Comprehensive Survey of Machine Learning Systems for COVID-19 Detection
Abstract
:1. Introduction
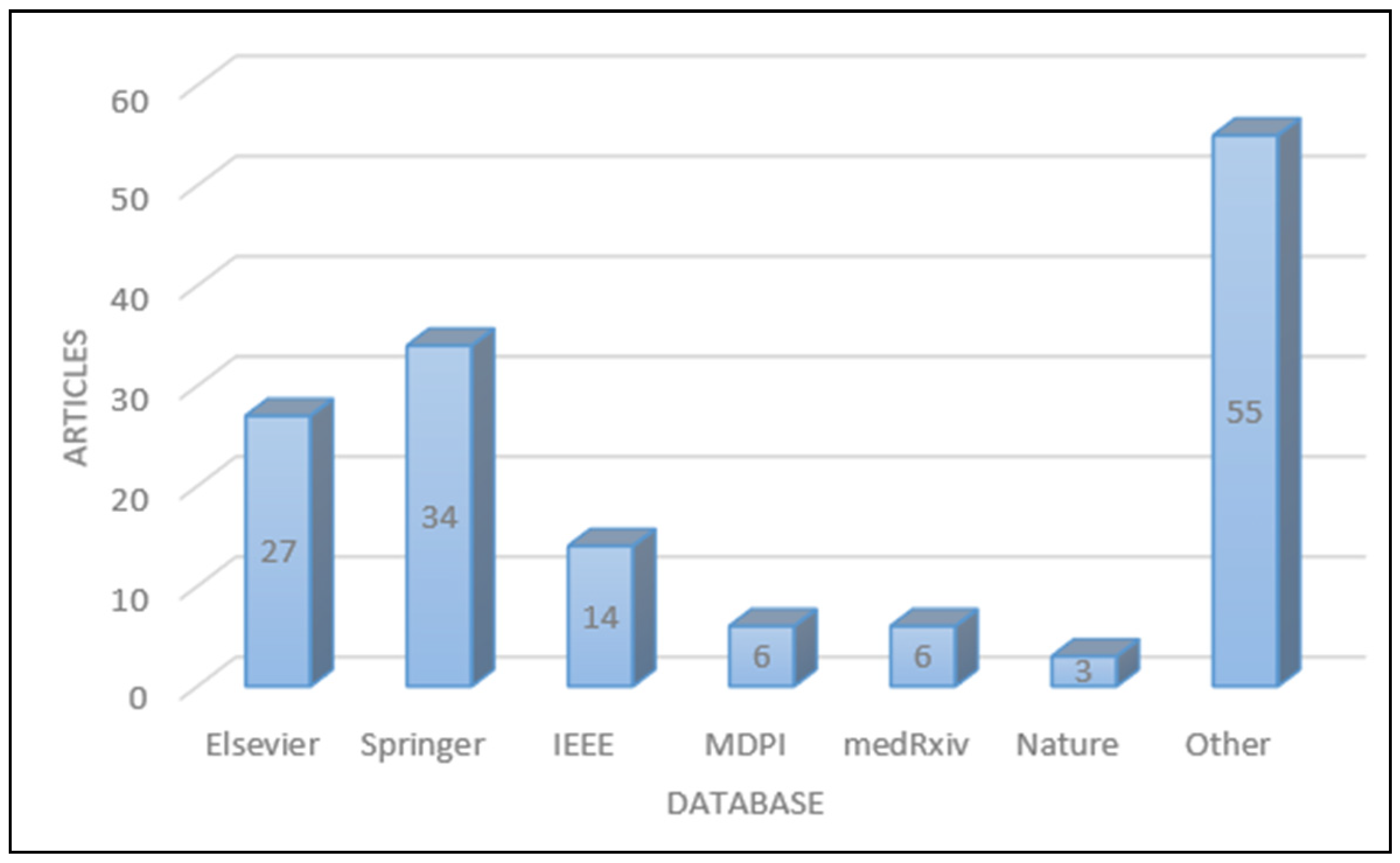
2. Searching Strategy
3. Materials
4. Data Augmentation
- Use stationary wavelets to split the training images into three levels;
- Apply shear operation using values [−30, 30];
- Apply rotation transformation within [−90, 90];
- Translate the pixels within [−10, 10].
- ±15° rotation;
- ±15% x-axis shift;
- ±15% y-axis shift;
- horizontal flipping;
- 85–115% scaling and shearing;
- mixup = 0.1.
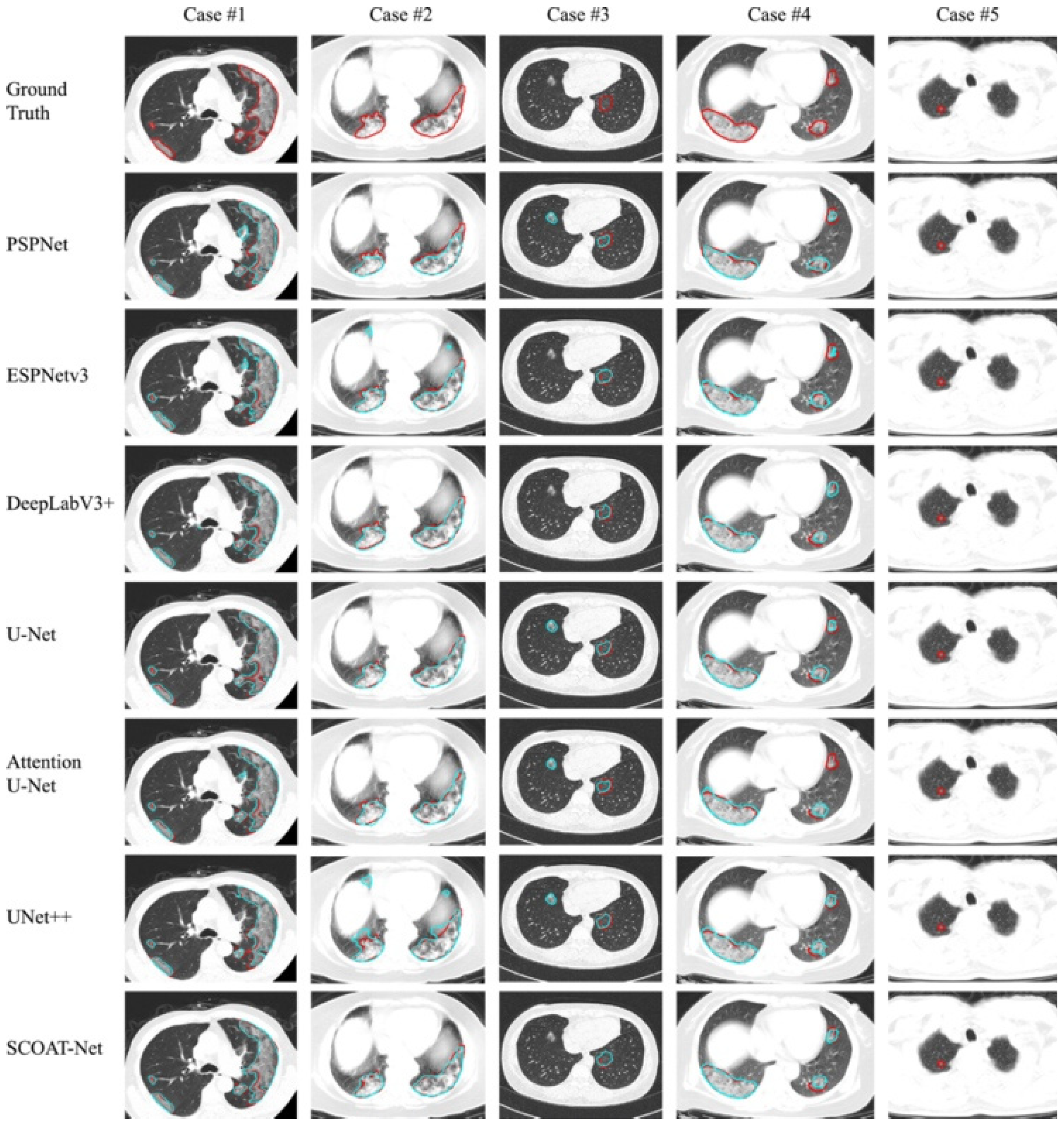
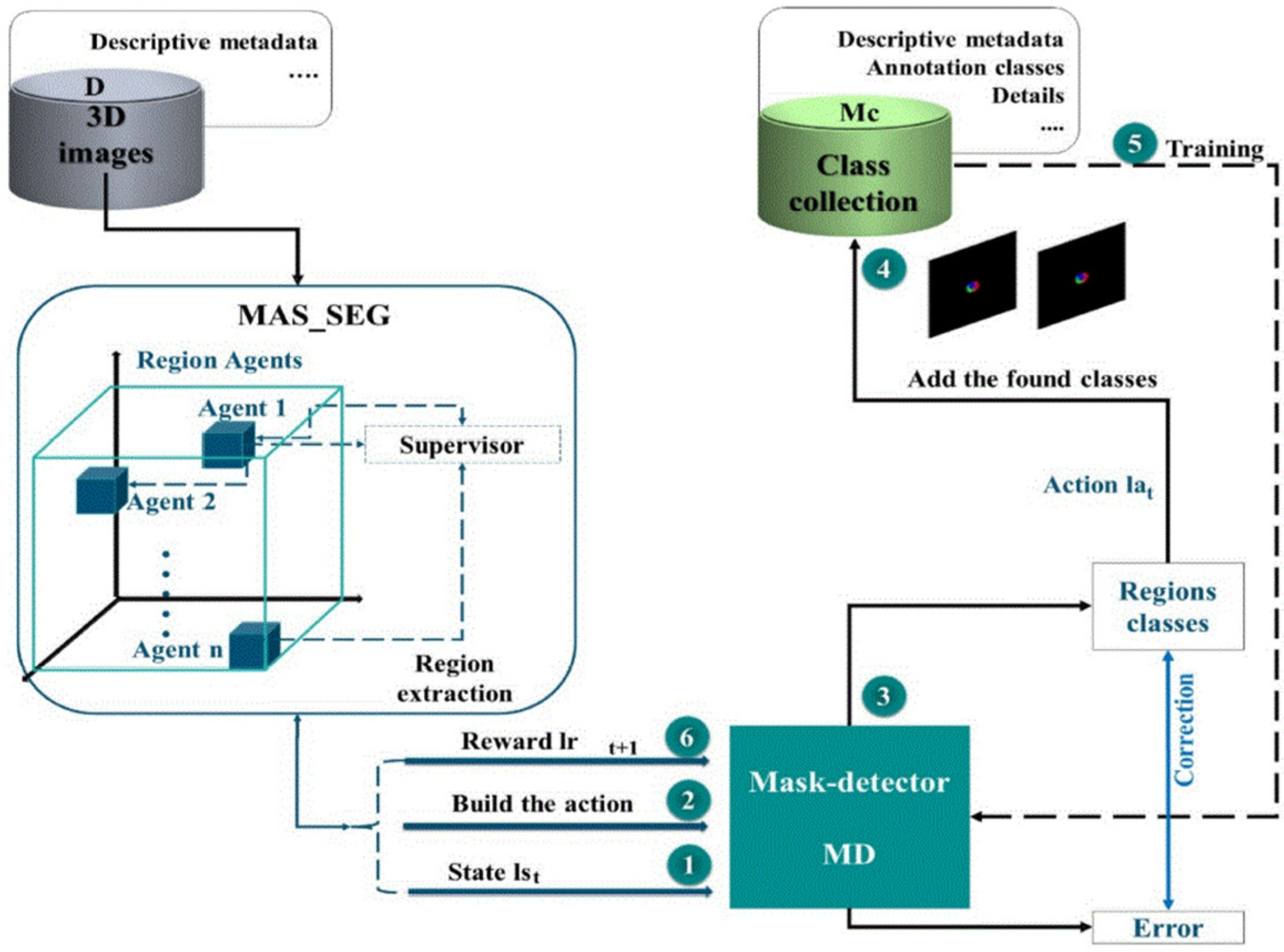
5. Segmentation
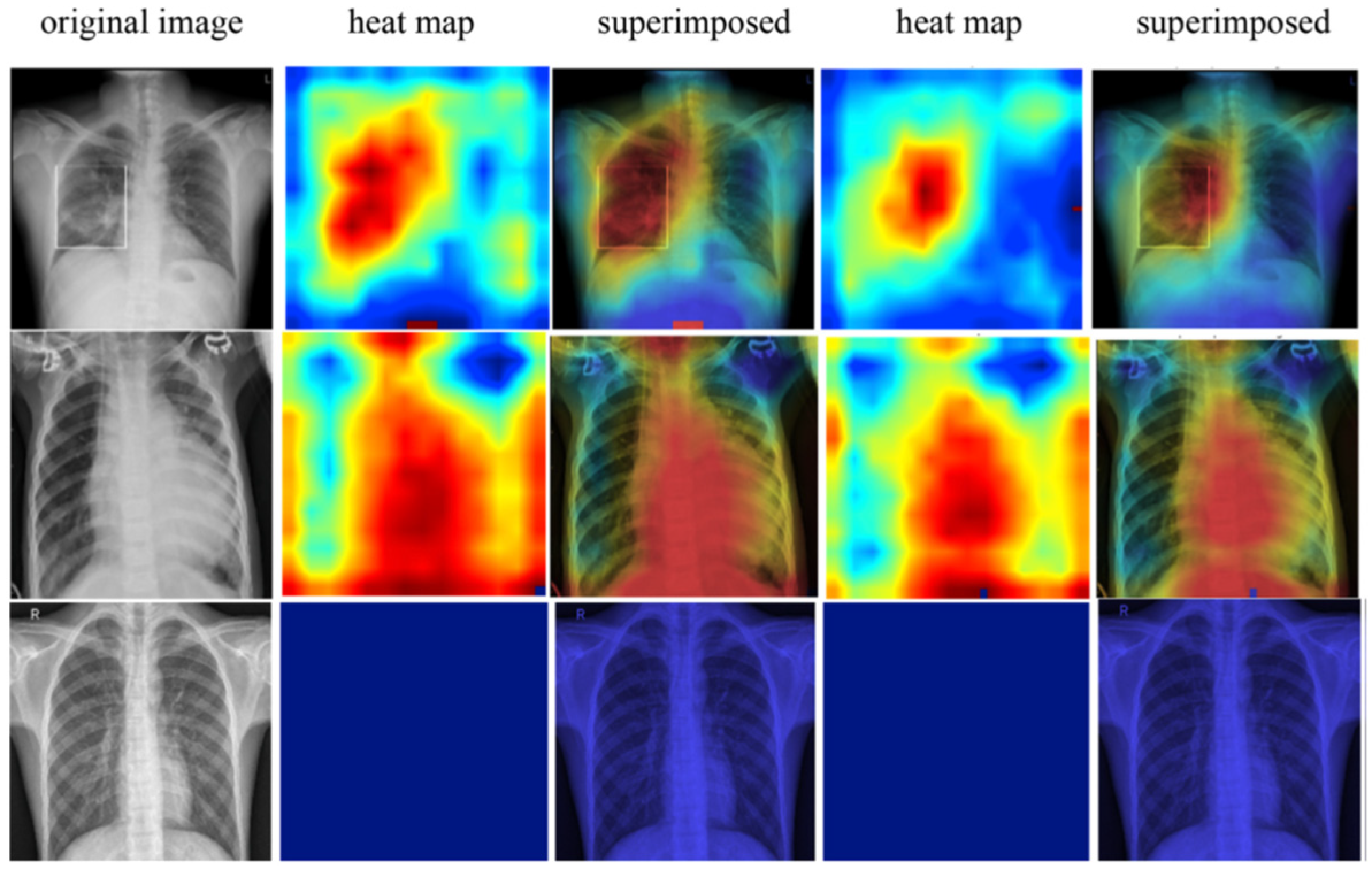
6. Classification
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Study | Year | Data Set | Methodology | Accuracy |
|---|---|---|---|---|
| A multi-dilation convolutional neural network for automatic COVID-19 and other pneumonia detection from chest X-ray images with transferable multi-receptive feature optimization [75] | 2020 | 5856 X-ray images | A deep neural network architecture namely CovXNet. | 97.4% COVID-19/Nomal 96.9% COVID-19/Viral pneumonia 94.7% COVID-19/Bacterial pneumonia 90.2% multiclass COVID-19/normal/ Vral/Bacterial pneumonias |
| COVID-19 detection in radiological text reports integrating entity recognition [76] | 2021 | CT Scan 295 anonymous CT scan reports | ML model NER system Five statistical parameters. | 90% |
| Deep-chest: Multi-classification deep learning model for diagnosing COVID-19, pneumonia, and lung cancer chest diseases [77] | 2021 | 33,676 X-ray and CT images | CNN and recurrent neural network (RNN) | The VGG19+CNN model achieved 98.05% accuracy (ACC) |
| COVID-19 cough classification using machine learning and global smartphone recordings [83] | 2021 | Data set | CNN | 95.3% |
| Automatic detection of COVID-19 using pruned GLCM-Based texture features and LDCRF classification [78] | 2021 | X-ray images 2300 | CAD methodology segmentation | 95.88% |
| Diagnosis and detection of infected tissue of COVID-19 patients based on lung X-ray image using convolutional neural network approaches [79] | 2020 | X-ray images 682 | Deep Neural Network (DNN) and Convolutional Neural Network (CNN) | CNN (93.2%) DNN 83.4% |
| Automatic method for classifying COVID-19 patients based on chest X-ray images, using deep features and PSO-optimized XGBoost [106] | 2021 | X-ray images 5586 | Extreme Gradient Boosting (XGBoost) optimized by particle swarm optimization (PSO). | 98.71% |
| COVID-19: Automatic Detection of the Novel Coronavirus Disease From CT Images Using an Optimized Convolutional Neural Network [107] | 2021 | CT Scan | Optimized Convolutional Neural Network | 95.7% |
| Automatic detection of COVID-19 from chest radiographs using deep learning [108] | 2020 | X-ray images 1428 | Deep Learning model | 96% |
| Deep learning approaches for COVID-19 detection based on chest X-ray images [82] | 2020 | 200 X-ray images | Deep Convolutional Neural Network | 91.6% |
| Automatic diagnosis of COVID-19 disease using deep convolutional neural network with multi-feature channel from respiratory sound data: Cough, voice, and breath [84] | 2021 | 4.5 k samples/web-based application. 2.5 k samples/ android based application | Multichannel Deep Convolutional Neural Network (DCNN) | 80% accuracy for respiratory-based sound classification 62% for audio-based classification |
| EMCNet: Automated COVID-19 diagnosis from X-ray images using convolutional neural network and ensemble of machine learning classifiers [109] | 2020 | 400 X-ray images | EMCNet | 98.91% |
| A combined deep CNN-LSTM network for the detection of novel coronavirus (COVID-19) using X-ray images [86] | 2020 | 613 X-ray images | Deep CNNLSTM network | 99.4% |
| Deep Net Model for Detection of COVID-19 using Radiographs based on ROC Analysis [87] | 2020 | 100 X-ray images | CNN | 98% |
| The Role of Artificial Intelligence in Management of Critical COVID-19 Patients [110] | 2020 | CT Scan | AI | - |
| Detection of coronavirus Disease (COVID-19) based on Deep Features and Support Vector Machine [88] | 2020 | 127 X-ray images | ResNet plus SVM model | 98.66%. |
| An automatic COVID-19 CT segmentation based on U-Net with attention mechanism [89] | 2020 | 100 CT Scan | U-Net based segmentation | - |
| A Critic Evaluation of Methods for COVID-19 Automatic Detection from X-ray Images [111] | 2020 | 108,948 X-ray images | - | 92% |
| Comparative study of deep learning methods for the automatic segmentation of lung lesion, and lesion type in CT scans of COVID-19 patients [90] | 2020 | 1103 CT Scan | Twelve deep learning methods | - |
| Automatic Deep Learning System for COVID-19 Infection Quantification in chest CT [91] | 2020 | 240,270 CT Scan | CNN | - |
| Improving Coronavirus (COVID-19) Diagnosis using Deep Transfer Learning [92] | 2020 | 19,200 X-ray | CNN | 98.7% |
| Fully automatic deep convolutional approaches for the analysis of COVID-19 using chest X-ray images [93] | 2020 | 5856 X-ray | CNN | 0.97% |
| Classification of COVID-19 from Chest X-ray images using Deep Convolutional Neural Networks [94] | 2020 | 315 X-ray | Deep Convolutional Neural Networks | 98% |
| Automatic Detection of COVID-19 Infection from Chest X-ray using Deep Learning [95] | 2020 | X-ray | Deep Convolutional Neural Network | 93% |
| An Automatic Computer-Based Method for Fast and Accurate COVID-19 Diagnosis [96] | 2020 | 195 CT Scan | CNN | 92.5% |
| Challenges of Deep Learning Methods for COVID-19 Detection Using Public Datasets [97] | 2020 | CT scan | Deep Neural Network | 98-99% |
| Automatic COVID-19 Detection from chest radiographic images using Convolutional Neural Network [98] | 2020 | 5740 X-ray | CNN | 99.45% |
| Classification of COVID-19 X-ray Images Using a Combination of Deep and Handcrafted Features [99] | 2021 | 5143 X-ray/CT Scan | SVM &CNN | 98.8% |
| DenResCov-19: A deep transfer learning network for robust automatic classification of COVID-19, pneumonia, and tuberculosis from X-rays [100] | 2021 | 3883 X-ray/CT Scan | Deep Learning Network | 86.4% |
| Automatic Diagnosis of COVID-19 from CT Images using CycleGAN and Transfer Learning [101] | 2021 | 1766 CT Scan | CycleGAN | 99.60% |
| An Automatic Classification of COVID with J48 and Simple K-Means using Weka [102] | 2020 | - | k-Means | 99.63% |
| Extracting Possibly Representative COVID-19 Biomarkers from X-ray Images with Deep Learning Approach and Image Data Related to Pulmonary Diseases [103] | 2020 | 3905 X-ray | Convolutional Neural Network | 99.18% |
| Automatic X-ray COVID-19 Lung Image Classification System based on Multi-Level Thresholding and Support Vector Machine [104] | 2020 | X-ray | SVM | 97.48% |
| Classification of the COVID-19 infected patients using DenseNet201 based deep transfer learning [105] | 2020 | 2492 CT Scan | CNN | 99.82% |
| ADOPT: automatic deep learning and optimization-based approach for detection of novel coronavirus COVID-19 disease using X-ray images [106] | 2021 | 50 X-ray | 11 different convolutional neural network-based (CNN) models | 98.54% |
| COVID-19: Automatic Detection of the Novel Coronavirus Disease From CT Images Using an Optimized Convolutional Neural Network [107] | 2021 | CT Scan | Optimized Convolutional Neural Network | 95.7% |
| An intelligent tool to support diagnosis of COVID-19 by texture analysis of X-ray images. Research on Biomedical Engineering [112] | 2020 | 6309 X-ray | IKONOS | 89.78% |
| An automatic approach based on CNN architecture to detect COVID-19 disease from chest X-ray images. [113] | 2020 | 8830 X-ray | CNN | 99.32% for binary class and 97.55% for multi-class |
| Automatic COVID-19 detection using exemplar hybrid deep features with X-ray images [114] | 2021 | 11,104 X-ray | COVID-19FclNet9 (CNN optimized) | 99.64% |
| An integrated feature frame work for automated segmentation of COVID-19 infection from lung CT images [115] | 2020 | 80 X-ray/CT | DNN Model | - |
| Automatic COVID-19 CT segmentation using U-Net integrated spatial and channel attention mechanism [116] | 2020 | 473 CT | U-Net Model | 83.1% |
| Transfer learning-based automatic detection of coronavirus disease 2019 (COVID-19) from chest X-ray images [117] | 2020 | 348 X-ray | Visual Geometry Group (VGG)-16, VGG-19, MobileNet, and InceptionResNetV2 (CNN optimized) | >90.0% |
| Deep convolutional neural networks for COVID-19 automatic diagnosis [118] | 2021 | 1954 X-ray | CNN | 99%, 99.12%, and 99.29% for ResNet18, ResNet50, and ResNet101, respectively. |
| Optimized genetic algorithm-extreme learning machine approach for automatic COVID-19 detection [119] | 2020 | 188 X-ray | Optimized Genetic Algorithm-Extreme Learning Machine (OGA-ELM) | 100.00% |
| Automatic evaluation of the lung condition of COVID-19 patients using X-ray images and convolutional neural networks [120] | 2021 | 185 X-ray | CNN | 96% |
| Cascaded deep learning classifiers for computer-aided diagnosis of COVID-19 and pneumonia diseases in X-ray scans [121] | 2020 | 306 X-ray | CNN: VGG ResNe | 99.9% |
| Evaluation of deep learning-based approaches for COVID-19 classification based on chest X-ray images [122] | 2021 | 760 X-ray | DCNN | 98.69% |
| Multi-task contrastive learning for automatic CT and X-ray diagnosis of COVID-19 [123] | 2021 | 4758 CT 5821 X-ray | Contrastive Multi-Task Convolutional Neural Network (CMT-CNN) | CT (5.49–6.45%) X-ray (0.96–2.42%) |
| Multi-task deep learning based CT imaging analysis for COVID-19 pneumonia: Classification and segmentation [124] | 2020 | 1369 CT | Multitask Deep Learning model | 97% |
| Automatic detection of coronavirus disease (COVID-19) using X-ray images and deep convolutional neural networks [125] | 2021 | 7065 X-ray | DCNN | 99.7% |
| Deep transfer learning with apache spark to detect COVID-19 in chest X-ray images [126] | 2020 | 320 X-ray | Deep Transfer Learning (DTL) Using (CCN) | 99.01% pre-trained InceptionV3 model 98.03% ResNet50 model |
| Implementation of convolutional neural network approach for COVID-19 disease detection [127] | 2020 | 4576 X-ray | DCNN | 98.92% |
| A novel approach of CT images feature analysis and prediction to screen for corona virus disease (COVID-19) [128] | 2020 | 51 CT | Composed Hybrid Feature Selection (CHFS) and Optimizes Genetic Algorithm (OGA) | 96.07% |
| Automatic Classification Approach for Detecting COVID-19 using Deep Convolutional Neural Networks [129] | 2020 | 1140 X-ray | DCNN | 92.54%, precision: 93.05%, recall: 92.81%, F1-score: 92.83%, specificity: 97.47% |
| Artificial intelligence distinguishes COVID-19 from community acquired pneumonia on chest CT. Radiology [130] | 2020 | 4356 CT | COVNet | 95% |
| A machine learning-based framework for diagnosis of COVID-19 from chest X-ray images [131] | 2021 | 500 X-ray | Logistic Regression (LR) and Convolutional Neural Networks (CNN) | 95.2–97.6% |
| Deep learning-based meta-classifier approach for COVID-19 classification using CT scan and chest X-ray images [132] | 2021 | 8055 CT 9544 X-ray | CNN | 99.48% |
| Rapid identification of COVID-19 severity in CT scans through classification of deep features [133] | 2020 | 729 CT Images | DNN (Deep Neural Network) | 95.34% |
| Machine-learning classification of texture features of portable chest X-ray accurately classifies COVID-19 lung infection [134] | 2020 | 250 CT Images | Deep Learning | - |
| Automatic classification between COVID-19 pneumonia, non-COVID-19 pneumonia, and the healthy on chest X-ray image: combination of data augmentation methods [65] | 2020 | 1248 X-ray images | Conventional Neural Network/data augmentation | >90% |
| The study of automatic machine learning base on radiomics of non-focus area in the first chest CT of different clinical types of COVID-19 pneumonia [135] | 2020 | 219 X-ray | Auto ML | 95% |
| A novel hand-crafted with deep learning features based fusion model for COVID-19 diagnosis and classification using chest X-ray images [136] | 2021 | 516 X-ray | FM-HCF-DLF model [Optimized CNN] | 94.08% |
| Classification of COVID-19 patients from chest CT images using multi-objective differential evolution–based convolutional neural networks [137] | 2020 | CT Images | CNN +ANN+ ANFIS | 92% proposed 91.7% CNN 91.4% ANFIS 89.5% ANN |
| Densely connected convolutional networks-based COVID-19 screening model [138] | 2021 | 11,494 CT Images | Densely Connected convolutional networks (DCCNs) [Optimized CNN] | 98.83% |
| End-to-end automatic differentiation of the coronavirus disease 2019 (COVID-19) from viral pneumonia based on chest CT [139] | 2021 | 448 CT Images | Large-scale bidirectional generative adversarial network (BigBiGAN) architecture | 92% |
| Toward real-time and efficient cardiovascular monitoring for COVID-19 patients by 5G-enabled wearable medical devices: a deep learning approach [140] | 2021 | - | Convolutional Neural Networks and long short-term memory networks model | 99.29% |
| Within the lack of chest COVID-19 X-ray dataset: a novel detection model based on GAN and deep transfer learning [141] | 2020 | 307 X-ray | GAN and Deep Transfer Learning | 99.9% |
| Automatic detection of COVID-19 infection using chest X-ray images through transfer learning [142] | 2021 | 194 X-ray | Different architectures of Convolutional Neural Networks (CNNs) | 98.5% |
| Automatic COVID-19 lung infected region segmentation and measurement using CT scans images [143] | 2020 | 275 CT | Automated tool of segmentation and measurement | 98% |
| Automatic detection of COVID-19 from chest X-ray images with convolutional neural networks [144] | 2021 | 165 X-ray | CNN | 97.56% |
| Auto-diagnosis of COVID-19 using lung CT images with semi-supervised shallow learning network [145] | 2021 | 2482 CT | CNN optimized | ----- |
| A deep learning-based COVID-19 automatic diagnostic framework using chest X-ray images [146] | 2021 | 6273 X-ray | Deep learning algorithm-based model | 97.11% |
| Performance evaluation of the NASNet convolutional network in the automatic identification of COVID-19 [147] | 2020 | 240 X-ray | Neural Architecture Search Network (NASNet) | 97% |
| A weakly-supervised framework for COVID-19 classification and lesion localization from chest CT [148] | 2020 | 530 CT images | DeCoVNet | 97.6% |
| Classification of COVID-19 in chest X-ray images using DeTraC deep convolutional neural network [149] | 2021 | 196 X-ray images | DeTraC deep CNN | 93.1% |
| Novel artificial intelligence algorithm for automatic detection of COVID-19 abnormalities in computed tomography images [150] | 2021 | 1581 CT images | artificial intelligence (AI) algorithm | 92.0% |
| CCBlock: an effective use of deep learning for automatic diagnosis of COVID-19 using X-ray images [151] | 2020 | 1828 X-ray images | enhancement of the classical visual geometry group (VGG) network | 95.34% |
| An effective deep residual network-based class attention layer with bidirectional LSTM for diagnosis and classification of COVID-19 [152] | 2020 | X-ray images | work (ResNet) based Class Attention Layer with Bidirectional LSTM called RCAL-BiLSTM for COVID-19 Diagnosis | 94.88% |
| COVID-caps: A capsule network-based framework for identification of COVID-19 cases from X-ray images [57] | 2020 | X-ray images | Capsule Networks | 95.7% |
| Automated detection of COVID-19 cases using deep neural networks with X-ray images [153] | 2020 | 127 X-ray | Deep Neural Networks | 98.08% |
| COVID-19: automatic detection from X-ray images utilizing transfer learning with convolutional neural networks [51] | 2020 | 1427 X-ray images | CNN | 96.78% |
| An open-source COVID-19 CT dataset with automatic lung tissue classification for radiomics [154] | 2021 | 62 CT images | ---- | |
| Explainable artificial intelligence-based edge fuzzy images for COVID-19 detection and identification [155] | 2022 | 5888 X-ray | Fuzzy CNNs | 95% |
| Ensemble Deep Learning and Internet of Things-Based Automated COVID-19 Diagnosis Framework [156] | 2022 | 12,146 CT scan | Deep learning and Internet of Things | 98.98% |
| Explainable Machine Learning for COVID-19 Pneumonia Classification With Texture-Based Features Extraction in Chest Radiography [157] | 2022 | 5222 X-ray | XGBoost (XGB) and Random Forest (RF). | 82% |
| A COVID-19 CXR image recognition method based on MSA-DDCovidNet [158] | 2022 | 5863 X-ray | multi-scale spatial attention mechanism with a convolutional neural network model (MSA-DDCovidNet) | 97.962% |
References
- World Health Organization. Coronavirus Disease 2019 (COVID-19): Situation Report, 73. 2020. Available online: https://apps.who.int/iris/handle/10665/331686 (accessed on 20 August 2021).
- Pokhrel, S.; Chhetri, R. A literature review on impact of COVID-19 pandemic on teaching and learning. High. Educ. Future 2021, 8, 133–141. [Google Scholar] [CrossRef]
- He, H.; Harris, L. The impact of COVID-19 pandemic on corporate social responsibility and marketing philosophy. J. Bus. Res. 2020, 116, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Ataguba, O.A.; Ataguba, J.E. Social determinants of health: The role of effective communication in the COVID-19 pandemic in developing countries. Glob. Health Action 2020, 13, 1788263. [Google Scholar] [CrossRef]
- Al-Hadidi, M.D.R.; AlSaaidah, B.; Al-Gawagzeh, M.Y. Glioblastomas brain tumour segmentation based on convolutional neural networks. Int. J. Electr. Comput. Eng. 2020, 10, 4738–4744. [Google Scholar] [CrossRef]
- Moh’d Rasoul, A.; Al-Gawagzeh, M.Y.; Alsaaidah, B.A. Solving mammography problems of breast cancer detection using artificial neural networks and image processing techniques. Indian J. Sci. Technol. 2012, 5, 2520–2528. [Google Scholar]
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.L. Machine learn- ing for medical imaging. Radiographics 2017, 37, 505–515. [Google Scholar] [CrossRef]
- Currie, G.; Hawk, K.E.; Rohren, E.; Vial, A.; Klein, R. Machine learning and deep learning in medical imaging: Intelligent imaging. J. Med. Imaging Radiat. Sci. 2019, 50, 477–487. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Samuel, A.L. Some studies in machine learning using the game of checkers. IBM J. Res. Dev. 1959, 3, 210–229. [Google Scholar] [CrossRef]
- Oravec, M. Feature extraction and classification by machine learning methods for biometric recognition of face and iris. In Proceedings of the ELMAR-2014, Zadar, Croatia, 10–12 September 2014; pp. 1–4. [Google Scholar]
- Amrane, M.; Oukid, S.; Gagaoua, I.; Ensari, T. Breast cancer classification using machine learning. In Proceedings of the 2018 Electric Electronics, Computer Science, Biomedical Engineerings’ Meeting (EBBT), Istanbul, Turkey, 18–19 April 2018; pp. 1–4. [Google Scholar]
- Dai, X.; Spasić, I.; Meyer, B.; Chapman, S.; Andres, F. Machine learning on mobile: An on-device inference app for skin cancer detection. In Proceedings of the 2019 Fourth International Conference on Fog and Mobile Edge Computing (FMEC), Rome, Italy, 10–13 June 2019; pp. 301–305. [Google Scholar]
- Al-Hadidi, M.R. Handwriting Recognition System Based on OCR. In Contemporary Engineering Sciences; HIKARI Ltd.: Rousse, Bulgaria, 2015; Volume 8, pp. 1399–1412. [Google Scholar]
- Alsaaidah, B.; Alarabeyyat, A.; Al-Hadidi, M.R. Voice Recognition System Using Wavelet Transform and Neural Networks. J. Comput. 2011, 3, 2151–9617. [Google Scholar]
- Mordohai, P.; Medioni, G. Tensor voting: A perceptual organization approach to computer vision and machine learning. In Synthesis Lectures on Image, Video, and Multimedia Processing; Springer: Cham, Switzerland, 2006; Volume 2, pp. 1–136. [Google Scholar]
- Lemley, J.; Bazrafkan, S.; Corcoran, P. Deep Learning for Consumer Devices and Services: Pushing the limits for machine learning, artificial intelligence, and computer vision. IEEE Consum. Electron. Mag. 2017, 6, 48–56. [Google Scholar] [CrossRef]
- Al-Saaidah, B.; Al-Nuaimy, W.; Al-Taee, M.; Al-Ataby, A.; Young, I.; Al-Jubouri, Q. Analysis of Embryonic Malformations in Zebrafish Larvae. In Proceedings of the 2016 9th International Conference on Developments in e Systems Engineering (DeSE), Liverpool, UK, 31 August–2 September 2016; pp. 29–34. [Google Scholar]
- Al-Saaidah, B.; Al-Nuaimy, W.; Al-Taee, M.; Young, I.; Al-Jubouri, Q. Identification of tail curvature malformation in zebrafish embryos. In Proceedings of the 2017 8th International Conference on Information Technology (ICIT), Amman, Jordan, 17–18 May 2017; pp. 588–593. [Google Scholar]
- Al-Saaidah, B.; Al-Nuaimy, W.; Al-Hadidi, M.R.; Young, I. Automatic counting system for zebrafish eggs using optical scanner. In Proceedings of the 2018 9th International Conference on Information and Communication Systems (ICICS), Irbid, Jordan, 3–5 April 2018; pp. 107–110. [Google Scholar]
- AlSaaidah, B.; Al-Nuaimy, W.; Al-Hadidi, M.R.; Young, I. Zebrafish Larvae Classification based on Decision Tree Model: A Comparative Analysis. Adv. Sci. Technol. Eng. Syst. J. 2018, 3, 347–353. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, B.; Warnell, G.; Stone, P. Motion control for mobile robot navigation using machine learning: A survey. arXiv 2020, arXiv:2011.13112. [Google Scholar] [CrossRef]
- Goil, A.; Derry, M.; Argall, B.D. Using machine learning to blend human and robot controls for assisted wheelchair navigation. In Proceedings of the 2013 IEEE 13th International Conference on Rehabilitation Robotics (ICORR), Seattle, WA, USA, 24–26 June 2013; pp. 1–6. [Google Scholar]
- Yates, E.J.; Yates, L.C.; Harvey, H. Machine learning “red dot”: Open-source, cloud, deep convolutional neural networks in chest radiograph binary normality classification. Clin. Radiol. 2018, 73, 827–831. [Google Scholar] [CrossRef] [PubMed]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Jiao, Z.; Hu, P.; Xu, H.; Wang, Q. Machine learning and deep learning in chemical health and safety: A systematic review of techniques and applications. ACS Chem. Health Saf. 2020, 27, 316–334. [Google Scholar] [CrossRef]
- Deng, L.; Yu, D. Deep learning: Methods and applications. Found. Trends Signal Process. 2014, 7, 197–387. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef]
- Ivakhnenko, A.G. Polynomial theory of complex systems. IEEE Trans. Syst. Man Cybern. 1971, SMC-1, 364–378. [Google Scholar] [CrossRef]
- Dechter, R. Learning while searching in constraint-satisfaction problems. In Proceedings of the 5th National Conference on Artificial Intelligence, Philadelphia, PA, USA, 11–15 August 1986; Volume 1. [Google Scholar]
- LeCun, Y.; Bottou, L.; Bengio, Y.; Haffner, P. Gradient-based learning applied to document recognition. Proc. IEEE 1998, 86, 2278–2324. [Google Scholar] [CrossRef]
- Long, W.; Lu, Z.; Cui, L. Deep learning-based feature engineering for stock price movement prediction. Knowl. Based Syst. 2019, 164, 163–173. [Google Scholar] [CrossRef]
- Domingos, P. A few useful things to know about machine learning. Commun. ACM 2012, 55, 78–87. [Google Scholar] [CrossRef]
- ”Patient Page”. ARRT—The American Registry of Radiologic Technologists. Archived from the Original on 9 November 2014. Available online: https://www.arrt.org/Patient-Public/Patient-Page (accessed on 12 July 2021).
- Meng, H.; Xiong, R.; He, R.; Lin, W.; Hao, B.; Zhang, L.; Lu, Z.; Shen, X.; Fan, T.; Jiang, W.; et al. CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. J. Infect. 2020, 81, 33–39. [Google Scholar] [CrossRef] [PubMed]
- AlJame, M.; Ahmad, I.; Imtiaz, A.; Mohammed, A. Ensemble learning model for diagnosing COVID-19 from routine blood tests. Inform Med Unlocked. 2020, 21, 100449. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Tsougenis, E.D.; Ho, J.W.K.; Chan, J.K.Y.; Chiu, K.W.H.; Fang, B.X.H.; Ng, M.Y.; Leung, S.T.; Lo, C.S.Y.; Wong, H.F.; et al. Machine learning application for the prediction of SARS-CoV-2 infection using blood tests and chest radiograph. Sci. Rep. 2021, 11, 14250. [Google Scholar] [CrossRef]
- Abayomi-Alli, O.O.; Damaševičius, R.; Maskeliūnas, R.; Misra, S. An Ensemble Learning Model for COVID-19 Detection from Blood Test Samples. Sensors 2022, 22, 2224. [Google Scholar] [CrossRef]
- Yang, H.S.; Hou, Y.; Vasovic, L.V.; Steel, P.A.; Chadburn, A.; Racine-Brzostek, S.E.; Velu, P.; Cushing, M.M.; Loda, M.; Kaushal, R.; et al. Routine laboratory blood tests predict SARS-CoV-2 infection using machine learning. Clin. Chem. 2020, 66, 1396–1404. [Google Scholar] [CrossRef]
- Alakus, T.B.; Turkoglu, I. Comparison of deep learning approaches to predict COVID-19 infection. Chaos Solitons Fractals 2020, 140, 110120. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, E.; Peng, S.; Chen, Q.; Li, D.; Lian, D. Using artificial intelligence technology to fight COVID-19: A review. Artif. Intell. Rev. 2022, 55, 4941–4977. [Google Scholar] [CrossRef]
- Garg, A.; Salehi, S.; La Rocca, M.; Garner, R.; Duncan, D. Efficient and visualizable convolutional neural networks for COVID-19 classification using Chest CT. Expert Syst. Appl. 2022, 195, 116540. [Google Scholar] [CrossRef]
- Agarwal, V.; Lohani, M.C.; Bist, A.S.; Harahap, E.P.; Khoirunisa, A. Analysis of Deep Learning Techniques for Chest X-ray Classification In Context Of COVID-19. ADI J. Recent Innov. 2022, 3, 208–216. [Google Scholar] [CrossRef]
- Alyasseri, Z.A.A.; Al-Betar, M.A.; Abu Doush, I.; Awadallah, M.A.; Abasi, A.K.; Makhadmeh, S.N.; Alomari, O.A.; Abdulkareem, K.H.; Adam, A.; Damasevicius, R.; et al. Review on COVID-19 diagnosis models based on machine learning and deep learning approaches. Expert Syst. 2021, 39, e12759. [Google Scholar] [CrossRef] [PubMed]
- Yasar, H.; Ceylan, M. A novel comparative study for detection of COVID-19 on CT lung images using texture analysis, machine learning, and deep learning methods. Multimed. Tools Appl. 2021, 80, 5423–5447. [Google Scholar] [CrossRef]
- Wu, Y.H.; Gao, S.H.; Mei, J.; Xu, J.; Fan, D.P.; Zhang, R.G.; Cheng, M.M. Jcs: An explainable COVID-19 diagnosis system by joint classification and segmentation. IEEE Trans. Image Process. 2021, 30, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Tong, L.; Zhu, Y.; Wang, M.D. COVID-19 Automatic Diagnosis with Radiographic Imaging: Explainable AttentionTransfer Deep Neural Networks. IEEE J. Biomed. Health Inform. 2021, 25, 2376–2387. [Google Scholar] [CrossRef] [PubMed]
- Goel, T.; Murugan, R.; Mirjalili, S.; Chakrabartty, D.K. Automatic screening of COVID-19 using an optimized generative adversarial network. Cogn. Comput. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Novelline, R. Squire’s Fundamentals of Radiology, 5th ed.; Harvard University Press: Cambridge, MA, USA, 1997. [Google Scholar]
- Muller, R.A. Physics and Technology for Future Presidents: An Introduction to the Essential Physics Every World Leader Needs to Know; Princeton University Press: Princeton, NJ, USA, 2010. [Google Scholar]
- US National Research Council. Health Risks from Low Levels of Ionizing Radiation, BEIR 7 Phase 2; National Academies Press: Washington, DC, USA, 2006; p. 5.
- Apostolopoulos, I.D.; Mpesiana, T.A. COVID-19: Automatic detection from x- ray images utilizing transfer learning with convolutional neural networks. Phys. Eng. Sci. Med. 2020, 43, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.E.; Rahman, T.; Khandakar, A.; Mazhar, R.; Kadir, M.A.; Mah-bub, Z.B.; Islam, K.R.; Khan, M.S.; Iqbal, A.; Al-Emadi, N.; et al. Can AI help in screening viral and COVID-19 pneumonia? arXiv 2020, arXiv:2003.13145. [Google Scholar] [CrossRef]
- Alom, M.Z.; Rahman, M.M.; Nasrin, M.S.; Taha, T.M.; Asari, V.K. COVIDMTNet: COVID–19 Detection with Multitask Deep Learning Approaches. arXiv 2020, arXiv:2004.03747. [Google Scholar]
- Basu, S.; Mitra, S. Deep Learning for Screening COVID-19 using Chest X-ray Images. arXiv 2020, arXiv:2004.10507. [Google Scholar]
- Brunese, L.; Mercaldo, F.; Reginelli, A.; Santone, A. Explainable Deep Learning for Pulmonary Disease and Coronavirus COVID-19 Detection from X-rays. Comput. Methods Programs Biomed. 2020, 196, 105608. [Google Scholar] [CrossRef] [PubMed]
- Tabik, S.; Gómez-Ríos, A.; Martin-Rodriguez, J.; Sevillano-Garcia, I.; Rey-Area, M.; Charte, D.; Guirado, E.; Suarez, J.; Luengo, J.; Valero-Gonzalez, M.; et al. COVIDGR dataset and COVID-SDNet methodology for predicting COVID-19 based on Chest X-ray images. arXiv 2020, arXiv:2006.01409. [Google Scholar] [CrossRef] [PubMed]
- Mobiny, A.; Cicalese, P.A.; Zare, S.; Yuan, P.; Abavisani, M.; Wu, C.C.; Ahuja, J.; de Groot, P.M.; Van Nguyen, H. Radiologist-Level COVID-19 Detection Using CT scans with Detail-Oriented Capsule Networks. arXiv 2020, arXiv:2004.07407. [Google Scholar]
- Afshar, P.; Heidarian, S.; Naderkhani, F.; Oikonomou, A.; Plataniotis, K.N.; Mohammadi, A. Covid-caps: A capsule network-based framework for identification of COVID-19 cases from X-ray images. arXiv 2020, arXiv:2004.02696. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Hafeez, A. Covid-resnet: A deep learning framework for screening of COVID-19 from radiographs. arXiv 2020, arXiv:2003.14395. [Google Scholar]
- Hemdan, E.E.; Shouman, M.A.; Karar, M.E. Covidx-net: A framework of deep learning classifiers to diagnose COVID-19 in X-ray images. arXiv 2020, arXiv:2003.11055. [Google Scholar]
- IEEE8023/Covid-Chestxray-Dataset. 2021. Available online: https://github.com/ieee8023/COVID-chestxray-dataset (accessed on 4 September 2021).
- Elpeltagy, M.; Sallam, H. Automatic prediction of COVID-19 from chest images using modified ResNet50. Multimed. Tools Appl. 2021, 80, 26451–26463. [Google Scholar] [CrossRef]
- Yoo, S.H.; Geng, H.; Chiu, T.L.; Yu, S.K.; Cho, D.C.; Heo, J.; Choi, M.S.; Choi, I.H.; Cung Van, C.; Nhung, N.V.; et al. Deep learning-based decision-tree classifier for COVID-19 diagnosis from chest X-ray imaging. Front. Med. 2020, 7, 427. [Google Scholar] [CrossRef]
- Ahuja, S.; Panigrahi, B.K.; Dey, N.; Rajinikanth, V.; Gandhi, T.K. Deep transfer learning-based automated detection of COVID-19 from lung CT scan slices. Appl. Intell. 2021, 51, 571–585. [Google Scholar] [CrossRef]
- Nishio, M.; Noguchi, S.; Matsuo, H.; Murakami, T. Automatic classification between COVID-19 pneumonia, non-COVID-19 pneumonia, and the healthy on chest X-ray image: Combination of data augmentation methods. Sci. Rep. 2020, 10, 147532. [Google Scholar] [CrossRef]
- Xin Tie, B.S.; Zhang, C.; Song, T.K.; Nadig, J.D.; Schiebler, M.L. Diagnosis of COVID-19 Pneumonia Using Chest Radiography: Value of Artificial Intelligence. Radiology 2020, 298, E88–E97. [Google Scholar]
- Waheed, A.; Goyal, M.; Gupta, D.; Khanna, A.; Al-Turjman, F.; Pin-heiro, P.R. Covidgan: Data augmentation using auxiliary classifier gan for improved COVID-19 detection. IEEE Access 2020, 8, 91916–91923. [Google Scholar] [CrossRef]
- Engstrom, L.; Tran, B.; Tsipras, D.; Schmidt, L.; Madry, A. A rotation and a translation suffice: Fooling CNNs with simple transformations. In Proceedings of the International Conference on Learning Representations, New Orleans, LA, USA, 6–9 May 2019. [Google Scholar]
- Goodfellow, I.; Pouget-Abadie, J.; Mirza, M.; Xu, B.; Warde-Farley, D.; Ozair, S.; Bengio, Y. Generative adversarial networks. Commun. ACM 2020, 63, 139–144. [Google Scholar] [CrossRef]
- Keshani, M.; Azimifar, Z.; Tajeripour, F.; Boostani, R. Lung nodule segmentation and recognition using SVM classifier and active contour modeling: A complete intelligent system. Comput. Biol. Med. 2013, 43, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; An, X.; Ge, C.; Yu, Z.; Chen, J.; Zhu, Q.; Dong, G.; He, J.; He, Z.; et al. Toward data-efficient learning: A benchmark for COVID-19 CT lung and infection segmentation. Med. Phys. 2021, 48, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.P.; Zhou, T.; Ji, G.P.; Zhou, Y.; Chen, G.; Fu, H.; Shen, J.; Shao, L. Inf-net: Automatic COVID-19 lung infection segmentation from ct images. IEEE Trans. Med. Imaging 2020, 39, 2626–2637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, Z.; Chen, Y.; Zhao, W.; Xie, X.; Liu, J.; Zhao, D.; Li, Y. SCOAT-Net: A novel network for segmenting COVID-19 lung opacification from CT images. Pattern Recognit. 2021, 119, 108109. [Google Scholar] [CrossRef]
- Shan, F.; Gao, Y.; Wang, J.; Shi, W.; Shi, N.; Han, M.; Xue, Z.; Shen, D.; Shi, Y. Lung infection quantification of COVID-19 in CT images with deep learning. arXiv 2020, arXiv:2003.04655. [Google Scholar]
- Ranjbarzadeh, R.; Jafarzadeh Ghoushchi, S.; Bendechache, M.; Amirabadi, A.; Ab Rahman, M.N.; Baseri Saadi, S.; Aghamohammadi, A.; Kooshki Forooshani, M. Lung infection segmentation for COVID-19 pneumonia based on a cascade convolutional network from CT images. BioMed. Res. Int. 2021, 2021, 5544742. [Google Scholar] [CrossRef]
- Teixeira, L.O.; Pereira, R.M.; Bertolini, D.; Oliveira, L.S.; Nanni, L.; Cavalcanti, G.D.; Costa, Y.M. Impact of lung segmentation on the diagnosis and explanation of COVID-19 in chest X-ray images. Sensors 2021, 21, 7116. [Google Scholar] [CrossRef]
- Mahmud, T.; Rahman, M.A.; Fattah, S.A. CovXNet: A multi-dilation convolutional neural network for automatic COVID-19 and other pneumonia detection from chest X-ray images with transferable multi-receptive feature optimization. Comput. Biol. Med. 2020, 122, 103869. [Google Scholar] [CrossRef] [PubMed]
- López-Úbeda, P.; Díaz-Galiano, M.C.; Martín-Noguerol, T.; Luna, A.; Ureña-López, L.A.; Martín-Valdivia, M.T. COVID-19 detection in radiological text reports integrating entity recognition. Comput. Biol. Med. 2020, 127, 104066. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D.M.; Elshennawy, N.M.; Sarhan, A.M. Deep-chest: Multi-classification deep learning model for diagnosing COVID-19, pneumonia, and lung cancer chest diseases. Comput. Biol. Med. 2021, 132, 104348. [Google Scholar] [CrossRef] [PubMed]
- Bakheet, S.; Al-Hamadi, A. Automatic detection of COVID-19 using pruned GLCM-Based texture features and LDCRF classification. Comput. Biol. Med. 2021, 137, 104781. [Google Scholar] [CrossRef]
- Hassantabar, S.; Ahmadi, M.; Sharifi, A. Diagnosis and detection of infected tissue of COVID-19 patients based on lung X-ray image using convolutional neural network approaches. Chaos Solitons Fractals 2020, 140, 110170. [Google Scholar] [CrossRef] [PubMed]
- Ismael, A.M.; Şengür, A. Deep learning approaches for COVID-19 detection based on chest X-ray images. Expert Syst. Appl. 2021, 164, 114054. [Google Scholar] [CrossRef]
- Lella, K.K.; Pja, A. Automatic diagnosis of COVID-19 disease using deep convolutional neural network with multi-feature channel from respiratory sound data: Cough, voice, and breath. Alex. Eng. J. 2022, 61, 1319–1334. [Google Scholar] [CrossRef]
- Pahar, M.; Klopper, M.; Warren, R.; Niesler, T. COVID-19 cough classification using machine learning and global smartphone recordings. Comput. Biol. Med. 2021, 135, 104572. [Google Scholar] [CrossRef]
- Islam, M.Z.; Islam, M.M.; Asraf, A. A combined deep CNN-LSTM network for the detection of novel coronavirus (COVID-19) using X-ray images. Inform. Med. Unlocked 2020, 20, 100412. [Google Scholar] [CrossRef]
- Dhaya, R. Deep net model for detection of COVID-19 using radiographs based on roc analysis. J. Innov. Image Process. 2020, 2, 135–140. [Google Scholar]
- Sethy, P.K.; Behera, S.K. Detection of Coronavirus Disease (COVID-19) Based on Deep Features. Preprints 2020, 2020030300. [Google Scholar] [CrossRef]
- Zhou, T.; Canu, S.; Ruan, S. An automatic COVID-19 CT segmentation based on U-Net with attention mechanism. arXiv 2020, arXiv:2004.06673. [Google Scholar]
- Tilborghs, S.; Dirks, I.; Fidon, L.; Willems, S.; Eelbode, T.; Bertels, J.; Ilsen, B.; Brys, A.; Dubbeldam, A.; Buls, N.; et al. Comparative study of deep learning methods for the automatic segmentation of lung, lesion and lesion type in CT scans of COVID-19 patients. arXiv 2020, arXiv:2007.15546. [Google Scholar]
- Alirr, O.I. Automatic deep learning system for COVID-19 infection quantification in chest CT. Multimed. Tools Appl. 2022, 81, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Naz, S.; Khan, A.; Zaib, A.; Razzak, I. Improving coronavirus (COVID-19) diagnosis using deep transfer learning. MedRxiv 2020. [Google Scholar] [CrossRef]
- De Moura, J.; Novo, J.; Ortega, M. Fully automatic deep convolutional approaches for the analysis of COVID-19 using chest X-ray images. Appl. Soft Comput. 2022, 115, 108190. [Google Scholar] [CrossRef]
- Asif, S.; Wenhui, Y.; Jin, H.; Jinhai, S. December. Classification of COVID-19 from chest X-ray images using deep convolutional neural network. In Proceedings of the 2020 IEEE 6th International Conference on Computer and Communications (ICCC), Chengdu, China, 11–14 December 2020; pp. 426–433. [Google Scholar]
- Medhi, K.; Jamil, M.; Hussain, M.I. Automatic detection of COVID-19 infection from chest X-ray using deep learning. Medrxiv 2020. [Google Scholar] [CrossRef]
- Jim, A.A.J.; Rafi, I.; Chowdhury, M.S.; Sikder, N.; Mahmud, M.P.; Rubaiee, S.; Masud, M.; Bairagi, A.K.; Bhakta, K.; Nahid, A.A. An automatic computer-based method for fast and accurate COVID-19 diagnosis. medRxiv 2020. [Google Scholar] [CrossRef]
- Hasan, M.K.; Alam, M.A.; Dahal, L.; Elahi, M.T.E.; Roy, S.; Wahid, S.R.; Martí, R.; Khanal, B. Challenges of deep learning methods for COVID-19 detection using public datasets. medRxiv 2020. [Google Scholar] [CrossRef]
- Asif, S.; Amjad, K. Automatic COVID-19 Detection from chest radiographic images using Convolutional Neural Network. medRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, W.; Pogorelsky, B.; Loveland, M.; Wolf, T. Classification of COVID-19 X-ray images using a combination of deep and handcrafted features. arXiv 2021, arXiv:2101.07866. [Google Scholar]
- Mamalakis, M.; Swift, A.J.; Vorselaars, B.; Ray, S.; Weeks, S.; Ding, W.; Clayton, R.H.; Mackenzie, L.S.; Banerjee, A. DenResCov-19: A deep transfer learning network for robust automatic classification of COVID-19, pneumonia, and tuberculosis from X-rays. Comput. Med. Imaging Graph. 2021, 94, 102008. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, N.; Shoeibi, A.; Khodatars, M.; Heras, J.; Rahimi, A.; Zare, A.; Pachori, R.B.; Gorriz, J.M. Automatic diagnosis of COVID-19 from ct images using cyclegan and transfer learning. arXiv 2021, arXiv:2104.11949. [Google Scholar]
- Chakkaravarthy, A.P.; Pugalenthi, R.; Ramya, J.; Dhanalakshmi, J. An Automatic Classification of COVID with J48 and Simple K-Means using Weka. Int. J. Future Gener. Commun. Netw. 2020, 13, 490–500. [Google Scholar]
- Apostolopoulos, I.D.; Aznaouridis, S.I.; Tzani, M.A. Extracting possibly representative COVID-19 biomarkers from X-ray images with deep learning approach and image data related to pulmonary diseases. J. Med. Biol. Eng. 2020, 40, 462–469. [Google Scholar] [CrossRef]
- Mahdy, L.N.; Ezzat, K.A.; Elmousalami, H.H.; Ella, H.A.; Hassanien, A.E. Automatic X-ray COVID-19 lung image classification system based on multi-level thresholding and support vector machine. MedRxiv 2020. [Google Scholar] [CrossRef]
- Jaiswal, A.; Gianchandani, N.; Singh, D.; Kumar, V.; Kaur, M. Classification of the COVID-19 infected patients using DenseNet201 based deep transfer learning. J. Biomol. Struct. Dyn. 2021, 39, 5682–5689. [Google Scholar] [CrossRef]
- Dhiman, G.; Chang, V.; Kant Singh, K.; Shankar, A. Adopt: Automatic deep learning and optimization-based approach for detection of novel coronavirus COVID-19 disease using X-ray images. J. Biomol. Struct. Dyn. 2021, 40, 5836–5847. [Google Scholar] [CrossRef]
- Castiglione, A.; Vijayakumar, P.; Nappi, M.; Sadiq, S.; Umer, M. COVID-19: Automatic detection of the novel coronavirus disease from ct images using an optimized convolutional neural network. IEEE Trans. Ind. Inform. 2021, 17, 6480–6488. [Google Scholar] [CrossRef]
- Pandit, M.K.; Banday, S.A.; Naaz, R.; Chishti, M.A. Automatic detection of COVID-19 from chest radiographs using deep learning. Radiography 2021, 27, 483–489. [Google Scholar] [CrossRef]
- Saha, P.; Sadi, M.S.; Islam, M.M. EMCNet: Automated COVID-19 diagnosis from X-ray images using convolutional neural network and ensemble of machine learning classifiers. Inform. Med. Unlocked 2021, 22, 100505. [Google Scholar] [CrossRef]
- Rahmatizadeh, S.; Valizadeh-Haghi, S.; Dabbagh, A. The role of artificial intelligence in management of critical COVID-19 patients. J. Cell. Mol. Anesth. 2020, 5, 16–22. [Google Scholar]
- Maguolo, G.; Nanni, L. A critic evaluation of methods for COVID-19 automatic detection from X-ray images. Inf. Fusion 2021, 76, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.C.; Barbosa, V.A.D.F.; Santana, M.A.; Bandeira, J.; Valença, M.J.S.; de Souza, R.E.; Ismael, A.M.; dos Santos, W.P. IKONOS: An intelligent tool to support diagnosis of COVID-19 by texture analysis of X-ray images. Res. Biomed. Eng. 2020, 38, 15–28. [Google Scholar] [CrossRef]
- Hira, S.; Bai, A.; Hira, S. An automatic approach based on CNN architecture to detect Covid-19 disease from chest X-ray images. Appl. Intell. 2021, 51, 2864–2889. [Google Scholar] [CrossRef] [PubMed]
- Barua, P.D.; Muhammad Gowdh, N.F.; Rahmat, K.; Ramli, N.; Ng, W.L.; Chan, W.Y.; Kuluozturk, M.; Dogan, S.; Baygin, M.; Yaman, O.; et al. Automatic COVID-19 detection using exemplar hybrid deep features with X-ray images. Int. J. Environ. Res. Public Health 2021, 18, 8052. [Google Scholar] [CrossRef]
- Selvaraj, D.; Venkatesan, A.; Mahesh, V.G.; Joseph Raj, A.N. An integrated feature frame work for automated segmentation of COVID-19 infection from lung CT images. Int. J. Imaging Syst. Technol. 2021, 31, 28–46. [Google Scholar] [CrossRef]
- Zhou, T.; Canu, S.; Ruan, S. Automatic COVID-19 CT segmentation using U-Net integrated spatial and channel attention mechanism. Int. J. Imaging Syst. Technol. 2021, 31, 16–27. [Google Scholar] [CrossRef]
- Mohammadi, R.; Salehi, M.; Ghaffari, H.; Rohani, A.A.; Reiazi, R. Transfer learning-based automatic detection of coronavirus disease 2019 (COVID-19) from chest X-ray images. J. Biomed. Phys. Eng. 2020, 10, 559. [Google Scholar] [CrossRef]
- Emara, H.M.; Shoaib, M.R.; Elwekeil, M.; El-Shafai, W.; Taha, T.E.; El-Fishawy, A.S.; El-Rabaie, E.S.M.; Alshebeili, S.A.; Dessouky, M.I.; Abd El-Samie, F.E. Deep convolutional neural networks for COVID-19 automatic diagnosis. Microsc. Res. Technol. 2021, 84, 2504–2516. [Google Scholar] [CrossRef]
- Albadr, M.A.A.; Tiun, S.; Ayob, M.; Al-Dhief, F.T.; Omar, K.; Hamzah, F.A. Optimised genetic algorithm-extreme learning machine approach for automatic COVID-19 detection. PLoS ONE 2020, 15, e0242899. [Google Scholar] [CrossRef] [PubMed]
- Lorencin, I.; Baressi Šegota, S.; Anđelić, N.; Blagojević, A.; Šušteršić, T.; Protić, A.; Arsenijević, M.; Ćabov, T.; Filipović, N.; Car, Z. Automatic evaluation of the lung condition of COVID-19 patients using X-ray images and convolutional neural networks. J. Pers. Med. 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Karar, M.E.; Hemdan, E.E.D.; Shouman, M.A. Cascaded deep learning classifiers for computer-aided diagnosis of COVID-19 and pneumonia diseases in X-ray scans. Complex Intell. Syst. 2021, 7, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Kc, K.; Yin, Z.; Wu, M.; Wu, Z. Evaluation of deep learning-based approaches for COVID-19 classification based on chest X-ray images. Signal Image Video Process. 2021, 15, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, G.; Tao, Y.; Zhai, P.; Chen, H.; He, H.; Cai, T. Multi-task contrastive learning for automatic CT and X-ray diagnosis of COVID-19. Pattern Recognit. 2021, 114, 107848. [Google Scholar] [CrossRef] [PubMed]
- Amyar, A.; Modzelewski, R.; Li, H.; Ruan, S. Multi-task deep learning based CT imaging analysis for COVID-19 pneumonia: Classification and segmentation. Comput. Biol. Med. 2020, 126, 104037. [Google Scholar] [CrossRef] [PubMed]
- Narin, A.; Kaya, C.; Pamuk, Z. Automatic detection of coronavirus disease (COVID-19) using X-ray images and deep convolutional neural networks. Pattern Anal. Appl. 2021, 24, 1207–1220. [Google Scholar] [CrossRef]
- Benbrahim, H.; Hachimi, H.; Amine, A. Deep transfer learning with apache spark to detect COVID-19 in chest X-ray images. Rom. J. Inf. Sci. Technol. 2020, 23, S117–S129. [Google Scholar]
- Irmak, E. Implementation of convolutional neural network approach for COVID-19 disease detection. Physiol. Genom. 2020, 52, 590–601. [Google Scholar] [CrossRef]
- Farid, A.A.; Selim, G.I.; Khater, H.A.A. A novel approach of CT images feature analysis and prediction to screen for corona virus disease (COVID-19). Int. J. Sci. Eng. Res. 2020, 11, 1141–1149. [Google Scholar] [CrossRef]
- Sarker, S.; Tan, L.; Wen Jie, M.; Shan Shan, R.; Ali, M.A.; Bilal, M.; Qiu, Z.; Kumar Mondal, S.; Tiwari, P. Automatic Classification Approach for Detecting COVID-19 using Deep Convolutional Neural Networks. Preprints 2020, 2020090524. [Google Scholar] [CrossRef]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Artificial intelligence distinguishes COVID-19 from community acquired pneumonia on chest CT. Radiology 2020, 296, E65–E71. [Google Scholar] [CrossRef]
- Rasheed, J.; Hameed, A.A.; Djeddi, C.; Jamil, A.; Al-Turjman, F. A machine learning-based framework for diagnosis of COVID-19 from chest X-ray images. Interdiscip. Sci. Comput. Life Sci. 2021, 13, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.; Narasimhan, H.; Chakraborty, C.; Pham, T.D. Deep learning-based meta-classifier approach for COVID-19 classification using CT scan and chest X-ray images. Multimed. Syst. 2021, 28, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, X.; Sun, H.; Wang, J.; Zhao, T.; Chen, H.; Ma, Y.; Zhu, S.; Xie, Z. Rapid identification of COVID-19 severity in CT scans through classification of deep features. BioMed. Eng. Online 2020, 19, 63. [Google Scholar] [CrossRef]
- Hussain, L.; Nguyen, T.; Li, H.; Abbasi, A.A.; Lone, K.J.; Zhao, Z.; Zaib, M.; Chen, A.; Duong, T.Q. Machine-learning classification of texture features of portable chest X-ray accurately classifies COVID-19 lung infection. BioMed. Eng. Online 2020, 19, 88. [Google Scholar] [CrossRef]
- Tan, H.B.; Xiong, F.; Jiang, Y.L.; Huang, W.C.; Wang, Y.; Li, H.H.; You, T.; Fu, T.T.; Lu, R.; Peng, B.W. The study of automatic machine learning base on radiomics of non-focus area in the first chest CT of different clinical types of COVID-19 pneumonia. Sci. Rep. 2020, 10, 18926. [Google Scholar] [CrossRef]
- Shankar, K.; Perumal, E. A novel hand-crafted with deep learning features based fusion model for COVID-19 diagnosis and classification using chest X-ray images. Complex Intell. Syst. 2021, 7, 1277–1293. [Google Scholar] [CrossRef]
- Singh, D.; Kumar, V.; Kaur, M. Classification of COVID-19 patients from chest CT images using multi-objective differential evolution–based convolutional neural networks. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Kumar, V.; Kaur, M. Densely connected convolutional networks-based COVID-19 screening model. Appl. Intell. 2021, 51, 3044–3051. [Google Scholar] [CrossRef]
- Song, J.; Wang, H.; Liu, Y.; Wu, W.; Dai, G.; Wu, Z.; Zhu, P.; Zhang, W.; Yeom, K.W.; Deng, K. End-to-end automatic differentiation of the coronavirus disease 2019 (COVID-19) from viral pneumonia based on chest CT. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2516–2524. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yu, K.; Bashir, A.K.; Cheng, X.; Ming, F.; Zhao, L.; Zhou, X. Toward real-time and efficient cardiovascular monitoring for COVID-19 patients by 5G-enabled wearable medical devices: A deep learning approach. Neural Comput. Appl. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Loey, M.; Smarandache, F.; MKhalifa, N.E. Within the lack of chest COVID-19 X-ray dataset: A novel detection model based on GAN and deep transfer learning. Symmetry 2020, 12, 651. [Google Scholar] [CrossRef]
- Ohata, E.F.; Bezerra, G.M.; das Chagas, J.V.S.; Neto, A.V.L.; Albuquerque, A.B.; de Albuquerque, V.H.C.; Reboucas Filho, P.P. Automatic detection of COVID-19 infection using chest X-ray images through transfer learning. IEEE/CAA J. Autom. Sin. 2020, 8, 239–248. [Google Scholar] [CrossRef]
- Oulefki, A.; Agaian, S.; Trongtirakul, T.; Laouar, A.K. Automatic COVID-19 lung infected region segmentation and measurement using CT-scans images. Pattern Recognit. 2021, 114, 107747. [Google Scholar] [CrossRef]
- Haque, K.F.; Haque, F.F.; Gandy, L.; Abdelgawad, A. Automatic detection of COVID-19 from chest X-ray images with convolutional neural networks. In Proceedings of the 2020 International Conference on Computing, Electronics & Communications Engineering (iCCECE), Southend, UK, 17–18 August 2020; pp. 125–130. [Google Scholar]
- Konar, D.; Panigrahi, B.K.; Bhattacharyya, S.; Dey, N.; Jiang, R. Auto-diagnosis of COVID-19 using lung CT images with semi-supervised shallow learning network. IEEE Access 2021, 9, 28716–28728. [Google Scholar] [CrossRef]
- Joshi, R.C.; Yadav, S.; Pathak, V.K.; Malhotra, H.S.; Khokhar, H.V.S.; Parihar, A.; Kohli, N.; Himanshu, D.; Garg, R.K.; Bhatt, M.L.B.; et al. A deep learning-based COVID-19 automatic diagnostic framework using chest X-ray images. Biocybern. Biomed. Eng. 2021, 41, 239–254. [Google Scholar] [CrossRef]
- Martínez, F.; Martínez, F.; Jacinto, E. Performance evaluation of the NASNet convolutional network in the automatic identification of COVID-19. Int. J. Adv. Sci. Eng. Inf. Technol. 2020, 10, 662. [Google Scholar] [CrossRef]
- Wang, X.; Deng, X.; Fu, Q.; Zhou, Q.; Feng, J.; Ma, H.; Liu, W.; Zheng, C. A weakly-supervised framework for COVID-19 classification and lesion localization from chest CT. IEEE Trans. Med. Imaging 2020, 39, 2615–2625. [Google Scholar] [CrossRef]
- Abbas, A.; Abdelsamea, M.M.; Gaber, M.M. Classification of COVID-19 in chest X-ray images using DeTraC deep convolutional neural network. Appl. Intell. 2021, 51, 854–864. [Google Scholar] [CrossRef]
- Bharadwaj, K.S.S.; Pawar, V.; Punia, V.; Apparao, M.L.V.; Mahajan, A. Novel artificial intelligence algorithm for automatic detection of COVID-19 abnormalities in computed tomography images. Cancer Res. Stat. Treat. 2021, 4, 256. [Google Scholar]
- Al-Bawi, A.; Al-Kaabi, K.; Jeryo, M.; Al-Fatlawi, A. CCBlock: An effective use of deep learning for automatic diagnosis of COVID-19 using X-ray images. Res. Biomed. Eng. 2020, 38, 49–58. [Google Scholar] [CrossRef]
- Pustokhin, D.A.; Pustokhina, I.V.; Dinh, P.N.; Phan, S.V.; Nguyen, G.N.; Joshi, G.P. An effective deep residual network based class attention layer with bidirectional LSTM for diagnosis and classification of COVID-19. J. Appl. Stat. 2020. [Google Scholar] [CrossRef]
- Ozturk, T.; Talo, M.; Yildirim, E.A.; Baloglu, U.B.; Yildirim, O.; Acharya, U.R. Automated detection of COVID-19 cases using deep neural networks with X-ray images. Comput. Biol. Med. 2020, 121, 103792. [Google Scholar] [CrossRef] [PubMed]
- Zaffino, P.; Marzullo, A.; Moccia, S.; Calimeri, F.; De Momi, E.; Bertucci, B.; Arcuri, P.P.; Spadea, M.F. An open-source COVID-19 ct dataset with automatic lung tissue classification for radiomics. Bioengineering 2021, 8, 26. [Google Scholar] [CrossRef]
- Hu, Q.; Gois, F.N.B.; Costa, R.; Zhang, L.; Yin, L.; Magaia, N.; de Albuquerque, V.H.C. Explainable artificial intelligence-based edge fuzzy images for COVID-19 detection and identification. Appl. Soft Comput. 2022, 123, 108966. [Google Scholar] [CrossRef]
- Kini, A.S.; Gopal Reddy, A.N.; Kaur, M.; Satheesh, S.; Singh, J.; Martinetz, T.; Alshazly, H. Ensemble deep learning and internet of things-based automated COVID-19 diagnosis framework. Contrast Media Mol. Imaging 2022, 10, 7377502. [Google Scholar] [CrossRef]
- De Moura, L.V.; Mattjie, C.; Dartora, C.M.; Barros, R.C.; da Silva, A.M.M. Explainable Machine Learning for COVID-19 Pneumonia Classification with Texture-Based Features Extraction in Chest Radiography. Front. Digit. Health 2021, 3, 662343. [Google Scholar] [CrossRef]
- Wang, W.; Huang, W.; Wang, X.; Zhang, P.; Zhang, N. A COVID-19 CXR image recognition method based on MSA-DDCovidNet. IET Image Process. 2022, 16, 2101–2113. [Google Scholar] [CrossRef]

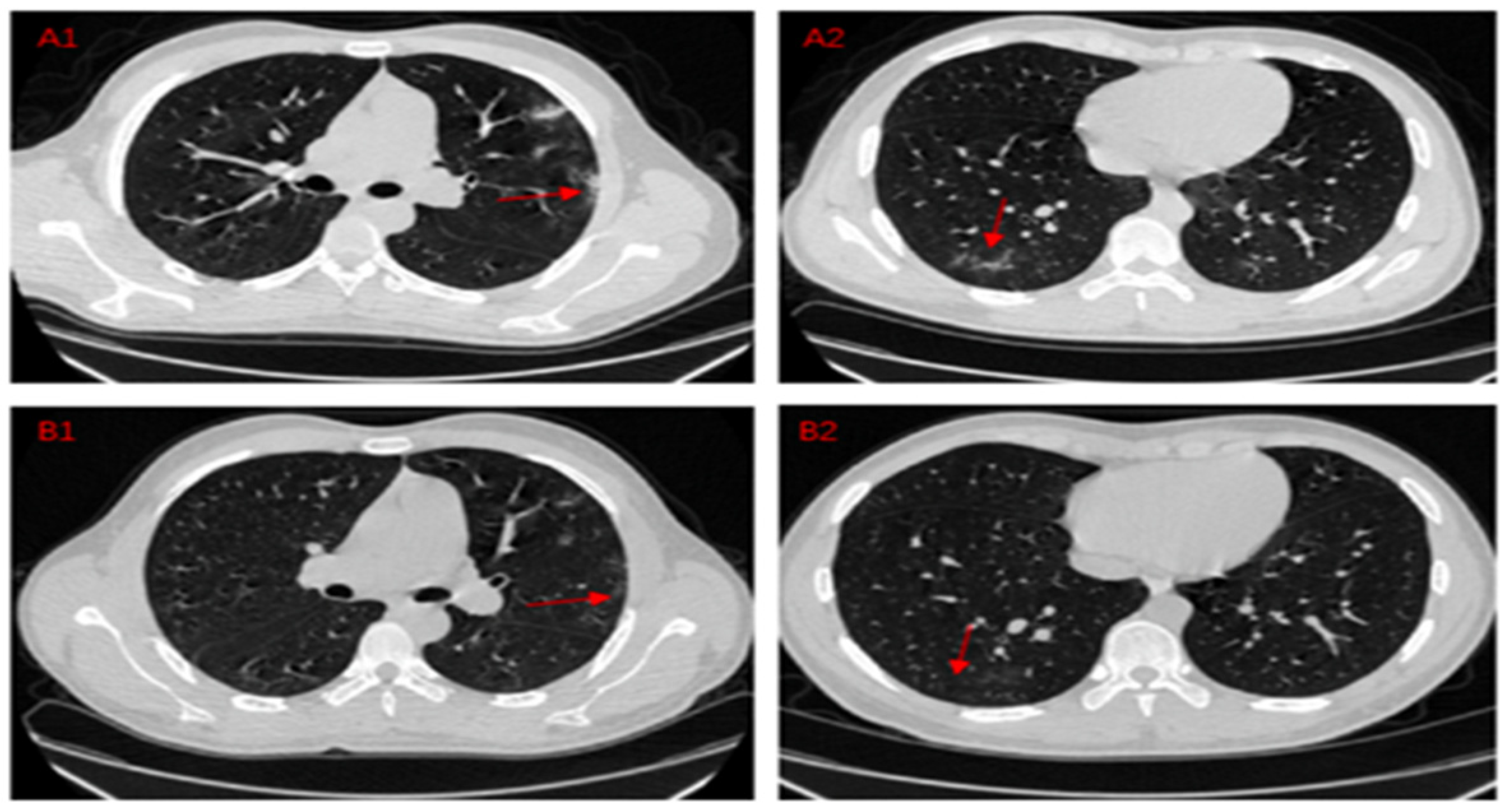
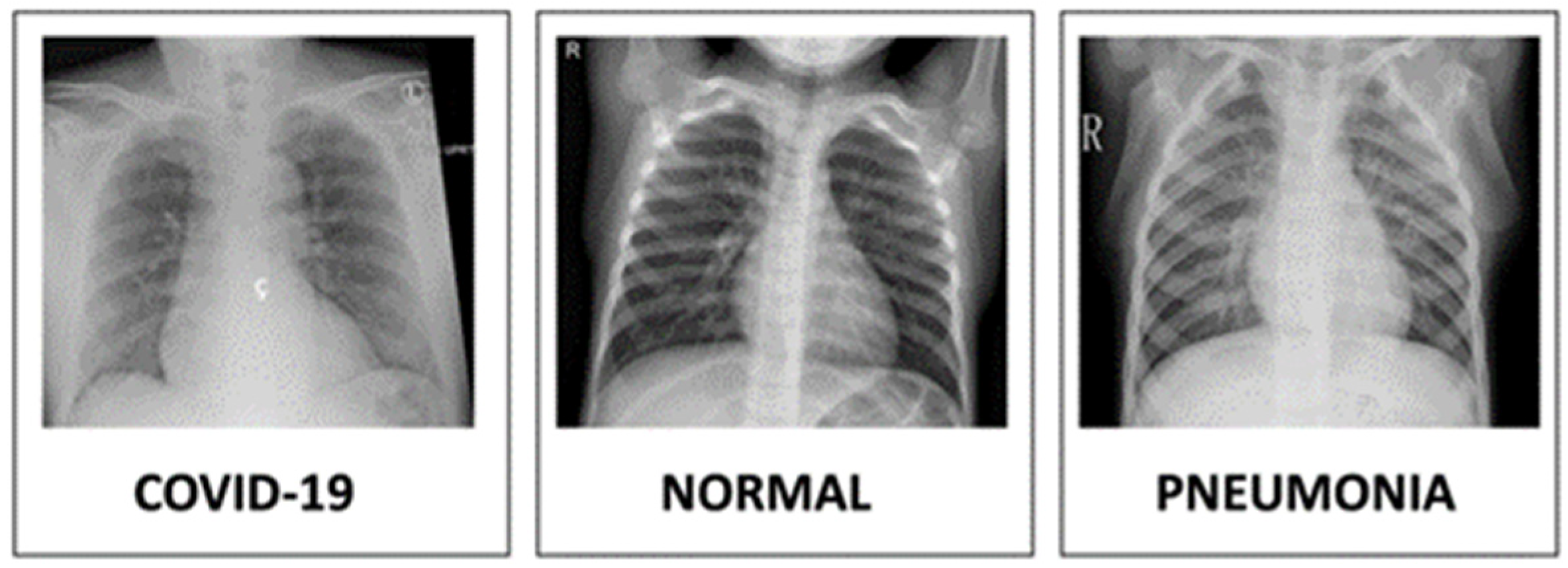

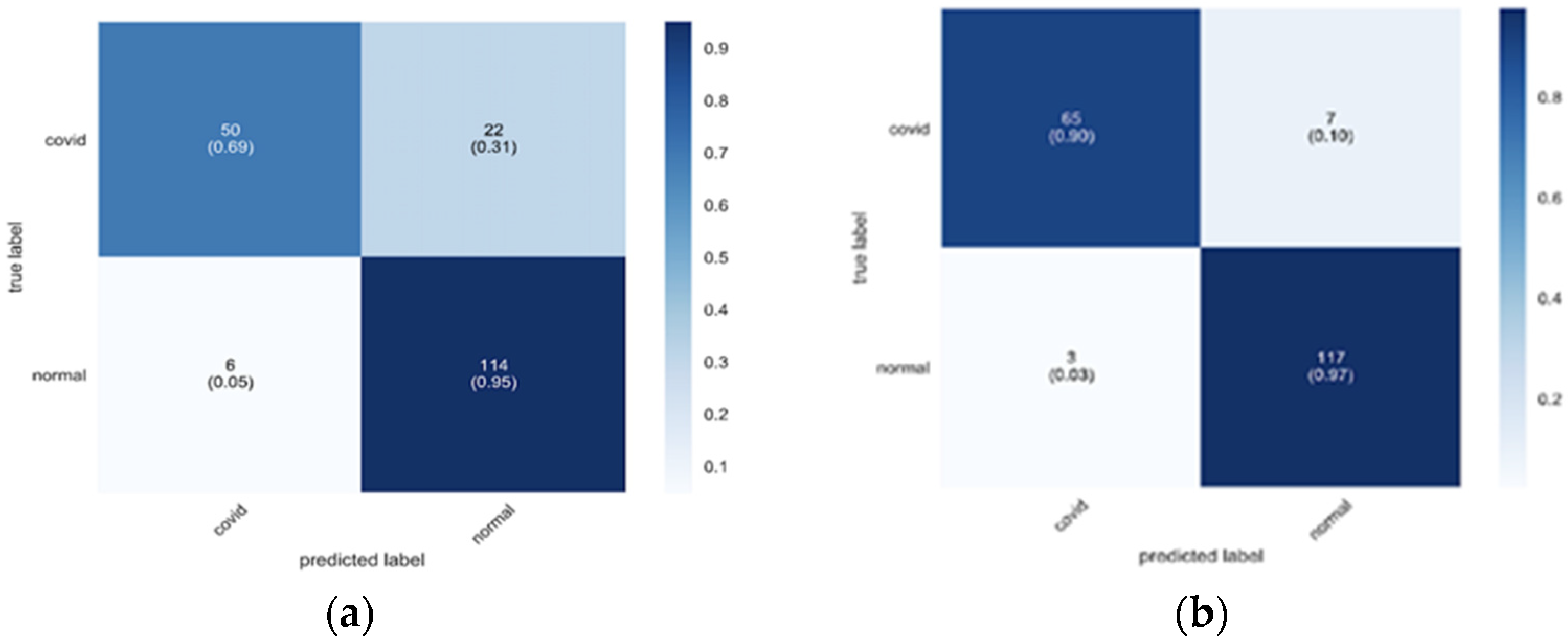



| Method | Setting |
|---|---|
| Rotation angle | 10 |
| Width shift | 0.2 |
| Height shift | 0.2 |
| Horizontal flip | True |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaaidah, B.; Al-Hadidi, M.R.; Al-Nsour, H.; Masadeh, R.; AlZubi, N. Comprehensive Survey of Machine Learning Systems for COVID-19 Detection. J. Imaging 2022, 8, 267. https://doi.org/10.3390/jimaging8100267
Alsaaidah B, Al-Hadidi MR, Al-Nsour H, Masadeh R, AlZubi N. Comprehensive Survey of Machine Learning Systems for COVID-19 Detection. Journal of Imaging. 2022; 8(10):267. https://doi.org/10.3390/jimaging8100267
Chicago/Turabian StyleAlsaaidah, Bayan, Moh’d Rasoul Al-Hadidi, Heba Al-Nsour, Raja Masadeh, and Nael AlZubi. 2022. "Comprehensive Survey of Machine Learning Systems for COVID-19 Detection" Journal of Imaging 8, no. 10: 267. https://doi.org/10.3390/jimaging8100267
APA StyleAlsaaidah, B., Al-Hadidi, M. R., Al-Nsour, H., Masadeh, R., & AlZubi, N. (2022). Comprehensive Survey of Machine Learning Systems for COVID-19 Detection. Journal of Imaging, 8(10), 267. https://doi.org/10.3390/jimaging8100267






