Cell Design for Improving Low-Temperature Performance of Lithium-Ion Batteries for Electric Vehicles
Abstract
:1. Introduction
2. Cell Fabrication/Structure Optimization
3. Material Optimization
3.1. Anode Materials
3.2. Cathode Materials
3.3. Electrolytes

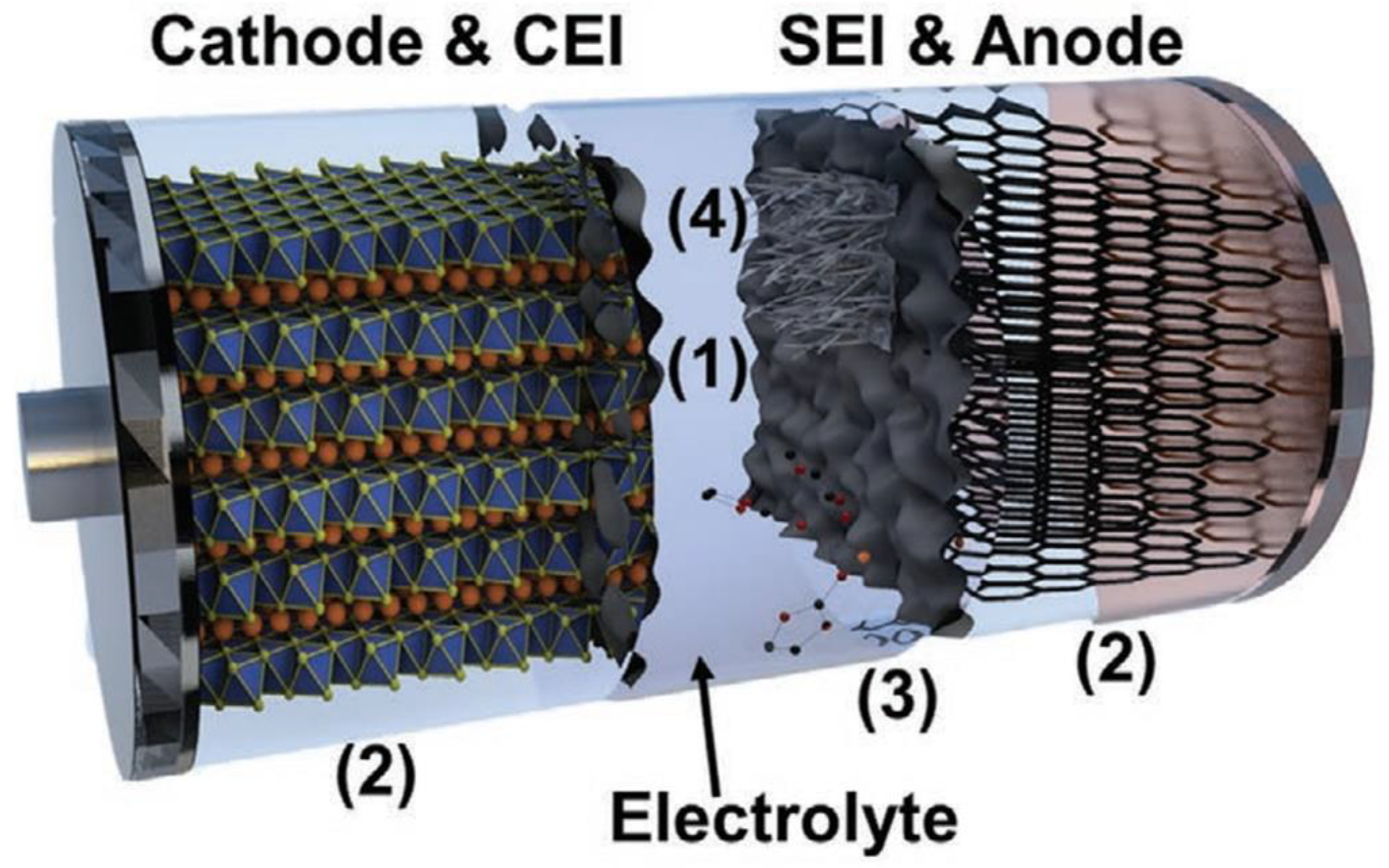
4. The Advantages and Disadvantages of Different Schemes
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full form/Chemical Name | Abbreviation | Full form/Chemical Name |
| ARLBs | Aqueous rechargeable lithium-ion batteries | LNCM | Li [NixCoyMn1−x−y] O2(0 < x < 1,0 < y < 1) |
| BE | Baseline electrolyte | LNMO | LiNi0.5Mn1.5O4 |
| BStSi | B–starch–Si | LP | 1 M LiPF6 in ethylene carbonate/diethyl carbonate |
| CA | carbon aerogel | LTO | Li4Ti5O12 |
| COFs | Covalent organic frameworks | LVP | Li3V2(PO4)3 |
| DCM | Dichloromethane | MA | Methyl acetate |
| DEC | Diethyl carbonate | MCMB | Graphitic mesocarbon microbead |
| DMC | Dimethyl carbonate | MOF | Metal–organic framework |
| EA | Ethyl acetate | NCA | LiNi0.80Co0.15Al0.05O2 |
| EC | Ethylene carbonate | NCM111 | LiNi1/3Co1/3Mn1/3O2 |
| EIS | Electrochemical impedance Spectroscopy | NCM622 | LiNi0.6Co0.2Mn0.2O2 |
| EMC | Ethyl methyl carbonate | PC | Propylene carbonate |
| EMIF | 1-ethyl-3-methylimidazolium Fluoride | PCS | 1,3-propanediolic sulfate |
| EMP | Electrospun MOF–PVA composite membranes | PP | Polypropylene |
| EtG | Ethylene glycol | PYR14TFSI | N-butyl-N-Methylpyrrolidiniumbis(trifluoromethanesulfonyl)imide |
| FEC | Fluoroethylene carbonate | SEAG | a-Si nano-layer |
| LATP | Li2O-Al2O3-TiO2-P2O5 | SEI | Solid-electrolyte interphase |
| LC | 1 M LiClO4 in propylene carbonate | SP | Smaller primary particle size |
| LFP | LiFePO4 | TFSI | Bis(trifluoromethanesulfonyl)imide |
| LIBs | Lithium-ion batteries | TMSP | Tris(trimethylsilyl) phosphite |
| LMO | LiMn2O4 | ||
References
- IEA. World Energy Outlook 2022; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/world-energy-outlook-2022 (accessed on 1 October 2022).
- UNEP. The Closing Window—Climate Crisis Calls for Rapid Transformation of Societies; UNEP: Nairobi, Kenya, 2022; Available online: https://www.unep.org/resources/emissions-gap-report-2022 (accessed on 27 October 2022).
- General Office of the State Council of China. Circular of The General Office of the State Council on Printing and Distributing the Development Plan for the New Energy Automobile Industry (2021–2035). Available online: http://www.gov.cn/zhengce/content/2020-11/02/content_5556716.htm (accessed on 20 October 2020).
- Xiong, R.; Chen, H.; Wang, C.; Sun, F. Towards a smarter hybrid energy storage system based on battery and ultracapacitor—A critical review on topology and energy management. J. Clean. Prod. 2018, 202, 1228–1240. [Google Scholar] [CrossRef]
- Li, Z.H.; Khajepour, A.; Song, J.C. A comprehensive review of the key technologies for pure electric vehicles. Energy 2019, 182, 824–839. [Google Scholar] [CrossRef]
- Fu, S.J.; Fu, H.J. A method to predict electric vehicles’ market penetration as well as its impact on energy saving and CO2 mitigation. Sci. Prog. 2021, 104, 00368504211040286. [Google Scholar] [CrossRef]
- Tong, F.; Azevedo, I.M.L. What are the best combinations of fuel-vehicle technologies to mitigate climate change and air pollution effects across the United States? Environ. Res. Lett. 2020, 15, 074046. [Google Scholar] [CrossRef]
- Ashok, B.; Kannan, C.; Mason, B.; Ashok, S.D.; Indragandhi, V.; Patel, D.; Wagh, A.S.; Jain, A.; Kavitha, C. Towards Safer and Smarter Design for Lithium-Ion-Battery-Powered Electric Vehicles: A Comprehensive Review on Control Strategy Architecture of Battery Management System. Energies 2022, 15, 4227. [Google Scholar] [CrossRef]
- Hemavathi, S. Overview of cell balancing methods for Li-ion battery technology. Energy Storage 2020, 3, e203. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Dobrzycki, A.; Filipiak, M.; Jajczyk, J. Changes in the range of electric vehicles during operation. In Proceedings of the Conference on Computer Applications in Electrical Engineering (ZkwE), Poznan, Poland, 15 April 2019. [Google Scholar]
- Xu, J.J.; Cai, X.Y.; Cai, S.M.; Shao, Y.X.; Hu, C.; Lu, S.R.; Ding, S.J. High-Energy Lithium-Ion Batteries: Recent Progress and a Promising Future in Applications. Energy Environ. Mater. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Van Ree, T. Electrolyte additives for improved lithium-ion battery performance and overcharge protection. Curr. Opin. Electrochem. 2020, 21, 22–30. [Google Scholar] [CrossRef]
- Li, Q.Y.; Jiao, S.H.; Luo, L.L.; Ding, M.S.; Zheng, J.M.; Cartmell, S.S.; Wang, C.M.; Xu, K.; Zhang, J.G.; Xu, W. Wide-Temperature Electrolytes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 18826–18835. [Google Scholar] [CrossRef]
- Jaguemont, J.; Boulon, L.; Dube, Y. Low Temperature Discharge Cycle Tests for a Lithium Ion Cell. In Proceedings of the 2014 IEEE Vehicle Power and Propulsion Conference (VPPC), Coimbra, Portugal, 27–30 October 2014. [Google Scholar]
- Hubble, D.; Brown, D.E.; Zhao, Y.; Fang, C.; Lau, J.; McCloskey, B.D.; Liu, G. Liquid electrolyte development for low-temperature lithium-ion batteries. Energy Environ. Sci. 2022, 15, 550–578. [Google Scholar] [CrossRef]
- Gupta, A.; Manthiram, A. Designing Advanced Lithium-Based Batteries for Low-Temperature Conditions. Adv. Energy Mater. 2020, 10, 2001972. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.-J.; Liu, Q.; Cohen, O.; Kim, M.; Persson, K.A.; Zhang, Z. Understanding the Role of SEI Layer in Low-Temperature Performance of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2022, 14, 11910–11918. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Song, L.; Wang, J.; Zhang, J.; Cui, X.; Wang, P.; Sun, J.; Cai, X.; Huang, J.; Zhang, N.; et al. Insight into the competitive reaction between LiDFP and LiFSI in lithium-ion battery at low temperature. J. Power Sources 2022, 549, 232147. [Google Scholar] [CrossRef]
- Zhang, N.; Deng, T.; Zhang, S.; Wang, C.; Chen, L.; Wang, C.; Fan, X. Critical Review on Low-Temperature Li-Ion/Metal Batteries. Adv. Mater. 2022, 34, 2107899. [Google Scholar] [CrossRef]
- Hu, D.; Chen, L.; Tian, J.; Su, Y.; Li, N.; Chen, G.; Hu, Y.; Dou, Y.; Chen, S.; Wu, F. Research Progress of Lithium Plating on Graphite Anode in Lithium-Ion Batteries. Chin. J. Chem. 2020, 39, 165–173. [Google Scholar] [CrossRef]
- Hu, A.; Li, F.; Chen, W.; Lei, T.; Li, Y.; Fan, Y.; He, M.; Wang, F.; Zhou, M.; Hu, Y.; et al. Ion Transport Kinetics in Low-Temperature Lithium Metal Batteries. Adv. Energy Mater. 2022, 12, 2202432. [Google Scholar] [CrossRef]
- Piao, N.; Gao, X.; Yang, H.; Guo, Z.; Hu, G.; Cheng, H.-M.; Li, F. Challenges and development of lithium-ion batteries for low temperature environments. Etransportation 2022, 11, 100145. [Google Scholar] [CrossRef]
- Zhang, M.; Chouchane, M.; Shojaee, S.A.; Winiarski, B.; Liu, Z.; Li, L.; Pelapur, R.; Shodiev, A.; Yao, W.; Doux, J.-M.; et al. Coupling of multiscale imaging analysis and computational modeling for understanding thick cathode degradation mechanisms. Joule 2022, 7, 201–220. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, P.; Xiong, T.; Yang, F.; Yang, H.; Huang, Y.; Li, D.; Deng, J.; Balogun, M.J.T. Sub-Thick Electrodes with Enhanced Transport Kinetics via In Situ Epitaxial Heterogeneous Interfaces for High Areal-Capacity Lithium Ion Batteries. Small 2021, 17, e2100778. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Ju, Z.; Yu, X.; Wang, Y.; Du, Y.; Bai, Z.; Dou, S.; Yu, G. Thickness-independent scalable high-performance Li-S batteries with high areal sulfur loading via electron-enriched carbon framework. Nat. Commun. 2021, 12, 4519. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Tian, G.; Li, S.; Han, Z.; Zhong, W.; Cheng, S.; Xie, J. Enhancing the Reversibility of Lithium Cobalt Oxide Phase Transition in Thick Electrode via Low Tortuosity Design. Nano Lett. 2022, 22, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Chen, C.; Kirsch, D.; Hu, L. Thick Electrode Batteries: Principles, Opportunities, and Challenges. Adv. Energy Mater. 2019, 9, 1901457. [Google Scholar] [CrossRef]
- Ju, Z.; Zhang, X.; Wu, J.; King, S.T.; Chang, C.-C.; Yan, S.; Xue, Y.; Takeuchi, K.J.; Marschilok, A.C.; Wang, L.; et al. Tortuosity Engineering for Improved Charge Storage Kinetics in High-Areal-Capacity Battery Electrodes. Nano Lett. 2022, 22, 6700–6708. [Google Scholar] [CrossRef]
- Sun, K.; Wei, T.-S.; Ahn, B.Y.; Seo, J.Y.; Dillon, S.J.; Lewis, J.A. 3D Printing of Interdigitated Li-Ion Microbattery Architectures. Adv. Mater. 2013, 25, 4539–4543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Shen, L.; Shen, J.; Liu, F.; Chen, G.; Tao, R.; Ma, S.; Peng, Y.; Lu, Y. Anion-Sorbent Composite Separators for High-Rate Lithium-Ion Batteries. Adv. Mater. 2019, 31, e1808338. [Google Scholar] [CrossRef]
- Sander, J.S.; Erb, R.M.; Li, L.; Gurijala, A.; Chiang, Y.-M. High-performance battery electrodes via magnetic templating. Nat. Energy 2016, 1, 16099. [Google Scholar] [CrossRef]
- Billaud, J.; Bouville, F.; Magrini, T.; Villevieille, C.; Studart, A.R. Correction: Corrigendum: Magnetically aligned graphite electrodes for high-rate performance Li-ion batteries. Nat. Energy 2017, 2, 17061. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Yuan, N.; Hu, B.; Ding, J.; Ge, S. Graphite-based lithium ion battery with ultrafast charging and discharging and excellent low temperature performance. J. Power Sources 2019, 430, 74–79. [Google Scholar] [CrossRef]
- Zhang, M.; Song, X.; Ou, X.; Tang, Y. Rechargeable batteries based on anion intercalation graphite cathodes. Energy Storage Mater. 2019, 16, 65–84. [Google Scholar] [CrossRef]
- Cai, W.; Yao, Y.-X.; Zhu, G.-L.; Yan, C.; Jiang, L.-L.; He, C.; Huang, J.-Q.; Zhang, Q. A review on energy chemistry of fast-charging anodes. Chem. Soc. Rev. 2020, 49, 3806–3833. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhou, L.; Ma, H.; Wu, Z.; Zhao, Q.; Li, H.; Zhang, K.; Chen, J. Challenges and advances in wide-temperature rechargeable lithium batteries. Energy Environ. Sci. 2022, 15, 1711–1759. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, B.; Dong, X.; Wang, Y.; Xia, Y. A Simple Prelithiation Strategy to Build a High-Rate and Long-Life Lithium-Ion Battery with Improved Low-Temperature Performance. Angew. Chem. Int. Ed. 2017, 56, 16606–16610. [Google Scholar] [CrossRef]
- Kim, N.; Chae, S.; Ma, J.; Ko, M.; Cho, J. Fast-charging high-energy lithium-ion batteries via implantation of amorphous silicon nanolayer in edge-plane activated graphite anodes. Nat. Commun. 2017, 8, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Ben, L.; Zhan, Y.; Huang, X. Nano-Sn embedded in expanded graphite as anode for lithium ion batteries with improved low temperature electrochemical performance. Electrochim. Acta 2016, 187, 186–192. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Hu, F.; Li, D.; Zhou, D.; Jing, P.; Du, F.; Qu, S. Carbon-Dots-Derived 3D Highly Nitrogen-Doped Porous Carbon Framework for High-Performance Lithium Ion Storage. ACS Sustain. Chem. Eng. 2019, 7, 9848–9856. [Google Scholar] [CrossRef]
- Mancini, M.; Nobili, F.; Dsoke, S.; D’amico, F.; Tossici, R.; Croce, F.; Marassi, R. Lithium intercalation and interfacial kinetics of composite anodes formed by oxidized graphite and copper. J. Power Sources 2009, 190, 141–148. [Google Scholar] [CrossRef]
- Markevich, E.; Salitra, G.; Aurbach, D. Low Temperature Performance of Amorphous Monolithic Silicon Anodes: Comparative Study of Silicon and Graphite Electrodes. J. Electrochem. Soc. 2016, 163, A2407–A2412. [Google Scholar] [CrossRef]
- Tan, L.; Hu, R.; Zhang, H.; Lan, X.; Liu, J.; Wang, H.; Yuan, B.; Zhu, M. Subzero temperature promotes stable lithium storage in SnO2. Energy Storage Mater. 2021, 36, 242–250. [Google Scholar] [CrossRef]
- Meng, Q.; Chen, F.; Hao, Q.; Li, N.; Sun, X. Nb-doped Li4Ti5O12-TiO2 hierarchical microspheres as anode materials for high-performance Li-ion batteries at low temperature. J. Alloy. Compd. 2021, 885, 160842. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, X.; Xu, J.; Wang, X.; Yuan, N.; Ge, S.; Ding, J. Excellent Rate and Low Temperature Performance of Lithium-Ion Batteries based on Binder-Free Li4Ti5O12 Electrode. Chemelectrochem 2020, 7, 716–722. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Xu, H.; Wang, L.; Lu, X.; He, X. Li4Ti5O12 spinel anode: Fundamentals and advances in rechargeable batteries. Infomat 2022, 4, e12228. [Google Scholar] [CrossRef]
- Umirov, N.; Yamada, Y.; Munakata, H.; Kim, S.-S.; Kanamura, K. Analysis of intrinsic properties of Li4Ti5O12 using single-particle technique. J. Electroanal. Chem. 2019, 855, 113514. [Google Scholar] [CrossRef]
- Pohjalainen, E.; Rauhala, T.; Valkeapää, M.; Kallioinen, J.; Kallio, T. Effect of Li4Ti5O12 Particle Size on the Performance of Lithium Ion Battery Electrodes at High C-Rates and Low Temperatures. J. Phys. Chem. C 2015, 119, 2277–2283. [Google Scholar] [CrossRef] [Green Version]
- Subburaj, T.; Brevet, W.; Farmakis, F.; Tsiplakides, D.; Balomenou, S.; Strataki, N.; Elmasides, C.; Samaniego, B.; Nestoridi, M. Silicon/LiNi0.8Co0.15Al0.05O2 lithium-ion pouch cells charging and discharging at 40 °C temperature. Electrochim. Acta 2020, 354, 136652. [Google Scholar] [CrossRef]
- Luo, H.; Wang, Y.; Feng, Y.-H.; Fan, X.-Y.; Han, X.; Wang, P.-F. Lithium-Ion Batteries under Low-Temperature Environment: Challenges and Prospects. Materials 2022, 15, 8166. [Google Scholar] [CrossRef]
- Parfeneva, A.V.; Rumyantsev, A.M.; Lozhkina, D.A.; Maximov, M.Y.; Astrova, E.V. Influence of Fluoroethylene Carbonate in the Composition of an Aprotic Electrolyte on the Electrochemical Characteristics of LIB&rsquos Anodes Based on Carbonized Nanosilicon. Batteries 2022, 8, 91. [Google Scholar]
- Miyazaki, R.; Hihara, T. Charge-discharge performances of Sn powder as a high capacity anode for all-solid-state lithium batteries. J. Power Sources 2019, 427, 15–20. [Google Scholar] [CrossRef]
- Liang, S.; Cheng, Y.-J.; Zhu, J.; Xia, Y.; Müller-Buschbaum, P. A Chronicle Review of Nonsilicon (Sn, Sb, Ge)-Based Lithium/Sodium-Ion Battery Alloying Anodes. Small Methods 2020, 4, 2000218. [Google Scholar] [CrossRef]
- Liu, K.; Meng, X.; Yan, L.; Fan, M.; Wu, Y.; Li, C.; Ma, T. Sn/SnOx core-shell structure encapsulated in nitrogen-doped porous carbon frameworks for enhanced lithium storage. J. Alloys Compd. 2022, 896, 163009. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Sundarrajan, S.; Chellappan, V.; Reddy, M.V.; Ramakrishna, S.; Zaghib, K. Recent Development in Carbon-LiFePO4 Cathodes for Lithium-Ion Batteries: A Mini Review. Batteries 2022, 8, 133. [Google Scholar] [CrossRef]
- Cao, R.; Fan, W.; Li, C. In Situ Carbon-Coated NCM622 through CFx for Lithium-Ion Batteries with High Cycling Stability. J. Electrochem. Soc. 2019, 166, A3348–A3353. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, H.; Liang, X.H. Understanding the roles of atomic layer deposition in improving the electrochemical performance of lithium-ion batteries. Appl. Phys. Rev. 2021, 8, 031301. [Google Scholar] [CrossRef]
- Asif, M.; Rashad, M.; Ali, Z.; Qiu, H.L.; Li, W.; Pan, L.J.; Hou, Y.L. Ni-doped MnO2/CNT nanoarchitectures as a cathode material for ultra-long life magnesium/lithium hybrid ion batteries. Mater. Today Energy 2018, 10, 108–117. [Google Scholar] [CrossRef]
- Zhao, B.; Xie, J.; Zhuang, H.; Liu, X.; Li, W.; Hu, X.; Jiang, Y.; Zhang, J. Improved low-temperature performance of surface modified lithium-rich Li1.2Ni0.13Co0.13Mn0.54O2 cathode materials for lithium ion batteries. Solid State Ion. 2020, 347, 115245. [Google Scholar] [CrossRef]
- Sun, Z.; Jiao, L.; Fan, Y.; Li, F.; Wang, D.; Han, D.; Niu, L. Industrialization of tailoring spherical cathode material towards high-capacity, cycling-stable and superior low temperature performance for lithium-ion batteries. RSC Adv. 2016, 6, 97818–97824. [Google Scholar] [CrossRef]
- Bi, K.; Zhao, S.-X.; Huang, C.; Nan, C.-W. Improving low-temperature performance of spinel LiNi0.5Mn1.5O4 electrode and LiNi0.5Mn1.5O4/Li4Ti5O12 full-cell by coating solid-state electrolyte Li-Al-Ti-P-O. J. Power Sources 2018, 389, 240–248. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, X.; Tang, Y.; Xia, H.; Zeng, Y.; Qiao, L.; Zhu, Z.; Lv, Z.; Zhang, Y.; Ge, X.; et al. Lowering Charge Transfer Barrier of LiMn2O4 via Nickel Surface Doping to Enhance Li+ Intercalation Kinetics at Subzero Temperatures. J. Am. Chem. Soc. 2019, 141, 14038–14042. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Zhao, C.; Yang, X.; Jiang, Q. Effects of carbon coating and metal ions doping on low temperature electrochemical properties of LiFePO4 cathode material. Electrochim. Acta 2012, 83, 341–347. [Google Scholar] [CrossRef]
- Nagasubramanian, G. Electrical characteristics of 18650 Li-ion cells at low temperatures. J. Appl. Electrochem. 2001, 31, 99–104. [Google Scholar] [CrossRef]
- Kim, J.W.; Jung, K.; Yim, T. Dually-functionalized Ni-rich layered oxides for high-capacity lithium-ion batteries. J. Mater. Sci. Technol. 2021, 86, 70–76. [Google Scholar] [CrossRef]
- Tan, S.; Wang, L.; Bian, L.; Xu, J.; Ren, W.; Hu, P.; Chang, A. Highly enhanced low temperature discharge capacity of LiNi1/3Co1/3Mn1/3O2 with lithium boron oxide glass modification. J. Power Sources 2015, 277, 139–146. [Google Scholar] [CrossRef]
- Li, G.; Huang, Z.; Zuo, Z.; Zhang, Z.; Zhou, H. Understanding the trace Ti surface doping on promoting the low temperature performance of LiNi1/3Co1/3Mn1/3O2 cathode. J. Power Sources 2015, 281, 69–76. [Google Scholar] [CrossRef]
- Bakierska, M.; Chudzik, K.; Świętosławski, M.; Klejna, S.; Kubicka, M.; Marciszko-Wiąckowska, M.; Gajewska, M.; Walas, S.; Molenda, M. Reversible Cation-Mediated Anionic Redox in Defect Spinel Structure for High Power Batteries. Adv. Funct. Mater. 2022, 32, 2108278. [Google Scholar] [CrossRef]
- Oh, H.H.; Joo, J. Colloidal synthesis of monodisperse ultrathin LiFePO4 nanosheets for Li-ion battery cathodes. Korean J. Chem. Eng. 2021, 38, 1052–1058. [Google Scholar] [CrossRef]
- Kim, C.; Yang, Y.; Ha, D.; Kim, D.H.; Kim, H. Crystal alignment of a LiFePO4 cathode material for lithium ion batteries using its magnetic properties. RSC Adv. 2019, 9, 31936–31942. [Google Scholar] [CrossRef]
- Zhu, Z.; Tang, Y.; Lv, Z.; Wei, J.; Zhang, Y.; Wang, R.; Zhang, W.; Xia, H.; Ge, M.; Chen, X. Fluoroethylene Carbonate Enabling a Robust LiF-rich Solid Electrolyte Interphase to Enhance the Stability of the MoS2 Anode for Lithium-Ion Storage. Angew. Chem. Int. Ed. 2018, 57, 3656–3660. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Zhang, Y.; Zheng, S. Lithium adsorption from brine by iron-doped titanium lithium ion sieves. Particuology 2018, 41, 40–47. [Google Scholar] [CrossRef]
- Alhammadi, A.S.; Yun, H.J.; Choi, D. Investigation of LiFePO4/MWCNT cathode-based half-cell lithium-ion batteries in subzero temperature environments. Ionics 2023, 29, 2163–2174. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Liang, F.; Yao, Y.; Zhang, K. Enhancing high rate performance and cyclability of LiFePO4 cathode materials for lithium ion batteries by boron doping. J. Alloys Compd. 2021, 880, 160560. [Google Scholar] [CrossRef]
- Cui, X.; Tuo, K.; Xie, Y.; Li, C.; Zhao, D.; Yang, L.; Fu, X.; Li, S. Investigation on electrochemical performance at the low temperature of LFP/C-P composite based on phosphorus doping carbon network. Ionics 2020, 26, 3795–3808. [Google Scholar] [CrossRef]
- Zhao, N.; Li, Y.; Zhao, X.; Zhi, X.; Liang, G. Effect of particle size and purity on the low temperature electrochemical performance of LiFePO4/C cathode material. J. Alloys Compd. 2016, 683, 123–132. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Yi, M.; Shen, Z.; Liu, L.; Liu, H.; Zhang, X. Coating LiFePO4 with Conductive Nanodots by Magnetron Sputtering: Toward High-Performance Cathode for Lithium-Ion Batteries. Energy Technol. 2019, 7, 1800634. [Google Scholar] [CrossRef]
- Peng, Y.; Tan, R.; Ma, J.; Li, Q.; Wang, T.; Duan, X. Electrospun Li3V2(PO4)3 nanocubes/carbon nanofibers as free-standing cathodes for high-performance lithium-ion batteries. J. Mater. Chem. A 2019, 7, 14681–14688. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, X.; Zhang, Y.; Pi, Y.; Zhao, Y.; Tian, X.; An, Q.; Wei, Q.; Mai, L. Hierarchical Carbon Decorated Li3V2(PO4)3 as a Bicontinuous Cathode with High-Rate Capability and Broad Temperature Adaptability. Adv. Energy Mater. 2014, 4, 1400107. [Google Scholar] [CrossRef]
- Dong, X.; Yang, Y.; Wang, B.; Cao, Y.; Wang, N.; Li, P.; Wang, Y.; Xia, Y. Low-Temperature Charge/Discharge of Rechargeable Battery Realized by Intercalation Pseudocapacitive Behavior. Adv. Sci. 2020, 7, 2000196. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Li, X.; Hu, X.; Cai, S.; Zheng, C. Recent progress in rate and cycling performance modifications of vanadium oxides cathode for lithium-ion batteries. J. Energy Chem. 2021, 59, 343–363. [Google Scholar] [CrossRef]
- Sides, C.R.; Martin, C.R. Nanostructured Electrodes and the Low-Temperature Performance of Li-Ion Batteries. Adv. Mater. 2005, 17, 125–128. [Google Scholar] [CrossRef]
- Von Wald Cresce, A.; Xu, K. Aqueous lithium-ion batteries. Carbon Energy 2021, 3, 721–751. [Google Scholar] [CrossRef]
- Chen, L.; Cao, L.; Ji, X.; Hou, S.; Li, Q.; Chen, J.; Yang, C.; Eidson, N.; Wang, C. Enabling safe aqueous lithium ion open batteries by suppressing oxygen reduction reaction. Nat. Commun. 2020, 11, 2638. [Google Scholar] [CrossRef]
- Ramanujapuram, A.; Yushin, G. Understanding the Exceptional Performance of Lithium-Ion Battery Cathodes in Aqueous Electrolytes at Subzero Temperatures. Adv. Energy Mater. 2018, 8, 1802624. [Google Scholar] [CrossRef]
- Tron, A.; Jeong, S.; Park, Y.D.; Mun, J. Aqueous Lithium-Ion Battery of Nano-LiFePO4 with Antifreezing Agent of Ethyleneglycol for Low-Temperature Operation. ACS Sustain. Chem. Eng. 2019, 7, 14531–14538. [Google Scholar] [CrossRef]
- Kühnel, R.-S.; Böckenfeld, N.; Passerini, S.; Winter, M.; Balducci, A. Mixtures of ionic liquid and organic carbonate as electrolyte with improved safety and performance for rechargeable lithium batteries. Electrochim. Acta 2011, 56, 4092–4099. [Google Scholar] [CrossRef]
- Ue, M.; Takeda, M.; Toriumi, A.; Kominato, A.; Hagiwara, R.; Ito, Y. Application of Low-Viscosity Ionic Liquid to the Electrolyte of Double-Layer Capacitors. J. Electrochem. Soc. 2003, 150, A499–A502. [Google Scholar] [CrossRef]
- Liu, B.; Li, Q.; Engelhard, M.H.; He, Y.; Zhang, X.; Mei, D.; Wang, C.; Zhang, J.-G.; Xu, W. Constructing Robust Electrode/Electrolyte Interphases to Enable Wide Temperature Applications of Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 21496–21505. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. A new approach toward improved low temperature performance of Li-ion battery. Electrochem. Commun. 2002, 4, 928–932. [Google Scholar] [CrossRef]
- Deiner, L.J.; Bezerra, C.A.G.; Howell, T.G.; Powell, A.S. Digital Printing of Solid-State Lithium-Ion Batteries. Adv. Eng. Mater. 2019, 21, 1900737. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Wang, Y.; Ren, G.; Yuan, C. Towards rational mechanical design of inorganic solid electrolytes for all-solid-state lithium ion batteries. Energy Storage Mater. 2020, 26, 313–324. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, J. Low-temperature all-solid-state lithium-ion batteries based on a di-cross-linked starch solid electrolyte. RSC Adv. 2019, 9, 34601–34606. [Google Scholar] [CrossRef]
- Li, X.; Hou, Q.; Huang, W.; Xu, H.-S.; Wang, X.; Yu, W.; Li, R.; Zhang, K.; Wang, L.; Chen, Z.; et al. Solution-Processable Covalent Organic Framework Electrolytes for All-Solid-State Li–Organic Batteries. ACS Energy Lett. 2020, 5, 3498–3506. [Google Scholar] [CrossRef]
- Tang, X.; Lv, S.; Jiang, K.; Zhou, G.; Liu, X. Recent development of ionic liquid-based electrolytes in lithium-ion batteries. J. Power Source 2022, 542, 231792. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, L.; Meng, T.; Zhou, S.; Sui, X.; Hu, X. Hybrid Ionogel Electrolytes for Advanced Lithium Secondary Batteries: Developments and Challenges. Chem.—Asian J. 2022, 17, e202200794. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.F.J.; Kyratzis, I.L.; Best, A.S. Lithium-Ion Battery Separators for Ionic-Liquid Electrolytes: A Review. Adv. Mater. 2020, 32, e1904205. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, L.; Scheers, J.; Matic, A. Enhanced low-temperature ionic conductivity via different Li+ solvated clusters in organic solvent/ionic liquid mixed electrolytes. Phys. Chem. Chem. Phys. 2016, 18, 25458–25464. [Google Scholar] [CrossRef]
- Chen, M.; Wu, J.; Ye, T.; Ye, J.; Zhao, C.; Bi, S.; Yan, J.; Mao, B.; Feng, G. Adding salt to expand voltage window of humid ionic liquids. Nat. Commun. 2020, 11, 5809. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, X.; Misra, R.K.; Cai, Q.; Zhao, Y. Progress in electrolytes for beyond-lithium-ion batteries. J. Mater. Sci. Technol. 2020, 44, 237–257. [Google Scholar] [CrossRef]
- Gao, Z.; Rao, S.; Zhang, T.; Li, W.; Yang, X.; Chen, Y.; Zheng, Y.; Ding, Y.; Dong, T.; Li, S. Design Strategies of Flame-Retardant Additives for Lithium Ion Electrolyte. J. Electrochem. Energy Convers. Storage 2022, 19, 030910. [Google Scholar] [CrossRef]
- Liu, Y.-K.; Zhao, C.-Z.; Du, J.; Zhang, X.-Q.; Chen, A.-B.; Zhang, Q. Research Progresses of Liquid Electrolytes in Lithium-Ion Batteries. Small 2023, 19, 2205315. [Google Scholar] [CrossRef]
- Dong, X.; Lin, Y.; Li, P.; Ma, Y.; Huang, J.; Bin, D.; Wang, Y.; Qi, Y.; Xia, Y. High-Energy Rechargeable Metallic Lithium Battery at −70 °C Enabled by a Cosolvent Electrolyte. Angew. Chem. Int. Engl. Ed. 2019, 58, 5623–5627. [Google Scholar] [CrossRef]
- Luo, D.; Li, M.; Zheng, Y.; Ma, Q.; Gao, R.; Zhang, Z.; Dou, H.; Wen, G.; Shui, L.; Yu, A.; et al. Electrolyte Design for Lithium Metal Anode-Based Batteries Toward Extreme Temperature Application. Adv. Sci. 2021, 8, e2101051. [Google Scholar] [CrossRef]
- Su, X.; Xu, Y.; Wu, Y.; Li, H.; Yang, J.; Liao, Y.; Qu, R.; Zhang, Z. Liquid electrolytes for low-temperature lithium batteries: Main limitations, current advances, and future perspectives. Energy Storage Mater. 2023, 56, 642–663. [Google Scholar] [CrossRef]
- Phiri, I.; Ko, S.; Kim, S.; Afrifah, V.A.; Lee, K.; Kim, S.H.; Ko, J.M. Zwitterionic osmolyte-inspired additives as scavengers and low temperature performance enhancers for lithium ion batteries. Mater. Lett. 2021, 288, 129366. [Google Scholar] [CrossRef]
- Xu, G.; Huang, S.; Cui, Z.; Du, X.; Wang, X.; Lu, D.; Shangguan, X.; Ma, J.; Han, P.; Zhou, X.; et al. Functional additives assisted ester-carbonate electrolyte enables wide temperature operation of a high-voltage (5 V-Class) Li-ion battery. J. Power Source 2019, 416, 29–36. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; Li, M.; Qiu, J.; Wen, Y.; Chen, J. Effect of fluoroethylene carbonate additive on the low–temperature performance of lithium–ion batteries. J. Electroanal. Chem. 2022, 925, 116870. [Google Scholar] [CrossRef]
- Klein, S.; Harte, P.; van Wickeren, S.; Borzutzki, K.; Röser, S.; Bärmann, P.; Nowak, S.; Winter, M.; Placke, T.; Kasnatscheew, J. Re-evaluating common electrolyte additives for high-voltage lithium ion batteries. Cell Rep. Phys. Sci. 2021, 2, 100521. [Google Scholar] [CrossRef]
- Kalantzopoulos, G.N.; Lundvall, F.; Checchia, S.; Lind, A.; Wragg, D.S.; Fjellvåg, H.; Arstad, B. In Situ Flow MAS NMR Spectroscopy and Synchrotron PDF Analyses of the Local Response of the Brønsted Acidic Site in SAPO-34 during Hydration at Elevated Temperatures. ChemPhysChem 2017, 19, 519–528. [Google Scholar] [CrossRef]
- Hu, X.; Zheng, Y.; Howey, D.A.; Perez, H.; Foley, A.; Pecht, M. Battery warm-up methodologies at subzero temperatures for automotive applications: Recent advances and perspectives. Prog. Energy Combust. Sci. 2020, 77, 100806. [Google Scholar] [CrossRef]
- He, F.; Li, X.; Zhang, G.; Zhong, G.; He, J. Experimental investigation of thermal management system for lithium ion batteries module with coupling effect by heat sheets and phase change materials. Int. J. Energy Res. 2018, 42, 3279–3288. [Google Scholar] [CrossRef]
- Ruan, H.; Sun, B.; Cruden, A.; Zhu, T.; Jiang, J.; He, X.; Su, X.; Ghoniem, E. Optimal external heating resistance enabling rapid compound self-heating for lithium-ion batteries at low temperatures. Appl. Therm. Eng. 2021, 200, 117536. [Google Scholar] [CrossRef]
- Wu, S.; Xiong, R.; Li, H.; Nian, V.; Ma, S. The state of the art on preheating lithium-ion batteries in cold weather. J. Energy Storage 2020, 27, 101059. [Google Scholar] [CrossRef]




| Methods | Chemistry | Test | Results | Ref. |
|---|---|---|---|---|
| Cell fabrication/ structure optimization | 1 M LiPF6 + DEC/EC (1:1 vol), 1 M (homemade) LiClO4 in PC | 30 °C, EIS | The ionic conductivity is 300% higher than before | [31] |
| Li|electrolyte|Li cells | Potentiostatic polarization at voltage bias of 20 mV Test: AC impedance and DC potentiostatic polarization measurements | The tLi+ is 460% higher than before | [31] | |
| half-cells | C/10 and 1 C, rate capability tests | Increase the specific charge capacity by threefold | [33] |
| Anode | Chemistry | Methods | Action Mechanism | Test | Results | Ref. |
|---|---|---|---|---|---|---|
| Carbon | 1 M LiPF6 + EMC/DMC/EC (1:1:1 vol), hard carbon/LVP cells | Prelithiation treatment | The lithium-ions needed to make the negative electrode form an SEI film are obtained from somewhere other than the positive electrode | −40 °C, 0.25 C, 3.5 to 4.3 V, charge–discharge test | Without improvement: cannot work After improvement: capacity retention rate exceeds 67% | [38] |
| 1 M LiPF6 + DMC/EC (1:1 vol), graphite CR2032-type coin cells | Embedding nano-Sn | The nano-Sn particles/graphene/2D graphene alternating stack structure will shorten the diffusion distance of lithium-ions | −20 °C, EIS | After improvement: the charge-transfer resistance is 104 Ω Without improvement: too large to measure | [40] | |
| LTO | 1 M LiPF6 + DMC/EC (1:1 wt), LFP/LTO full cells | Reducing the particle size | Smaller particle sizes will increase the number of surface sites for lithium insertion, shorten the diffusion path of lithium-ions, and may form denser composite structures | −20 °C, 0.1 C, 1 to 2.6 V, charge–discharge test | The capacity of small particles is about 31% higher than that of large particles | [49] |
| Si | 1 M LiPF6 + FEC/DMC (1:4 vol), Si/Li cells | Use silicon instead of graphite | The charge-transfer resistance of silicon material is smaller than that of graphite at zero temperature, and the diffusion rate of lithium-ion is faster | −30 °C, 0.25 C, 0.24 mA cm−2, charge capacity test | The capacity of Si anode is 28.8-times that of graphite anode | [43] |
| SnO2 | 1 M LiPF6 + EC/PC/EMC (1:1:2 vol) + FEC (5 wt%), SnO2 2016 coin-type half-cells | Use SnO2 instead of graphite | The low temperature inhibits Sn coarsening to maintain the high reversibility of the SnO2 alloying and conversion reactions, ensuring a stable capacity | −10 °C, capacity retention rate test | The capacity retention is 680% higher than that of graphite anode | [44] |
| Cathode | Chemistry | Methods | Action Mechanism | Test | Results | Ref. |
|---|---|---|---|---|---|---|
| Layered oxides | 1 M LiPF6 + EC/PC/EMC, NCM111 CR2025-type coin cells | Coating Li3BO3 | Li3BO3 coating can reduce the direct contact between the active substance and the electrolyte and can effectively reduce the charge-transfer resistance at low temperatures | −40 °C, 0.2 C, 2 to 4.8 V, discharge capacity test | The discharge capacity increased by 173.9% | [67] |
| 1 M LiPF6 + DMC/EC (1:1 wt), NCM111 CR2025-type coin cells | Coating AlF3 | The spinel structure produced by aluminum fluoride coating improves ion migration and enhances lithium diffusion kinetics | −20 °C, 0.1 C, 2.5 to 4.5 V, discharge capacity test | The discharge capacity increased by about 100% | [60] | |
| 1 M LiPF6 + DMC/EC (1:1 vol), NCM111 2032 coin-type half-cells | Reducing the particle size | The layered nanorods assembled with a small particle size have a stable structure, fast ion transport, a large surface area, full contact between the electrolyte and cathode, and good capacity reversibility | 0 °C, 1 C, 3 to 4.4 V, discharge capacity test | The discharge capacity of the minimum particle size is 27.6% higher than that of the maximum particle size | [61] | |
| 1 M LiPF6 + EMC/DMC/EC (1:1:1 vol) Li/NCM111 R2032-type coin cells | Doping Ti | Ti doping can change the lattice parameters of cathodes during charging and dis-charging and reduce impedance | −20 °C, 1 C, 2.8 to 4.25 V, discharge capacity test | The discharge capacity increased by 16.0% | [68] | |
| Spinel-structured oxides | 1 M LiPF6 + EC/DEC (3:7 vol), Li/LNMO CR2032-type coin cells | Coating LATP | LATP coating can reduce polarization and charge-transfer resistance at low temperatures, which is conducive to lithium-ion diffusion | −20 °C, 0.1 C, 3.5 to 5 V, discharge capacity test | The discharge capacity increased by 71.0% | [62] |
| Li/LMO coin cells | Doping Ni | Ni doping and the change in the ratio of Mn4+–O2− bond reduces the energy barrier during charge-transfer by about 20%, alleviating the energy loss at lower temperatures | −20 °C, 0.2 C capacity retention rate test | The capacity retention rate increased by 38.5% | [63] | |
| Polyanionic-type compounds | 1 M LiPF6 + EC/DEC (1:1 vol), Li/LFP 2032-type coin cells | Doping La and Mg | Cationic defects caused by doping elements can increase the conductivity, and the porous structure formed by doping shorts the solid-state diffusion path | −20 °C, 10 C, 2.5 to 4.2 V, discharge capacity test | The discharge capacity increased by 379.8% | [64] |
| 1 M LiPF6 + EMC/DMC/EC (1:1:1 vol), LFP 2025-type coin cells | Coating C and doping P | C coating and P doping can alleviate the corrosion of the electrolyte on the cathode and can also establish a certain number of interconnecting channels to shorten the diffusion path of lithium-ions | −25 °C, 1.5 C, 2.7 to 4.2 V, discharge capacity test | The discharge capacity increased by 35.0% | [76] |
| Cathode | Chemistry | Methods | Test | Results at Low Temperatures | Results at Higher Temperatures | Ref. |
|---|---|---|---|---|---|---|
| Layered oxides | 1 M LiPF6 + EC/PC/EMC, NCM111 CR2025-type coin cells | Coating Li3BO3 | −40 °C, 20 °C, 0.2 C, 2 to 4.5 V, discharge capacity test | The discharge capacity increased by 173.9% | The discharge capacity increased by 6.3% | [67] |
| 1 M LiPF6 + DMC/EC (1:1 wt), NCM111 CR2025-type coin cells | Coating AlF3 | −20 °C, 25 °C, 0.1 C, 2.5 to 4.5 V, discharge capacity test | The discharge capacity increased by about 100% | The discharge capacity increased by 4.5% | [60] | |
| 1 M LiPF6 + EMC/DMC/EC (1:1:1 vol) Li/NCM111 R2032-type coin cells | Doping Ti | −20 °C, 25 °C, 1 C, 2.8 to 4.25 V, discharge capacity test | The discharge capacity increased by 16.0% | Little improvement | [68] | |
| Spinel-structured oxides | 1 M LiPF6 + EC/DEC (3:7 vol), Li/LNMO CR2032-type coin cells | Coating LATP | −20 °C, 25 °C, 0.1 C, 3.5 to 5 V, discharge capacity test | The discharge capacity increased by 71.0% | The discharge capacity increased by 6.4% | [62] |
| Li/LMO coin cells | Doping Ni | −20 °C, 25 °C, 0.2 C capacity retention rate test | The capacity retention rate increased by 38.5% | Little improvement | [63] | |
| Polyanionic-type compounds | 1 M LiPF6 + EC/DEC (1:1 vol), Li/LFP 2032-type coin cells | Doping La and Mg | −20 °C, 20 °C, 10 C, 2.5 to 4.2 V, discharge capacity test | The discharge capacity increased by 379.8% | The capacity is not mentioned, but the charge-transfer resistance is only about 30Ω higher than at −20 °C | [64] |
| 1 M LiPF6 + EMC/DMC/EC (1:1:1 vol), LFP 2025-type coin cells | Coating C and doping P | −25 °C, 0 °C, 1.5 C, 2.7 to 4.2 V, discharge capacity test | The discharge capacity increased by 35.0% | The discharge capacity increased by 15.0% | [76] |
| Electrolyte | Chemistry | Methods | Test | Results | Ref. |
|---|---|---|---|---|---|
| Aqueous electrolyte | saturated LiCl aqueous electrolyte solutions, LiCoO2 cells | Adding inorganic salt | −40 °C, 0.2 C, capacity retention rate test | Without improvement: cannot work After improvement: capacity retention rate is 72% | [86] |
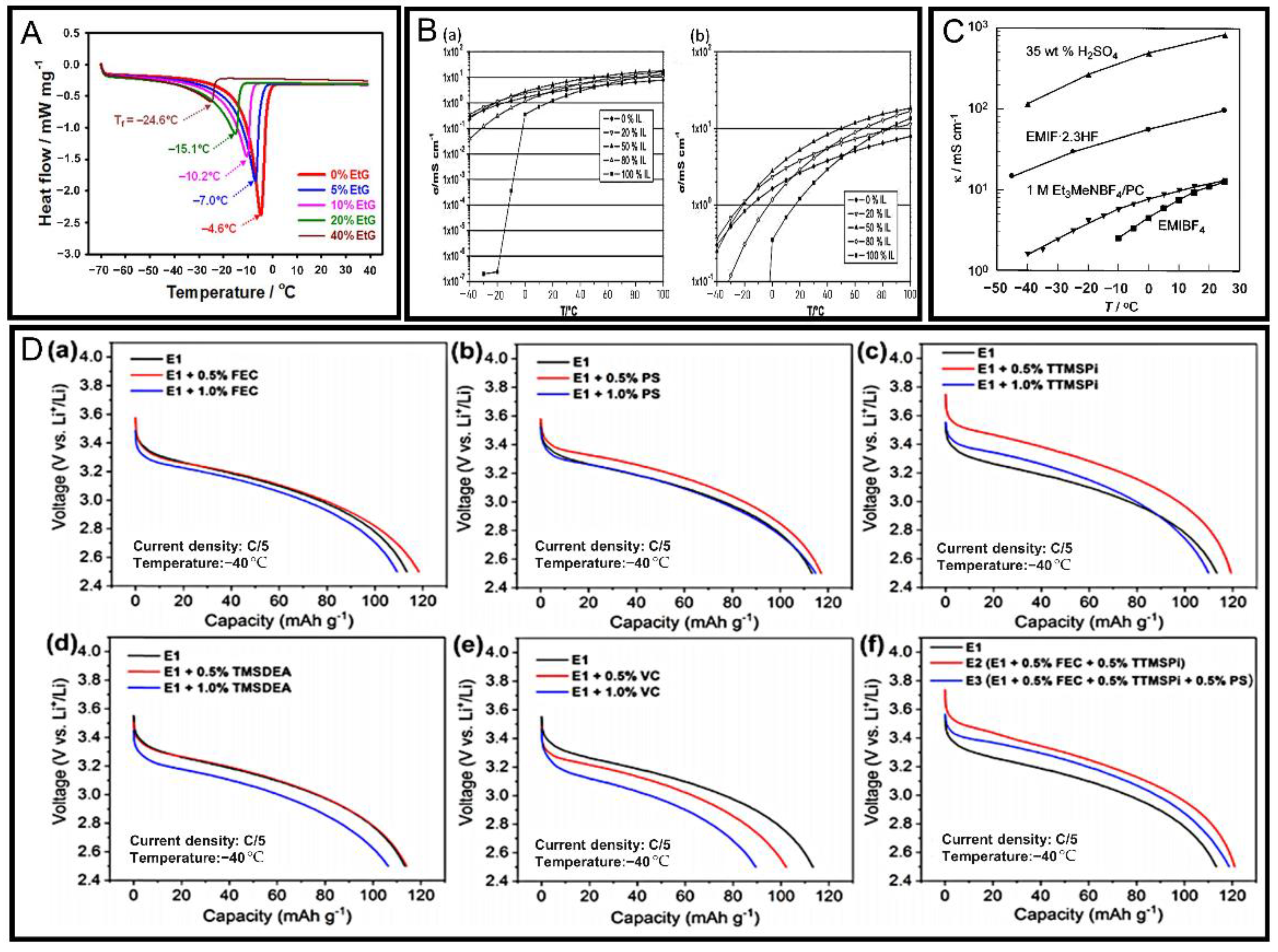
| 1 M Li2SO4 aqueous electrolyte solutions | Adding EtG | Cooling/heating rate: 5 °C min−1 40 °C to −70 °C, differential scanning calorimeter analysis | The crystallization temperature is reduced to −20 °C | [87] | |
| Cryogenic ionic liquid electrolyte | PYR14TFSI ionic liquid electrolyte, LFP cells | mixing PC | −20 °C, conductivity test | The conductivity is increased by more than 106 times | [88] |
| EMIF·2.3 HF ionic liquid electrolyte, 2032-type coin cells | Ionic liquid EMIF·2.3 HF | −40 °C, conductivity test | The conductivity is about 10-times higher than that of 1 M Et3MeNBF4/PC electrolyte | [89] | |
| Organic electrolyte | 5 M LiTFSI/EA + DCM (1:4 vol), Li/P cells | Adding low melting point cosolvent | −70 °C, conductivity test | The conductivity is 60-times higher than that of ordinary carbonate-based electrolyte | [104] |
| 1 M LiPF6 + MA/DEC/EC/EMC (3:1:1:1 vol), MCMB/LNMO full-cells | Adding TMSP and PCS additive | −5 °C, 0.3 C, 3.5 V to 4.9 V, capacity retention rate test | The capacity retention rate increased by 34.2% | [108] | |
| 1 M LiPF6 + EP/EMC/EC (4:1:1 wt), Li/LiCoO2 cells | Adding FEC additive | −40 °C, ionic conductivity test | The ionic conductivity increased by 333.6% | [109] | |
| 1 M LiPF6 + EP/EMC/EC (4:1:1 wt), Li/LiCoO2 cells | Adding FEC additive | −40 °C, 0.2 C, 3.0 V to 4.2 V, capacity retention rate test | The capacity retention rate increased by 16.8% | [109] |
| Scheme | Perspective | Concrete Measures | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|---|
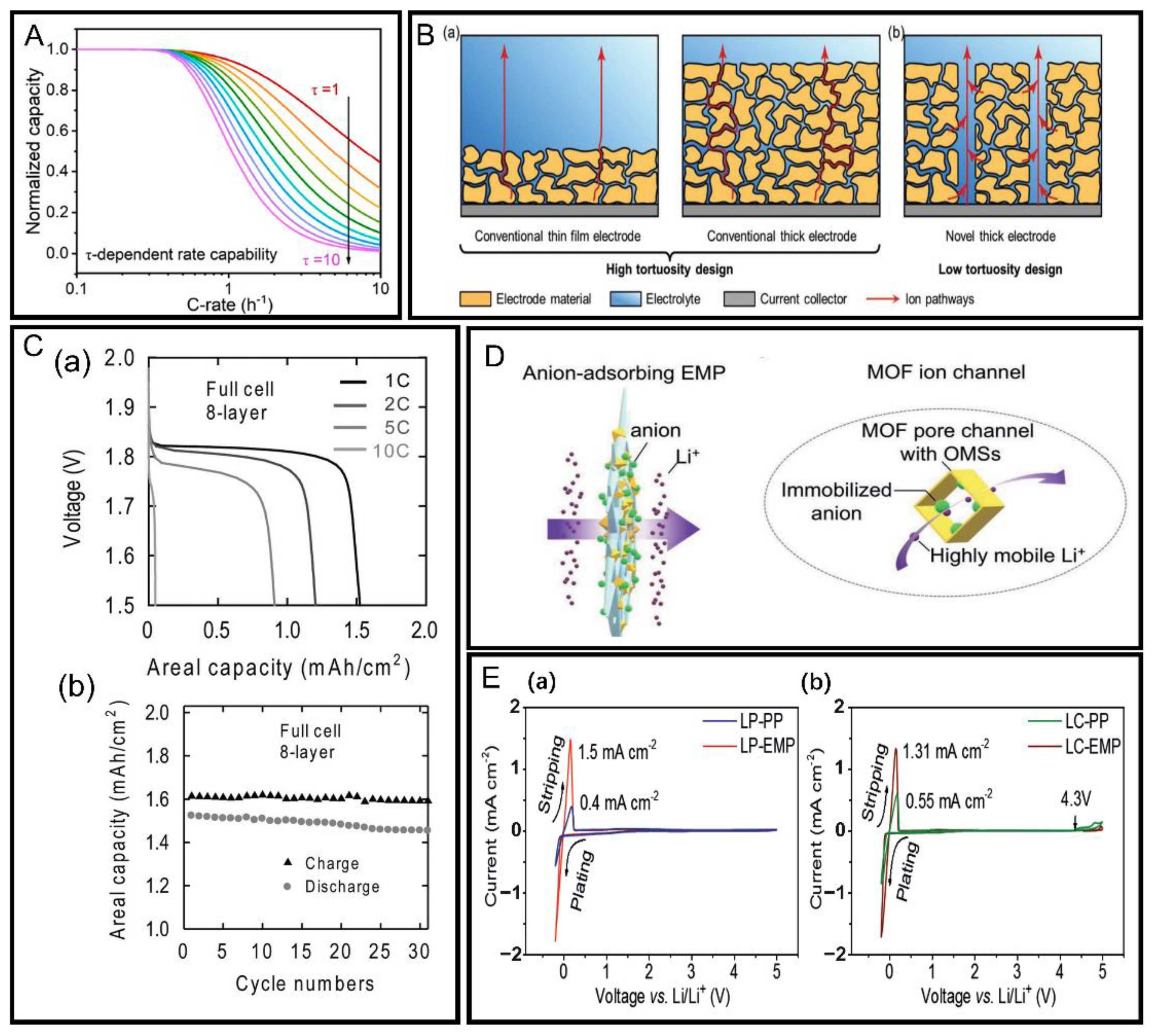
| Cell fabrication/structure optimization | Low tortuosity | 3D printing | Improves lithium-ion transport efficiency Increases cycle life Reduces electrode tortuosity | Cannot be used easily for large-scale industrial manufacturing | [30] |
| Use of new composite separator | Significantly increases lithium-ion transference number and lithium-ion conductivity Reduces interface resistance between electrolyte and electrode Significantly improves rate performance and cycle durability | Not mentioned | [31] | ||
| High porosity | Removal of solvent components from electrode | Improves ionic transport efficiency in electrode Has low cost | Increases electrode tortuosity | [28] | |
| Introduction of orderly directional pore structures into electrode | Effectively reduces tortuosity of electrode structure Improves hole regularity Improves lithium-ion transport efficiency | Has high complexity Needs more methods of directional introduction of holes | [28,29,30,31,32] | ||
| Electrode materials | Anode | Prelithiation treatment | Alleviates lithium plating | Cannot increase ionic conductivity Affects improvement effect of prelithiation through low ionic conductivity of electrolytes at low temperatures | [38] |
| Element doping | Increases battery capacity Improves rate capacity and cycle stability | May negatively affect battery capacity at room-temperature | [40,41,42] | ||
| Particle size reduction | Effectively reduces transport paths of ions and electrons Improves solid diffusion kinetics | May cause side effects due to increased surface area | [49,77] | ||
| Development of next generation of negative electrode materials, such as Si | Has high theoretical capacity, low possibility of lithium plating, good safety, and low cost (Si) | Leads to severe volume expansion | [43,50,51] | ||
| Cathode | Addition of coatings | Protects cathode surface from liquid electrolytes and unwanted side reactions Reduces battery polarization | May hinder lithium-ion transport due to thick coating, affecting storage of lithium-ions | [60,67] | |
| Particle size reduction | Same as those for anode materials | ||||
| Element doping | Same as those for anode materials | ||||
| Electrolytes | Aqueous electrolytes | Addition of inorganic salt | Reduces freezing point of solution Improves performance at low temperatures | Not mentioned | [86] |
| Addition of antifreeze | Prevents solution crystallization | May easily generate acidic substances, corroding metal | [37,96] | ||
| Solid electrolytes | Use of starch-based solid electrolyte | Improves transport ability of lithium-ions and working performance of LIBs at low temperatures and high pressures | Requires difficult electrolyte preparation Has high cost | [94] | |
| Use of COF solid electrolyte | Promotes single-ion conduction Rapidly combines with and separates from lithium-ions Improves ionic conductivity | Has high preparation cost Exhibits poor long-term use stability | [95] | ||
| Cryogenic ionic liquid electrolytes | Mixture of ionic liquid with traditional electrolyte | Reduces freezing point of electrolyte Improves low-temperature performance of battery | Not mentioned | [99,100] | |
| Organic electrolytes | Addition of low-melting-point cosolvent | Improves ionic conductivity at low temperatures | Can easily cause environmental pollution due to chemical composition | [37,104] | |
| Lithium salt anion modification | Improves ionic mobility | May decrease electrolyte conductivity due to low solubility of some modified lithium salts in aprotic solvents | [105] | ||
| Addition of additives | Improves electrochemical performance of electrolyte | May cause oxidation reaction on cathode (for some reducing additives) Increases difficulty of determining additive amount May cause graphite electrode to form thick SEI film (for certain electrolyte additives) | [107,109] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, J.; Deng, Y.; Ren, J.; Gao, Y.; Liu, Y.; Rao, S.; Li, W.; Gao, Z. Cell Design for Improving Low-Temperature Performance of Lithium-Ion Batteries for Electric Vehicles. Batteries 2023, 9, 373. https://doi.org/10.3390/batteries9070373
Zhan J, Deng Y, Ren J, Gao Y, Liu Y, Rao S, Li W, Gao Z. Cell Design for Improving Low-Temperature Performance of Lithium-Ion Batteries for Electric Vehicles. Batteries. 2023; 9(7):373. https://doi.org/10.3390/batteries9070373
Chicago/Turabian StyleZhan, Jincheng, Yifei Deng, Jiaoyi Ren, Yaohui Gao, Yuang Liu, Shun Rao, Weifeng Li, and Zhenhai Gao. 2023. "Cell Design for Improving Low-Temperature Performance of Lithium-Ion Batteries for Electric Vehicles" Batteries 9, no. 7: 373. https://doi.org/10.3390/batteries9070373
APA StyleZhan, J., Deng, Y., Ren, J., Gao, Y., Liu, Y., Rao, S., Li, W., & Gao, Z. (2023). Cell Design for Improving Low-Temperature Performance of Lithium-Ion Batteries for Electric Vehicles. Batteries, 9(7), 373. https://doi.org/10.3390/batteries9070373








