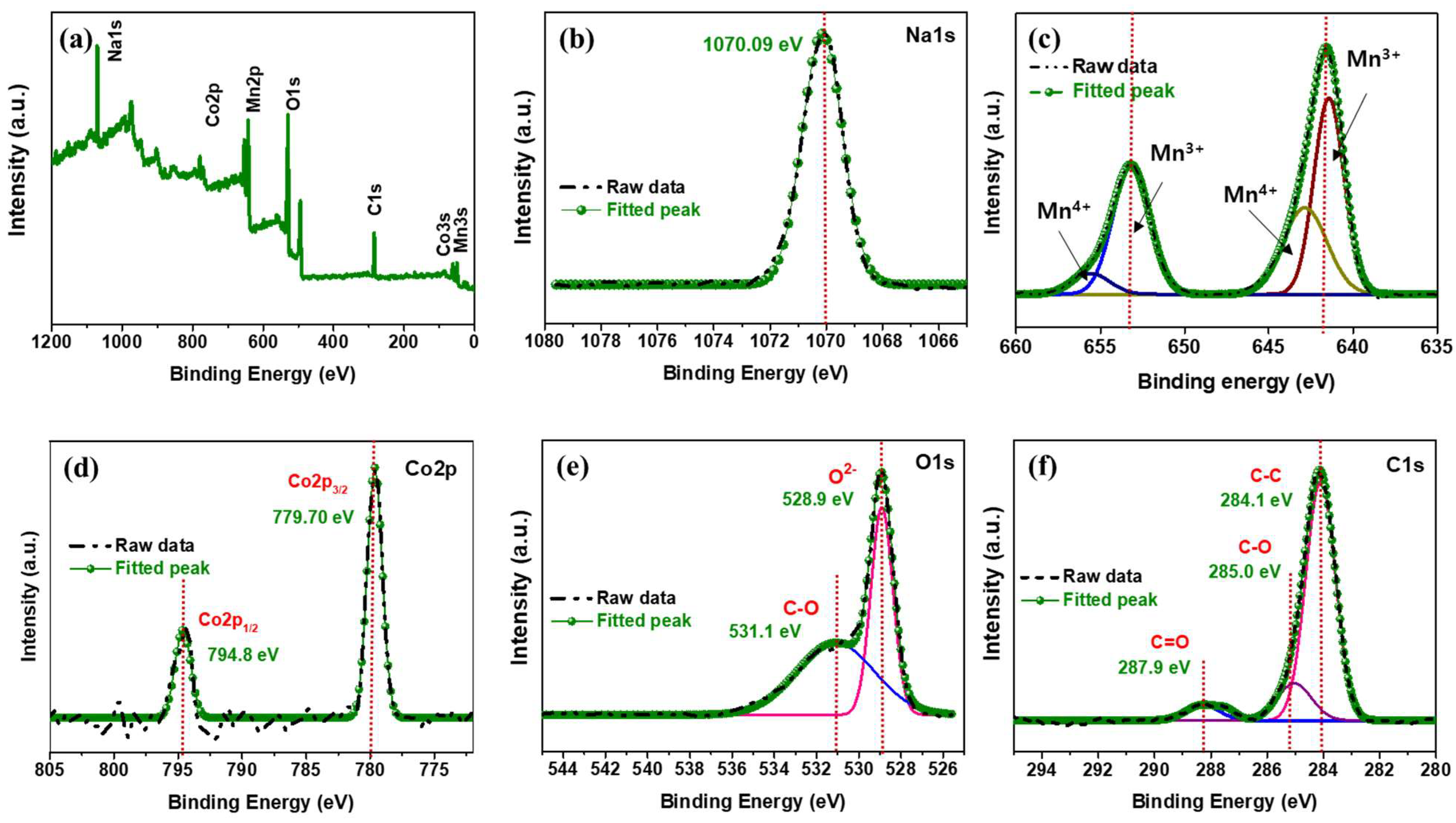
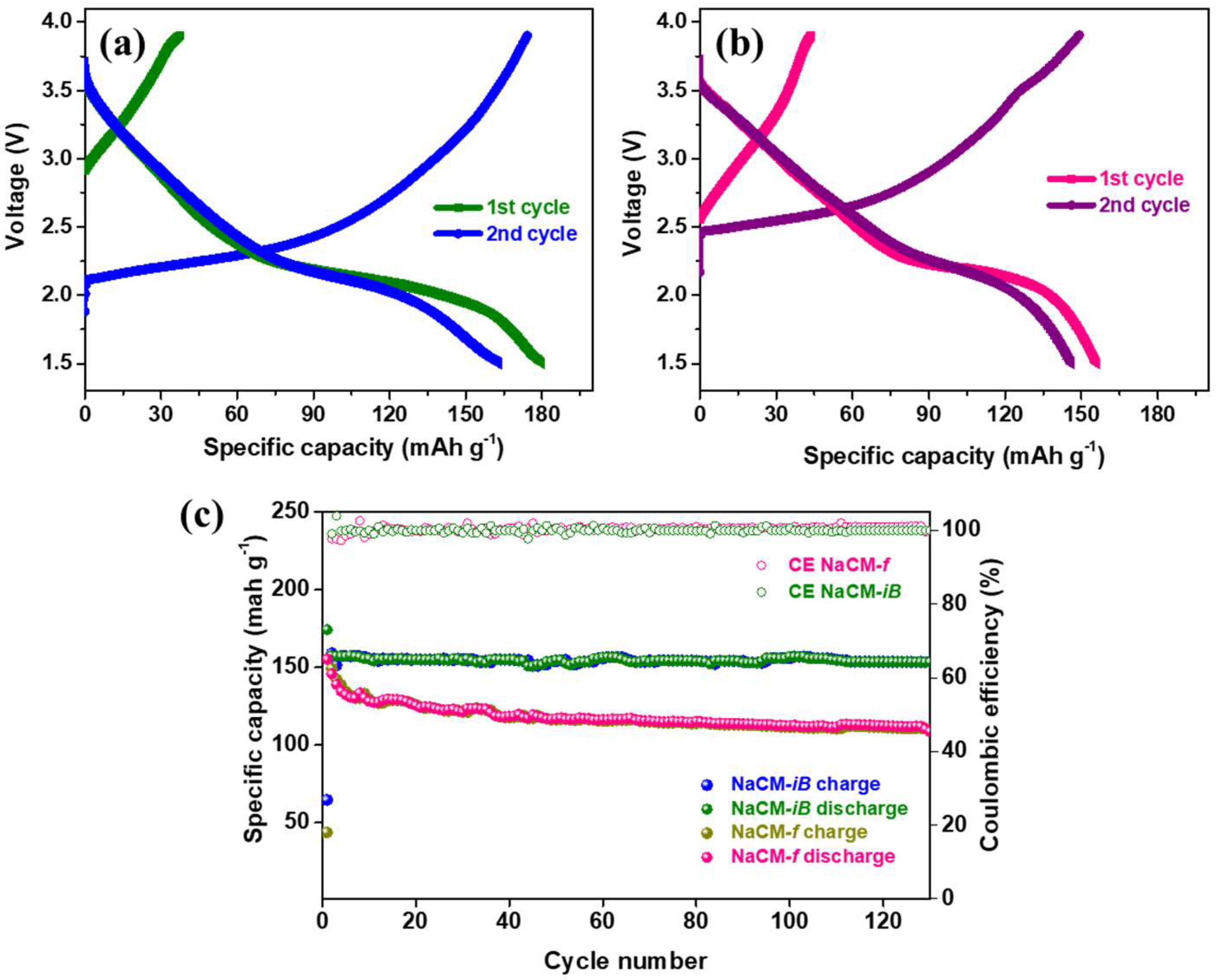
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Tarascon, J.M. Is lithium the new gold? Nat. Chem. 2010, 2, 510. [Google Scholar] [CrossRef]
- Kim, S.-W.; Seo, D.-H.; Ma, X.; Ceder, G.; Kang, K. Electrode Materials for Rechargeable Sodium-Ion Batteries: Potential Alternatives to Current Lithium-Ion Batteries. Adv. Energy Mater. 2012, 2, 710–721. [Google Scholar] [CrossRef]
- Wang, L.P.; Yu, L.; Wang, X.; Srinivasan, M.; Xu, Z.J. Recent developments in electrode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 9353–9378. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Yu, S.; Wang, B.; Li, Y.; Sun, W.; Lu, Y.; Yan, M.; Song, B.; Dou, S. Prussian Blue@C Composite as an Ultrahigh-Rate and Long-Life Sodium-Ion Battery Cathode. Adv. Funct. Mater. 2016, 26, 5315–5321. [Google Scholar] [CrossRef]
- Yue, Y.; Binder, A.J.; Guo, B.; Zhang, Z.; Qiao, Z.A.; Tian, C.; Dai, S. Mesoporous Prussian blue analogues: Template-free synthesis and sodium-ion battery applications. Angew. Chem. 2014, 53, 3134–3137. [Google Scholar] [CrossRef]
- Fan, X.; Hu, E.; Ji, X.; Zhu, Y.; Han, F.; Hwang, S.; Liu, J.; Bak, S.; Ma, Z.; Gao, T.; et al. High energy-density and reversibility of iron fluoride cathode enabled via an intercalation-extrusion reaction. Nat. Commun. 2018, 9, 2324. [Google Scholar] [CrossRef] [Green Version]
- Euchner, H.; Clemens, O.; Reddy, M.A. Unlocking the potential of weberite-type metal fluorides in electrochemical energy storage. Npj Comput. Mater. 2019, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Ni, Q.; Bai, Y.; Wu, F.; Wu, C. Polyanion-Type Electrode Materials for Sodium-Ion Batteries. Adv. Sci. 2017, 4, 1600275. [Google Scholar] [CrossRef]
- Barpanda, P.; Lander, L.; Nishimura, S.-I.; Yamada, A. Polyanionic Insertion Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1703055. [Google Scholar] [CrossRef]
- Han, M.H.; Gonzalo, E.; Singh, G.; Rojo, T. A comprehensive review of sodium layered oxides: Powerful cathodes for Na-ion batteries. Energy Environ. Sci. 2015, 8, 81–102. [Google Scholar] [CrossRef]
- Chagas, L.G.; Buchholz, D.; Vaalma, C.; Wu, L.; Passerini, S. P-type NaxNi0.22Co0.11Mn0.66O2 materials: Linking synthesis with structure and electrochemical performance. J. Mater. Chem. A 2014, 2, 20263–20270. [Google Scholar] [CrossRef] [Green Version]
- Hasa, I.; Buchholz, D.; Passerini, S.; Scrosati, B.; Hassoun, J. High Performance Na0.5[Ni0.23Fe0.13Mn0.63]O2 Cathode for Sodium-Ion Batteries. Adv. Energy Mater. 2014, 4, 1400083. [Google Scholar] [CrossRef]
- Delmas, C.; Fouassier, C.; Hagenmuller, P. Structural classification and properties of the layered oxides. Phys. B+C 1980, 99, 81–85. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kajiyama, M.; Iwatate, J.; Nishikawa, H.; Hitomi, S.; Okuyama, R.; Usui, R.; Yamada, Y.; Komaba, S. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 2012, 11, 512–517. [Google Scholar] [CrossRef]
- Mortemard de Boisse, B.; Carlier, D.; Guignard, M.; Delmas, C. Structural and Electrochemical Characterizations of P2 and New O3-NaxMn1-yFeyO2Phases Prepared by Auto-Combustion Synthesis for Na-Ion Batteries. J. Electrochem. Soc. 2013, 160, A569–A574. [Google Scholar] [CrossRef]
- Luo, C.; Langrock, A.; Fan, X.; Liang, Y.; Wang, C. P2-type transition metal oxides for high performance Na-ion battery cathodes. J. Mater. Chem. A 2017, 5, 18214–18220. [Google Scholar] [CrossRef]
- Kalluri, S.; Seng, K.H.; Pang, W.K.; Guo, Z.; Chen, Z.; Liu, H.K.; Dou, S.X. Electrospun P2-type Na2/3(Fe1/2Mn1/2)O2 hierarchical nanofibers as cathode material for sodium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 8953–8958. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, Y.; Lei, K.; Yang, Q.; Liu, Z.; Jiang, K.; Li, F.; Lu, Q.; Mikhailova, D.; Zheng, S. Na+/vacancy disordered manganese-based oxide cathode with ultralow strain enabled by tuning charge distribution. J. Mater. Chem. A 2022, 10, 10391–10399. [Google Scholar] [CrossRef]
- Baster, D.; Zając, W.; Kondracki, Ł.; Hartman, F.; Molenda, J. Improvement of electrochemical performance of Na0.7Co1−yMnyO2—Cathode material for rechargeable sodium-ion batteries. Solid State Ion. 2016, 288, 213–218. [Google Scholar] [CrossRef]
- Hemalatha, K.; Jayakumar, M.; Prakash, A.S. Influence of the manganese and cobalt content on the electrochemical performance of P2-Na0.67MnxCo1-xO2 cathodes for sodium-ion batteries. Dalton Trans. 2018, 47, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Bucher, N.; Hartung, S.; Franklin, J.B.; Wise, A.M.; Lim, L.Y.; Chen, H.-Y.; Weker, J.N.; Toney, M.F.; Srinivasan, M. P2–NaxCoyMn1–yO2 (y = 0, 0.1) as Cathode Materials in Sodium-Ion Batteries—Effects of Doping and Morphology To Enhance Cycling Stability. Chem. Mater. 2016, 28, 2041–2051. [Google Scholar] [CrossRef]
- Fu, C.C.; Wang, J.; Li, Y.; Liu, G.; Deng, T. Explore the effect of Co Doping on P2-Na0.67MnO2 prepared by hydrothermal method as cathode materials for sodium ion batteries. J. Alloy. Compd. 2022, 918, 165569. [Google Scholar] [CrossRef]
- Li, H.; Bai, Y.; Wu, F.; Ni, Q.; Wu, C. Na3V2(PO4)3/C nanorods as advanced cathode material for sodium ion batteries. Solid State Ion. 2015, 278, 281–286. [Google Scholar] [CrossRef]
- Mai, L.; Xu, L.; Han, C.; Xu, X.; Luo, Y.; Zhao, S.; Zhao, Y. Electrospun ultralong hierarchical vanadium oxide nanowires with high performance for lithium ion batteries. Nano Lett. 2010, 10, 4750–4755. [Google Scholar] [CrossRef]
- Haridas, A.K.; Heo, J.; Li, X.; Ahn, H.-J.; Zhao, X.; Deng, Z.; Agostini, M.; Matic, A.; Ahn, J.-H. A flexible and free-standing FeS/sulfurized polyacrylonitrile hybrid anode material for high-rate sodium-ion storage. Chem. Eng. J. 2019, 385, 123453. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Qian, Q.; Huang, H.; Chen, Y.; Wang, Z.; Chen, Q.; Yang, J.; Li, J.; Mai, Y.W. Electrospinning-Based Strategies for Battery Materials. Adv. Energy Mater. 2020, 11, 2000845. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Chung, S.-H. A design of the cathode substrate for high-loading polysulfide cathodes in lean-electrolyte lithium-sulfur cells. Chem. Eng. J. 2021, 422, 130363. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Correia, D.M.; Fidalgo-Marijuan, A.; Gonçalves, R.; Fernandes, M.; de Zea Bermudez, V.; Silva, M.M.; Lanceros-Mendez, S.; Costa, C.M. Sustainable Lithium-Ion Battery Separators Based on Poly(3-Hydroxybutyrate-Co-Hydroxyvalerate) Pristine and Composite Electrospun Membranes. Energy Technol. 2021, 10, 2100761. [Google Scholar] [CrossRef]
- Joshi, B.; Samuel, E.; Kim, Y.-i.; Yarin, A.L.; Swihart, M.T.; Yoon, S.S. Progress and potential of electrospinning-derived substrate-free and binder-free lithium-ion battery electrodes. Chem. Eng. J. 2022, 430, 132876. [Google Scholar] [CrossRef]
- Haridas, A.K.; Sharma, C.S.; Rao, T.N. Caterpillar-like sub-micron LiNi0.5Mn1.5O4 structures with site disorder and excess Mn3+ as high performance cathode material for lithium ion batteries. Electrochim. Acta 2016, 212, 500–509. [Google Scholar] [CrossRef]
- Fu, B.; Zhou, X.; Wang, Y. High-rate performance electrospun Na0.44MnO2 nanofibers as cathode material for sodium-ion batteries. J. Power Sources 2016, 310, 102–108. [Google Scholar] [CrossRef]
- Li, J.-Y.; Lü, H.-Y.; Zhang, X.-H.; Xing, Y.-M.; Wang, G.; Guan, H.-Y.; Wu, X.-L. P2-type Na0.53MnO2 nanorods with superior rate capabilities as advanced cathode material for sodium ion batteries. Chem. Eng. J. 2017, 316, 499–505. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Manuel, J.; Chauhan, G.S.; Ahn, H.J.; Kim, K.W.; Cho, K.K.; Ahn, J.H. Nitrogen-Doped Mesoporous Carbon: A Top-Down Strategy to Promote Sulfur Immobilization for Lithium-Sulfur Batteries. ChemSusChem 2015, 8, 3234–3241. [Google Scholar] [CrossRef]
- Kumar Gupta, N.; Bae, J.; Soo Kim, K. Metal-organic framework-derived NaMnxOy hexagonal microsheets for superior adsorptive-oxidative removal of hydrogen sulfide in ambient conditions. Chem. Eng. J. 2022, 427, 130909. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, X.; Hu, M.; Liang, J.; Wu, D.; Wei, J.; Zhou, Z. Stable layered P3/P2 Na0.66Co0.5Mn0.5O2 cathode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 20708–20714. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Wang, S.; Wang, W.; Xiang, Y.; Dai, C.; Liu, Z.; He, Z.; Wu, X. Surfactant-assisted solvothermal synthesis of NiCo2O4 as an anode for lithium-ion batteries. RSC Adv. 2017, 7, 36909–36916. [Google Scholar] [CrossRef] [Green Version]
- Haridas, A.K.; Sadan, M.K.; Liu, Y.; Jung, H.Y.; Lee, Y.; Ahn, H.-J.; Ahn, J.-H. Simple and scalable gelatin-mediated synthesis of a novel iron sulfide/graphitic carbon nanoarchitecture for sustainable sodium-ion storage. J. Alloy. Compd. 2022, 928, 167125. [Google Scholar] [CrossRef]
- Zhou, Y.-T.; Sun, X.; Zou, B.-K.; Liao, J.-Y.; Wen, Z.-Y.; Chen, C.-H. Cobalt-substituted Na0.44Mn1−xCoxO2: Phase evolution and a high capacity positive electrode for sodium-ion batteries. Electrochim. Acta 2016, 213, 496–503. [Google Scholar] [CrossRef]
- Bucher, N.; Hartung, S.; Nagasubramanian, A.; Cheah, Y.L.; Hoster, H.E.; Madhavi, S. Layered NaxMnO2+z in sodium ion batteries-influence of morphology on cycle performance. ACS Appl. Mater. Interfaces 2014, 6, 8059–8065. [Google Scholar] [CrossRef]
- Wang, X.; Tamaru, M.; Okubo, M.; Yamada, A. Electrode Properties of P2–Na2/3MnyCo1–yO2 as Cathode Materials for Sodium-Ion Batteries. J. Phys. Chem. C 2013, 117, 15545–15551. [Google Scholar] [CrossRef]
- Wang, C.; Liu, L.; Zhao, S.; Liu, Y.; Yang, Y.; Yu, H.; Lee, S.; Lee, G.H.; Kang, Y.M.; Liu, R.; et al. Tuning local chemistry of P2 layered-oxide cathode for high energy and long cycles of sodium-ion battery. Nat. Commun. 2021, 12, 2256. [Google Scholar] [CrossRef]
- Luo, R.; Wu, F.; Xie, M.; Ying, Y.; Zhou, J.; Huang, Y.; Ye, Y.; Li, L.; Chen, R. Habit plane-driven P2-type manganese-based layered oxide as long cycling cathode for Na-ion batteries. J. Power Sources 2018, 383, 80–86. [Google Scholar] [CrossRef]
- Konarov, A.; Kim, H.J.; Voronina, N.; Bakenov, Z.; Myung, S.T. P2-Na2/3MnO2 by Co Incorporation: As a Cathode Material of High Capacity and Long Cycle Life for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 28928–28933. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haridas, A.K.; Sadan, M.K.; Kim, J.-H.; Lee, Y.; Ahn, J.-H. Electrospun Interconnected Bead-Like P2-NaxCoyMn1−yO2 (x = 0.66, y = 0.1) Cathode Material for Stable Sodium-Ion Storage. Batteries 2022, 8, 237. https://doi.org/10.3390/batteries8110237
Haridas AK, Sadan MK, Kim J-H, Lee Y, Ahn J-H. Electrospun Interconnected Bead-Like P2-NaxCoyMn1−yO2 (x = 0.66, y = 0.1) Cathode Material for Stable Sodium-Ion Storage. Batteries. 2022; 8(11):237. https://doi.org/10.3390/batteries8110237
Chicago/Turabian StyleHaridas, Anupriya K., Milan K. Sadan, Joo-Hyung Kim, Younki Lee, and Jou-Hyeon Ahn. 2022. "Electrospun Interconnected Bead-Like P2-NaxCoyMn1−yO2 (x = 0.66, y = 0.1) Cathode Material for Stable Sodium-Ion Storage" Batteries 8, no. 11: 237. https://doi.org/10.3390/batteries8110237
APA StyleHaridas, A. K., Sadan, M. K., Kim, J.-H., Lee, Y., & Ahn, J.-H. (2022). Electrospun Interconnected Bead-Like P2-NaxCoyMn1−yO2 (x = 0.66, y = 0.1) Cathode Material for Stable Sodium-Ion Storage. Batteries, 8(11), 237. https://doi.org/10.3390/batteries8110237






