Longitudinal Imaging of Injured Spinal Cord Myelin and White Matter with 3D Ultrashort Echo Time Magnetization Transfer (UTE-MT) and Diffusion MRI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal and Surgery
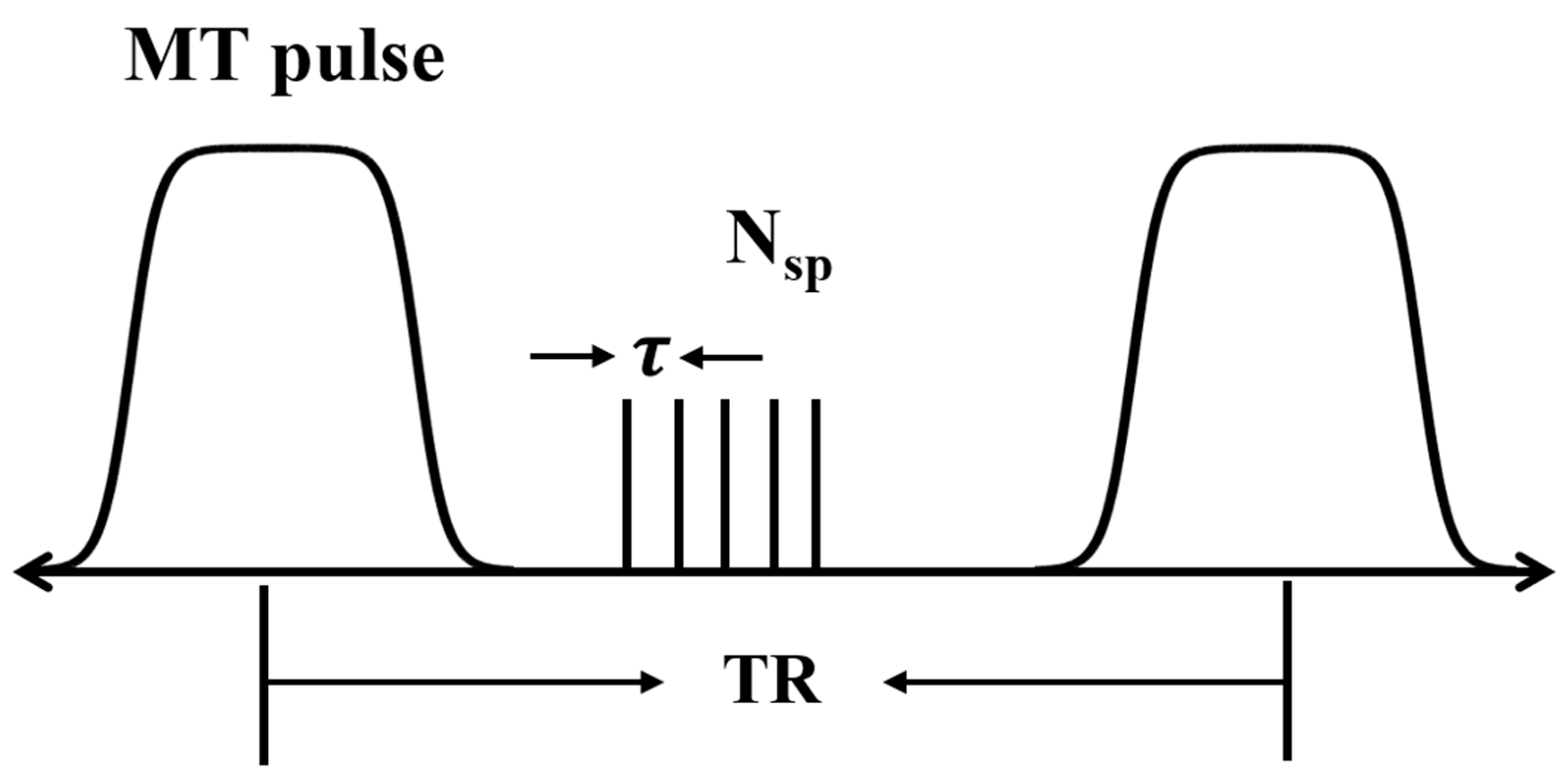
2.2. UTE-MT MRI and MTR Measurements
2.3. Diffusion-Weighted MRI and ADC Measurements
√(ADC_X2 + ADC_Y2 + ADC_Z2))
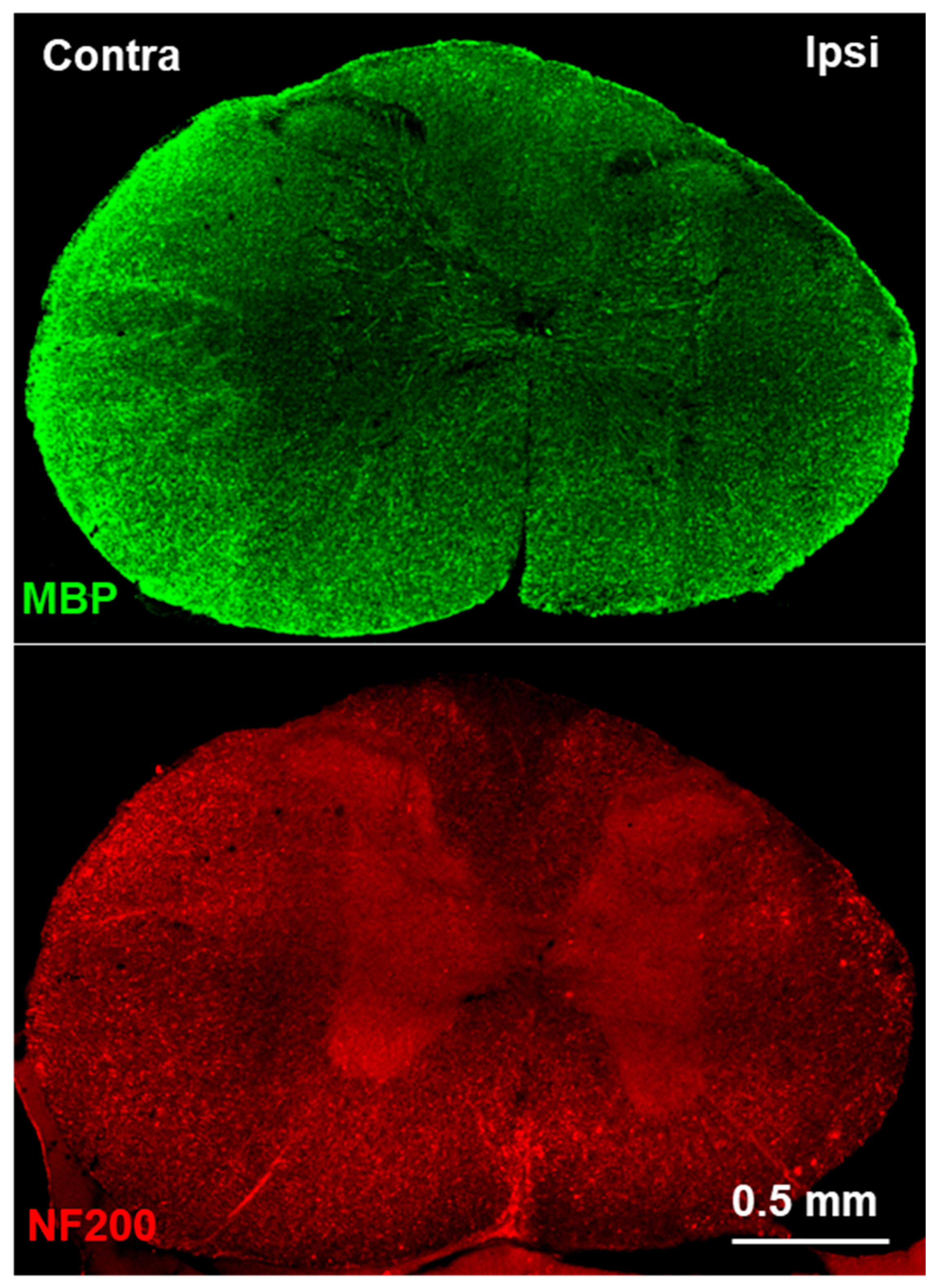
2.4. Immunofluorescence Staining
2.5. Statistical Analysis
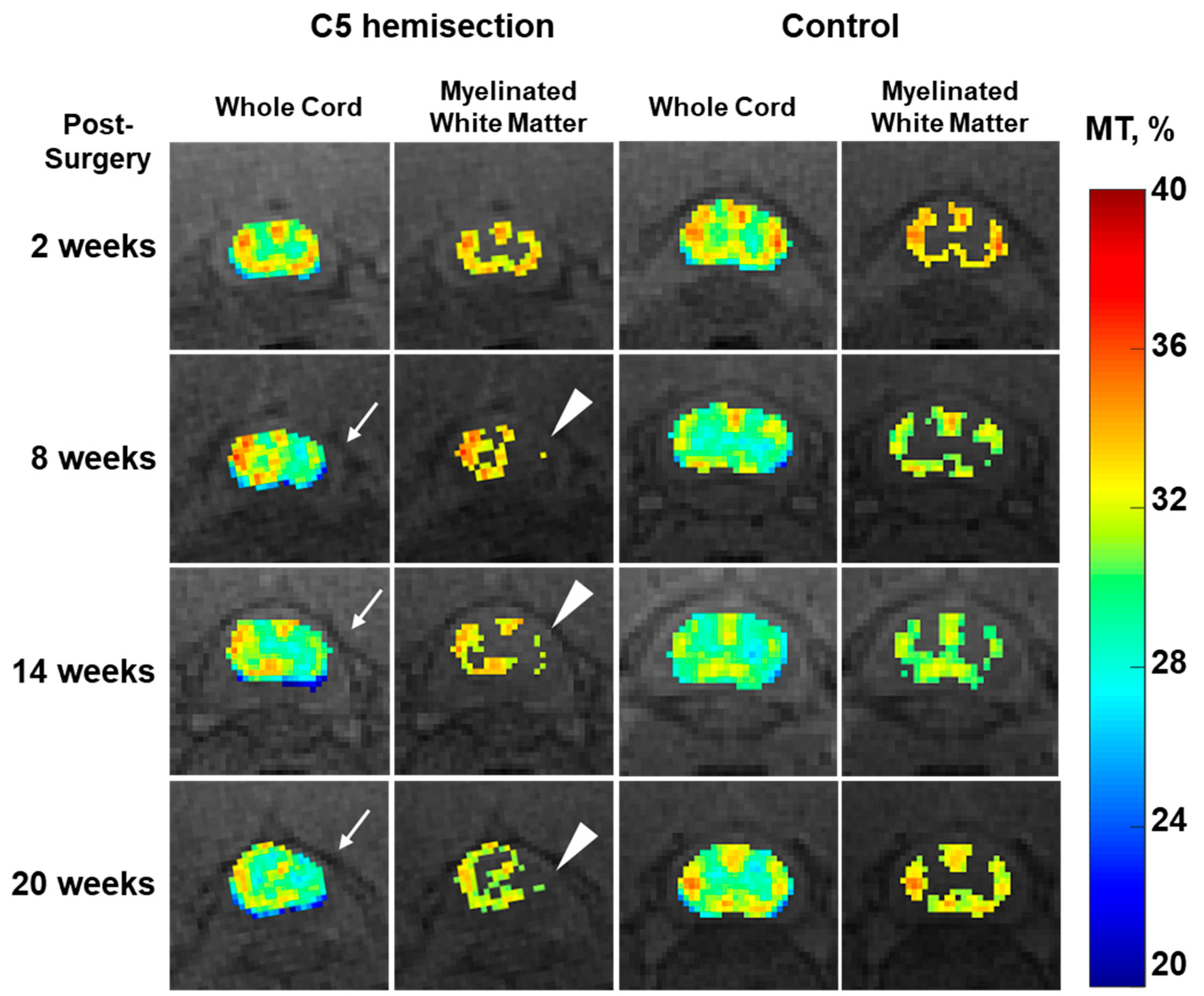
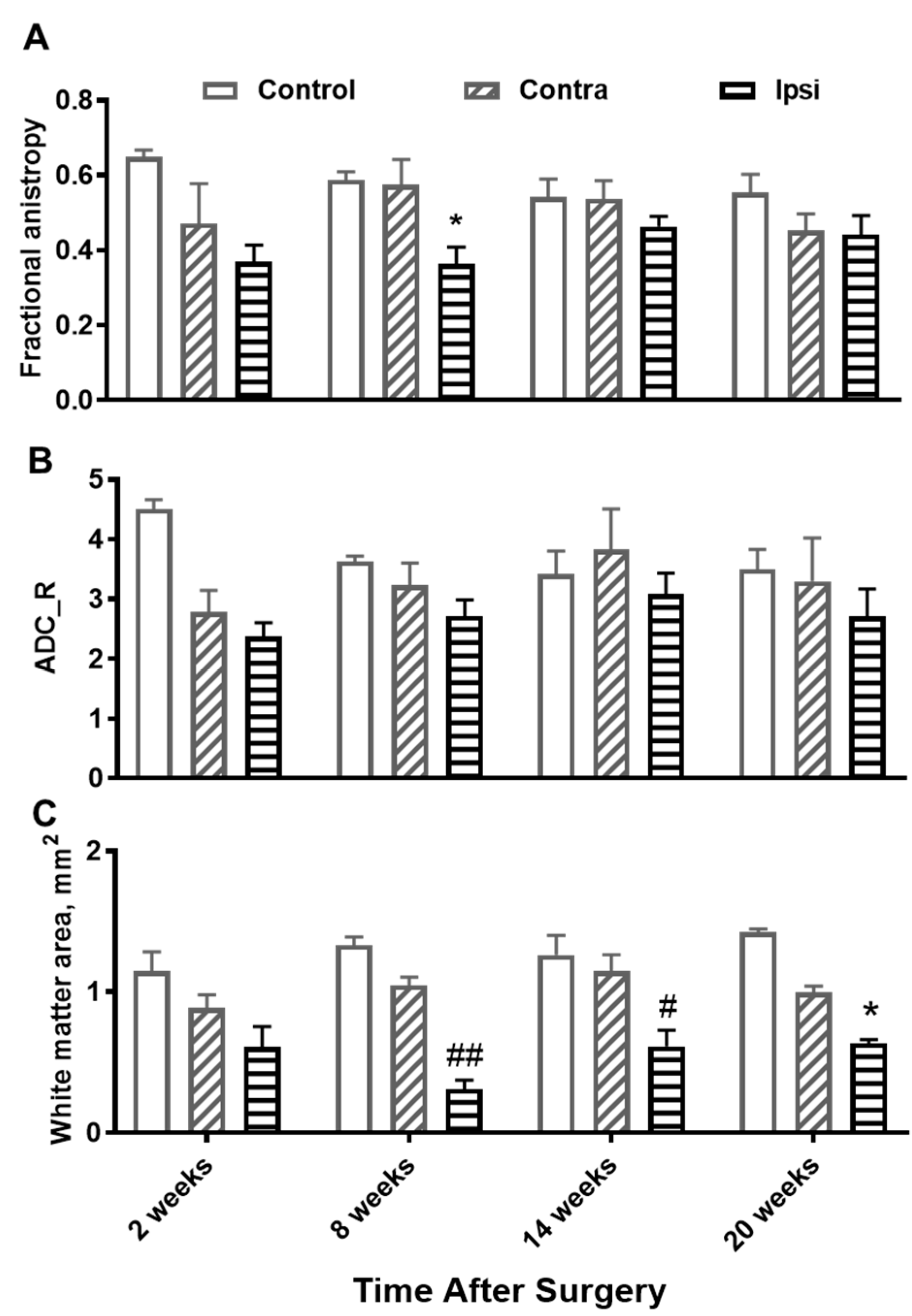
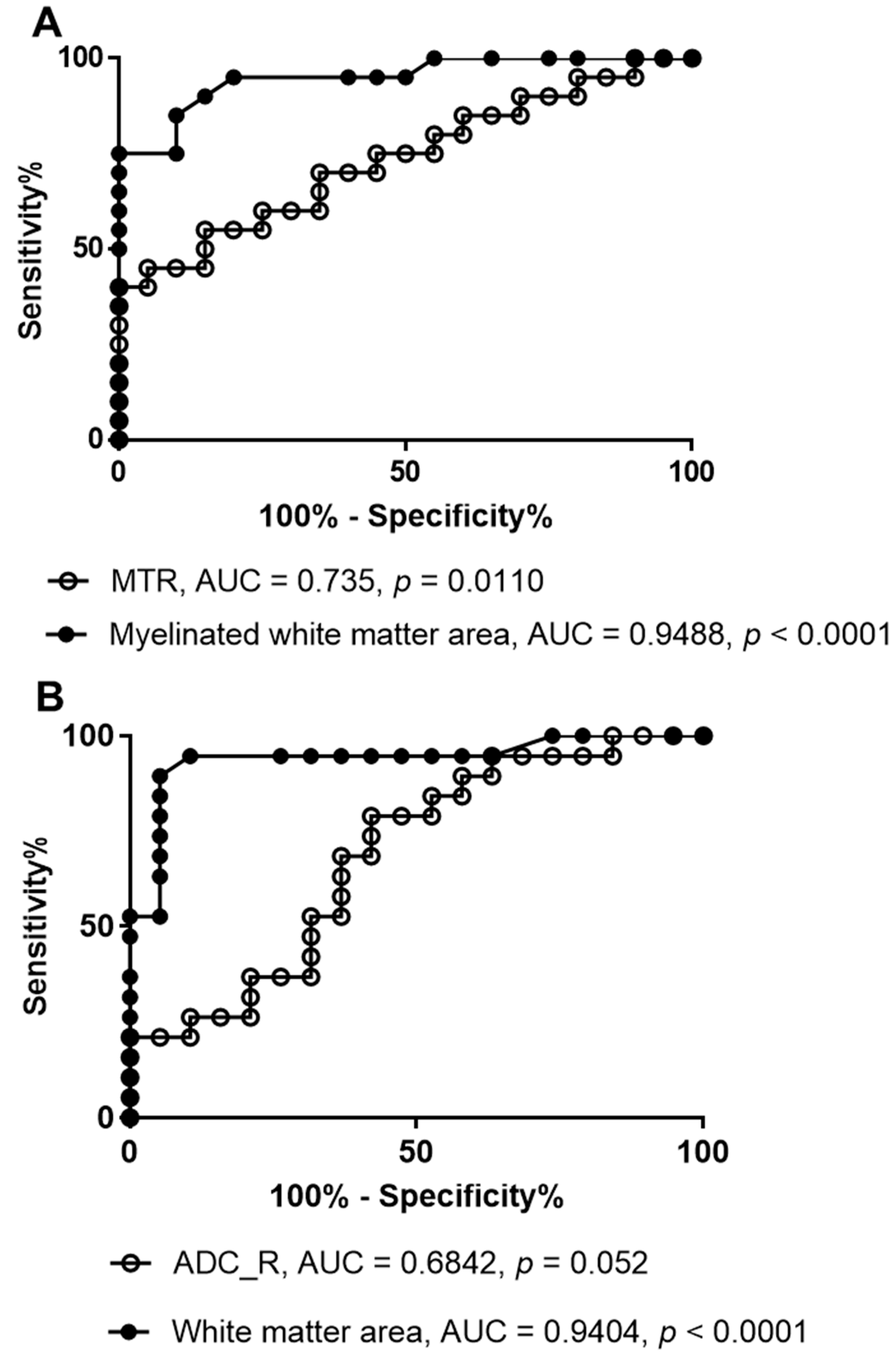
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shah, L.M.; Ross, J.S. Imaging of Spine Trauma. Neurosurgery 2016, 79, 626–642. [Google Scholar] [CrossRef]
- Freund, P.; Seif, M.; Weiskopf, N.; Friston, K.; Fehlings, M.G.; Thompson, A.J.; Curt, A. MRI in traumatic spinal cord injury: From clinical assessment to neuroimaging biomarkers. Lancet Neurol. 2019, 18, 1123–1135. [Google Scholar] [CrossRef]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef]
- Ramani, A.; Dalton, C.; Miller, D.H.; Tofts, P.S.; Barker, G.J. Precise estimate of fundamental in-vivo MT parameters in human brain in clinically feasible times. Magn. Reson. Imaging 2002, 20, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Horch, R.A.; Gore, J.C.; Does, M.D. Origins of the ultrashort-T2 1H NMR signals in myelinated nerve: A direct measure of myelin content? Magn. Reson. Med. 2011, 66, 24–31. [Google Scholar] [CrossRef]
- Wolff, S.D.; Balaban, R.S. Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn. Reson. Med. 1989, 10, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, R.M.; Stanisz, G.J.; Graham, S.J. Magnetization transfer in MRI: A review. NMR Biomed. 2001, 14, 57–64. [Google Scholar] [CrossRef]
- Guglielmetti, C.; Boucneau, T.; Cao, P.; Van der Linden, A.; Larson, P.E.Z.; Chaumeil, M.M. Longitudinal evaluation of demyelinated lesions in a multiple sclerosis model using ultrashort echo time magnetization transfer (UTE-MT) imaging. Neuroimage 2020, 208, 116415. [Google Scholar] [CrossRef]
- Laule, C.; Vavasour, I.M.; Kolind, S.H.; Li, D.K.; Traboulsee, T.L.; Moore, G.R.; MacKay, A.L. Magnetic resonance imaging of myelin. Neurotherapeutics 2007, 4, 460–484. [Google Scholar] [CrossRef]
- Wilhelm, M.J.; Ong, H.H.; Wehrli, S.L.; Li, C.; Tsai, P.H.; Hackney, D.B.; Wehrli, F.W. Direct magnetic resonance detection of myelin and prospects for quantitative imaging of myelin density. Proc. Natl. Acad. Sci. USA 2012, 109, 9605–9610. [Google Scholar] [CrossRef]
- Brennan, F.H.; Cowin, G.J.; Kurniawan, N.D.; Ruitenberg, M.J. Longitudinal assessment of white matter pathology in the injured mouse spinal cord through ultra-high field (16.4 T) in vivo diffusion tensor imaging. Neuroimage 2013, 82, 574–585. [Google Scholar] [CrossRef]
- Chen, L.M.; Wang, F.; Mishra, A.; Yang, P.F.; Sengupta, A.; Reed, J.L.; Gore, J.C. Longitudinal multiparametric MRI of traumatic spinal cord injury in animal models. Magn. Reson. Imaging 2023, 102, 184–200. [Google Scholar] [CrossRef]
- Deo, A.A.; Grill, R.J.; Hasan, K.M.; Narayana, P.A. In vivo serial diffusion tensor imaging of experimental spinal cord injury. J. Neurosci. Res. 2006, 83, 801–810. [Google Scholar] [CrossRef]
- Herrera, J.J.; Chacko, T.; Narayana, P.A. Histological correlation of diffusion tensor imaging metrics in experimental spinal cord injury. J. Neurosci. Res. 2008, 86, 443–447. [Google Scholar] [CrossRef]
- Sundberg, L.M.; Herrera, J.J.; Narayana, P.A. In vivo longitudinal MRI and behavioral studies in experimental spinal cord injury. J. Neurotrauma 2010, 27, 1753–1767. [Google Scholar] [CrossRef]
- Vallotton, K.; David, G.; Hupp, M.; Pfender, N.; Cohen-Adad, J.; Fehlings, M.G.; Samson, R.S.; Wheeler-Kingshott, C.; Curt, A.; Freund, P.; et al. Tracking White and Gray Matter Degeneration along the Spinal Cord Axis in Degenerative Cervical Myelopathy. J. Neurotrauma 2021, 38, 2978–2987. [Google Scholar] [CrossRef]
- Wang, F.; Li, K.; Mishra, A.; Gochberg, D.; Min Chen, L.; Gore, J.C. Longitudinal assessment of spinal cord injuries in nonhuman primates with quantitative magnetization transfer. Magn. Reson. Med. 2016, 75, 1685–1696. [Google Scholar] [CrossRef]
- Brown, A.R.; Martinez, M. Ipsilesional Motor Cortex Plasticity Participates in Spontaneous Hindlimb Recovery after Lateral Hemisection of the Thoracic Spinal Cord in the Rat. J. Neurosci. 2018, 38, 9977–9988. [Google Scholar] [CrossRef]
- Filli, L.; Zorner, B.; Weinmann, O.; Schwab, M.E. Motor deficits and recovery in rats with unilateral spinal cord hemisection mimic the Brown-Sequard syndrome. Brain 2011, 134, 2261–2273. [Google Scholar] [CrossRef]
- Lu, P.; Blesch, A.; Graham, L.; Wang, Y.; Samara, R.; Banos, K.; Haringer, V.; Havton, L.; Weishaupt, N.; Bennett, D.; et al. Motor axonal regeneration after partial and complete spinal cord transection. J. Neurosci. 2012, 32, 8208–8218. [Google Scholar] [CrossRef]
- Lopez-Dolado, E.; Lucas-Osma, A.M.; Collazos-Castro, J.E. Dynamic motor compensations with permanent, focal loss of forelimb force after cervical spinal cord injury. J. Neurotrauma 2013, 30, 191–210. [Google Scholar] [CrossRef]
- Ghosh, A.; Sydekum, E.; Haiss, F.; Peduzzi, S.; Zorner, B.; Schneider, R.; Baltes, C.; Rudin, M.; Weber, B.; Schwab, M.E. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J. Neurosci. 2009, 29, 12210–12219. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Fujito, Y.; Satomi, H.; Kurosawa, Y.; Kasaba, T. The possible role of collateral sprouting in the functional restitution of corticospinal connections after spinal hemisection. Neurosci. Res. 1986, 3, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, E.S.; Courtine, G.; Jindrich, D.L.; Brock, J.H.; Ferguson, A.R.; Strand, S.C.; Nout, Y.S.; Roy, R.R.; Miller, D.M.; Beattie, M.S.; et al. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat. Neurosci. 2010, 13, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Adad, J.; El Mendili, M.M.; Lehericy, S.; Pradat, P.F.; Blancho, S.; Rossignol, S.; Benali, H. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 2011, 55, 1024–1033. [Google Scholar] [CrossRef]
- Ma, Y.J.; Chang, E.Y.; Carl, M.; Du, J. Quantitative magnetization transfer ultrashort echo time imaging using a time-efficient 3D multispoke Cones sequence. Magn. Reson. Med. 2018, 79, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Afshari, R.; Santini, F.; Heule, R.; Meyer, C.H.; Pfeuffer, J.; Bieri, O. One-minute whole-brain magnetization transfer ratio imaging with intrinsic B(1)-correction. Magn. Reson. Med. 2021, 85, 2686–2695. [Google Scholar] [CrossRef]
- Manzanera Esteve, I.V.; Wang, F.; Reed, J.L.; Qi, H.X.; Thayer, W.; Gore, J.C.; Chen, L.M. Model-based parcellation of diffusion MRI of injured spinal cord predicts hand use impairment and recovery in squirrel monkeys. Behav. Brain Res. 2024, 459, 114808. [Google Scholar] [CrossRef]
- Murray, E.; Cho, J.H.; Goodwin, D.; Ku, T.; Swaney, J.; Kim, S.Y.; Choi, H.; Park, Y.G.; Park, J.Y.; Hubbert, A.; et al. Simple, Scalable Proteomic Imaging for High-Dimensional Profiling of Intact Systems. Cell 2015, 163, 1500–1514. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef]
- Hill, C.E.; Beattie, M.S.; Bresnahan, J.C. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp. Neurol. 2001, 171, 153–169. [Google Scholar] [CrossRef]
- Ugorji, C.O.; Samson, R.S.; Liechti, M.D.; Panicker, J.N.; Miller, D.H.; Wheeler-Kingshott, C.A.; Yiannakas, M.C. Grey and White Matter Magnetisation Transfer Ratio Measurements in the Lumbosacral Enlargement: A Pilot In Vivo Study at 3T. PLoS ONE 2015, 10, e0134495. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Ma, Y.; Cheng, Q.; Wu, Y.; Chen, J.; Du, J.; Lu, P.; Chang, E.Y. Longitudinal Imaging of Injured Spinal Cord Myelin and White Matter with 3D Ultrashort Echo Time Magnetization Transfer (UTE-MT) and Diffusion MRI. J. Imaging 2024, 10, 213. https://doi.org/10.3390/jimaging10090213
Tang Q, Ma Y, Cheng Q, Wu Y, Chen J, Du J, Lu P, Chang EY. Longitudinal Imaging of Injured Spinal Cord Myelin and White Matter with 3D Ultrashort Echo Time Magnetization Transfer (UTE-MT) and Diffusion MRI. Journal of Imaging. 2024; 10(9):213. https://doi.org/10.3390/jimaging10090213
Chicago/Turabian StyleTang, Qingbo, Yajun Ma, Qun Cheng, Yuanshan Wu, Junyuan Chen, Jiang Du, Pengzhe Lu, and Eric Y. Chang. 2024. "Longitudinal Imaging of Injured Spinal Cord Myelin and White Matter with 3D Ultrashort Echo Time Magnetization Transfer (UTE-MT) and Diffusion MRI" Journal of Imaging 10, no. 9: 213. https://doi.org/10.3390/jimaging10090213





