Polymer Electrolytes for Lithium-Sulfur Batteries: Progress and Challenges
Abstract
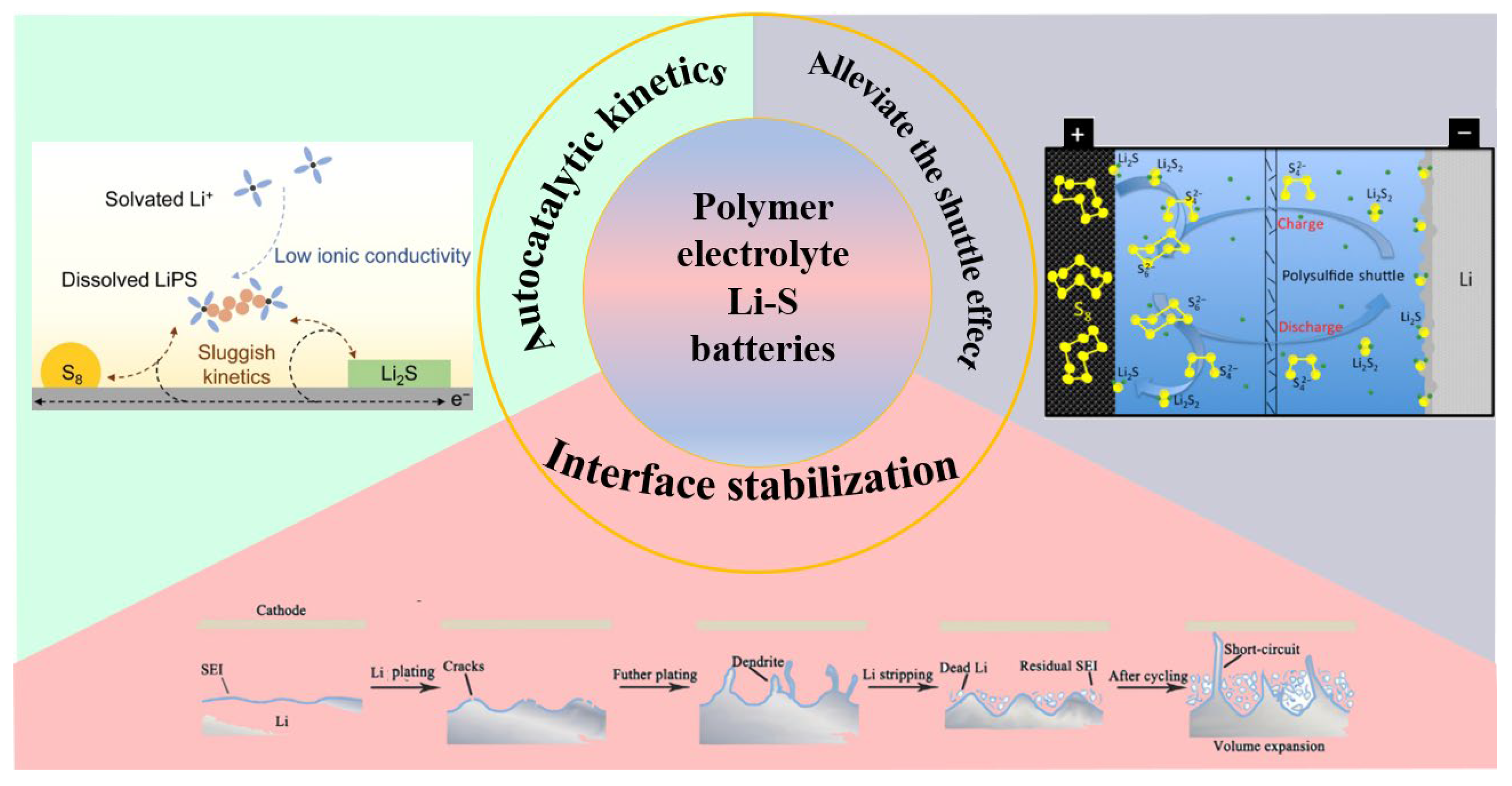
:1. Introduction
2. Classification of Polymer Electrolyte
2.1. Gel Polymer Electrolyte (GPE)
2.2. Solid Polymer Electrolyte (SPE)
3. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Basar, S.; Tosun, B. Environmental Pollution Index and economic growth: Evidence from OECD countries. Environ. Sci. Pollut. Res. Int. 2021, 28, 36870–36879. [Google Scholar] [CrossRef] [PubMed]
- Fretz, S.J.; Pal, U.; Girard, G.M.A.; Howlett, P.C.; Palmqvist, A.E.C. Lithium Sulfonate Functionalization of Carbon Cathodes as a Substitute for Lithium Nitrate in the Electrolyte of Lithium–Sulfur Batteries. Adv. Funct. Mater. 2020, 30, 2002485. [Google Scholar] [CrossRef]
- Li, C.; Ge, W.; Qi, S.; Zhu, L.; Huang, R.; Zhao, M.; Qian, Y.; Xu, L. Manipulating Electrocatalytic Polysulfide Redox Kinetics by 1D Core–Shell Like Composite for Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103915. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, L.; Hou, Y. MXenes: Synthesis strategies and lithium-sulfur battery applications. eScience 2022, 2, 164–182. [Google Scholar] [CrossRef]
- Feng, L.; Yu, P.; Fu, X.; Zhang, Z.M.; Davey, K.; Wang, Y.; Guo, Z.; Yang, W. Regulating Polysulfide Diffusion and Deposition via Rational Design of Core-Shell Active Materials in Li-S Batteries. ACS Nano 2022, 16, 7982–7992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Zhang, C.; Pan, J.L.; Sun, G.W.; Shi, Z.; Li, C.; Chang, X.; Sun, G.Z.; Zhou, J.Y.; Cabot, A. Surface strain-enhanced MoS2 as a high-performance cathode catalyst for lithium–sulfur batteries. eScience 2022, 2, 405–415. [Google Scholar] [CrossRef]
- Ji, L.; Wang, X.; Jia, Y.; Qin, X.; Sui, Y.; Yan, H.; Niu, Z.; Liu, J.; Zhang, Y. Oxygen and nitrogen tailoring carbon fiber aerogel with platinum electrocatalysis interfaced lithium/sulfur (Li/S) batteries. Chin. Chem. Lett. 2023, 34, 107123. [Google Scholar] [CrossRef]
- Wang, Z.; Ge, H.; Liu, S.; Li, G.; Gao, X. High-Entropy Alloys to Activate the Sulfur Cathode for Lithium–Sulfur Batteries. Energy Environ. Mater. 2022, 6, e12358. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.; Zhang, B.; Zeng, S.; Tian, F.; Dou, J.; Qian, Y.; Xu, L. Niobium Diboride Nanoparticles Accelerating Polysulfide Conversion and Directing Li2S Nucleation Enabled High Areal Capacity Lithium-Sulfur Batteries. ACS Nano 2022, 16, 4947–4960. [Google Scholar] [CrossRef]
- Zhang, H.; Ono, L.K.; Tong, G.; Liu, Y.; Qi, Y. Long-life lithium-sulfur batteries with high areal capacity based on coaxial CNTs@TiN-TiO2 sponge. Nat. Commun. 2021, 12, 4738. [Google Scholar] [CrossRef]
- Xue, P.; Zhu, K.; Gong, W.; Pu, J.; Li, X.; Guo, C.; Wu, L.; Wang, R.; Li, H.; Sun, J.; et al. “One Stone Two Birds” Design for Dual-Functional TiO2-TiN Heterostructures Enabled Dendrite-Free and Kinetics-Enhanced Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2200308. [Google Scholar] [CrossRef]
- Li, Z.; Sami, I.; Yang, J.; Li, J.; Kumar, R.V.; Chhowalla, M. Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium–sulfur batteries. Nat. Energy 2023, 8, 84–93. [Google Scholar] [CrossRef]
- Xu, J.; Xu, L.; Zhang, Z.; Sun, B.; Jin, Y.; Jin, Q.; Liu, H.; Wang, G. Heterostructure ZnSe-CoSe2 embedded with yolk-shell conductive dodecahedral as Two-in-one hosts for cathode and anode protection of Lithium–Sulfur full batteries. Energy Storage Mater. 2022, 47, 223–234. [Google Scholar] [CrossRef]
- Wu, T.; Ye, J.; Li, T.; Liu, Y.; Jia, L.; Sun, L.; Liu, J.; Xie, H. Tetrathiafulvalene as a multifunctional electrolyte additive for simultaneous interface amelioration, electron conduction, and polysulfide redox regulation in lithium-sulfur batteries. J. Power Sources 2022, 536, 231482. [Google Scholar] [CrossRef]
- Wu, T.; Sun, G.; Lu, W.; Zhao, L.; Mauger, A.; Julien, C.M.; Sun, L.; Xie, H.; Liu, J. A polypyrrole/black-TiO2/S double-shelled composite fixing polysulfides for lithium-sulfur batteries. Electrochim. Acta 2020, 353, 136529. [Google Scholar] [CrossRef]
- De Luna, Y.; Abdullah, M.; Dimassi, S.N.; Bensalah, N. All-solid lithium-sulfur batteries: Present situation and future progress. Ionics 2021, 27, 4937–4960. [Google Scholar] [CrossRef]
- AbdelHamid, A.A.; Cheong, J.L.; Ying, J.Y. Metal oxide-mediated differential chalcogen morphogenesis for Li-chalcogen battery application. Nano Energy 2021, 84, 105842. [Google Scholar] [CrossRef]
- Pervez, S.A.; Vinayan, B.P.; Cambaz, M.A.; Melinte, G.; Diemant, T.; Braun, T.; Karkera, G.; Behm, R.J.; Fichtner, M. Electrochemical and compositional characterization of solid interphase layers in an interface-modified solid-state Li–sulfur battery. J. Mater. Chem. A 2020, 8, 16451–16462. [Google Scholar] [CrossRef]
- Yan, C.; Zhou, Y.; Cheng, H.; Orenstein, R.; Zhu, P.; Yildiz, O.; Bradford, P.; Jur, J.; Wu, N.; Dirican, M.; et al. Interconnected cathode-electrolyte double-layer enabling continuous Li-ion conduction throughout solid-state Li-S battery. Energy Storage Mater. 2022, 44, 136–144. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, X.; Song, Z.; Rui, K.; Wang, Q.; Gu, S.; Yang, J.; Xiu, T.; Badding, M.E.; Wen, Z. Highly stable garnet solid electrolyte based Li-S battery with modified anodic and cathodic interfaces. Energy Storage Mater. 2018, 15, 282–290. [Google Scholar] [CrossRef]
- Huang, X.; Liu, C.; Lu, Y.; Xiu, T.; Jin, J.; Badding, M.E.; Wen, Z. A Li-Garnet composite ceramic electrolyte and its solid-state Li-S battery. J. Power Sources 2018, 382, 190–197. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, K.; Lang, J.; Jiang, X.; Zheng, Z.; Su, Q.; Huang, Z.; Long, Y.; Wang, C.A.; Wu, H.; et al. High-Energy-Density Solid-Electrolyte-Based Liquid Li-S and Li-Se Batteries. Joule 2020, 4, 262–274. [Google Scholar] [CrossRef]
- Wan, H.; Cai, L.; Han, F.; Mwizerwa, J.P.; Wang, C.; Yao, X. Construction of 3D Electronic/Ionic Conduction Networks for All-Solid-State Lithium Batteries. Small 2019, 15, e1905849. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Hayashi, A.; Tatsumisago, M. Sulfur–carbon composite electrode for all-solid-state Li/S battery with Li2S–P2S5 solid electrolyte. Electrochim. Acta 2011, 56, 6055–6059. [Google Scholar] [CrossRef]
- Nagao, M.; Hayashi, A.; Tatsumisago, M. High-capacity Li2S–nanocarbon composite electrode for all-solid-state rechargeable lithium batteries. J. Mater. Chem. 2012, 19, 10015–10020. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Shen, Y.; Lin, Y.; Nan, C.-W. Synergistic effect of processing and composition x on conductivity of xLi2S-(100−x) P2S5 electrolytes. Solid State Ion. 2017, 305, 1–6. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Yu, X.; Chen, L.; Li, H. Approaching practically accessible solid-state batteries: Stability issues related to solid electrolytes and interfaces. Chem. Rev. 2019, 120, 6820–6877. [Google Scholar] [CrossRef]
- Liu, G.; Sun, Q.; Li, Q.; Zhang, J.; Ming, J. Electrolyte issues in lithium–sulfur batteries: Development, prospect, and challenges. Energy Fuels 2021, 35, 10405–10427. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.W.; Appleby, A.J. Solvent-free composite PEO-ceramic fiber/mat electrolytes for lithium secondary cells. J. Electrochem. Soc. 2004, 152, A205. [Google Scholar] [CrossRef]
- Yu, J.; Kwok, S.C.; Lu, Z.; Effat, M.B.; Lyu, Y.Q.; Yuen, M.M.; Ciucci, F. A ceramic-PVDF composite membrane with modified interfaces as an ion-conducting electrolyte for solid-state lithium-ion batteries operating at room temperature. ChemElectroChem 2018, 5, 2873–2881. [Google Scholar] [CrossRef]
- Kachmar, A.; Carignano, M.; Laino, T.; Iannuzzi, M.; Hutter, J. Mapping the free energy of lithium solvation in the Protic ionic liquid ethylammonuim nitrate: A metadynamics study. ChemSusChem 2017, 10, 3083–3090. [Google Scholar] [CrossRef]
- Prasanth, R.; Aravindan, V.; Srinivasan, M. Novel polymer electrolyte based on cob-web electrospun multi component polymer blend of polyacrylonitrile/poly (methyl methacrylate)/polystyrene for lithium ion batteries—Preparation and electrochemical characterization. J. Power Sources 2012, 202, 299–307. [Google Scholar] [CrossRef]
- Liang, B.; Tang, S.; Jiang, Q.; Chen, C.; Chen, X.; Li, S.; Yan, X. Preparation and characterization of PEO-PMMA polymer composite electrolytes doped with nano-AlO. Electrochim. Acta 2015, 169, 334–341. [Google Scholar] [CrossRef]
- Lu, Y.; Tu, Z.; Archer, L.A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 2014, 13, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Qin, X.; Ruan, Y.; Li, W.; Xu, Y.; Zhang, D.; Thokchom, J. Enhanced performance of solid-state lithium-air batteries with continuous 3D garnet network added composite polymer electrolyte. J. Power Sources 2020, 461, 228146. [Google Scholar] [CrossRef]
- Han, X.; Gong, Y.; Fu, K.K.; He, X.; Hitz, G.T.; Dai, J.; Hu, L. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 2016, 16, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pang, Y.; Zhu, T.; Wang, Y.; Xia, Y. A gel polymer electrolyte based lithium-sulfur battery with low self-discharge. Solid State Ion. 2018, 318, 82–87. [Google Scholar] [CrossRef]
- Nagajothi, A.J.; Kannan, R.; Rajashabala, S. Studies on electrochemical properties of poly (ethylene oxide)-based gel polymer electrolytes with the effect of chitosan for lithium–sulfur batteries. Polym. Bull. 2017, 74, 4887–4897. [Google Scholar] [CrossRef]
- Hao, X.; Wenren, H.; Wang, X.; Xia, X.; Tu, J. A gel polymer electrolyte based on PVDF-HFP modified double polymer matrices via ultraviolet polymerization for lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 558, 145–154. [Google Scholar] [CrossRef]
- Chen, L.; Fan, L.Z. Dendrite-free Li metal deposition in all-solid-state lithium sulfur batteries with polymer-in-salt polysiloxane electrolyte. Energy Storage Mater. 2018, 15, 37–45. [Google Scholar] [CrossRef]
- Zhang, J.; Si, Y.; Huang, Q.; Yang, T.; Wang, C.; Ji, K.; Wang, J.; Chen, M. Gradient distribution of functional components in PAN-based electrolytes to endow solid-state lithium sulfur batteries with long cycle life. Sustain. Energy Fuels 2022, 6, 4240–4247. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, H.R.; Ren, Y.X.; Zhou, D.; Kang, F.Y.; Zhao, T.S. In-situ Fabrication of a Freestanding Acrylate-based Hierarchical Electrolyte for Lithium-sulfur Batteries. Electrochim. Acta 2016, 213, 871–878. [Google Scholar] [CrossRef]
- Aldalur, I.; Zhang, H.; Piszcz, M.; Oteo, U.; Rodriguez-Martinez, L.M.; Shanmukaraj, D.; Rojo, T.; Armand, M. Jeffamine® based polymers as highly conductive polymer electrolytes and cathode binder materials for battery application. J. Power Sources 2017, 347, 37–46. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, H.; Liu, J.; Qian, T.; Yan, C. A new high ionic conductive gel polymer electrolyte enables highly stable quasi-solid-state lithium sulfur battery. Energy Storage Mater. 2019, 22, 256–264. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Feng, J.; Ma, Z.; Shao, G. High capacity and cycle stability Rechargeable Lithium–Sulfur batteries by sandwiched gel polymer electrolyte. Electrochim. Acta 2016, 210, 71–78. [Google Scholar] [CrossRef]
- Song, J.; Noh, H.; Lee, H.; Lee, J.-N.; Lee, D.J.; Lee, Y.; Kim, C.H.; Lee, Y.M.; Park, J.-K.; Kim, H.-T. Polysulfide rejection layer from alpha-lipoic acid for high performance lithium–sulfur battery. J. Mater. Chem. A 2015, 3, 323–330. [Google Scholar] [CrossRef]
- Han, D.-D.; Liu, S.; Liu, Y.-T.; Zhang, Z.; Li, G.-R.; Gao, X.-P. Lithiophilic gel polymer electrolyte to stabilize the lithium anode for a quasi-solid-state lithium–sulfur battery. J. Mater. Chem. A 2018, 6, 18627–18634. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, D.; He, Y.-B.; Fu, Y.; Qin, X.; Miao, C.; Du, H.; Li, B.; Yang, Q.-H.; Lin, Z.; et al. Novel gel polymer electrolyte for high-performance lithium–sulfur batteries. Nano Energy 2016, 22, 278–289. [Google Scholar] [CrossRef]
- Du, H.; Li, S.; Qu, H.; Lu, B.; Wang, X.; Chai, J.; Zhang, H.; Ma, J.; Zhang, Z.; Cui, G. Stable cycling of lithium-sulfur battery enabled by a reliable gel polymer electrolyte rich in ester groups. J. Membr. Sci. 2018, 550, 399–406. [Google Scholar] [CrossRef]
- Yang, Y.J.; Wang, R.; Xue, J.X.; Liu, F.Q.; Yan, J.; Jia, S.X.; Xiang, T.Q.; Huo, H.; Zhou, J.J.; Li, L. In situ forming asymmetric bi-functional gel polymer electrolyte in lithium–sulfur batteries. J. Mater. Chem. A 2021, 9, 27390–27397. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, F.; Mei, Y.; Ding, Y.; Pang, H.; Zhang, P. Gel polymer electrolyte based on hydrophilic–lipophilic TiO2-modified thermoplastic polyurethane for high-performance Li-ion batteries. J. Mater. Sci. 2020, 56, 2474–2485. [Google Scholar] [CrossRef]
- Karim, J.; Aminah Mohd Noor, S.; Zuliana Dzulkipli, M.; Ahmad, A.; Sukor Su’ait, M.; Hasyareeda Hassan, N. Influence of Electron beam radiation on the properties of Surface-Modified Titania-Filled gel polymer electrolytes using vinyltriethoxysilane (VTES) for lithium battery application. Results Chem. 2022, 4, 100383. [Google Scholar] [CrossRef]
- Guo, J.; Chen, Y.; Xiao, Y.; Xi, C.; Xu, G.; Li, B.; Yang, C.; Yu, Y. Flame-retardant composite gel polymer electrolyte with a dual acceleration conduction mechanism for lithium ion batteries. Chem. Eng. J. 2021, 422, 130526. [Google Scholar] [CrossRef]
- Ahn, J.H.; You, T.-S.; Lee, S.-M.; Esken, D.; Dehe, D.; Huang, Y.-C.; Kim, D.-W. Hybrid separator containing reactive, nanostructured alumina promoting in-situ gel electrolyte formation for lithium-ion batteries with good cycling stability and enhanced safety. J. Power Sources 2020, 472, 228519. [Google Scholar] [CrossRef]
- Wu, F.; Chen, N.; Chen, R.; Wang, L.; Li, L. Organically modified silica-supported ionogels electrolyte for high temperature lithium-ion batteries. Nano Energy 2017, 31, 9–18. [Google Scholar] [CrossRef]
- Li, Y.J.; Guo, C.; Yue, L.S.; Qu, W.J.; Chen, N.; Dai, Y.-J.; Chen, R.-J.; Wu, F. Organosilicon-group-derived silica-ionogel electrolyte for lithium ion batteries. Rare Met. 2018, 37, 504–509. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Zhang, Z.; Huang, J.; Yang, L.; Hirano, S.i. High-performance polymeric ionic liquid–silica hybrid ionogel electrolytes for lithium metal batteries. J. Mater. Chem. A 2016, 4, 13822–13829. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Chen, Y.M.; Hou, S.S.; Jan, J.S.; Lee, Y.L.; Teng, H. Diode-like gel polymer electrolytes for full-cell lithium ion batteries. J. Mater. Chem. A 2017, 5, 17476–17481. [Google Scholar] [CrossRef]
- Pei, F.; Dai, S.; Guo, B.; Xie, H.; Zhao, C.; Cui, J.; Fang, X.; Chen, C.; Zheng, N. Titanium–oxo cluster reinforced gel polymer electrolyte enabling lithium–sulfur batteries with high gravimetric energy densities. Energy Environ. Sci. 2021, 14, 975–985. [Google Scholar] [CrossRef]
- Xie, P.; Yang, R.; Zhou, Y.; Zhang, B.; Tian, X. Rationally designing composite gel polymer electrolyte enables high sulfur utilization and stable lithium anode. Chem. Eng. J. 2022, 450, 138195. [Google Scholar] [CrossRef]
- Jiang, H.; Han, Y.; Wang, H.; Zhu, Y.; Guo, Q.; Jiang, H.; Zheng, C.; Xie, K. LiS-LiPS (LPS) Composite Synthesized by Liquid-Phase Shaking for All-Solid-State Lithium-Sulfur Batteries with High Performance. Energy Technol. 2020, 8, 2000023. [Google Scholar] [CrossRef]
- Hakari, T.; Hayashi, A.; Tatsumisago, M. LiS-Based Solid Solutions as Positive Electrodes with Full Utilization and Superlong Cycle Life in All-Solid-State Li/S Batteries. Adv. Sustain. Syst. 2017, 1, 1700017. [Google Scholar] [CrossRef]
- Phuc, N.H.H.; Takaki, M.; Kazuhiro, H.; Hiroyuki, M.; Atsunori, M. Dual effect of MgS addition on LiS ionic conductivity and all-solid-state Li–S cell performance. SN Appl. Sci. 2020, 2, 1803. [Google Scholar] [CrossRef]
- Gamo, H.; Maeda, T.; Hikima, K.; Deguchi, M.; Fujita, Y.; Kawasaki, Y.; Sakuda, A.; Muto, H.; Phuc, N.H.H.; Hayashi, A.; et al. Synthesis of an AlI-doped LiS positive electrode with superior performance in all-solid-state batteries. Mater. Adv. 2022, 3, 2488–2494. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, Z.; Dudney, N.J.; Liang, C. Lithium Superionic Sulfide Cathode for All-Solid Lithium-Sulfur Batteries. ACS Nano 2013, 7, 2829–2833. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Wang, S.; Xiao, M.; Meng, Y. Polymer electrolytes for lithium polymer batteries. J. Mater. Chem. A 2016, 4, 10038–10069. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, S.; Huang, Z.; Wei, Z.; Shen, L.; Gu, H.; Xu, X.; Yao, X. High conductivity polymer electrolyte with comb-like structure via a solvent-free UV-cured method for large-area ambient all-solid-sate lithium batteries. J. Mater. 2019, 5, 195–203. [Google Scholar] [CrossRef]
- Jayathilaka, P.; Dissanayake, M.; Albinsson, I.; Mellander, B.-E. Effect of nano-porous AlO on thermal, dielectric and transport properties of the (PEO) LiTFSI polymer electrolyte system. Electrochim. Acta 2002, 47, 3257–3268. [Google Scholar] [CrossRef]
- Watanabe, M.; Endo, T.; Nishimoto, A.; Miura, K.; Yanagida, M. High ionic conductivity and electrode interface properties of polymer electrolytes based on high molecular weight branched polyether. J. Power Source 1999, 81–82, 786–789. [Google Scholar] [CrossRef]
- Kim, S.-C.; Oh, T.H.; Kim, D.W.; Lee, C.; Kang, Y. Ion-conducting hyperbranched PEG electrolytes derived from poly(glycidol). Macromol. Res. 2009, 17, 141–143. [Google Scholar] [CrossRef]
- Imholt, L.; Dörr, T.S.; Zhang, P.; Ibing, L.; Cekic-Laskovic, I.; Winter, M.; Brunklaus, G. Grafted polyrotaxanes as highly conductive electrolytes for lithium metal batteries. J. Power Source 2019, 409, 148–158. [Google Scholar] [CrossRef]
- Song, Y.X.; Shi, Y.; Wan, J.; Lang, S.-Y.; Hu, X.C.; Yan, H.J.; Liu, B.; Guo, Y.G.; Wen, R.; Wan, L.J. Direct tracking of the polysulfide shuttling and interfacial evolution in all-solid-state lithium–sulfur batteries: A degradation mechanism study. Energy Environ. Sci. 2019, 12, 2496–2506. [Google Scholar] [CrossRef]
- Borodin, O.; Smith Grant, D. Mechanism of Ion Transport in Amorphous Poly(ethylene oxide)/LiTFSI from Molecular Dynamics Simulations. Macromolecules 2006, 39, 1620–1629. [Google Scholar] [CrossRef]
- Stolwijk, N.A.; Heddier, C.; Reschke, M.; Wiencierz, M.; Bokeloh, J.; Wilde, G. Salt-Concentration Dependence of the Glass Transition Temperature in PEO–NaI and PEO–LiTFSI Polymer Electrolytes. Macromolecules 2013, 46, 8580–8588. [Google Scholar] [CrossRef]
- Marceau, H.; Kim, C.-S.; Paolella, A.; Ladouceur, S.; Lagacé, M.; Chaker, M.; Vijh, A.; Guerfi, A.; Julien, C.M.; Mauger, A.; et al. In operando scanning electron microscopy and ultraviolet–visible spectroscopy studies of lithium/sulfur cells using all solid-state polymer electrolyte. J. Power Sources 2016, 319, 247–254. [Google Scholar] [CrossRef]
- Fang, R.; Xu, H.; Xu, B.; Li, X.; Li, Y.; Goodenough, J.B. Reaction Mechanism Optimization of Solid-State Li–S Batteries with a PEO-Based Electrolyte. Adv. Funct. Mater. 2020, 31, 2001812. [Google Scholar] [CrossRef]
- Sheng, O.; Jin, C.; Luo, J.; Yuan, H.; Fang, C.; Huang, H.; Gan, Y.; Zhang, J.; Xia, Y.; Liang, C.; et al. Ionic conductivity promotion of polymer electrolyte with ionic liquid grafted oxides for all-solid-state lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 12934–12942. [Google Scholar] [CrossRef]
- Wei, B.; Huang, S.; Song, Y.; Wang, X.; Liu, M.; Jin, H.; Cao, G. A three-in-one C60-integrated PEO-based solid polymer electrolyte enables superior all-solid-state lithium–sulfur batteries. J. Mater. Chem. A 2023, 11, 11426–11435. [Google Scholar] [CrossRef]
- Liu, S.M.; Chen, M.X.; Xie, Y.; Liu, D.H.; Zheng, J.F.; Xiong, X.; Jiang, H.; Wang, L.C.; Luo, H.; Han, K. Nb2CTx MXene boosting PEO polymer electrolyte for all-solid-state Li-S batteries: Two birds with one stone strategy to enhance Li+ conductivity and polysulfide adsorptivity. Rare Met. 2023, 42, 2562–2576. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Geng, Y.; Shi, Z.; Zhu, S.; Xu, Q.; Min, Y. A multifunctional nano filler for solid polymer electrolyte toward stable cycling for lithium-metal anodes in lithium–sulfur batteries. Chem. Eng. J. 2022, 444, 136328. [Google Scholar] [CrossRef]
- Wang, P.F.; He, X.; Lv, Z.C.; Song, H.; Song, X.; Yi, T.F.; Xu, N.; He, P.; Zhou, H. Light-Driven Polymer-Based All-Solid-State Lithium-Sulfur Battery Operating at Room Temperature. Adv. Funct. Mater. 2023, 33, 2211074. [Google Scholar] [CrossRef]
- Song, X.; Wang, M.; Wang, S.; Cheng, Z.; Zhang, T.; Zhu, T.; Song, H.; Yu, L.; Xu, J.; Chen, K. A wide temperature solid-state Li–S battery enabled by a plasmon-enhanced copper–silicon nanowire photothermal current collector. J. Mater. Chem. A 2022, 10, 22584–22591. [Google Scholar] [CrossRef]
- Li, M.; Frerichs, J.E.; Kolek, M.; Sun, W.; Zhou, D.; Huang, C.J.; Hwang, B.J.; Hansen, M.R.; Winter, M.; Bieker, P. Solid-State Lithium–Sulfur Battery Enabled by Thio-LiSICON/Polymer Composite Electrolyte and Sulfurized Polyacrylonitrile Cathode. Adv. Funct. Mater. 2020, 30, 191023. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, S.; Xiao, M.; Liu, W.; Han, D.; Li, Z.; Qin, J.; Li, Y.; Zhang, S.; Huang, S.; et al. Addressing interface elimination: Boosting comprehensive performance of all-solid-state Li-S battery. Energy Storage Mater. 2021, 41, 563–570. [Google Scholar] [CrossRef]
- Gao, J.; Sun, C.; Xu, L.; Chen, J.; Wang, C.; Guo, D.; Chen, H. Lithiated Nafion as polymer electrolyte for solid-state lithium sulfur batteries using carbon-sulfur composite cathode. J. Power Source 2018, 382, 179–189. [Google Scholar] [CrossRef]





| Type | Materials | Liquid Electrolyte | Conductivity | Specific Discharge Capacity | Advantages | Refs. |
|---|---|---|---|---|---|---|
| PEGDE | PEGDE/PEI | DOL/DME + 1 M LiTFSI | 0.75 × 10−3 S cm−1 at 30 °C or 2.2 × 10−3 S cm−1 at 60 °C | 950 mAh g−1 (0.2 C) | high ionic conductivity adsorbable LiPSs high capacity retention rate | [44] |
| PVDF | PMMA | DOL/DME + 1 M LiTFSI | 1.95 × 10−3 S cm−1 at RM | 1711.8 mAh g−1 (0.1 C) | high initial discharge capacity high capacity retention rate | [45] |
| PVDF | PDA | DOL/DME + 1 M LiTFSI | - | 1215.4 mAh g−1 (0.1 C) | high capacity retention rate protect lithium anode | [47] |
| PETEA | AIBN | DOL/DME + 1 M LiTFSI + 1 wt% LiNO3 | 1.13 × 10−2 S cm−1 at RM | 1219.8 mAh g−1 (0.1 C) | high ionic conductivity high flexibility protect lithium anode | [48] |
| DOL(PDXL) | PVA-CN | DOL/DME + 1 M LiTFSI | 3.23 × 10−3 S cm−1 at RM | 1130 mAh g−1 (0.1 C) | promote lithium deposition long cycle stability | [50] |
| PVFH | TOC/PEG | DOL/DME + 1 M LiTFSI + 2 wt% LiNO3 | 8 × 10−3 S cm−1 at RM | 1103 mAh g−1 (0.1 C) | support low E/S ratio and low N/P ratio. high cycling stability | [59] |
| PEO/PAN | LLZO | DOL/DME + 1 M LiTFSI + 1 wt% LiNO3 | 2.01 × 10−3 S cm−1 at RM | 1459 mAh g−1 (0.1 C) | good interface stability long cycle stability | [60] |
| Type | Materials | Conductivity | Specific Discharge Capacity | Advantages | Refs. |
|---|---|---|---|---|---|
| PEO | PVDF | - | 825 mAh g−1 (0.05 C) | long cycle stability high capacity retention rate | [76] |
| EO | IL@NPs/ZrO2 | 4.95 × 10−4 S cm−1 at 50 °C or 2.32 × 10−4 S cm−1 at 37 °C | 986 mAh g−1 | high ionic conductivity | [77] |
| PEO | C60 | 1.27 × 10−4 S cm−1 at 60 °C | 1125 mAh g−1 (0.1 C) | long cycle stability inhibit dendritic | [78] |
| PEO | Nb2CTx MXene | 2.63 × 10−4 S cm−1 at 60 °C | 1149 mAh g−1 (0.5 C) | long cycle stability high ionic conductivity | [79] |
| PEO | In2O3 | - | 1227 mAh g−1 (0.2 C) | long cycle stability inhibit dendritic | [80] |
| PVDF-HFP | DMF | 7.87 × 10−3 S cm−1 at 60 °C | 1089 mAh g−1 (0.2 C) | high ionic conductivity | [81] |
| PEO | LGPS | 0.42 × 10−3 S cm−1 at RM | 1183 mAh g−1 (0.2 C) | high ionic conductivity high initial discharge capacity | [83] |
| Li-Nafion | PC | 2.1 × 10−4 S cm−1 at 70 °C | 1072.8 mAh g−1 (0.05 C) | high capacity retention rate | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, M.; Li, T.; Yang, D.; Lu, L.; Duan, L.; Liu, J.; Wu, T. Polymer Electrolytes for Lithium-Sulfur Batteries: Progress and Challenges. Batteries 2023, 9, 488. https://doi.org/10.3390/batteries9100488
Jia M, Li T, Yang D, Lu L, Duan L, Liu J, Wu T. Polymer Electrolytes for Lithium-Sulfur Batteries: Progress and Challenges. Batteries. 2023; 9(10):488. https://doi.org/10.3390/batteries9100488
Chicago/Turabian StyleJia, Mingxun, Tunan Li, Daotong Yang, Luhua Lu, Limei Duan, Jinghai Liu, and Tong Wu. 2023. "Polymer Electrolytes for Lithium-Sulfur Batteries: Progress and Challenges" Batteries 9, no. 10: 488. https://doi.org/10.3390/batteries9100488





