Advanced Metal-Organic Frameworks Based on Anthraquinone-2,3-Dicarboxylate Ligands as Cathode for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Anthraquinone 2,3-Dicarboxylate Zinc (or Manganese) Coordination Polymer (ZnAQDC, or MnAQDC)
2.2. Material Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Structural Characterization of MnAQDC and ZnAQDC
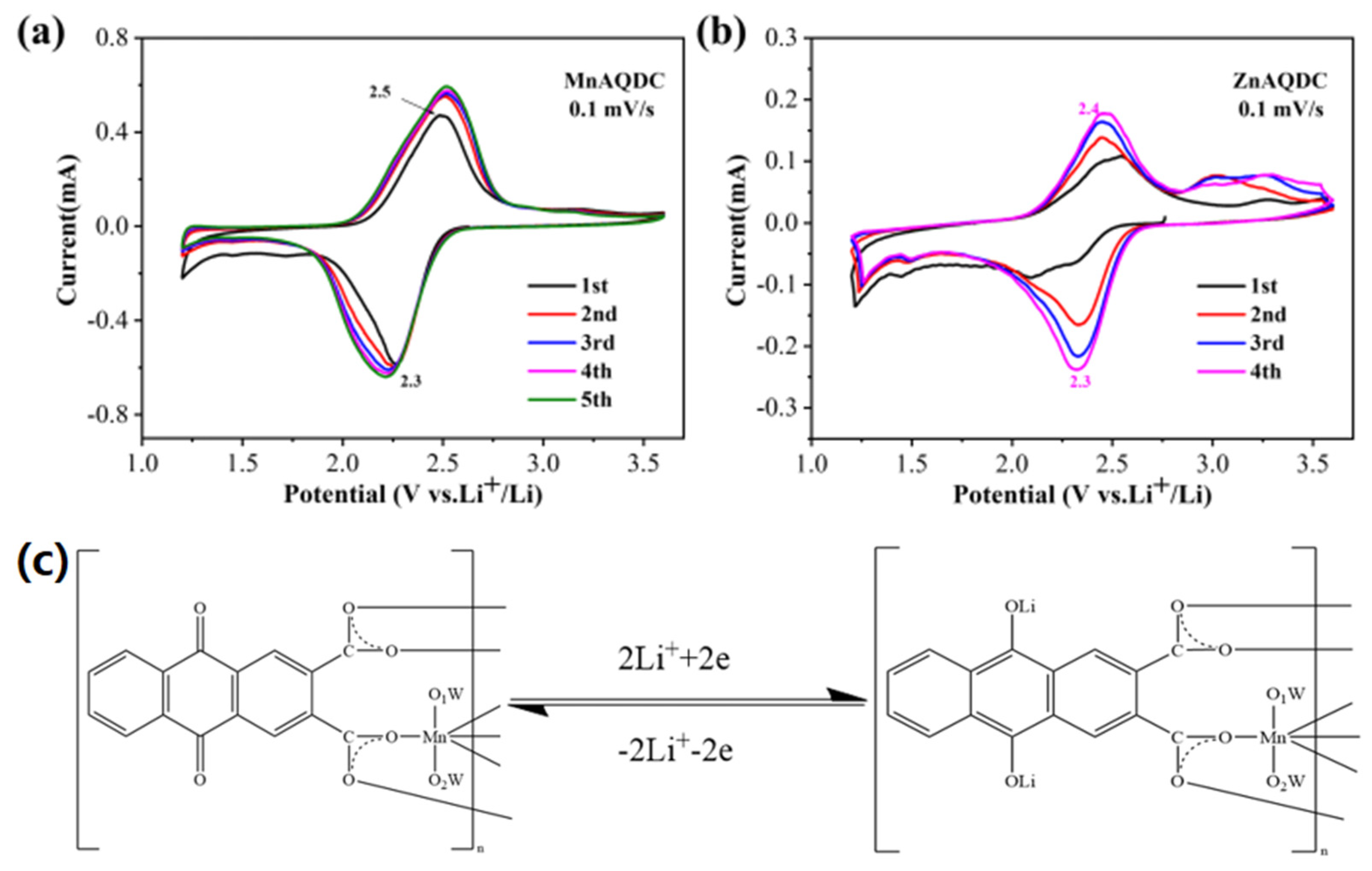
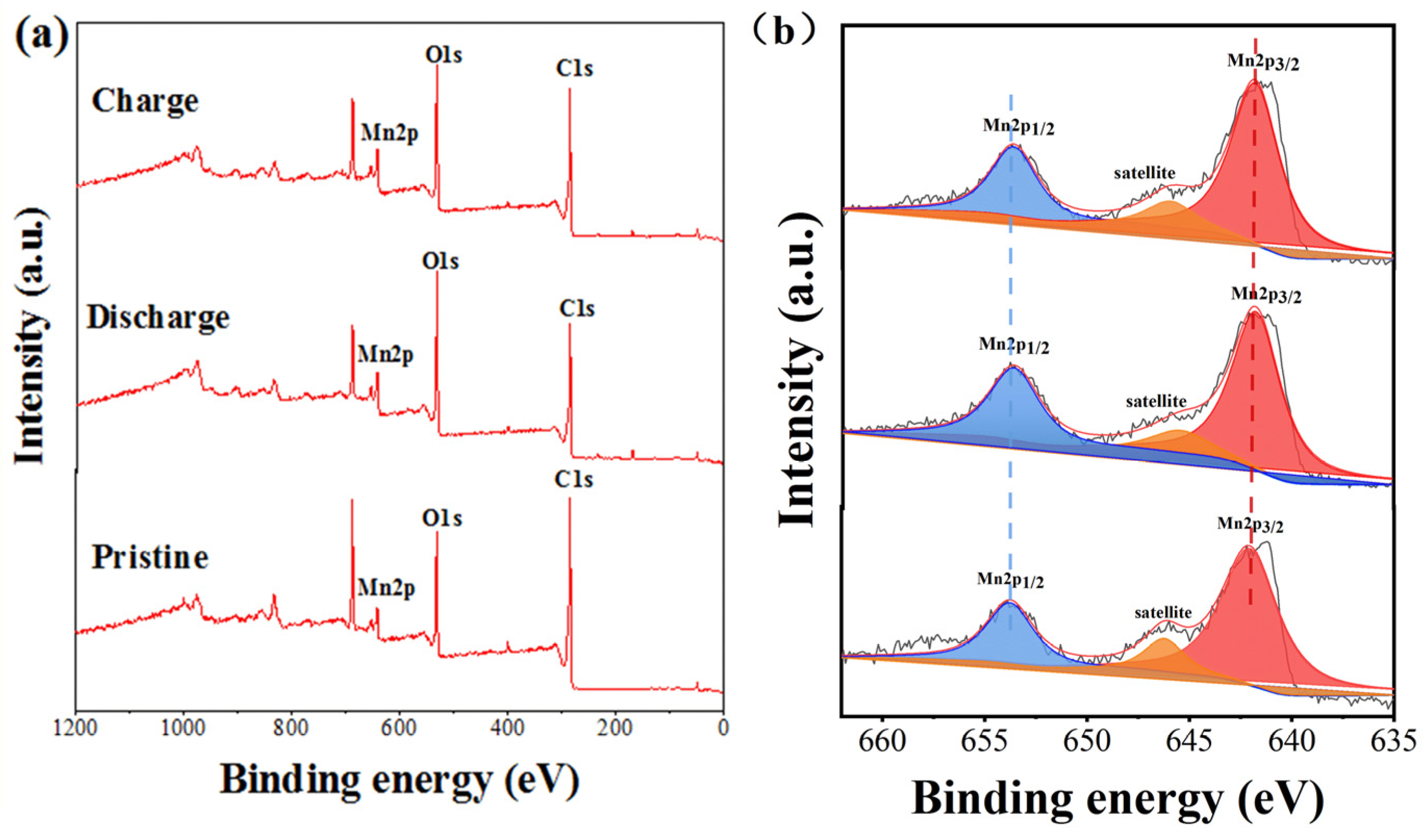
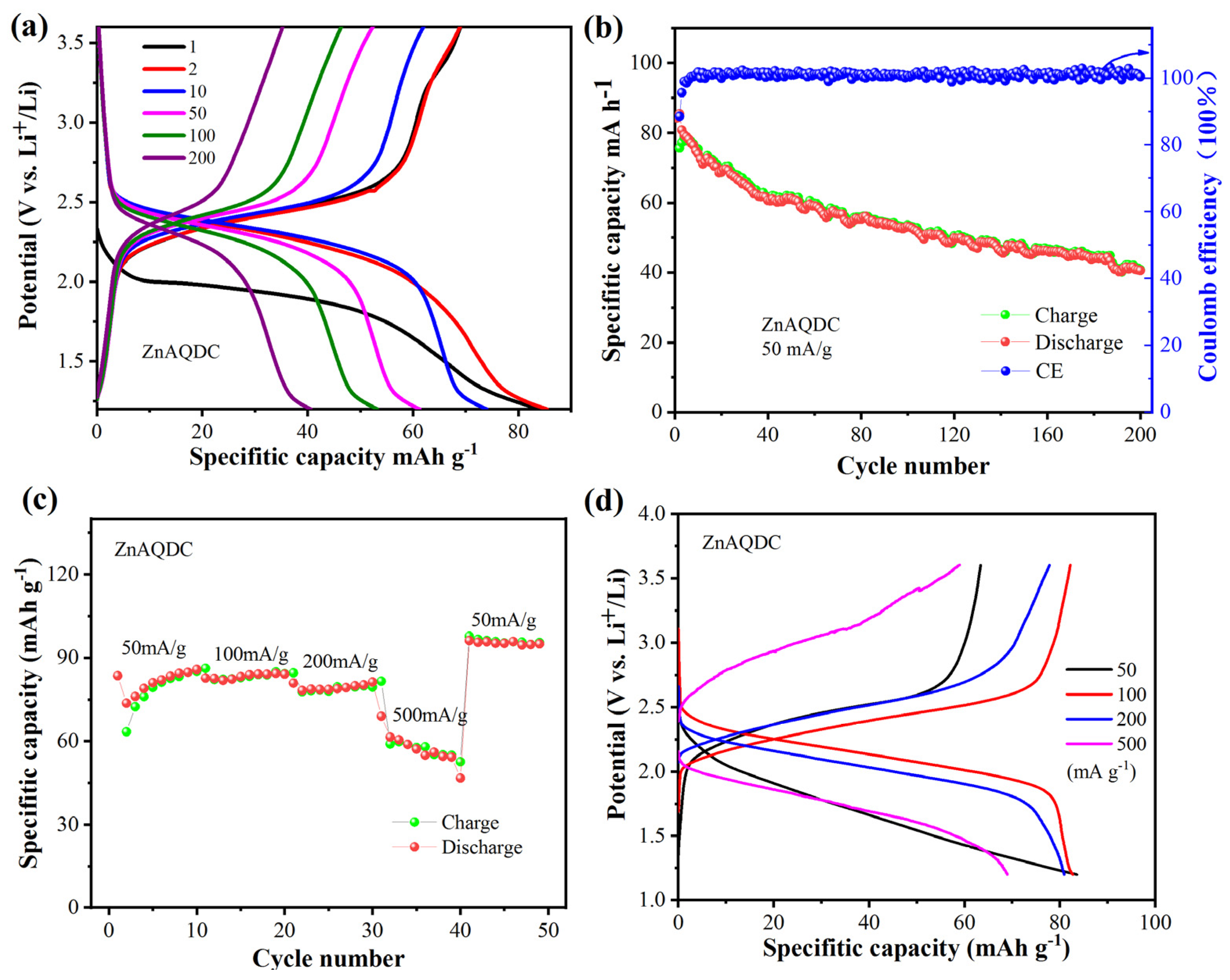
3.2. Electrochemical Performance of MnAQDC and ZnAQDC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, M.J.; Han, J.; Lee, K.; Lee, Y.J.; Kim, B.G.; Jung, K.-N.; Kim, B.J.; Lee, S.W. Elastomeric Electrolytes for High-Energy Solid-State Lithium Batteries. Nature 2022, 601, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-L.; Zhao, X.-X.; Li, W.-H.; Liang, H.-J.; Gu, Z.-Y.; Liu, Y.; Du, M.; Wu, X.-L. Advanced Cathode for Dual-ion Batteries: Waste-to-Wealth Reuse of Spent Graphite from Lithium-Ion Batteries. eScience 2022, 2, 95–101. [Google Scholar] [CrossRef]
- Huang, T.; Gao, H.; Chen, J.; Liu, H.; Wu, D.; Wang, G. A Book-Like Organic Based Electrode with High Areal Capacity for High Performance Flexible Lithium/Sodium-Ion Batteries. Chem. Commun. 2022, 58, 10158–10161. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Dai, H.; Zhu, S.; Wu, Y.; Sun, M.; Chen, Y.; Fan, K.; Zhang, C.; Wang, C. A Branched Dihydrophenazine-Based Polymer as a Cathode Material to Achieve Dual-Ion Batteries with High Energy and Power Density. eScience 2021, 12, 60–68. [Google Scholar] [CrossRef]
- Khetan, A. High-Throughput Virtual Screening of Quinones for Aqueous Redox Flow Batteries: Status and Perspectives. Batteries 2023, 9, 24. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Kosaka, W.; Yamagishi, K.; Hori, A.; Sato, H.; Matsuda, R.; Kitagawa, S.; Takata, M.; Miyasaka, H. Selective NO Trapping in the Pores of Chain-Type Complex Assemblies Based on Electronically Activated Paddlewheel-Type [Ru2II,II]/[Rh2II,II] Dimers. J. Am. Chem. Soc. 2013, 135, 18469–18480. [Google Scholar] [CrossRef]
- Inokuma, Y.; Yoshioka, S.; Ariyoshi, J.; Arai, T.; Hitora, Y.; Takada, K.; Matsunaga, S.; Rissanen, K.; Fujita, M. X-ray Analysis on the Nanogram to Microgram Scale Using Porous Complexes. Nature 2013, 495, 461–466. [Google Scholar] [CrossRef]
- Cho, S.-H.; Ma, B.; Nguyen, S.B.T.; Hupp, J.T.; Albrecht-Schmitt, T.E. a Metal–Organic Framework Material That Functions as an Enantioselective Catalyst for Olefin Epoxidation. Chem. Commun. 2006, 24, 2563–2565. [Google Scholar] [CrossRef]
- Li, X.; Cheng, F.; Zhang, S.; Chen, J. Shape-Controlled Synthesis and Lithium-Storage Study of Metal-Organic Frameworks Zn4O(1,3,5-benzenetribenzoate)2. J. Power Sources 2006, 160, 542–547. [Google Scholar] [CrossRef]
- Combarieu, G.D.; Morcrette, M.; Millange, F.; Guillou, N.; Cabana, J.; Grey, C.; Margiolaki, I.; Férey, G.; Tarascon, J. Influence of the Benzoquinone Sorption on the Structure and Electrochemical Performance of the MIL-53(Fe) Hybrid Porous Material in a Lithium-Ion Battery. Chem. Mater. 2009, 21, 1602–1611. [Google Scholar] [CrossRef]
- Reddy, R.C.K.; Lin, J.; Chen, Y.; Zeng, C.; Lin, X.; Cai, Y.; Su, C.-Y. Progress of Nanostructured Metal Oxides Derived from Metal–Organic Frameworks as Anode Materials for Lithium–Ion Batteries. Coord. Chem. Rev. 2020, 420, 213434. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-Organic Framework Functionalization and Design Strategies for Advanced Electrochemical Energy Storage Devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Batteries. Joule 2018, 2, 2235–2259. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, C.; Zeng, R.; Liu, Y.; Zhang, W.; Wang, Y.; Liu, Q.; Huang, Y. In Situ-Formed Hierarchical Metal–Organic Flexible Cathode for High-Energy Sodium-Ion Batteries. ChemSusChem 2017, 10, 4704–4708. [Google Scholar] [CrossRef]
- An, T.; Wang, Y.; Tang, J.; Wang, Y.; Zhang, L.; Zheng, G. a Flexible Ligand-Based Wavy Layered Metal–Organic Framework for Lithium-Ion Storage. J. Colloid Interface Sci. 2015, 445, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cheng, F.; Shi, W.; Chen, J.; Cheng, P. Transition-Metal-Triggered High-Efficiency Lithium Ion Storage via Coordination Interactions with Redox-Active Croconate in One-Dimensional Metal–Organic Anode Materials. ACS Appl. Mater. Interfaces 2018, 10, 6398–6406. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, M.; Luo, Y.; Pang, B.; Su, X.; Zhou, M.; Han, L. Redox Active Azo-Based Metal–Organic Frameworks as Anode Materials for Lithium-Ion Batteries. New J. Chem. 2019, 43, 1710–1715. [Google Scholar] [CrossRef]
- Huang, Q.; Wei, T.; Zhang, M.; Dong, L.-Z.; Zhang, A.-M.; Li, S.-L.; Liu, W.-J.; Liu, J.; Lan, Y.-Q. A Highly Stable Polyoxometalate-Based Metal–Organic Framework with π–π Stacking for Enhancing Lithium Ion Battery Performance. J. Mater. Chem. A 2017, 5, 8477–8483. [Google Scholar] [CrossRef]
- Sun, L.; Xie, J.; Chen, Z.; Wu, J.; Li, L. Reversible Lithium Storage In a Porphyrin-Based MOF (PCN-600) with Exceptionally High Capacity and Stability. Dalton Trans. 2018, 47, 9989–9993. [Google Scholar] [CrossRef]
- Wang, P.; Lou, X.; Li, C.; Hu, X.; Yang, Q.; Hu, B. One-Pot Synthesis of Co-Based Coordination Polymer Nanowire for Li-Ion Batteries with Great Capacity and Stable Cycling Stability. Nanomicro Lett. 2017, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Maiti, S.; Pramanik, A.; Manju, U.; Mahanty, S. Reversible Lithium Storage in Manganese 1,3,5-Benzenetricarboxylate Metal-Organic Framework with High Capacity and Rate Performance. ACS Appl. Mater. Interfaces 2015, 7, 16357–16363. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Shen, L.; Wang, J.; Wang, C. Reversible Lithiation–Delithiation Chemistry in Cobalt Based Metal Organic Framework Nanowire Electrode Engineering for Advanced Lithium-Ion Batteries. J. Mater. Chem. A 2016, 4, 15411–15419. [Google Scholar] [CrossRef]
- Gan, Q.; He, H.; Zhao, K.; He, Z.; Liu, S. Morphology-Dependent Electrochemical Performance of Ni-1,3,5-Benzenetricarboxylate Metal-Organic Frameworks as an Anode Material for Li-ion Batteries. J. Colloid Interface Sci. 2018, 530, 127–136. [Google Scholar] [CrossRef]
- Meng, C.; Chen, T.; Fang, C.; Huang, Y.; Hu, P.; Tong, Y.; Bian, T.; Zhang, J.; Wang, Z.; Yuan, A. Multiple Active Sites: Lithium Storage Mechanism of Cu-TCNQ as an Anode Material for Lithium-Ion Batteries. Chem. Asian J. 2019, 14, 4289–4295. [Google Scholar] [CrossRef]
- Jiang, Q.; Xiong, P.; Liu, J.; Xie, Z.; Wang, Q.; Yang, X.-Q.; Hu, E.; Cao, Y.; Sun, J.; Xu, Y.; et al. a Redox-Active 2D Metal–Organic Framework for Efficient Lithium Storage with Extraordinary High Capacity. Angew. Chem. Int. Ed. 2020, 59, 5273–5277. [Google Scholar] [CrossRef]
- Zhang, Z.; Yoshikawa, H.; Awaga, K. Monitoring the solid-state electrochemistry of Cu(2,7-AQDC) (AQDC = anthraquinone dicarboxylate) in a lithium battery: Coexistence of metal and ligand redox activities in a metal-organic framework. J. Am. Chem. Soc. 2014, 136, 16112–16115. [Google Scholar] [CrossRef]
- Zhang, Z.; Yoshikawa, H.; Awaga, K. Discovery of a “Bipolar Charging” Mechanism in the Solid-State Electrochemical Process of a Flexible Metal–Organic Framework. Chem. Mater. 2016, 28, 1298–1303. [Google Scholar] [CrossRef]
- Wada, K.; Sakaushi, K.; Sasaki, S.; Nishihara, H. Multielectron-Transfer-Based Rechargeable Energy Storage of Two-Dimensional Coordination Frameworks with Non-Innocent Ligands. Angew. Chem. Int. Ed. 2018, 57, 8886–8890. [Google Scholar] [CrossRef]
- Gu, S.; Bai, Z.; Majumder, S.; Huang, B.; Chen, G. Conductive Metal–Organic Framework with Redox Metal Center as Cathode for High Rate Performance Lithium Ion Battery. J. Power Sources 2019, 429, 22–29. [Google Scholar] [CrossRef]
- Shin, J.; Kim, M.; Cirera, J.; Chen, S.; Halder, G.J.; Yersak, T.A.; Paesani, F.; Cohen, S.M.; Meng, Y.S. MIL-101(Fe) as a Lithium-ion Battery Electrode Material: Relaxation and Intercalation Mechanism During Lithium Insertion. J. Mater. Chem. A 2015, 3, 4738–4744. [Google Scholar] [CrossRef]
- Férey, G.; Millange, F.; Morcrette, M.; Serre, C.; Doublet, M.-L.; Grenèche, J.-M.; Tarascon, J.-M. Mixed-Valence Li/Fe-Based Metal-Organic Frameworks with Both Reversible Redox and Sorption Properties. Angew. Chem. Int. Ed. 2007, 119, 3323–3327. [Google Scholar] [CrossRef]
- Tian, B.; Ning, G.H.; Gao, Q.; Tan, L.M.; Tang, W.; Chen, Z.; Su, C.; Loh, K.P. Crystal Engineering of Naphthalenediimide-Based Metal-Organic Frameworks: Structure-Dependent Lithium Storage. ACS Appl. Mater. Interfaces 2016, 8, 31067–31075. [Google Scholar] [CrossRef]
- Fateeva, A.; Horcajada, P.; Devic, T.; Serre, C.; Marrot, J.; Grenèche, J.M.; Morcrette, M.; Tarascon, J.M.; Maurin, G.; Férey, G. Synthesis, Structure, Characterization, and Redox Properties of the Porous Mil-68(Fe) Solid. Eur. J. Inorg. Chem. 2010, 2010, 3789–3794. [Google Scholar] [CrossRef]
- Yamada, T.; Shiraishi, K.; Kitagawa, H.; Kimizuka, N. Applicability of Mil-101(Fe) as a Cathode of Lithium Ion Batteries. Chem. Commun. 2017, 53, 8215–8218. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Yi, X.; Liu, Z.; Shang, J.; Wang, D. Triphenylamine-Based Metal-Organic Frameworks as Cathode Materials in Lithium-Ion Batteries with Coexistence of Redox Active Sites, High Working Voltage, and High Rate Stability. ACS Appl. Mater. Interfaces 2016, 8, 14578–14585. [Google Scholar] [CrossRef] [PubMed]
- Furman, J.D.; Burwood, R.P.; Tang, M.; Mikhailovsky, A.A.; Cheetham, A.K. Understanding Ligand-Centred Photoluminescence through Flexibility and Bonding of Anthraquinone Inorganic–Organic Frameworks. J. Mater. Chem. 2011, 21, 6595–6601. [Google Scholar] [CrossRef]
- Bruker AXS Inc. APEXII Software, Version 6.12; Bruker AXS Inc.: Madison, WI, USA, 2004. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for X-ray Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Maiti, S.; Pramanik, A.; Dhawa, T.; Sreemany, M.; Mahanty, S. Bi-Metal Organic Framework Derived Nickel Manganese Oxide Spinel for Lithium-Ion Battery Anode. Mater. Sci. Eng. B 2018, 229, 27–36. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Hu, X.; Tian, H.; Zhang, Y.; Yang, X. Realization of Ti Doping by Electrostatic Assembly to Improve the Stability of LiCoO2 Cycled to 4.5 V. J. Electrochem. Soc. 2019, 166, A1793–A1798. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Reissig, F.; Frankenstein, L.; Heidbüchel, M.; Winter, M.; Placke, T.; Schmuch, R. Magnesium Substitution in Ni-Rich NMC Layered Cathodes for High-Energy Lithium Ion Batteries. Adv. Energy Mater. 2022, 12, 2103045. [Google Scholar] [CrossRef]
- Zhao, M.; Fu, Y.; Xu, N.; Li, G.; Wu, M.; Gao, X.J. High Performance LiMnPO4/C Prepared by a Crystallite Size Control Method. J. Mater. Chem. A 2014, 2, 15070–15077. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, Y.; Liu, Y.; Luo, C.; Wang, C. Comparison of Electrochemical Performances of Olivine Nafepo4 in Sodium-Ion Batteries And Olivine Lifepo4 In Lithium-Ion Batteries. Nanoscale 2013, 5, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Pagot, G.; Bandiera, M.; Vezzù, K.; Migliori, A.; Bertoncello, R.; Negro, E.; Morandi, V.; Di Noto, V. High Valence Transition Metal-Doped Olivine Cathodes for Superior Energy and Fast Cycling Lithium Batteries. J. Mater. Chem. A 2020, 8, 25727–25738. [Google Scholar] [CrossRef]
- Amine, K. Olivine LiCoPO4 as 4.8 V Electrode Material for Lithium Batteries. Electrochem. Solid-State Lett. 1999, 3, 178. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, M.; Zhang, D.; Chen, F.; Lin, X.; Qiu, A.; Lei, C.; Liang, J.; Liang, J.; Li, J.; Wang, Q.; et al. Advanced Metal-Organic Frameworks Based on Anthraquinone-2,3-Dicarboxylate Ligands as Cathode for Lithium-Ion Batteries. Batteries 2023, 9, 247. https://doi.org/10.3390/batteries9050247
Lai M, Zhang D, Chen F, Lin X, Qiu A, Lei C, Liang J, Liang J, Li J, Wang Q, et al. Advanced Metal-Organic Frameworks Based on Anthraquinone-2,3-Dicarboxylate Ligands as Cathode for Lithium-Ion Batteries. Batteries. 2023; 9(5):247. https://doi.org/10.3390/batteries9050247
Chicago/Turabian StyleLai, Minjie, Dongying Zhang, Fenghua Chen, Xiaoying Lin, Ankun Qiu, Chenxi Lei, Jiaying Liang, Junfeng Liang, Jianhui Li, Qunfang Wang, and et al. 2023. "Advanced Metal-Organic Frameworks Based on Anthraquinone-2,3-Dicarboxylate Ligands as Cathode for Lithium-Ion Batteries" Batteries 9, no. 5: 247. https://doi.org/10.3390/batteries9050247




