Ex Vivo Simultaneous H215O Positron Emission Tomography and Magnetic Resonance Imaging of Porcine Kidneys—A Feasibility Study
Abstract
:1. Introduction
2. Materials and Methods
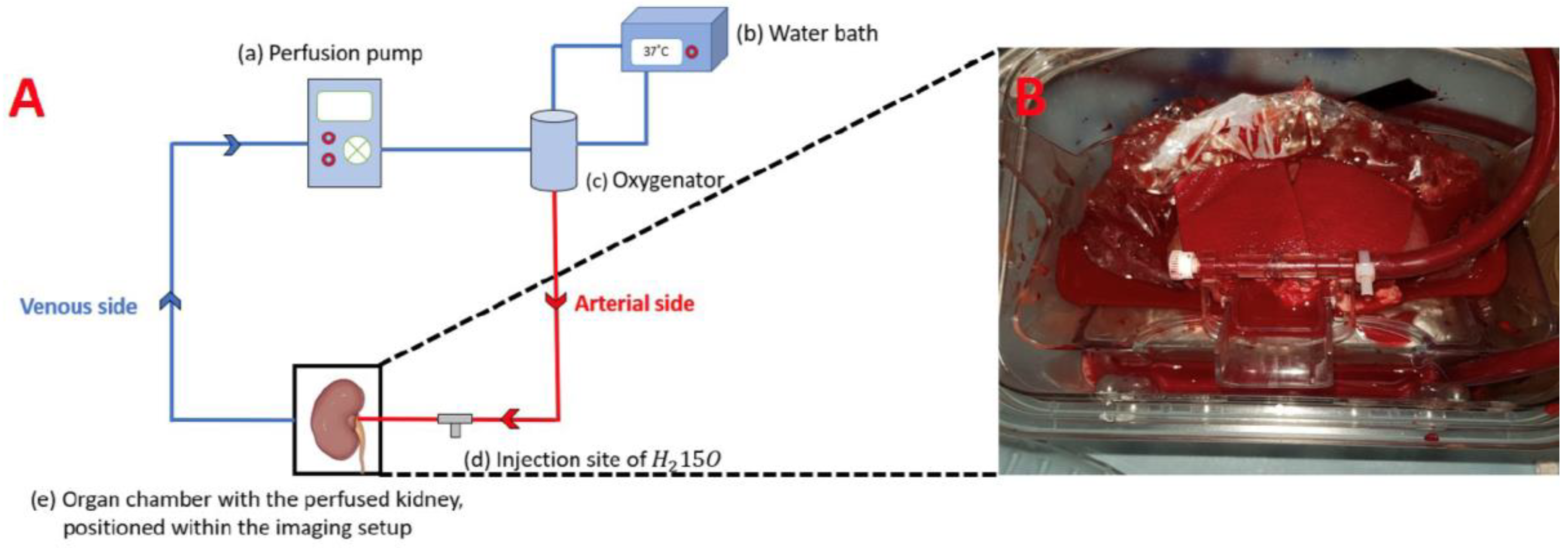
2.1. Ex Vivo Kidney Perfusion
2.2. PET/MRI Examination
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Vaidya, S.R.; Aeddula, N.R. Chronic Renal Failure; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Khatir, D.S.; Pedersen, M.; Jespersen, B.; Buus, N.H. Reproducibility of MRI renal artery blood flow and BOLD measurements in patients with chronic kidney disease and healthy controls. J. Magn. Reson. Imaging 2013, 40, 1091–1098. [Google Scholar] [CrossRef]
- Green, M.A.; Hutchins, G.D. Positron Emission Tomography (PET) Assessment of Renal Perfusion. Semin. Nephrol. 2011, 31, 291–299. [Google Scholar] [CrossRef]
- Päivärinta, J.; Koivuviita, N.; Oikonen, V.; Iida, H.; Liukko, K.; Manner, I.; Löyttyniemi, E.; Nuutila, P.; Metsärinne, K. The renal blood flow reserve in healthy humans and patients with atherosclerotic renovascular disease measured by positron emission tomography using [15O]H2O. EJNMMI Res. 2018, 8, 45. [Google Scholar] [CrossRef]
- Eckerbom, P.; Hansell, P.; Cox, E.F.; Buchanan, C.; Weis, J.; Palm, F.; Francis, S.T.; Liss, P. Multiparametric assessment of renal physiology in healthy volunteers using noninvasive magnetic resonance imaging. Am. J. Physiol. Physiol. 2019, 316, F693–F702. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Textor, S.C.; Lerman, L.O. Renal Relevant Radiology. Clin. J. Am. Soc. Nephrol. 2014, 9, 395–405. [Google Scholar] [CrossRef]
- de Bazelaire, C.; Alsop, D.C.; George, D.; Pedrosa, I.; Wang, Y.; Michaelson, M.D.; Rofsky, N.M. Magnetic Resonance Imaging–Measured Blood Flow Change after Antiangiogenic Therapy with PTK787/ZK 222584 Correlates with Clinical Outcome in Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2008, 14, 5548–5554. [Google Scholar] [CrossRef]
- Gloviczki, M.L.; Glockner, J.F.; Lerman, L.O.; McKusick, M.A.; Misra, S.; Grande, J.P.; Textor, S.C. Preserved Oxygenation Despite Reduced Blood Flow in Poststenotic Kidneys in Human Atherosclerotic Renal Artery Stenosis. Hypertension 2010, 55, 961–966. [Google Scholar] [CrossRef]
- Schutter, R.; van Varsseveld, O.C.; Lantinga, V.A.; Pool, M.B.F.; Hamelink, T.H.; Potze, J.H.; Leuvenink, H.G.D.; Laustsen, C.; Borra, R.J.H.; Moers, C. Magnetic resonance imaging during warm ex vivo kidney perfusion. Artif. Organs 2022, 47, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Frisch, K.; Mortensen, F.V.; Munk, O.L.; Gormsen, L.C.; Alstrup, A.K.O. N-(4-[18F]fluorobenzyl)cholylglycine, a potential tracer for positron emission tomography of enterohepatic circulation and drug-induced inhibition of ileal bile acid transport. A proof-of-concept PET/CT study in pigs. Nucl. Med. Biol. 2022, 114–115, 49–57. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Exp. Physiol. 2020, 105, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-P.; Halter, S.; Prasad, P.V. Blood Oxygen Level-Dependent MR Imaging of the Kidneys. Magn. Reson. Imaging Clin. N. Am. 2008, 16, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Bane, O.; Mendichovszky, I.A.; Milani, B.; Dekkers, I.A.; Deux, J.-F.; Eckerbom, P.; Grenier, N.; Hall, M.E.; Inoue, T.; Laustsen, C.; et al. Consensus-based technical recommendations for clinical translation of renal BOLD MRI. Magn. Reson. Mater. Phys. Biol. Med. 2019, 33, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Niendorf, T.; Pohlmann, A.; Arakelyan, K.; Flemming, B.; Cantow, K.; Hentschel, J.; Grosenick, D.; Ladwig, M.; Reimann, H.; Klix, S.; et al. How bold is blood oxygenation level-dependent (BOLD) magnetic resonance imaging of the kidney? Opportunities, challenges and future directions. Acta Physiol. 2014, 213, 19–38. [Google Scholar] [CrossRef]
- Kudomi, N.; Koivuviita, N.; Liukko, K.E.; Oikonen, V.J.; Tolvanen, T.; Iida, H.; Tertti, R.; Metsärinne, K.; Iozzo, P.; Nuutila, P. Parametric renal blood flow imaging using [15O]H2O and PET. Eur. J. Nucl. Med. 2008, 36, 683–691. [Google Scholar] [CrossRef]
- Mariager, C.; Hansen, E.S.S.; Bech, S.K.; Munk, A.; Kjærgaard, U.; Lyhne, M.D.; Søberg, K.; Nielsen, P.F.; Ringgaard, S.; Laustsen, C. Graft assessment of the ex vivo perfused porcine kidney using hyperpolarized [1-13C]pyruvate. Magn. Reson. Med. 2020, 84, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Woolen, S.A.; Shankar, P.R.; Gagnier, J.J.; MacEachern, M.P.; Singer, L.; Davenport, M.S. Risk of Nephrogenic Systemic Fibrosis in Patients With Stage 4 or 5 Chronic Kidney Disease Receiving a Group II Gadolinium-Based Contrast Agent. JAMA Intern. Med. 2020, 180, 223–230. [Google Scholar] [CrossRef]
- Evans, R.G.; Ince, C.; Joles, J.A.; Smith, D.W.; May, C.N.; O’Connor, P.M.; Gardiner, B.S. Haemodynamic influences on kidney oxygenation: Clinical implications of integrative physiology. Clin. Exp. Pharmacol. Physiol. 2013, 40, 106–122. [Google Scholar] [CrossRef]
- Espes, D.; Manell, E.; Rydén, A.; Carlbom, L.; Weis, J.; Jensen-Waern, M.; Jansson, L.; Eriksson, O. Pancreatic perfusion and its response to glucose as measured by simultaneous PET/MRI. Acta Diabetol. 2019, 56, 1113–1120. [Google Scholar] [CrossRef]
- Puig, O.; Henriksen, O.M.; Vestergaard, M.B.; E Hansen, A.; Andersen, F.L.; Ladefoged, C.N.; Rostrup, E.; Larsson, H.B.; Lindberg, U.; Law, I. Comparison of simultaneous arterial spin labeling MRI and 15O-H2O PET measurements of regional cerebral blood flow in rest and altered perfusion states. J. Cereb. Blood Flow Metab. 2019, 40, 1621–1633. [Google Scholar] [CrossRef]
- Geist, B.K.; Baltzer, P.; Fueger, B.; Hamboeck, M.; Nakuz, T.; Papp, L.; Rasul, S.; Sundar, L.K.S.; Hacker, M.; Staudenherz, A. Assessing the kidney function parameters glomerular filtration rate and effective renal plasma flow with dynamic FDG-PET/MRI in healthy subjects. EJNMMI Res. 2018, 8, 37. [Google Scholar] [CrossRef]
- Geist, B.K.; Baltzer, P.; Fueger, B.; Hamboeck, M.; Nakuz, T.; Papp, L.; Rasul, S.; Sundar, L.K.S.; Hacker, M.; Staudenherz, A. Assessment of the kidney function parameters split function, mean transit time, and outflow efficiency using dynamic FDG-PET/MRI in healthy subjects. Eur. J. Hybrid Imaging 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Zhang, K.; Herzog, H.; Mauler, J.; Filss, C.; Okell, T.W.; Kops, E.R.; Tellmann, L.; Fischer, T.; Brocke, B.; Sturm, W.; et al. Comparison of Cerebral Blood Flow Acquired by Simultaneous [15O]Water Positron Emission Tomography and Arterial Spin Labeling Magnetic Resonance Imaging. J. Cereb. Blood Flow Metab. 2014, 34, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Cutajar, M.; Thomas, D.L.; Hales, P.W.; Banks, T.; Clark, C.A.; Gordon, I. Comparison of ASL and DCE MRI for the non-invasive measurement of renal blood flow: Quantification and reproducibility. Eur. Radiol. 2014, 24, 1300–1308. [Google Scholar] [CrossRef]
- Winter, J.D.; Lawrence, K.S.S.; Cheng, H.M. Quantification of renal perfusion: Comparison of arterial spin labeling and dynamic contrast-enhanced MRI. J. Magn. Reson. Imaging 2011, 34, 608–615. [Google Scholar] [CrossRef]
- Dalal, R.; Bruss, Z.S.; Sehdev, J.S. Physiology, Renal Blood Flow and Filtration; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hannon, J.P.; Bossone, C.A.; Wade, C.E. Normal physiological values for conscious pigs used in biomedical research. Lab. Anim. Sci. 1990, 40, 293–298. [Google Scholar]
- Alpert, N.M.; Rabito, C.A.; Correia, D.J.A.; Babich, J.W.; Littman, B.H.; Tompkins, R.G.; Rubin, N.T.; Rubin, R.H.; Fischman, A.J. Mapping of local renal blood flow with PET and H(2)(15)O. J. Nucl. Med. 2002, 43, 470–475. [Google Scholar]
- Milani, B.; Ansaloni, A.; Sousa-Guimaraes, S.; Vakilzadeh, N.; Piskunowicz, M.; Vogt, B.; Stuber, M.; Burnier, M.; Pruijm, M. Reduction of cortical oxygenation in chronic kidney disease: Evidence obtained with a new analysis method of blood oxygenation level-dependent magnetic resonance imaging. Nephrol. Dial. Transplant. 2016, 32, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Juillard, L.; Janier, M.F.; Fouque, D.; Lionnet, M.; Le Bars, D.; Cinotti, L.; Barthez, P.; Gharib, C.; Laville, M. Renal blood flow measurement by positron emission tomography using 15O-labeled water. Kidney Int. 2000, 57, 2511–2518. [Google Scholar] [CrossRef] [PubMed]

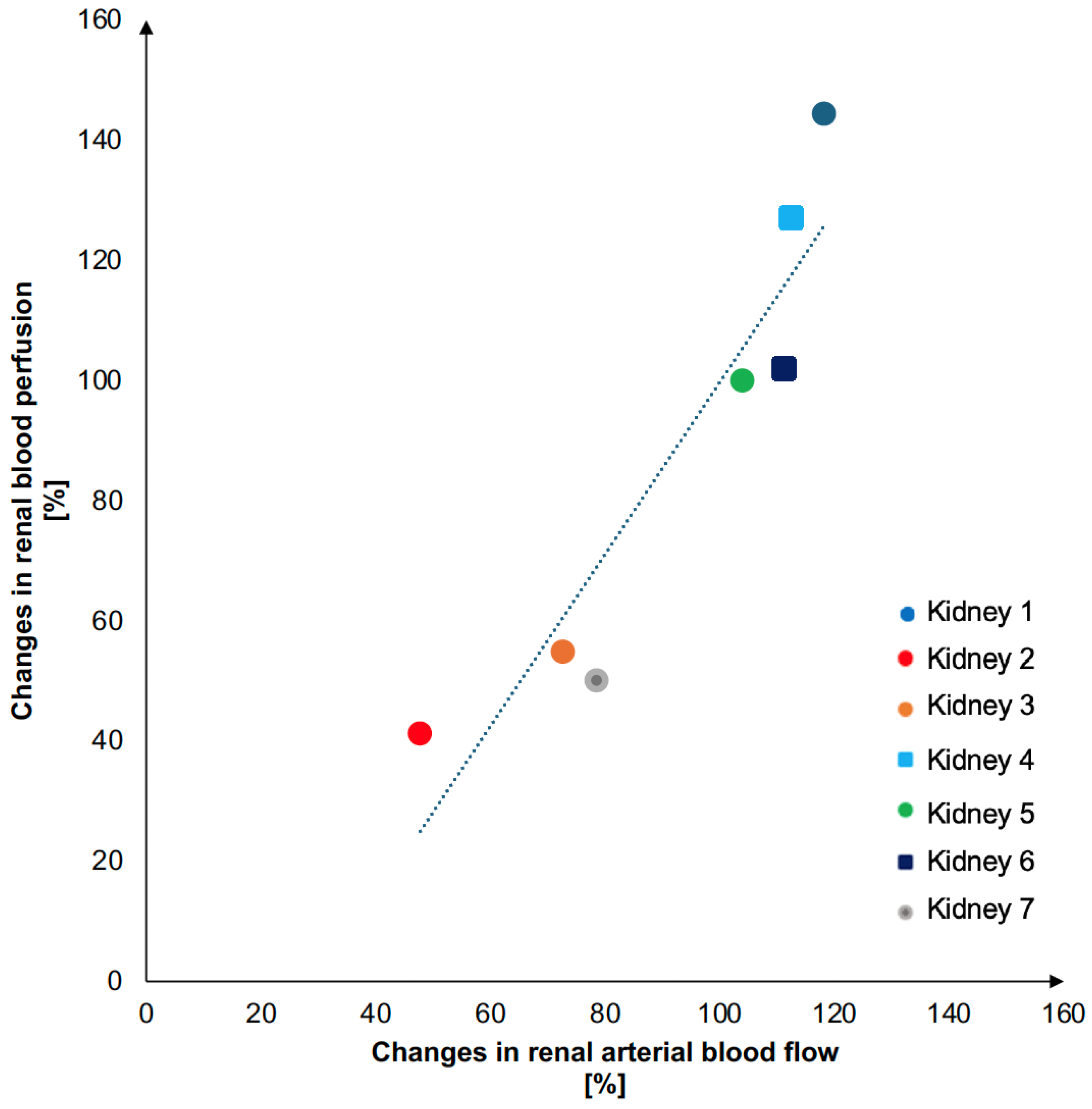

| Kidney (Number) | Weight (g) | Pump Flow Increase (%) | Renal Blood Perfusion Increase (%) |
|---|---|---|---|
| 1 | 107 | 79 | 50 |
| 2 | 165 | 48 | 41 |
| 3 | 170 | 73 | 54 |
| 4 | 169 | 113 | 127 |
| 5 | 190 | 104 | 100 |
| 6 | 242 | 111 | 102 |
| 7 | 213 | 118 | 144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arildsen, M.M.; Mariager, C.Ø.; Overgaard, C.V.; Vorre, T.; Bøjesen, M.; Moeslund, N.; Alstrup, A.K.O.; Tolbod, L.P.; Vendelbo, M.H.; Ringgaard, S.; et al. Ex Vivo Simultaneous H215O Positron Emission Tomography and Magnetic Resonance Imaging of Porcine Kidneys—A Feasibility Study. J. Imaging 2024, 10, 209. https://doi.org/10.3390/jimaging10090209
Arildsen MM, Mariager CØ, Overgaard CV, Vorre T, Bøjesen M, Moeslund N, Alstrup AKO, Tolbod LP, Vendelbo MH, Ringgaard S, et al. Ex Vivo Simultaneous H215O Positron Emission Tomography and Magnetic Resonance Imaging of Porcine Kidneys—A Feasibility Study. Journal of Imaging. 2024; 10(9):209. https://doi.org/10.3390/jimaging10090209
Chicago/Turabian StyleArildsen, Maibritt Meldgaard, Christian Østergaard Mariager, Christoffer Vase Overgaard, Thomas Vorre, Martin Bøjesen, Niels Moeslund, Aage Kristian Olsen Alstrup, Lars Poulsen Tolbod, Mikkel Holm Vendelbo, Steffen Ringgaard, and et al. 2024. "Ex Vivo Simultaneous H215O Positron Emission Tomography and Magnetic Resonance Imaging of Porcine Kidneys—A Feasibility Study" Journal of Imaging 10, no. 9: 209. https://doi.org/10.3390/jimaging10090209





