Rural Urban Nutrient Partnership (RUN): Life Cycle Assessment of Multi Nutrient Recovery from Kitchen Waste and Blackwater
Abstract
:1. Introduction
2. Results
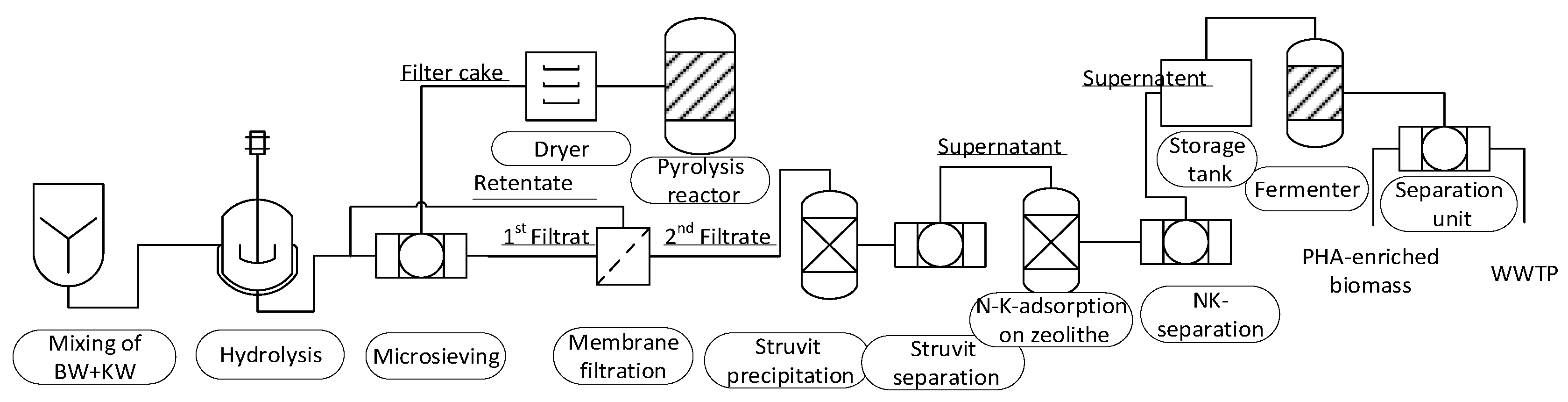
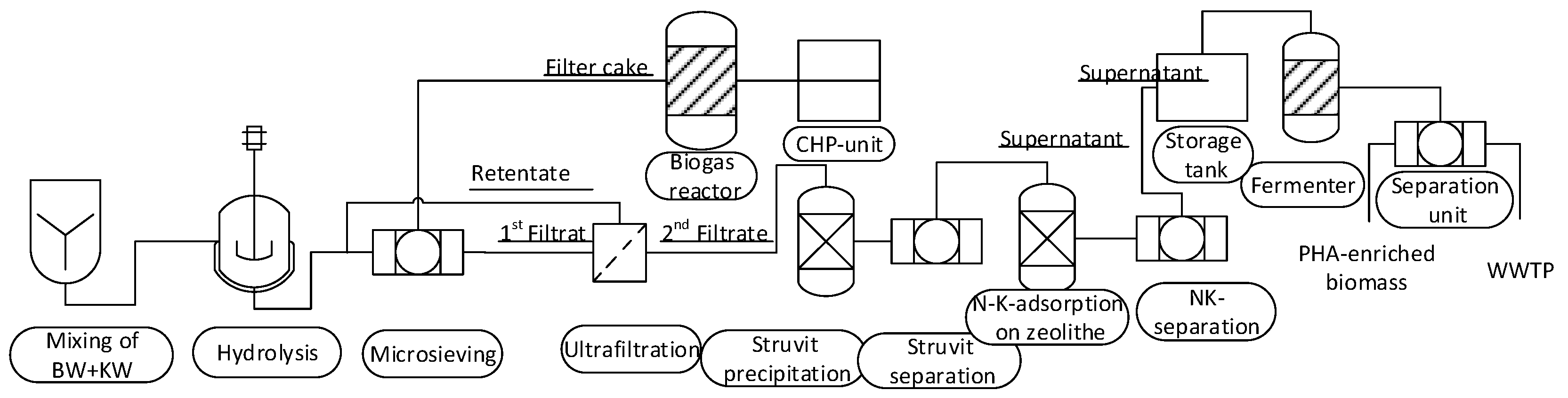
2.1. System
2.2. Identification of Substitution Products
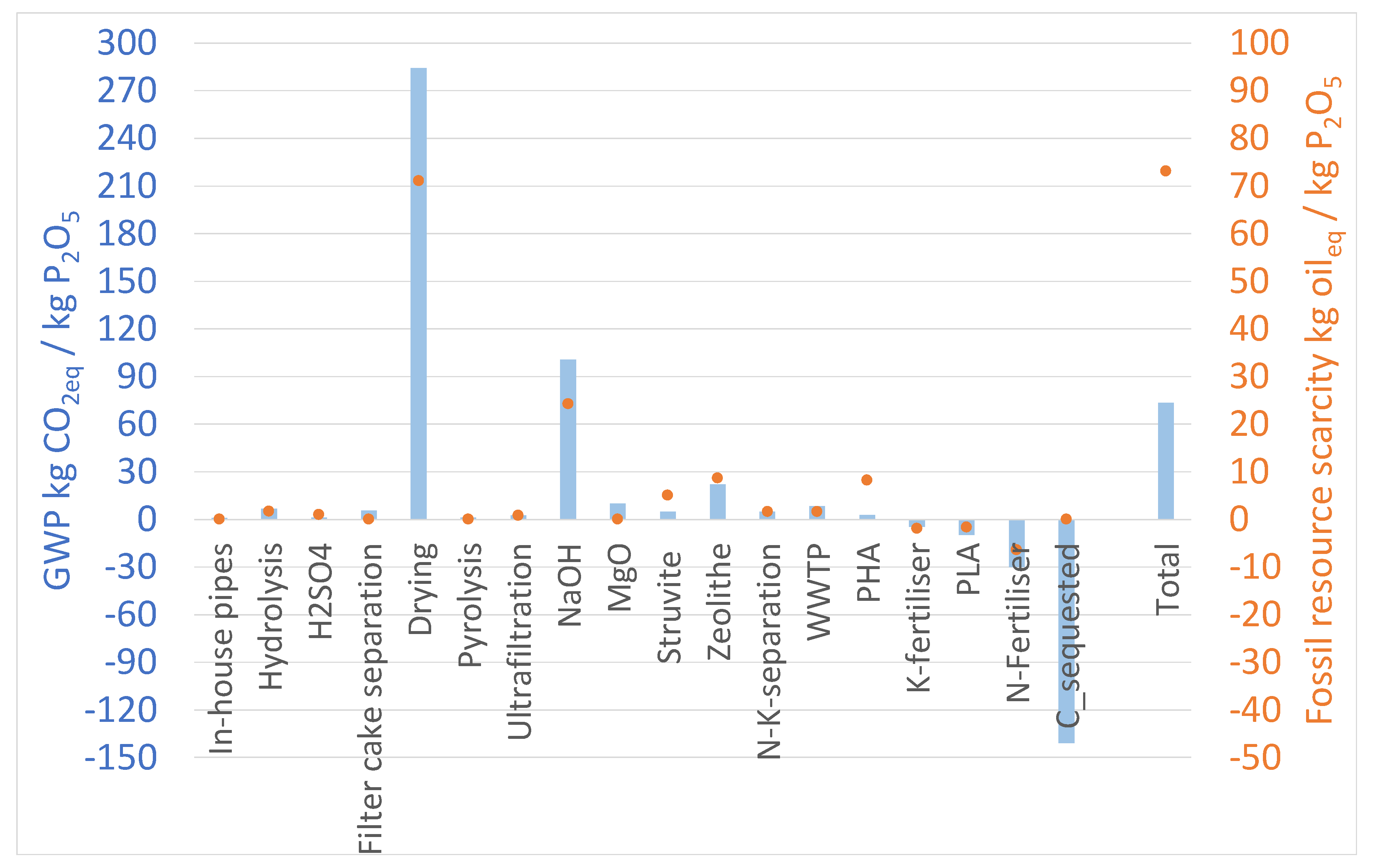
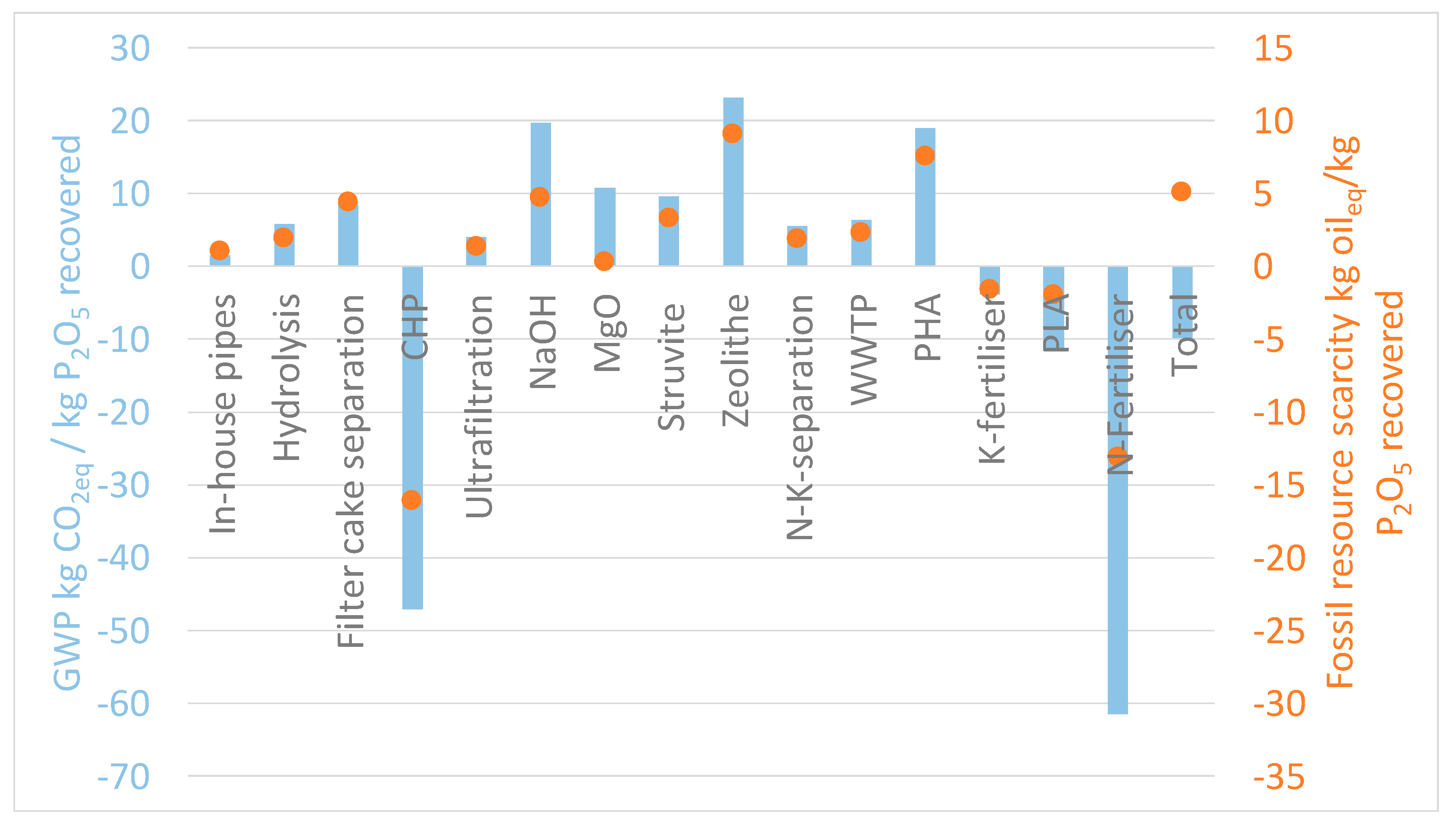
2.3. LCA Results at Laboratory and Technical Scale
3. Discussion
4. Materials and Methods
System Boundary
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Rockstrom, J.; Steffen, W.; Noone, K.; Persson, A.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; de Vries, W.; de Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.M.; Beare, D.J.; Bennett, E.M.; Hall-Spencer, J.M.; Ingram, J.S.I.; Jaramillo, F.; Ortiz, R.; Ramankutty, N.; Sayer, J.A.; Shindell, D. Agriculture production as a major driver of the Earth system exceeding planetary boundaries. Ecol. Soc. 2017, 22. [Google Scholar] [CrossRef]
- Conijn, J.G.; Bindraban, P.S.; Schröder, J.J.; Jongschaap, R.E.E. Can our global food system meet food demand within planetary boundaries? Agric. Ecosyst. Environ. 2018, 251, 244–256. [Google Scholar] [CrossRef]
- Springmann, M.; Wiebe, K.; Mason-D’Croz, D.; Sulser, T.B.; Rayner, M.; Scarborough, P. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: A global modelling analysis with country-level detail. Lancet Planet. Health 2018, 2, e451–e461. [Google Scholar] [CrossRef] [PubMed]
- Lenton, T.M.; Xu, C.; Abrams, J.F.; Ghadiali, A.; Loriani, S.; Sakschewski, B.; Zimm, C.; Ebi, K.L.; Dunn, R.R.; Svenning, J.-C.; et al. Quantifying the human cost of global warming. Nat. Sustain. 2023, 6, 1237–1247. [Google Scholar] [CrossRef]
- Sena, M.; Hicks, A. Life cycle assessment review of struvite precipitation in wastewater treatment. Resour. Conserv. Recycl. 2018, 139, 194–204. [Google Scholar] [CrossRef]
- Gaidajis, G.; Kakanis, I. Life Cycle Assessment of Nitrate and Compound Fertilizers Production—A Case Study. Sustainability 2021, 13, 148. [Google Scholar] [CrossRef]
- Hasler, K.; Bröring, S.; Omta, S.W.F.; Olfs, H.W. Life cycle assessment (LCA) of different fertilizer product types. Eur. J. Agron. 2015, 69, 41–51. [Google Scholar] [CrossRef]
- Makhlouf, A.; Serradj, T.; Cheniti, H. Life cycle impact assessment of ammonia production in Algeria: A comparison with previous studies. Environ. Impact Assess. Rev. 2015, 50, 35–41. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, G.; Frison, N.; Vázquez-Padín, J.R.; Hospido, A.; Garrido, J.M.; Fatone, F.; Bolzonella, D.; Moreira, M.T.; Feijoo, G. Life cycle assessment of nutrient removal technologies for the treatment of anaerobic digestion supernatant and its integration in a wastewater treatment plant. Sci. Total Environ. 2014, 490, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Ding, A.; Zhang, R.; Ngo, H.H.; He, X.; Ma, J.; Nan, J.; Li, G. Life cycle assessment of sewage sludge treatment and disposal based on nutrient and energy recovery: A review. Sci. Total Environ. 2021, 769, 144451. [Google Scholar] [CrossRef] [PubMed]
- Bisinella, V.; Schmidt, S.; Varling, A.S.; Laner, D.; Christensen, T.H. Waste LCA and the future. Waste Manage. 2024, 174, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Laratte, B.; Guillaume, B.; Kim, J.; Birregah, B. Modeling cumulative effects in life cycle assessment: The case of fertilizer in wheat production contributing to the global warming potential. Sci. Total Environ. 2014, 481, 588–595. [Google Scholar] [CrossRef]
- Ravi, R.; Beyers, M.; Bruun, S.; Meers, E. Life cycle assessment of struvite recovery and wastewater sludge end-use: A Flemish illustration. Resour. Conserv. Recycl. 2022, 182, 106325. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Xia, R.; Li, W.P.; Zhang, Y.; Zhang, K.; Tong, S.L.; Jia, R.N.; Hu, Q.; Wang, L.; et al. Phosphorus—The main limiting factor in riverine ecosystems in China. Sci. Total Environ. 2023, 870, 61613. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, F.; de Sario, S.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous removal and recovery from urban wastewater: Current practices and new directions. Sci. Total Environ. 2022, 823, 153750. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Boiarkina, I.; Yu, W.; Huang, H.M.; Munir, T.; Wang, G.Q.; Young, B.R. Phosphorous recovery through struvite crystallization: Challenges for future design. Sci. Total Environ. 2019, 648, 1244–1256. [Google Scholar] [CrossRef]
- Aguado, D.; Barat, R.; Bouzas, A.; Seco, A.; Ferrer, J. P-recovery in a pilot-scale struvite crystallisation reactor for source separated urine systems using seawater and magnesium chloride as magnesium sources. Sci. Total Environ. 2019, 672, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Manoukian, L.; Metson, G.S.; Herández, E.M.; Vaneeckhaute, C.; Frigon, D.; Omelon, S. Forging a cohesive path: Integrating life cycle assessments of primary-origin phosphorus fertilizer production and secondary-origin recovery from municipal wastewater. Resour. Conserv. Recycl. 2023, 199, 7260. [Google Scholar] [CrossRef]
- Mayer, B.K.; Baker, L.A.; Boyer, T.H.; Drechsel, P.; Gifford, M.; Hanjra, M.A.; Parameswaran, P.; Stoltzfus, J.; Westerhoff, P.; Rittmann, B.E. Total Value of Phosphorus Recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef] [PubMed]
- Sena, M.; Seib, M.; Noguera, D.R.; Hicks, A. Environmental impacts of phosphorus recovery through struvite precipitation in wastewater treatment. J. Clean. Prod. 2021, 280, 124222. [Google Scholar] [CrossRef]
- Igos, E.; Besson, M.; Navarrete Gutiérrez, T.; Bisinella de Faria, A.B.; Benetto, E.; Barna, L.; Ahmadi, A.; Spérandio, M. Assessment of environmental impacts and operational costs of the implementation of an innovative source-separated urine treatment. Water Res. 2017, 126, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Bisinella de Faria, A.B.; Spérandio, M.; Ahmadi, A.; Tiruta-Barna, L. Evaluation of new alternatives in wastewater treatment plants based on dynamic modelling and life cycle assessment (DM-LCA). Water Res. 2015, 84, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.; Bhandari, R.; Gäth, S.A. Life cycle assessment of prospective sewage sludge treatment paths in Germany. J. Environ. Manage. 2021, 290, 112557. [Google Scholar] [CrossRef]
- Meyer, C.; Preyl, V.; Steinmetz, H.; Maier, W.; Mohn, R.E.; Schönberger, H. The Stuttgart process. In Phosphorous Recovery and Recycling; Ohtake, H., Tsuneda, S., Eds.; Springer: Singapore, 2018. [Google Scholar]
- Hospido, A.; Moreira, M.T.; Fernández-Couto, M.; Feijoo, G. Environmental performance of a municipal wastewater treatment plant. Int. J. LCA 2004, 9, 261–271. [Google Scholar] [CrossRef]
- Corominas, L.; Byrne, D.M.; Guest, J.S.; Hospido, A.; Roux, P.; Shaw, A.; Short, M.D. The application of life cycle assessment (LCA) to wastewater treatment: A best practice guide and critical review. Water Res. 2020, 184, 116058. [Google Scholar] [CrossRef] [PubMed]
- Corominas, L.; Foley, J.; Guest, J.S.; Hospido, A.; Larsen, H.F.; Morera, S.; Shaw, A. Life cycle assessment applied to wastewater treatment: State of the art. Water Res. 2013, 47, 5480–5492. [Google Scholar] [CrossRef] [PubMed]
- Remy, C. Life Cycle Assessment of Selected Processes for P Recovery from Sewage Sludge, Sludge Liquor, or Ash, Sustainable Sewage Sludge Management Fostering Phosphorus Recovery and Energy Efficiency (P-REX). 2015. Available online: https://zenodo.org/records/242550 (accessed on 1 April 2024).
- Joseph, B.S.H. Influences of Management Practices and Methodological Choices on Life Cycle Assessment Results of Composting Mixtures of Biowaste and Green Cuts. Waste 2023, 1, 919–934. [Google Scholar] [CrossRef]
- Tonini, D.; Hamelin, L.; Wenzel, H.; Astrup, T. Bioenergy Production from Perennial Energy Crops: A Consequential LCA of 12 Bioenergy Scenarios including Land Use Changes. Environ. Sci. Technol. 2012, 46, 13521–13530. [Google Scholar] [CrossRef] [PubMed]
- Petersen, B.M. Life Cycle Assessment of Slurry Management Technologies; Miljøstyrelsen: Odense, Danmark, 2009. [Google Scholar]
- De Vries, J.W.; Groenestein, C.M.; De Boer, I.J.M. Environmental consequences of processing manure to produce mineral fertilizer and bio-energy. J. Environ. Manage. 2012, 102, 173–183. [Google Scholar] [CrossRef]
- Ahlgren, S.B.A.; Bernesson, S.; Nordberg, Å.; Norén, O.; Hansson, P.-A. Green Nitrogen-Possibilities for Production of Mineral Nitrogen Fertilisers Based on Renewable Resources in Sweden; Rapport (Institutionen för energi och teknik, SLU): Uppsala, Sweden, 2011; ISSN 1654–9406. [Google Scholar]
- Ahlgren, S.; Baky, A.; Bernesson, S.; Nordberg, Ã.; Norèn, O.; Hansson, P.-A. Ammonium nitrate fertiliser production based on biomass - environmental effects from a life cycle perspective. Bioresour. Technol. 2008, 99, 8034–8041. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Zoboli, O.; Krampe, J.; Rechberger, H.; Zessner, M.; Egle, L. Environmental impacts of phosphorus recovery from municipal wastewater. Resour. Conserv. Recycl. 2018, 130, 127–139. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char Sequestration in Terrestrial Ecosystems A Review. Mitig. Adapt. Strateg. Glob. Change 2006, 11, 395–419. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental Benefits of Biochar. J. Environ. Qual. 2012, 41, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sainju, U.M. Soil Carbon and Nitrogen Fractions and Crop Yields Affected by Residue Placement and Crop Types. PLoS ONE 2014, 9, e105039. [Google Scholar] [CrossRef] [PubMed]
- ISO 14044; Life Cycle Assessment—Requirements and Guidelines. Environ Manage. ISO: Geneva, Switzerland, 2006.
- ISO 14040; Environmental Management—Life Cycle Assessment—Principles and Framework. ISO: Geneva, Switzerland, 2006.
- Huijbregts, M.A.J.; Steinmann, Z.J.N.; Elshout, P.M.F.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; van Zelm, R. ReCiPe2016: A harmonised life cycle impact assessment method at midpoint and endpoint level. Int. J. LCA 2017, 22, 138–147. [Google Scholar] [CrossRef]
- Liu, J.; Nauta, J.; van Eekert, M.H.A.; Chen, W.-S.; Buisman, C.J.N. Integrated life cycle assessment of biotreatment and agricultural use of domestic organic residues: Environmental benefits, trade-offs, and impacts on soil application. Sci. Total Environ. 2023, 897, 165372. [Google Scholar] [CrossRef] [PubMed]
| N Fertilizer | K Fertilizer |
|---|---|
| Ammonium Nitrate (35% N) | Potassium Chloride (60% K2O) |
| Ammonium Sulphate (21% N) | Potassium Sulphate (50% K2O) |
| Calcium Ammonium Nitrate (26.5% N) | |
| Calcium Nitrate (12% N) | |
| Urea Ammonium Nitrate (32% N) | |
| Urea (46%) |
| Impact Category | Reference Unit | Lab Scale Total Incl. Credits | Lab Scale Chemicals | Tech Scale Total Incl. Credits | Tech Scale Chemicals |
|---|---|---|---|---|---|
| Fine particulate matter formation | kg PM2.5 eq | 2.0 × 10−1 | 1.5 × 10−1 | 7.1 × 10−2 | 3.5 × 10−2 |
| Fossil resource scarcity | kg oileq | 7.3 × 10 | 2.8 × 10 | 5.2 | 1.4 × 10 |
| Freshwater ecotoxicity | kg 1.4-DCB | 2.0 × 10 | 1.5 × 10 | −1.3 × 10 | 2.5 |
| Freshwater eutrophication | kg P eq | 1.6 × 10−1 | 1.4 × 10−1 | 1.3 × 10−2 | 2.8 × 10−2 |
| Global warming (GWP) | kg CO2 eq | 7.3 × 10 | 1.2 × 102 | −9.8 | 5.4 × 10 |
| Human carcinogenic toxicity | kg 1.4-DCB | 1.1 × 10 | 9.5 | 1.5 | 3.2 |
| Human non-carcinogenic toxicity | kg 1.4-DCB | 3.2 × 102 | 3.1 × 102 | −7.4 × 10 | 9.7 × 10 |
| Ionizing radiation | kBq Co-60 eq | 9.8 | 1.6 × 10 | 2.2 | 1.7 |
| Land use | m2 annual cropeq | −1.8 × 10 | 4.4 | −8.4 | −2.5 |
| Marine ecotoxicity | kg 1.4-DCB | 2.5 × 10 | 1.9 × 10 | −1.6 × 10 | 3.5 |
| Marine eutrophication | kg Neq | 1.2 × 10−2 | 1.5 × 10−2 | 1.3 × 10−3 | 2.2 × 10−3 |
| Mineral resource scarcity | kg Cueq | 7.3 × 10−1 | 6.1 × 10−1 | −3.6 × 10−1 | 1.5 × 10−1 |
| Ozone formation. Human health | kg NOx eq | 1.7 × 10−1 | 1.6 × 10−1 | −1.1 × 10−1 | 4.7 × 10−2 |
| Ozone formation. Terrestrial ecosystems | kg NOx eq | 1.8 × 10−1 | 1.7 × 10−1 | −1.1 × 10−1 | 4.8 × 10−2 |
| Stratospheric ozone depletion | kg CFC11 eq | −2.9 × 10−4 | 1.5 × 10−4 | −1.1 × 10−3 | 3.6 × 10−5 |
| Terrestrial acidification | kg SO2 eq | 4.8 × 10−1 | 3.3 × 10−1 | 2.3 × 10−1 | 6.2 × 10−2 |
| Terrestrial ecotoxicity | kg 1.4-DCB | 4.8 × 102 | 5.3 × 102 | −5.2 × 102 | 1.0 × 102 |
| Water consumption | m3 | −1.3 × 10 | 3.0 | 1.6 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stichnothe, H.; Joseph, B.; Preyl, V.; Meyer, C. Rural Urban Nutrient Partnership (RUN): Life Cycle Assessment of Multi Nutrient Recovery from Kitchen Waste and Blackwater. Recycling 2024, 9, 31. https://doi.org/10.3390/recycling9020031
Stichnothe H, Joseph B, Preyl V, Meyer C. Rural Urban Nutrient Partnership (RUN): Life Cycle Assessment of Multi Nutrient Recovery from Kitchen Waste and Blackwater. Recycling. 2024; 9(2):31. https://doi.org/10.3390/recycling9020031
Chicago/Turabian StyleStichnothe, Heinz, Ben Joseph, Volker Preyl, and Carsten Meyer. 2024. "Rural Urban Nutrient Partnership (RUN): Life Cycle Assessment of Multi Nutrient Recovery from Kitchen Waste and Blackwater" Recycling 9, no. 2: 31. https://doi.org/10.3390/recycling9020031





