Solid Electrolyte Interphase Layer Formation on the Si-Based Electrodes with and without Binder Studied by XPS and ToF-SIMS Analysis
Abstract
:1. Introduction
2. Experimental Part
2.1. Si-Based Electrodes
2.2. Galvanostatic Tests
2.3. Morphology Characterizations
2.4. XPS and ToF-SIMS Characterizations
2.4.1. XPS and ToF-SIMS Experimental Conditions
2.4.2. Sample Preparation for XPS and ToF-SIMS
- -
- -
- -
- -
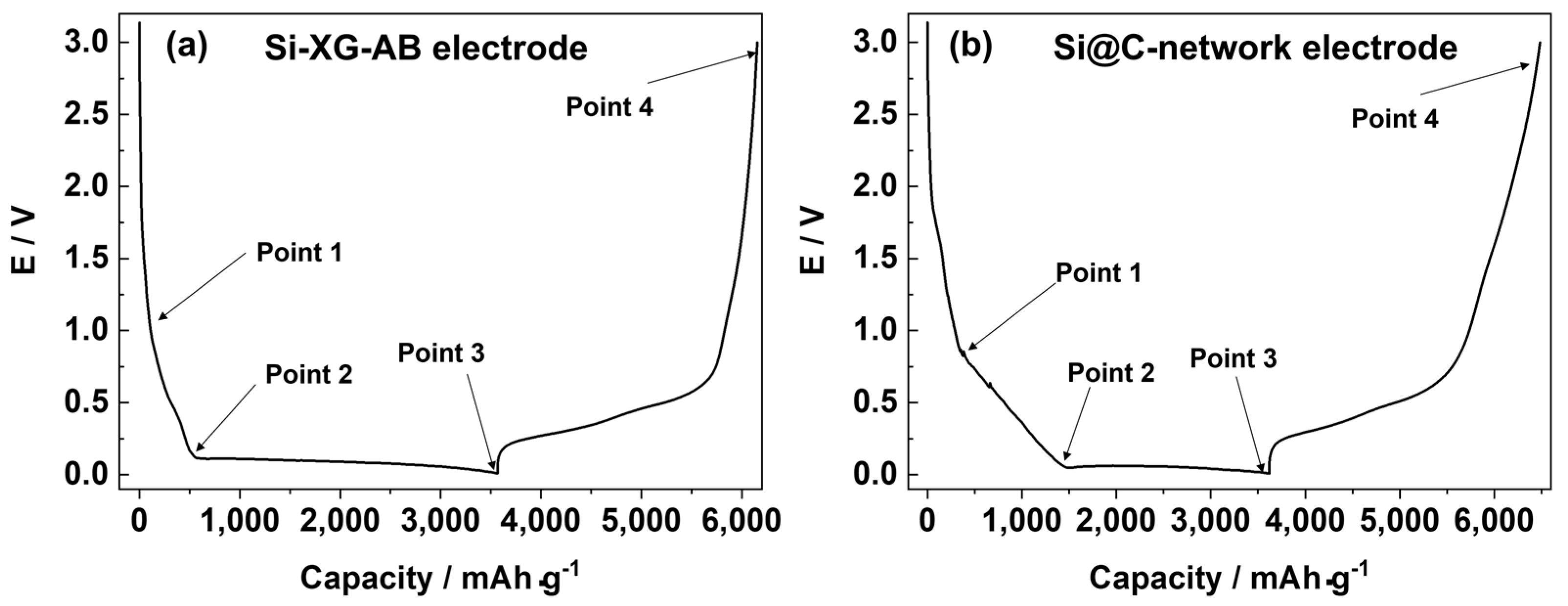
- point 4 (3.0 V) corresponding to the full delithiated state of the electrode.
3. Results and Discussion
3.1. Morphological Characterization of Electrodes by SEM
3.2. Galvanostatic Tests
3.3. Surface Characterizations
3.3.1. XPS Surface Characterizations of the Si-XG-AB Electrode
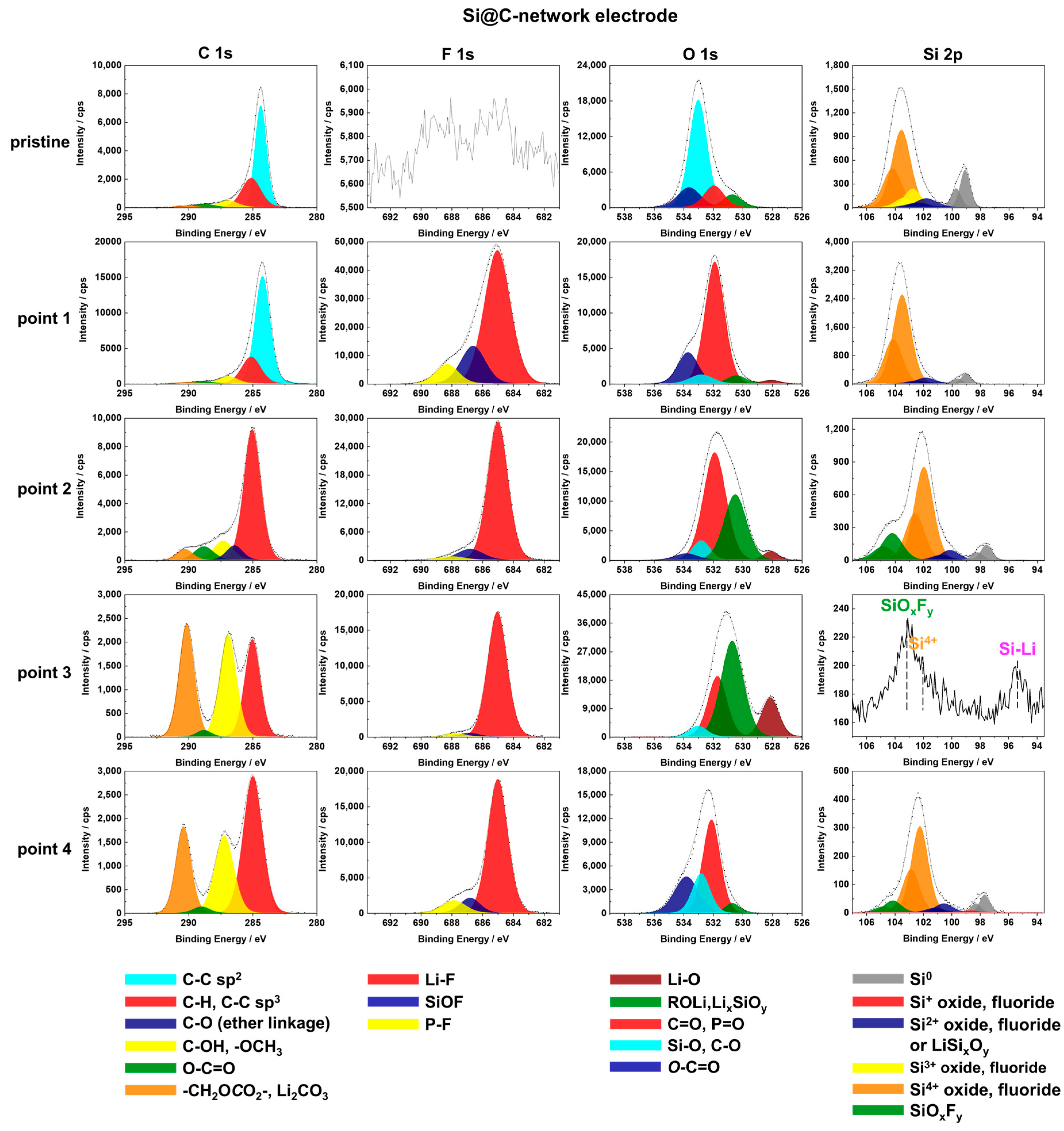
3.3.2. XPS Surface Characterizations of the Si@C-Network Electrode
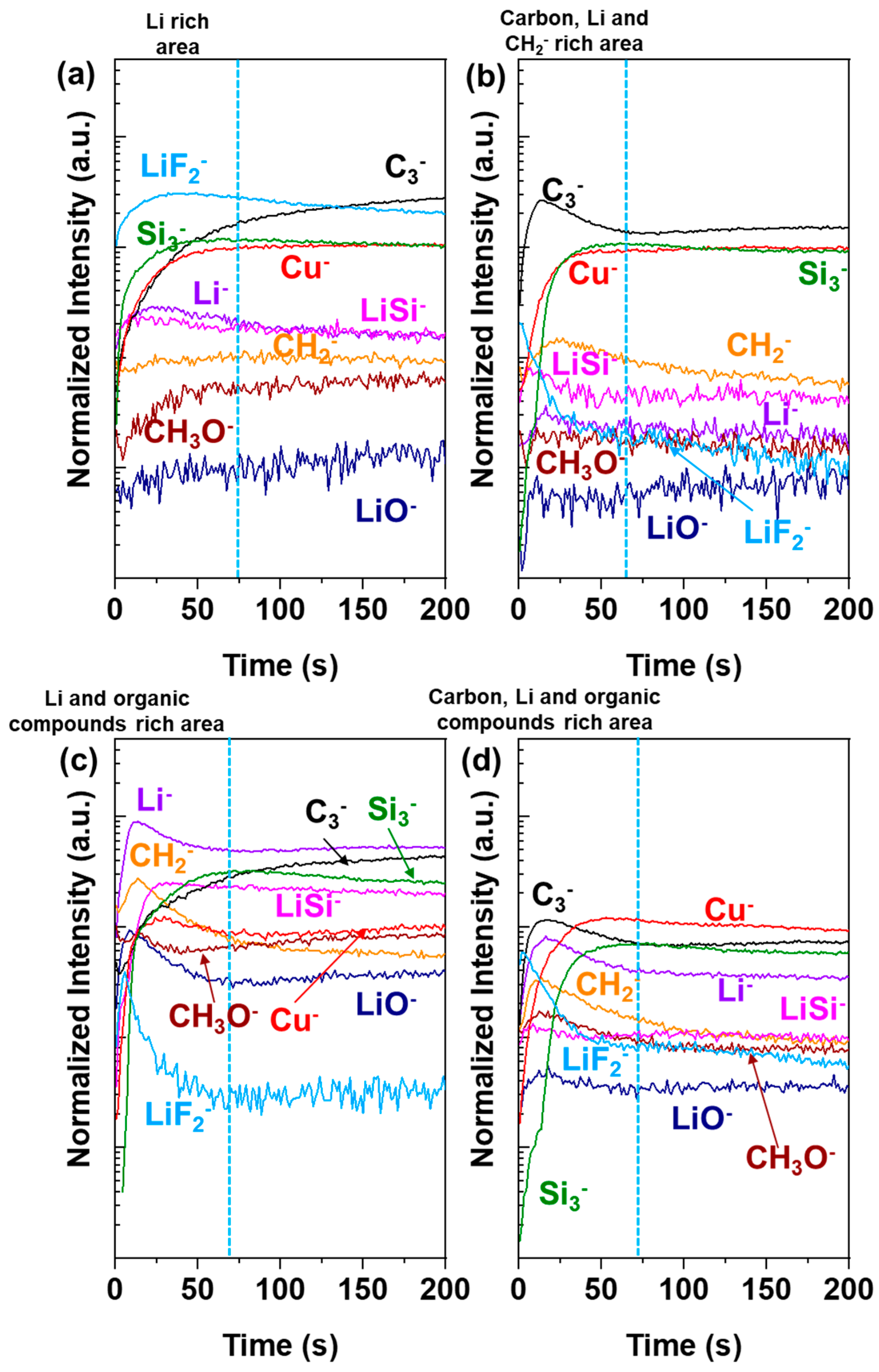
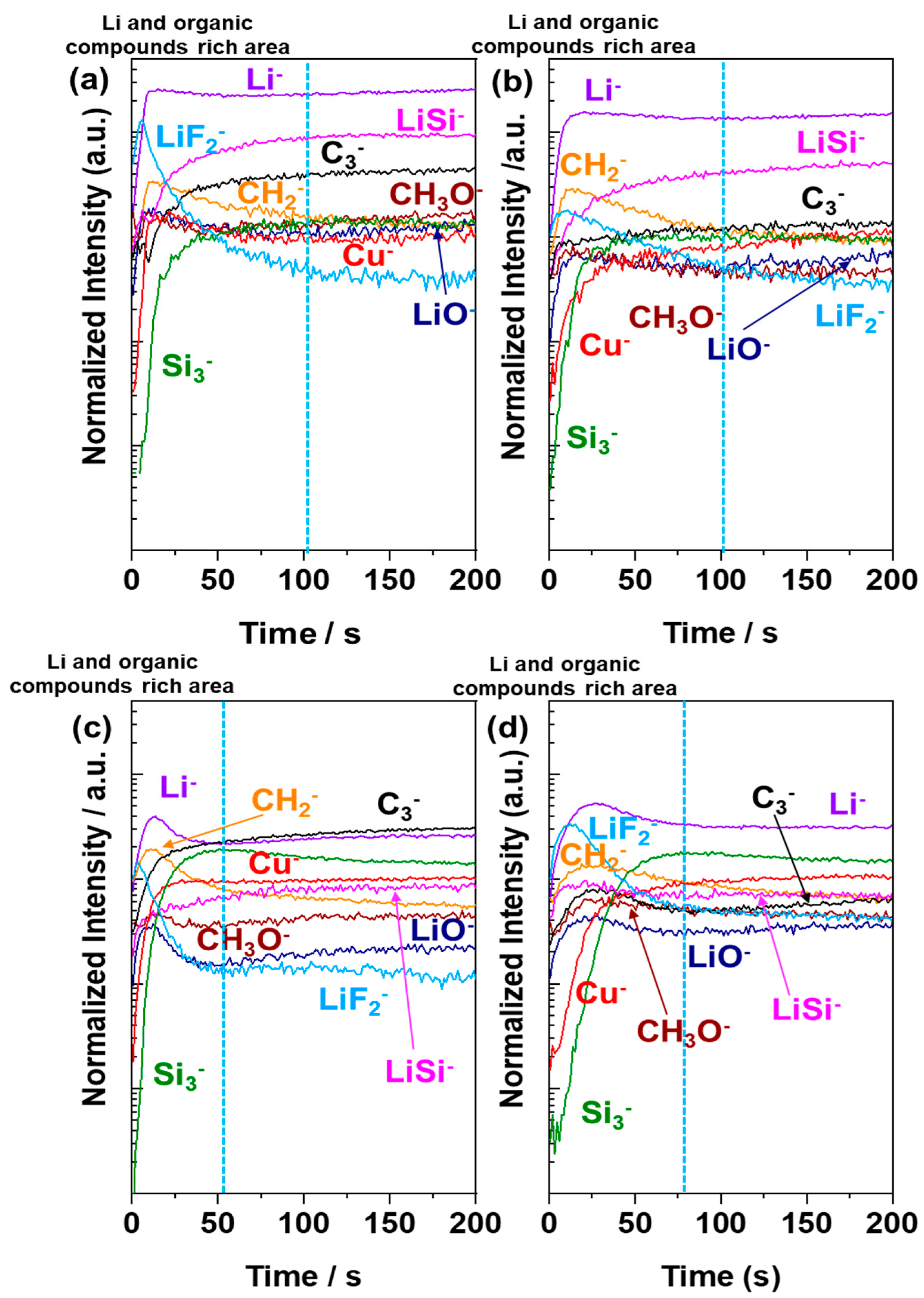
3.4. ToF-SIMS Surface and Bulk Characterizations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Slade, T.; Stolt, M.J.; Li, L.; Girard, S.N.; Mai, L.; Jin, S. Low-Temperature Molten-Salt Production of Silicon Nanowires by the Electrochemical Reduction of CaSiO3. Angew. Chem. Int. Ed. 2017, 129, 14645–14649. [Google Scholar] [CrossRef]
- Sun, L.; Wang, F.; Su, T.; Du, H. Room-Temperature Solution Synthesis of Mesoporous Silicon for Lithium Ion Battery Anodes. ACS Appl. Mater. Interfaces 2017, 9, 40386–40393. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Li, W.; Wang, L.; Fan, Y.; Jiang, W.; Luo, W.; Wang, Y.; Kong, B.; Selomulya, C.; et al. Amorphous TiO2 Shells: A Vital Elastic Buffering Layer on Silicon Nanoparticles for High-Performance and Safe Lithium Storage. Adv. Mater. 2017, 29, 1700523. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zu, L.; Liu, Y.; Meng, R.; Feng, Y.; Peng, C.; Zhu, F.; Hao, T.; Ru, J.; Wang, Y.; et al. Space-Confined Atomic Clusters Catalyze Superassembly of Silicon Nanodots within Carbon Frameworks for Use in Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2020, 132, 3161–3166. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, G.; Lv, G.; Mu, Y.; Zhao, Y.; Wang, Y.; Li, X.; Yao, P.; Deng, Y.; Cui, Y.; et al. Minimized lithium trapping by isovalent isomorphism for high initial Coulombic efficiency of silicon anodes. Sci. Adv. 2019, 5, eaax0651. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.; Miao, C.; Mou, H.; Xiao, W. Facile synthesis of Si@TiO2@rGO composite with sandwich-like nanostructure as superior performance anodes for lithium ion batteries. J. Alloys Compd. 2020, 818, 152884. [Google Scholar] [CrossRef]
- Deng, L.; Wu, Z.-Y.; You, J.; Yin, Z.; Ren, W.; Zhang, P.; Xu, B.; Zhou, Y.; Li, J. The Si@C-Network Electrode Prepared by an In Situ Carbonization Strategy with Enhanced Cycle Performance. ChemElectroChem 2020, 7, 4999–5004. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward Efficient Binders for Li-Ion Battery Si-Based Anodes: Polyacrylic Acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Q.; Zhang, T.; Li, J.-T.; Huang, L.; Sun, S.-G. A Robust Ion-Conductive Biopolymer as a Binder for Si Anodes of Lithium-Ion Batteries. Adv. Funct. Mater. 2015, 25, 3599–3605. [Google Scholar] [CrossRef]
- Li, J.-T.; Wu, Z.-Y.; Lu, Y.-Q.; Zhou, Y.; Huang, Q.-S.; Huang,, L.; Sun, S.-G. Water Soluble Binder, an Electrochemical Performance Booster for Electrode Materials with High Energy Density. Adv. Energy Mater. 2017, 7, 1701185. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, F.; Li, S.; Zhang, Y.; Tian, Z.; Xu, Q.; Xin, S.; Guo, Y. Advances of polymer binders for silicon-based anodes in high energy density lithium-ion batteries. InfoMat 2021, 3, 460–501. [Google Scholar] [CrossRef]
- Chen, L.; Xie, X.; Xie, J.; Wang, K.; Yang, J. Binder effect on cycling performance of silicon/carbon composite anodes for lithium ion batteries. J. Appl. Electrochem. 2006, 36, 1099–1104. [Google Scholar] [CrossRef]
- Hochgatterer, N.S.; Schweiger, M.R.; Koller, S.; Raimann, P.R.; Wöhrle, T.; Wurm, C.; Winter, M. Silicon/Graphite Composite Electrodes for High-Capacity Anodes: Influence of Binder Chemistry on Cycling Stability. Electrochem. Solid State Lett. 2008, 11, A76. [Google Scholar] [CrossRef]
- Chou, S.-L.; Pan, Y.; Wang, J.-Z.; Liu, H.-K.; Dou, S.-X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Choi, J.W.; Coskun, A. The emerging era of supramolecular polymeric binders in silicon anodes. Chem. Soc. Rev. 2018, 47, 2145–2164. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Wang, K.; Xie, X.; Xie, J. Effect of vinylene carbonate (VC) as electrolyte additive on electrochemical performance of Si film anode for lithium ion batteries. J. Power Sources 2007, 174, 538–543. [Google Scholar] [CrossRef]
- Etacheri, V.; Haik, O.; Goffer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D. Effect of Fluoroethylene Carbonate (FEC) on the Performance and Surface Chemistry of Si-Nanowire Li-Ion Battery Anodes. Langmuir 2012, 28, 965–976. [Google Scholar] [CrossRef]
- Li, Y.; Xu, G.; Yao, Y.; Xue, L.; Zhang, S.; Lu, Y.; Toprakci, O.; Zhang, X. Improvement of cyclability of silicon-containing carbon nanofiber anodes for lithium-ion batteries by employing succinic anhydride as an electrolyte additive. J. Solid State Electrochem. 2013, 17, 1393–1399. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Lu, Y.-Q.; Li, J.-T.; Zanna, S.; Seyeux, A.; Huang, L.; Sun, S.-G.; Marcus, P.; Światowska, J. Influence of Carbonate Solvents on Solid Electrolyte Interphase Composition over Si Electrodes Monitored by In Situ and Ex Situ Spectroscopies. ACS Omega 2021, 6, 27335–27350. [Google Scholar] [CrossRef] [PubMed]
- Philippe, B.; Dedryvère, R.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Improved Performances of Nanosilicon Electrodes Using the Salt LiFSI: A Photoelectron Spectroscopy Study. J. Am. Chem. Soc. 2013, 135, 9829–9842. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Nabais, C.; Światowska, J.; Chagnes, A.; Ozanam, F.; Gohier, A.; Tran-Van, P.; Cojocaru, C.-S.; Cassir, M.; Marcus, P. Interphase chemistry of Si electrodes used as anodes in Li-ion batteries. Appl. Surf. Sci. 2013, 266, 5–16. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Liao, C.; Dogan, F.; Trask, S.E.; Lapidus, S.H.; Vaughey, J.T.; Key, B. Using Mixed Salt Electrolytes to Stabilize Silicon Anodes for Lithium-Ion Batteries via in Situ Formation of Li–M–Si Ternaries (M = Mg, Zn, Al, Ca). ACS Appl. Mater. Interfaces 2019, 11, 29780–29790. [Google Scholar] [CrossRef] [Green Version]
- Ren, W.-F.; Zhou, Y.; Li, J.-T.; Huang, L.; Sun, S.-G. Si anode for next-generation lithium-ion battery. Curr. Opin. Electrochem. 2019, 18, 46–54. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Figgemeier, E. Confronting the Challenges of Next-Generation Silicon Anode-Based Lithium-Ion Batteries: Role of Designer Electrolyte Additives and Polymeric Binders. ChemSusChem 2019, 12, 2515–2539. [Google Scholar] [CrossRef]
- Dalavi, S.; Guduru, P.; Lucht, B.L. Performance Enhancing Electrolyte Additives for Lithium Ion Batteries with Silicon Anodes. J. Electrochem. Soc. 2012, 159, A642. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Fluoroethylene Carbonate as an Important Component for the Formation of an Effective Solid Electrolyte Interphase on Anodes and Cathodes for Advanced Li-Ion Batteries. ACS Energy Lett. 2017, 2, 1337–1345. [Google Scholar] [CrossRef] [Green Version]
- Choi, N.-S.; Yew, K.H.; Kim, H.; Kim, S.S.; Choi, W.-U. Surface layer formed on silicon thin-film electrode in lithium bis(oxalato) borate-based electrolyte. J. Power Sources 2007, 172, 404–409. [Google Scholar] [CrossRef]
- Ma, L.; Glazier, S.L.; Petibon, R.; Xia, J.; Peters, J.M.; Liu, Q.; Allen, J.; Doig, R.N.C.; Dahn, J.R. A Guide to Ethylene Carbonate-Free Electrolyte Making for Li-Ion Cells. J. Electrochem. Soc. 2016, 164, A5008–A5018. [Google Scholar] [CrossRef]
- Pereira-Nabais, C.; Światowska, J.; Chagnes, A.; Gohier, A.; Zanna, S.; Seyeux, A.; Tran-Van, P.; Cojocaru, C.-S.; Cassir, M.; Marcus, P. Insight into the Solid Electrolyte Interphase on Si Nanowires in Lithium-Ion Battery: Chemical and Morphological Modifications upon Cycling. J. Phys. Chem. C 2014, 118, 2919–2928. [Google Scholar] [CrossRef]
- Pereira-Nabais, C.; Światowska, J.; Rosso, M.; Ozanam, F.; Seyeux, A.; Gohier, A.; Tran-Van, P.; Cassir, M.; Marcus, P. Effect of Lithiation Potential and Cycling on Chemical and Morphological Evolution of Si Thin Film Electrode Studied by ToF-SIMS. ACS Appl. Mater. Interfaces 2014, 6, 13023–13033. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Koo, B.M.; Seyeux, A.; Światowska, J.; Henry de Villeneuve, C.; Rosso, M.; Ozanam, F. ToF-SIMS Li Depth Profiling of Pure and Methylated Amorphous Silicon Electrodes After Their Partial Lithiation. ACS Appl. Mater. Interfaces 2022, 14, 35716–35725. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Ruffo, R.; Hong, S.S.; Cui, Y. Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes. J. Power Sources 2009, 189, 1132–1140. [Google Scholar] [CrossRef]
- Bryngelsson, H.; Stjerndahl, M.; Gustafsson, T.; Edström, K. How dynamic is the SEI? J. Power Sources 2007, 174, 970–975. [Google Scholar] [CrossRef]
- Rezvani, S.J.; Nobili, F.; Gunnella, R.; Ali, M.; Tossici, R.; Passerini, S.; Di Cicco, A. SEI Dynamics in Metal Oxide Conversion Electrodes of Li-Ion Batteries. J. Phys. Chem. C 2017, 121, 26379–26388. [Google Scholar] [CrossRef]
- Hou, T.; Yang, G.; Rajput, N.N.; Self, J.; Park, S.-W.; Nanda, J.; Persson, K.A. The influence of FEC on the solvation structure and reduction reaction of LiPF6/EC electrolytes and its implication for solid electrolyte interphase formation. Nano Energy 2019, 64, 103881. [Google Scholar] [CrossRef]
- Jeschull, F.; Lindgren, F.; Lacey, M.J.; Björefors, F.; Edström, K.; Brandell, D. Influence of inactive electrode components on degradation phenomena in nano-Si electrodes for Li-ion batteries. J. Power Sources 2016, 325, 513–524. [Google Scholar] [CrossRef]
- Radvanyi, E.; De Vito, E.; Porcher, W.; Jouanneau Si Larbi, S. An XPS/AES comparative study of the surface behaviour of nano-silicon anodes for Li-ion batteries. J. Anal. At. Spectrom. 2014, 29, 1120–1131. [Google Scholar] [CrossRef]
- Bordes, A.; De Vito, E.; Haon, C.; Secouard, C.; Montani, A.; Marcus, P. Investigation of Lithium Insertion Mechanisms of a Thin-Film Si Electrode by Coupling Time-of-Flight Secondary-Ion Mass Spectrometry, X-ray Photoelectron Spectroscopy, and Focused-Ion-Beam/SEM. ACS Appl. Mater. Interfaces 2015, 7, 27853–27862. [Google Scholar] [CrossRef]
- Philippe, B.; Dedryvère, R.; Allouche, J.; Lindgren, F.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Nanosilicon Electrodes for Lithium-Ion Batteries: Interfacial Mechanisms Studied by Hard and Soft X-ray Photoelectron Spectroscopy. Chem. Mater. 2012, 24, 1107–1115. [Google Scholar] [CrossRef]
- Philippe, B.; Dedryvère, R.; Gorgoi, M.; Rensmo, H.; Gonbeau, D.; Edström, K. Role of the LiPF6 Salt for the Long-Term Stability of Silicon Electrodes in Li-Ion Batteries—A Photoelectron Spectroscopy Study. Chem. Mater. 2013, 25, 394–404. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Song, S.-W. Characterization of SEI layer formed on high performance Si–Cu anode in ionic liquid battery electrolyte. Electrochem. Commun. 2010, 12, 1593–1595. [Google Scholar] [CrossRef]
- Aurbach, D.; Levi, M.D.; Levi, E.; Schechter, A. Failure and Stabilization Mechanisms of Graphite Electrodes. J. Phys. Chem. B 1997, 101, 2195–2206. [Google Scholar] [CrossRef]
- Ota, H.; Sakata, Y.; Inoue, A.; Yamaguchi, S. Analysis of Vinylene Carbonate Derived SEI Layers on Graphite Anode. J. Electrochem. Soc. 2004, 151, A1659. [Google Scholar] [CrossRef]
- Hu, Y.; Kong, W.; Li, H.; Huang, X.; Chen, L. Experimental and theoretical studies on reduction mechanism of vinyl ethylene carbonate on graphite anode for lithium ion batteries. Electrochem. Commun. 2004, 6, 126–131. [Google Scholar] [CrossRef]
- Cui, L.-F.; Ruffo, R.; Chan, C.K.; Peng, H.; Cui, Y. Crystalline-Amorphous Core-Shell Silicon Nanowires for High Capacity and High Current Battery Electrodes. Nano Lett. 2009, 9, 491–495. [Google Scholar] [CrossRef]
- Laïk, B.; Ung, D.; Caillard, A.; Sorin Cojocaru, C.; Pribat, D.; Pereira-Ramos, J.-P. An electrochemical and structural investigation of silicon nanowires as negative electrode for Li-ion batteries. J. Solid State Electrochem. 2010, 14, 1835–1839. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, M.; Wang, J.; Hu, S.; Liu, X.; Shao, Z. High yield and low-cost ball milling synthesis of nano-flake Si@SiO2 with small crystalline grains and abundant grain boundaries as a superior anode for Li-ion batteries. J. Alloys Compd. 2015, 639, 27–35. [Google Scholar] [CrossRef]
- Światowska-Mrowiecka, J.; Maurice, V.; Zanna, S.; Klein, L.; Marcus, P. XPS study of Li ion intercalation in V2O5 thin films prepared by thermal oxidation of vanadium metal. Electrochim. Acta 2007, 52, 5644–5653. [Google Scholar] [CrossRef]
- Verma, P.; Maire, P.; Novák, P. A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim. Acta 2010, 55, 6332–6341. [Google Scholar] [CrossRef]
- Konstadinidis, K.; Zhang, P.; Opila, R.L.; Allara, D.L. An in-situ X-ray photoelectron study of the interaction between vapor-deposited Ti atoms and functional groups at the surfaces of self-assembled monolayers. Surf. Sci. 1995, 338, 300–312. [Google Scholar] [CrossRef]
- Philippe, B.; Hahlin, M.; Edström, K.; Gustafsson, T.; Siegbahn, H.; Rensmo, H. Photoelectron Spectroscopy for Lithium Battery Interface Studies. J. Electrochem. Soc. 2015, 163, A178–A191. [Google Scholar] [CrossRef] [Green Version]
- Malmgren, S.; Ciosek, K.; Hahlin, M.; Gustafsson, T.; Gorgoi, M.; Rensmo, H.; Edström, K. Comparing anode and cathode electrode/electrolyte interface composition and morphology using soft and hard X-ray photoelectron spectroscopy. Electrochim. Acta 2013, 97, 23–32. [Google Scholar] [CrossRef]
- Xu, K.; von Cresce, A. Interfacing electrolytes with electrodes in Li ion batteries. J. Mater. Chem. 2011, 21, 9849–9864. [Google Scholar] [CrossRef]
- Etacheri, V.; Geiger, U.; Gofer, Y.; Roberts, G.A.; Stefan, I.C.; Fasching, R.; Aurbach, D. Exceptional Electrochemical Performance of Si-Nanowires in 1,3-Dioxolane Solutions: A Surface Chemical Investigation. Langmuir 2012, 28, 6175–6184. [Google Scholar] [CrossRef] [PubMed]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal Decomposition of LiPF6-Based Electrolytes for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A2327. [Google Scholar] [CrossRef]
- Wilken, S.; Treskow, M.; Scheers, J.; Johansson, P.; Jacobsson, P. Initial stages of thermal decomposition of LiPF6-based lithium ion battery electrolytes by detailed Raman and NMR spectroscopy. RSC Adv. 2013, 3, 16359–16364. [Google Scholar] [CrossRef]
- Dedryvère, R.; Gireaud, L.; Grugeon, S.; Laruelle, S.; Tarascon, J.-M.; Gonbeau, D. Characterization of Lithium Alkyl Carbonates by X-ray Photoelectron Spectroscopy: Experimental and Theoretical Study. J. Phys. Chem. B 2005, 109, 15868–15875. [Google Scholar] [CrossRef]
- Ermolieff, A.; Martin, F.; Amouroux, A.; Marthon, S.; Westendorp, J.F.M. Surface composition analysis of HF vapour cleaned silicon by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 1991, 48, 178–184. [Google Scholar] [CrossRef]
- Cardinaud, C.; Rhounna, A.; Turban, G.; Grolleau, B. Analyse XPS des surfaces de Si et SiO2 exposées aux plasmas de CHF3 et CHF3—C2F6. Polymérisation et gravure. Rev. de Phys. Appliquée 1989, 24, 309–321. [Google Scholar] [CrossRef]
- Gruntz, K.J.; Ley, L.; Johnson, R.L. Photoelectron spectra of fluorinated amorphous silicon (a −Si:F). Phys. Rev. B 1981, 24, 2069–2080. [Google Scholar] [CrossRef]
- Zazzera, L.A. XPS and SIMS Study of Anhydrous HF and UV/Ozone-Modified Silicon (100) Surfaces. J. Electrochem. Soc. 1989, 136, 484. [Google Scholar] [CrossRef]
- Andersson, A.M.; Edström, K. Chemical Composition and Morphology of the Elevated Temperature SEI on Graphite. J. Electrochem. Soc. 2001, 148, A1100. [Google Scholar] [CrossRef]
- Dupré, N.; Moreau, P.; De Vito, E.; Quazuguel, L.; Boniface, M.; Bordes, A.; Rudisch, C.; Bayle-Guillemaud, P.; Guyomard, D. Multiprobe Study of the Solid Electrolyte Interphase on Silicon-Based Electrodes in Full-Cell Configuration. Chem. Mater. 2016, 28, 2557–2572. [Google Scholar] [CrossRef] [Green Version]
- Bordes, A.; Eom, K.; Fuller, T.F. The effect of fluoroethylene carbonate additive content on the formation of the solid-electrolyte interphase and capacity fade of Li-ion full-cell employing nano Si–graphene composite anodes. J. Power Sources 2014, 257, 163–169. [Google Scholar] [CrossRef]
- Gauthier, M.; Danet, J.; Lestriez, B.; Roué, L.; Guyomard, D.; Moreau, P. Nanoscale compositional changes during first delithiation of Si negative electrodes. J. Power Sources 2013, 227, 237–242. [Google Scholar] [CrossRef]
- Lehner, A.; Steinhoff, G.; Brandt, M.S.; Eickhoff, M.; Stutzmann, M. Hydrosilylation of crystalline silicon (111) and hydrogenated amorphous silicon surfaces: A comparative X-ray photoelectron spectroscopy study. J. Appl. Phys. 2003, 94, 2289–2294. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-Y.; Deng, L.; Li, J.-T.; Zanna, S.; Seyeux, A.; Huang, L.; Sun, S.-G.; Marcus, P.; Światowska, J. Solid Electrolyte Interphase Layer Formation on the Si-Based Electrodes with and without Binder Studied by XPS and ToF-SIMS Analysis. Batteries 2022, 8, 271. https://doi.org/10.3390/batteries8120271
Wu Z-Y, Deng L, Li J-T, Zanna S, Seyeux A, Huang L, Sun S-G, Marcus P, Światowska J. Solid Electrolyte Interphase Layer Formation on the Si-Based Electrodes with and without Binder Studied by XPS and ToF-SIMS Analysis. Batteries. 2022; 8(12):271. https://doi.org/10.3390/batteries8120271
Chicago/Turabian StyleWu, Zhan-Yu, Li Deng, Jun-Tao Li, Sandrine Zanna, Antoine Seyeux, Ling Huang, Shi-Gang Sun, Philippe Marcus, and Jolanta Światowska. 2022. "Solid Electrolyte Interphase Layer Formation on the Si-Based Electrodes with and without Binder Studied by XPS and ToF-SIMS Analysis" Batteries 8, no. 12: 271. https://doi.org/10.3390/batteries8120271
APA StyleWu, Z.-Y., Deng, L., Li, J.-T., Zanna, S., Seyeux, A., Huang, L., Sun, S.-G., Marcus, P., & Światowska, J. (2022). Solid Electrolyte Interphase Layer Formation on the Si-Based Electrodes with and without Binder Studied by XPS and ToF-SIMS Analysis. Batteries, 8(12), 271. https://doi.org/10.3390/batteries8120271







