Effect of Water-Soluble CMC/SBR Binder Ratios on Si-rGO Composites Using µm- and nm-Sized Silicon as Anode Materials for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of prGO and rGO
2.2. Synthesis of Si-rGO Composite Material and Milled rGO
2.3. Composite Electrode Preparation
2.4. Cell Fabrication
2.5. Electrochemical Testing
2.6. Analytics
3. Results and Discussion
3.1. Characterization of GO, prGO, and rGO
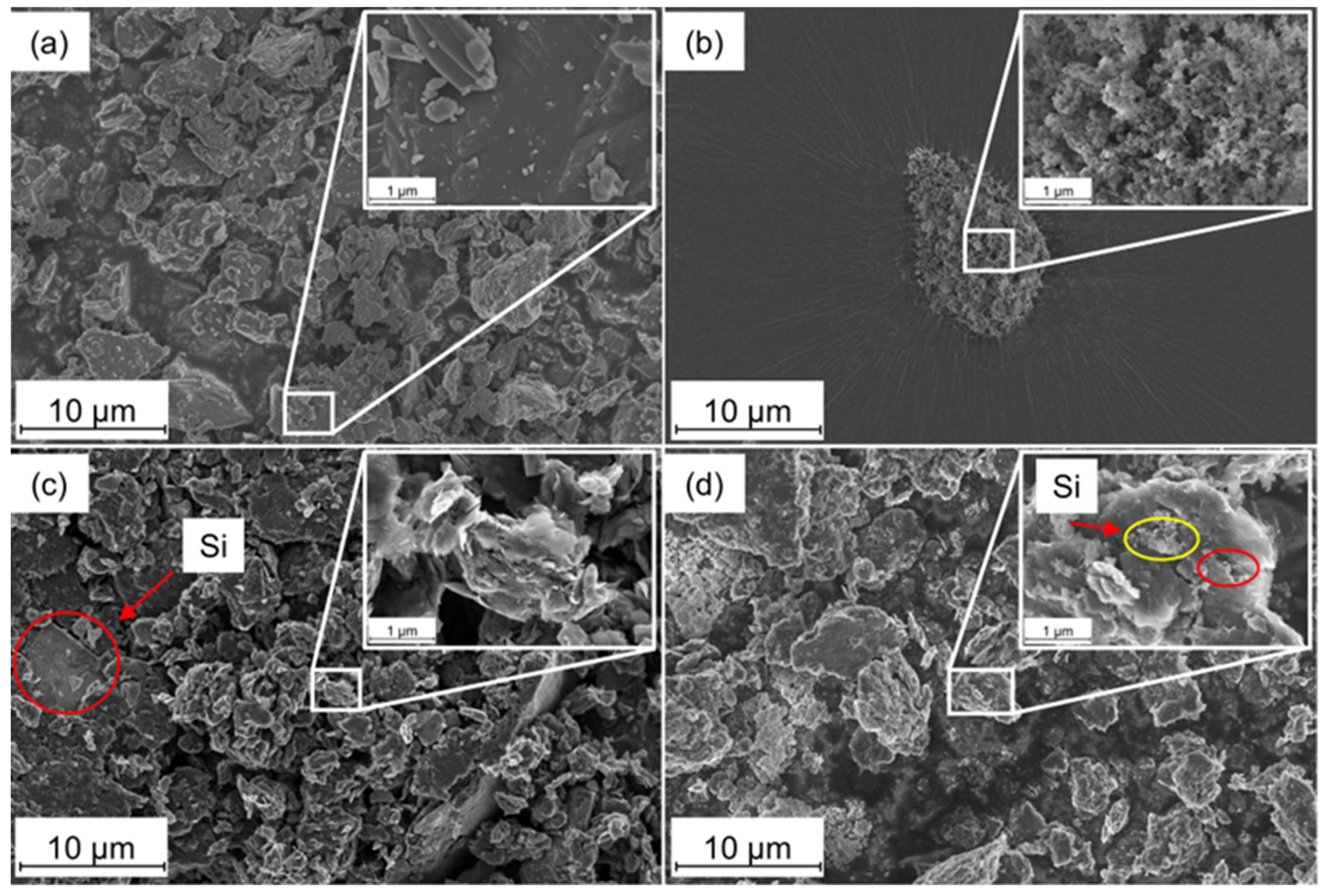
3.2. Characterization of the Si-rGO Composite Material
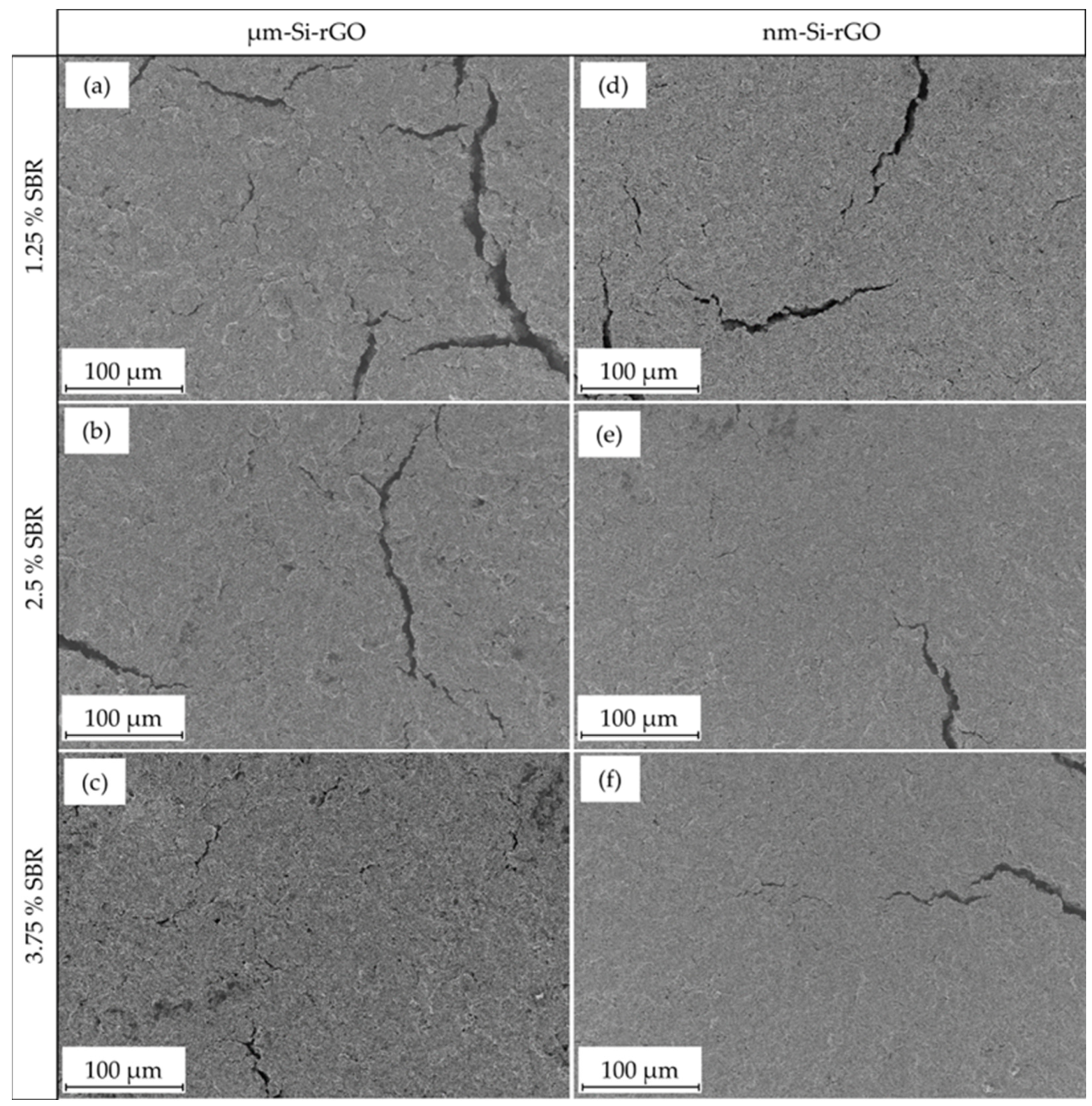
3.3. Characterization of Si-rGO Composite Electrodes with Variations in CMC-/SBR-Binder Content
3.4. Electrochemical Performance
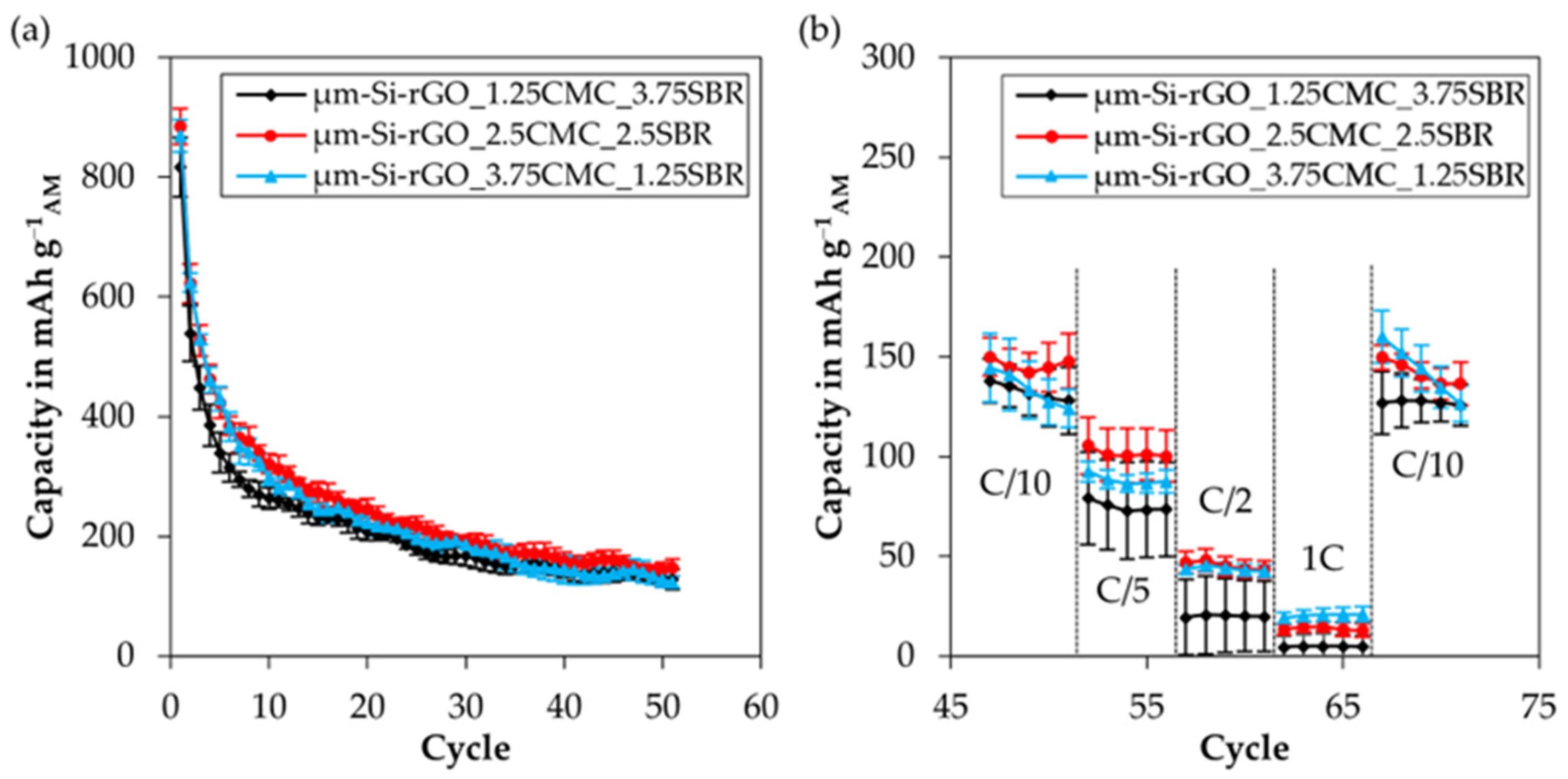
3.4.1. Influence of Binder Composition on Electrode Performance for µm-Si-rGO as an Active Material
3.4.2. Influence of Binder Composition on Electrode Performance for nm-Si-rGO as an Active Material
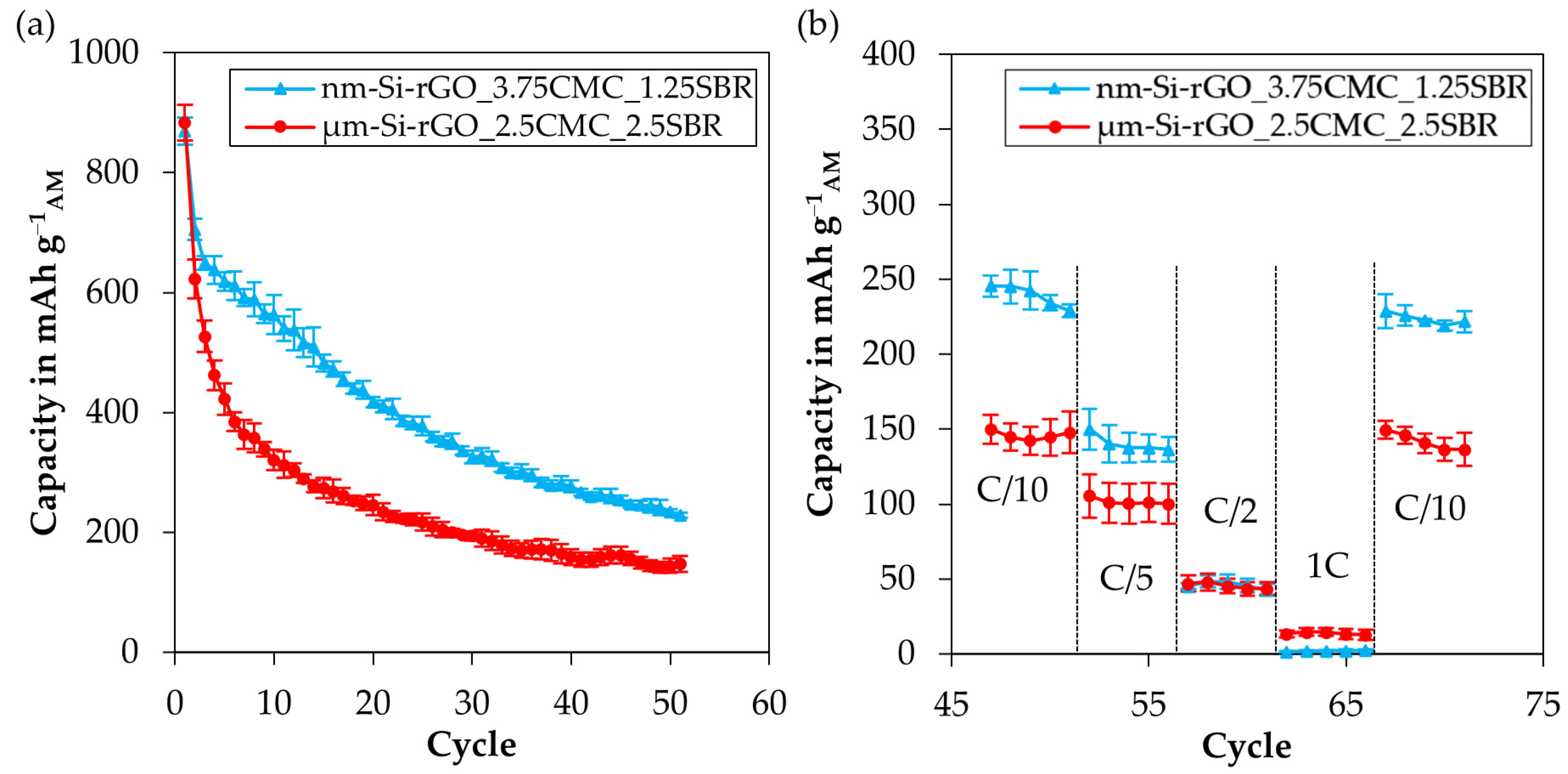
3.4.3. Influence of the Silicon Particle Size on the Electrode Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obrovac, M.N.; Christensen, L. Structural Changes in Silicon Anodes during Lithium Insertion/Extraction. Electrochem. Solid-State Lett. 2004, 7, A93. [Google Scholar] [CrossRef]
- Cui, Y. Silicon anodes. Nat. Energy 2021, 6, 995–996. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Yim, C.-H.; Karkar, Z.; Abu-Lebdeh, Y. On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review. Batteries 2022, 8, 70. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotech. 2014, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.-W.; Choi, J.W.; Coskun, A. The emerging era of supramolecular polymeric binders in silicon anodes. Chem. Soc. Rev. 2018, 47, 2145–2164. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-dependent fracture of silicon nanoparticles during lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef]
- Müllner, S.; Held, T.; Schmidt-Rodenkirchen, A.; Gerdes, T.; Roth, C. Reactive Spray Drying as a One-Step Synthesis Approach towards Si/rGO Anode Materials for Lithium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 120545. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Qiu, X.; Ma, Y.; Kong, D.; Müllen, K.; Li, X.; Zhi, L. Stable high-capacity and high-rate silicon-based lithium battery anodes upon two-dimensional covalent encapsulation. Nat. Commun. 2020, 11, 3826. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhao, X.; Wu, J.; Jang, H.D.; Kung, H.H.; Huang, J. Crumpled Graphene-Encapsulated Si Nanoparticles for Lithium Ion Battery Anodes. J. Phys. Chem. Lett. 2012, 3, 1824–1829. [Google Scholar] [CrossRef]
- Deng, L.; Zheng, Y.; Zheng, X.; Or, T.; Ma, Q.; Qian, L.; Deng, Y.; Yu, A.; Li, J.; Chen, Z. Design Criteria for Silicon-Based Anode Binders in Half and Full Cells. Adv. Energy Mater. 2022, 12, 2200850. [Google Scholar] [CrossRef]
- Chen, H.; Ling, M.; Hencz, L.; Ling, H.Y.; Li, G.; Lin, Z.; Liu, G.; Zhang, S. Exploring Chemical, Mechanical, and Electrical Functionalities of Binders for Advanced Energy-Storage Devices. Chem. Rev. 2018, 118, 8936–8982. [Google Scholar] [CrossRef] [PubMed]
- Jeschull, F.; Brandell, D.; Wohlfahrt-Mehrens, M.; Memm, M. Water-Soluble Binders for Lithium-Ion Battery Graphite Electrodes: Slurry Rheology, Coating Adhesion, and Electrochemical Performance. Energy Technol. 2017, 5, 2108–2118. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Chen, Y.; Zheng, P.; Yi, H.; Deng, Y.; Yang, Y.; Wang, C. Reversible cross-linked phosphorylate binder for recyclable lithium-sulfur batteries. Chem. Eng. J. 2023, 452, 139128. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, X.; Li, B.; Jin, M.; Shen, X.; Luo, Z.; Tian, Z.; Yuan, L.; Deng, J.; Dai, Z.; et al. Design of high-energy-dissipation, deformable binder for high-areal-capacity silicon anode in lithium-ion batteries. Chem. Eng. J. 2021, 420, 129991. [Google Scholar] [CrossRef]
- Ding, N.; Xu, J.; Yao, Y.; Wegner, G.; Lieberwirth, I.; Chen, C. Improvement of cyclability of Si as anode for Li-ion batteries. J. Power Sources 2009, 192, 644–651. [Google Scholar] [CrossRef]
- Lee, J.-H.; Paik, U.; Hackley, V.A.; Choi, Y.-M. Effect of Carboxymethyl Cellulose on Aqueous Processing of Natural Graphite Negative Electrodes and their Electrochemical Performance for Lithium Batteries. J. Electrochem. Soc. 2005, 152, A1763. [Google Scholar] [CrossRef]
- Buqa, H.; Holzapfel, M.; Krumeich, F.; Veit, C.; Novák, P. Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J. Power Sources 2006, 161, 617–622. [Google Scholar] [CrossRef]
- Liu, W.-R.; Yang, M.-H.; Wu, H.-C.; Chiao, S.M.; Wu, N.-L. Enhanced Cycle Life of Si Anode for Li-Ion Batteries by Using Modified Elastomeric Binder. Electrochem. Solid-State Lett. 2005, 8, A100. [Google Scholar] [CrossRef]
- Li, J.; Lewis, R.B.; Dahn, J.R. Sodium Carboxymethyl Cellulose: A Potential Binder for Si Negative Electrodes for Li-Ion Batteries. Electrochem. Solid-State Lett. 2007, 10, A17. [Google Scholar] [CrossRef]
- Hochgatterer, N.S.; Schweiger, M.R.; Koller, S.; Raimann, P.R.; Wöhrle, T.; Wurm, C.; Winter, M. Silicon/Graphite Composite Electrodes for High-Capacity Anodes: Influence of Binder Chemistry on Cycling Stability. Electrochem. Solid-State Lett. 2008, 11, A76. [Google Scholar] [CrossRef]
- Zhao, Y.-M.; Yue, F.-S.; Li, S.-C.; Zhang, Y.; Tian, Z.-R.; Xu, Q.; Xin, S.; Guo, Y.-G. Advances of polymer binders for silicon-based anodes in high energy density lithium-ion batteries. InfoMat 2021, 3, 460–501. [Google Scholar] [CrossRef]
- Wang, R.; Feng, L.; Yang, W.; Zhang, Y.; Zhang, Y.; Bai, W.; Liu, B.; Zhang, W.; Chuan, Y.; Zheng, Z.; et al. Effect of Different Binders on the Electrochemical Performance of Metal Oxide Anode for Lithium-Ion Batteries. Nanoscale Res. Lett. 2017, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Fitz, O.; Ingenhoven, S.; Bischoff, C.; Gentischer, H.; Birke, K.P.; Saracsan, D.; Biro, D. Comparison of Aqueous- and Non-Aqueous-Based Binder Polymers and the Mixing Ratios for Zn//MnO2 Batteries with Mildly Acidic Aqueous Electrolytes. Batteries 2021, 7, 40. [Google Scholar] [CrossRef]
- Li, T.; Yang, J.; Lu, S. Effect of modified elastomeric binders on the electrochemical properties of silicon anodes for lithium-ion batteries. Int. J. Miner. Metall. Mater. 2012, 19, 752–756. [Google Scholar] [CrossRef]
- Zuo, P.; Yang, W.; Cheng, X.; Yin, G. Enhancement of the electrochemical performance of silicon/carbon composite material for lithium ion batteries. Ionics 2011, 17, 87–90. [Google Scholar] [CrossRef]
- Fang, C.; Xiao, H.; Zheng, T.; Bai, H.; Liu, G. Organic Solvent Free Process to Fabricate High Performance Silicon/Graphite Composite Anode. J. Compos. Sci. 2021, 5, 188. [Google Scholar] [CrossRef]
- Shu, Z.X.; McMillan, R.S.; Murray, J.J. Electrochemical Intercalation of Lithium into Graphite. J. Electrochem. Soc. 1993, 140, 922–927. [Google Scholar] [CrossRef]
- Klemeyer, L.; Park, H.; Huang, J. Geometry-Dependent Thermal Reduction of Graphene Oxide Solid. ACS Mater. Lett. 2021, 3, 511–515. [Google Scholar] [CrossRef]
- Chen, C.-M.; Huang, J.-Q.; Zhang, Q.; Gong, W.-Z.; Yang, Q.-H.; Wang, M.-Z.; Yang, Y.-G. Annealing a graphene oxide film to produce a free standing high conductive graphene film. Carbon 2012, 50, 659–667. [Google Scholar] [CrossRef]
- Silva, K.K.H.d.; Huang, H.-H.; Joshi, R.; Yoshimura, M. Restoration of the graphitic structure by defect repair during the thermal reduction of graphene oxide. Carbon 2020, 166, 74–90. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Braga, G.B.; Tarley, C.R.T.; Pereira, A.C. Thermally reduced graphene oxide: Synthesis, studies and characterization. J. Mater. Sci 2018, 53, 12005–12015. [Google Scholar] [CrossRef]
- Pan, Q.; Chung, C.-C.; He, N.; Jones, J.L.; Gao, W. Accelerated Thermal Decomposition of Graphene Oxide Films in Air via in Situ X-ray Diffraction Analysis. J. Phys. Chem. C 2016, 120, 14984–14990. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Han, J.; Bai, S.; Tan, J.; Liu, J.; Li, F. Challenges and Recent Progress on Silicon-Based Anode Materials for Next-Generation Lithium-Ion Batteries. Small Struct. 2021, 2, 2100009. [Google Scholar] [CrossRef]
- Chong, J.; Xun, S.; Zheng, H.; Song, X.; Liu, G.; Ridgway, P.; Wang, J.Q.; Battaglia, V.S. A comparative study of polyacrylic acid and poly(vinylidene difluoride) binders for spherical natural graphite/LiFePO4 electrodes and cells. J. Power Sources 2011, 196, 7707–7714. [Google Scholar] [CrossRef]
- Park, J.; Willenbacher, N.; Ahn, K.H. How the interaction between styrene-butadiene-rubber (SBR) binder and a secondary fluid affects the rheology, microstructure and adhesive properties of capillary-suspension-type graphite slurries used for Li-ion battery anodes. Colloids Surf. A 2019, 579, 123692. [Google Scholar] [CrossRef]
- Gordon, R.; Orias, R.; Willenbacher, N. Effect of carboxymethyl cellulose on the flow behavior of lithium-ion battery anode slurries and the electrical as well as mechanical properties of corresponding dry layers. J. Mater. Sci 2020, 55, 15867–15881. [Google Scholar] [CrossRef]
- Liu, J.; Lee, S.Y.; Yoo, J.; Kim, S.; Kim, J.-H.; Cho, H. Real-Time Observation of Mechanical Evolution of Micro-Sized Si Anodes by In Situ Atomic Force Microscopy. ACS Mater. Lett. 2022, 4, 840–846. [Google Scholar] [CrossRef]
- Kumar, P.; Berhaut, C.L.; Zapata Dominguez, D.; de Vito, E.; Tardif, S.; Pouget, S.; Lyonnard, S.; Jouneau, P.-H. Nano-Architectured Composite Anode Enabling Long-Term Cycling Stability for High-Capacity Lithium-Ion Batteries. Small 2020, 16, e1906812. [Google Scholar] [CrossRef]
- Gutierrez, M.; Morcrette, M.; Monconduit, L.; Oudart, Y.; Lemaire, P.; Davoisne, C.; Louvain, N.; Janot, R. Towards a better understanding of the degradation mechanisms of Li-ion full cells using Si/C composites as anode. J. Power Sources 2022, 533, 231408. [Google Scholar] [CrossRef]
- Lee, J.; Moon, J.; Han, S.A.; Kim, J.; Malgras, V.; Heo, Y.-U.; Kim, H.; Lee, S.-M.; Liu, H.K.; Dou, S.X.; et al. Everlasting Living and Breathing Gyroid 3D Network in Si@SiOx/C Nanoarchitecture for Lithium Ion Battery. ACS Nano 2019, 13, 9607–9619. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chae, O.B.; Lucht, B.L. Perspective—Structure and Stability of the Solid Electrolyte Interphase on Silicon Anodes of Lithium-ion Batteries. J. Electrochem. Soc. 2021, 168, 30521. [Google Scholar] [CrossRef]
- Lee, J.G.; Kim, J.; Lee, J.B.; Park, H.; Kim, H.-S.; Ryu, J.H.; Jung, D.S.; Kim, E.K.; Oh, S.M. Mechanical Damage of Surface Films and Failure of Nano-Sized Silicon Electrodes in Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A6103–A6109. [Google Scholar] [CrossRef]
- Vorauer, T.; Kumar, P.; Berhaut, C.L.; Chamasemani, F.F.; Jouneau, P.-H.; Aradilla, D.; Tardif, S.; Pouget, S.; Fuchsbichler, B.; Helfen, L.; et al. Multi-scale quantification and modeling of aged nanostructured silicon-based composite anodes. Commun. Chem. 2020, 3, 141. [Google Scholar] [CrossRef] [PubMed]






| Sample | Parameters | ||||
|---|---|---|---|---|---|
| BET in m2 g−1 | d10 in µm | d50 in µm | d90 in µm | Si in wt.% | |
| GO | 36.5 | 1.7 | 4.4 | 9.3 | - |
| prGO | 474.3 | 2.4 | 5.2 | 10.6 | - |
| rGO | 512.0 | 2.3 | 5.2 | 11.0 | - |
| rGO_milled | 175.5 | 2.0 | 4.7 | 10.2 | - |
| rGO_milled_45µm | 161.0 | 1.9 | 4.4 | 11.4 | - |
| µm-Si | 2.9 | 1.1 | 4.6 | 9.2 | - |
| nm-Si | 47.7 | 0.2 | 0.7 | 1.6 | - |
| µm-Si-rGO | 129.3 | 1.4 | 4.4 | 14.4 | 17.4 |
| nm-Si-rGO | 162.0 | 1.1 | 3.6 | 10.7 | 15.1 |
| Sample | Parameter | ||
|---|---|---|---|
| Total Delithiation Capacity (51 Cycles) in | CE (1st Cycle) in % | Average CE (Cycle 2–51) in % | |
| µm-Si-rGO_1.25CMC_3.75SBR | 11.1 ± 0.8 | 46.22 ± 1.13 | 97.64 ± 0.06 |
| µm-Si-rGO_2.5CMC_2.5SBR | 13.0 ± 0.7 | 51.01 ± 2.60 | 97.51 ± 0.08 |
| µm-Si-rGO_3.75CMC_1.25SBR | 12.2 ± 0.5 | 51.15 ± 1.47 | 97.23 ± 0.17 |
| nm-Si-rGO_1.25CMC_3.75SBR | 17.1 ± 0.7 | 48.89 ± 1.95 | 97.09 ± 0.16 |
| nm-Si-rGO_2.5CMC_2.5SBR | 17.4 ± 0.3 | 50.27 ± 0.79 | 96.83 ± 0.03 |
| nm-Si-rGO_3.75CMC_1.25SBR | 20.6 ± 0.6 | 52.88 ± 0.45 | 96.95 ± 0.07 |
| rGO_2.5CMC_2.5SBR | 12.7 ± 0.1 | 31.78 ± 1.03 | 98.05 ± 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müllner, S.; Michlik, T.; Reichel, M.; Held, T.; Moos, R.; Roth, C. Effect of Water-Soluble CMC/SBR Binder Ratios on Si-rGO Composites Using µm- and nm-Sized Silicon as Anode Materials for Lithium-Ion Batteries. Batteries 2023, 9, 248. https://doi.org/10.3390/batteries9050248
Müllner S, Michlik T, Reichel M, Held T, Moos R, Roth C. Effect of Water-Soluble CMC/SBR Binder Ratios on Si-rGO Composites Using µm- and nm-Sized Silicon as Anode Materials for Lithium-Ion Batteries. Batteries. 2023; 9(5):248. https://doi.org/10.3390/batteries9050248
Chicago/Turabian StyleMüllner, Sebastian, Tobias Michlik, Michael Reichel, Tilo Held, Ralf Moos, and Christina Roth. 2023. "Effect of Water-Soluble CMC/SBR Binder Ratios on Si-rGO Composites Using µm- and nm-Sized Silicon as Anode Materials for Lithium-Ion Batteries" Batteries 9, no. 5: 248. https://doi.org/10.3390/batteries9050248
APA StyleMüllner, S., Michlik, T., Reichel, M., Held, T., Moos, R., & Roth, C. (2023). Effect of Water-Soluble CMC/SBR Binder Ratios on Si-rGO Composites Using µm- and nm-Sized Silicon as Anode Materials for Lithium-Ion Batteries. Batteries, 9(5), 248. https://doi.org/10.3390/batteries9050248







