Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications
Abstract
:1. Introduction
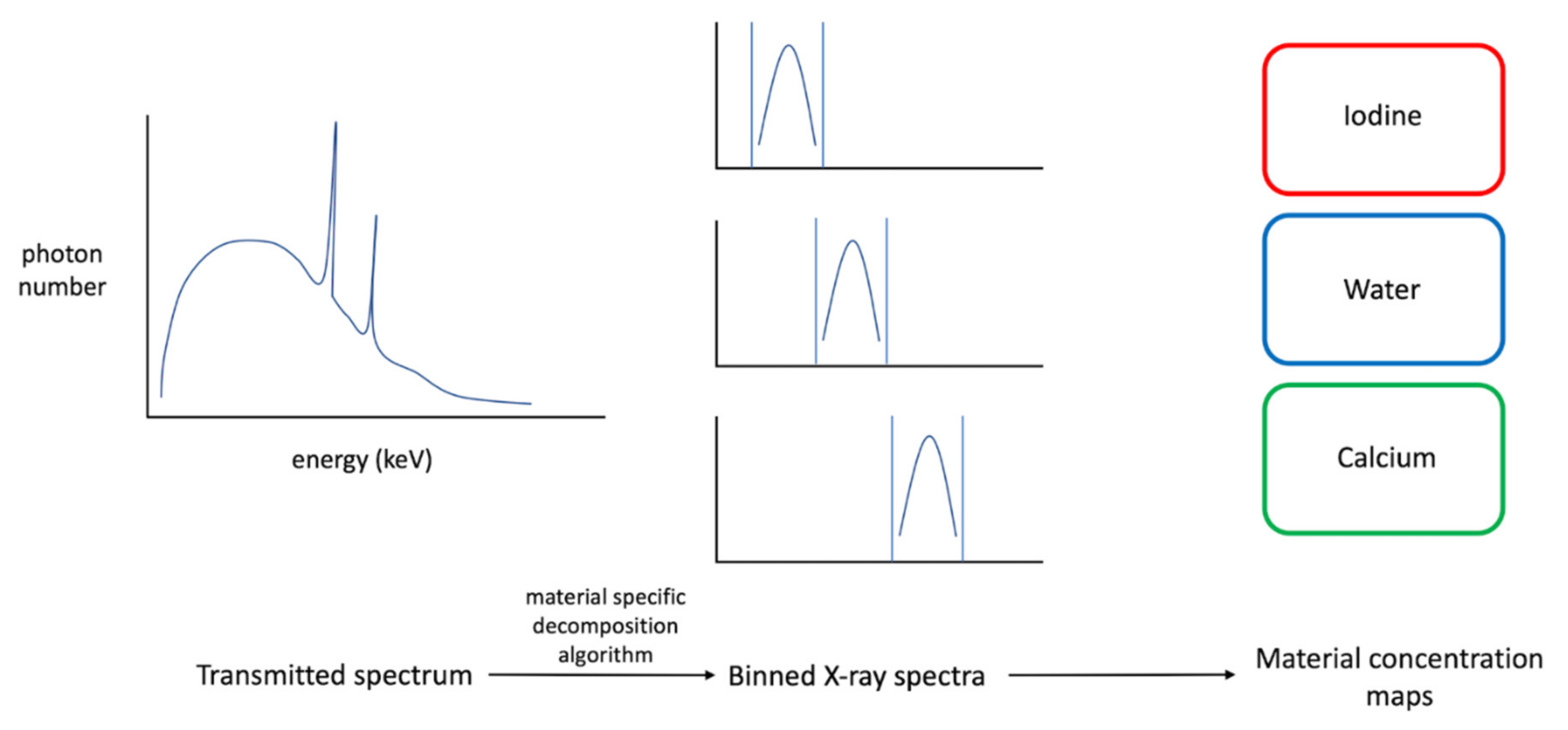
2. Technical Principles
3. Clinical Applications
3.1. Head and Neck Imaging
3.2. Temporal Bone Imaging
3.3. Chest Imaging
3.4. Breast Imaging
3.5. Cardiovascular Imaging
3.6. Abdominal Imaging
3.7. Musculoskeletal Imaging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flohr, T.; Ulzheimer, S.; Petersilka, M.; Schmidt, B. Basic principles and clinical potential of photon-counting detector CT. Chin. J. Acad. Radiol. 2020, 3, 19–34. [Google Scholar] [CrossRef] [Green Version]
- Vliegenthart, R.; Pelgrim, G.J.; Ebersberger, U.; Rowe, G.W.; Oudkerk, M.; Schoepf, U.J. Dual-Energy CT of the Heart. Am. J. Roentgenol. 2012, 199, S54–S63. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.M.; Zhao, Y.; Zhang, L.J.; Schoepf, U.J. Dual-Energy CT of the Lung. Am. J. Roentgenol. 2012, 199, S40–S53. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.; Boll, D.T.; Mileto, A.; Nelson, R.C. State of the Art: Dual-Energy CT of the Abdomen. Radiology 2014, 271, 327–342. [Google Scholar] [CrossRef]
- Mallinson, P.I.; Coupal, T.M.; McLaughlin, P.D.; Nicolaou, S.; Munk, P.L.; Ouellette, H.A. Dual-Energy CT for the Musculoskeletal System. Radiology 2016, 281, 690–707. [Google Scholar] [CrossRef]
- Siegel, M.J.; Ramirez-Giraldo, J.C. Dual-Energy CT in Children: Imaging Algorithms and Clinical Applications. Radiology 2019, 291, 286–297. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Leng, S.; Bruesewitz, M.; Tao, S.; Rajendran, K.; Halaweish, A.F.; Campeau, N.G.; Fletcher, J.G.; McCollough, C.H. Photon-counting Detector CT: System Design and Clinical Applications of an Emerging Technology. RadioGraphics 2019, 39, 729–743. [Google Scholar] [CrossRef]
- Flohr, T.G.; Stierstorfer, K.; Süß, C.; Schmidt, B.; Primak, A.N.; McCollough, C.H. Novel ultrahigh resolution data acquisition and image reconstruction for multi-detector row CT. Med. Phys. 2007, 34, 1712–1723. [Google Scholar] [CrossRef]
- Engel, K.J.; Spies, L.; Vogtmeier, G.; Luhta, R. Impact of CT detector pixel-to-pixel crosstalk on image quality. In Medical Imaging 2006: Physics of Medical Imaging; Flynn, M.J., Hsieh, J., Eds.; SPIE: Bellingham, WA, USA, 2006; p. 61422F. [Google Scholar]
- Danielsson, M.; Persson, M.; Sjölin, M. Photon-counting x-ray detectors for CT. Phys. Med. Biol. 2021, 66, 03TR01. [Google Scholar] [CrossRef]
- Taguchi, K.; Iwanczyk, J.S. Vision 20/20: Single photon counting x-ray detectors in medical imaging. Med. Phys. 2013, 40, 100901. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Danielsson, M.; Bornefalk, H. Evaluation of Energy Loss and Charge Sharing in Cadmium Telluride Detectors for Photon-Counting Computed Tomography. IEEE Trans. Nucl. Sci. 2011, 58, 614–625. [Google Scholar] [CrossRef]
- Shikhaliev, P.M.; Fritz, S.G.; Chapman, J.W. Photon counting multienergy x-ray imaging: Effect of the characteristic x rays on detector performance. Med. Phys. 2009, 36, 5107–5119. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.J.B.; Schmidt, T.G. Experimental study of photon-counting CT neural network material decomposition under conditions of pulse pileup. J. Med. Imaging 2021, 8, 013502. [Google Scholar] [CrossRef]
- Alvarez, R.E. Signal to noise ratio of energy selective x-ray photon counting systems with pileup. Med. Phys. 2014, 41, 111909. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Zhang, M.; Frey, E.C.; Wang, X.; Iwanczyk, J.S.; Nygard, E.; Hartsough, N.E.; Tsui, B.M.W.; Barber, W.C. Modeling the performance of a photon counting x-ray detector for CT: Energy response and pulse pileup effects. Med. Phys. 2011, 38, 1089–1102. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.S.; Harrison, D.; Lobastov, V.; Tkaczyk, J.E. Pulse pileup statistics for energy discriminating photon counting x-ray detectors. Med. Phys. 2011, 38, 4265–4275. [Google Scholar] [CrossRef]
- Baek, J.; Pineda, A.R.; Pelc, N.J. To bin or not to bin? The effect of CT system limiting resolution on noise and detectability. Phys. Med. Biol. 2013, 58, 1433–1446. [Google Scholar] [CrossRef]
- Pourmorteza, A.; Symons, R.; Reich, D.S.; Bagheri, M.; Cork, T.E.; Kappler, S.; Ulzheimer, S.; Bluemke, D.A. Photon-Counting CT of the Brain: In Vivo Human Results and Image-Quality Assessment. Am. J. Neuroradiol. 2017, 38, 2257–2263. [Google Scholar] [CrossRef] [Green Version]
- Shikhaliev, P.M. Beam hardening artefacts in computed tomography with photon counting, charge integrating and energy weighting detectors: A simulation study. Phys. Med. Biol. 2005, 50, 5813–5827. [Google Scholar] [CrossRef]
- Kalluri, K.S.; Mahd, M.; Glick, S.J. Investigation of energy weighting using an energy discriminating photon counting detector for breast CT. Med. Phys. 2013, 40, 081923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, T.G. Optimal “image-based” weighting for energy-resolved CT. Med. Phys. 2009, 36, 3018–3027. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.G. CT energy weighting in the presence of scatter and limited energy resolution. Med. Phys. 2010, 37, 1056–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giersch, J.; Niederlöhner, D.; Anton, G. The influence of energy weighting on X-ray imaging quality. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2004, 531, 68–74. [Google Scholar] [CrossRef]
- Alvarez, R.E.; Macovski, A. Energy-selective reconstructions in X-ray computerised tomography. Phys. Med. Biol. 1976, 21, 002. [Google Scholar] [CrossRef]
- Leng, S.; Zhou, W.; Yu, Z.; Halaweish, A.; Krauss, B.; Schmidt, B.; Yu, L.; Kappler, S.; McCollough, C. Spectral performance of a whole-body research photon counting detector CT: Quantitative accuracy in derived image sets. Phys. Med. Biol. 2017, 62, 7216–7232. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Christner, J.A.; Leng, S.; Wang, J.; Fletcher, J.G.; McCollough, C.H. Virtual monochromatic imaging in dual-source dual-energy CT: Radiation dose and image quality. Med. Phys. 2011, 38, 6371–6379. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.R.C.; Krauß, B.; Sedlmair, M.; Grasruck, M.; Bruder, H.; Morhard, D.; Fink, C.; Weckbach, S.; Lenhard, M.; Schmidt, B.; et al. Material differentiation by dual energy CT: Initial experience. Eur. Radiol. 2007, 17, 1510–1517. [Google Scholar] [CrossRef]
- Pourmorteza, A.; Symons, R.; Henning, A.; Ulzheimer, S.; Bluemke, D.A. Dose Efficiency of Quarter-Millimeter Photon-Counting Computed Tomography. Investig. Radiol. 2018, 53, 365–372. [Google Scholar] [CrossRef]
- Ballabriga, R.; Alozy, J.; Bandi, F.N.; Campbell, M.; Egidos, N.; Fernandez-Tenllado, J.M.; Heijne, E.H.M.; Kremastiotis, I.; Llopart, X.; Madsen, B.J.; et al. Photon Counting Detectors for X-Ray Imaging with Emphasis on CT. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 5, 422–440. [Google Scholar] [CrossRef]
- Fornaro, J.; Leschka, S.; Hibbeln, D.; Butler, A.; Anderson, N.; Pache, G.; Scheffel, H.; Wildermuth, S.; Alkadhi, H.; Stolzmann, P. Dual- and multi-energy CT: Approach to functional imaging. Insights Imaging 2011, 2, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müllner, M.; Schlattl, H.; Hoeschen, C.; Dietrich, O. Feasibility of spectral CT imaging for the detection of liver lesions with gold-based contrast agents—A simulation study. Phys. Med. 2015, 31, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Cormode, D.P.; Skajaa, T.; Fayad, Z.A.; Mulder, W.J.M. Nanotechnology in Medical Imaging. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 992–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si-Mohamed, S.; Cormode, D.P.; Bar-Ness, D.; Sigovan, M.; Naha, P.C.; Langlois, J.-B.; Chalabreysse, L.; Coulon, P.; Blevis, I.; Roessl, E.; et al. Evaluation of spectral photon counting computed tomography K-edge imaging for determination of gold nanoparticle biodistribution in vivo. Nanoscale 2017, 9, 18246–18257. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef]
- Goodsitt, M.M.; Christodoulou, E.G.; Larson, S.C. Accuracies of the synthesized monochromatic CT numbers and effective atomic numbers obtained with a rapid kVp switching dual energy CT scanner. Med. Phys. 2011, 38, 2222–2232. [Google Scholar] [CrossRef] [Green Version]
- Pelgrim, G.J.; van Hamersvelt, R.W.; Willemink, M.J.; Schmidt, B.T.; Flohr, T.; Schilham, A.; Milles, J.; Oudkerk, M.; Leiner, T.; Vliegenthart, R. Accuracy of iodine quantification using dual energy CT in latest generation dual source and dual layer CT. Eur. Radiol. 2017, 27, 3904–3912. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Yu, L.; Primak, A.N.; McCollough, C.H. Quantitative imaging of element composition and mass fraction using dual-energy CT: Three-material decomposition. Med. Phys. 2009, 36, 1602–1609. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.; Fessler, J.A. Multi-Material Decomposition Using Statistical Image Reconstruction for Spectral CT. IEEE Trans. Med. Imaging 2014, 33, 1614–1626. [Google Scholar] [CrossRef] [Green Version]
- Schlomka, J.P.; Roessl, E.; Dorscheid, R.; Dill, S.; Martens, G.; Istel, T.; Bäumer, C.; Herrmann, C.; Steadman, R.; Zeitler, G.; et al. Experimental feasibility of multi-energy photon-counting K-edge imaging in pre-clinical computed tomography. Phys. Med. Biol. 2008, 53, 4031–4047. [Google Scholar] [CrossRef]
- Roessl, E.; Brendel, B.; Engel, K.-J.; Schlomka, J.-P.; Thran, A.; Proksa, R. Sensitivity of Photon-Counting Based Edge Imaging in X-ray Computed Tomography. IEEE Trans. Med. Imaging 2011, 30, 1678–1690. [Google Scholar] [CrossRef] [PubMed]
- Roessl, E.; Proksa, R. K-edge imaging in x-ray computed tomography using multi-bin photon counting detectors. Phys. Med. Biol. 2007, 52, 4679–4696. [Google Scholar] [CrossRef] [PubMed]
- Si-Mohamed, S.; Bar-Ness, D.; Sigovan, M.; Tatard-Leitman, V.; Cormode, D.P.; Naha, P.C.; Coulon, P.; Rascle, L.; Roessl, E.; Rokni, M.; et al. Multicolour imaging with spectral photon-counting CT: A phantom study. Eur. Radiol. Exp. 2018, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Symons, R.; Reich, D.S.; Bagheri, M.; Cork, T.E.; Krauss, B.; Ulzheimer, S.; Kappler, S.; Bluemke, D.A.; Pourmorteza, A. Photon-Counting Computed Tomography for Vascular Imaging of the Head and Neck. Investig. Radiol. 2018, 53, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kuno, H.; Onaya, H.; Iwata, R.; Kobayashi, T.; Fujii, S.; Hayashi, R.; Otani, K.; Ojiri, H.; Yamanaka, T.; Satake, M. Evaluation of Cartilage Invasion by Laryngeal and Hypopharyngeal Squamous Cell Carcinoma with Dual-Energy CT. Radiology 2012, 265, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Yu, Z.; Halaweish, A.; Kappler, S.; Hahn, K.; Henning, A.; Li, Z.; Lane, J.; Levin, D.L.; Jorgensen, S.; et al. A high-resolution imaging technique using a whole-body, research photon counting detector CT system. In Medical Imaging 2016: Physics of Medical Imaging; Kontos, D., Flohr, T.G., Lo, J.Y., Eds.; SPIE: Bellingham, WA, USA, 2016; p. 97831I. [Google Scholar]
- Rajendran, K.; Voss, B.A.; Zhou, W.; Tao, S.; DeLone, D.R.; Lane, J.I.; Weaver, J.M.; Carlson, M.L.; Fletcher, J.G.; McCollough, C.H.; et al. Dose Reduction for Sinus and Temporal Bone Imaging Using Photon-Counting Detector CT With an Additional Tin Filter. Investig. Radiol. 2020, 55, 91–100. [Google Scholar] [CrossRef]
- Zhou, W.; Lane, J.I.; Carlson, M.L.; Bruesewitz, M.R.; Witte, R.J.; Koeller, K.K.; Eckel, L.J.; Carter, R.E.; McCollough, C.H.; Leng, S. Comparison of a Photon-Counting-Detector CT with an Energy-Integrating-Detector CT for Temporal Bone Imaging: A Cadaveric Study. Am. J. Neuroradiol. 2018, 39, 1733–1738. [Google Scholar] [CrossRef] [Green Version]
- Kopp, F.K.; Daerr, H.; Si-Mohamed, S.; Sauter, A.P.; Ehn, S.; Fingerle, A.A.; Brendel, B.; Pfeiffer, F.; Roessl, E.; Rummeny, E.J.; et al. Evaluation of a preclinical photon-counting CT prototype for pulmonary imaging. Sci. Rep. 2018, 8, 17386. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Miailhes, J.; Rodesch, P.-A.; Boccalini, S.; Lacombe, H.; Leitman, V.; Cottin, V.; Boussel, L.; Douek, P. Spectral Photon-Counting CT Technology in Chest Imaging. J. Clin. Med. 2021, 10, 5757. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Greffier, J.; Miailhes, J.; Boccalini, S.; Rodesch, P.-A.; Vuillod, A.; van der Werf, N.; Dabli, D.; Racine, D.; Rotzinger, D.; et al. Comparison of image quality between spectral photon-counting CT and dual-layer CT for the evaluation of lung nodules: A phantom study. Eur. Radiol. 2022, 32, 524–532. [Google Scholar] [CrossRef]
- Symons, R.; Cork, T.E.; Sahbaee, P.; Fuld, M.K.; Kappler, S.; Folio, L.R.; Bluemke, D.A.; Pourmorteza, A. Low-dose lung cancer screening with photon-counting CT: A feasibility study. Phys. Med. Biol. 2017, 62, 202–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watadani, T.; Sakai, F.; Johkoh, T.; Noma, S.; Akira, M.; Fujimoto, K.; Bankier, A.A.; Lee, K.S.; Müller, N.L.; Song, J.-W.; et al. Interobserver Variability in the CT Assessment of Honeycombing in the Lungs. Radiology 2013, 266, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Ferda, J.; Vendiš, T.; Flohr, T.; Schmidt, B.; Henning, A.; Ulzheimer, S.; Pecen, L.; Ferdová, E.; Baxa, J.; Mírka, H. Computed tomography with a full FOV photon-counting detector in a clinical setting, the first experience. Eur. J. Radiol. 2021, 137, 109614. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.J.; Koo, C.W.; Bartholmai, B.J.; Rajendran, K.; Weaver, J.M.; Halaweish, A.F.; Leng, S.; McCollough, C.H.; Fletcher, J.G. High-Resolution Chest Computed Tomography Imaging of the Lungs. Investig. Radiol. 2019, 54, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Taniguchi, Y.; Matsuoka, Y.; Yanaka, K.; Izawa, Y.; Tsuboi, Y.; Mori, S.; Kono, A.; Nakayama, K.; Emoto, N.; et al. Evaluation of microvasculopathy using dual-energy computed tomography in patients with chronic thromboembolic pulmonary hypertension. Pulm. Circ. 2021, 11, 2045894020983162. [Google Scholar] [CrossRef] [PubMed]
- Rößler, A.-C.; Kalender, W.; Kolditz, D.; Steiding, C.; Ruth, V.; Preuss, C.; Peter, S.C.; Brehm, B.; Hammon, M.; Schulz-Wendtland, R.; et al. Performance of Photon-Counting Breast Computed Tomography, Digital Mammography, and Digital Breast Tomosynthesis in Evaluating Breast Specimens. Acad. Radiol. 2017, 24, 184–190. [Google Scholar] [CrossRef]
- Shikhaliev, P.M. Soft tissue imaging with photon counting spectroscopic CT. Phys. Med. Biol. 2015, 60, 2453–2474. [Google Scholar] [CrossRef]
- Van der Werf, N.R.; Si-Mohamed, S.; Rodesch, P.A.; van Hamersvelt, R.W.; Greuter, M.J.W.; Boccalini, S.; Greffier, J.; Leiner, T.; Boussel, L.; Willemink, M.J.; et al. Coronary calcium scoring potential of large field-of-view spectral photon-counting CT: A phantom study. Eur. Radiol. 2022, 32, 152–162. [Google Scholar] [CrossRef]
- Symons, R.; Sandfort, V.; Mallek, M.; Ulzheimer, S.; Pourmorteza, A. Coronary artery calcium scoring with photon-counting CT: First in vivo human experience. Int. J. Cardiovasc. Imaging 2019, 35, 733–739. [Google Scholar] [CrossRef]
- Symons, R.; Cork, T.E.; Lakshmanan, M.N.; Evers, R.; Davies-Venn, C.; Rice, K.A.; Thomas, M.L.; Liu, C.-Y.; Kappler, S.; Ulzheimer, S.; et al. Dual-contrast agent photon-counting computed tomography of the heart: Initial experience. Int. J. Cardiovasc. Imaging 2017, 33, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Maintz, D.; Juergens, K.-U.; Wichter, T.; Grude, M.; Heindel, W.; Fischbach, R. Imaging of coronary artery stents using multislice computed tomography: In vitro evaluation. Eur. Radiol. 2003, 13, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Mahnken, A.H.; Buecker, A.; Wildberger, J.E.; Ruebben, A.; Stanzel, S.; Vogt, F.; Günther, R.W.; Blindt, R. Coronary Artery Stents in Multislice Computed Tomography. Investig. Radiol. 2004, 39, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Maintz, D.; Seifarth, H.; Raupach, R.; Flohr, T.; Rink, M.; Sommer, T.; Özgün, M.; Heindel, W.; Fischbach, R. 64-slice multidetector coronary CT angiography: In vitro evaluation of 68 different stents. Eur. Radiol. 2006, 16, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Maintz, D.; Burg, M.C.; Seifarth, H.; Bunck, A.C.; Özgün, M.; Fischbach, R.; Jürgens, K.U.; Heindel, W. Update on multidetector coronary CT angiography of coronary stents: In vitro evaluation of 29 different stent types with dual-source CT. Eur. Radiol. 2009, 19, 42–49. [Google Scholar] [CrossRef]
- Mannil, M.; Hickethier, T.; von Spiczak, J.; Baer, M.; Henning, A.; Hertel, M.; Schmidt, B.; Flohr, T.; Maintz, D.; Alkadhi, H. Photon-Counting CT. Investig. Radiol. 2018, 53, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Bratke, G.; Hickethier, T.; Bar-Ness, D.; Bunck, A.C.; Maintz, D.; Pahn, G.; Coulon, P.; Si-Mohamed, S.; Douek, P.; Sigovan, M. Spectral Photon-Counting Computed Tomography for Coronary Stent Imaging. Investig. Radiol. 2020, 55, 61–67. [Google Scholar] [CrossRef]
- Stettler, C.; Wandel, S.; Allemann, S.; Kastrati, A.; Morice, M.C.; Schömig, A.; Pfisterer, M.E.; Stone, G.W.; Leon, M.B.; de Lezo, J.S.; et al. Outcomes associated with drug-eluting and bare-metal stents: A collaborative network meta-analysis. Lancet 2007, 370, 937–948. [Google Scholar] [CrossRef]
- Smits, P.C.; Vlachojannis, G.J.; McFadden, E.P.; Royaards, K.-J.; Wassing, J.; Joesoef, K.S.; van Mieghem, C.; van de Ent, M. Final 5-Year Follow-Up of a Randomized Controlled Trial of Everolimus- and Paclitaxel-Eluting Stents for Coronary Revascularization in Daily Practice. JACC Cardiovasc. Interv. 2015, 8, 1157–1165. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.S.; John, J.M.; Chew, D.P.; Lee, D.S.; Ellis, S.G.; Bhatt, D.L. Bare metal stent restenosis is not a benign clinical entity. Am. Heart J. 2006, 151, 1260–1264. [Google Scholar] [CrossRef]
- Dangelmaier, J.; Bar-Ness, D.; Daerr, H.; Muenzel, D.; Si-Mohamed, S.; Ehn, S.; Fingerle, A.A.; Kimm, M.A.; Kopp, F.K.; Boussel, L.; et al. Experimental feasibility of spectral photon-counting computed tomography with two contrast agents for the detection of endoleaks following endovascular aortic repair. Eur. Radiol. 2018, 28, 3318–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belghiti, J.; Cauchy, F.; Paradis, V.; Vilgrain, V. Diagnosis and management of solid benign liver lesions. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Murakami, T.; Takahashi, S.; Tsuda, K.; Tomoda, K.; Narumi, Y.; Oi, H.; Sakon, M.; Nakamura, H. Optimal phases of dynamic CT for detecting hepatocellular carcinoma: Evaluation of unenhanced and triple-phase images. Abdom. Imaging 1999, 24, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Soyer, P.; Poccard, M.; Boudiaf, M.; Abitbol, M.; Hamzi, L.; Panis, Y.; Valleur, P.; Rymer, R. Detection of Hypovascular Hepatic Metastases at Triple-Phase Helical CT: Sensitivity of Phases and Comparison with Surgical and Histopathologic Findings. Radiology 2004, 231, 413–420. [Google Scholar] [CrossRef]
- Muenzel, D.; Daerr, H.; Proksa, R.; Fingerle, A.A.; Kopp, F.K.; Douek, P.; Herzen, J.; Pfeiffer, F.; Rummeny, E.J.; Noël, P.B. Simultaneous dual-contrast multi-phase liver imaging using spectral photon-counting computed tomography: A proof-of-concept study. Eur. Radiol. Exp. 2017, 1, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Si-Mohamed, S.; Tatard-Leitman, V.; Laugerette, A.; Sigovan, M.; Pfeiffer, D.; Rummeny, E.J.; Coulon, P.; Yagil, Y.; Douek, P.; Boussel, L.; et al. Spectral Photon-Counting Computed Tomography (SPCCT): In-vivo single-acquisition multi-phase liver imaging with a dual contrast agent protocol. Sci. Rep. 2019, 9, 8458. [Google Scholar] [CrossRef]
- Chua, T.C.; Moran, B.J.; Sugarbaker, P.H.; Levine, E.A.; Glehen, O.; Gilly, F.N.; Baratti, D.; Deraco, M.; Elias, D.; Sardi, A.; et al. Early- and Long-Term Outcome Data of Patients With Pseudomyxoma Peritonei From Appendiceal Origin Treated by a Strategy of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. J. Clin. Oncol. 2012, 30, 2449–2456. [Google Scholar] [CrossRef]
- Van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef]
- Bonnot, P.-E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.-M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery with or Without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Dohan, A.; Hobeika, C.; Najah, H.; Pocard, M.; Rousset, P.; Eveno, C. Preoperative assessment of peritoneal carcinomatosis of colorectal origin. J. Visc. Surg. 2018, 155, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H.; Sardi, A.; Brown, G.; Dromain, C.; Rousset, P.; Jelinek, J.S. Concerning CT features used to select patients for treatment of peritoneal metastases, a pictoral essay. Int. J. Hyperth. 2017, 33, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Dohan, A.; Hoeffel, C.; Soyer, P.; Jannot, A.S.; Valette, P.-J.; Thivolet, A.; Passot, G.; Glehen, O.; Rousset, P. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br. J. Surg. 2017, 104, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Laghi, A.; Bellini, D.; Rengo, M.; Accarpio, F.; Caruso, D.; Biacchi, D.; Di Giorgio, A.; Sammartino, P. Diagnostic performance of computed tomography and magnetic resonance imaging for detecting peritoneal metastases: Systematic review and meta-analysis. Radiol. Med. 2017, 122, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Torkzad, M.; Casta, N.; Bergman, A.; Ahlström, H.; Påhlman, L.; Mahteme, H. Comparison between MRI and CT in prediction of peritoneal carcinomatosis index (PCI) in patients undergoing cytoreductive surgery in relation to the experience of the radiologist. J. Surg. Oncol. 2015, 111, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.-L.; Yan, T.D.; Glenn, D.; Morris, D.L. Evaluation of Preoperative Computed Tomography in Estimating Peritoneal Cancer Index in Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2009, 16, 327–333. [Google Scholar] [CrossRef]
- Marin, D.; Catalano, C.; Baski, M.; Di Martino, M.; Geiger, D.; Di Giorgio, A.; Sibio, S.; Passariello, R. 64-Section multi-detector row CT in the preoperative diagnosis of peritoneal carcinomatosis: Correlation with histopathological findings. Abdom. Imaging 2010, 35, 694–700. [Google Scholar] [CrossRef]
- Thivolet, A.; Si-Mohamed, S.; Bonnot, P.-E.; Blanchet, C.; Képénékian, V.; Boussel, L.; Douek, P.; Rousset, P. Spectral photon-counting CT imaging of colorectal peritoneal metastases: Initial experience in rats. Sci. Rep. 2020, 10, 13394. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Quinn, T.M.; Häuselmann, H.-J. Quantitative structural organization of normal adult human articular cartilage. Osteoarthr. Cartil. 2002, 10, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Hosseininia, S.; Lindberg, L.R.; Dahlberg, L.E. Cartilage collagen damage in hip osteoarthritis similar to that seen in knee osteoarthritis: A case-control study of relationship between collagen, glycosaminoglycan and cartilage swelling. BMC Musculoskelet. Disord. 2013, 14, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andriacchi, T.P.; Mündermann, A.; Smith, R.L.; Alexander, E.J.; Dyrby, C.O.; Koo, S. A Framework for the in Vivo Pathomechanics of Osteoarthritis at the Knee. Ann. Biomed. Eng. 2004, 32, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, A.; Honkanen, J.T.J.; Myller, K.A.H.; Prakash, M.; Korhonen, M.; Saukko, A.E.A.; Virén, T.; Joukainen, A.; Patwa, A.N.; Kröger, H.; et al. Quantitative Dual Contrast CT Technique for Evaluation of Articular Cartilage Properties. Ann. Biomed. Eng. 2018, 46, 1038–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paakkari, P.; Inkinen, S.I.; Honkanen, M.K.M.; Prakash, M.; Shaikh, R.; Nieminen, M.T.; Grinstaff, M.W.; Mäkelä, J.T.A.; Töyräs, J.; Honkanen, J.T.J. Quantitative dual contrast photon-counting computed tomography for assessment of articular cartilage health. Sci. Rep. 2021, 11, 5556. [Google Scholar] [CrossRef]
- Turkiewicz, A.; Petersson, I.F.; Björk, J.; Hawker, G.; Dahlberg, L.E.; Lohmander, L.S.; Englund, M. Current and future impact of osteoarthritis on health care: A population-based study with projections to year 2032. Osteoarthr. Cartil. 2014, 22, 1826–1832. [Google Scholar] [CrossRef] [Green Version]
- Bourne, R.B.; Chesworth, B.M.; Davis, A.M.; Mahomed, N.N.; Charron, K.D.J. Patient Satisfaction after Total Knee Arthroplasty: Who is Satisfied and Who is Not? Clin. Orthop. Relat. Res. 2010, 468, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Mont, M.A.; Serna, F.K.; Krackow, K.A.; Hungerford, D.S. Exploration of Radiographically Normal Total Knee Replacements for Unexplained Pain. Clin. Orthop. Relat. Res. 1996, 331, 216–220. [Google Scholar] [CrossRef]
- Flierl, M.A.; Sobh, A.H.; Culp, B.M.; Baker, E.A.; Sporer, S.M. Evaluation of the Painful Total Knee Arthroplasty. J. Am. Acad. Orthop. Surg. 2019, 27, 743–751. [Google Scholar] [CrossRef]
- Lau, L.C.M.; Lee, W.Y.W.; Butler, A.P.H.; Chernoglazov, A.I.; Chung, K.Y.; Ho, K.K.W.; Griffith, J.; Butler, P.H.; Yung, P.S.H. Multi-energy spectral photon-counting computed tomography (MARS) for detection of arthroplasty implant failure. Sci. Rep. 2021, 11, 1554. [Google Scholar] [CrossRef]
- Kellock, T.T.; Nicolaou, S.; Kim, S.S.Y.; Al-Busaidi, S.; Louis, L.J.; O’Connell, T.W.; Ouellette, H.A.; McLaughlin, P.D. Detection of Bone Marrow Edema in Nondisplaced Hip Fractures: Utility of a Virtual Noncalcium Dual-Energy CT Application. Radiology 2017, 284, 798–805. [Google Scholar] [CrossRef] [Green Version]
- Bernabei, I.; Sayous, Y.; Raja, A.Y.; Amma, M.R.; Viry, A.; Steinmetz, S.; Falgayrac, G.; van Heeswijk, R.B.; Omoumi, P.; Pascart, T.; et al. Multi-energy photon-counting computed tomography versus other clinical imaging techniques for the identification of articular calcium crystal deposition. Rheumatology 2021, 60, 2483–2485. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E. Clinical Features of Metastatic Bone Disease and Risk of Skeletal Morbidity. Clin. Cancer Res. 2006, 12, 6243s–6249s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennecke, H.; Yerushalmi, R.; Woods, R.; Cheang, M.C.U.; Voduc, D.; Speers, C.H.; Nielsen, T.O.; Gelmon, K. Metastatic Behavior of Breast Cancer Subtypes. J. Clin. Oncol. 2010, 28, 3271–3277. [Google Scholar] [CrossRef] [PubMed]
- Sathiakumar, N.; Delzell, E.; Morrisey, M.A.; Falkson, C.; Yong, M.; Chia, V.; Blackburn, J.; Arora, T.; Brill, I.; Kilgore, M.L. Mortality following bone metastasis and skeletal-related events among women with breast cancer: A population-based analysis of U.S. Medicare beneficiaries, 1999–2006. Breast Cancer Res. Treat. 2012, 131, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, F.; Matsuura, M.; Kuhn, V.; Priemel, M.; Müller, R.; Link, T.M.; Lochmüller, E.-M. Sex Differences of Human Trabecular Bone Microstructure in Aging Are Site-Dependent. J. Bone Miner. Res. 2007, 22, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Wehrse, E.; Sawall, S.; Klein, L.; Glemser, P.; Delorme, S.; Schlemmer, H.-P.; Kachelrieß, M.; Uhrig, M.; Ziener, C.H.; Rotkopf, L.T. Potential of ultra-high-resolution photon-counting CT of bone metastases: Initial experiences in breast cancer patients. npj Breast Cancer 2021, 7, 3. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. J. Imaging 2022, 8, 112. https://doi.org/10.3390/jimaging8040112
Tortora M, Gemini L, D’Iglio I, Ugga L, Spadarella G, Cuocolo R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. Journal of Imaging. 2022; 8(4):112. https://doi.org/10.3390/jimaging8040112
Chicago/Turabian StyleTortora, Mario, Laura Gemini, Imma D’Iglio, Lorenzo Ugga, Gaia Spadarella, and Renato Cuocolo. 2022. "Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications" Journal of Imaging 8, no. 4: 112. https://doi.org/10.3390/jimaging8040112
APA StyleTortora, M., Gemini, L., D’Iglio, I., Ugga, L., Spadarella, G., & Cuocolo, R. (2022). Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. Journal of Imaging, 8(4), 112. https://doi.org/10.3390/jimaging8040112







