Bay Leaf Extract-Based Near-Infrared Fluorescent Probe for Tissue and Cellular Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Cell Culture
2.2. Leaf Extraction Procedure
2.3. Fluorescence Imaging of Leaf Extracts
2.4. Spectral Analysis
2.5. Agarose Phantom Gel Imaging
2.6. Ex Vivo NIR Imaging
2.7. Cellular Uptake
2.8. Physicochemical Characterization
2.9. Statistical Analysis
3. Results
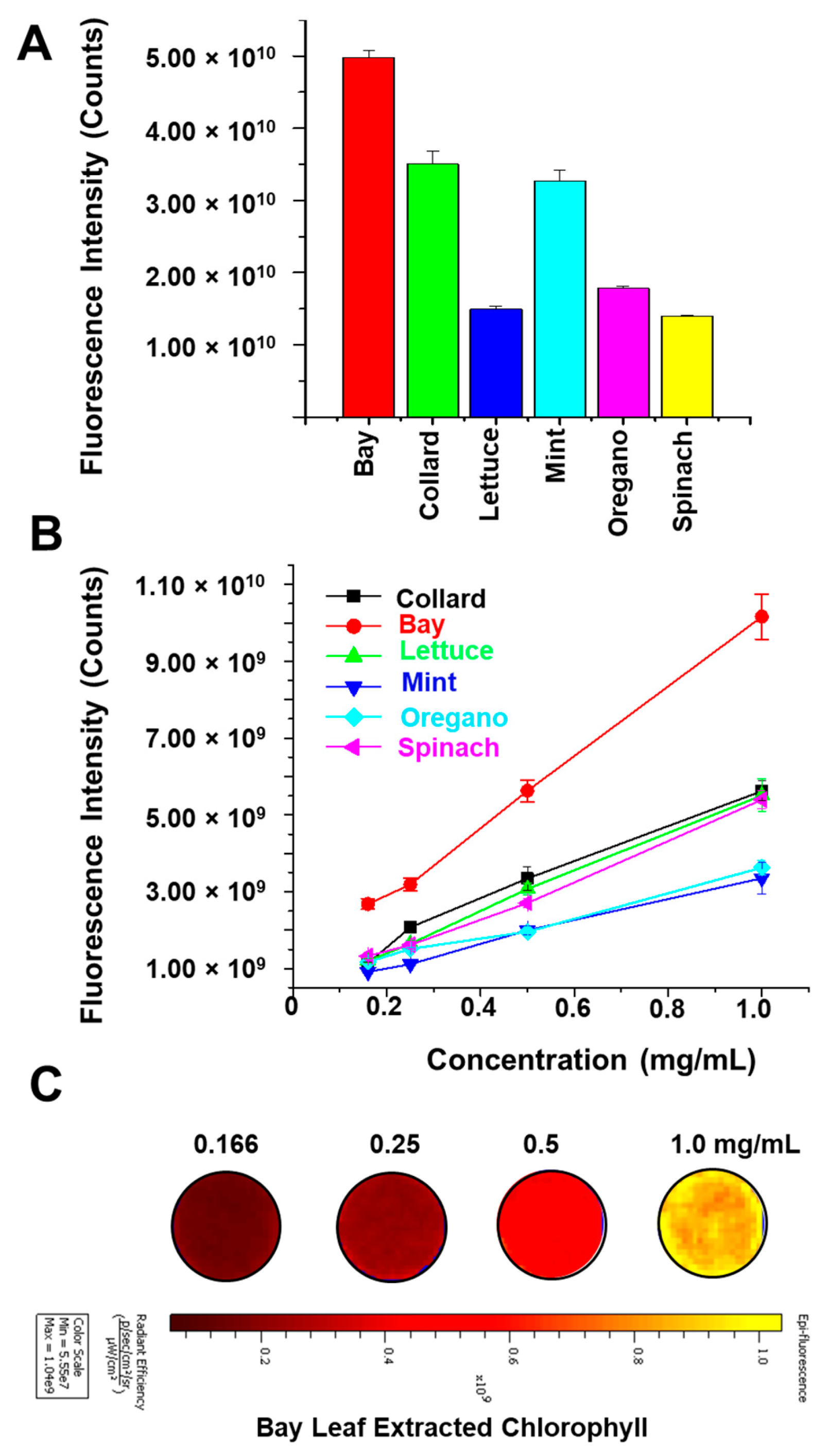
3.1. NIR Fluorescence Measurement in Dietary Leaves
3.2. Bay Leaf Extract Exhibits Superior NIR Fluorescence Properties
3.3. Bay Leaf Extract Demonstrates Optical Characteristics
3.4. Bay Leaf Extract Demonstrates Optical Characteristics
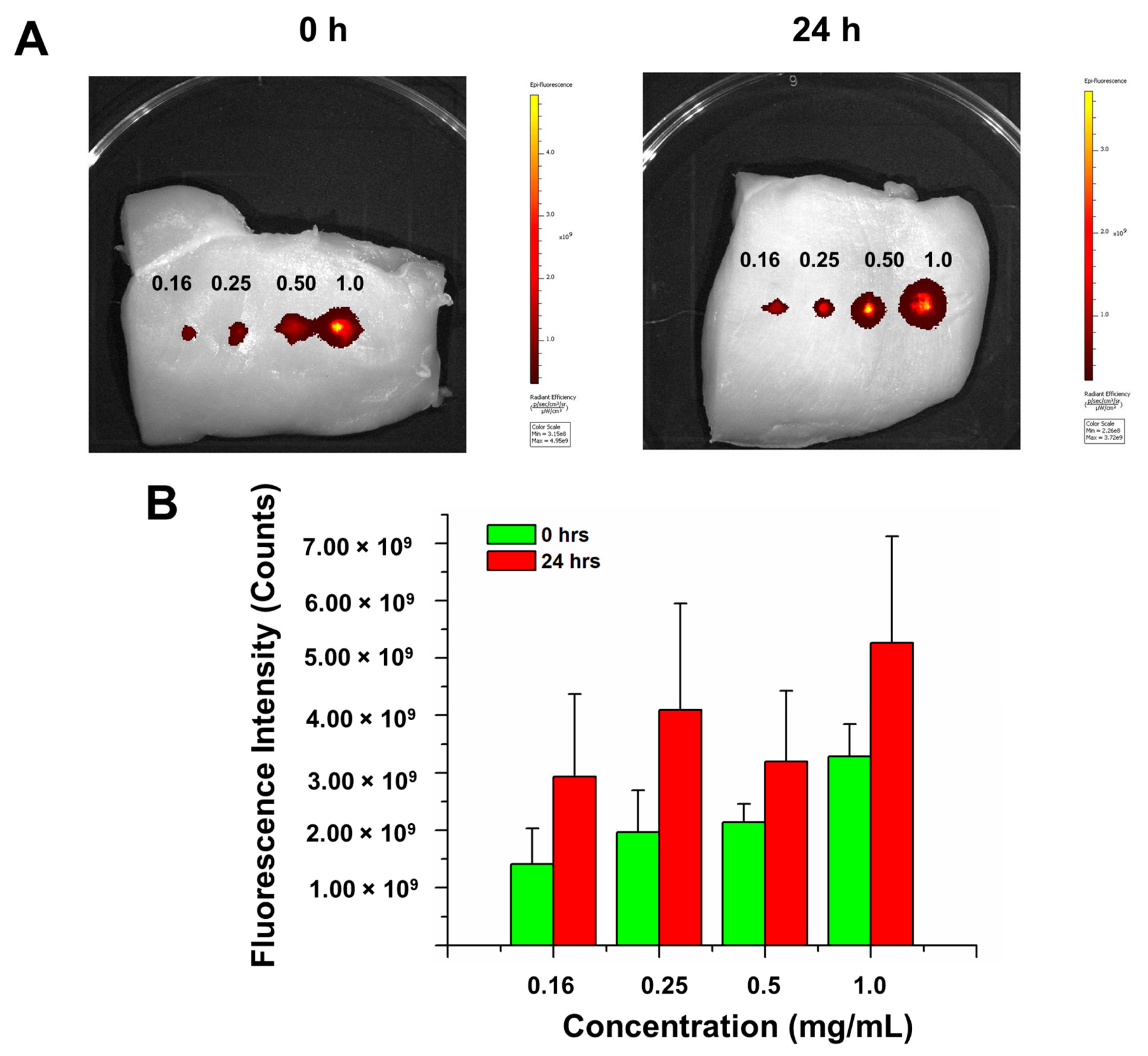
3.5. Bay Leaf Extracts Exhibit Tissue NIR Imaging
3.6. Bay Leaf Extract Facilitates Visualization of Cancer Cells
3.7. Characterization of Bay Leaf Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Fass, L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef] [PubMed]
- Choy, G.; Choyke, P.; Libutti, S.K. Current advances in molecular imaging: Noninvasive in vivo bioluminescent and fluorescent optical imaging in cancer research. Mol. Imaging 2003, 2, 303–312. [Google Scholar] [CrossRef]
- Lu, H.; Su, F.; Mei, Q.; Tian, Y.; Tian, W.; Johnson, R.H.; Meldrum, D.R. Using fluorine-containing amphiphilic random copolymers to manipulate the quantum yields of aggregation-induced emission fluorophores in aqueous solutions and the use of these polymers for fluorescent bioimaging. J. Mater. Chem. 2012, 22, 9890–9900. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Zhang, R.R.; Schroeder, A.B.; Grudzinski, J.J.; Rosenthal, E.L.; Warram, J.M.; Pinchuk, A.N.; Eliceiri, K.W.; Kuo, J.S.; Weichert, J.P. Beyond the margins: Real-time detection of cancer using targeted fluorophores. Nat. Reviews. Clin. Oncol. 2017, 14, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Schaafsma, B.E.; Mieog, J.S.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.J.; Löwik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Tao, Z.; Hong, G.; Shinji, C.; Chen, C.; Diao, S.; Antaris, A.L.; Zhang, B.; Zou, Y.; Dai, H. Biological imaging using nanoparticles of small organic molecules with fluorescence emission at wavelengths longer than 1000 nm. Angew. Chem. (Int. Ed. Engl.) 2013, 52, 13002–13006. [Google Scholar] [CrossRef]
- Chen, Z.-L.; Yu, Z.-L.; Zhou, M.-M.; Zhang, S.; Zhang, B.; Liu, Y.; Zhao, Y.-F.; Cao, H.-M.; Lin, Y.; Zhang, Z.-L.; et al. Chlorophyll-Based Near-Infrared Fluorescent Nanocomposites: Preparation and Optical Properties. ACS Omega 2020, 5, 14261–14266. [Google Scholar] [CrossRef]
- Chen, H.; Li, B.; Zhang, M.; Sun, K.; Wang, Y.; Peng, K.; Ao, M.; Guo, Y.; Gu, Y. Characterization of tumor-targeting Ag2S quantum dots for cancer imaging and therapy in vivo. Nanoscale 2014, 6, 12580–12590. [Google Scholar] [CrossRef] [PubMed]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Chu, M.; Li, H.; Wu, Q.; Wo, F.; Shi, D.J.B. Pluronic-encapsulated natural chlorophyll nanocomposites for in vivo cancer imaging and photothermal/photodynamic therapies. Biomaterials 2014, 35, 8357–8373. [Google Scholar] [CrossRef] [PubMed]
- Chiu, L.C.-M.; Kong, C.K.-L.; Ooi, V.E.-C. The chlorophyllin-induced cell cycle arrest and apoptosis in human breast cancer MCF-7 cells is associated with ERK deactivation and Cyclin D1 depletion. Int. J. Mol. Med. 2005, 16, 735–740. [Google Scholar] [PubMed]
- Li, L.; Zhang, R.; Lu, C.; Sun, J.; Wang, L.; Qu, B.; Li, T.; Liu, Y.; Li, S. In situ synthesis of NIR-light emitting carbon dots derived from spinach for bio-imaging applications. J. Mater. Chem. B 2017, 5, 7328–7334. [Google Scholar] [CrossRef]
- Shao, X.R.; Wei, X.Q.; Song, X.; Hao, L.Y.; Cai, X.X.; Zhang, Z.R.; Peng, Q.; Lin, Y.F. Independent effect of polymeric nanoparticle zeta potential/surface charge, on their cytotoxicity and affinity to cells. Cell Prolif. 2015, 48, 465–474. [Google Scholar] [CrossRef]
- Frangioni, J.V. New technologies for human cancer imaging. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 4012–4021. [Google Scholar] [CrossRef]
- Yi, X.; Wang, F.; Qin, W.; Yang, X.; Yuan, J. Near-infrared fluorescent probes in cancer imaging and therapy: An emerging field. Int. J. Nanomed. 2014, 9, 1347–1365. [Google Scholar] [CrossRef] [Green Version]
- Kosaka, N.; Ogawa, M.; Choyke, P.L.; Kobayashi, H. Clinical implications of near-infrared fluorescence imaging in cancer. Future Oncol. 2009, 5, 1501–1511. [Google Scholar] [CrossRef] [Green Version]
- Takami, T.; Naito, K.; Yamagata, T.; Shimokawa, N.; Ohata, K. Benefits and Limitations of Indocyanine Green Fluorescent Image-Guided Surgery for Spinal Intramedullary Tumors. Oper. Neurosurg. 2017, 13, 746–754. [Google Scholar] [CrossRef]
- Sevieri, M.; Silva, F.; Bonizzi, A.; Sitia, L.; Truffi, M.; Mazzucchelli, S.; Corsi, F. Indocyanine Green Nanoparticles: Are They Compelling for Cancer Treatment? Front. Chem. 2020, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Bacheti, R.K.; Husen, A. Medicinal Uses of Chlorophyll: A critical overview. In Chlorophyll: Structure, Function and Medicinal Uses; Le, H., Salcedo, E., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 177–196. [Google Scholar]





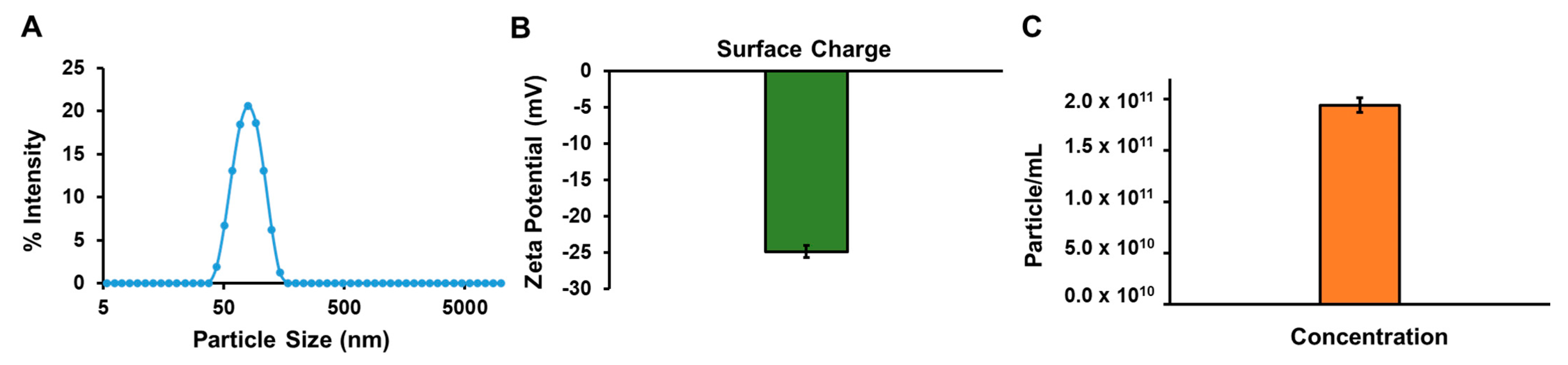
| Samples | Size (nm) | Charge (mV) | Concentration (Particles/mL) |
|---|---|---|---|
| Bay leaf | 62.72 ± 5.97 | −24.76 ± 1.52 | 1.95 × 1011 |
| Collard | 170.70 ± 9.88 | −21.68 ± 1.47 | 4.60 × 1010 |
| Lettuce | 131.07 ± 1.82 | −25.04 ± 1.25 | 5.01 × 1019 |
| Mint | 51.8 ± 0.45 | −20.87 ± 0.61 | 2.13 × 1011 |
| Oregano | 227.07 ± 2.5 | −25.36 ± 0.73 | 9.26 × 109 |
| Spinach | 75.61 ± 3.95 | −21.93 ± 0.14 | 5.29 × 1010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adriano, B.; Cotto, N.M.; Chauhan, N.; Karumuru, V.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Bay Leaf Extract-Based Near-Infrared Fluorescent Probe for Tissue and Cellular Imaging. J. Imaging 2021, 7, 256. https://doi.org/10.3390/jimaging7120256
Adriano B, Cotto NM, Chauhan N, Karumuru V, Jaggi M, Chauhan SC, Yallapu MM. Bay Leaf Extract-Based Near-Infrared Fluorescent Probe for Tissue and Cellular Imaging. Journal of Imaging. 2021; 7(12):256. https://doi.org/10.3390/jimaging7120256
Chicago/Turabian StyleAdriano, Benilde, Nycol M. Cotto, Neeraj Chauhan, Vinita Karumuru, Meena Jaggi, Subhash C. Chauhan, and Murali M. Yallapu. 2021. "Bay Leaf Extract-Based Near-Infrared Fluorescent Probe for Tissue and Cellular Imaging" Journal of Imaging 7, no. 12: 256. https://doi.org/10.3390/jimaging7120256
APA StyleAdriano, B., Cotto, N. M., Chauhan, N., Karumuru, V., Jaggi, M., Chauhan, S. C., & Yallapu, M. M. (2021). Bay Leaf Extract-Based Near-Infrared Fluorescent Probe for Tissue and Cellular Imaging. Journal of Imaging, 7(12), 256. https://doi.org/10.3390/jimaging7120256










