Prefabrication of a Lithium Fluoride Interfacial Layer to Enable Dendrite-Free Lithium Deposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of LiF-Coated Li Metal
2.2. Material Characterizations
2.3. Electrochemical Evaluations
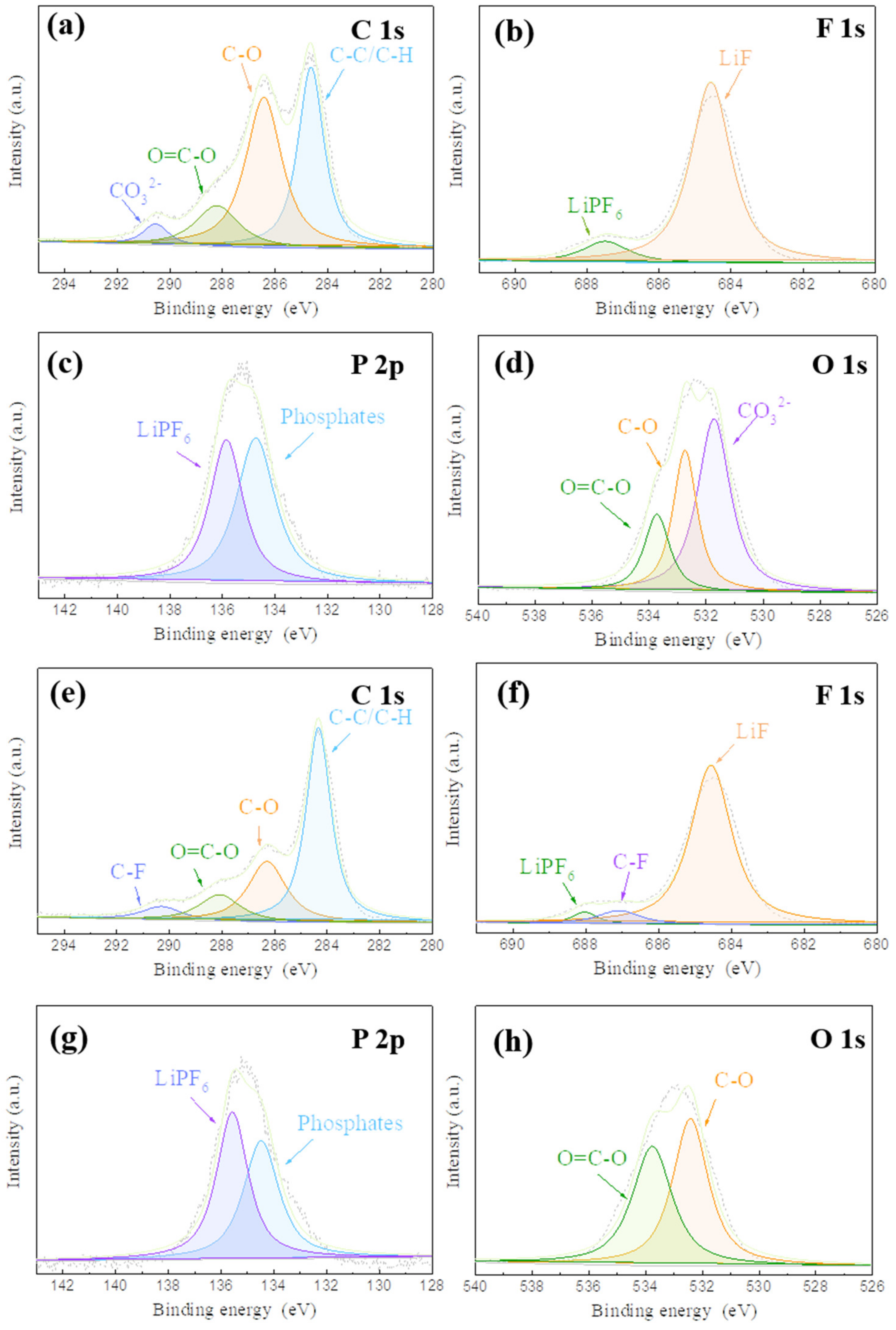
3. Results and Discussion
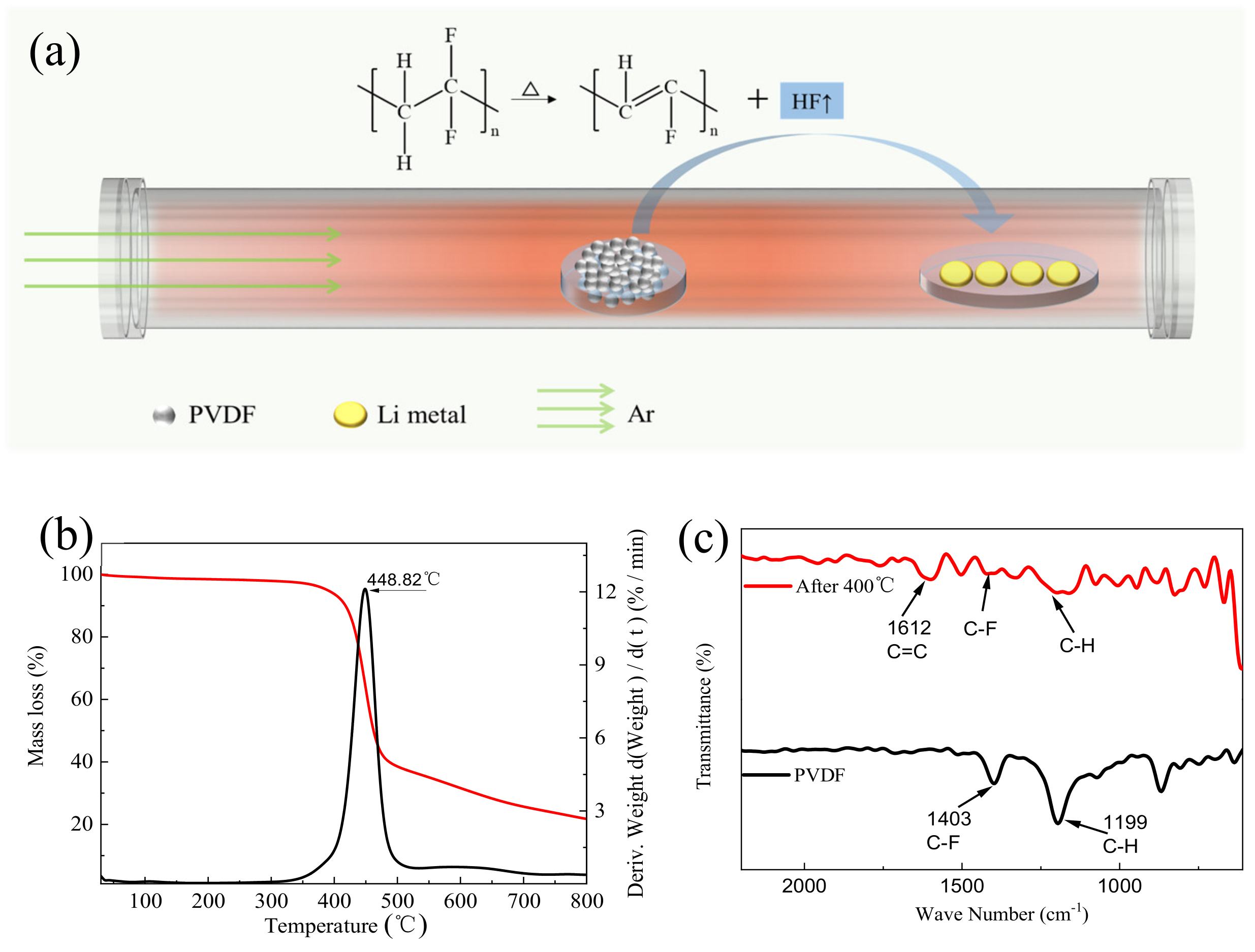
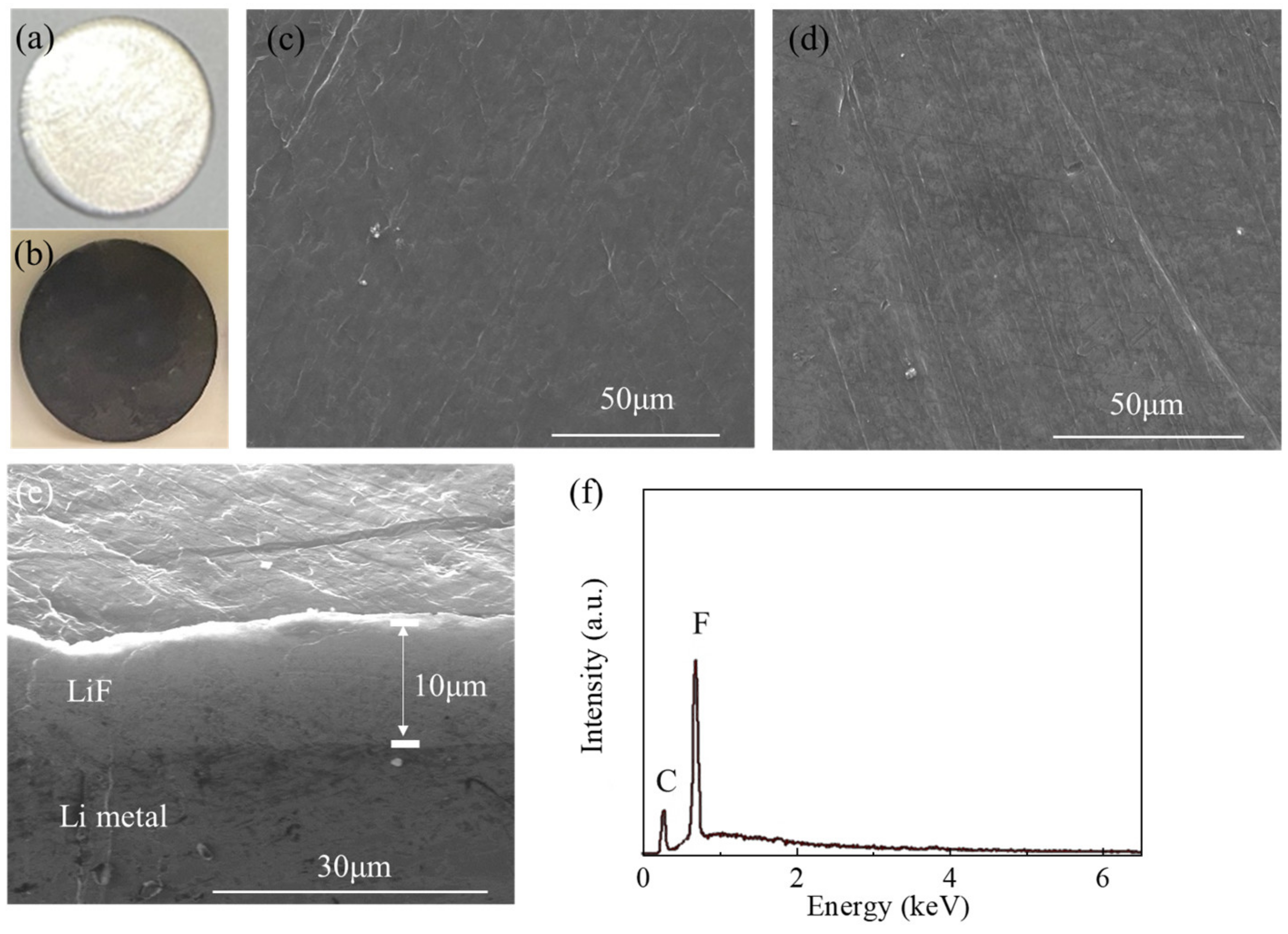
3.1. Fabrication and Characterizationof LiF-Coated Li
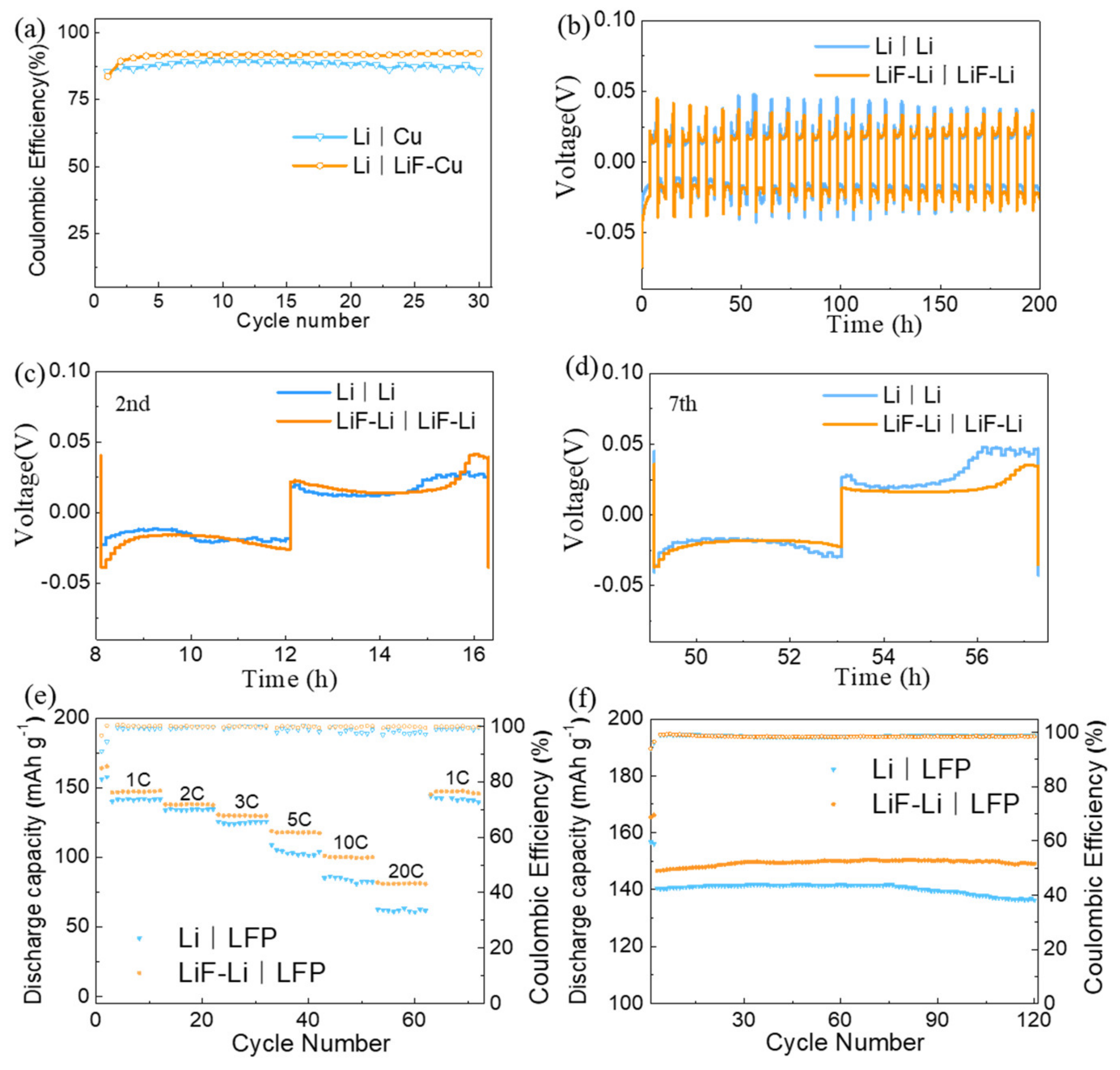
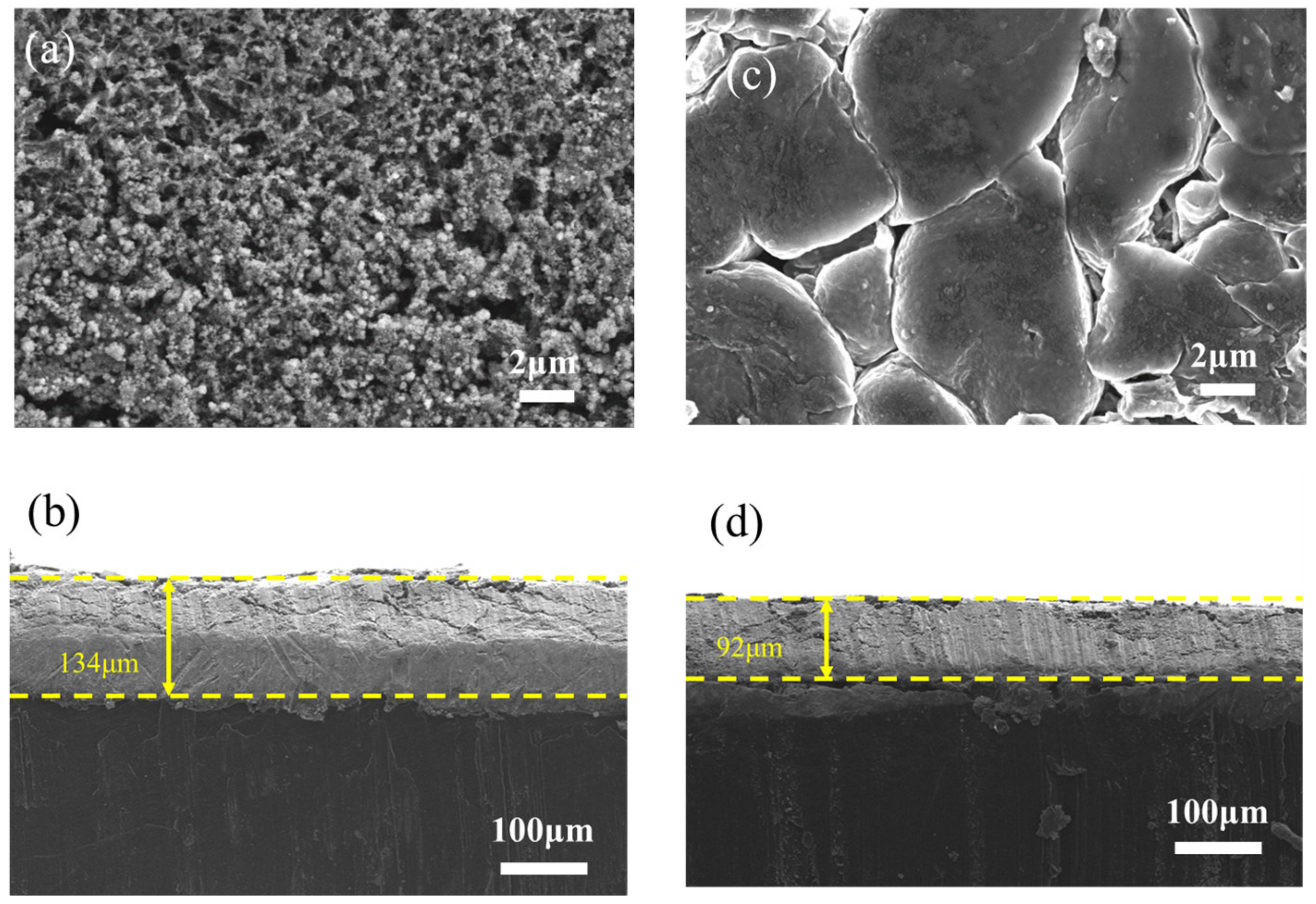
3.2. Effect of LiFCoating on Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing Lithium Metal Batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; Sun, Z.; Sun, C.; Qi, F.; An, B.; Li, F.; Cheng, H. Key Aspects of Lithium Metal Anodes for Lithium Metal Batteries. Small 2019, 15, e1900687. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Lu, Y.; Tu, Z.; Archer, L.A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 2014, 13, 961–969. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, X.; Tian, Y.; Chen, X.; Zhang, X.; Li, W.; Huang, J.; Zhang, Q. Dual-Layered Film Protected Lithium Metal Anode to Enable Dendrite-Free Lithium Deposition. Adv. Mater. 2018, 30, e1707629. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.R.; Chen, X.; Cheng, X.B.; Zhang, X.Q.; Yan, C.; Zhang, Q. Lithiophilic Sites in Doped Graphene Guide Uniform Lithium Nucleation for Dendrite-Free Lithium Metal Anodes. Angew. Chem. Int. Ed. Engl. 2017, 56, 7764–7768. [Google Scholar] [CrossRef]
- Aurbach, D. Review of selected electrode-solution interactions which determine the performance of Li and Li ion batteries. J. Power Sources 2000, 89, 206–218. [Google Scholar] [CrossRef]
- Harry, K.J.; Hallinan, D.; Parkinson, D.Y.; MacDowell, A.A.; Balsara, N.P. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 2014, 13, 69–73. [Google Scholar] [CrossRef]
- Wood, K.N.; Kazyak, E.; Chadwick, A.F.; Chen, K.-H.; Zhang, J.-G.; Thornton, K.; Dasgupta, N.P. Dendrites and Pits: Untangling the Complex Behavior of Lithium Metal Anodes through Operando Video Microscopy. ACS Cent. Sci. 2016, 2, 790–801. [Google Scholar] [CrossRef]
- Liu, F.; Xiao, Q.; Bin Wu, H.; Shen, L.; Xu, D.; Cai, M.; Lu, Y. Fabrication of Hybrid Silicate Coatings by a Simple Vapor Deposition Method for Lithium Metal Anodes. Adv. Energy Mater. 2018, 8, 1701744. [Google Scholar] [CrossRef]
- Xu, R.; Xiao, Y.; Zhang, R.; Cheng, X.; Zhao, C.; Zhang, X.; Yan, C.; Zhang, Q.; Huang, J. Dual-Phase Single-Ion Pathway Interfaces for Robust Lithium Metal in Working Batteries. Adv. Mater. 2019, 31, e1808392. [Google Scholar] [CrossRef]
- Calderón, C.A.; Vizintin, A.; Bobnar, J.; Barraco, D.E.; Leiva, E.P.; Visintin, A.; Fantini, S.; Fischer, F.; Dominko, R. Lithium Metal Protection by a Cross-Linked Polymer Ionic Liquid and Its Application in Lithium Battery. ACS Appl. Energy Mater. 2020, 3, 2020–2027. [Google Scholar] [CrossRef]
- Chen, L.; Connell, J.G.; Nie, A.; Huang, Z.; Zavadil, K.R.; Klavetter, K.C.; Yuan, Y.; Sharifi-Asl, S.; Shahbazian-Yassar, R.; Libera, J.A.; et al. Lithium metal protected by atomic layer deposition metal oxide for high performance anodes. J. Mater. Chem. A 2017, 5, 12297–12309. [Google Scholar] [CrossRef]
- Alaboina, P.K.; Rodrigues, S.; Rottmayer, M.; Cho, S.-J. In Situ Dendrite Suppression Study of Nanolayer Encapsulated Li Metal Enabled by Zirconia Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2018, 10, 32801–32808. [Google Scholar] [CrossRef]
- Jie, Y.; Ren, X.; Cao, R.; Cai, W.; Jiao, S. Advanced Liquid Electrolytes for Rechargeable Li Metal Batteries. Adv. Funct. Mater. 2020, 30, 1910777. [Google Scholar] [CrossRef]
- Borodin, O. Challenges with prediction of battery electrolyte electrochemical stability window and guiding the electrode—Electrolyte stabilization. Curr. Opin. Electrochem. 2019, 13, 86–93. [Google Scholar] [CrossRef]
- Zheng, Q.; Yamada, Y.; Shang, R.; Ko, S.; Lee, Y.-Y.; Kim, K.; Nakamura, E.; Yamada, A. A cyclic phosphate-based battery electrolyte for high voltage and safe operation. Nat. Energy 2020, 5, 291–298. [Google Scholar] [CrossRef]
- Ni, J.; Jin, L.; Xue, M.; Xiao, Q.; Zheng, J.; Zheng, J.P.; Zhang, C. TiO2 microbox/carbon nanotube composite-modified separator for high-performance lithium-sulfur batteries. J. Solid. State Electrochem. 2020, 25, 949–961. [Google Scholar] [CrossRef]
- Ji, M.; Ni, J.; Liang, X.; Cheng, Q.; Gao, G.; Wu, G.; Xiao, Q. Biomimetic Synthesis of VO x @C Yolk-Shell Nanospheres and Their Application in Li—S Batteries. Adv. Funct. Mater. 2022, 32, 2206589. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Lee, S.-Y. Closely packed SiO2 nanoparticles/poly(vinylidene fluoride-hexafluoropropylene) layers-coated polyethylene separators for lithium-ion batteries. J. Power Sources 2011, 196, 6716–6722. [Google Scholar] [CrossRef]
- Song, Y.H.; Wu, K.J.; Zhang, T.W.; Lu, L.-L.; Guan, Y.; Zhou, F.; Wang, X.-X.; Yin, Y.-C.; Tan, Y.-H.; Li, F.; et al. A Nacre-Inspired Separator Coating for Impact-Tolerant Lithium Batteries. Adv. Mater. 2019, 31, e1905711. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhou, Z. Towards practical lithium-metal anodes. Chem. Soc. Rev. 2020, 49, 3040–3071. [Google Scholar] [CrossRef]
- Jiang, G.; Li, K.; Yu, F.; Li, X.; Mao, J.; Jiang, W.; Sun, F.; Dai, B.; Li, Y. Robust Artificial Solid-Electrolyte Interfaces with Biomimetic Ionic Channels for Dendrite-Free Li Metal Anodes. Adv. Energy Mater. 2020, 11, 2003496. [Google Scholar] [CrossRef]
- Zhu, B.; Jin, Y.; Hu, X.; Zheng, Q.; Zhang, S.; Wang, Q.; Zhu, J. Poly(dimethylsiloxane) Thin Film as a Stable Interfacial Layer for High-Performance Lithium-Metal Battery Anodes. Adv. Mater. 2017, 29, 1701568. [Google Scholar] [CrossRef]
- Zhai, P.; Liu, L.; Gu, X.; Wang, T.; Gong, Y. Interface Engineering for Lithium Metal Anodes in Liquid Electrolyte. Adv. Energy Mater. 2020, 10, 2001257. [Google Scholar] [CrossRef]
- Ozhabes, Y.; Gunceler, D.; Arias, T.A. Stability and surface diffusion at lithium-electrolyte interphases with connections to dendrite suppression. arXiv 2015, preprint. arXiv:1504.05799. [Google Scholar]
- Lin, D.; Liu, Y.; Chen, W.; Zhou, G.; Liu, K.; Dunn, B.; Cui, Y. Conformal Lithium Fluoride Protection Layer on Three-Dimensional Lithium by Nonhazardous Gaseous Reagent Freon. Nano Lett. 2017, 17, 3731–3737. [Google Scholar] [CrossRef]
- Yu, T.; Zhao, T.; Zhang, N.; Xue, T.; Chen, Y.; Ye, Y.; Wu, F.; Chen, R. Spatially Confined LiF Nanoparticles in an Aligned Polymer Matrix as the Artificial SEI Layer for Lithium Metal Anodes. Nano Lett. 2023, 23, 276–282. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, Z.; Ma, F.; Wang, X.; Wu, Y.; Liu, M.; He, R.; Cheng, S.; Xie, J. Juggling Formation of HF and LiF to Reduce Crossover Effects in Carbonate Electrolyte with Fluorinated Cosolvents for High-Voltage Lithium Metal Batteries. Adv. Funct. Mater. 2022, 33, 2212000. [Google Scholar] [CrossRef]
- Choudhury, S.; Archer, L.A. Lithium Fluoride Additives for Stable Cycling of Lithium Batteries at High Current Densities. Adv. Electron. Mater. 2016, 2, 1500246. [Google Scholar] [CrossRef]
- Shadike, Z.; Lee, H.; Borodin, O.; Cao, X.; Fan, X.; Wang, X.; Lin, R.; Bak, S.-M.; Ghose, S.; Xu, K.; et al. Identification of LiH and nanocrystalline LiF in the solid-electrolyte interphase of lithium metal anodes. Nat. Nanotechnol. 2021, 16, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Jurng, S.; Brown, Z.L.; Kim, J.; Lucht, B.L. Effect of electrolyte on the nanostructure of the solid electrolyte interphase (SEI) and performance of lithium metal anodes. Energy Environ. Sci. 2018, 11, 2600–2608. [Google Scholar] [CrossRef]
- Liu, Z.; He, B.; Zhang, Z.; Deng, W.; Dong, D.; Xia, S.; Zhou, X.; Liu, Z. Lithium/Graphene Composite Anode with 3D Structural LiF Protection Layer for High-Performance Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 2871–2880. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, Y.S.; Yoo, J. An in situ formed LiF protective layer on a Li metal anode with solvent-less cross-linking. Sustain. Energy Fuels 2020, 4, 3282–3287. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, F.; Bai, Y.; Li, Y.; Chen, G.; Wang, Z.; Wu, C. Regulating Li deposition by constructing LiF-rich host for dendrite-free lithium metal anode. Energy Storage Mater. 2019, 16, 411–418. [Google Scholar] [CrossRef]
- Lang, J.; Long, Y.; Qu, J.; Luo, X.; Wei, H.; Huang, K.; Zhang, H.; Qi, L.; Zhang, Q.; Li, Z.; et al. One-pot solution coating of high quality LiF layer to stabilize Li metal anode. Energy Storage Mater. 2019, 16, 85–90. [Google Scholar] [CrossRef]
- Zhao, J.; Liao, L.; Shi, F.; Lei, T.; Chen, G.; Pei, A.; Sun, J.; Yan, K.; Zhou, G.; Xie, J.; et al. Surface Fluorination of Reactive Battery Anode Materials for Enhanced Stability. J. Am. Chem. Soc. 2017, 139, 11550–11558. [Google Scholar] [CrossRef]
- Xie, J.; Liao, L.; Gong, Y.; Li, Y.; Shi, F.; Pei, A.; Sun, J.; Zhang, R.; Kong, B.; Subbaraman, R.; et al. Stitching h-BN by atomic layer deposition of LiF as a stable interface for lithium metal anode. Sci. Adv. 2017, 3, eaao3170. [Google Scholar] [CrossRef]
- Chen, L.; Chen, K.-S.; Chen, X.; Ramierez, G.; Huang, Z.; Geise, N.R.; Steinrück, H.-G.; Fisher, B.L.; Shahbazian-Yassar, R.; Toney, M.F.; et al. Novel ALD Chemistry Enabled Low-Temperature Synthesis of Lithium Fluoride Coatings for Durable Lithium Anodes. ACS Appl. Mater. Interfaces 2018, 10, 26972–26981. [Google Scholar] [CrossRef]
- Ko, J.; Yoon, Y.S. Lithium fluoride layer formed by thermal evaporation for stable lithium metal anode in rechargeable batteries. Thin Solid. Film. 2019, 673, 119–125. [Google Scholar] [CrossRef]
- Fan, L.; Zhuang, H.L.; Gao, L.; Lu, Y.; Archer, L.A. Regulating Li deposition at artificial solid electrolyte interphases. J. Mater. Chem. A 2017, 5, 3483–3492. [Google Scholar] [CrossRef]
- Vernardou, D. Special Issue: Advances in Chemical Vapor Deposition. Materials 2020, 13, 4167. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Ma, X.; Wu, J.; Deng, X.; Xiao, X.; Liu, J.; Nan, J. Self-supporting ethyl cellulose/poly(vinylidene fluoride) blended gel polymer electrolyte for 5 V high-voltage lithium-ion batteries. Electrochim. Acta 2018, 271, 582–590. [Google Scholar] [CrossRef]
- Tian, X.; Xin, B.; Gao, W.; Jin, S.; Chen, Z. Preparation and characterization of polyvinylidene fluoride/polysulfone-amide composite nanofiber mats. J. Text. Inst. 2018, 110, 815–821. [Google Scholar] [CrossRef]
- Singha, S.; Jana, T. Effect of composition on the properties of PEM based on polybenzimidazole and poly(vinylidene fluoride) blends. Polymer 2014, 55, 594–601. [Google Scholar] [CrossRef]
- Sanad, M.M.S.; Meselhy, N.K.; El-Boraey, H.A.; Toghan, A. Controllable engineering of new ZnAl2O4-decorated LiNi0·8Mn0·1Co0·1O2 cathode materials for high performance lithium-ion batteries. J. Mater. Res. Technol. 2023, 23, 1528–1542. [Google Scholar] [CrossRef]
- Moustafa, M.G.; Sanad, M.M.S. Green fabrication of ZnAl2O4-coated LiFePO4 nanoparticles for enhanced electrochemical performance in Li-ion batteries. J. Alloy. Compd. 2022, 903, 163910. [Google Scholar] [CrossRef]
- Heine, J.; Krüger, S.; Hartnig, C.; Wietelmann, U.; Winter, M.; Bieker, P. Coated Lithium Powder (CLiP) Electrodes for Lithium-Metal Batteries. Adv. Energy Mater. 2014, 4, 1300815. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Yu, Y.; Shen, C.; Zhou, C.; Dong, C.; Zhao, T.; Xu, X. One-Pot Preparation of Lithium Compensation Layer, Lithiophilic Layer, and Artificial Solid Electrolyte Interphase for Lean-Lithium Metal Anode. ACS Appl. Mater. Interfaces 2022, 14, 19437–19447. [Google Scholar] [CrossRef]
- Zheng, J.; Yan, P.; Cao, R.; Xiang, H.; Engelhard, M.H.; Polzin, B.J.; Wang, C.; Zhang, J.-G.; Xu, W. Effects of Propylene Carbonate Content in CsPF(6)-Containing Electrolytes on the Enhanced Performances of Graphite Electrode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 5715–5722. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Pan, Q.; Li, C.Y. Polymerized Ionic Liquid-Containing Interpenetrating Network Solid Polymer Electrolytes for All-Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2019, 11, 34904–34912. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, Y.; Chai, S.; Usman, I.; Qiao, Y.; Luo, S.; Xie, X.; Chen, J.; Liang, S.; Pan, A.; et al. Incorporation of LiF into functionalized polymer fiber networks enabling high capacity and high rate cycling of lithium metal composite anodes. Chem. Eng. J. 2021, 404, 126508. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, J.; Lei, Y.; Han, Y.; Zhang, Y.; Zhang, C.; Geng, Z.; Xiao, Q. Prefabrication of a Lithium Fluoride Interfacial Layer to Enable Dendrite-Free Lithium Deposition. Batteries 2023, 9, 283. https://doi.org/10.3390/batteries9050283
Ni J, Lei Y, Han Y, Zhang Y, Zhang C, Geng Z, Xiao Q. Prefabrication of a Lithium Fluoride Interfacial Layer to Enable Dendrite-Free Lithium Deposition. Batteries. 2023; 9(5):283. https://doi.org/10.3390/batteries9050283
Chicago/Turabian StyleNi, Jie, Yike Lei, Yongkang Han, Yingchuan Zhang, Cunman Zhang, Zhen Geng, and Qiangfeng Xiao. 2023. "Prefabrication of a Lithium Fluoride Interfacial Layer to Enable Dendrite-Free Lithium Deposition" Batteries 9, no. 5: 283. https://doi.org/10.3390/batteries9050283
APA StyleNi, J., Lei, Y., Han, Y., Zhang, Y., Zhang, C., Geng, Z., & Xiao, Q. (2023). Prefabrication of a Lithium Fluoride Interfacial Layer to Enable Dendrite-Free Lithium Deposition. Batteries, 9(5), 283. https://doi.org/10.3390/batteries9050283





