Advances in Optical Contrast Agents for Medical Imaging: Fluorescent Probes and Molecular Imaging
Abstract
1. Introduction
2. Principle of Optical Imaging
3. Contrast Agents
4. Fluorescence Imaging
Parts of Fluorescence Imaging System
5. Types of Fluorescence Imaging
5.1. Widefield Fluorescence Imaging
5.2. Confocal Fluorescence Imaging
5.3. Multiphoton Fluorescence Imaging
5.4. Fluorescence Lifetime Imaging Microscopy (FLIM)
5.5. Super-Resolution Fluorescence Imaging
5.6. In-Vivo Fluorescence Imaging
6. Fluorescent Probe Designing and Synthesis
7. Applications of Fluorescence Probes
7.1. Molecular Imaging and Cancer Detection
7.2. Brain and Cardiovascular Imaging
7.3. Clinical Translation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luker, G.D.; Luker, K.E. Optical imaging: Current applications and future directions. J. Nucl. Med. 2008, 49, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Licha, K. Contrast Agents for Optical Imaging. In Contrast Agents II. Topics in Current Chemistry; Krause, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; Volume 222. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Boppart, S.A. Review of optical coherence tomography in oncology. J. Biomed. Opt. 2017, 22, 121711. [Google Scholar] [CrossRef] [PubMed]
- Bremer, C.; Ntziachristos, V.; Weissleder, R. Optical-based molecular imaging: Contrast agents and potential medical applications. Eur. Radiol. 2003, 13, 231–243. [Google Scholar] [CrossRef]
- Gotfried, J. Endoscopy—Gastrointestinal Disorders—Merck Manual Professional Edition. Merck Manuals. 2023. Available online: https://www.merckmanuals.com/professional/gastrointestinal-disorders/diagnostic-and-therapeutic-gastrointestinal-procedures/endoscopy (accessed on 1 September 2024).
- Nguyen, V.X.; Le Nguyen, V.T.; Nguyen, C.C. Appropriate use of endoscopy in the diagnosis and treatment of gastrointestinal diseases: Up-to-date indications for primary care providers. Int. J. Gen. Med. 2010, 3, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Sivak, M.V. Gastrointestinal endoscopy: Past and future. Gut 2006, 55, 1061–1064. [Google Scholar] [CrossRef]
- Kaller, M.O.; An, J. Contrast Agent Toxicity. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Omeh, D.J.; Shlofmitz, E. Angiography. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Lu, S.; Dai, Z.; Cui, Y.; Kong, D.M. Recent Development of Advanced Fluorescent Molecular Probes for Organelle-Targeted Cell Imaging. Biosensors 2023, 13, 360. [Google Scholar] [CrossRef]
- Hellebust, A.; Richards-Kortum, R. Advances in molecular imaging: Targeted optical contrast agents for cancer diagnostics. Nanomedicine 2012, 7, 429–445. [Google Scholar] [CrossRef]
- Rehman, S.; Brennan, P.M.; Lilienkampf, A.; Bradley, M. Approved and investigational fluorescent optical imaging agents for disease detection in surgery. Int. J. Surg. 2023, 109, 2378–2387. [Google Scholar] [CrossRef]
- Hintz, S.R.; Cheong, W.F.; van Houten, J.P.; Stevenson, D.K.; Benaron, D.A. Bedside imaging of intracranial hemorrhage in the neonate using light: Comparison with ultrasound, computed tomography, and magnetic resonance imaging. Pediatr. Res. 1999, 45, 54–59. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, W.; Zhang, Y.; Chen, S.; Yang, D. Harnessing the Power of Hybrid Light Propagation Model for Three-Dimensional Optical Imaging in Cancer Detection. Front. Oncol. 2021, 11, 750764. [Google Scholar] [CrossRef]
- Moriyama, E.H.; Zheng, G.; Wilson, B.C. Optical molecular imaging: From single cell to patient. Clin. Pharmacol. Ther. 2008, 84, 267–271. [Google Scholar] [CrossRef]
- Rodriguez, E.A.; Tran, G.N.; Tsien, R.Y. Optical Imaging for Diagnostics—Nature; Nature Publishing Group: New York, NY, USA, 2016; Available online: https://www.nature.com/collections/njfvwrzbtv (accessed on 1 January 2025).
- Lakowicz, J.R.; Lakowicz, J.R. Introduction to fluorescence. In Principles of Fluorescence Spectroscopy; Plenum Press: New York, NY, USA, 1999; pp. 1–23. [Google Scholar]
- King, H. Single-Molecule Photochemical Catalysis on Titanium Dioxide@ Gold Nanorods. Master’s Thesis, Kent State University, Kent, OH, USA, 2022. [Google Scholar]
- Chen, Y. Recent Advances in Excimer-Based Fluorescence Probes for Biological Applications. Molecules 2022, 27, 8628. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.; Du, J.; Peng, X. Fluorescent Probes for Sensing and Imaging within Specific Cellular Organelles. Acc. Chem. Res. 2016, 49, 2115–2126. [Google Scholar] [CrossRef]
- Seah, D.; Cheng, Z.; Vendrell, M. Fluorescent Probes for Imaging in Humans: Where Are We Now? ACS Nano 2023, 17, 19478–19490. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10604082/ (accessed on 3 October 2024). [CrossRef]
- Liu, Z.; Liu, J.; Wang, X.; Mi, F.; Wang, D.; Wu, C. Fluorescent Bioconjugates for Super-Resolution Optical Nanoscopy. Bioconjugate Chem. 2020, 31, 1857–1872. [Google Scholar] [CrossRef]
- Dean, K.M.; Palmer, A.E. Advances in fluorescence labeling strategies for dynamic cellular imaging. Nat. Chem. Biol. 2014, 10, 512–523. [Google Scholar] [CrossRef]
- Pirovano, G.; Roberts, S.; Kossatz, S.; Reiner, T. Optical Imaging Modalities: Principles and Applications in Preclinical Research and Clinical Settings. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 1419–1427. [Google Scholar] [CrossRef]
- Wang, D.; Xia, J. Optics based biomedical imaging: Principles and applications. J. Appl. Phys. 2019, 125, 191101. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, P.; Bao, Z.; Pang, W.; Ding, S.; Yin, M.J.; Li, P.; Gu, B. Fundamentals of Optical Imaging. Adv. Exp. Med. Biol. 2021, 3233, 1–22. [Google Scholar] [CrossRef]
- Han, S.; Lee, D.; Kim, S.; Kim, H.H.; Jeong, S.; Kim, J. Contrast Agents for Photoacoustic Imaging: A Review Focusing on the Wavelength Range. Biosensors 2022, 12, 594. [Google Scholar] [CrossRef]
- Hadzima, M.; Faucher, F.F.; Blažková, K.; Yim, J.J.; Guerra, M.; Chen, S.; Woods, E.C.; Park, K.W.; Šácha, P.; Šubr, V.; et al. Polymer-Tethered Quenched Fluorescent Probes for Enhanced Imaging of Tumor-Associated Proteases. ACS Sens. 2024, 9, 3720–3729. [Google Scholar] [CrossRef]
- Park, J.H.; Dumani, D.S.; Arsiwala, A.; Emelianov, S.; Kane, R.S. Tunable aggregation of gold-silica janus nanoparticles to enable contrast-enhanced multiwavelength photoacoustic imaging in vivo. Nanoscale 2018, 10, 15365–15370. [Google Scholar] [CrossRef]
- Miao, Q.; Pu, K. Organic Semiconducting Agents for Deep-Tissue Molecular Imaging: Second Near-Infrared Fluorescence, Self-Luminescence, and Photoacoustics. Adv. Mater. (Deerfield Beach Fla.) 2018, 30, e1801778. [Google Scholar] [CrossRef]
- Gottschalk, H.M.; Wecker, T.; Khattab, M.H.; Fischer, C.V.; Callizo, J.; Rehfeldt, F.; Lubjuhn, R.; Russmann, C.; Hoerauf, H.; van Oterendorp, C. Lipid Emulsion-Based OCT Angiography for Ex Vivo Imaging of the Aqueous Outflow Tract. Investig. Ophthalmol. Vis. Sci. 2019, 60, 397–406. [Google Scholar] [CrossRef]
- Hui, X.; Malik, M.O.A.; Pramanik, M. Looking deep inside tissue with photoacoustic molecular probes: A review. J. Biomed. Opt. 2022, 27, 070901. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Qian, W.; Wang, X.; Paulus, Y.M. Functionalized contrast agents for multimodality photoacoustic microscopy, optical coherence tomography, and fluorescence microscopy molecular retinal imaging. Methods Enzymol. 2021, 657, 443–480. [Google Scholar] [CrossRef]
- Lemaster, J.E.; Jokerst, J.V. What is new in nanoparticle-based photoacoustic imaging? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1404. [Google Scholar] [CrossRef]
- Contrast Agents Market Size by Segments, Share, Regulatory, Reimbursement and Forecast to 2033. Market Research Reports & Consulting|GlobalData UK Ltd. 2024. Available online: https://www.globaldata.com/store/report/contrast-agents-devices-market-analysis/ (accessed on 27 September 2024).
- Contrast Media Market Size, Share & Growth Report, 2030. 2024. Available online: https://www.grandviewresearch.com/industry-analysis/contrast-media-contrast-agents-market (accessed on 3 September 2024).
- Study on Global MRI Contrast Media Agents Market Size to ... 2023. Available online: https://www.fnfresearch.com/news/global-mri-contrast-media-agents-market (accessed on 3 November 2024).
- Lee, J.H.; Park, G.; Hong, G.H.; Choi, J.; Choi, H.S. Design considerations for targeted optical contrast agents. Quant. Imaging Med. Surg. 2012, 2, 266–273. [Google Scholar] [CrossRef]
- Nakata, E.; Gerelbaatar, K.; Komatsubara, F.; Morii, T. Stimuli-Responsible SNARF Derivatives as a Latent Ratiometric Fluorescent Probe. Molecules 2022, 27, 7181. [Google Scholar] [CrossRef]
- Guo, L.; Liu, F.; Cai, C.; Liu, J.; Zhang, G. 3D deep encoder-decoder network for fluorescence molecular tomography. Opt. Lett. 2019, 44, 1892–1895. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, L.; Zhou, M.; Alifu, N. Application of organic fluorescent probe-assisted near infrared fluorescence imaging in cervical cancer diagnosis. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2021, 37, 2678–2687. [Google Scholar] [CrossRef]
- Boutorine, A.S.; Novopashina, D.S.; Krasheninina, O.A.; Nozeret, K.; Venyaminova, A.G. Fluorescent probes for nucleic Acid visualization in fixed and live cells. Molecules 2013, 18, 15357–15397. [Google Scholar] [CrossRef]
- Chen, G.; Li, C.; Zhang, Y.; Wang, Q. Whole-Body Fluorescence Imaging in the Near-Infrared Window. Adv. Exp. Med. Biol. 2021, 3233, 83–108. [Google Scholar] [CrossRef]
- Heeman, W.; Vonk, J.; Ntziachristos, V.; Pogue, B.W.; Dierckx, R.A.J.O.; Kruijff, S.; van Dam, G.M. A Guideline for Clinicians Performing Clinical Studies with Fluorescence Imaging. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2022, 63, 640–645. [Google Scholar] [CrossRef]
- Si, K.; Fiolka, R.; Cui, M. Fluorescence imaging beyond the ballistic regime by ultrasound pulse guided digital phase conjugation. Nat. Photonics 2012, 6, 657–661. [Google Scholar] [CrossRef]
- Nguyen, J.Q.M.; McWade, M.; Thomas, G.; Beddard, B.T.; Herington, J.L.; Paria, B.C.; Schwartz, H.S.; Halpern, J.L.; Holt, G.E.; Mahadevan-Jansen, A. Development of a modular fluorescence overlay tissue imaging system for wide-field intraoperative surgical guidance. J. Med. Imaging (Bellingham Wash.) 2018, 5, 021220. [Google Scholar] [CrossRef]
- Karki, P. Fluorescence Microscope: Principle, Parts, Uses, Examples. Microbe Notes. 2024. Available online: https://microbenotes.com/fluorescence-microscope-principle-instrumentation-applications-advantages-limitations/ (accessed on 25 June 2024).
- Mubaid, F.; Kaufman, D.; Wee, T.L.; Nguyen-Huu, D.S.; Young, D.; Anghelopoulou, M.; Brown, C.M. Fluorescence microscope light source stability. Histochem. Cell Biol. 2019, 151, 357–366. [Google Scholar] [CrossRef]
- Ettinger, A.; Wittmann, T. Fluorescence live cell imaging. Methods Cell Biol. 2014, 123, 77–94. [Google Scholar] [CrossRef]
- Combs, C.A. Fluorescence microscopy: A concise guide to current imaging methods. Curr. Protoc. Neurosci. 2010, 50, 2.1.1–2.1.14. [Google Scholar] [CrossRef]
- Scribano, F.J.; Engevik, K.A.; Gebert, J.T.; Hyser, J.M. Live-Cell Fluorescence Imaging for Virus-Host Interactions. In Host-Pathogen Interactions: Methods and Protocols; Springer US: New York, NY, USA, 2024; pp. 33–46. [Google Scholar]
- Agard, D.A.; Hiraoka, Y.; Sedat, J.W. Three-dimensional light microscopy of diploid Drosophila chromosomes. Cell Motil. Cytoskelet. 1988, 10, 18–27. [Google Scholar] [CrossRef]
- van der Valk, M.J.M.; Handgraaf, H.J.M.; Sibinga-Mulder, B.G.; Hilling, D.E.; van Dam, G.M.; Vahrmeijer, A.L. Intraoperatieve beeldvorming met fluorescentie, 5 jaar later [Intraoperative imaging using fluorescence, 5 years later]. Ned. Tijdschr. Voor Geneeskd. 2018, 162, D2067. [Google Scholar]
- Li, C.; Gao, L.; Liu, Y.; Wang, L.V. Optical sectioning by wide-field photobleaching imprinting microscopy. Appl. Phys. Lett. 2013, 103, 183703. [Google Scholar] [CrossRef]
- Forsgren, E.; Edlund, C.; Oliver, M.; Barnes, K.; Sjögren, R.; Jackson, T.R. High-throughput widefield fluorescence imaging of 3D samples using deep learning for 2D projection image restoration. PLoS ONE 2022, 17, e0264241. [Google Scholar] [CrossRef]
- Xiao, D.; Zang, Z.; Wang, Q.; Jiao, Z.; Rocca, F.M.D.; Chen, Y.; Li, D.D.U. Smart Wide-field Fluorescence Lifetime Imaging System with CMOS Single-photon Avalanche Diode Arrays. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; Volume 2022, pp. 1887–1890. [Google Scholar] [CrossRef]
- Crowe, S.E.; Ellis-Davies, G.C. Longitudinal in vivo two-photon fluorescence imaging. J. Comp. Neurol. 2014, 522, 1708–1727. [Google Scholar] [CrossRef]
- Park, S.; Zhang, J.; Reyer, M.A.; Zareba, J.; Troy, A.A.; Fei, J. Conducting Multiple Imaging Modes with One Fluorescence Microscope. J. Vis. Exp. JoVE 2018, 58320. [Google Scholar] [CrossRef]
- Pacheco, S.; Wang, C.; Chawla, M.K.; Nguyen, M.; Baggett, B.K.; Utzinger, U.; Barnes, C.A.; Liang, R. High resolution, high speed, long working distance, large field of view confocal fluorescence microscope. Sci. Rep. 2017, 7, 13349. [Google Scholar] [CrossRef]
- Yan, W.; Peng, X.; Qi, J.; Gao, J.; Fan, S.; Wang, Q.; Qu, J.; Niu, H. Dynamic fluorescence lifetime imaging based on acousto-optic deflectors. J. Biomed. Opt. 2014, 19, 116004. [Google Scholar] [CrossRef] [PubMed]
- Botchway, S.W.; Scherer, K.M.; Hook, S.; Stubbs, C.D.; Weston, E.; Bisby, R.H.; Parker, A.W. A series of flexible design adaptations to the Nikon E-C1 and E-C2 confocal microscope systems for UV, multiphoton and FLIM imaging. J. Microsc. 2015, 258, 68–78. [Google Scholar] [CrossRef]
- Ishikawa-Ankerhold, H.C.; Ankerhold, R.; Drummen, G.P. Advanced fluorescence microscopy techniques—FRAP, FLIP, FLAP, FRET and FLIM. Molecules 2012, 17, 4047–4132. [Google Scholar] [CrossRef]
- Schäferling, M. The art of fluorescence imaging with chemical sensors. Angew. Chem. (Int. Ed. Engl.) 2012, 51, 3532–3554. [Google Scholar] [CrossRef]
- Datta, R.; Heaster, T.M.; Sharick, J.T.; Gillette, A.A.; Skala, M.C. Fluorescence lifetime imaging microscopy: Fundamentals and advances in instrumentation, analysis, and applications. J. Biomed. Opt. 2020, 25, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Fluorescence Resonance Energy Transfer. 2021. Available online: https://www.gu.se/en/core-facilities/fluorescence-resonance-energy-transfer (accessed on 7 November 2024).
- Stanisavljevic, M.; Krizkova, S.; Vaculovicova, M.; Kizek, R.; Adam, V. Quantum dots-fluorescence resonance energy transfer-based nanosensors and their application. Biosens. Bioelectron. 2015, 74, 562–574. [Google Scholar] [CrossRef]
- Balconi, M.; Crivelli, D. Veridical and false feedback sensitivity and punishment-reward system (BIS/BAS): ERP amplitude and theta frequency band analysis. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2010, 121, 1502–1510. [Google Scholar] [CrossRef]
- Kumar, N.; Bhalla, V.; Kumar, M. Resonance energy transfer-based fluorescent probes for Hg2+, Cu2+ and Fe2+/Fe3+ ions. Analyst 2014, 139, 543–558. [Google Scholar] [CrossRef]
- Zhu, F.Y.; Mei, L.J.; Tian, R.; Li, C.; Wang, Y.L.; Xiang, S.L.; Zhu, M.Q.; Tang, B.Z. Recent advances in super-resolution optical imaging based on aggregation-induced emission. Chem. Soc. Rev. 2024, 53, 3350–3383. [Google Scholar] [CrossRef]
- Emptage, N.J. Fluorescent imaging in living systems. Curr. Opin. Pharmacol. 2001, 1, 521–525. [Google Scholar] [CrossRef]
- Ntziachristos, V.; Turner, G.; Dunham, J.; Windsor, S.; Soubret, A.; Ripoll, J.; Shih, H.A. Planar fluorescence imaging using normalized data. J. Biomed. Opt. 2005, 10, 064007. [Google Scholar] [CrossRef]
- Hugelier, S.; Colosi, P.L.; Lakadamyali, M. Quantitative Single-Molecule Localization Microscopy. Annu. Rev. Biophys. 2023, 52, 139–160. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, G.; Wang, J. In Vivo Fluorescence Imaging-Guided Development of Near-Infrared AIEgens. Chem. Asian J. 2023, 18, e202201251. [Google Scholar] [CrossRef]
- Yu, M.; Ward, M.B.; Franke, A.; Ambrose, S.L.; Whaley, Z.L.; Bradford, T.M.; Gorden, J.D.; Beyers, R.J.; Cattley, R.C.; Ivanović-Burmazović, I.; et al. Adding a Second Quinol to a Redox-Responsive MRI Contrast Agent Improves Its Relaxivity Response to H2O2. Inorg. Chem. 2017, 56, 2812–2826. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Xu, S.; Li, C.; Dong, B. Recent Progress in Fluorescent Probes For Metal Ion Detection. Front. Chem. 2022, 10, 875241. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, L.; Carroll, S.L.; Chen, J.; Wang, M.C.; Wang, J. Challenges and Opportunities for Small-Molecule Fluorescent Probes in Redox Biology Applications. Antioxid. Redox Signal. 2018, 29, 518–540. [Google Scholar] [CrossRef]
- He, G.; Liu, C.; Liu, X.; Wang, Q.; Fan, A.; Wang, S.; Qian, X. Design and synthesis of a fluorescent probe based on naphthalene anhydride and its detection of copper ions. PLoS ONE 2017, 12, e0186994. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Liu, C.; Yang, N.; Yang, L.; He, G. Design and Synthesis of a Fluorescent Probe Based on Copper Complex for Selective Detection of Hydrogen Sulfide. J. Sens. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Jacoby-Morris, K.; Patterson, G.H. Choosing Fluorescent Probes and Labeling Systems. Methods Mol. Biol. 2021, 2304, 37–64. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, H.; Peng, B.; Huang, X.; Hu, Y.; Zheng, C.; Zhang, Z. Illuminating the future of precision cancer surgery with fluorescence imaging and artificial intelligence convergence. npj Precis. Oncol. 2024, 8, 196. [Google Scholar] [CrossRef]
- Glatz, J.; Symvoulidis, P.; Garcia-Allende, P.B.; Ntziachristos, V. Robust overlay schemes for the fusion of fluorescence and color channels in biological imaging. J. Biomed. Opt. 2014, 19, 040501. [Google Scholar] [CrossRef]
- Okubo, Y. Investigation of Brain Functions with Fluorescence Imaging Techniques. Juntendo Iji Zasshi = Juntendo Med. J. 2022, 68, 157–162. [Google Scholar] [CrossRef]
- Malik, M.M.U.D.; Alqahtani, M.M.; Hadadi, I.; Kanbayti, I.; Alawaji, Z.; Aloufi, B.A. Molecular Imaging Biomarkers for Early Cancer Detection: A Systematic Review of Emerging Technologies and Clinical Applications. Diagnostics 2024, 14, 2459. [Google Scholar] [CrossRef]
- Sun, J.; Miller, J.P.; Hathi, D.; Zhou, H.; Achilefu, S.; Shokeen, M.; Akers, W.J. Enhancing in vivo tumor boundary delineation with structured illumination fluorescence molecular imaging and spatial gradient mapping. J. Biomed. Opt. 2016, 21, 80502. [Google Scholar] [CrossRef]
- Natarajan, V.; Thirumalaivasan, N.; Wu, S.P.; Sivan, V. A far-red to NIR emitting ultra-sensitive probe for the detection of endogenous HOCl in zebrafish and the RAW 264.7 cell line. Org. Biomol. Chem. 2019, 17, 3538–3544. [Google Scholar] [CrossRef]
- Bai, J.W.; Qiu, S.Q.; Zhang, G.J. Molecular and functional imaging in cancer-targeted therapy: Current applications and future directions. Signal Transduct. Target. Ther. 2023, 8, 89. [Google Scholar] [CrossRef]
- Sosnovik, D.E.; Nahrendorf, M.; Weissleder, R. Targeted imaging of myocardial damage. Nat. Clin. Practice. Cardiovasc. Med. 2008, 5 (Suppl. 2), S63–S70. [Google Scholar] [CrossRef]
- Duprée, A.; Rieß, H.; Detter, C.; Debus, E.S.; Wipper, S.H. Utilization of indocynanine green fluorescent imaging (ICG-FI) for the assessment of microperfusion in vascular medicine. Innov. Surg. Sci. 2018, 3, 193–201. [Google Scholar] [CrossRef]
- Che, F.; Zhao, X.; Wang, X.; Li, P.; Tang, B. Fluorescent Imaging Agents for Brain Diseases. Targets 2023, 10, 3. [Google Scholar] [CrossRef]
- Hong, G.; Diao, S.; Chang, J.; Antaris, A.L.; Chen, C.; Zhang, B.; Zhao, S.; Atochin, D.N.; Huang, P.L.; Andreasson, K.I.; et al. Through-skull fluorescence imaging of the brain in a new near-infrared window. Nat. Photonics 2014, 8, 723–730. [Google Scholar] [CrossRef]
- Kim, T.H.; Schnitzer, M.J. Fluorescence imaging of large-scale neural ensemble dynamics. Cell 2022, 185, 9–41. [Google Scholar] [CrossRef]
- Mashalchi, S.; Pahlavan, S.; Hejazi, M. A novel fluorescent cardiac imaging system for preclinical intraoperative angiography. BMC Med. Imaging 2021, 21, 37. [Google Scholar] [CrossRef]
- Yu, X.; Feng, Z.; Cai, Z.; Jiang, M.; Xue, D.; Zhu, L.; Zhang, Y.; Liu, J.; Que, B.; Yang, W.; et al. Deciphering of cerebrovasculatures via ICG-assisted NIR-II fluorescence microscopy. J. Mater. Chem. B 2019, 7, 6623–6629. [Google Scholar] [CrossRef]
- Jun, Y.W.; Kim, H.R.; Reo, Y.J.; Dai, M.; Ahn, K.H. Addressing the autofluorescence issue in deep tissue imaging by two-photon microscopy: The significance of far-red emitting dyes. Chem. Sci. 2017, 8, 7696–7704. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhong, X.; Dennis, A.M. Minimizing near-infrared autofluorescence in preclinical imaging with diet and wavelength selection. J. Biomed. Opt. 2023, 28, 094805. [Google Scholar] [CrossRef] [PubMed]
- Schouw, H.M.; Huisman, L.A.; Janssen, Y.F.; Slart, R.H.J.A.; Borra, R.J.H.; Willemsen, A.T.M.; Brouwers, A.H.; van Dijl, J.M.; Dierckx, R.A.; van Dam, G.M.; et al. Targeted optical fluorescence imaging: A meta-narrative review and future perspectives. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4272–4292. [Google Scholar] [CrossRef]
- de Moliner, F.; Nadal-Bufi, F.; Vendrell, M. Recent advances in minimal fluorescent probes for optical imaging. Curr. Opin. Chem. Biol. 2024, 80, 102458. [Google Scholar] [CrossRef]
- Huang, J.; Pu, K. Activatable Molecular Probes for Second Near-Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging. Angew. Chem. (Int. Ed. Engl.) 2020, 59, 11717–11731. [Google Scholar] [CrossRef]
- Taruttis, A.; Ntziachristos, V. Translational optical imaging. AJR. Am. J. Roentgenol. 2012, 199, 263–271. [Google Scholar] [CrossRef]
- Dang, X.; Bardhan, N.M.; Qi, J.; Gu, L.; Eze, N.A.; Lin, C.W.; Kataria, S.; Hammond, P.T.; Belcher, A.M. Deep-tissue optical imaging of near cellular-sized features. Sci. Rep. 2019, 9, 3873. [Google Scholar] [CrossRef]
- Zhou, L.; Gan, Y.; Wu, Y.; Xue, D.; Hu, J.; Zhang, Y.; Liu, Y.; Ma, S.; Zhou, J.; Luo, G.; et al. Indocyanine Green Fluorescence Imaging in the Surgical Management of Skin Squamous Cell Carcinoma. Clin. Cosmet. Investig. Dermatol. 2023, 16, 3309–3320. [Google Scholar] [CrossRef]
- Achterberg, F.B.; Deken, M.M.; Meijer, R.P.J.; Mieog, J.S.D.; Burggraaf, J.; van de Velde, C.J.H.; Swijnenburg, R.J.; Vahrmeijer, A.L. Clinical translation and implementation of optical imaging agents for precision image-guided cancer surgery. Eur. J. Nucl. Med. Mol. Imaging 2020, 48, 332–339. [Google Scholar] [CrossRef]
- Rosenthal, E.L.; Warram, J.M.; de Boer, E.; Basilion, J.P.; Biel, M.A.; Bogyo, M.; Bouvet, M.; Brigman, B.E.; Colson, Y.L.; DeMeester, S.R.; et al. Successful Translation of Fluorescence Navigation During Oncologic Surgery: A Consensus Report. J. Nucl. Med. 2015, 57, 144–150. [Google Scholar] [CrossRef]
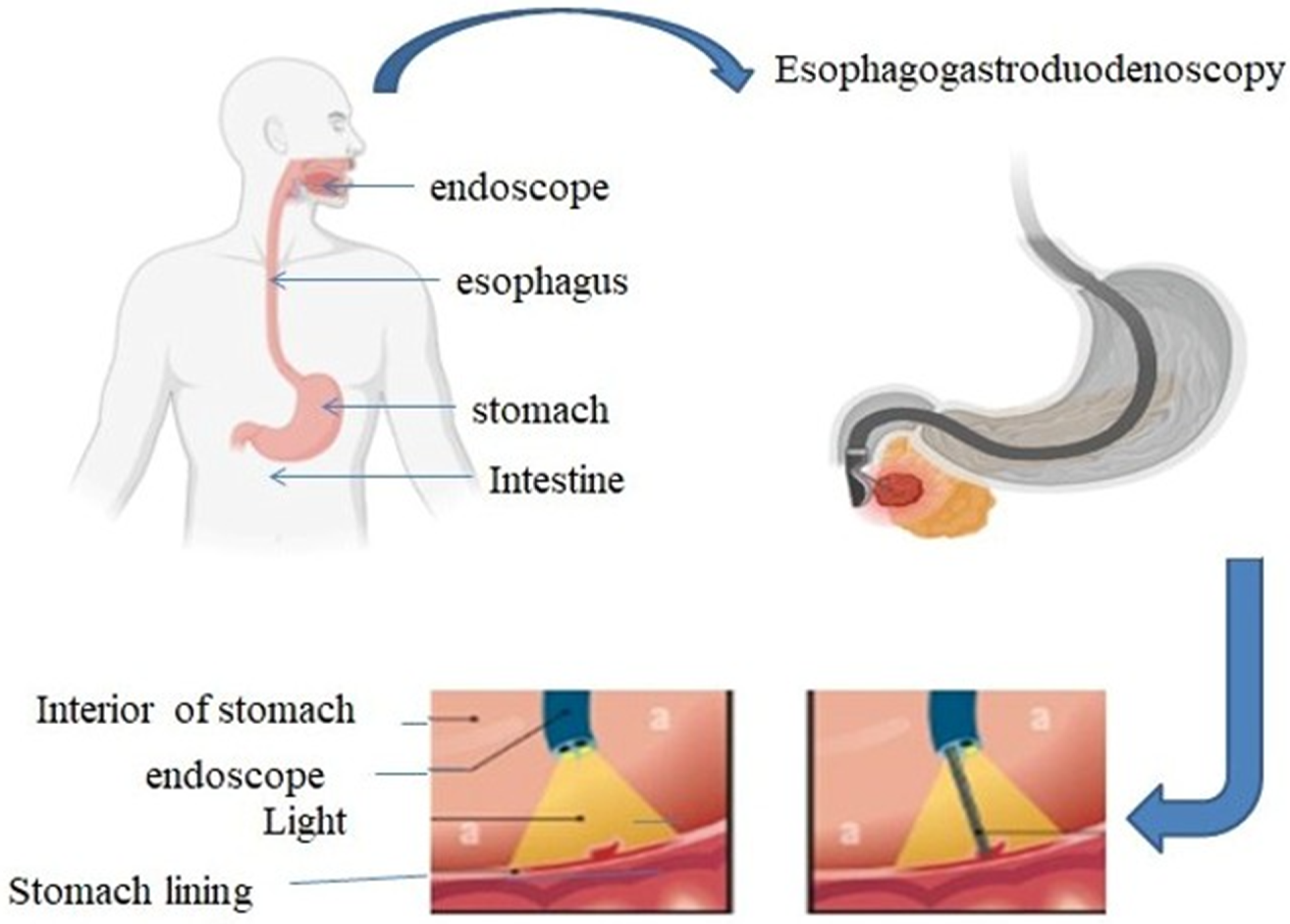
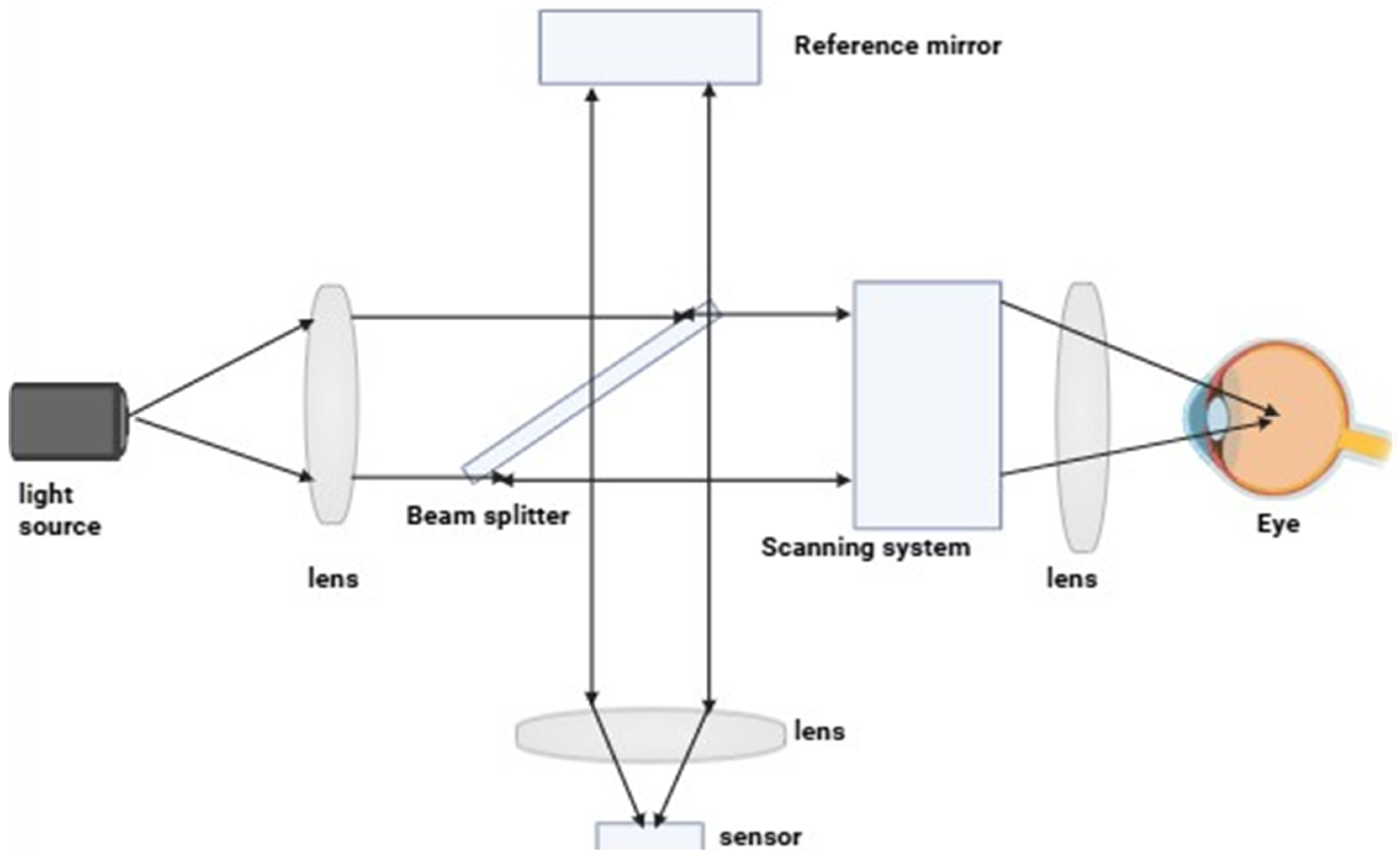

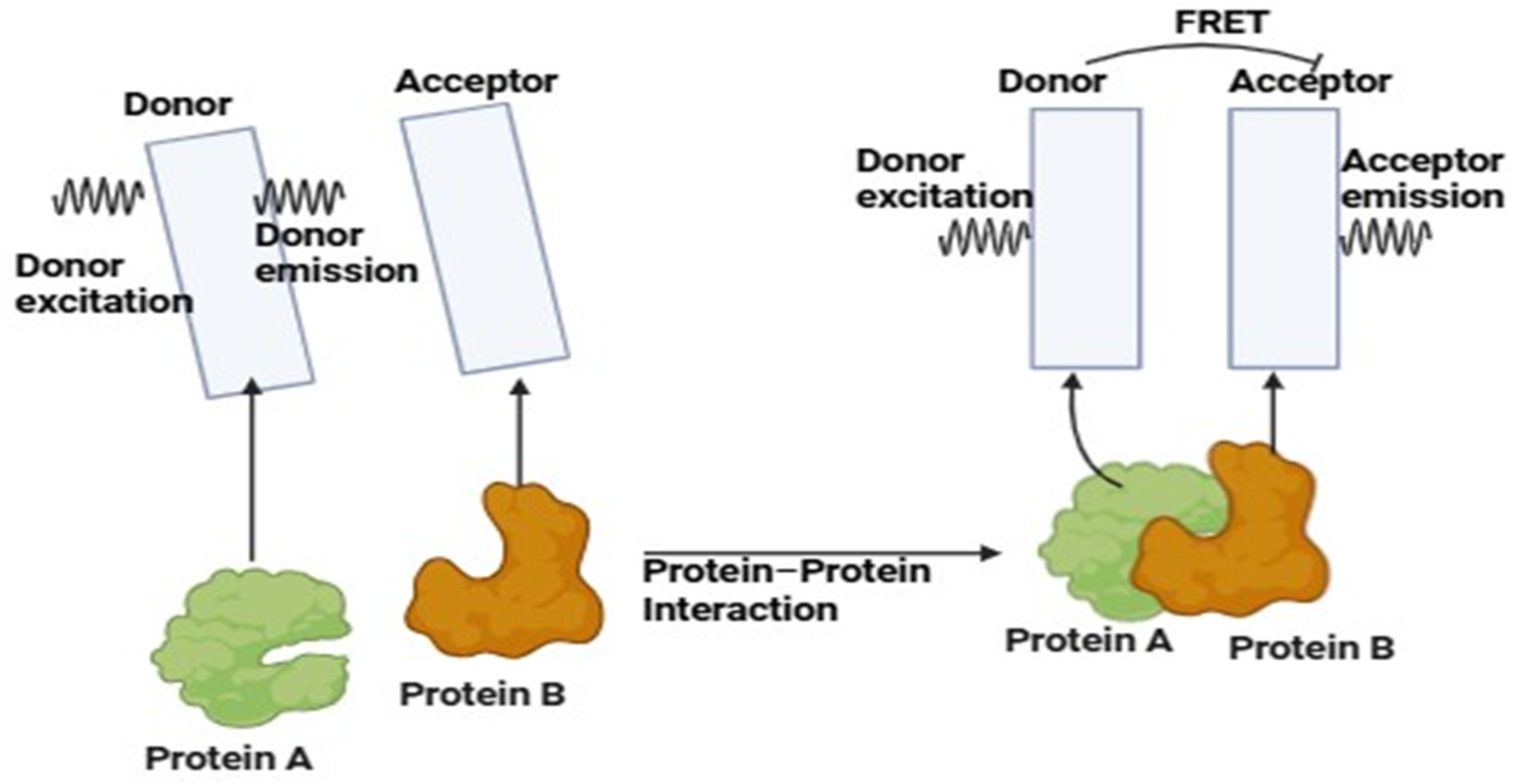
| Application | Description |
|---|---|
| Fluorescence molecular imaging | A non-invasive technique for tracking illnesses, researching biological processes, and learning about how drugs work [80]. |
| Cancer identification | Improves tumor border delineation with sophisticated imaging techniques; uses tumor-avid probes for high specificity and sensitivity in detecting malignancies [81]. |
| Development of probes | The development of fluorescent probes that glow in the far-red to near-infrared spectrum and are sensitive to specific targets like HOCl has allowed for deep tissue penetration and high sensitivity. |
| Real-time imaging | With the use of visualization techniques and adaptive procedures to improve accuracy, fluorescence imaging is increasingly being used in clinical settings. |
| Surgical guidance | Enhance endoscopic and surgical imaging by continuously providing feedback during the procedure. Motion artifacts are minimized by using methods such optical flow correction. |
| Near–infrared imaging | Provides superior real-time display and spatial resolution for cancer diagnosis, making up for the drawbacks of conventional imaging modalities in a range of applications. |
| Brain imaging | Enables the cellular and molecular analysis of brain activity, using specialized optics and fluorescent markers to examine neurotransmission and synaptic communication [82]. |
| Vascular imaging | Non-invasive cerebral vasculature observation is vital to comprehending disorders such as stroke since it tracks anomalies in blood vessels in real time [83]. |
| Cardiovascular imaging | To assess vascular anatomy and detect cerebrovascular diseases, employ near-infrared fluorescence imaging, which offers deep tissue penetration and great spatial resolution [84]. |
| Benefits | Difficulties |
|---|---|
| Real-time imaging: Fluorescent probes enable the visualization physiological conditions and real-time cellular operations [93]. | Background signals: Fluorescence signals resulting from natural cofactors within living cells might provide a problem for imaging research [94]. |
| High sensitivity: The detection of specific biomolecules is made possible by the high sensitivity and specificity of fluorescent probes [94]. | Elevated background signals: They can diminish signal contrast in intact tissue and multi-cell systems [94]. |
| Non-invasive imaging: Highly precise non-invasive imaging of cellular events is made possible by tiny fluorophores [93]. | Challenges with in vivo cancer imaging: Creating fluorescent nanoparticle probes presents difficulties. |
| Increased functionality: Optimal optical characteristics for certain subcellular locations are provided by small-molecule fluorescent probes. | Complex photo physical schemes: The complex photo physical schemes of certain fluorescent probes influence their bio-analytical responses. |
| Targeted therapy: Fluorescent probes can help medications be delivered in a specific manner in targeted therapy. | Inadequate signal-to-background ratios hinder the clinical application of optical molecular imaging. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathi, D.; Hardaniya, M.; Pande, S.; Maity, D. Advances in Optical Contrast Agents for Medical Imaging: Fluorescent Probes and Molecular Imaging. J. Imaging 2025, 11, 87. https://doi.org/10.3390/jimaging11030087
Tripathi D, Hardaniya M, Pande S, Maity D. Advances in Optical Contrast Agents for Medical Imaging: Fluorescent Probes and Molecular Imaging. Journal of Imaging. 2025; 11(3):87. https://doi.org/10.3390/jimaging11030087
Chicago/Turabian StyleTripathi, Divya, Mayurakshi Hardaniya, Suchita Pande, and Dipak Maity. 2025. "Advances in Optical Contrast Agents for Medical Imaging: Fluorescent Probes and Molecular Imaging" Journal of Imaging 11, no. 3: 87. https://doi.org/10.3390/jimaging11030087
APA StyleTripathi, D., Hardaniya, M., Pande, S., & Maity, D. (2025). Advances in Optical Contrast Agents for Medical Imaging: Fluorescent Probes and Molecular Imaging. Journal of Imaging, 11(3), 87. https://doi.org/10.3390/jimaging11030087






