Special Types of Breast Cancer: Clinical Behavior and Radiological Appearance
Abstract
:1. Introduction
2. Lobular (Classic, Pleomorphic and Florid Types)
3. Tubular
4. Mucinous
5. Mucinous Cystadenocarcinoma
6. Medullary
7. Cribriform
8. Papillary and Micropapillary
9. Apocrine
10. Metaplastic
11. Neuroendocrine Carcinoma
12. Rare and Salivary-Gland-Type Tumors
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rakha, E.A.; Reis-Filho, J.S.; Baehner, F.; Dabbs, D.J.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Breast Cancer Prognostic Classification in the Molecular Era: The Role of Histological Grade. Breast Cancer Res. BCR 2010, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Ellis, I.O.; Galea, M.; Broughton, N.; Locker, A.; Blamey, R.W.; Elston, C.W. Pathological Prognostic Factors in Breast Cancer. II. Histological Type. Relationship with Survival in a Large Study with Long-Term Follow-Up. Histopathology 1992, 20, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.; Kachur, M.E.; Rechache, K.; Wells, J.M.; Lipkowitz, S. Rare Breast Cancer Subtypes. Curr. Oncol. Rep. 2021, 23, 54. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Sahoo, S.; Saluja, K. Invasive Breast Carcinoma of No Special Type, Microinvasive Carcinoma, Tubular Carcinoma, and Cribriform Carcinoma. In A Comprehensive Guide to Core Needle Biopsies of the Breast, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 391–443. ISBN 978-3-031-05531-7. [Google Scholar]
- World Health Organization. WHO Classification of Tumours: Breast Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; Volume 2, Available online: https://tumourclassification.iarc.who.int/welcome/ (accessed on 12 March 2022).
- Kufel, J.; Bargieł, K.; Koźlik, M.; Czogalik, Ł.; Dudek, P.; Jaworski, A.; Cebula, M.; Gruszczyńska, K. Application of Artificial Intelligence in Diagnosing COVID-19 Disease Symptoms on Chest X-rays: A Systematic Review. Int. J. Med. Sci. 2022, 19, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Shin, S.; Yang, S.-A.; Park, E.K.; Kim, K.H.; Cho, S.I.; Ock, C.-Y.; Kim, S. Artificial Intelligence in Breast Cancer Diagnosis and Personalized Medicine. J. Breast Cancer 2023, 26, 405–435. [Google Scholar] [CrossRef] [PubMed]
- Yoen, H.; Jang, M.J.; Yi, A.; Moon, W.K.; Chang, J.M. Artificial Intelligence for Breast Cancer Detection on Mammography: Factors Related to Cancer Detection. Acad. Radiol. 2024, 31, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Calisto, F.M. Human-Centered Design of Personalized Intelligent Agents in Medical Imaging Diagnosis. Ph.D. Thesis, University of Lisbon, Lisbon, Portugal, 2024. [Google Scholar]
- Calisto, F.M.; Nunes, N.; Nascimento, J.C. Modeling Adoption of Intelligent Agents in Medical Imaging. Int. J. Hum.-Comput. Stud. 2022, 168, 102922. [Google Scholar] [CrossRef]
- Cserni, G. Histological Type and Typing of Breast Carcinomas and the WHO Classification Changes over Time. Pathol.—J. Ital. Soc. Anat. Pathol. Diagn. Cytopathol. 2020, 112, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Mouabbi, J.A.; Hassan, A.; Lim, B.; Hortobagyi, G.N.; Tripathy, D.; Layman, R.M. Invasive Lobular Carcinoma: An Understudied Emergent Subtype of Breast Cancer. Breast Cancer Res. Treat. 2022, 193, 253–264. [Google Scholar] [CrossRef]
- Wen, X.; Yu, Y.; Yu, X.; Cheng, W.; Wang, Z.; Liu, L.; Zhang, L.; Qin, L.; Tian, J. Correlations between Ultrasonographic Findings of Invasive Lobular Carcinoma of the Breast and Intrinsic Subtypes. Ultraschall Med.-Eur. 2019, 40, 764–770. [Google Scholar] [CrossRef]
- McCart Reed, A.E.; Kalinowski, L.; Simpson, P.T.; Lakhani, S.R. Invasive Lobular Carcinoma of the Breast: The Increasing Importance of This Special Subtype. Breast Cancer Res. 2021, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Fusco, N.; Guerini-Rocco, E.; Leonardi, M.C.; Criscitiello, C.; Zagami, P.; Nicolò, E.; Mazzarol, G.; La Vecchia, C.; Pesapane, F.; et al. Invasive Lobular Breast Cancer: Focus on Prevention, Genetics, Diagnosis, and Treatment. Semin. Oncol. 2024; in press. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Tse, G.M.; Quinn, C.M. An Update on the Pathological Classification of Breast Cancer. Histopathology 2023, 82, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Manning, P.; Fazeli, S.; Lim, V.; Ladd, W.A.; Eghtedari, M.; Chong, A.; Rakow-Penner, R.; Ojeda-Fournier, H. Invasive Lobular Carcinoma: A Multimodality Imaging Primer. RadioGraphics 2022, 42, E115–E116. [Google Scholar] [CrossRef] [PubMed]
- Danzinger, S.; Pöckl, K.; Kronawetter, G.; Pfeifer, C.; Behrendt, S.; Gscheidlinger, P.; Harrasser, L.; Mühlböck, H.; Dirschlmayer, W.; Schauer, C.; et al. Axillary Lymph Node Status and Invasive Lobular Breast Cancer. Wien. Klin. Wochenschr. 2023, 135, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Sarma, D.; Hwang, E.S. Lobular Breast Cancer Series: Imaging. Breast Cancer Res. BCR 2015, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Berg, W.A.; Gutierrez, L.; NessAiver, M.S.; Carter, W.B.; Bhargavan, M.; Lewis, R.S.; Ioffe, O.B. Diagnostic Accuracy of Mammography, Clinical Examination, US, and MR Imaging in Preoperative Assessment of Breast Cancer. Radiology 2004, 233, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Savaridas, S.L.; Bristow, G.D.; Cox, J. Invasive Lobular Cancer of the Breast: A Pictorial Essay of Imaging Findings on Mammography, Sonography, and Magnetic Resonance Imaging. Can. Assoc. Radiol. J. 2016, 67, 263–276. [Google Scholar] [CrossRef]
- Onega, T.; Abraham, L.; Miglioretti, D.L.; Lee, C.I.; Henderson, L.M.; Kerlikowske, K.; Tosteson, A.N.A.; Weaver, D.; Sprague, B.L.; Bowles, E.J.A.; et al. Digital Mammography and Digital Breast Tomosynthesis for Detecting Invasive Lobular and Ductal Carcinoma. Breast Cancer Res. Treat. 2023, 202, 505–514. [Google Scholar] [CrossRef]
- Romanucci, G.; Zantedeschi, L.; Ventriglia, A.; Mercogliano, S.; Bisighin, M.V.; Cugola, L.; Bricolo, P.; Rella, R.; Mandarà, M.; Benassuti, C.; et al. Lobular Breast Cancer Conspicuity on Digital Breast Tomosynthesis Compared to Synthesized 2D Mammography: A Multireader Study. J. Imaging 2021, 7, 185. [Google Scholar] [CrossRef]
- Grubstein, A.; Rapson, Y.; Morgenstern, S.; Gadiel, I.; Haboosheh, A.; Yerushalmi, R.; Cohen, M. Invasive Lobular Carcinoma of the Breast: Appearance on Digital Breast Tomosynthesis. Breast Care 2016, 11, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cha, E.S.; Park, C.S.; Kang, B.J.; Whang, I.Y.; Lee, A.W.; Song, B.J.; Park, J. Imaging Features of Invasive Lobular Carcinoma: Comparison with Invasive Ductal Carcinoma. Jpn. J. Radiol. 2011, 29, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Watermann, D.O.; Tempfer, C.; Hefler, L.A.; Parat, C.; Stickeler, E. Ultrasound Morphology of Invasive Lobular Breast Cancer Is Different Compared with Other Types of Breast Cancer. Ultrasound Med. Biol. 2005, 31, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Selinko, V.L.; Middleton, L.P.; Dempsey, P.J. Role of Sonography in Diagnosing and Staging Invasive Lobular Carcinoma. J. Clin. Ultrasound JCU 2004, 32, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Dołęga-Kozierowski, B.; Lis, M.; Marszalska-Jacak, H.; Koziej, M.; Celer, M.; Bandyk, M.; Kasprzak, P.; Szynglarewicz, B.; Matkowski, R. Multimodality Imaging in Lobular Breast Cancer: Differences in Mammography, Ultrasound, and MRI in the Assessment of Local Tumor Extent and Correlation with Molecular Characteristics. Front. Oncol. 2022, 12, 855519. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Kuhl, C.K.; Kinkel, K.; Boetes, C. Breast MRI: Guidelines from the European Society of Breast Imaging. Eur. Radiol. 2008, 18, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, A.; Birdwell, R.L.; Daniel, B.L.; Nowels, K.W.; Jeffrey, S.S.; Agoston, T.A.; Herfkens, R.J. MR Imaging Features of Infiltrating Lobular Carcinoma of the Breast: Histopathologic Correlation. AJR Am. J. Roentgenol. 2002, 178, 1227–1232. [Google Scholar] [CrossRef]
- Szabó, B.K.; Aspelin, P.; Wiberg, M.K.; Boné, B. Dynamic MR Imaging of the Breast. Analysis of Kinetic and Morphologic Diagnostic Criteria. Acta Radiol. 2003, 44, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Amato, F.; Bicchierai, G.; Cirone, D.; Depretto, C.; Di Naro, F.; Vanzi, E.; Scaperrotta, G.; Bartolotta, T.V.; Miele, V.; Nori, J. Preoperative Loco-Regional Staging of Invasive Lobular Carcinoma with Contrast-Enhanced Digital Mammography (CEDM). Radiol. Med. 2019, 124, 1229–1237. [Google Scholar] [CrossRef]
- Patel, B.K.; Davis, J.; Ferraro, C.; Kosiorek, H.; Hasselbach, K.; Ocal, T.; Pockaj, B. Value Added of Preoperative Contrast-Enhanced Digital Mammography in Patients With Invasive Lobular Carcinoma of the Breast. Clin. Breast Cancer 2018, 18, e1339–e1345. [Google Scholar] [CrossRef]
- Lobbes, M.B.I.; Neeter, L.M.F.H.; Raat, F.; Turk, K.; Wildberger, J.E.; van Nijnatten, T.J.A.; Nelemans, P.J. The Performance of Contrast-Enhanced Mammography and Breast MRI in Local Preoperative Staging of Invasive Lobular Breast Cancer. Eur. J. Radiol. 2023, 164, 110881. [Google Scholar] [CrossRef] [PubMed]
- Diab, S.G.; Clark, G.M.; Osborne, C.K.; Libby, A.; Allred, D.C.; Elledge, R.M. Tumor Characteristics and Clinical Outcome of Tubular and Mucinous Breast Carcinomas. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 1442–1448. [Google Scholar] [CrossRef]
- Rakha, E.A.; Lee, A.H.S.; Evans, A.J.; Menon, S.; Assad, N.Y.; Hodi, Z.; Macmillan, D.; Blamey, R.W.; Ellis, I.O. Tubular Carcinoma of the Breast: Further Evidence to Support Its Excellent Prognosis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 99–104. [Google Scholar] [CrossRef]
- Wasif, N.; McCullough, A.E.; Gray, R.J.; Pockaj, B.A. Influence of Uncommon Histology on Breast Conservation Therapy for Breast Cancer-Biology Dictates Technique? J. Surg. Oncol. 2012, 105, 586–590. [Google Scholar] [CrossRef]
- Yang, M.; Bao, W.; Zhang, X.; Kang, Y.; Haffty, B.; Zhang, L. Short-Term and Long-Term Clinical Outcomes of Uncommon Types of Invasive Breast Cancer. Histopathology 2017, 71, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-W.; Wu, S.-G.; Ling, Y.-H.; Sun, J.-Y.; Long, Z.-Q.; Hua, X.; Dong, Y.; Li, F.-Y.; He, Z.-Y.; Lin, H.-X. Clinicopathologic Characteristics and Clinical Outcomes of Pure Type and Mixed Type of Tubular Carcinoma of the Breast: A Single-Institution Cohort Study. Cancer Manag. Res. 2018, 10, 4509–4515. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Bae, S.Y.; Lee, H.-C.; Lee, J.H.; Kim, M.; Kim, J.; Lee, S.K.; Kil, W.H.; Kim, S.W.; Lee, J.E.; et al. Tubular Carcinoma of the Breast: Clinicopathologic Features and Survival Outcome Compared with Ductal Carcinoma in Situ. J. Breast Cancer 2013, 16, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A. Unusual Breast Cancers: Useful Clues to Expanding the Differential Diagnosis. Radiology 2007, 242, 683–694. [Google Scholar] [CrossRef]
- Sheppard, D.G.; Whitman, G.J.; Huynh, P.T.; Sahin, A.A.; Fornage, B.D.; Stelling, C.B. Tubular Carcinoma of the Breast: Mammographic and Sonographic Features. AJR Am. J. Roentgenol. 2000, 174, 253–257. [Google Scholar] [CrossRef]
- Caumo, F.; Romanucci, G.; Hunter, K.; Zorzi, M.; Brunelli, S.; Macaskill, P.; Houssami, N. Comparison of Breast Cancers Detected in the Verona Screening Program Following Transition to Digital Breast Tomosynthesis Screening with Cancers Detected at Digital Mammography Screening. Breast Cancer Res. Treat. 2018, 170, 391–397. [Google Scholar] [CrossRef]
- Linda, A.; Zuiani, C.; Girometti, R.; Londero, V.; Machin, P.; Brondani, G.; Bazzocchi, M. Unusual Malignant Tumors of the Breast: MRI Features and Pathologic Correlation. Eur. J. Radiol. 2010, 75, 178–184. [Google Scholar] [CrossRef]
- Ghai, S.; Muradali, D.; Bukhanov, K.; Kulkarni, S. Nonenhancing Breast Malignancies on MRI: Sonographic and Pathologic Correlation. Am. J. Roentgenol. 2005, 185, 481–487. [Google Scholar] [CrossRef]
- Yılmaz, R.; Bayramoğlu, Z.; Emirikçi, S.; Önder, S.; Salmaslıoğlu, A.; Dursun, M.; Acunaş, G.; Özmen, V. MR Imaging Features of Tubular Carcinoma: Preliminary Experience in Twelve Masses. Eur. J. Breast Health 2018, 14, 39–45. [Google Scholar] [CrossRef]
- Evans, A.; Sim, Y.T.; Thomson, K.; Jordan, L.; Purdie, C.; Vinnicombe, S.J. Shear Wave Elastography of Breast Cancer: Sensitivity According to Histological Type in a Large Cohort. Breast 2016, 26, 115–118. [Google Scholar] [CrossRef]
- Di Saverio, S.; Gutierrez, J.; Avisar, E. A Retrospective Review with Long Term Follow up of 11,400 Cases of Pure Mucinous Breast Carcinoma. Breast Cancer Res. Treat. 2008, 111, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Budzik, M.P.; Fudalej, M.M.; Badowska-Kozakiewicz, A.M. Histopathological Analysis of Mucinous Breast Cancer Subtypes and Comparison with Invasive Carcinoma of No Special Type. Sci. Rep. 2021, 11, 5770. [Google Scholar] [CrossRef] [PubMed]
- Limaiem, F.; Ahmad, F. Mucinous Breast Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pintican, R.; Duma, M.; Chiorean, A.; Fetica, B.; Badan, M.; Bura, V.; Szep, M.; Feier, D.; Dudea, S. Mucinous versus Medullary Breast Carcinoma: Mammography, Ultrasound, and MRI Findings. Clin. Radiol. 2020, 75, 483–496. [Google Scholar] [CrossRef]
- Lam, W.W.M.; Chu, W.C.W.; Tse, G.M.; Ma, T.K. Sonographic Appearance of Mucinous Carcinoma of the Breast. AJR Am. J. Roentgenol. 2004, 182, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Larribe, M.; Thomassin-Piana, J.; Jalaguier-Coudray, A. Breast Cancers with Round Lumps: Correlations between Imaging and Anatomopathology. Diagn. Interv. Imaging 2014, 95, 37–46. [Google Scholar] [CrossRef]
- Chaudhry, A.R.; El Khoury, M.; Gotra, A.; Eslami, Z.; Omeroglu, A.; Omeroglu-Altinel, G.; Chaudhry, S.H.; Mesurolle, B. Imaging Features of Pure and Mixed Forms of Mucinous Breast Carcinoma with Histopathological Correlation. Br. J. Radiol. 2019, 92, 20180810. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, N.; Han, L.; Yang, L.; Xu, W.; Chen, W. Comparative Analysis of Imaging and Pathology Features of Mucinous Carcinoma of the Breast. Clin. Breast Cancer 2015, 15, e147–e154. [Google Scholar] [CrossRef] [PubMed]
- Caumo, F.; Zorzi, M.; Brunelli, S.; Romanucci, G.; Rella, R.; Cugola, L.; Bricolo, P.; Fedato, C.; Montemezzi, S.; Houssami, N. Digital Breast Tomosynthesis with Synthesized Two-Dimensional Images versus Full-Field Digital Mammography for Population Screening: Outcomes from the Verona Screening Program. Radiology 2018, 287, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Memis, A.; Ozdemir, N.; Parildar, M.; Ustun, E.E.; Erhan, Y. Mucinous (Colloid) Breast Cancer: Mammographic and US Features with Histologic Correlation. Eur. J. Radiol. 2000, 35, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-J.; Kim, S.-H.; Cha, E.-S.; Kim, H.-S.; Kang, B.-J.; Choi, J.-J.; Lee, J.-H.; Lee, A.-W. Imaging Features of Mucinous Breast Carcinoma. Investig. Magn. Reson. Imaging 2010, 14, 21–30. [Google Scholar] [CrossRef]
- Perkins, G.; Babiera, G.; Bedrosian, I.; Gonzalez-Angulo, A.; Whitman, G.; Yang, W.; Strom, E.; Woodward, W.; Tereffe, W.; Yu, T.; et al. Mucinous Breast Carcinoma: Occult Multifocality/Multicentricity in a Favorable Disease. Cancer Res. 2009, 69, 4117. [Google Scholar] [CrossRef]
- Bitencourt, A.G.V.; Graziano, L.; Osório, C.A.B.T.; Guatelli, C.S.; Souza, J.A.; Mendonça, M.H.S.; Marques, E.F. MRI Features of Mucinous Cancer of the Breast: Correlation with Pathologic Findings and Other Imaging Methods. AJR Am. J. Roentgenol. 2016, 206, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Monzawa, S.; Yokokawa, M.; Sakuma, T.; Takao, S.; Hirokaga, K.; Hanioka, K.; Adachi, S. Mucinous Carcinoma of the Breast: MRI Features of Pure and Mixed Forms with Histopathologic Correlation. AJR Am. J. Roentgenol. 2009, 192, W125–W131. [Google Scholar] [CrossRef]
- Kulka, J.; Madaras, L.; Floris, G.; Lax, S.F. Papillary Lesions of the Breast. Virchows Arch. 2022, 480, 65. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.; Shi, Y.Q.; Chen, T.B. Mammary Mucinous Cystadenocarcinoma with Long-Term Follow-Up: Molecular Information and Literature Review. Diagn. Pathol. 2023, 18, 13. [Google Scholar]
- Jain, E.; Kumar, A.; Jain, R.; Sharma, S. Primary Mucinous Cystadenocarcinoma of the Breast: A Rare Case Report with Review of Literature. Int. J. Surg. Pathol. 2021, 29, 740–746. [Google Scholar] [CrossRef]
- Seong, M.; Ko, E.Y.; Han, B.-K.; Cho, S.Y.; Cho, E.Y.; Lee, S.K.; Lee, J.E. Radiologic Findings of Primary Mucinous Cystadenocarcinoma of the Breast: A Report of Two Cases and a Literature Review. J. Breast Cancer 2016, 19, 330–333. [Google Scholar] [CrossRef]
- Vegni, F.; D’Alessandris, N.; Santoro, A.; Angelico, G.; Scaglione, G.; Carlino, A.; Arciuolo, D.; Valente, M.; Sfregola, S.; Natale, M.; et al. Primary Mucinous Cystadenocarcinoma of the Breast Intermixed with Pleomorphic Invasive Lobular Carcinoma: The First Report of This Rare Association. J. Pers. Med. 2023, 13, 948. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Zhao, P.; Jia, H.; Dong, X.; Zhang, L.; Wang, C. Primary Mucinous Cystadenocarcinoma of the Breast: A Clinicopathologic Analysis of One Case and Review of the Literature. Int. J. Clin. Exp. Pathol. 2020, 13, 2562–2568. [Google Scholar] [PubMed]
- Deng, Y.; Xue, D.; Wang, X.; Xu, S.; Ao, Q.; Hu, Z.; Wang, G. Mucinous Cystadenocarcinoma of the Breast with a Basal-like Immunophenotype. Pathol. Int. 2012, 62, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Kim, J.; Kim, M.; Bae, S.Y.; Lee, S.K.; Kil, W.H.; Lee, J.E.; Nam, S.J. Comparison of the Characteristics of Medullary Breast Carcinoma and Invasive Ductal Carcinoma. J. Breast Cancer 2013, 16, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, A.; Odabas, H.; Kaya, S.; Bozkurt, O.; Degirmenci, M.; Topcu, T.O.; Aytekin, A.; Arpaci, E.; Avci, N.; Pilanci, K.N.; et al. Hormone Receptor Status and Survival of Medullary Breast Cancer Patients. Saudi Med. J. 2017, 38, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Limaiem, F.; Mlika, M. Medullary Breast Carcinoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Huober, J.; Gelber, S.; Goldhirsch, A.; Coates, A.S.; Viale, G.; Öhlschlegel, C.; Price, K.N.; Gelber, R.D.; Regan, M.M.; Thürlimann, B. Prognosis of Medullary Breast Cancer: Analysis of 13 International Breast Cancer Study Group (IBCSG) Trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 2843–2851. [Google Scholar] [CrossRef] [PubMed]
- Matheus, V.S.; Kestelman, F.P.; Canella, E.D.O.; Djahjah, M.C.R.; Koch, H.A. Medullary Breast Carcinoma: Anatomo-Radiological Correlation. Radiol. Bras. 2008, 41, 379–383. [Google Scholar] [CrossRef]
- Yilmaz, E.; Lebe, B.; Balci, P.; Sal, S.; Canda, T. Comparison of Mammographic and Sonographic Findings in Typical and Atypical Medullary Carcinomas of the Breast. Clin. Radiol. 2002, 57, 640–645. [Google Scholar] [CrossRef]
- Wang, X.; Xu, P.; Wang, Y.; Grant, E.G. Contrast-Enhanced Ultrasonographic Findings of Different Histopathologic Types of Breast Cancer. Acta Radiol. 2011, 52, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, J.; Hama, H.; Kimura, N.; Takahashi, S. MR Imaging of Medullary Carcinoma of the Breast. Eur. J. Radiol. 2009, 70, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Akın, Y.; Uğurlu, M.Ü.; Kaya, H.; Arıbal, E. Diagnostic Value of Diffusion-Weighted Imaging and Apparent Diffusion Coefficient Values in the Differentiation of Breast Lesions, Histpathologic Subgroups and Correlatıon with Prognostıc Factors Using 3.0 Tesla MR. J. Breast Health 2016, 12, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Lim, H.S.; Lee, J.S.; Park, M.H.; Yoon, J.H.; Park, J.G.; Kang, H.K. Medullary Carcinoma of the Breast: MRI Findings. AJR Am. J. Roentgenol. 2012, 198, W482–W487. [Google Scholar] [CrossRef]
- Demir, S.; Sezgin, G.; Sari, A.A.; Kucukzeybek, B.B.; Yigit, S.; Etit, D.; Yazici, A.; Kucukzeybek, Y. Clinicopathological Analysis of Invasive Cribriform Carcinoma of the Breast, with Review of the Literature. Ann. Diagn. Pathol. 2021, 54, 151794. [Google Scholar] [CrossRef]
- Mo, C.-H.; Ackbarkhan, Z.; Gu, Y.-Y.; Chen, G.; Pang, Y.-Y.; Dang, Y.-W.; Feng, Z.-B. Invasive Cribriform Carcinoma of the Breast: A Clinicopathological Analysis of 12 Cases with Review of Literature. Int. J. Clin. Exp. Pathol. 2017, 10, 9917–9924. [Google Scholar] [PubMed]
- Lee, Y.J.; Choi, B.B.; Suh, K.S. Invasive Cribriform Carcinoma of the Breast: Mammographic, Sonographic, MRI, and 18 F-FDG PET-CT Features. Acta Radiol. 2015, 56, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Balci, P.; Başara Akin, I.; Köremezli, N.; Güray Durak, M.; Altay, C.; Gezer, N.S.; Sevinç, A.İ. Evaluation and Comparison of Radiologic-Pathologic Findings in Invasive Cribriform Carcinoma of the Breast. Turk. J. Med. Sci. 2017, 47, 738–747. [Google Scholar] [CrossRef]
- Jagmohan, P.; Pool, F.J.; Putti, T.C.; Wong, J. Papillary Lesions of the Breast: Imaging Findings and Diagnostic Challenges. Diagn. Interv. Radiol. Ank. Turk. 2013, 19, 471–478. [Google Scholar] [CrossRef]
- Kestelman, F.P.; Gomes, C.F.A.; Fontes, F.B.; Marchiori, E. Imaging Findings of Papillary Breast Lesions: A Pictorial Review. Clin. Radiol. 2014, 69, 436–441. [Google Scholar] [CrossRef]
- Gültekin, M.A.; Yabul, F.Ç.; Temur, H.O.; Sari, L.; Yilmaz, T.F.; Toprak, H.; Yildiz, S. Papillary Lesions of the Breast: Addition of DWI and TIRM Sequences to Routine Breast MRI Could Help in Differentiation Benign from Malignant. Curr. Med. Imaging 2022, 18, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Durur-Subasi, I. DW-MRI of the Breast: A Pictorial Review. Insights Imaging 2019, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Deman, F.; Punie, K.; Laenen, A.; Neven, P.; Oldenburger, E.; Smeets, A.; Nevelsteen, I.; Van Ongeval, C.; Baten, A.; Faes, T.; et al. Assessment of Stromal Tumor Infiltrating Lymphocytes and Immunohistochemical Features in Invasive Micropapillary Breast Carcinoma with Long-Term Outcomes. Breast Cancer Res. Treat. 2020, 184, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Acs, G.; Paragh, G.; Chuang, S.-T.; Laronga, C.; Zhang, P.J. The Presence of Micropapillary Features and Retraction Artifact in Core Needle Biopsy Material Predicts Lymph Node Metastasis in Breast Carcinoma. Am. J. Surg. Pathol. 2009, 33, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Nangong, J.; Cheng, Z.; Yu, L.; Zheng, X.; Ding, G. Invasive Micropapillary Breast Carcinoma: A Retrospective Study on the Clinical Imaging Features and Pathologic Findings. Front. Surg. 2022, 9, 1011773. [Google Scholar] [CrossRef] [PubMed]
- Adrada, B.; Arribas, E.; Gilcrease, M.; Yang, W.T. Invasive Micropapillary Carcinoma of the Breast: Mammographic, Sonographic, and MRI Features. AJR Am. J. Roentgenol. 2009, 193, W58–W63. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.N.; Guimaraes, L.S.; Reynolds, C.A.; Ghosh, K.; Degnim, A.C.; Glazebrook, K.N. Invasive Micropapillary Carcinoma of the Breast: Imaging Features with Clinical and Pathologic Correlation. AJR Am. J. Roentgenol. 2013, 200, 689–695. [Google Scholar] [CrossRef]
- Günhan-Bilgen, I.; Zekioglu, O.; Ustün, E.E.; Memis, A.; Erhan, Y. Invasive Micropapillary Carcinoma of the Breast: Clinical, Mammographic, and Sonographic Findings with Histopathologic Correlation. AJR Am. J. Roentgenol. 2002, 179, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.U.; Choi, B.B.; Shu, K.S.; Kim, S.M.; Seo, Y.D.; Lee, J.S.; Chang, E.S. Imaging Findings of Invasive Micropapillary Carcinoma of the Breast. J. Breast Cancer 2012, 15, 57–64. [Google Scholar] [CrossRef]
- Alsharif, S.; Daghistani, R.; Kamberoğlu, E.A.; Omeroglu, A.; Meterissian, S.; Mesurolle, B. Mammographic, Sonographic and MR Imaging Features of Invasive Micropapillary Breast Cancer. Eur. J. Radiol. 2014, 83, 1375–1380. [Google Scholar] [CrossRef]
- Kamitani, K.; Kamitani, T.; Ono, M.; Toyoshima, S.; Mitsuyama, S. Ultrasonographic Findings of Invasive Micropapillary Carcinoma of the Breast: Correlation between Internal Echogenicity and Histological Findings. Breast Cancer 2012, 19, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.A.; El-Ayat, G.A. Non-Operative Breast Pathology: Apocrine Lesions. J. Clin. Pathol. 2007, 60, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Vranic, S.; Gatalica, Z. An Update on the Molecular and Clinical Characteristics of Apocrine Carcinoma of the Breast. Clin. Breast Cancer 2022, 22, e576–e585. [Google Scholar] [CrossRef]
- Provenzano, E.; Gatalica, Z.; Vranic, S. Carcinoma with Apocrine Differentiation; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-832-4500-1. [Google Scholar]
- D’Arcy, C.; Quinn, C. Apocrine Lesions of the Breast: Part 1 of a Two-Part Review: Benign, Atypical and in Situ Apocrine Proliferations of the Breast. J. Clin. Pathol. 2019, 72, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, S.W.; Lee, J.E.; Jeong, W.G.; Lee, J.S.; Park, M.H.; Lim, H.S. Malignant Apocrine Lesions of the Breast: Multimodality Imaging Findings and Biologic Features. J. Breast Cancer 2022, 25, 513–521. [Google Scholar] [CrossRef]
- Gilles, R.; Lesnik, A.; Guinebretière, J.M.; Tardivon, A.; Masselot, J.; Contesso, G.; Vanel, D. Apocrine Carcinoma: Clinical and Mammographic Features. Radiology 1994, 190, 495–497. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, S.Y.; Oh, M.H.; Lee, J.E. A Rare Case of Invasive Apocrine Carcinoma of the Breast with Unusual Radiologic Findings. Iran. J. Radiol. 2016, 13, e35298. [Google Scholar] [CrossRef]
- Seo, K.-J.; An, Y.Y.; Whang, I.Y.; Chang, E.D.; Kang, B.J.; Kim, S.H.; Park, C.S.; Kim, J.S.; Hong, H. Sonography of Invasive Apocrine Carcinoma of the Breast in Five Cases. Korean J. Radiol. 2015, 16, 1006–1011. [Google Scholar] [CrossRef]
- Gokalp, G.; Topal, U.; Haholu, A.; Kizilkaya, E. Apocrine Carcinoma of the Breast: Mammography and Ultrasound Findings. Eur. J. Radiol. Extra 2006, 60, 55–59. [Google Scholar] [CrossRef]
- Onoue, S.; Katoh, T.; Chigira, H.; Matsuo, K.; Suzuki, M.; Shibata, Y.; Maeda, M. A Case of Apocrine Carcinoma of the Breast Presenting as Two Cysts. Breast Cancer 1997, 4, 193–196. [Google Scholar] [CrossRef]
- Jia, Y.; He, C.; Liu, L.; Sun, L.; Chen, Y.; Luo, Y.; Yu, T. A Retrospective Study of the Imaging and Pathological Features of Metaplastic Breast Carcinoma and Review of the Literature. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 248–258. [Google Scholar] [CrossRef]
- Esbah, O.; Turkoz, F.P.; Turker, I.; Durnali, A.; Ekinci, A.S.; Bal, O.; Sonmez, O.U.; Budakoglu, B.; Arslan, U.Y.; Oksuzoglu, B. Metaplastic Breast Carcinoma: Case Series and Review of the Literature. Asian Pac. J. Cancer Prev. APJCP 2012, 13, 4645–4649. [Google Scholar] [CrossRef]
- Tse, G.M.; Tan, P.H.; Putti, T.C.; Lui, P.C.W.; Chaiwun, B.; Law, B.K.B. Metaplastic Carcinoma of the Breast: A Clinicopathological Review. J. Clin. Pathol. 2006, 59, 1079–1083. [Google Scholar] [CrossRef]
- Toumi, Z.; Bullen, C.; Tang, A.C.S.; Dalal, N.; Ellenbogen, S. Metaplastic Breast Carcinoma: A Case Report and Systematic Review of the Literature. Pathol. Int. 2011, 61, 582–588. [Google Scholar] [CrossRef]
- Wang, S.; Lou, J.; Zou, Q.; Jiang, Y.; Wang, S.; Shi, H. Metaplastic Carcinoma of the Breast: MRI Features with Clinical and Histopathologic Correlation. Acad. Radiol. 2023, 30, 1786–1793. [Google Scholar] [CrossRef]
- Pezzi, C.M.; Patel-Parekh, L.; Cole, K.; Franko, J.; Klimberg, V.S.; Bland, K. Characteristics and Treatment of Metaplastic Breast Cancer: Analysis of 892 Cases from the National Cancer Data Base. Ann. Surg. Oncol. 2007, 14, 166–173. [Google Scholar] [CrossRef]
- Donato, H.; Candelária, I.; Oliveira, P.; Gonçalo, M.; Caseiro-Alves, F. Imaging Findings of Metaplastic Carcinoma of the Breast with Pathologic Correlation. J. Belg. Soc. Radiol. 2018, 102, 46. [Google Scholar] [CrossRef]
- Choi, B.B.; Shu, K.S. Metaplastic Carcinoma of the Breast: Multimodality Imaging and Histopathologic Assessment. Acta Radiol. 2012, 53, 5–11. [Google Scholar] [CrossRef]
- Park, J.M.; Han, B.K.; Moon, W.K.; Choe, Y.H.; Ahn, S.H.; Gong, G. Metaplastic Carcinoma of the Breast: Mammographic and Sonographic Findings. J. Clin. Ultrasound JCU 2000, 28, 179–186. [Google Scholar] [CrossRef]
- Yang, W.T.; Hennessy, B.; Broglio, K.; Mills, C.; Sneige, N.; Davis, W.G.; Valero, V.; Hunt, K.K.; Gilcrease, M.Z. Imaging Differences in Metaplastic and Invasive Ductal Carcinomas of the Breast. AJR Am. J. Roentgenol. 2007, 189, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.; Santamaría, G.; Ganau, S.; Farrús, B.; Zanón, G.; Romagosa, C.; Fernández, P.L. MRI of Metaplastic Carcinoma of the Breast. AJR Am. J. Roentgenol. 2005, 184, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, B.; Albarracin, C.T.; Hu, J.; Abraham, S.C.; Wu, Y. Invasive Neuroendocrine Carcinoma of the Breast: A Population-Based Study from the Surveillance, Epidemiology and End Results (SEER) Database. BMC Cancer 2014, 14, 147. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, M.; Menet, E.; Tille, J.-C.; Lae, M.; Fuhrmann, L.; Bonneau, C.; Deniziaut, G.; Melaabi, S.; Ng, C.C.K.; Marchiò, C.; et al. Comprehensive Clinical and Molecular Analyses of Neuroendocrine Carcinomas of the Breast. Mod. Pathol. 2018, 31, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Pareja, F.; D’Alfonso, T.M. Neuroendocrine Neoplasms of the Breast: A Review Focused on the Updated World Health Organization (WHO) 5th Edition Morphologic Classification. Breast J. 2020, 26, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization Classification of Tumours of the Breast. Histopathology 2020, 77, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Wu, Y.; Wei, W.; Yang, W.T. Primary Neuroendocrine Carcinoma of the Breast: Clinical, Imaging, and Histologic Features. AJR Am. J. Roentgenol. 2014, 203, W221–W230. [Google Scholar] [CrossRef]
- Park, S.E.; Cho, K.R.; Song, S.E.; Woo, O.H.; Seo, B.K.; Lee, J. Mammographic, Sonographic, and MRI Features of Primary Neuroendocrine Carcinoma of the Breast: A Case Report. J. Korean Soc. Radiol. 2021, 82, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.H.; Kim, S.M.; Jang, M.; Yun, B.L.; Ahn, H.S.; Kim, S.-W.; Kang, E.; Park, S.Y. Clinical and Radiologic Features of Neuroendocrine Breast Carcinomas. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2014, 33, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Irshad, A.; Ackerman, S.J.; Pope, T.L.; Moses, C.K.; Rumboldt, T.; Panzegrau, B. Rare Breast Lesions: Correlation of Imaging and Histologic Features with WHO Classification. RadioGraphics 2008, 28, 1399–1414. [Google Scholar] [CrossRef]
- Bergstrom, C.; Porembka, J.; Fang, Y.; Sarode, V.; Syed, S. Primary Neuroendocrine Carcinoma of the Breast. Breast J. 2019, 25, 519–520. [Google Scholar] [CrossRef]
- Richardson, E.T.; Jo, V.Y.; Schnitt, S.J. Salivary Gland–like Tumors of the Breast: A Comparison of Clinicopathologic Features and Molecular Pathogenesis with Their Salivary Gland Counterparts. Arch. Pathol. Lab. Med. 2023, 147, 1014–1024. [Google Scholar] [CrossRef]
- Jacob, J.D.; Hodge, C.; Franko, J.; Pezzi, C.M.; Goldman, C.D.; Klimberg, V.S. Rare Breast Cancer: 246 Invasive Secretory Carcinomas from the National Cancer Data Base. J. Surg. Oncol. 2016, 113, 721–725. [Google Scholar] [CrossRef]
- Hoda, R.S.; Brogi, E.; Pareja, F.; Nanjangud, G.; Murray, M.P.; Weigelt, B.; Reis-Filho, J.S.; Wen, H.Y. Secretory Carcinoma of the Breast: Clinicopathologic Profile of 14 Cases Emphasising Distant Metastatic Potential. Histopathology 2019, 75, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xiao, X.; Yang, W.; Shui, R.; Tu, X.; Lu, H.; Shi, D. Secretory Breast Carcinoma: A Clinicopathological and Immunophenotypic Study of 15 Cases with a Review of the Literature. Mod. Pathol. 2012, 25, 567–575. [Google Scholar] [CrossRef]
- Horowitz, D.P.; Sharma, C.S.; Connolly, E.; Gidea-Addeo, D.; Deutsch, I. Secretory Carcinoma of the Breast: Results from the Survival, Epidemiology and End Results Database. Breast 2012, 21, 350–353. [Google Scholar] [CrossRef]
- Amott, D.H.; Masters, R.; Moore, S. Secretory Carcinoma of the Breast. Breast J. 2006, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Paeng, M.H.; Choi, H.-Y.; Sung, S.H.; Moon, B.I.; Shim, S.S. Secretory Carcinoma of the Breast. J. Clin. Ultrasound 2003, 31, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Ko, E.Y.; Han, B.-K.; Shin, J.H.; Kim, S.J.; Cho, E.Y. Secretory Carcinoma of the Breast: Sonographic Features. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2008, 27, 947–954. [Google Scholar] [CrossRef]
- Guldogan, N.; Esen, G.; Kayadibi, Y.; Taskin, F.; Alfatli, A.O.; Boy, F.N.S.; Balci, P.; Bugdayci, O.; Tokat, F.; Ozturk, T.; et al. Adenoid Cystic Carcinoma of the Breast: Multimodality Imaging Findings and Review of the Literature. Acad. Radiol. 2023, 30, 1107–1117. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Yang, H.; Jin, F.; Zheng, A. Breast Adenoid Cystic Carcinoma: A Report of Seven Cases and Literature Review. BMC Surg. 2022, 22, 113. [Google Scholar] [CrossRef]
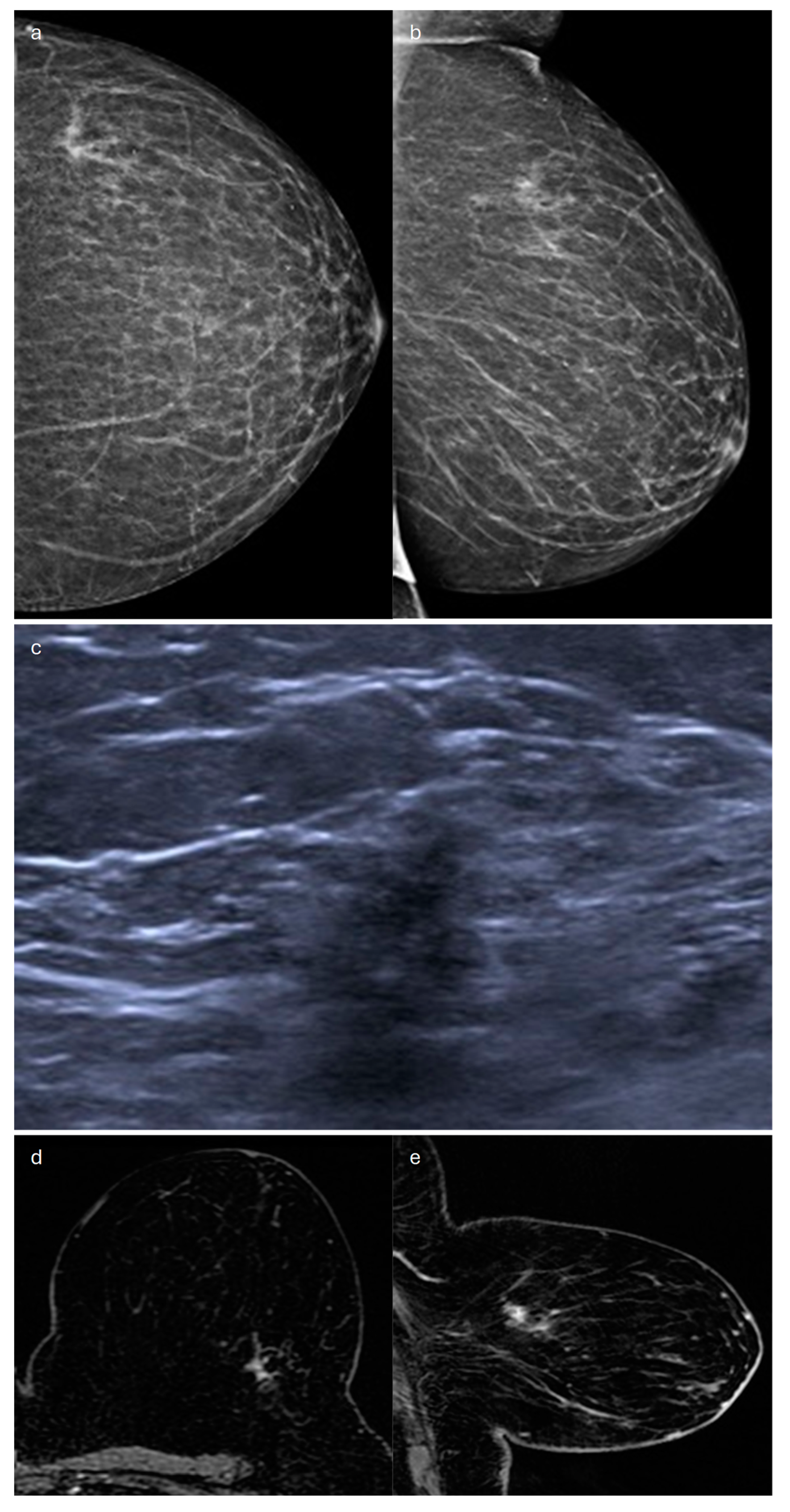
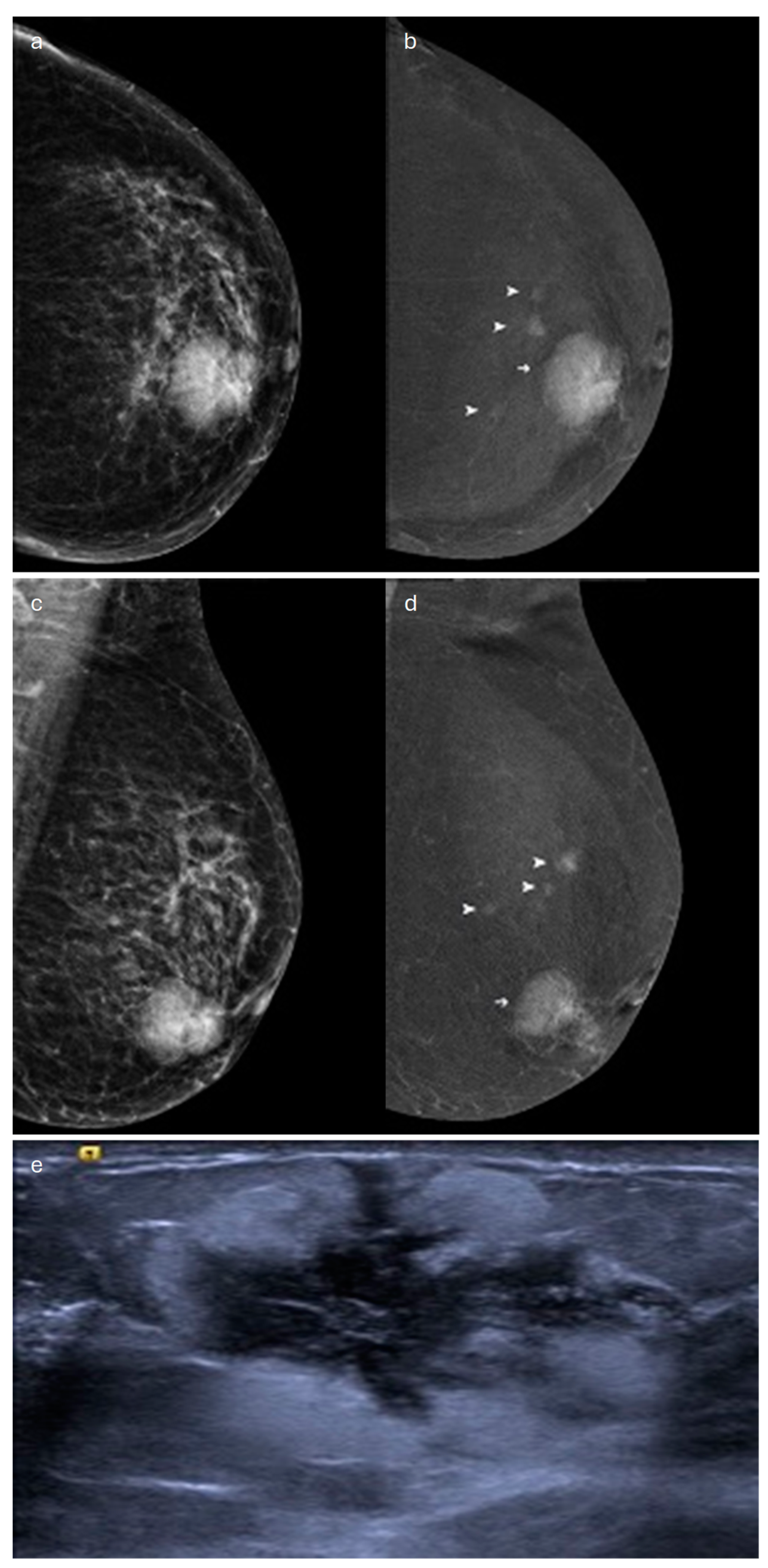


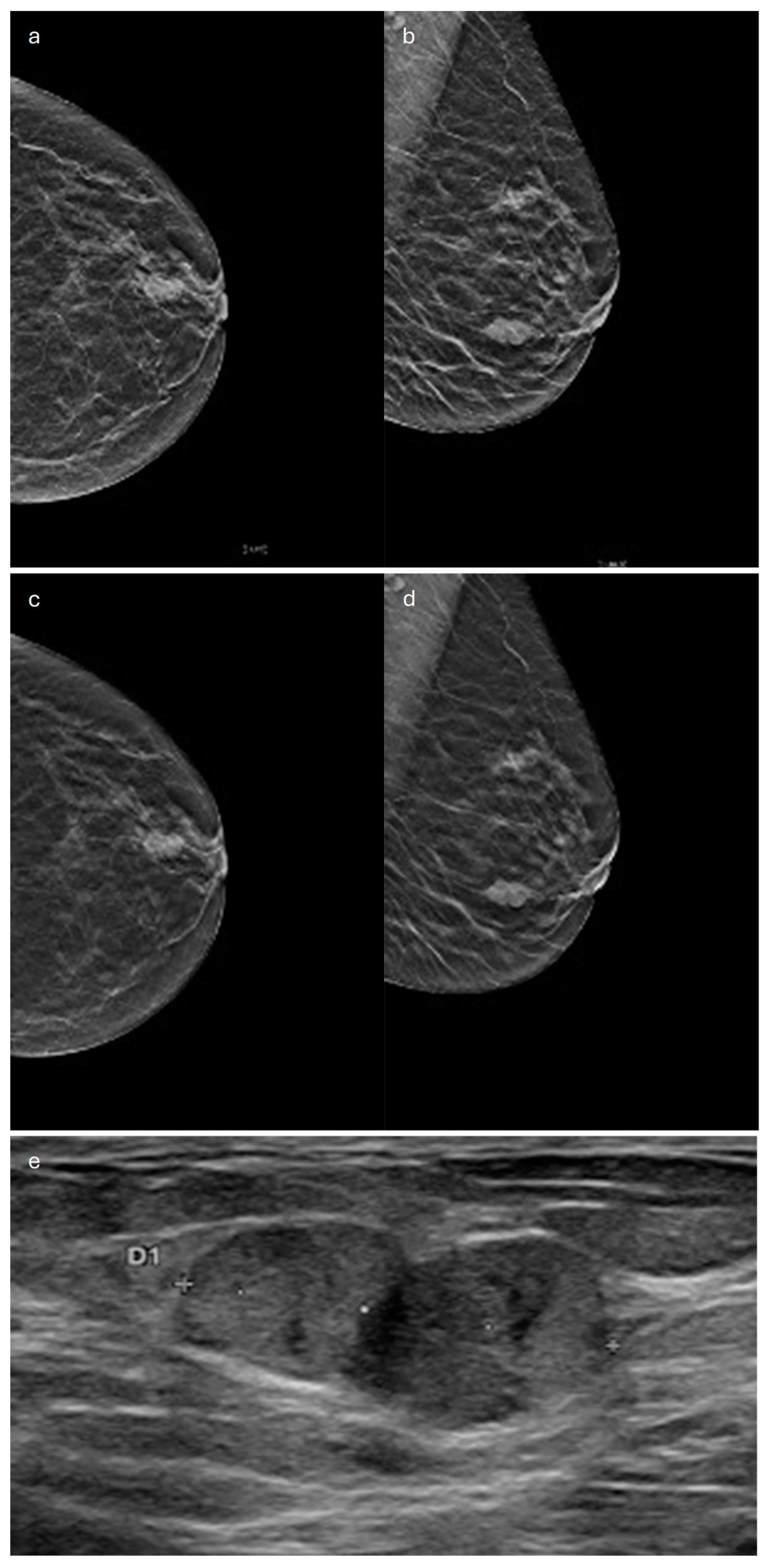
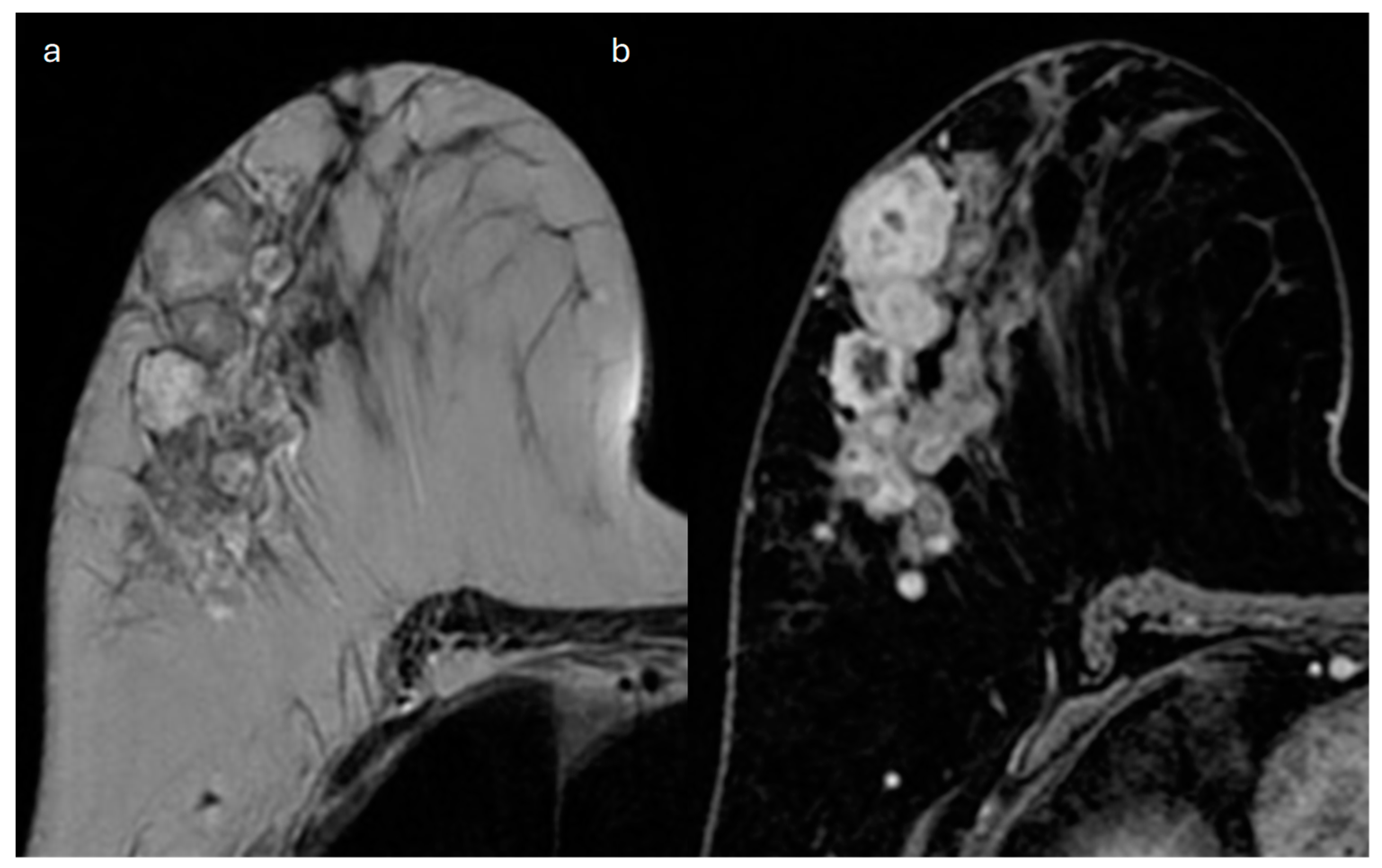

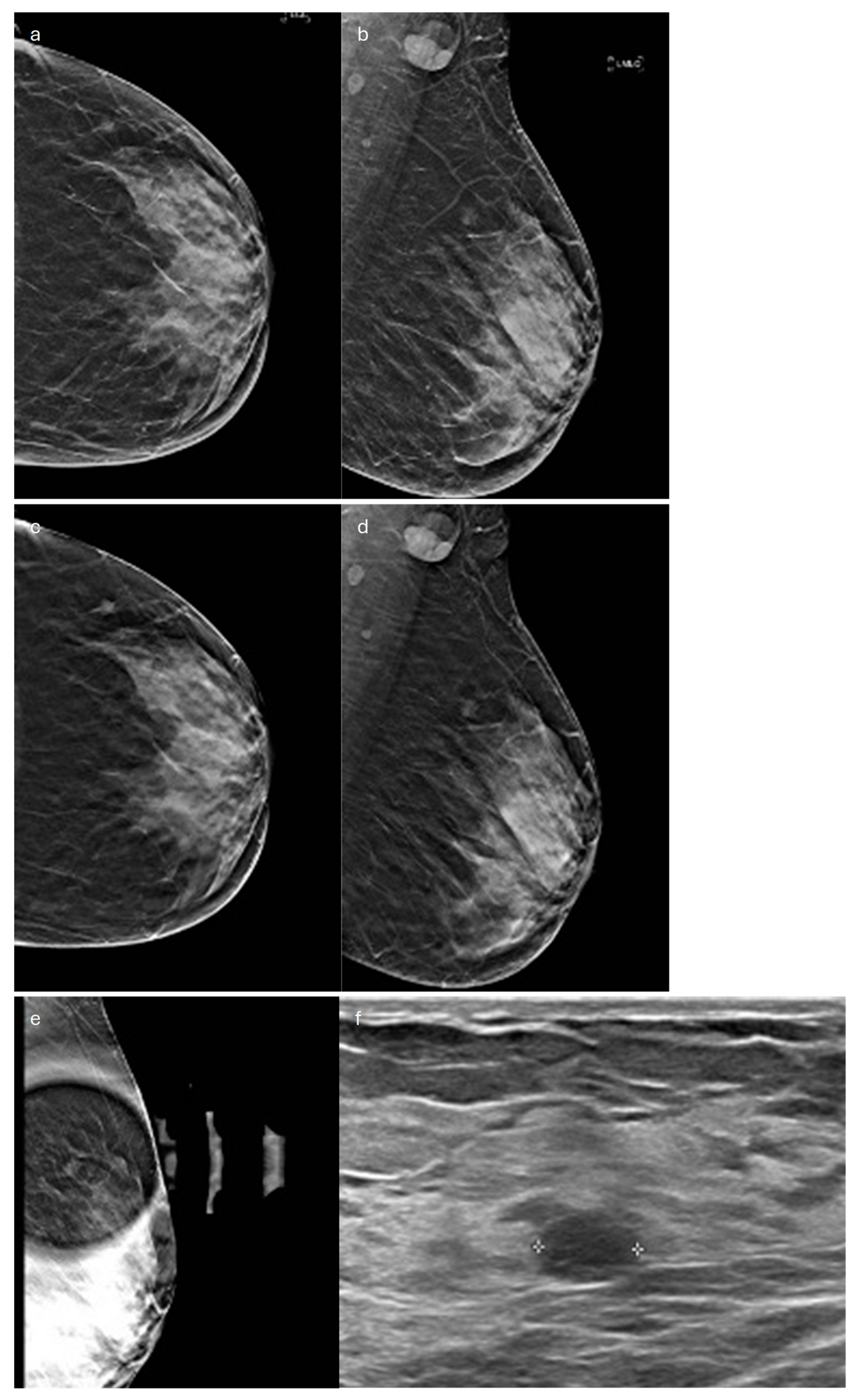
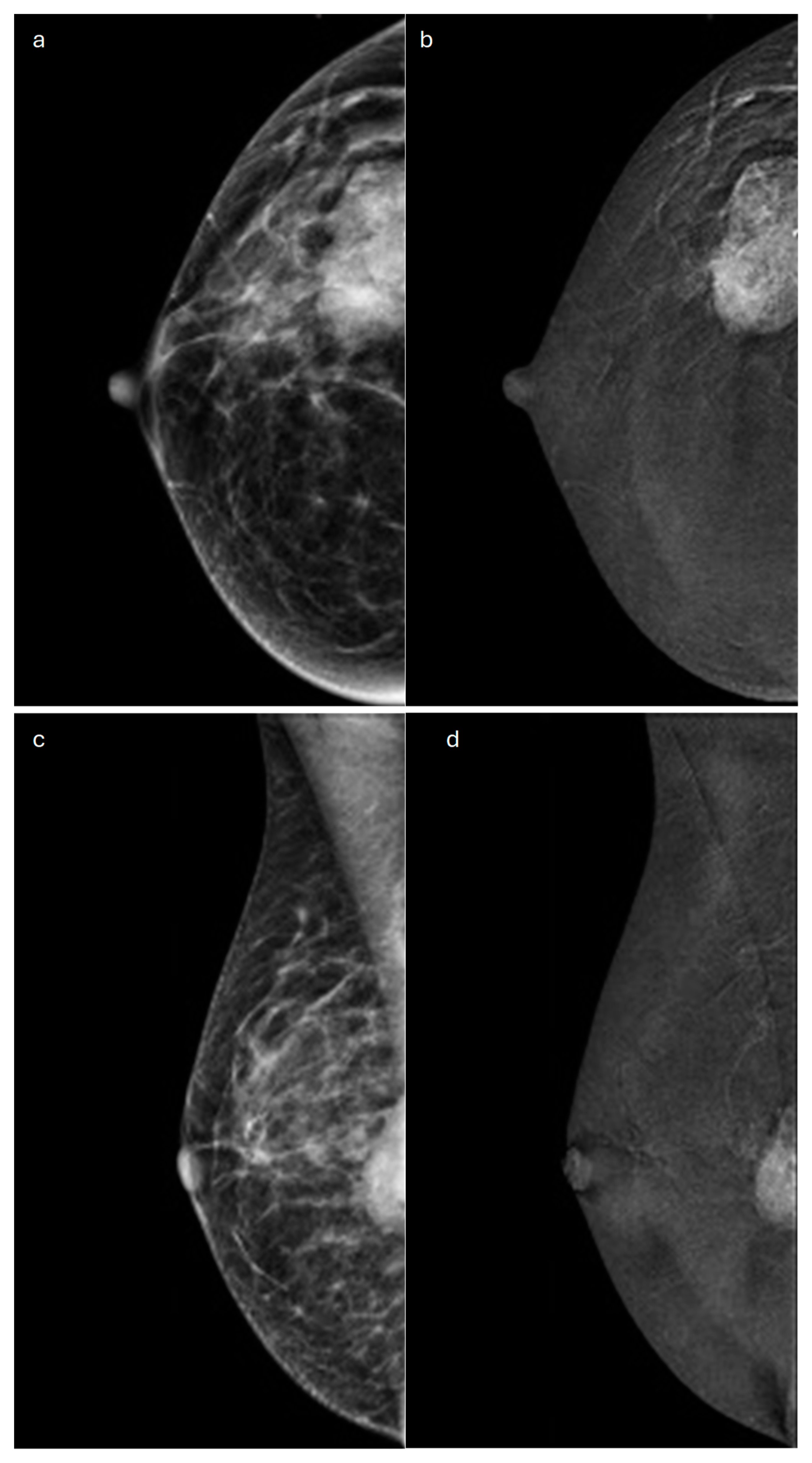

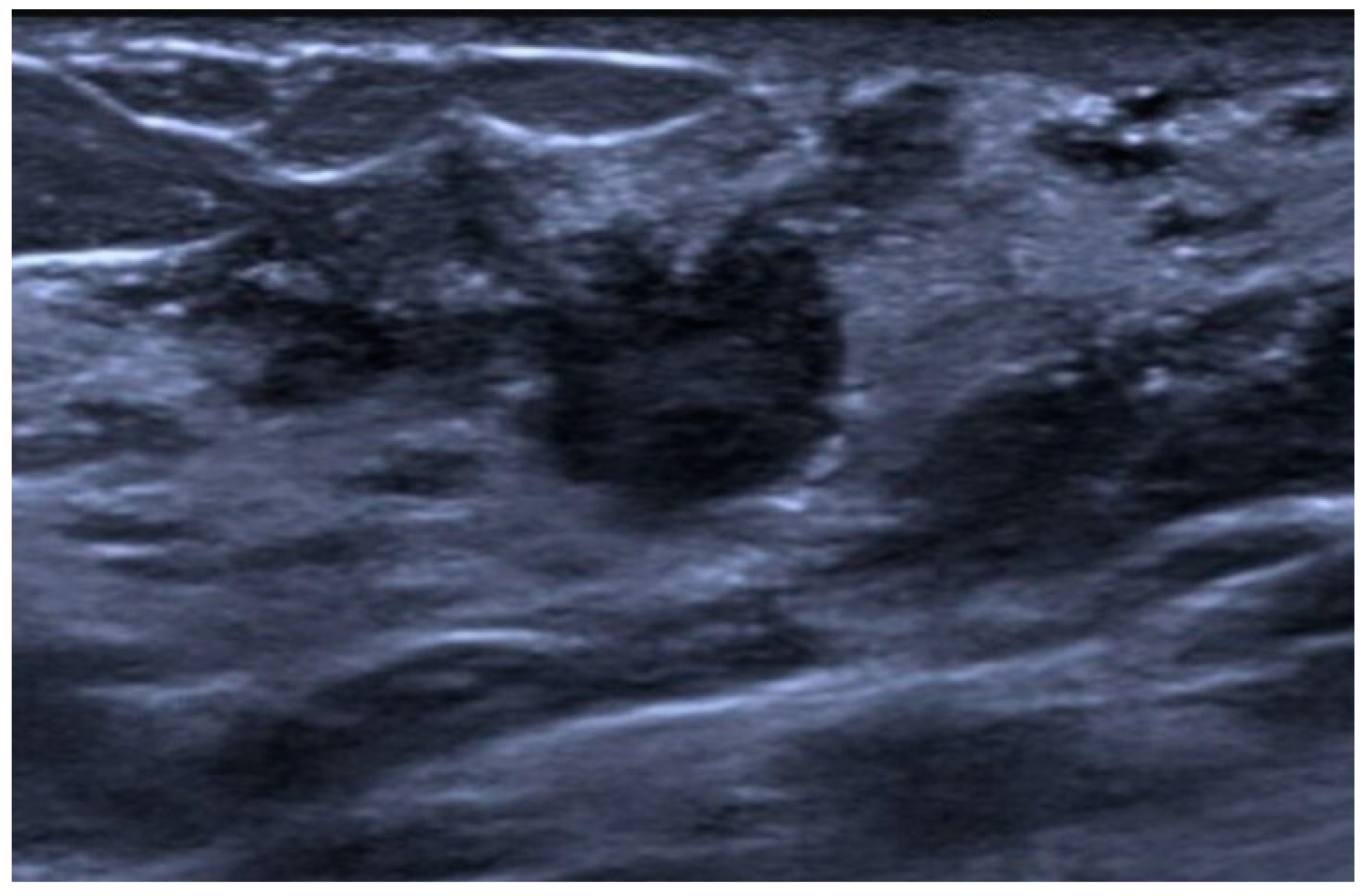
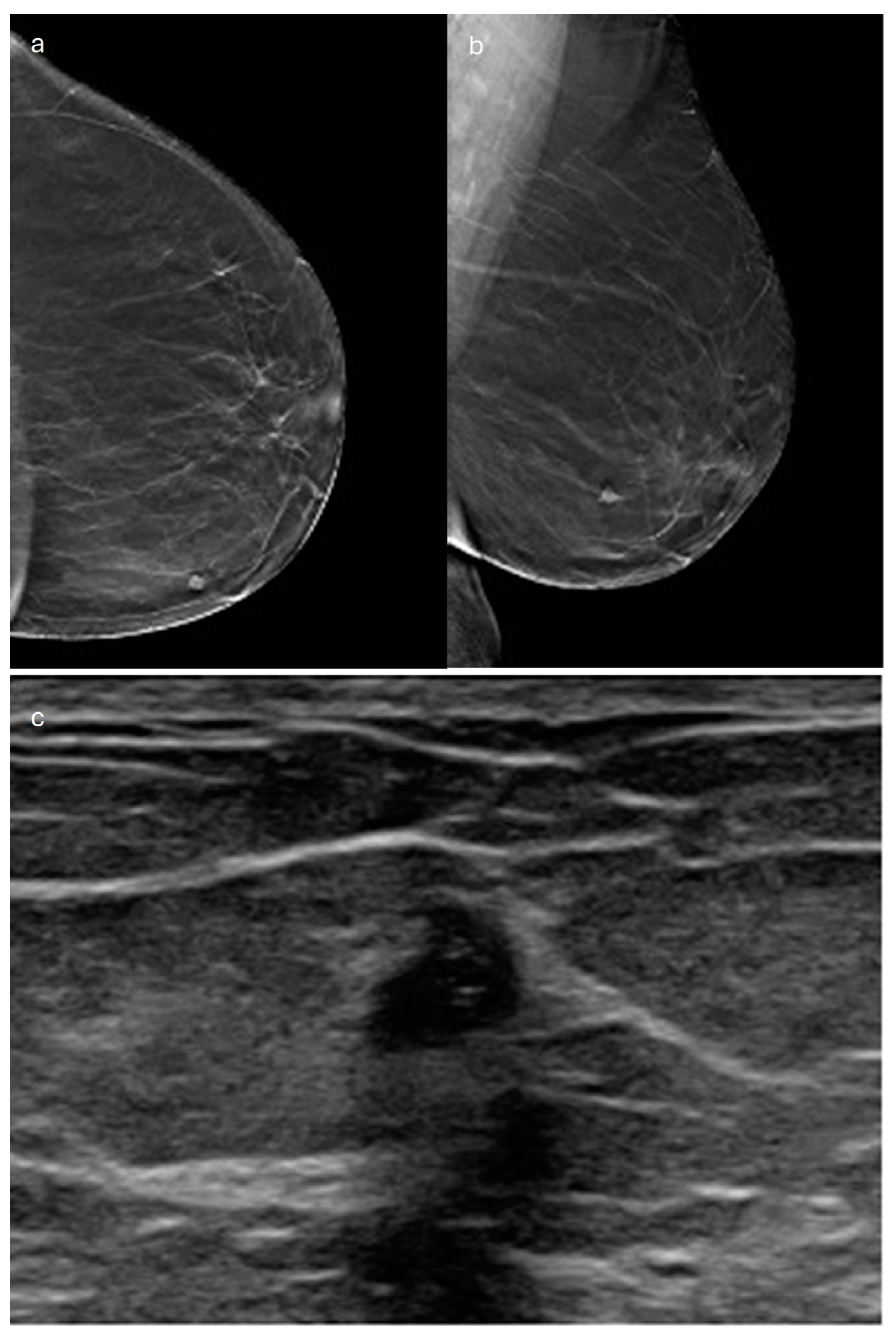
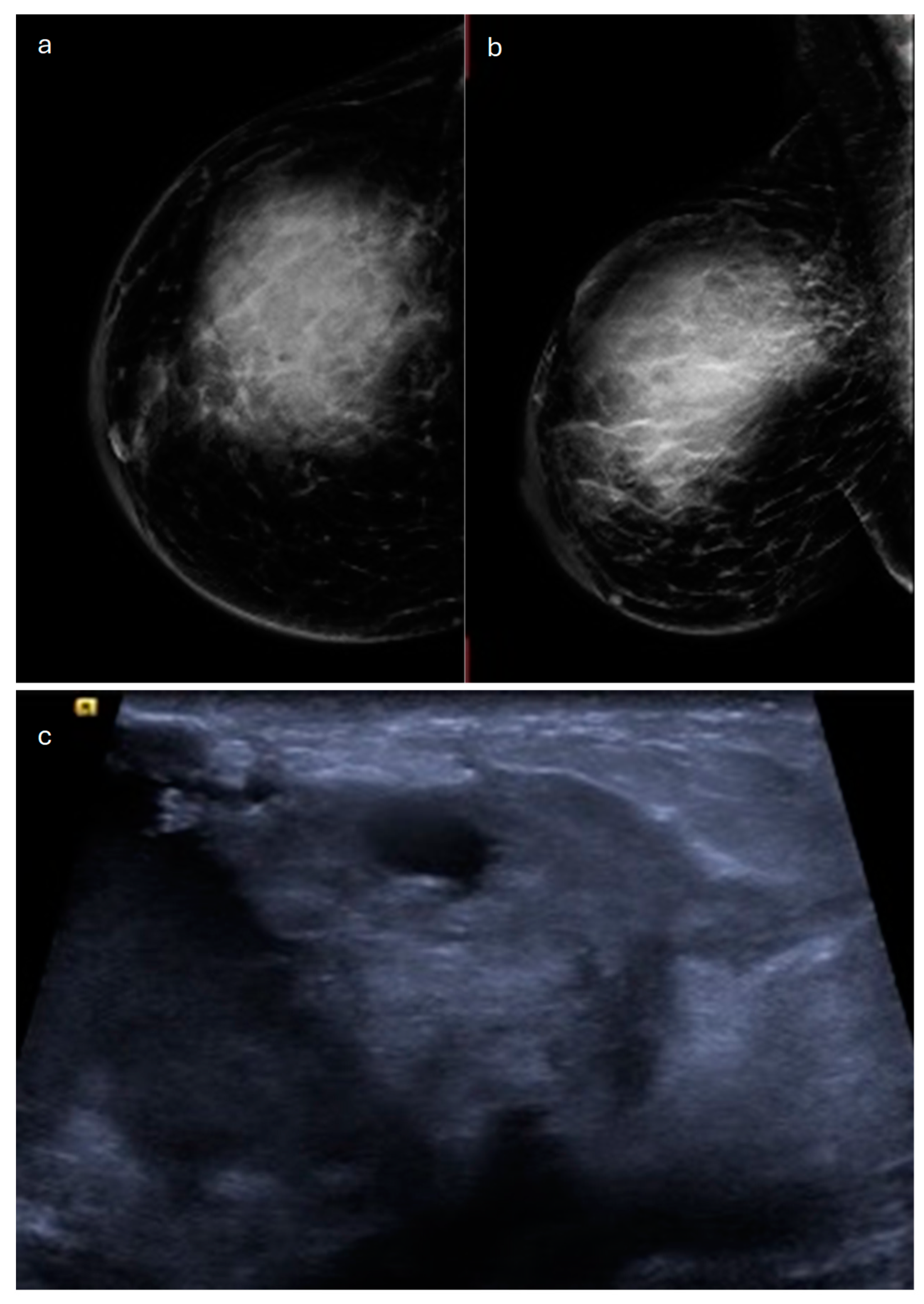
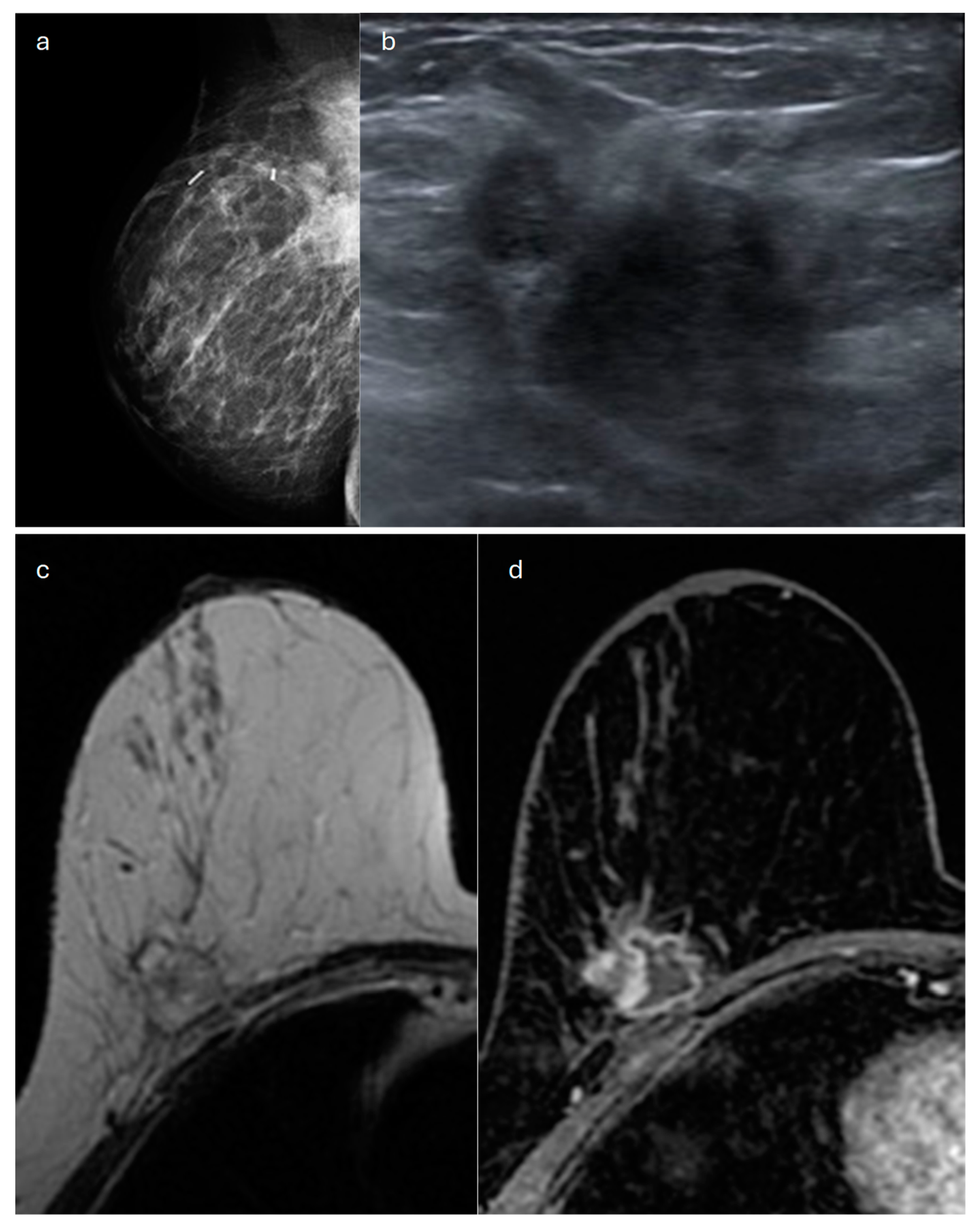
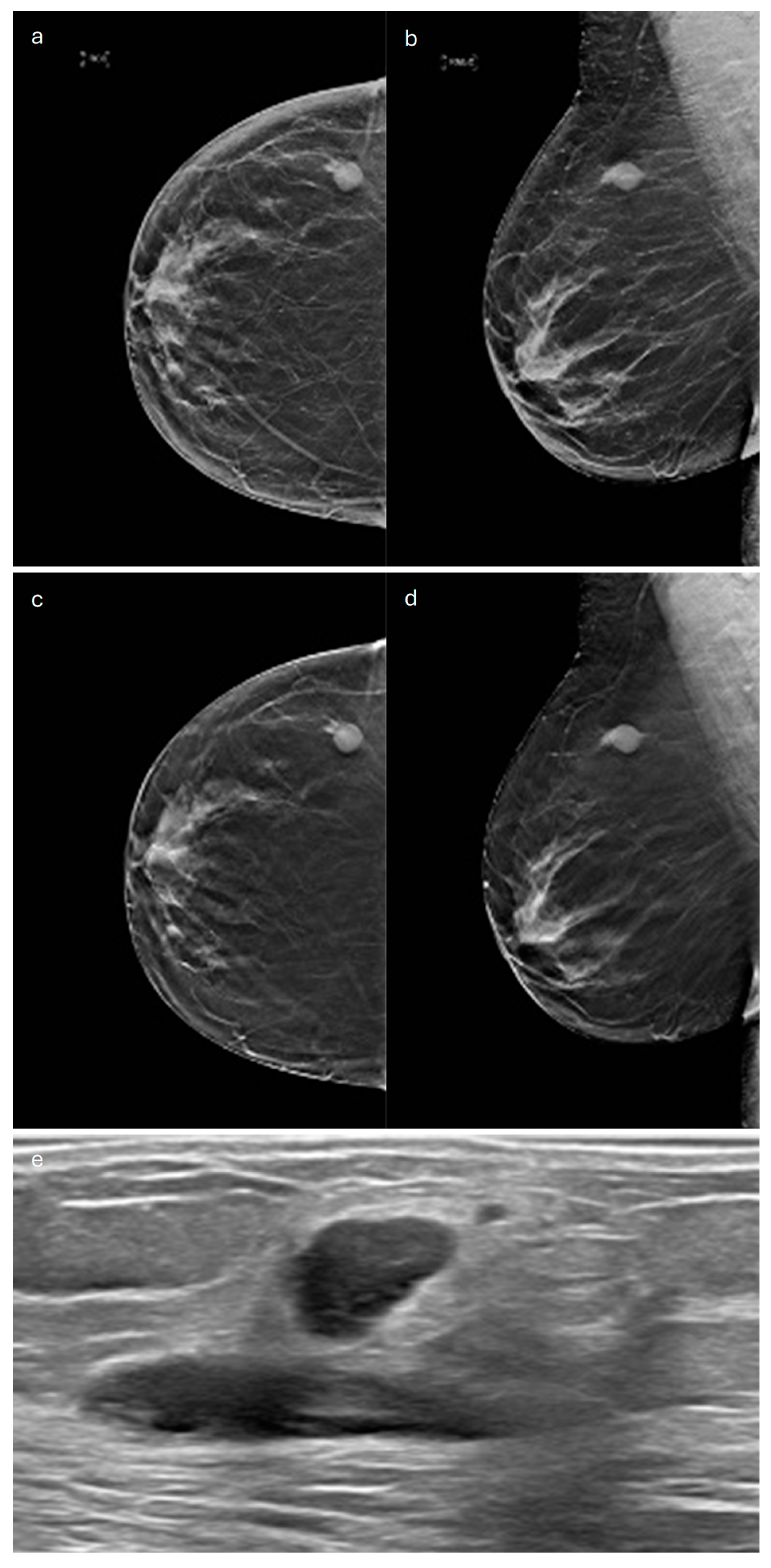
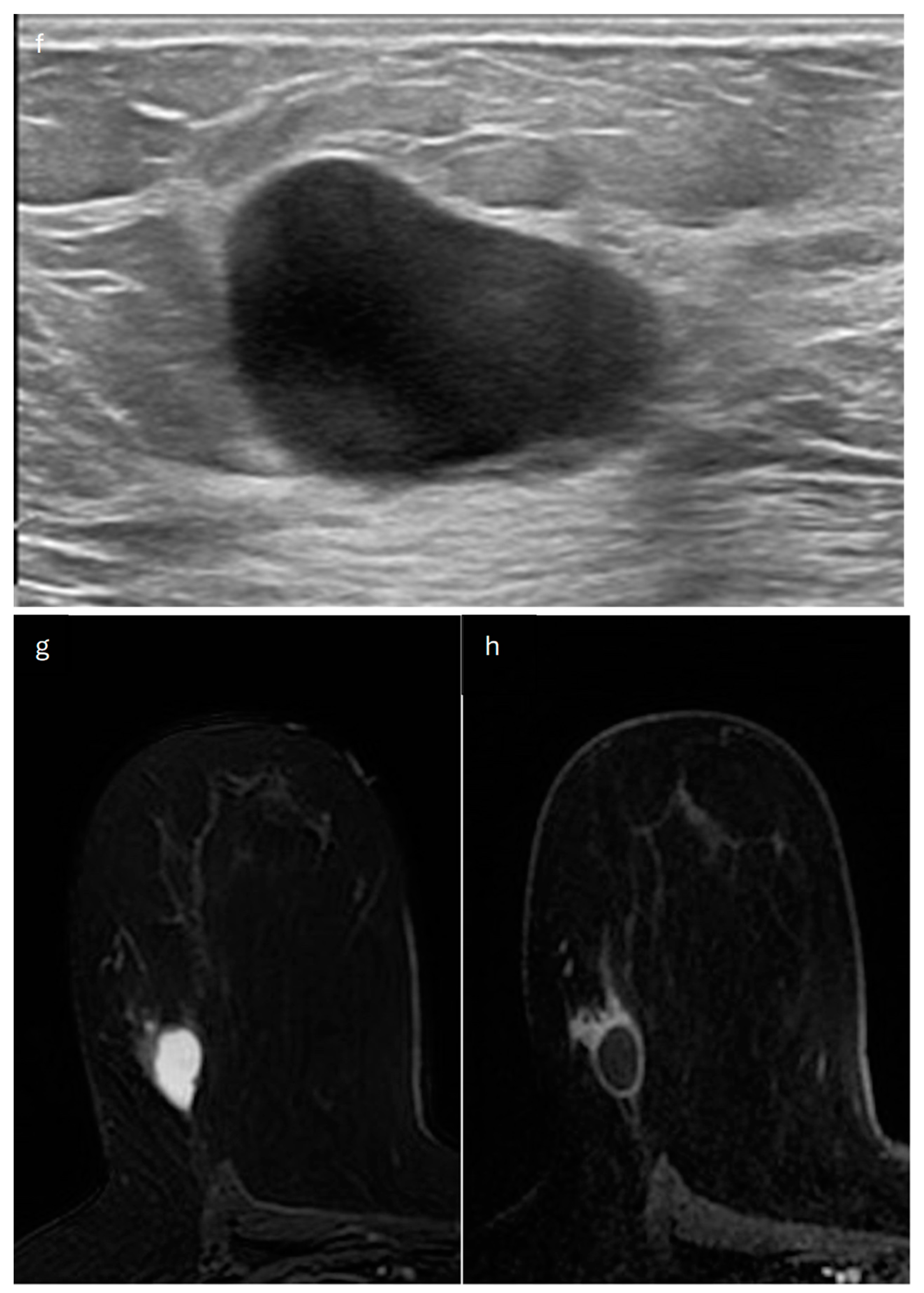
| Type | Mammography | Ultrasound | MRI |
|---|---|---|---|
| Lobular |
|
|
|
| Tubular |
|
|
|
| Mucinous |
|
|
|
| Mucinous cystadenocarcinoma |
|
|
|
| Medullary |
|
|
|
| Cribriform |
|
|
|
| Papillary and Micropapillary | Papillary:
| Papillary:
| Papillary:
|
| Apocrine |
|
|
|
| Metaplastic |
|
|
|
| Neuroendocrine |
|
|
|
| Secretory |
|
|
|
| Adenoid cystic |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, M.; Morciano, F.; Amodeo, S.; Gori, E.; Romanucci, G.; Belli, P.; Tommasini, O.; Fornasa, F.; Rella, R. Special Types of Breast Cancer: Clinical Behavior and Radiological Appearance. J. Imaging 2024, 10, 182. https://doi.org/10.3390/jimaging10080182
Conti M, Morciano F, Amodeo S, Gori E, Romanucci G, Belli P, Tommasini O, Fornasa F, Rella R. Special Types of Breast Cancer: Clinical Behavior and Radiological Appearance. Journal of Imaging. 2024; 10(8):182. https://doi.org/10.3390/jimaging10080182
Chicago/Turabian StyleConti, Marco, Francesca Morciano, Silvia Amodeo, Elisabetta Gori, Giovanna Romanucci, Paolo Belli, Oscar Tommasini, Francesca Fornasa, and Rossella Rella. 2024. "Special Types of Breast Cancer: Clinical Behavior and Radiological Appearance" Journal of Imaging 10, no. 8: 182. https://doi.org/10.3390/jimaging10080182
APA StyleConti, M., Morciano, F., Amodeo, S., Gori, E., Romanucci, G., Belli, P., Tommasini, O., Fornasa, F., & Rella, R. (2024). Special Types of Breast Cancer: Clinical Behavior and Radiological Appearance. Journal of Imaging, 10(8), 182. https://doi.org/10.3390/jimaging10080182







