Strategies and Challenge of Thick Electrodes for Energy Storage: A Review
Abstract
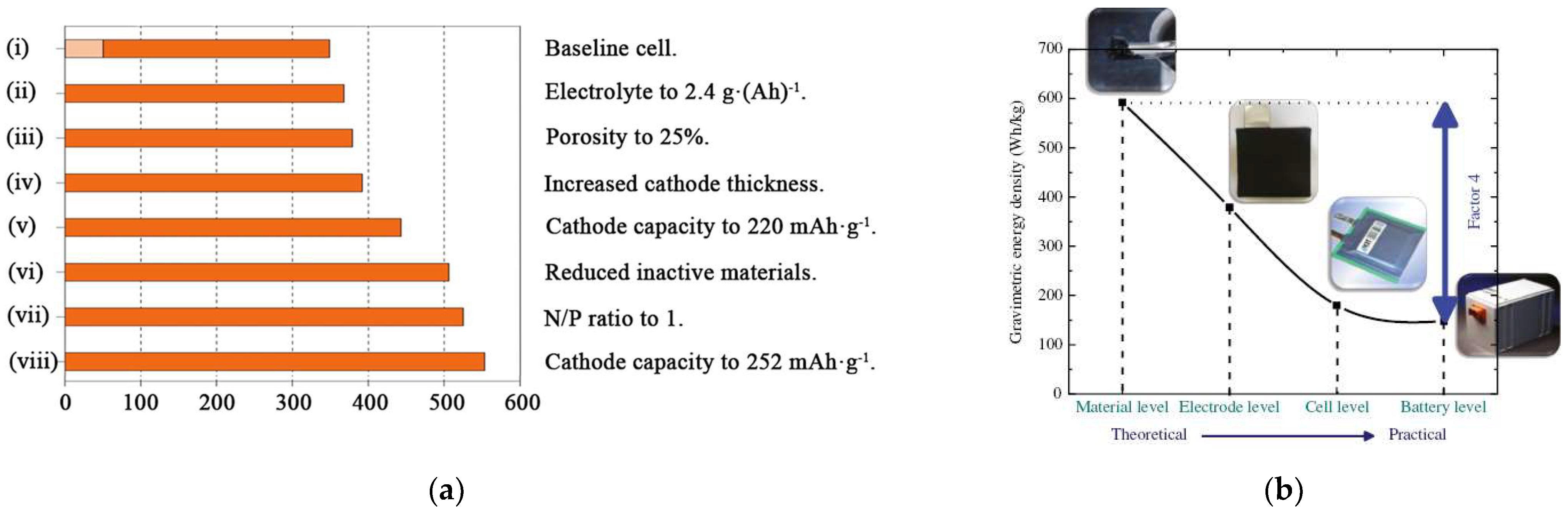
:1. Introduction
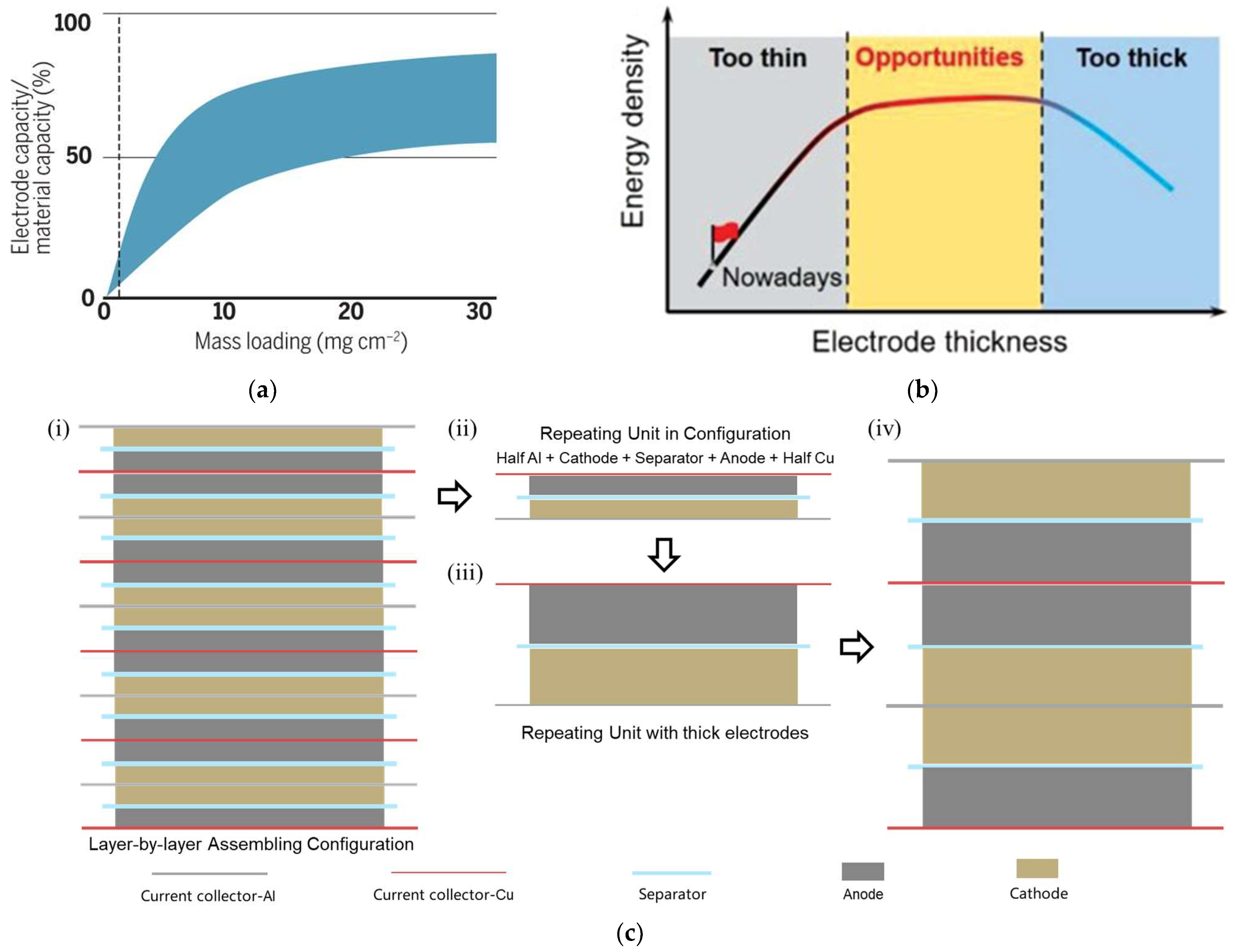
2. The Challenge of Thick Electrodes
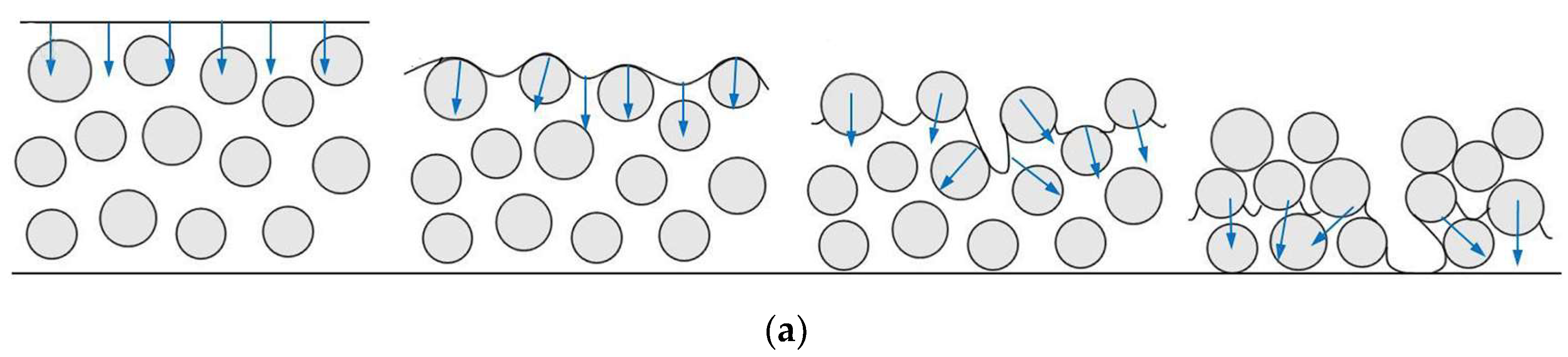
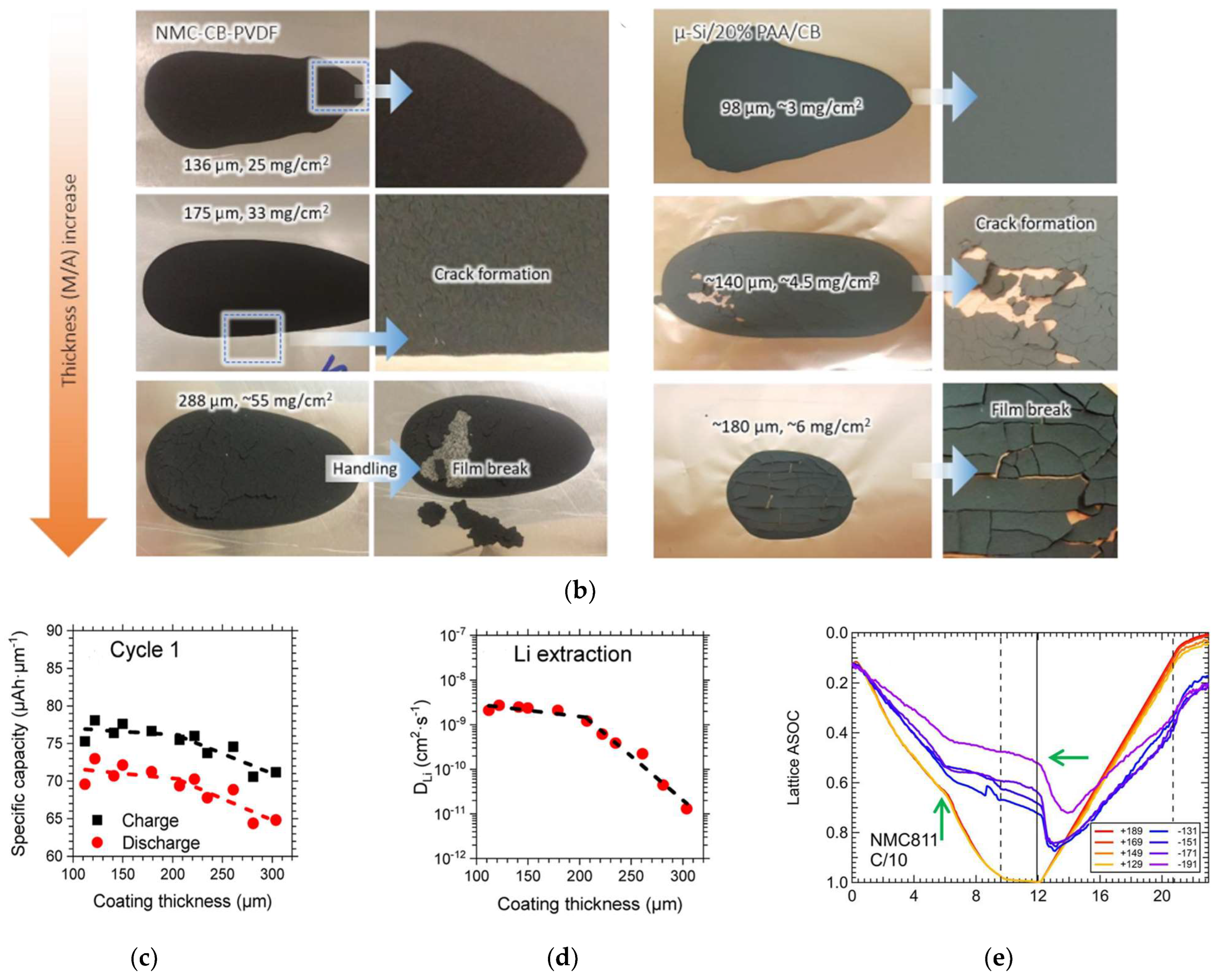
2.1. The Critical Cracking Thickness (CCT)
2.2. The Limited Penetration Depth (LPD)
3. Strategies for Increasing Electrode Thickness
3.1. Increasing the CCT
3.1.1. Decreasing Generated Stresses
3.1.2. Utilizing 3D Frameworks
3.1.3. Taking New Technology
3.2. Increasing the LPD
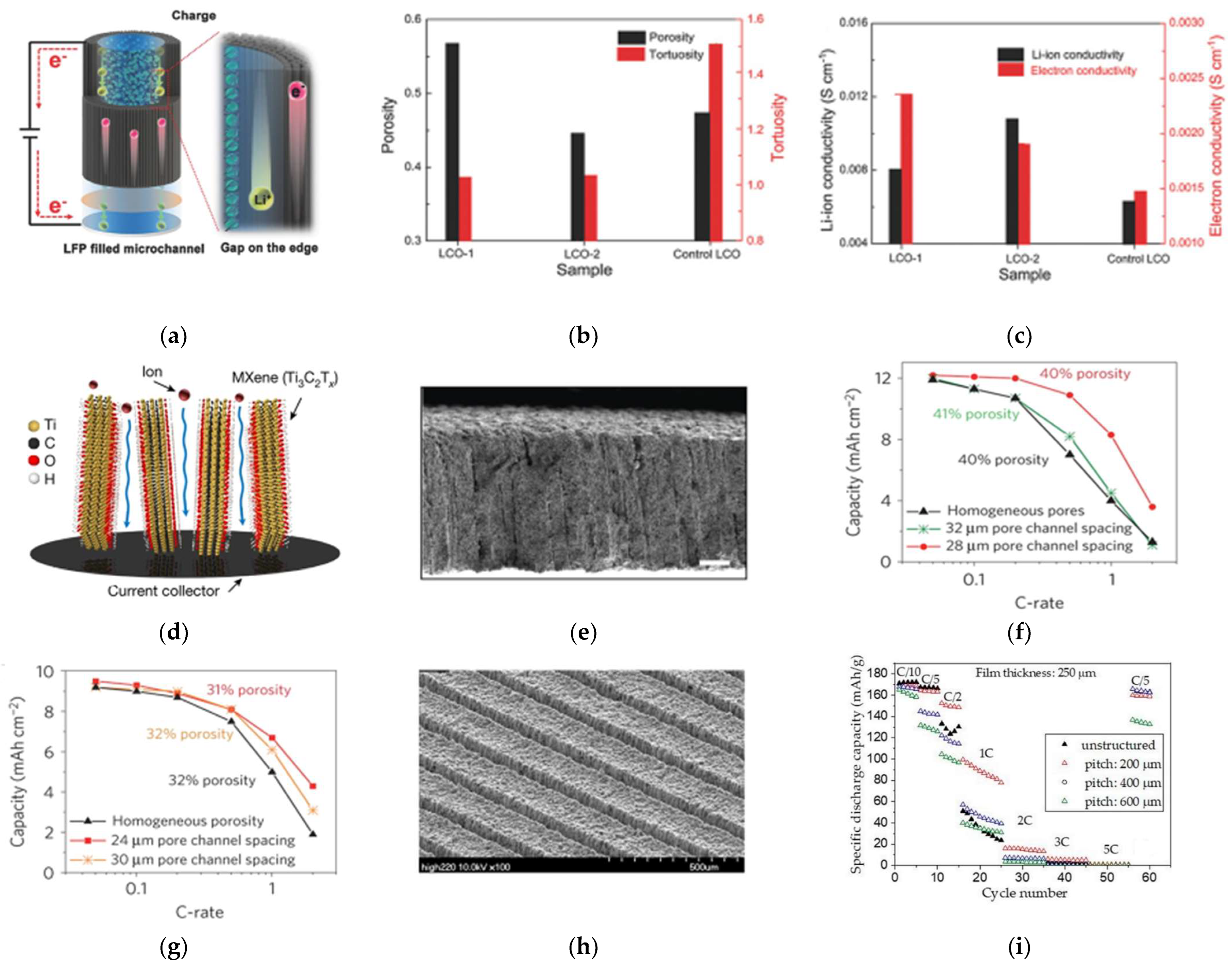
3.2.1. Optimizing Electrode Porosity
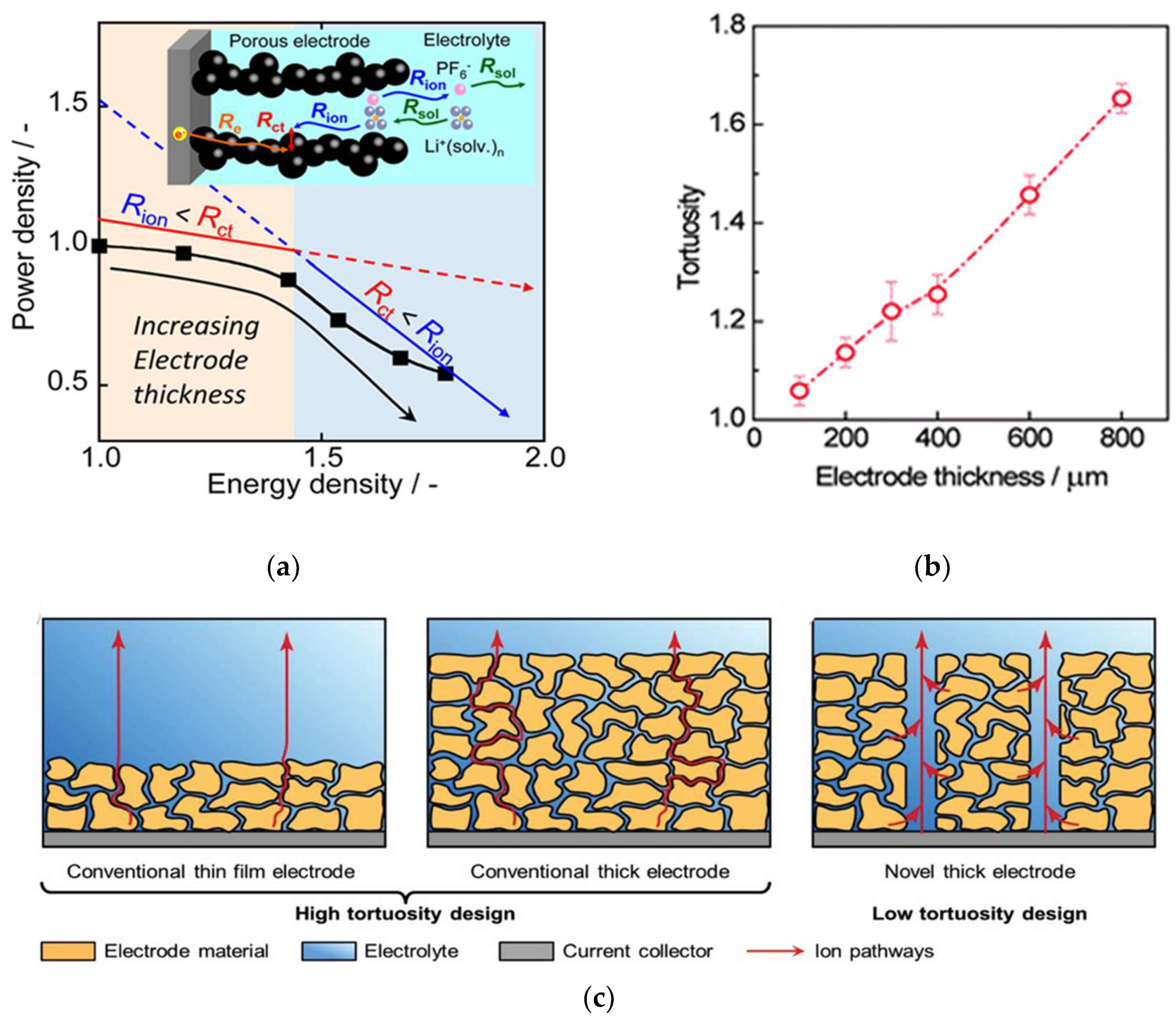
3.2.2. Decreasing Electrode Tortuosity
3.3. Summary
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rempel, J. Vehicle Technologies Office Merit Review 2015: High Energy High Power Battery Exceeding PHEV-40 Requirements. Available online: https://www.energy.gov/sites/prod/files/2015/06/f23/es209_rempel_2015_p.pdf (accessed on 30 January 2023).
- Gallagher, K.G.; Trask, S.E.; Bauer, C.; Woehrle, T.; Lux, S.F.; Tschech, M.; Lamp, P.; Polzin, B.J.; Ha, S.; Long, B.; et al. Optimizing Areal Capacities through Understanding the Limitations of Lithium-Ion Electrodes. J. Electrochem. Soc. 2015, 163, A138–A149. [Google Scholar] [CrossRef]
- Gröger, O.; Gasteiger, H.A.; Suchsland, J.-P. Review—Electromobility: Batteries or Fuel Cells? J. Electrochem. Soc. 2015, 162, A2605–A2622. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.-J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- China Society of Automotive Engineers. Technology Roadmap of Clean and New Energy Vehicles 2.0. Available online: http://zhishi.sae-china.org/ppt.html?id=2100 (accessed on 30 January 2023).
- Xia, C.; Kwok, C.; Nazar, L. A high-energy-density lithium-oxygen battery based on a reversible four-electron conversion to lithium oxid. Science 2018, 361, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, P.F.; Bai, P.; Wan, H.; Liu, S.; Hou, S.; Pu, X.; Xia, J.; Zhang, W.; Wang, Z.; et al. Interfacial Design for a 4.6 V High-Voltage Single-Crystalline LiCoO2 Cathode. Adv. Mater. 2022, 34, e2108353. [Google Scholar] [CrossRef]
- Du, K.; Tao, R.; Guo, C.; Li, H.; Liu, X.; Guo, P.; Wang, D.; Liang, J.; Li, J.; Dai, S.; et al. In-situ synthesis of porous metal fluoride@carbon composite via simultaneous etching/fluorination enabled superior Li storage performance. Nano Energy 2022, 103, 107862. [Google Scholar] [CrossRef]
- Shi, B.; Shang, Y.; Pei, Y.; Pei, S.; Wang, L.; Heider, D.; Zhao, Y.Y.; Zheng, C.; Yang, B.; Yarlagadda, S.; et al. Low Tortuous, Highly Conductive, and High-Areal-Capacity Battery Electrodes Enabled by Through-thickness Aligned Carbon Fiber Framework. Nano Lett. 2020, 20, 5504–5512. [Google Scholar] [CrossRef] [PubMed]
- Pfleging, W. A review of laser electrode processing for development and manufacturing of lithium-ion batteries. Nanophotonics 2018, 7, 549–573. [Google Scholar] [CrossRef]
- Liu, J.; Bao, Z.; Cui, Y.; Dufek, E.J.; Goodenough, J.B.; Khalifah, P.; Li, Q.; Liaw, B.Y.; Liu, P.; Manthiram, A.; et al. Pathways for practical high-energy long-cycling lithium metal batteries. Nat. Energy 2019, 4, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Li, K. Charge delivery goes the distance. Science 2017, 356, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Chen, C.; Kirsch, D.; Hu, L. Thick Electrode Batteries: Principles, Opportunities, and Challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Zheng, H.; Li, J.; Song, X.; Liu, G.; Battaglia, V. A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes. Electrochim. Acta 2012, 71, 258–265. [Google Scholar] [CrossRef]
- Wang, J.S.; Liu, P.; Sherman, E.; Verbrugge, M.; Tataria, H. Formulation and characterization of ultra-thick electrodes for high energy lithium-ion batteries employing tailored metal foams. J. Power Sources 2011, 196, 8714–8718. [Google Scholar] [CrossRef]
- Singh, K.; Tirumkudulu, M. Cracking in drying colloidal films. Phys. Rev. Lett. 2007, 98, 218302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, R.; Garino, T.; Cima, M. Drying of Granular Ceramic Films: I, Effect of Processing Variables on Cracking Behavior. J. Am. Ceram. Soc. 1993, 76, 2257–2264. [Google Scholar] [CrossRef]
- Slowik, V.; Ju, J. Discrete modeling of plastic cement paste subjected to drying. Cem. Concr. Compos. 2011, 33, 925–935. [Google Scholar] [CrossRef]
- Tirumkudulu, M.; Russel, W. Cracking in drying latex films. Langmuir 2005, 21, 4938–4948. [Google Scholar] [CrossRef] [PubMed]
- Tambio, S.; Cadiou, F.; Maire, E.; Besnard, N.; Deschamps, M.; Lestriez, B. The Concept of Effective Porosity in the Discharge Rate Performance of High-Density Positive Electrodes for Automotive Application. J. Electrochem. Soc. 2020, 167, 160509. [Google Scholar] [CrossRef]
- Lu, C.; Huang, Q.; Chen, X. High-performance silicon nanocomposite based ionic actuators. J. Mater. Chem. A 2020, 8, 9228–9238. [Google Scholar] [CrossRef]
- Yan, L.; Yudong, L.; Ting’an, Z.; Naixiang, F. Research on the Penetration Depth in Aluminum Reduction Cell with New Type of Anode and Cathode Structures. JOM 2014, 66, 1202–1209. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Hou, P.; Cheng, M.; Wang, S.; Cheng, H.; Liu, C.; Li, F. 3D Interconnected Electrode Materials with Ultrahigh Areal Sulfur Loading for Li-S Batteries. Adv. Mater. 2016, 28, 3374–3382. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ouyang, T.; Xiong, T.; Jiang, Z.; Adekoya, D.; Wu, Y.; Huang, Y.; Balogun, M. All-carbon-frameworks enabled thick electrode with exceptional high-areal-capacity for Li-Ion storage. Carbon 2021, 174, 1–9. [Google Scholar] [CrossRef]
- Woodward, R.; Markoulidis, F.; Luca, F.D.; Anthony, D.; Malko, D.; McDonald, T.; Shaffer, M.; Bismarck, A. Carbon foams from emulsion-templated reduced graphene oxide polymer composites: Electrodes for supercapacitor devices. J. Mater. Chem. A 2018, 6, 1840–1849. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Pham, H.Q.; Kang, D.-H.; Park, H.-Y.; Song, S.-W. Improved rate capability of highly loaded carbon fiber-interwoven LiNi 0.6 Co 0.2 Mn 0.2 O 2 cathode material for high-power Li-ion batteries. J. Alloys Compd. 2016, 657, 464–471. [Google Scholar] [CrossRef]
- Shen, F.; Luo, W.; Dai, J.; Yao, Y.; Zhu, M.; Hitz, E.; Tang, Y.; Chen, Y.; Sprenkle, V.L.; Li, X.; et al. Ultra-Thick, Low-Tortuosity, and Mesoporous Wood Carbon Anode for High-Performance Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600377. [Google Scholar] [CrossRef]
- Ebner, M.; Chung, D.-W.; García, R.E.; Wood, V. Tortuosity Anisotropy in Lithium-Ion Battery Electrodes. Adv. Energy Mater. 2014, 4, 1301278. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, Y.; Wang, Y.; Song, L.; Li, M.; Yang, H.; Huang, Z.; Li, D.; Zhou, H. Scalable Manufacture of High-Performance Battery Electrodes Enabled by a Template-Free Method. Small Methods 2021, 5, 2100280. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, Y.; Chen, Y.; Li, M.; Liu, P.; Wang, C.; Wang, P.; Lu, H. Designing vertical channels with expanded interlayers for Li-ion batteries. Chem. Commun. 2019, 55, 4258–4261. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Mathis, T.S.; Zhao, M.Q.; Anasori, B.; Dang, A.; Zhou, Z.; Cho, H.; Gogotsi, Y.; Yang, S. Thickness-independent capacitance of vertically aligned liquid-crystalline MXenes. Nature 2018, 557, 409–412. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Thick Electrodes for High Energy Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1196–A1201. [Google Scholar] [CrossRef]
- Kato, Y.; Shiotani, S.; Morita, K.; Suzuki, K.; Hirayama, M.; Kanno, R. All-Solid-State Batteries with Thick Electrode Configurations. J. PhysIcal Chem. Lett. 2018, 9, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Kubanska, A.; Castro, L.; Tortet, L.; Dollé, M.; Bouchet, R. Effect of composite electrode thickness on the electrochemical performances of all-solid-state li-ion batteries. J. Electroceram. 2017, 38, 189–196. [Google Scholar] [CrossRef]
- Hong, S.-B.; Lee, Y.-J.; Kim, U.-H.; Bak, C.; Lee, Y.M.; Cho, W.; Hah, H.J.; Sun, Y.-K.; Kim, D.-W. All-Solid-State Lithium Batteries: Li+-Conducting Ionomer Binder for Dry-Processed Composite Cathodes. ACS Energy Lett. 2022, 7, 1092–1100. [Google Scholar] [CrossRef]
- Kukay, A.; Sahore, R.; Parejiya, A.; Hawley, W.; Li, J.; Wood, D. Aqueous Ni-rich-cathode dispersions processed with phosphoric acid for lithium-ion batteries with ultra-thick electrodes. J. Colloid Interface Sci. 2021, 581, 635–643. [Google Scholar] [CrossRef]
- Li, H. Practical Evaluation of Li-Ion Batteries. Joule 2019, 3, 911–914. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.; Santamarina, J. Desiccation cracks in saturated fine-grained soils: Particle-level phenomena and effective-stress analysis. Géotechnique 2011, 61, 961–972. [Google Scholar] [CrossRef]
- Lura, P.; Pease, B.; Mazzotta, G.B.; Rajabipour, F.; Weiss, J. Influence of Shrinkage-Reducing Admixtures on Development of Plastic Shrinkage Cracks. ACI Mater. J. 2007, 104, 187–194. [Google Scholar] [CrossRef]
- Du, Z.; Rollag, K.M.; Li, J.; An, S.J.; Wood, M.; Sheng, Y.; Mukherjee, P.P.; Daniel, C.; Wood, D.L. Enabling aqueous processing for crack-free thick electrodes. J. Power Sources 2017, 354, 200–206. [Google Scholar] [CrossRef] [Green Version]
- Dufresne, E.R.; Corwin, E.I.; Greenblatt, N.A.; Ashmore, J.; Wang, D.Y.; Dinsmore, A.D.; Cheng, J.X.; Xie, X.S.; Hutchinson, J.W.; Weitz, D.A. Flow and fracture in drying nanoparticle suspensions. Phys. Rev. Lett. 2003, 91, 224501. [Google Scholar] [CrossRef] [Green Version]
- Birk-Braun, N.; Yunus, K.; Rees, E.J.; Schabel, W.; Routh, A.F. Generation of strength in a drying film: How fracture toughness depends on dispersion properties. Phys. Rev. Lett. 2017, 95, 022610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibing, L.; Gallasch, T.; Schneider, P.; Niehoff, P.; Hintennach, A.; Winter, M.; Schappacher, F.M. Towards water based ultra-thick Li ion battery electrodes—A binder approach. J. Power Sources 2019, 423, 183–191. [Google Scholar] [CrossRef]
- Kumberg, J.; Müller, M.; Diehm, R.; Spiegel, S.; Wachsmann, C.; Bauer, W.; Scharfer, P.; Schabel, W. Drying of Lithium-Ion Battery Anodes for Use in High-Energy Cells: Influence of Electrode Thickness on Drying Time, Adhesion, and Crack Formation. Energy Technol. 2019, 7, 1900722. [Google Scholar] [CrossRef]
- Park, S.-H.; King, P.J.; Tian, R.; Boland, C.S.; Coelho, J.; Zhang, C.; McBean, P.; McEvoy, N.; Kremer, M.P.; Daly, D.; et al. High areal capacity battery electrodes enabled by segregated nanotube networks. Nat. Energy 2019, 4, 560–567. [Google Scholar] [CrossRef]
- Gao, H.; Wu, Q.; Hu, Y.; Zheng, J.P.; Amine, K.; Chen, Z. Revealing the Rate-Limiting Li-Ion Diffusion Pathway in Ultrathick Electrodes for Li-Ion Batteries. J. Phys. Chem. Lett. 2018, 9, 5100–5104. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, B.; Cao, X.; Bi, Y.; Chae, S.; Niu, C.; Xiao, B.; Tao, J.; Zhang, J.; Xiao, J. Evolution of the rate-limiting step: From thin film to thick Ni-rich cathodes. J. Power Sources 2020, 454, 227966. [Google Scholar] [CrossRef]
- Appiah, W.; Park, J.; Song, S.; Byun, S.; Ryou, M.; Lee, Y. Design optimization of LiNi0.6Co0.2Mn0.2O2/graphite lithium-ion cells based on simulation and experimental data. J. Power Sources 2016, 319, 147–158. [Google Scholar] [CrossRef]
- Li, Z.; Yin, L.; Mattei, G.S.; Cosby, M.R.; Lee, B.-S.; Wu, Z.; Bak, S.-M.; Chapman, K.W.; Yang, X.-Q.; Liu, P.; et al. Synchrotron Operando Depth Profiling Studies of State-of-Charge Gradients in Thick Li(Ni0.8Mn0.1Co0.1)O2 Cathode Films. Chem. Mater. 2020, 32, 6358–6364. [Google Scholar] [CrossRef]
- Xu, C.; Li, Q.; Wang, Q.; Kou, X.; Fang, H.-T.; Yang, L. Femtosecond laser drilled micro-hole arrays in thick and dense 2D nanomaterial electrodes toward high volumetric capacity and rate performance. J. Power Sources 2021, 492, 229638. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Li, Y.; Kuang, Y.; Song, J.; Luo, W.; Wang, Y.; Yao, Y.; Pastel, G.; Xie, J.; et al. Highly Conductive, Lightweight, Low-Tortuosity Carbon Frameworks as Ultrathick 3D Current Collectors. Adv. Energy Mater. 2017, 7, 1700595. [Google Scholar] [CrossRef]
- Sahore, R.; Wood, D.L.; Kukay, A.; Grady, K.M.; Li, J.; Belharouak, I. Towards Understanding of Cracking during Drying of Thick Aqueous-Processed LiNi0.8Mn0.1Co0.1O2 Cathodes. ACS Sustain. Chem. Eng. 2020, 8, 3162–3169. [Google Scholar] [CrossRef]
- Yang, G.F.; Song, K.Y.; Joo, S.K. A metal foam as a current collector for high power and high capacity lithium iron phosphate batteries. J. Mater. Chem. A 2014, 2, 19648–19652. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Tian, J.; Ye, Z.; Jin, Y.; Cui, C.; Xie, Q.; Wang, J.; Zhang, G.; Dong, Z.; Miao, Y.; et al. High energy and high power density supercapacitor with 3D Al foam-based thick graphene electrode: Fabrication and simulation. Energy Storage Mater. 2020, 33, 18–25. [Google Scholar] [CrossRef]
- Yang, G.-F.; Song, K.-Y.; Joo, S.-K. Ultra-thick Li-ion battery electrodes using different cell size of metal foam current collectors. RSC Adv. 2015, 5, 16702–16706. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; La Mantia, F.; Wu, H.; Xie, X.; McDonough, J.; Pasta, M.; Cui, Y. Lithium-Ion Textile Batteries with Large Areal Mass Loading. Adv. Energy Mater. 2011, 1, 1012–1017. [Google Scholar] [CrossRef]
- Al-Shroofy, M.; Zhang, Q.; Xu, J.; Chen, T.; Kaur, A.P.; Cheng, Y.-T. Solvent-free dry powder coating process for low-cost manufacturing of LiNi1/3Mn1/3Co1/3O2 cathodes in lithium-ion batteries. J. Power Sources 2017, 352, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Park, D.-W.; Cañas, N.A.; Wagner, N.; Friedrich, K.A. Novel solvent-free direct coating process for battery electrodes and their electrochemical performance. J. Power Sources 2016, 306, 758–763. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Wang, X.; Xie, J.; Wen, L. Hierarchically porous and conductive LiFePO4 bulk electrode: Binder-free and ultrahigh volumetric capacity Li-ion cathode. J. Mater. Chem. 2011, 21, 12444–12448. [Google Scholar] [CrossRef]
- Elango, R.; Demortière, A.; Andrade, V.; Morcrette, M.; Seznec, V. Thick Binder-Free Electrodes for Li–Ion Battery Fabricated Using Templating Approach and Spark Plasma Sintering Reveals High Areal Capacity. Adv. Energy Mater. 2018, 8, 1703031. [Google Scholar] [CrossRef]
- Sun, C.; Liu, S.; Shi, X.; Lai, C.; Liang, J.; Chen, Y. 3D printing nanocomposite gel-based thick electrode enabling both high areal capacity and rate performance for lithium-ion battery. Chem. Eng. J. 2020, 381, 122641. [Google Scholar] [CrossRef]
- Tang, X.; Zhou, H.; Cai, Z.; Cheng, D.; He, P.; Xie, P.; Zhang, D.; Fan, T. Generalized 3D Printing of Graphene-Based Mixed-Dimensional Hybrid Aerogels. ACS Nano 2018, 12, 3502–3511. [Google Scholar] [CrossRef]
- Gao, X.; Yang, X.; Sun, Q.; Luo, J.; Liang, J.; Li, W.; Wang, J.; Wang, S.; Li, M.; Li, R.; et al. Converting a thick electrode into vertically aligned “Thin electrodes” by 3D-Printing for designing thickness independent Li-S cathode. Energy Storage Mater. 2020, 24, 682–688. [Google Scholar] [CrossRef]
- Sajadi, S.M.; Enayat, S.; Vásárhelyi, L.; Alabastri, A.; Lou, M.; Sassi, L.M.; Kutana, A.; Bhowmick, S.; Durante, C.; Kukovecz, Á.; et al. Three-dimensional printing of complex graphite structures. Carbon 2021, 181, 260–269. [Google Scholar] [CrossRef]
- Sotomayor, M.; Torre-Gamarra, C.; Bucheli, W.; Amarilla, J.; Varez, A.; LevenfelD, B.; Sanchez, J. Additive-free Li4Ti5O12 thick electrodes for Li-ion batteries with high electrochemical performance. J. Mater. Chem. A 2018, 6, 5952–5961. [Google Scholar] [CrossRef]
- de la Torre-Gamarra, C.; Sotomayor, M.E.; Sanchez, J.-Y.; Levenfeld, B.; Várez, A.; Laïk, B.; Pereira-Ramos, J.-P. High mass loading additive-free LiFePO4 cathodes with 500 μm thickness for high areal capacity Li-ion batteries. J. Power Sources 2020, 458, 228033. [Google Scholar] [CrossRef]
- Sotomayor, M.E.; Torre-Gamarra, C.d.l.; Levenfeld, B.; Sanchez, J.-Y.; Varez, A.; Kim, G.-T.; Varzi, A.; Passerini, S. Ultra-thick battery electrodes for high gravimetric and volumetric energy density Li-ion batteries. J. Power Sources 2019, 437, 226923. [Google Scholar] [CrossRef]
- Duong, H.; Shin, J.; Yudi, Y. Dry Electrode Coating Technology; Maxwell Technologies, Inc.: San Diego, CA, USA, 2018. [Google Scholar]
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-ion Batteries. Sci. Rep. 2016, 6, 23150. [Google Scholar] [CrossRef] [Green Version]
- Fu, K.; Yao, Y.; Dai, J.; Hu, L. Progress in 3D Printing of Carbon Materials for Energy-Related Applications. Adv. Mater. 2017, 29, 1603486. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, K.; Kwon, S.; Seo, S.; Yoo, H.; Kim, S.; Shin, Y.; Park, Y.; Kim, D.; Choi, J.Y.; et al. Vertical alignments of graphene sheets spatially and densely piled for fast ion diffusion in compact supercapacitors. ACS Nano 2014, 8, 4580–4590. [Google Scholar] [CrossRef]
- Johns, P.A.; Roberts, M.R.; Wakizaka, Y.; Sanders, J.H.; Owen, J.R. How the electrolyte limits fast discharge in nanostructured batteries and supercapacitors. Electrochem. Commun. 2009, 11, 2089–2092. [Google Scholar] [CrossRef] [Green Version]
- Ogihara, N.; Itou, Y.; Sasaki, T.; Takeuchi, Y. Impedance Spectroscopy Characterization of Porous Electrodes under Different Electrode Thickness Using a Symmetric Cell for High-Performance Lithium-Ion Batteries. J. Phys. Chem. C 2015, 119, 4612–4619. [Google Scholar] [CrossRef]
- Vijayaraghavan, B.; Ely, D.R.; Chiang, Y.-M.; García-García, R.; Garcia, R.E. An Analytical Method to Determine Tortuosity in Rechargeable Battery Electrodes. J. Electrochem. Soc. 2012, 159, A548–A552. [Google Scholar] [CrossRef]
- Harris, S.; Lu, P. Effects of Inhomogeneities—Nanoscale to Mesoscale—On the Durability of Li-Ion Batteries. J. Phys. Chem. C 2013, 117, 6481–6492. [Google Scholar] [CrossRef]
- Ebner, M.; Wood, V. Tool for Tortuosity Estimation in Lithium Ion Battery Porous Electrodes. J. Electrochem. Soc. 2015, 162, A3064–A3070. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Tao, Y.; Zheng, X.; Luo, J.; Kang, F.; Cheng, H.-M.; Yang, Q.-H. Ultra-thick graphene bulk supercapacitor electrodes for compact energy storage. Energy Environ. Sci. 2016, 9, 3135–3142. [Google Scholar] [CrossRef]
- Xiao, Q.; Gu, M.; Yang, H.; Li, B.; Zhang, C.; Liu, Y.; Liu, F.; Dai, F.; Yang, L.; Liu, Z.; et al. Inward lithium-ion breathing of hierarchically porous silicon anodes. Nat. Commun. 2015, 6, 8844. [Google Scholar] [CrossRef] [Green Version]
- Ramadesigan, V.; Methekar, R.N.; Latinwo, F.; Braatz, R.D.; Subramanian, V.R. Optimal Porosity Distribution for Minimized Ohmic Drop across a Porous Electrode. J. Electrochem. Soc. 2010, 157, A1328–A1334. [Google Scholar] [CrossRef]
- Golmon, S.; Maute, K.; Dunn, M.L. Multiscale design optimization of lithium ion batteries using adjoint sensitivity analysis. Int. J. Numer. Methods Eng. 2012, 92, 475–494. [Google Scholar] [CrossRef]
- Golmon, S.; Maute, K.; Dunn, M. A design optimization methodology for Li+ batteries. J. Power Sources 2014, 253, 239–250. [Google Scholar] [CrossRef]
- Qi, Y.; Jang, T.; Ramadesigan, V.; Schwartz, D.T.; Subramanian, V.R. Is There a Benefit in Employing Graded Electrodes for Lithium-Ion Batteries? J. Electrochem. Soc. 2017, 164, A3196–A3207. [Google Scholar] [CrossRef] [Green Version]
- Bitsch, B.; Gallasch, T.; Schroeder, M.; Börner, M.; Winter, M.; Willenbacher, N. Capillary suspensions as beneficial formulation concept for high energy density Li-ion battery electrodes. J. Power Sources 2016, 328, 114–123. [Google Scholar] [CrossRef]
- Wang, C.P.; Lopatin, S.D.; Bachrach, R.Z.; Sikha, G. Graded Electrode Technologies for High Energy Lithium-Ion Batteries. U.S. Patent 20110168550, 21 July 2011. [Google Scholar]
- Huang, C.; Dontigny, M.; Zaghib, K.; Grant, P.S. Low-tortuosity and graded lithium ion battery cathodes by ice templating. J. Mater. Chem. A 2019, 7, 21421–21431. [Google Scholar] [CrossRef] [Green Version]
- Kolosnitsyn, V.; Karaseva, E. Improvements relating to electrode structures in batteries. European Patent 2006010894, 02 February 2006. [Google Scholar]
- Huebsch, N.; Mooney, D.J. Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Yao, D.; Liang, H.; Yin, J.; Xia, Y.; Zuo, K.; Zeng, Y.-P. Ultra-thick wood biochar monoliths with hierarchically porous structure from cotton rose for electrochemical capacitor electrodes. Electrochim. Acta 2020, 352, 136452. [Google Scholar] [CrossRef]
- Liu, K.; Mo, R.; Dong, W.; Zhao, W.; Huang, F. Nature-derived, structure and function integrated ultra-thick carbon electrode for high-performance supercapacitors. J. Mater. Chem. A 2020, 8, 20072–20081. [Google Scholar] [CrossRef]
- Lv, Z.; Yue, M.; Ling, M.; Zhang, H.; Yan, J.; Zheng, Q.; Li, X. Controllable Design Coupled with Finite Element Analysis of Low-Tortuosity Electrode Architecture for Advanced Sodium-Ion Batteries with Ultra-High Mass Loading. Adv. Energy Mater. 2021, 11, 2003725. [Google Scholar] [CrossRef]
- Lu, L.L.; Lu, Y.Y.; Xiao, Z.J.; Zhang, T.W.; Zhou, F.; Ma, T.; Ni, Y.; Yao, H.B.; Yu, S.H.; Cui, Y. Wood-Inspired High-Performance Ultrathick Bulk Battery Electrodes. Adv. Mater. 2018, 30, e1706745. [Google Scholar] [CrossRef]
- Behr, S.; Amin, R.; Chiang, Y.M.; Tomsia, A.P. Highly structured, additive free lithium-ion cathodes by freeze-casting technology. Ceram. Forum Int. 2015, 92, E39–E43. [Google Scholar]
- Li, L.; Erb, R.M.; Wang, J.; Wang, J.; Chiang, Y.M. Fabrication of Low-Tortuosity Ultrahigh-Area-Capacity Battery Electrodes through Magnetic Alignment of Emulsion-Based Slurries. Adv. Energy Mater. 2018, 9, 1802472. [Google Scholar] [CrossRef]
- Sander, J.S.; Erb, R.M.; Li, L.; Gurijala, A.; Chiang, Y.M. High-performance battery electrodes via magnetic templating. Nat. Energy 2016, 1, 16099. [Google Scholar] [CrossRef]
- Billaud, J.; Bouville, F.; Magrini, T.; Villevieille, C.; Studart, A.R. Magnetically aligned graphite electrodes for high-rate performance Li-ion batteries. Nat. Energy 2016, 1, 16097. [Google Scholar] [CrossRef]
- Ma, J.; Qiao, Y.; Huang, M.; Shang, H.; Zhou, H.; Li, T.; Liu, W.; Qu, M.; Zhang, H.; Peng, G. Low tortuosity thick cathode design in high loading lithium sulfur batteries enabled by magnetic hollow carbon fibers. Appl. Surf. Sci. 2021, 542, 148664. [Google Scholar] [CrossRef]
- Pan, G.; Hu, L.; Zhang, F.; Chen, Q. Out-of-Plane Alignment of Conjugated Semiconducting Polymers by Horizontal Rotation in a High Magnetic Field. J. Phys. Chem. Lett. 2021, 12, 3476–3484. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Liu, C.; Cai, J.; Zhang, Y.; Jiang, Y.; Zhang, D. High-Efficiency Alignment of 3D Biotemplated Helices via Rotating Magnetic Field for Terahertz Chiral Metamaterials. Adv. Opt. Mater. 2019, 7, 1900247. [Google Scholar] [CrossRef]
- Chen, K.-H.; Namkoong, M.J.; Goel, V.; Yang, C.; Kazemiabnavi, S.; Mortuza, S.M.; Kazyak, E.; Mazumder, J.; Thornton, K.; Sakamoto, J.; et al. Efficient fast-charging of lithium-ion batteries enabled by laser-patterned three-dimensional graphite anode architectures. J. Power Sources 2020, 471, 228475. [Google Scholar] [CrossRef]
- Park, J.; Hyeon, S.; Jeong, S.; Kim, H.-J. Performance enhancement of Li-ion battery by laser structuring of thick electrode with low porosity. J. Ind. Eng. Chem. 2019, 70, 178–185. [Google Scholar] [CrossRef]
- Park, J.; Jeon, C.; Kim, W.; Bong, S.; Jeong, S.; Kim, H. Challenges, laser processing and electrochemical characteristics on application of ultra-thick electrode for high-energy lithium-ion battery. J. Power Sources 2021, 482, 228948. [Google Scholar] [CrossRef]
- Mangang, M.; Seifert, H.J.; Pfleging, W. Influence of laser pulse duration on the electrochemical performance of laser structured LiFePO4 composite electrodes. J. Power Sources 2016, 304, 24–32. [Google Scholar] [CrossRef]
- Pfleging, W.; Pröll, J. A new approach for rapid electrolyte wetting in tape cast electrodes for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14918–14926. [Google Scholar] [CrossRef]
- Zhu, P.; Seifert, H.J.; Pfleging, W. The Ultrafast Laser Ablation of Li(Ni0.6Mn0.2Co0.2)O2 Electrodes with High Mass Loading. Appl. Sci. 2019, 9, 4067. [Google Scholar] [CrossRef] [Green Version]
- Mottay, E.; Liu, X.; Zhang, H.; Mazur, E.; Sanatinia, R.; Pfleging, W. Industrial applications of ultrafast laser processing. MRS Bull. 2016, 41, 984–992. [Google Scholar] [CrossRef]
- Habedank, J.B.; Kraft, L.; Rheinfeld, A.; Krezdorn, C.; Jossen, A.; Zaeh, M.F. Increasing the Discharge Rate Capability of Lithium-Ion Cells with Laser-Structured Graphite Anodes: Modeling and Simulation. J. Electrochem. Soc. 2018, 165, A1563–A1573. [Google Scholar] [CrossRef]
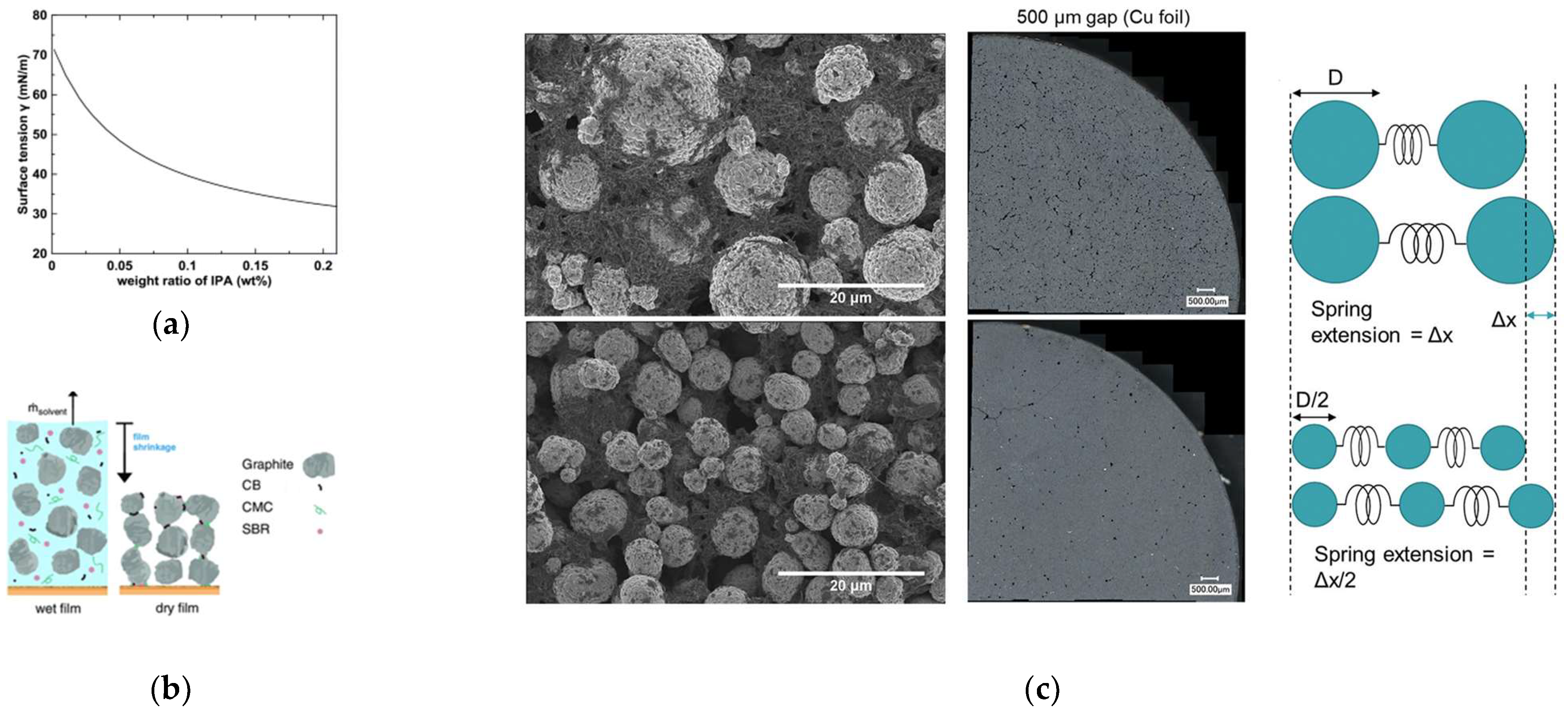

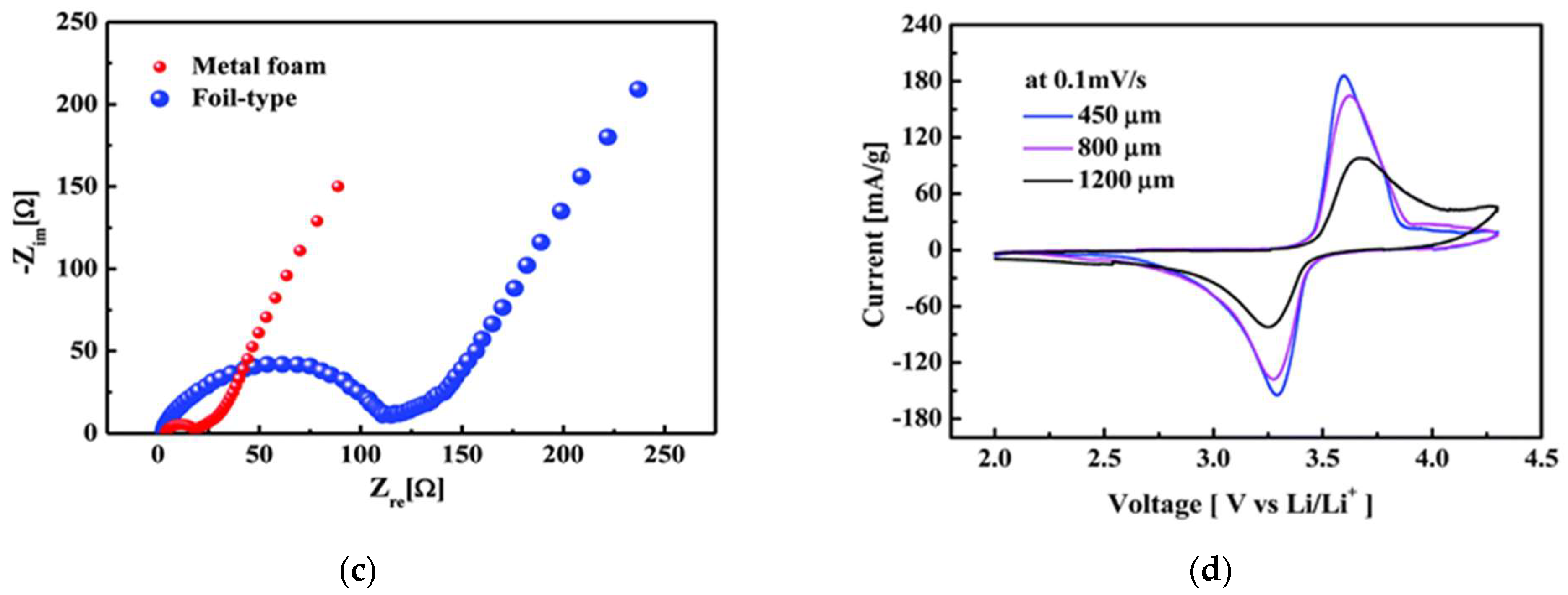
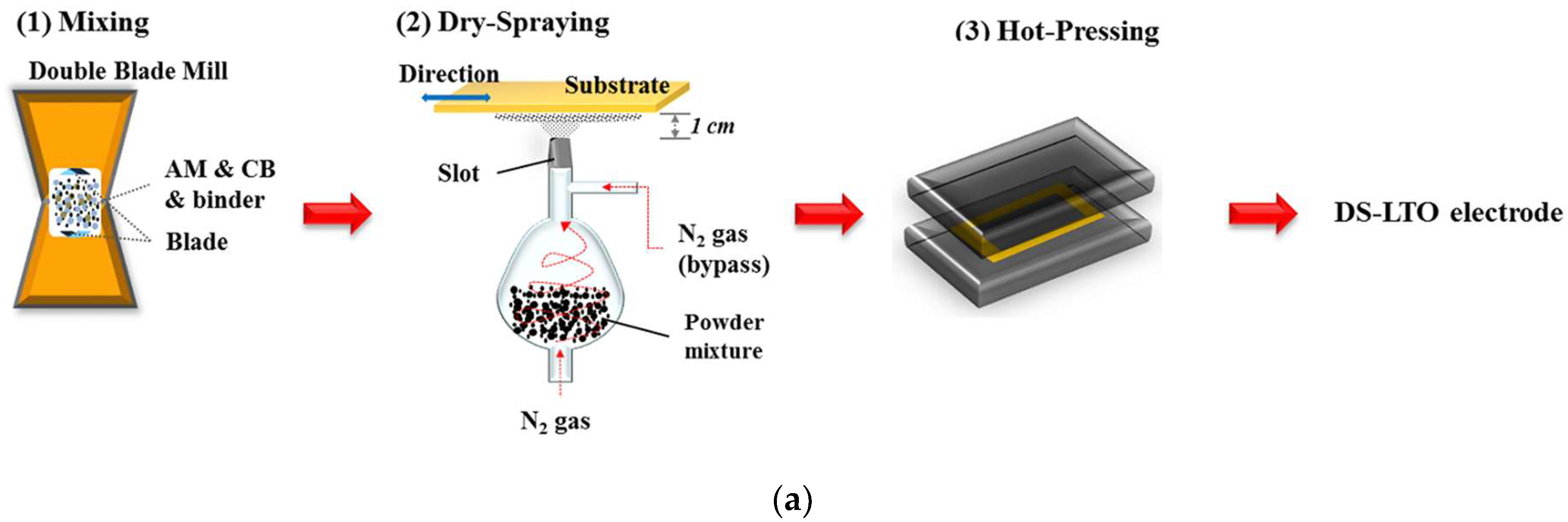
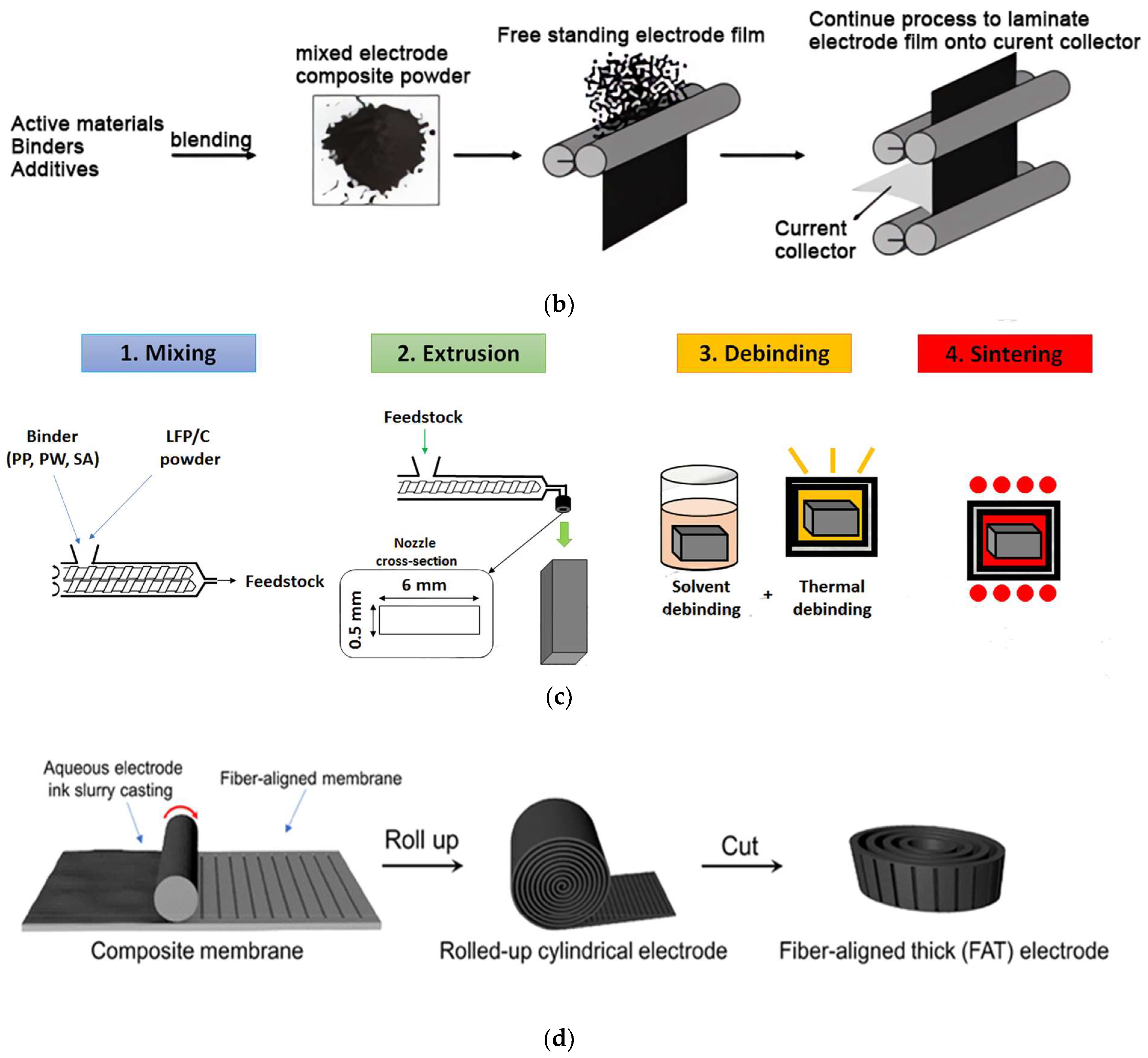

| Active Materials | Thickness/μm | Mass Loading/mg·cm−2 | Areal Capacity/ mAh·cm−2@mA·cm−2 | Volumetric Capacity/mAh·cm−3 | Reference |
|---|---|---|---|---|---|
| NMC622 | 154 | 37.6 | 6.58@0.38 | 427.3 | [2] |
| Graphite | 182 | 23.4 | 7.84@0.82 | 430.8 | |
| LFP | 1000 | 128 | 19.6@1 | 196 | [9] |
| Graphite | 1200 | 50 | 17.25@0.93 | 143.8 | [15] |
| NMC532 | 240 | 30 | 5.84@1.15 | 228.3 | [30] |
| NMC111 | 320 | 72 | 9.86@1.12 | 308.3 | [33] |
| Graphite | 320 | 43 | 11.23@1.62 | 352.1 | |
| LCO | 600 | 115.4 | 15.7@0.5 | 261.7 | [34] |
| NMC111 | 322 | 60 | 5.1@1.8 | 158.4 | [44] |
| NMC811 | 740 | 155 | 29@1.47 | 391.9 | [46] |
| Si | 210 | 15 | 45@1.79 | 2142 | |
| LFP | 800 | 60 | 5.7@1 | 71.3 | [52] |
| LFP/C | 240 | 12 | 1.86@1.02 | 77.5 | [54] |
| LFP | 430 | 46.5 | 7.2@1 | 167.4 | [56] |
| LTO | 600 | 168 | 26.5@1.68 | 441.7 | [57] |
| LTO | ~1500 | 30 | 4.74@1.06 | 31.6 | [62] |
| LTO | 475 | 138 | 15.2@1.02 | 319 | [66] |
| LTO | 550 | 110 | 11.11@1.6 | 202 | [68] |
| LFP | 500 | 90 | 11.07@1.23 | 221.4 | |
| Graphite | 240 | 16.5 | 3.79@1.23 | 158.1 | [84] |
| LCO | 1500 | 206 | 24.5@1.44 | 163.8 | [92] |
| NCA | 600 | 73.8 | 13@1.48 | 216.7 | [93] |
| LCO | 440 | 100.5 | 13.6@1.41 | 309.1 | [94] |
| S | 300 | 6 | 6.9@1 | 230 | [97] |
| LCO | 700 | 172 | 20.1@1.21 | 287.1 | [102] |
| NMC622 | 250 | 51.7 | 8.79@0.93 | 351.6 | [105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Xing, G.; Jin, L.; Lu, Y.; Qin, N.; Gao, S.; Zheng, J.P. Strategies and Challenge of Thick Electrodes for Energy Storage: A Review. Batteries 2023, 9, 151. https://doi.org/10.3390/batteries9030151
Zheng J, Xing G, Jin L, Lu Y, Qin N, Gao S, Zheng JP. Strategies and Challenge of Thick Electrodes for Energy Storage: A Review. Batteries. 2023; 9(3):151. https://doi.org/10.3390/batteries9030151
Chicago/Turabian StyleZheng, Junsheng, Guangguang Xing, Liming Jin, Yanyan Lu, Nan Qin, Shansong Gao, and Jim P. Zheng. 2023. "Strategies and Challenge of Thick Electrodes for Energy Storage: A Review" Batteries 9, no. 3: 151. https://doi.org/10.3390/batteries9030151
APA StyleZheng, J., Xing, G., Jin, L., Lu, Y., Qin, N., Gao, S., & Zheng, J. P. (2023). Strategies and Challenge of Thick Electrodes for Energy Storage: A Review. Batteries, 9(3), 151. https://doi.org/10.3390/batteries9030151









