A Study of Li3.8Ge0.9S0.1O4 Solid Electrolyte Stability Relative to Electrode Materials of Lithium Power Sources
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Samples Characterization
2.3. Conductivity Measurement
3. Results and Discussion
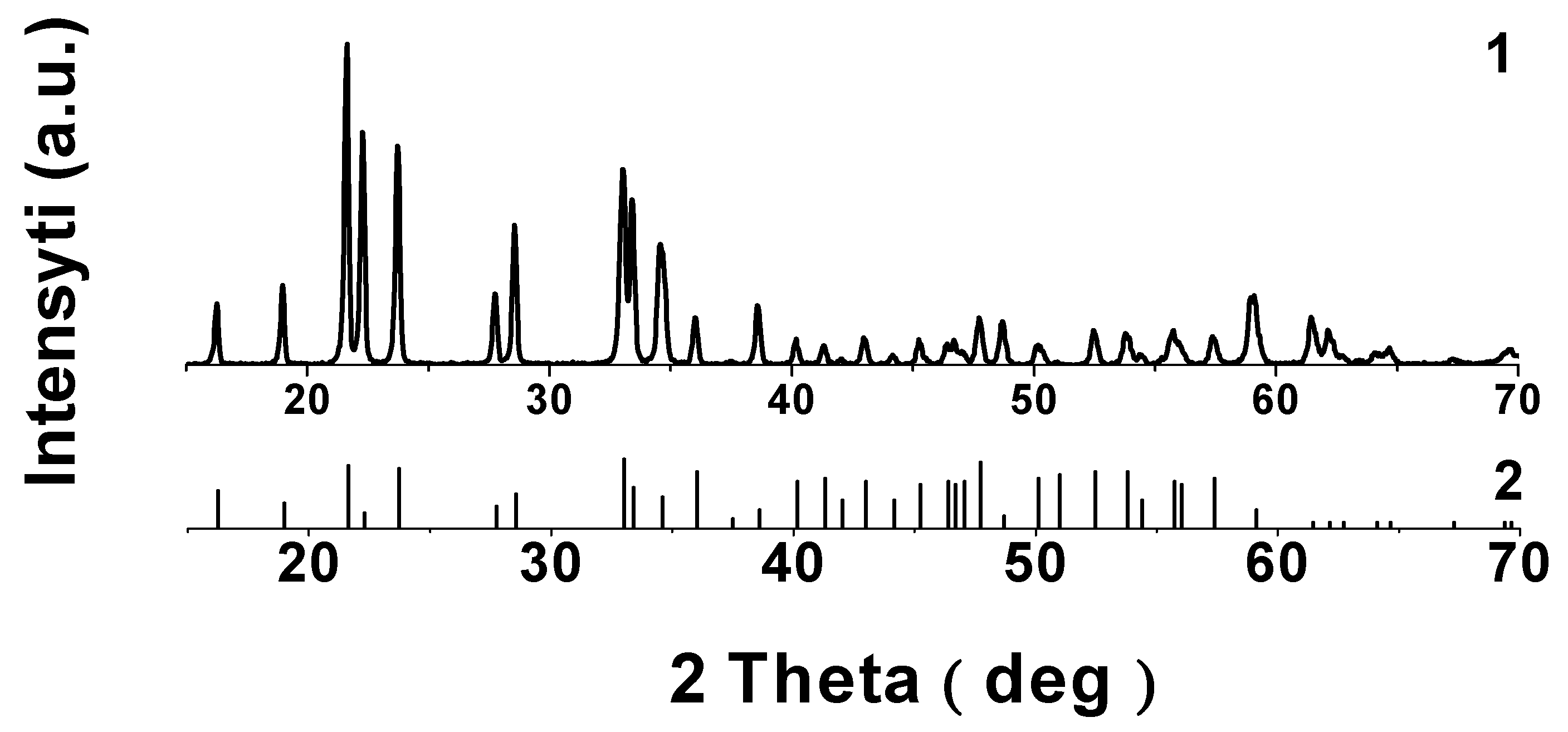
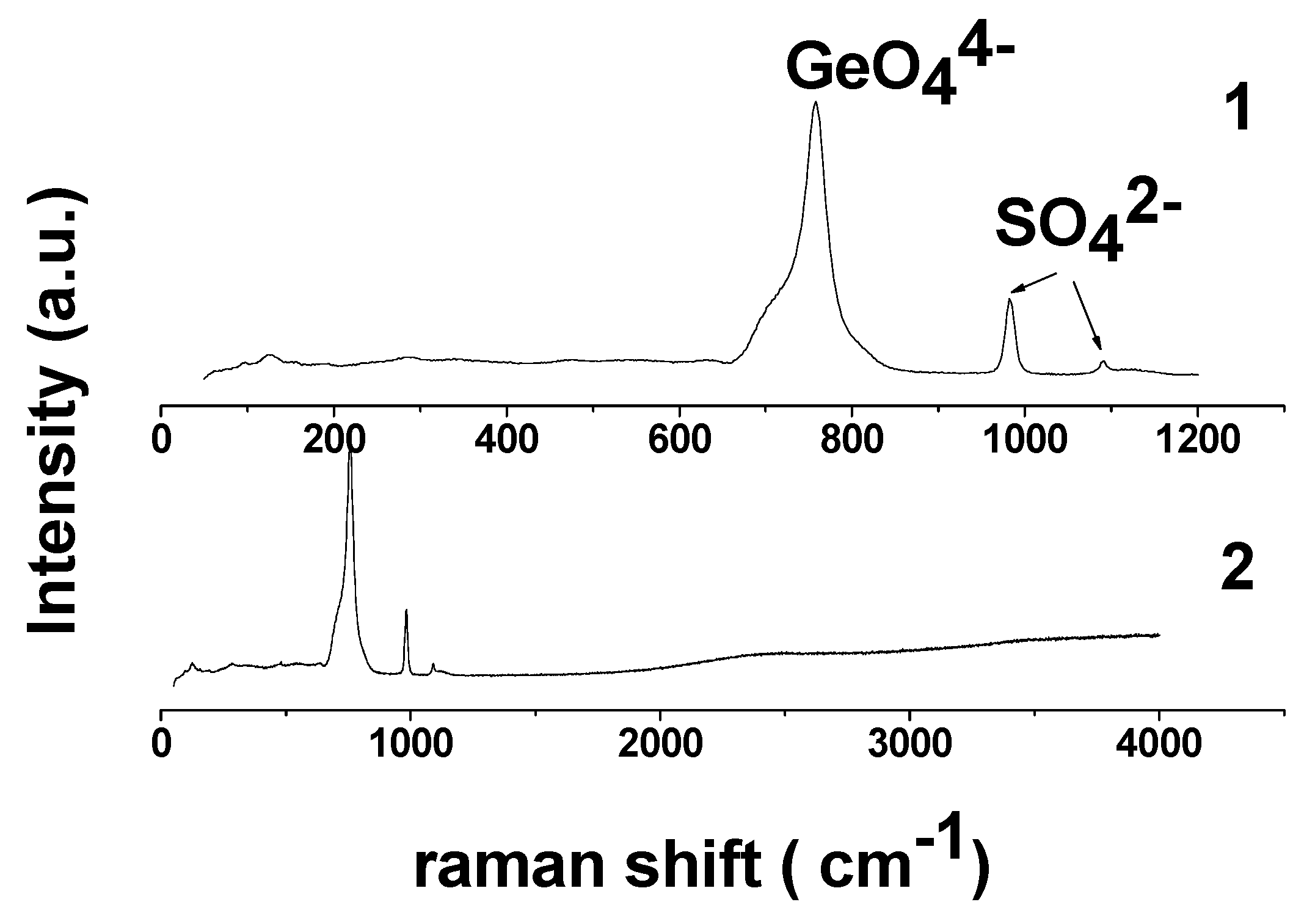
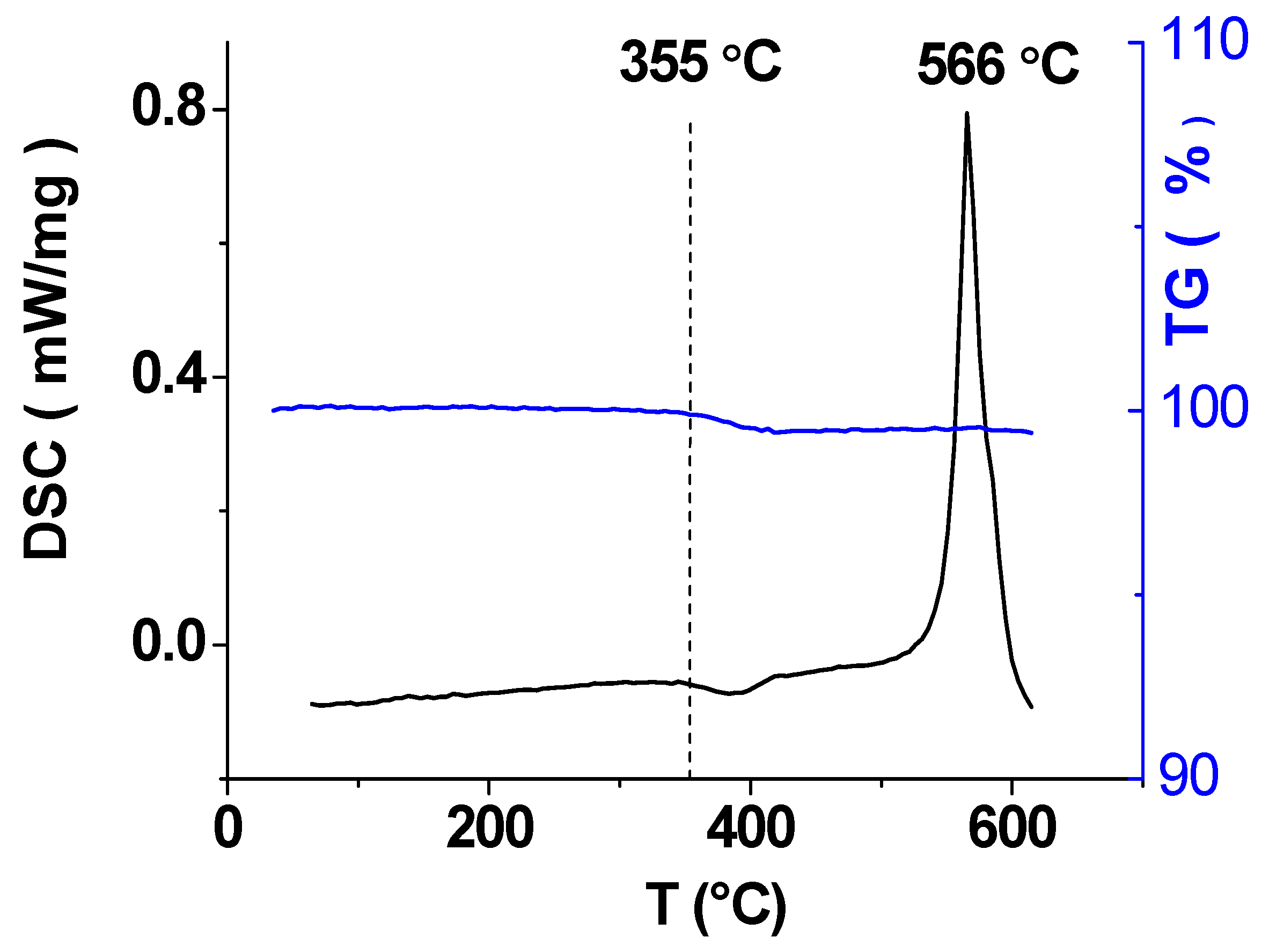
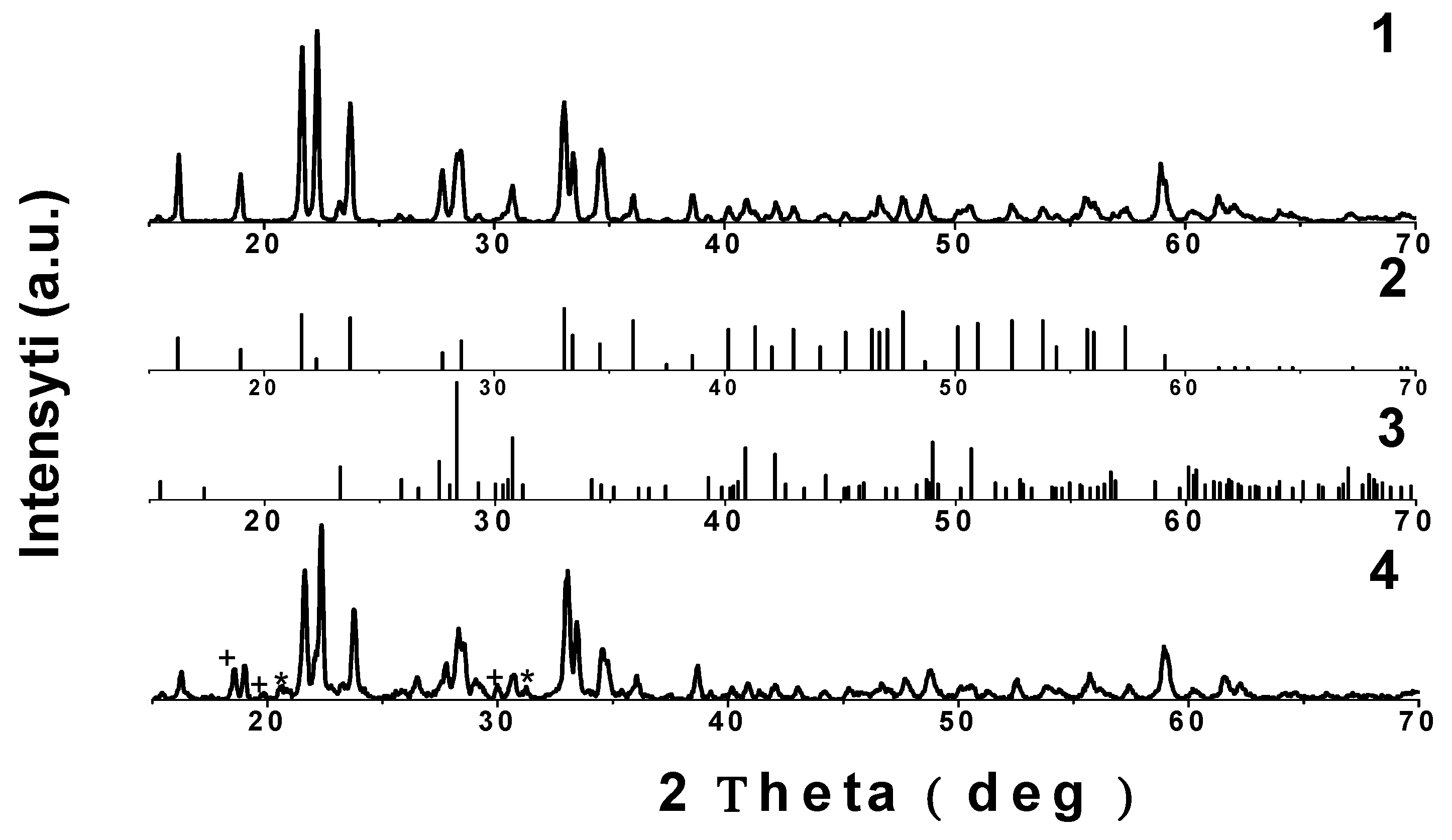
3.1. Characterization of Li3.8Ge0.9S0.1O4
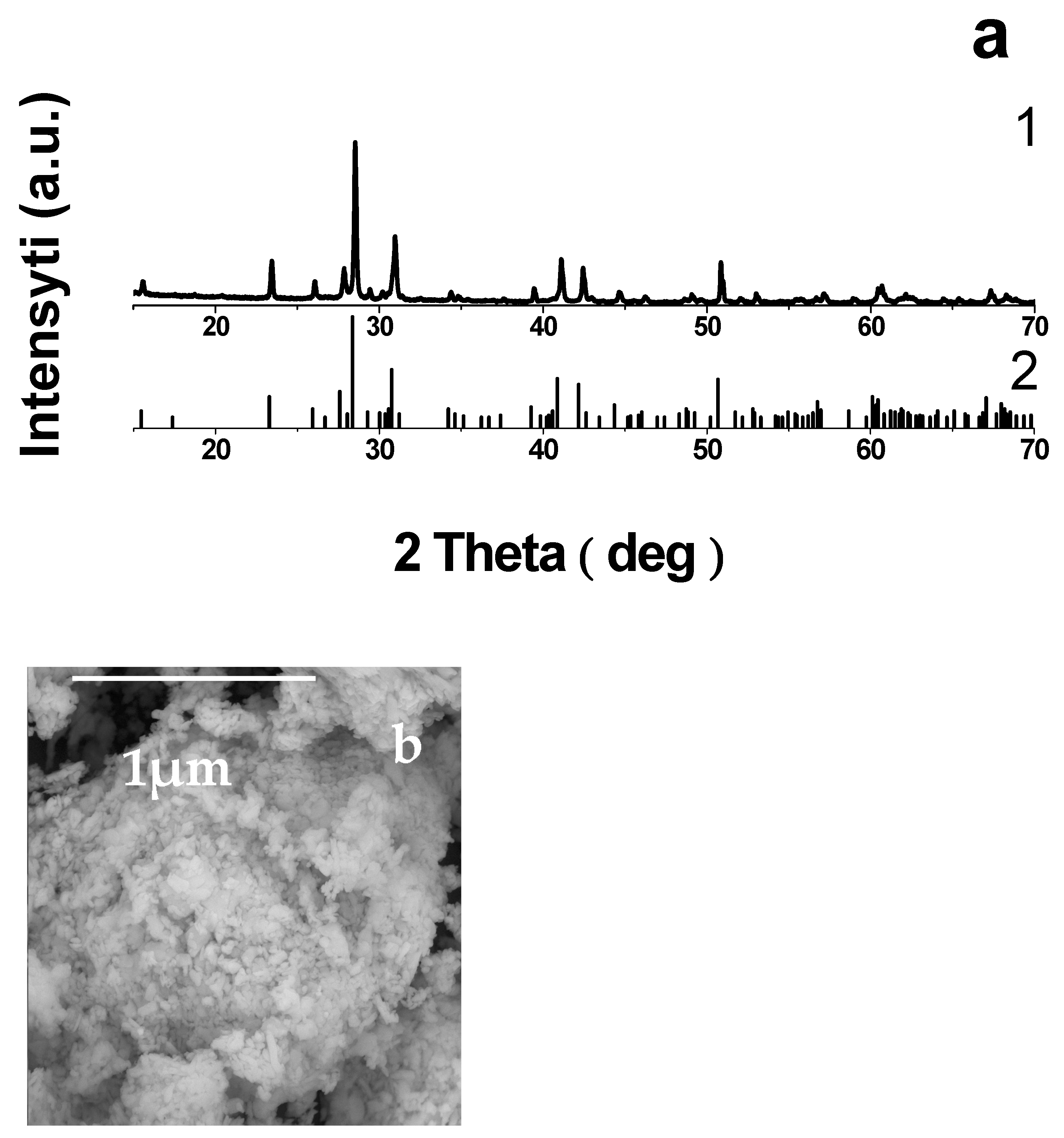
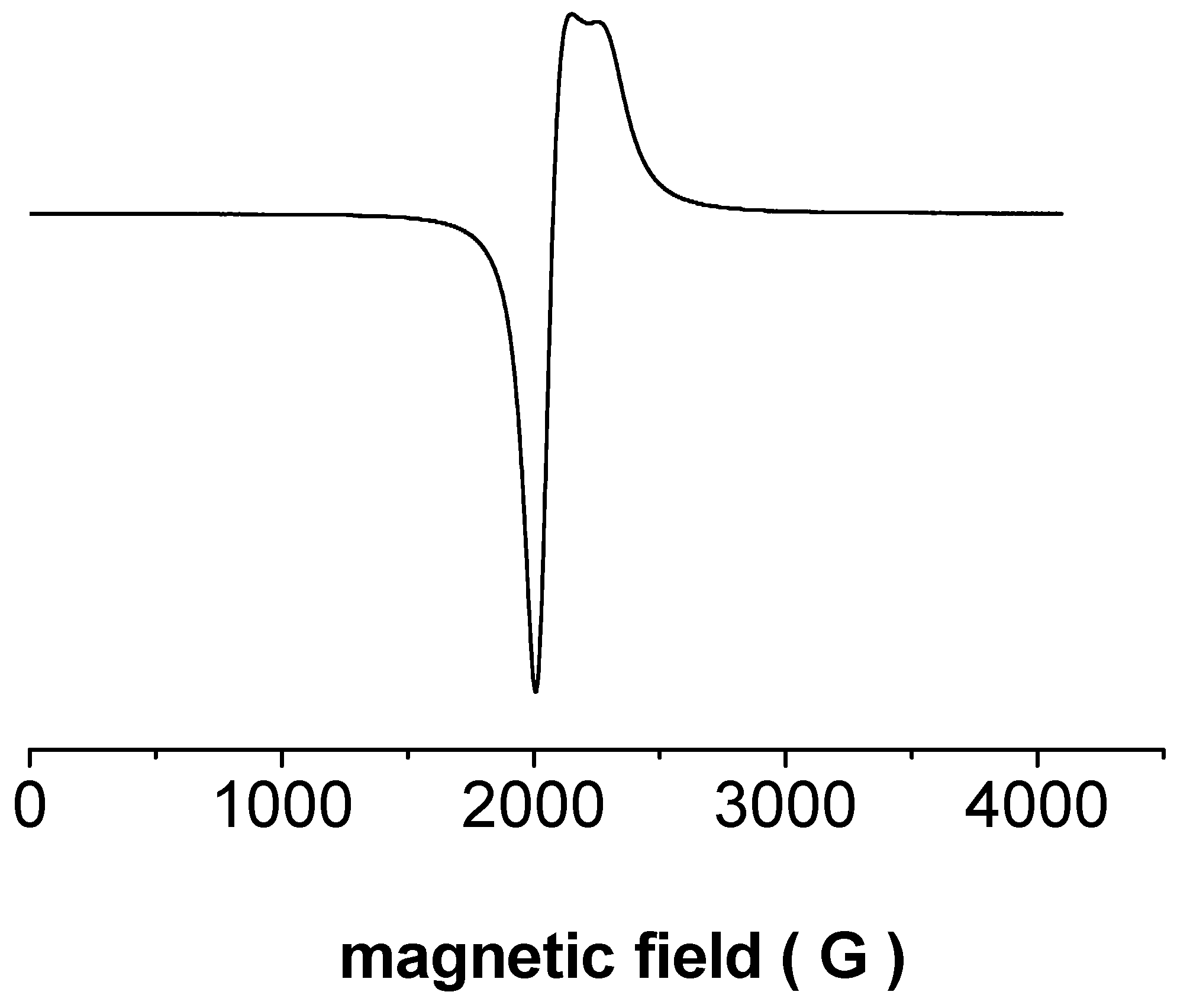
3.2. Characterization of Li1.3V3O8 and Determination of V4+ Content
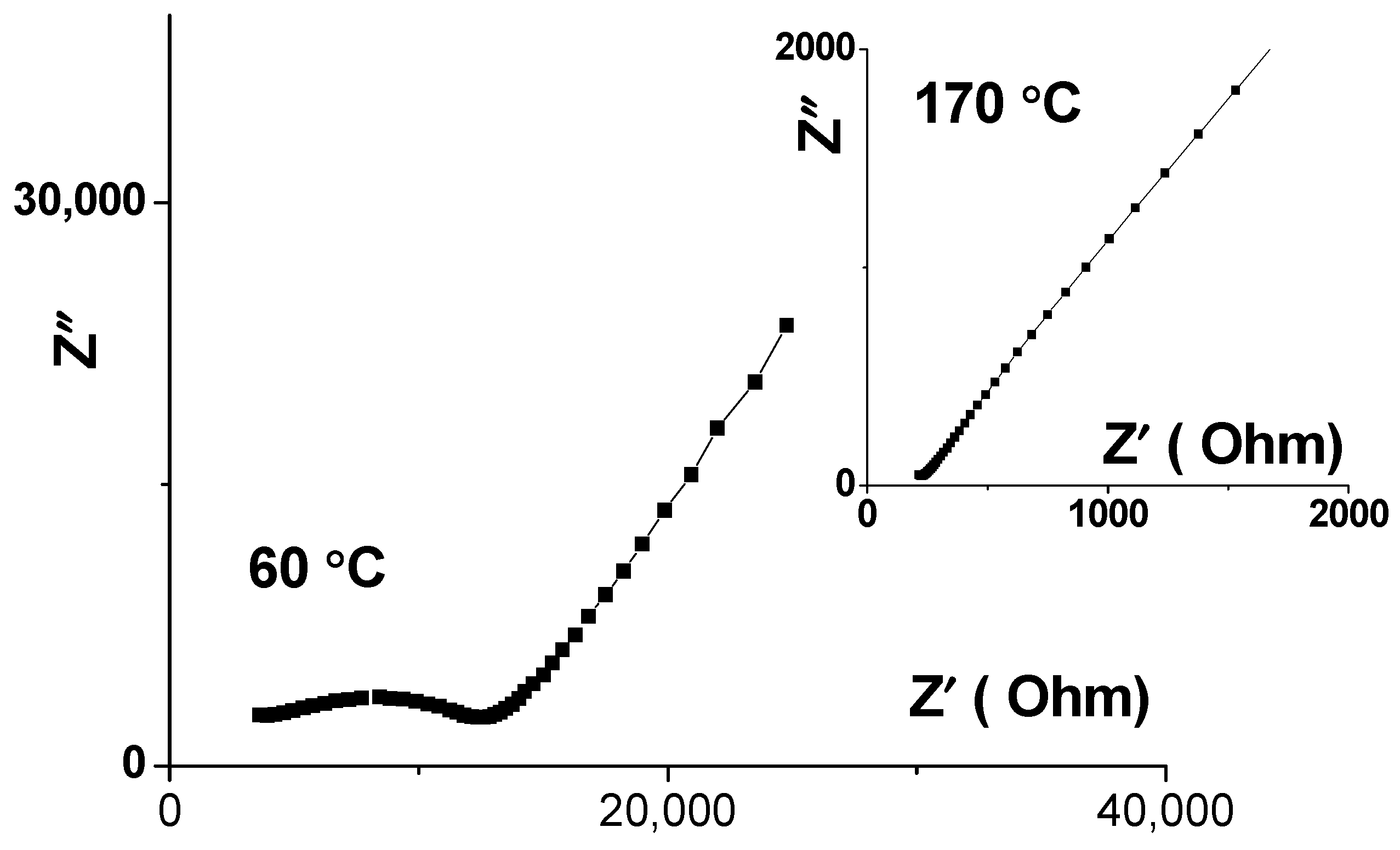
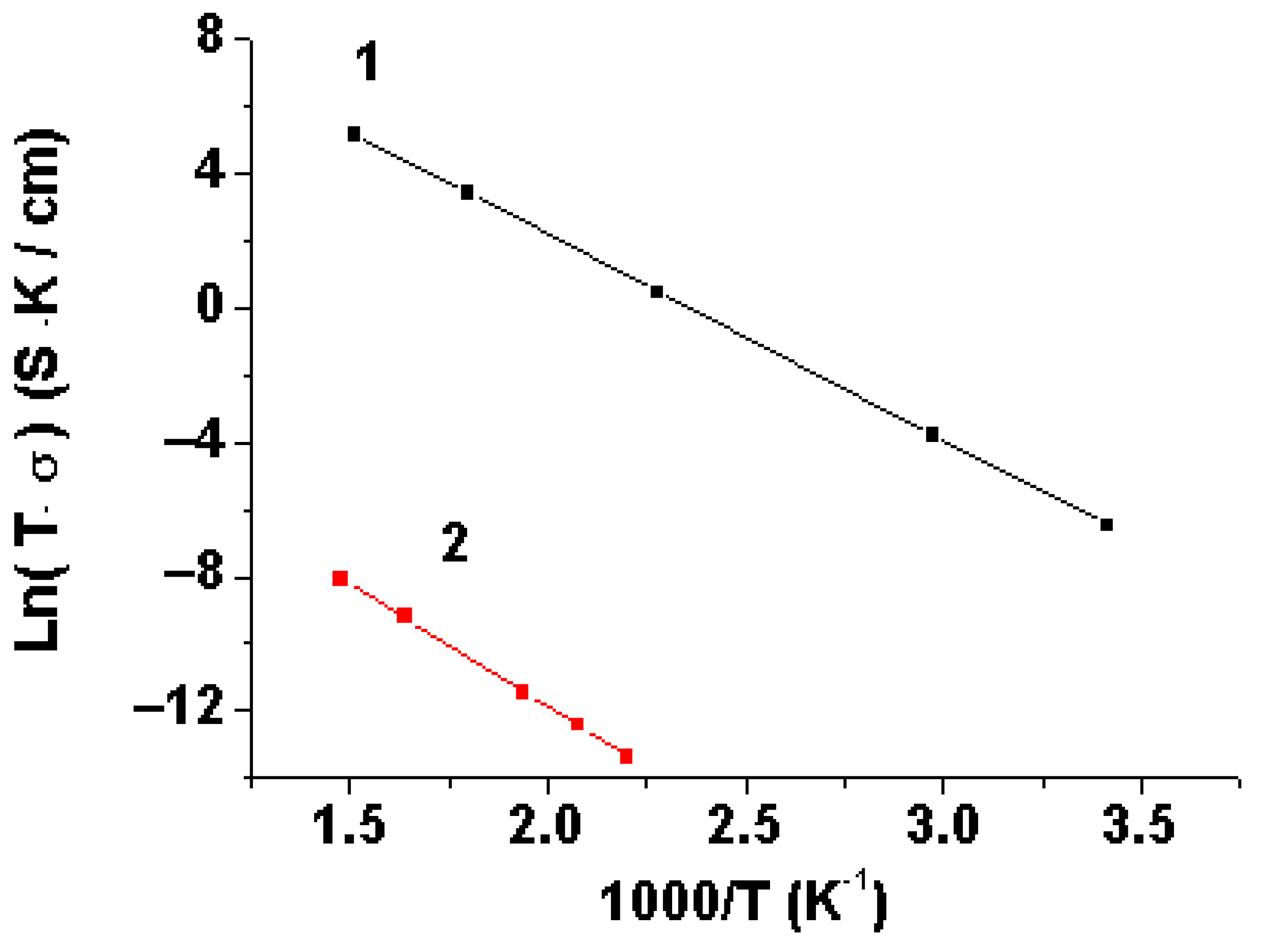
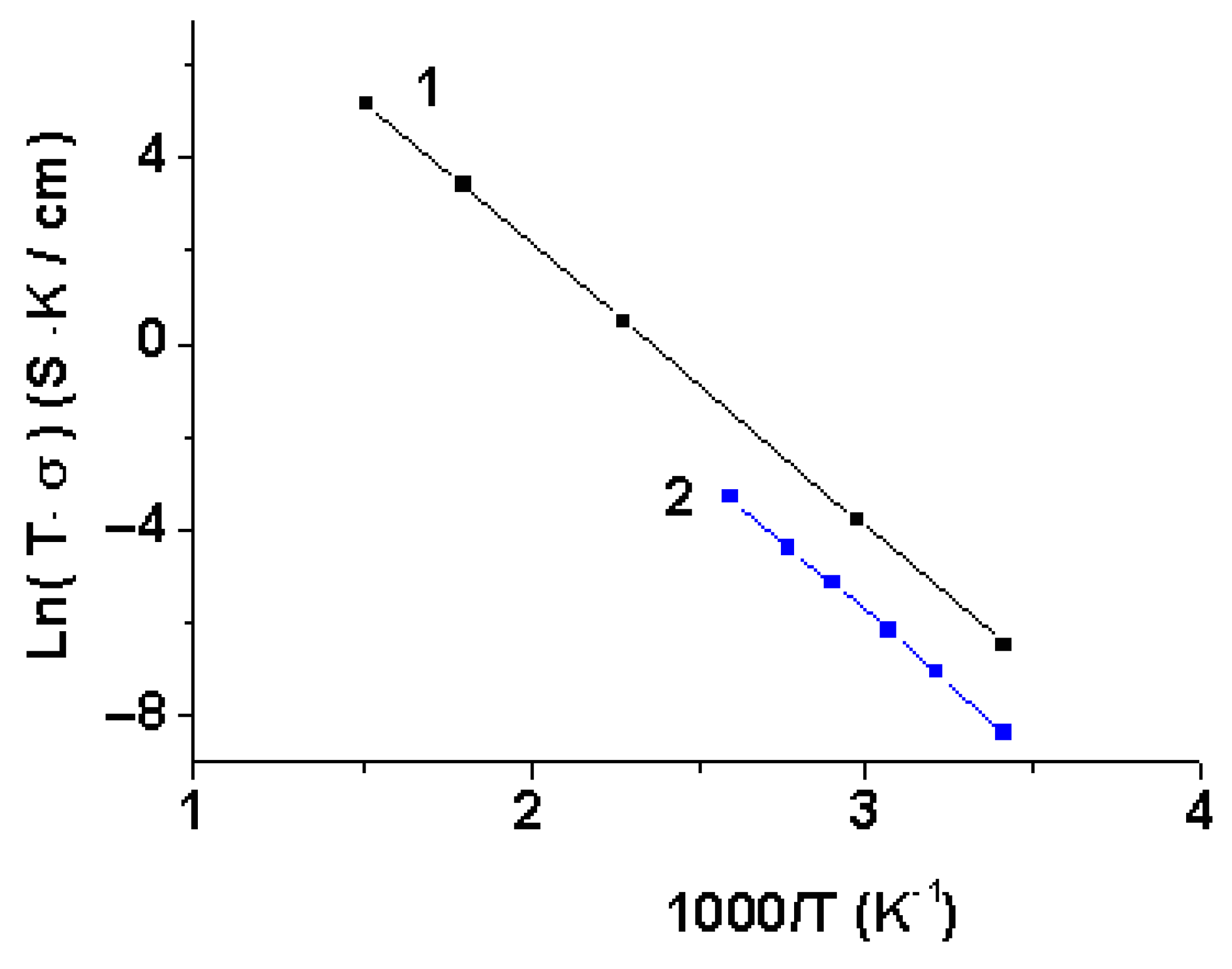
3.3. The Conductivity of Li3.8Ge0.9S0.1O4
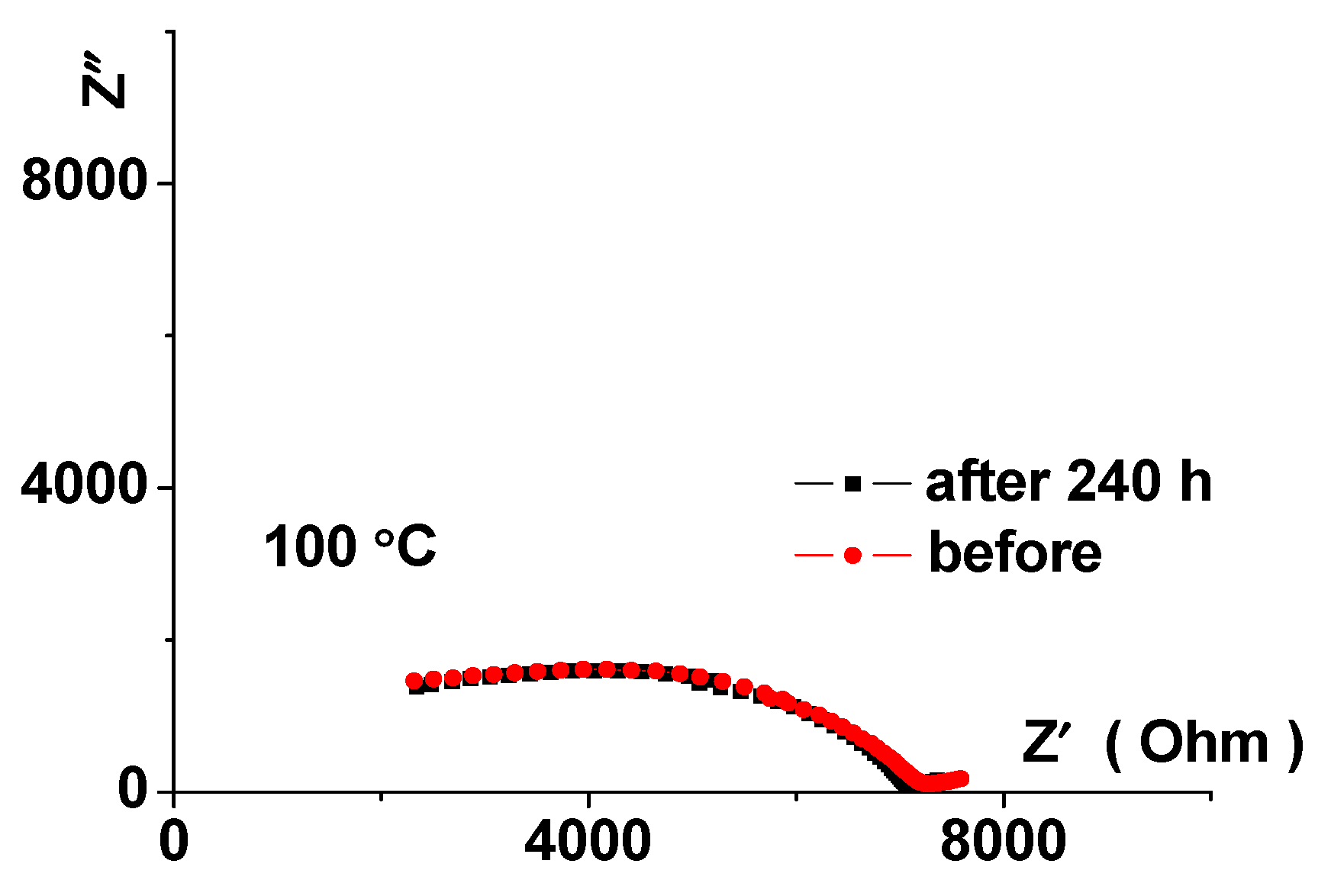
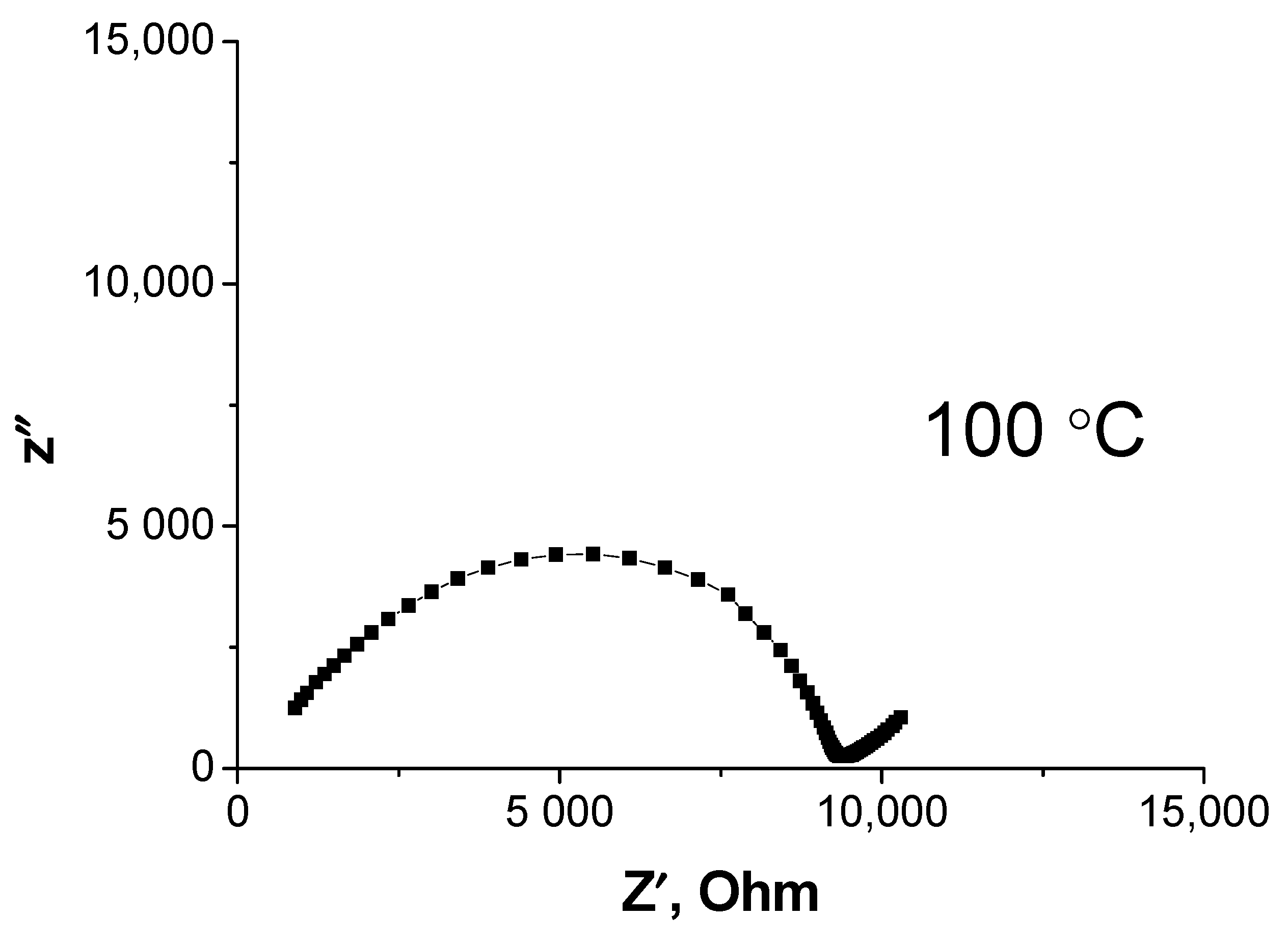
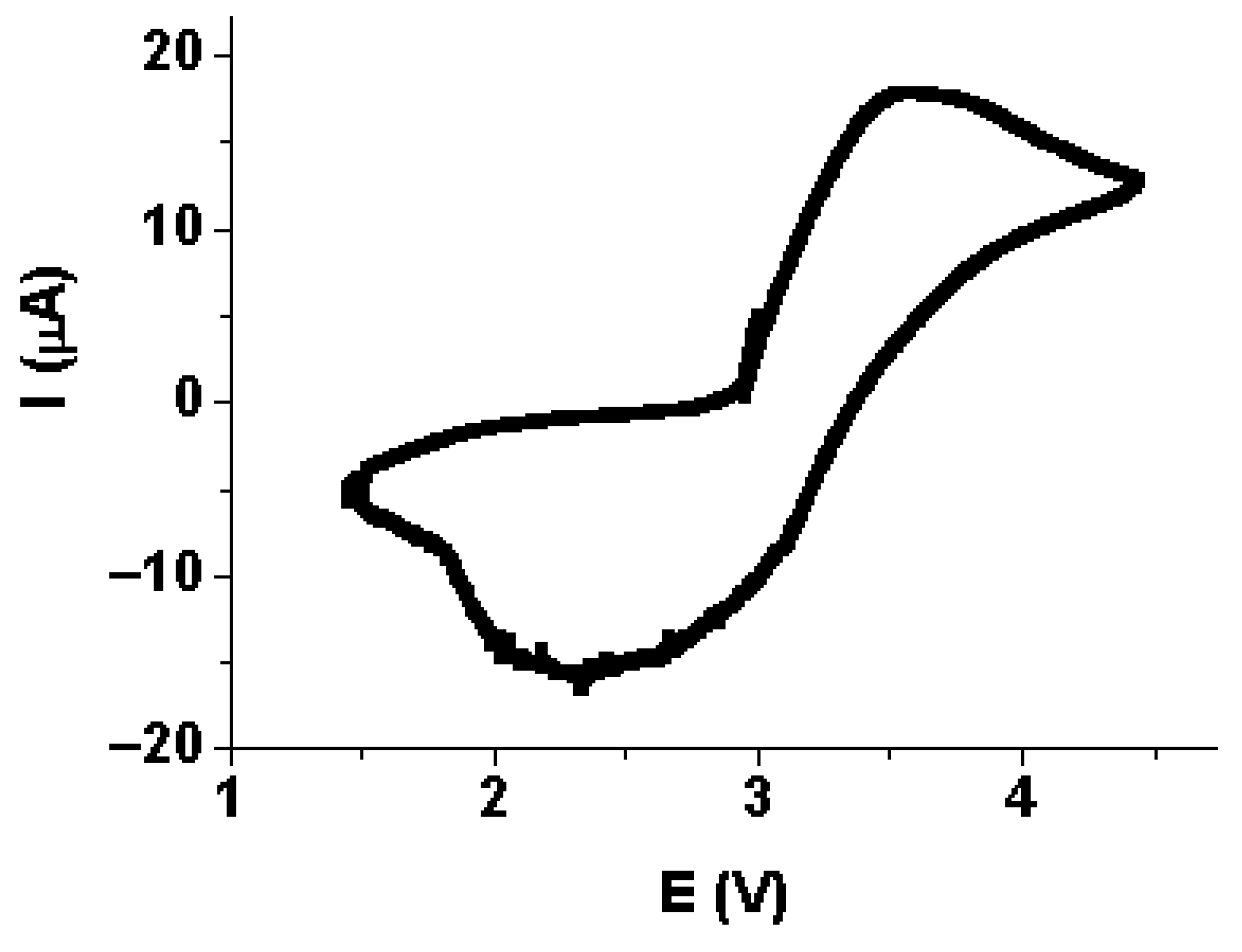
3.4. Stability of Li3.8Ge0.9S0.1O4in Contact with Lithium-Vanadium Oxide Li1.3V3O8
3.5. Stability of Li3.8Ge0.9S0.1O4in Contact with Metallic Lithium
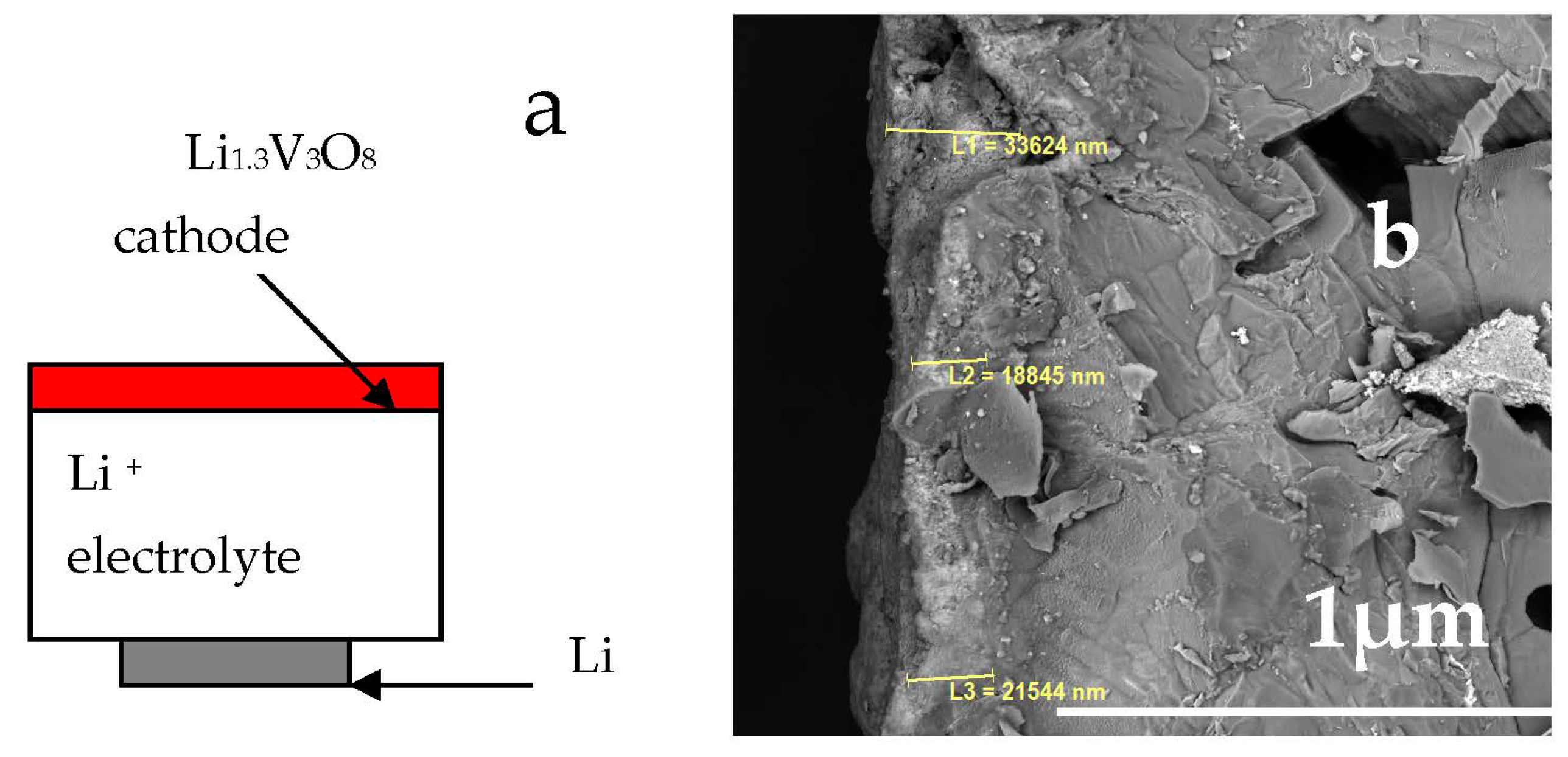
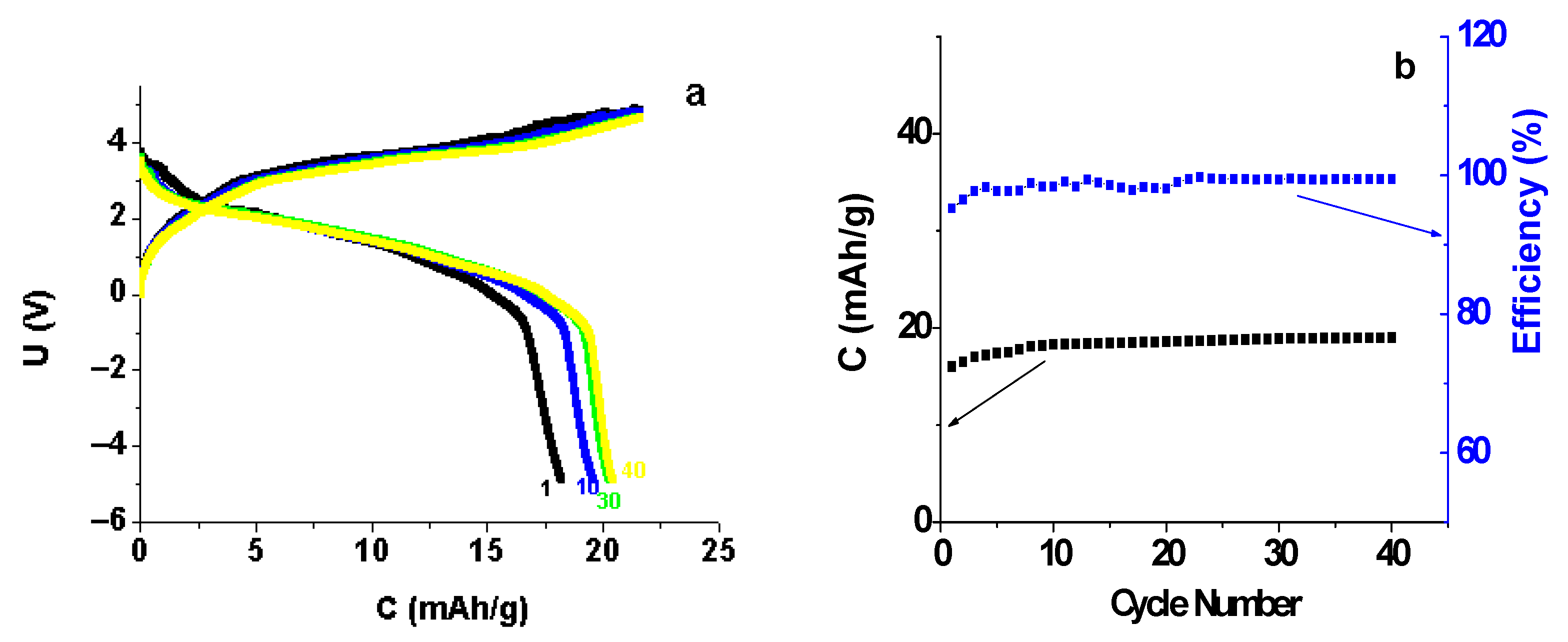
3.6. Testing the Li|Li3.8Ge0.9S0.1O4|Li1.3V3O8 Cell Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zubi, G.; Dufo-Lopez, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Hu, S.; Sun, W.; He, Y. On the sustainability of lithium ion battery industry—A review and perspective. Energy Storage Mater. 2020, 36, 182–212. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, B.; Stoliarov, S.I.; Sun, J. A review of lithium ion battery failure mechanisms and fire prevention strategies. Prog. Energy Combust. Sci. 2019, 73, 95–131. [Google Scholar] [CrossRef]
- Luy, P.; Liu, X.; Qu, J.; Zhao, J.; Huo, Y.; Qu, Z.; Rao, Z. Recent advances on thermal safety of lithium ion battery for energy storage. Energy Storage Mater. 2020, 31, 195–220. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium ion battery safety. Sci. Adv. 2018, 4, 9820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, Y.J.; Oh, D.Y.; Jung, S.H.; Jung, Y.S. Towards practical all-solid-state lithium-ion batteries with high energy density and safety: Comparative study for electrodes fabricated by dry- and slurry-mixing processes. J. Power Sources 2018, 375, 93–101. [Google Scholar] [CrossRef]
- Ferraresi, G.; El Kazzi, M.; Czornomaz, L.; Tsai, C.-L.; Uhlenbruck, S.; Villevielle, C. Electrochemical Performance of All-Solid-State Li-Ion Batteries Based on Garnet Electrolyte Using Silicon as Model Electrode. ACS Energy. Lett. 2018, 3, 1006–1012. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, J.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy. 2017, 33, 363–386. [Google Scholar] [CrossRef] [Green Version]
- Schnell, J.; Gunther, T.; Knoche, T.; Vieider, C.; Kohler, L.; Just, A.; Keller, M.; Passerini, S.; Reinhart, G. All-solid-state lithium-ion and lithium-meal batteries–paving the way to large-scale production. J. Power Sources 2018, 382, 160–175. [Google Scholar] [CrossRef]
- Kammampata, S.P.; Thangadurai, V. Cruising in ceramics–discovering new structures for all-solid-state batteries–fundamentals, materials and performances. Ionics 2018, 24, 639–660. [Google Scholar] [CrossRef]
- Cao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, Challenges, and Recent Progress of Inorganic Solid-State Electrolytes for All-Solid-State Lithium Batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, S.; Song, M.; Lai, M.O.; Lu, l. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Z.; Yoshida, A.; Wang, Z.; Xao, X.; Abudula, A.; Guan, G. Utmost limits of various solid electrolytes in all-solid-state lithium batteries: A critical review. Renew. Sustain. Energy Rev. 2019, 109, 367–385. [Google Scholar] [CrossRef]
- Shoji, M.; Munakata, H.; Kanamura, K. Fabrication of All-Solid-State Lithium-Ion Cells using Three-Dimensionally Structured Solid Electrolyte Li7La3Zr2O12 Pellets. Front. Energy. Res. 2016, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Geng, Z.; Han, C.; Fu, Y.; Li, S.; He, Y.-B.; Kang, F.; Li, B. Challenges and perspectives of garnet solid electrolytes for all-solid-state lithium batteries. J. Power Sources 2018, 389, 120–134. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714. [Google Scholar] [CrossRef]
- Feng, J.K.; Yan, B.J.; Liu, J.S.; Lai, M.O.; Li, L. All solid state lithium ion rechargeable batteries using NASICON structured electrolyte. Mater. Technol. 2013, 28, 276–279. [Google Scholar] [CrossRef]
- Hou, M.; Liang, F.; Chen, K.; Dai, Y.; Xue, D. Challenges and perspectives of NASICON-type solid electrolytes for all-solid-sates lithium batteries. Nanotechnology 2020, 31, 132003. [Google Scholar] [CrossRef]
- Anantharamulu, N.; Rao, K.K.; Rambabu, G.; Kumar, B.V.; Radha, V.; Vihal, M. A wide-ranging review on NASICON type materials. J. Mater. Sci. 2011, 46, 2821–2837. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y. Perovskite-type Li-ion solid electrolytes: A review. J. Mater. Sci. Mater. Electron. 2021, 32, 9736–9754. [Google Scholar] [CrossRef]
- Cao, C.; Li, Z.-B.; Wang, X.-L.; Zhao, X.-B.; Han, W.-Q. Recent advances in inorganic solid electrolytes for lithium batteries. Front. Energy Res. 2014, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.Y.-P. Crystal structure and ionic conductivity of Li14Zn(GeO4)4 and other new Li+ superionic conductors. Mat. Res. Bull. 1978, 13, 117–124. [Google Scholar] [CrossRef]
- Bachman, J.K.; Muy, S.; Grimaud, A.; Chang, H.H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic Solid-Sate Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Wang, Y.; Ren, G.; Yuan, C. Towards rational mechanical design of inorganic solid electrolytes for all-solid-state lithium ion batteries. Energy Storage Mater. 2020, 26, 313–324. [Google Scholar] [CrossRef]
- Whitacre, J.; West, W.C. Crystalline Li4SiO4/Li3PO4 solid solutions as an electrolyte for film batteries using sputtered cathode layers. Solid State Ion. 2004, 175, 251–255. [Google Scholar] [CrossRef]
- Deng, Y.; Eames, C.; Chotard, J.-N.; Larele, F.; Seznec, V.; Emge, S.; Pecher, O.; Grey, C.P.; Masquelier, C.; Islam, M.S. Structural and Mechanistic Insights into Fast Lithium-Ion Conduction in Li4SiO4–Li3PO4 Solid Electrolytes. J. Am. Chem. Soc. 2015, 137, 9136–9145. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-W.; Lee, J.-W. Improved Li-ion conductivity of 0.7Li4SiO4-0.3Li3PO4 by aluminium doping. Solid State Ion. 2016, 289, 173–179. [Google Scholar] [CrossRef]
- Mahreen, A.; Kong, X.Y. A lithium ion conductor in Li4SiO4-Li3PO4-LiBO2 ternary system. Solid State Ion. 2016, 293, 72–76. [Google Scholar] [CrossRef]
- Shchelkanova, M.S.; Shekhman, G.S.; Druzhinin, K.V.; Pankratov, A.A.; Pryakhina, V.I. The study of lithium vanadium oxide LiV3O8 as electrode material for all-solid-state lithium-ion batteries with solid electrolyte Li3.4Si0.4P0.6O4. Electrochim. Acta 2019, 320, 134570. [Google Scholar] [CrossRef]
- Yoneda, Y.; Shigeno, M.; Kimura, T.; Nagao, K.; Hotehama, C.; Sakuda, A.; Tatsumisago, M.; Hayashi, A. Preparation and characterization of hexagonal Li4GeO4-based glass-ceramic electrolytes. Solid State Ion. 2011, 363, 115605. [Google Scholar] [CrossRef]
- Taminato, S.; Okumura, T.; Takeuchi, T.; Kobayashi, H. Fabrication and charge-discharge reaction of all solid-state lithium battery using Li4−2xGe1−xSxO4 electrolyte. Solid State Ion. 2018, 326, 52–57. [Google Scholar] [CrossRef]
- Dissanayake, M.A.K.L.; Gunavardane, R.P.; West, A.R.; Senadeera, G.K.R.; Bandaranayke, P.W.S.K.; Careem, M.A. Lithium ion conducting Li4−2xGe1−xSxO4 solid electrolytes. Solid State Ion. 1993, 62, 217–223. [Google Scholar] [CrossRef]
- West, K.; Zachau-Christiansen, B.; Ostergard, M.J.L.; Jacobsen, T. Vanadium oxides as electrode materials for rechargeable lithium cells. J. Power Sources 1987, 20, 165–172. [Google Scholar] [CrossRef]
- West, K.; Zachau-Christiansen, B.; Skaarup, S.; Saidi, Y.; Barker, J.; Olsen, I.I.; Pynenburg, R.; Koksbang, R. Comparison of LiV3O8 Cathode Materials Prepared by Different Methods. J. Electrochem. Soc. 1996, 143, 820–824. [Google Scholar] [CrossRef] [Green Version]
- Bensalah, N.; Dawood, H. Review on Synthesis, Characterization and Electrochemical Properties of Cathode Materials for Lithium Ion Batteries. J. Material Sci. Eng. 2016, 5, 1000258. [Google Scholar] [CrossRef] [Green Version]
- Fomichev, V.V.; Proskuryakova, E.V. Vibrational spectra and energy characteristics of the superionics Li4SiO4 and Li4GeO4. J. Solid State Chem. 1997, 232–237. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 3rd ed.; Wiley: New York, NY, USA, 1978. [Google Scholar]
- Brooker, M.H.; Bates, J.B. Raman and Infrared Spectral Studies of Anhydrous Li2CO3 and Na2CO3. J. Chem. Phys. 1971, 54, 4788. [Google Scholar] [CrossRef]
- Wadsley, A.D. Crystal chemistry of non-stoichiometric pentavalent vandadium oxides: Crystal structure of Li1+xV3O8. J. Acta Crystallogr. 1957, 10, 261. [Google Scholar] [CrossRef]
- Kera, Y. Infrared study of alkali tri-and hexavanadates as formed from their melts. J. Solid State Chem. 1984, 51, 205–211. [Google Scholar] [CrossRef]
- Magon, C.J.; Lima, J.F.; Donoso, J.P.; Lavayen, V.; Benavenete, E.; Navas, D.; Gonzales, G. Deconvolution of the EPR spectra of vanadium oxide nanotubes. J. Magn. Reson. 2012, 222, 26–33. [Google Scholar] [CrossRef]
| Li3.8Ge0.9S0.1O4 | |
|---|---|
| t, °C | σ, S/cm |
| 25 | 5 × 10 −6 |
| 60 | 6 × 10 −5 |
| 170 | 4 × 10 −3 |
| 390 | 4 × 10 −1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shchelkanova, M.; Shekhtman, G.; Pershina, S. A Study of Li3.8Ge0.9S0.1O4 Solid Electrolyte Stability Relative to Electrode Materials of Lithium Power Sources. Batteries 2023, 9, 66. https://doi.org/10.3390/batteries9020066
Shchelkanova M, Shekhtman G, Pershina S. A Study of Li3.8Ge0.9S0.1O4 Solid Electrolyte Stability Relative to Electrode Materials of Lithium Power Sources. Batteries. 2023; 9(2):66. https://doi.org/10.3390/batteries9020066
Chicago/Turabian StyleShchelkanova, Mariya, Georgiy Shekhtman, and Svetlana Pershina. 2023. "A Study of Li3.8Ge0.9S0.1O4 Solid Electrolyte Stability Relative to Electrode Materials of Lithium Power Sources" Batteries 9, no. 2: 66. https://doi.org/10.3390/batteries9020066
APA StyleShchelkanova, M., Shekhtman, G., & Pershina, S. (2023). A Study of Li3.8Ge0.9S0.1O4 Solid Electrolyte Stability Relative to Electrode Materials of Lithium Power Sources. Batteries, 9(2), 66. https://doi.org/10.3390/batteries9020066





