Quantitative Comparison of Color-Coded Parametric Imaging Technologies Based on Digital Subtraction and Digital Variance Angiography: A Retrospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Image Acquisition
2.3. Image Processing
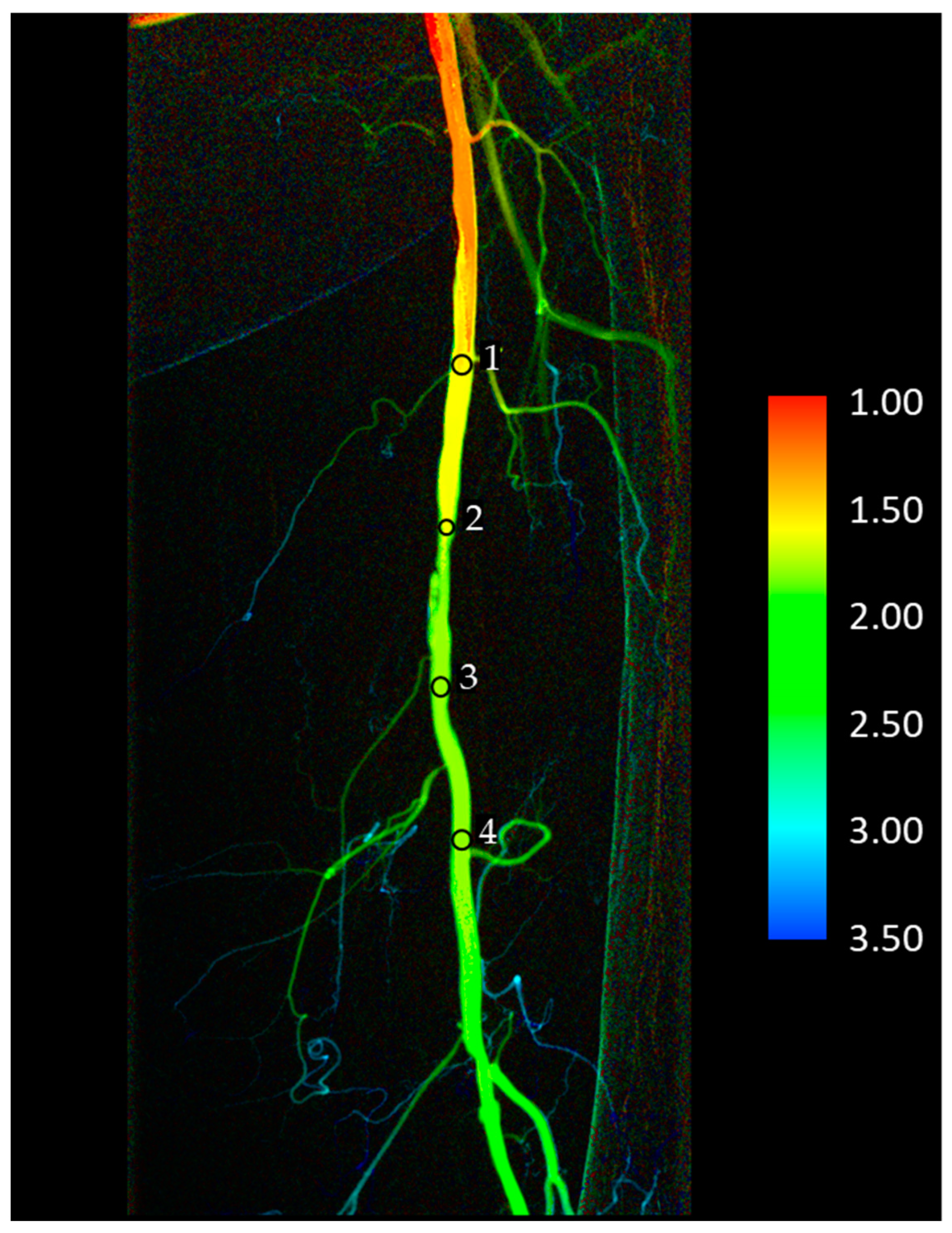
2.4. Data Collection
2.5. Data Analysis
3. Results
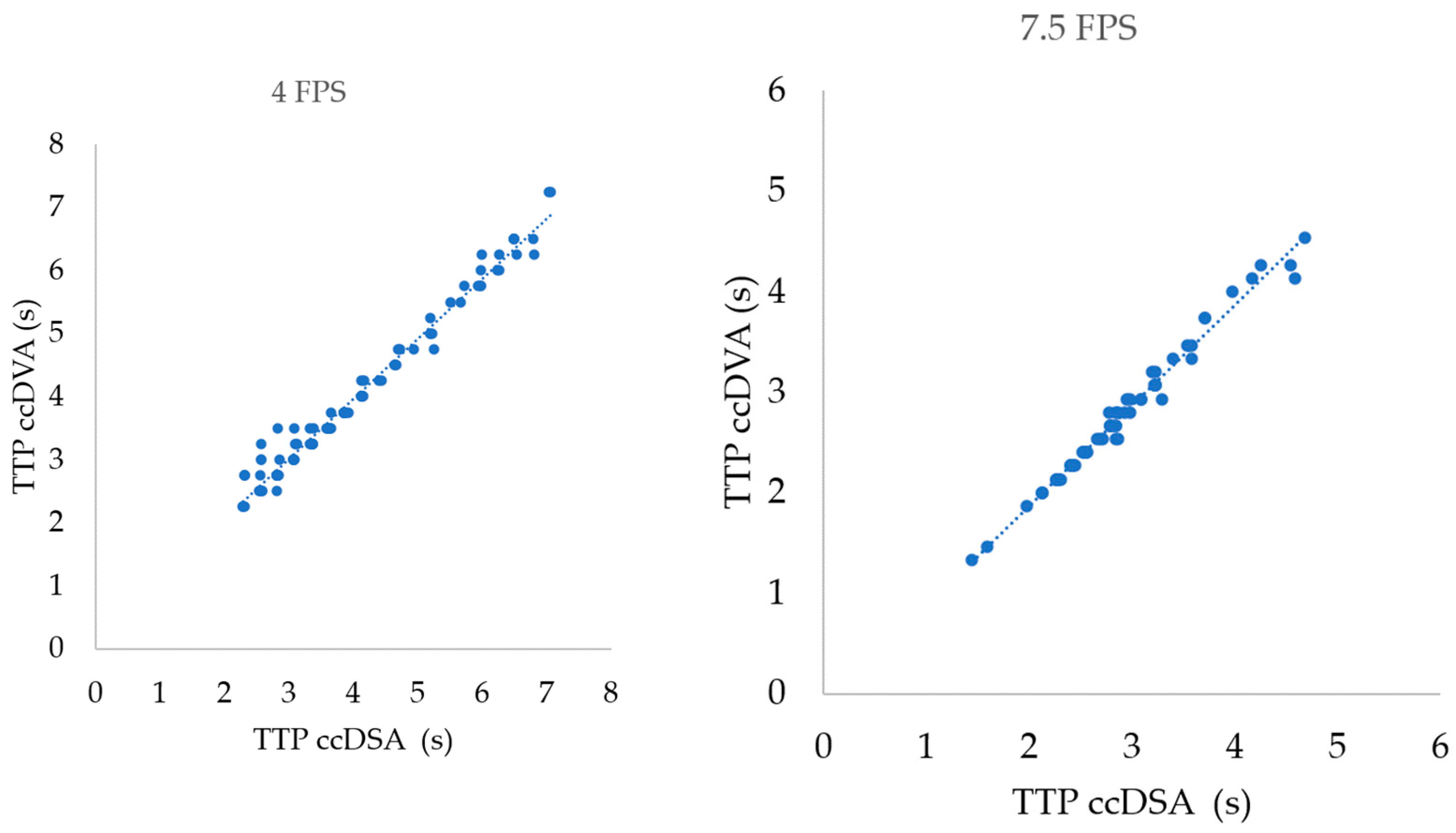
3.1. Correlation of TTP Parameters in Different Acquisition Protocols
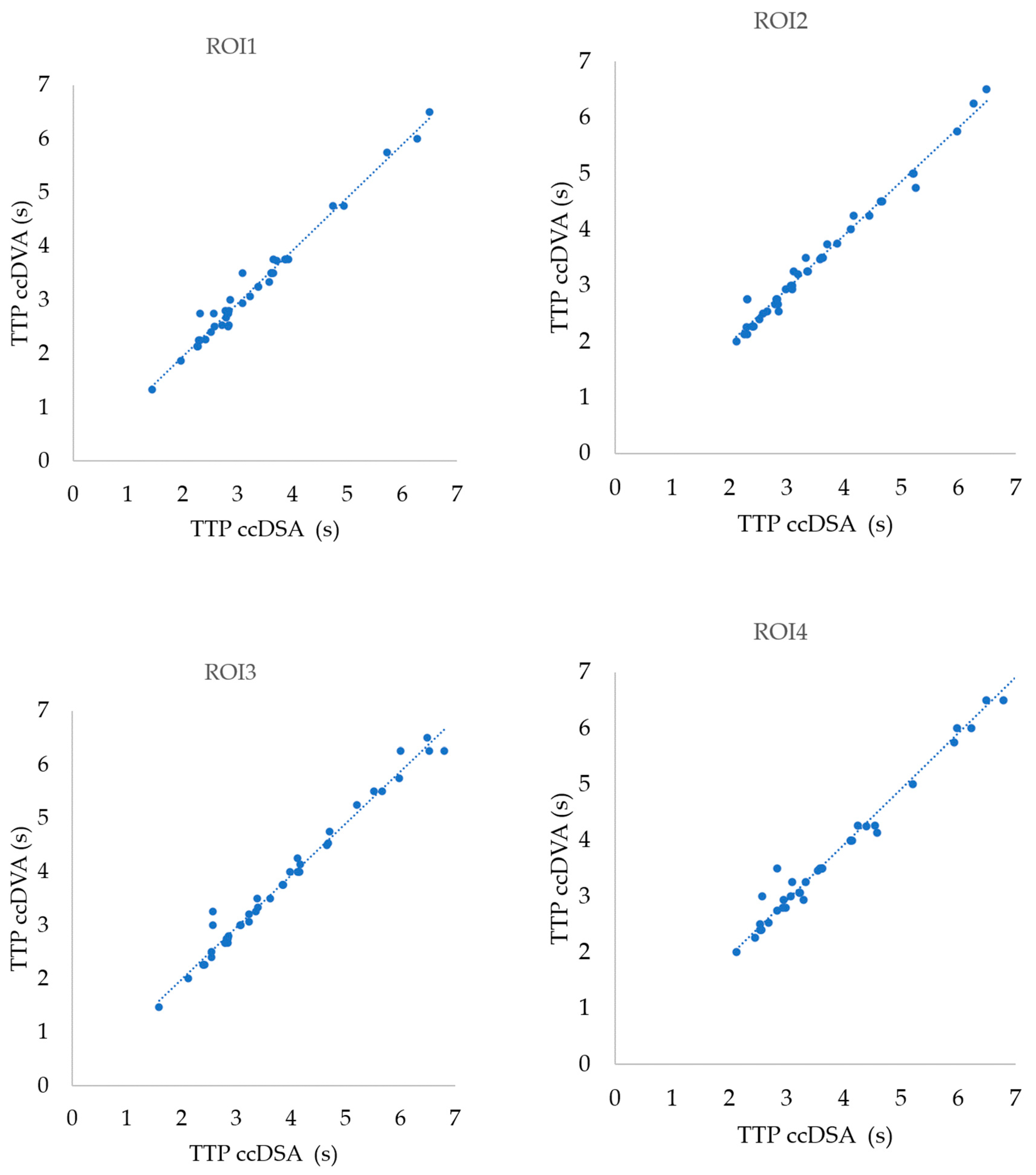
3.2. Correlation of TTP Parameters in Different ROI Positions
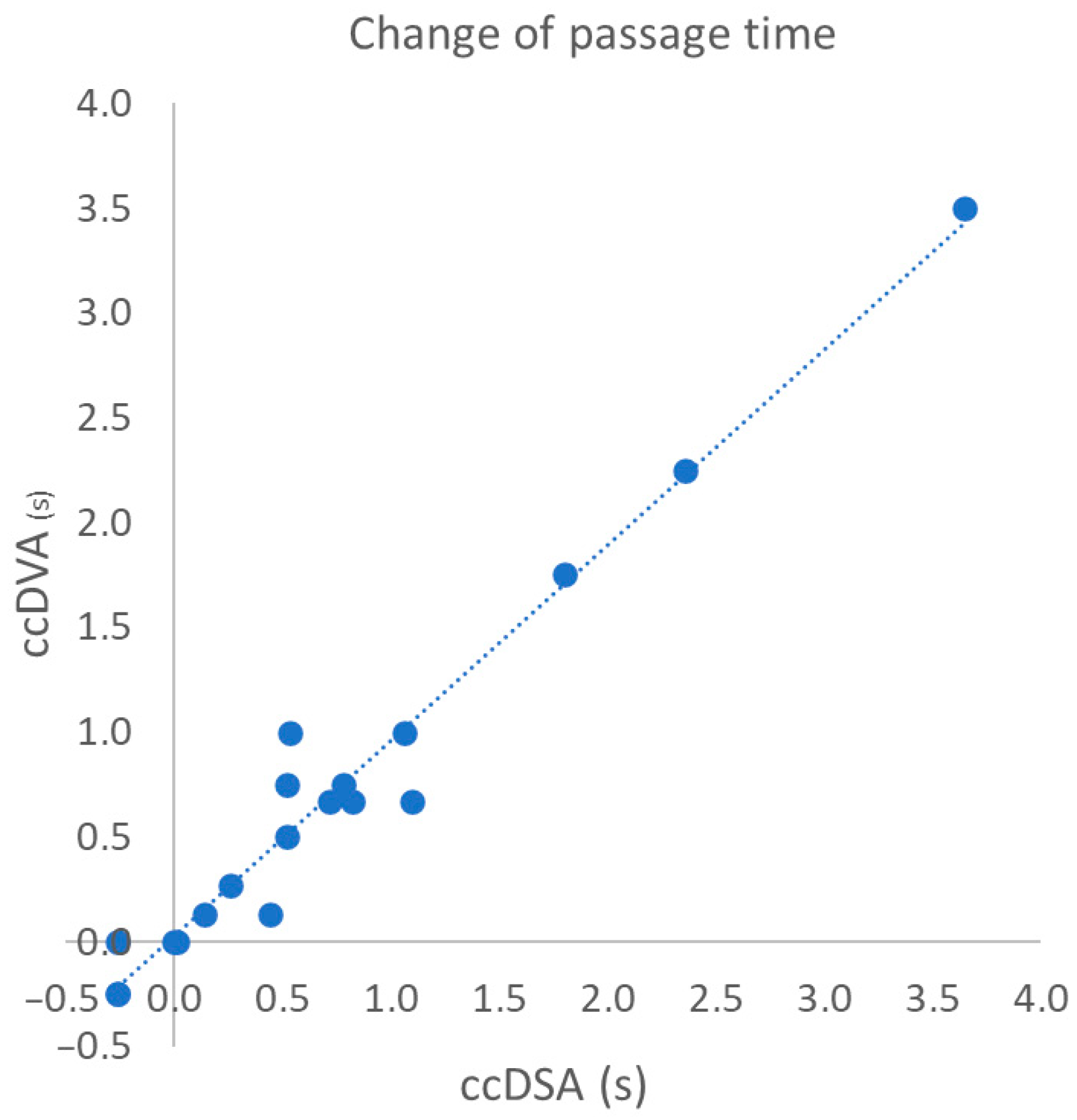
3.3. Correlation of Change in Passage Time Following the Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.J.; Keung, J.J.; McCarragher, B. Interventional Radiology: Indications and Best Practices. Am. Fam. Physician 2019, 99, 547–556. [Google Scholar] [PubMed]
- Jeans, W.D. The development and use of digital subtraction angiography. Br. J. Radiol. 1990, 63, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Haendle, H.; Haendle, J.; Maass, W.; Finkler, K. Digitale Subtraktionsangiographie mit dynamischen Farbfunktionsbildern [Digital subtraction angiography with dynamic color function images]. Digit. Bilddiagn. 1984, 4, 153–157. [Google Scholar]
- Hunter, G.J.; Hunter, J.V.; Brown, N.J. Parametric imaging using digital subtraction angiography. Br. J. Radiol. 1986, 59, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Strother, C.M.; Bender, F.; Deuerling-Zheng, Y.; Royalty, K.; Pulfer, K.A.; Baumgart, J.; Zellerhoff, M.; Aagaard-Kienitz, B.; Niemann, D.B.; Lindstrom, M.L. Parametric color coding of digital subtraction angiography. AJNR Am. J. Neuroradiol. 2010, 31, 919–924. [Google Scholar] [CrossRef]
- Jens, S.; Marquering, H.A.; Koelemay, M.J.; Reekers, J.A. Perfusion angiography of the foot in patients with critical limb ischemia: Description of the technique. Cardiovasc. Interv. Radiol. 2015, 38, 201–205. [Google Scholar] [CrossRef]
- Wen, W.L.; Fang, Y.B.; Yang, P.F.; Zhang, Y.W.; Wu, Y.N.; Shen, H.; Ge, J.J.; Xu, Y.; Hong, B.; Huang, Q.H.; et al. Parametric Digital Subtraction Angiography Imaging for the Objective Grading of Collateral Flow in Acute Middle Cerebral Artery Occlusion. World Neurosurg. 2016, 88, 119–125. [Google Scholar] [CrossRef]
- Gölitz, P.; Muehlen, I.; Gerner, S.T.; Knossalla, F.; Doerfler, A. Ultraearly assessed reperfusion status after middle cerebral artery recanalization predicting clinical outcome. Acta Neurol. Scand. 2018, 137, 609–617. [Google Scholar] [CrossRef]
- Gölitz, P.; Struffert, T.; Lücking, H.; Rösch, J.; Knossalla, F.; Ganslandt, O.; Deuerling-Zheng, Y.; Doerfler, A. Parametric color coding of digital subtraction angiography in the evaluation of carotid cavernous fistulas. Clin. Neuroradiol. 2013, 23, 113–120. [Google Scholar] [CrossRef]
- Kostrzewa, M.; Kara, K.; Pilz, L.; Mueller-Muertz, H.; Rathmann, N.; Schoenberg, S.O.; Diehl, S.J. Treatment Evaluation of Flow-Limiting Stenoses of the Superficial Femoral and Popliteal Artery by Parametric Color-Coding Analysis of Digital Subtraction Angiography Series. Cardiovasc. Interv. Radiol. 2017, 40, 1147–1154. [Google Scholar] [CrossRef]
- Ng, J.J.; Papadimas, E.; Dharmaraj, R.B. Assessment of Flow after Lower Extremity Endovascular Revascularisation: A Feasibility Study Using Time Attenuation Curve Analysis of Digital Subtraction Angiography. EJVES Short. Rep. 2019, 45, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, G.; Minelli, F.; De Nigris, F.; Vincenzoni, C.; Filipponi, M.; Bruno, P.; Massetti, M.; Flex, A.; Iezzi, R. The potential role of quantitative digital subtraction angiography in evaluating type B chronic aortic dissection during TEVAR: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 516–522. [Google Scholar] [PubMed]
- Chen, Y.; Xu, W.; Liu, J.; Zhao, C.; Cao, X.; Wang, R.; Feng, D.; Zhang, R.; Zhou, X. Color-coded parametric imaging support display of vessel hemorrhage-an in vitro experiment and clinical validation study. Front. Cardiovasc. Med. 2024, 11, 1387421. [Google Scholar] [CrossRef] [PubMed]
- Badar, W.; Anitescu, M.; Ross, B.; Wallace, S.; Uy-Palmer, R.; Ahmed, O. Quantifying Change in Perfusion after Genicular Artery Embolization with Parametric Analysis of Intraprocedural Digital Subtraction Angiograms. J. Vasc. Interv. Radiol. 2023, 34, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Meine, T.C.; Maschke, S.K.; Kirstein, M.M.; Jaeckel, E.; Lena, B.S.; Werncke, T.; Dewald, C.L.A.; Wacker, F.K.; Meyer, B.C.; Hinrichs, J.B. Evaluation of perfusion changes using a 2D Parametric Parenchymal Blood Flow technique with automated vessel suppression following partial spleen embolization in patients with hypersplenism and portal hypertension. Medicine 2021, 100, e24783. [Google Scholar] [CrossRef]
- Rivera, R.; Sordo, J.G.; Echeverria, D.; Badilla, L.; Pinto, C.; Merino-Osorio, C. Quantitative evaluation of arteriovenous malformation hemodynamic changes after endovascular treatment using parametric color coding: A case series study. Interv. Neuroradiol. 2017, 23, 650–655. [Google Scholar] [CrossRef]
- Gölitz, P.; Struffert, T.; Rösch, J.; Ganslandt, O.; Knossalla, F.; Doerfler, A. Cerebral aneurysm treatment using flow-diverting stents: In-vivo visualization of flow alterations by parametric colour coding to predict aneurysmal occlusion: Preliminary results. Eur. Radiol. 2015, 25, 428–435. [Google Scholar] [CrossRef]
- Hussein, A.E.; Shownkeen, M.; Thomas, A.; Stapleton, C.; Brunozzi, D.; Nelson, J.; Naumgart, J.; Linninger, A.; Atwal, G.; Alaraj, A. 2D parametric contrast time-density analysis for the prediction of complete aneurysm occlusion at six months’ post-flow diversion stent. Interv. Neuroradiol. 2020, 26, 468–475. [Google Scholar] [CrossRef]
- Szigeti, K.; Mathe, D.; Osvath, S. Motion based X-ray imaging modality. IEEE Trans. Med. Imaging 2014, 33, 2031–2038. [Google Scholar] [CrossRef]
- Gyánó, M.; Góg, I.; Óriás, V.I.; Ruzsa, Z.; Nemes, B.; Csobay-Novák, C.; Oláh, Z.; Nagy, Z.; Merkely, B.; Szigeti, K.; et al. Kinetic imaging in lower extremity arteriography: Comparison to digital subtraction angiography. Radiology 2019, 290, 246–253. [Google Scholar] [CrossRef]
- Óriás, V.I.; Gyánó, M.; Góg, I.; Szöllősi, D.; Veres, D.S.; Nagy, Z.; Csobay-Novák, C.; Zoltán, O.; Kiss, J.P.; Osváth, S.; et al. Digital Variance Angiography as a Paradigm Shift in Carbon Dioxide Angiography. Investig. Radiol. 2019, 54, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Bastian, M.B.; König, A.M.; Viniol, S.; Gyánó, M.; Szöllősi, D.; Góg, I.; Kiss, J.P.; Osvath, S.; Szigeti, K.; Mahnken, A.H.; et al. Digital Variance Angiography in Lower-Limb Angiography with Metal Implants. Cardiovasc. Interv. Radiol. 2021, 44, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.P.; Bastian, M.B.; Viniol, S.; König, A.M.; Amin, S.S.; Eldergash, O.; Schnabel, J.; Gyánó, M.; Szöllősi, D.; Góg, I.; et al. Digital variance angiography in selective lower limb interventions. J. Vasc. Interv. Radiol. 2022, 33, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Óriás, V.I.; Szöllősi, D.; Gyánó, M.; Veres, D.S.; Nardai, S.; Csobay-Novák, C.; Nemes, B.; Kiss, J.P.; Szigeti, K.; Osváth, S.; et al. Initial evidence of a 50% reduction of contrast media using digital variance angiography in endovascular carotid interventions. Eur. J. Radiol. Open 2020, 7, 100288. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, L.S.; Gyánó, M.; Góg, I.; Szigeti, K.; Osváth, S.; Kiss, J.P.; Yel, I.; Koch, V.; Grünewald, L.D.; Vogl, T.J.; et al. Initial experience using Digital Variance Angiography in context of Prostatic Artery Embolization in comparison with Digital Subtraction Angiography. Acad. Radiol. 2022, 30, 689–697. [Google Scholar] [CrossRef]
- Lucatelli, P.; Rocco, B.; Ciaglia, S.; Teodoli, L.; Argirò, R.; Guiu, B.; Saba, L.; Vallati, G.; Spiliopoulos, S.; Patrone, L.; et al. Possible use of digital variance angiography in liver transarterial chemoembolization: A retrospective observational study. Cardiovasc. Interv. Radiol. 2023, 46, 635–642. [Google Scholar] [CrossRef]
- Gyánó, M.; Berczeli, M.; Csobay-Novák, C.; Szöllősi, D.; Óriás, V.I.; Góg, I.; Kiss, J.P.; Veres, D.S.; Szigeti, K.; Osváth, S.; et al. Digital variance angiography allows about 70% decrease of DSA-related radiation exposure in lower limb X-ray angiography. Sci. Rep. 2021, 11, 21790. [Google Scholar] [CrossRef]
- Sótonyi, P.; Berczeli, M.; Gyánó, M.; Legeza, P.; Mihály, Z.; Csobay-Novák, C.; Pataki, Á.; Juhász, V.; Góg, I.; Szigeti, K.; et al. Radiation Exposure Reduction by Digital Variance Angiography in Lower Limb Angiography: A Randomized Controlled Trial. J. Cardiovasc. Dev. Dis. 2023, 10, 198. [Google Scholar] [CrossRef]

| Sex | Number of Patients | Age 1 (Years) | BMI 1 | GFR 1 (mL/min/1.73 m2) |
|---|---|---|---|---|
| Male | 8 | 69.6 ± 7.0 | 25.0 ± 4.5 | 65.3 ± 26.7 |
| Female | 11 | 67.9 ± 8.8 | 26.6 ± 4.6 | 55.8 ± 26.2 |
| Correlation Group (n) | Pearson r | 95% Confidence Interval | R2 | Two-Tailed p |
|---|---|---|---|---|
| TTP 4 FPS (106) | 0.9889 | 0.9837–0.9924 | 0.9779 | <0.0001 |
| TTP 7.5 FPS (64) | 0.9917 | 0.9864–0.9950 | 0.9835 | <0.0001 |
| TTP ROI1 (44) | 0.9899 | 0.9814–0.9845 | 0.9799 | <0.0001 |
| TTP ROI2 (44) | 0.9903 | 0.9822–0.9947 | 0.9807 | <0.0001 |
| TTP ROI3 (44) | 0.9902 | 0.9819–0.9947 | 0.9804 | <0.0001 |
| TTP ROI4 (38) | 0.9904 | 0.9814–0.9950 | 0.9808 | <0.0001 |
| TTP all ROI (170) | 0.9905 | 0.9871–0.9930 | 0.9811 | <0.0001 |
| Δ passage time 1 (19) | 0.9806 | 0.9491–0.9927 | 0.9615 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Góg, I.; Sótonyi, P.; Nemes, B.; Kiss, J.P.; Szigeti, K.; Osváth, S.; Gyánó, M. Quantitative Comparison of Color-Coded Parametric Imaging Technologies Based on Digital Subtraction and Digital Variance Angiography: A Retrospective Observational Study. J. Imaging 2024, 10, 260. https://doi.org/10.3390/jimaging10100260
Góg I, Sótonyi P, Nemes B, Kiss JP, Szigeti K, Osváth S, Gyánó M. Quantitative Comparison of Color-Coded Parametric Imaging Technologies Based on Digital Subtraction and Digital Variance Angiography: A Retrospective Observational Study. Journal of Imaging. 2024; 10(10):260. https://doi.org/10.3390/jimaging10100260
Chicago/Turabian StyleGóg, István, Péter Sótonyi, Balázs Nemes, János P. Kiss, Krisztián Szigeti, Szabolcs Osváth, and Marcell Gyánó. 2024. "Quantitative Comparison of Color-Coded Parametric Imaging Technologies Based on Digital Subtraction and Digital Variance Angiography: A Retrospective Observational Study" Journal of Imaging 10, no. 10: 260. https://doi.org/10.3390/jimaging10100260
APA StyleGóg, I., Sótonyi, P., Nemes, B., Kiss, J. P., Szigeti, K., Osváth, S., & Gyánó, M. (2024). Quantitative Comparison of Color-Coded Parametric Imaging Technologies Based on Digital Subtraction and Digital Variance Angiography: A Retrospective Observational Study. Journal of Imaging, 10(10), 260. https://doi.org/10.3390/jimaging10100260






