Multiple Papillomas of the Breast: A Review of Current Evidence and Challenges
Abstract
:1. Introduction
2. Materials and Methods
3. Results
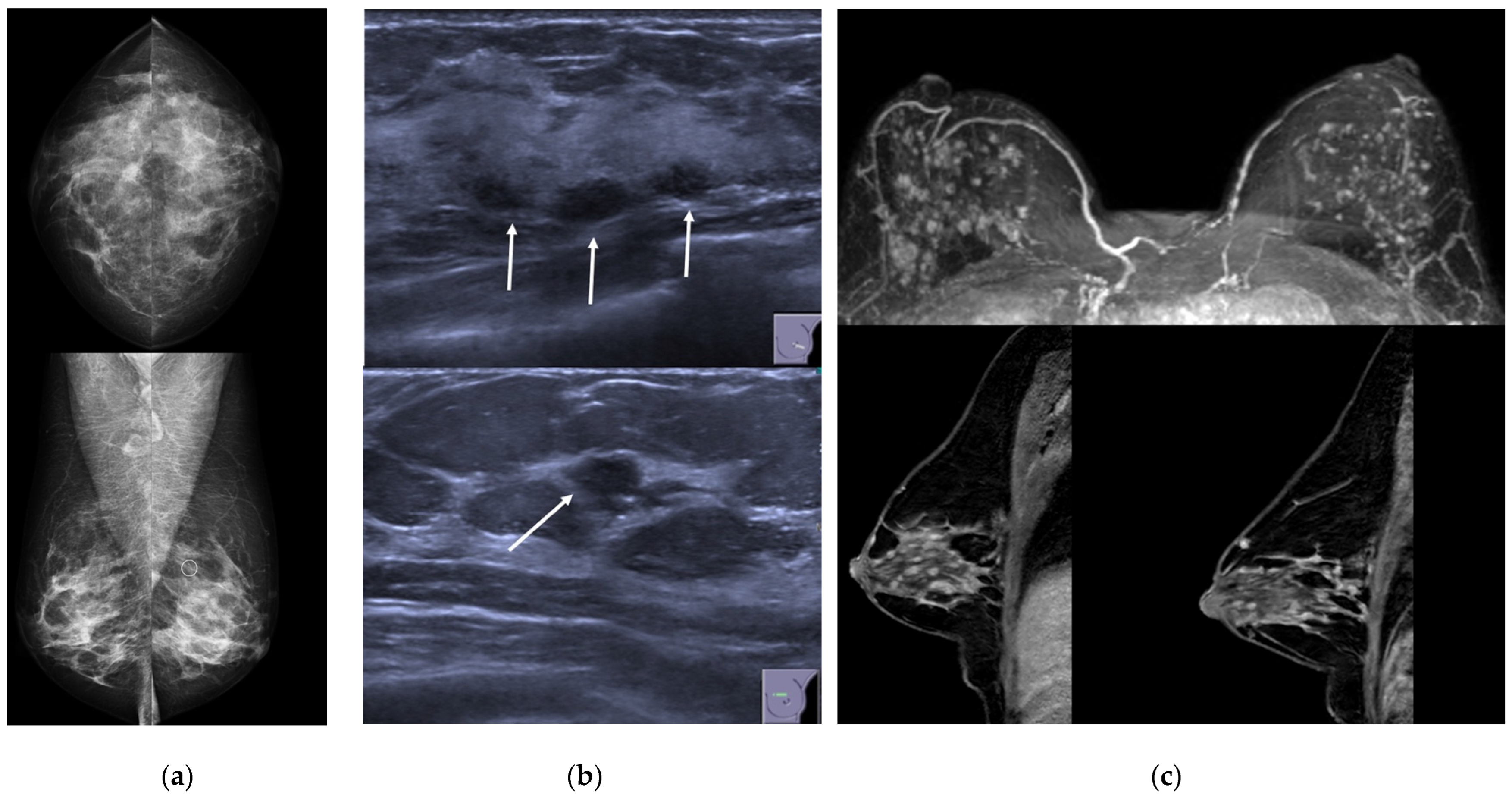
3.1. Clinical and Imaging Findings
3.2. MP and Malignancy
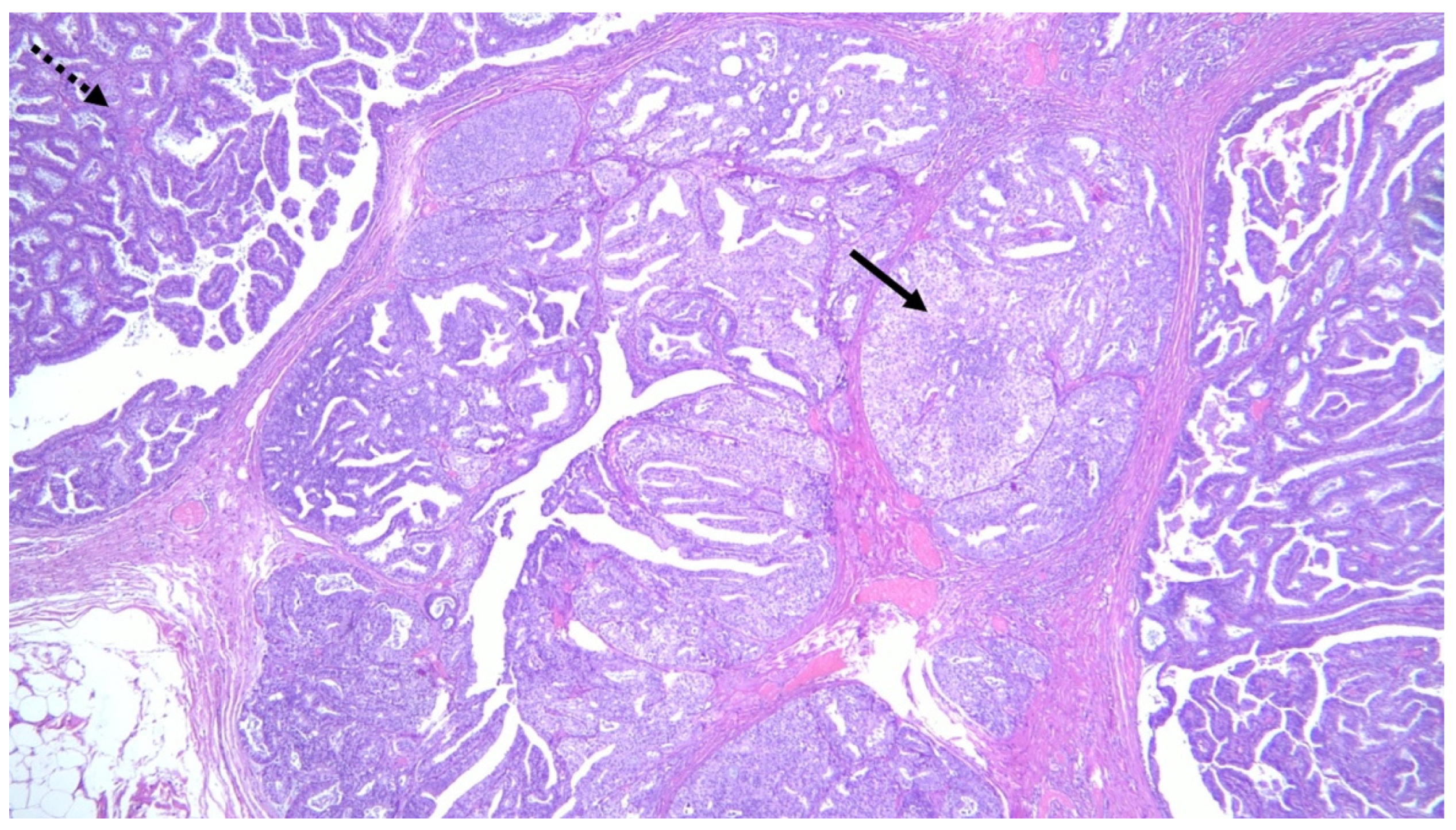
3.2.1. MP and Malignancy in Core Needle Biopsy (CNB) and Vacuum-Assisted Biopsy (VAB)
3.2.2. MP and Association with Premalignant/Malignant Lesions in Surrounding Parenchyma
3.2.3. MP and Risk for Breast Cancer Development
3.3. Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanby, A.M.; Walker, C.; Tavassoli, F.A.; Devilee, P. Pathology and Genetics: Tumours of the Breast and Female Genital Organs. WHO Classification of Tumours series—volume IV. Breast Cancer Res. 2004, 6, 133. [Google Scholar] [CrossRef] [Green Version]
- Dahnert, W. Breast disorders. In Radiology Review Manual, 4th ed.; Williams and Wilkins: Philadelphia, PA, USA, 1999; pp. 458–474. [Google Scholar]
- Muttarak, M.; Lerttumnongtum, P.; Chaiwun, B.; Peh, W.C. Spectrum of papillary lesions of the breast: Clinical, imaging, and pathologic correlation. Am. J. Roentgenol. 2008, 191, 700–707. [Google Scholar] [CrossRef]
- Lewis, J.T.; Hartmann, L.C.; Vierkant, R.A.; Maloney, S.D.; Shane Pankratz, V.; Allers, T.M.; Frost, M.H.; Visscher, D.W. An analysis of breast cancer risk in women with single, multiple, and atypical papilloma. Am. J. Surg. Pathol. 2006, 30, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Carder, P.J.; Khan, T.; Burrows, P.; Sharma, N. Large volume “mammotome” biopsy may reduce the need for diagnostic surgery in papillary lesions of the breast. J. Clin. Pathol. 2008, 61, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Ali-Fehmi, R.; Carolin, K.; Wallis, T.; Visscher, D.W. Clinicopathologic analysis of breast lesions associated with multiple papillomas. Hum. Pathol. 2003, 34, 234–239. [Google Scholar] [CrossRef]
- Carter, D. Intraductal papillary tumors of the breast: A study of 78 cases. Cancer 1977, 39, 1689–1692. [Google Scholar] [CrossRef]
- Han, S.H.; Kim, M.; Chung, Y.R.; Yun, B.; Jang, M.; Kim, S.M.; Kang, E.; Kim, E.K.; Park, S.Y. Benign intraductal papilloma without atypia on core needle biopsy has a low rate of upgrading to malignancy after excision. J. Breast Cancer 2018, 21, 80–86. [Google Scholar] [CrossRef]
- Harjit, K.; Willsher, P.C.; Bennett, M.; Jackson, L.R.; Metcalf, C.; Saunders, C.M. Multiple papillomas of the breast: Is current management adequate? Breast 2006, 15, 777–781. [Google Scholar] [CrossRef]
- Murad, T.M.; Contesso, G.; Mouriesse, H. Papillary tumors of large lactiferous ducts. Cancer 1981, 48, 122–133. [Google Scholar] [CrossRef]
- Ohuchi, N.; Abe, R.; Kasai, M. Possible cancerous change of intraductal papillomas of the breast. A 3-D reconstruction study of 25 cases. Cancer 1984, 54, 605–611. [Google Scholar] [CrossRef]
- Papotti, M.; Gugliotta, P.; Ghiringhello, B.; Bussolati, G. Association of breast carcinoma and multiple intraductal papillomas: An histological and immunohistochemical investigation. Histopathology 1984, 8, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Pellettiere, E.V., II. The clinical and pathologic aspects of papillomatous disease of the breast: A follow-up study of 97 patients treated by local excision. Am. J. Clin. Pathol. 1971, 55, 740–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raju, U.; Vertes, D. Breast papillomas with atypical ductal hyperplasia: A clinicopathologic study. Hum. Pathol. 1996, 27, 1231–1238. [Google Scholar] [CrossRef]
- Chang, J.M.; Han, W.; Moon, W.K.; Cho, N.; Noh, D.Y.; Park, I.A.; Jung, E.J. Papillary lesions initially diagnosed at ultrasound-guided vacuum-assisted breast biopsy: Rate of malignancy based on subsequent surgical excision. Ann. Surg. Oncol. 2011, 18, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Ciatto, S.; Andreoli, C.; Cirillo, A.; Bonardi, R.; Bianchi, S.; Santoro, G.; Farante, G.; Magni, A.; Campa, T.; Costa, A.; et al. The risk of breast cancer subsequent to histologic diagnosis of benign intraductal papilloma follow-up study of 339 cases. Tumori J. 1991, 77, 41–43. [Google Scholar] [CrossRef]
- Fu, C.-Y.; Chen, T.-W.; Hong, Z.-J.; Chan, D.-C.; Young, C.-Y.; Chen, C.-J.; Hsieh, C.-B.; Hsu, H.-H.; Peng, Y.-J.; Lu, H.-E.; et al. Papillary breast lesions diagnosed by core biopsy require complete excision. Eur. J. Surg. Oncol. 2012, 38, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Gendler, L.S.; Feldman, S.M.; Balassanian, R.; Riker, M.A.; Frencher, S.K.; Whelan, D.B.; Anne, S.; Gross, J.D.; Cohen, J.M.; Boolbol, S.K. Association of breast cancer with papillary lesions identified at percutaneous image-guided breast biopsy. Am. J. Surg. 2004, 188, 365–370. [Google Scholar] [CrossRef]
- Kabat, G.C.; Jones, J.G.; Olson, N.; Negassa, A.; Duggan, C.; Ginsberg, M.; Kandel, R.A.; Glass, A.G.; Rohan, T.E. A multi-center prospective cohort study of benign breast disease and risk of subsequent breast cancer. Cancer Causes Control 2010, 21, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Koo, J.S.; Han, K.; Kim, M.J.; Moon, H.J.; Kim, E.K.; Park, B.W. Can additional immunohistochemistry staining replace the surgical excision for the diagnosis of papillary breast lesions classified as benign on 14-gauge core needle biopsy? Breast Cancer Res. Treat. 2013, 137, 797–806. [Google Scholar] [CrossRef]
- Liberman, L.; Tornos, C.; Huzjan, R.; Bartella, L.; Morris, E.A.; Dershaw, D.D. Is surgical excision warranted after benign, concordant diagnosis of papilloma at percutaneous breast biopsy? Am. J. Roentgenol. 2006, 186, 1328–1334. [Google Scholar] [CrossRef]
- Sohn, Y.M.; Park, S.H. Comparison of sonographically guided core needle biopsy and excision in breast papillomas: Clinical and sonographic featuRes. predictive of malignancy. J. Ultrasound Med. 2013, 32, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Cardenosa, G.; Eklund, G.W. Benign papillary neoplasms of the breast: Mammographic findings. Radiology 1991, 181, 751–755. [Google Scholar] [CrossRef]
- Manganaro, L.; D’Ambrosio, I.; Gigli, S.; Di Pastena, F.; Giraldi, G.; Tardioli, S.; Framarino, M.; Porfiri, L.M.; Ballesio, L. Breast MRI in patients with unilateral bloody and serous-bloody nipple discharge: A comparison with galactography. BioMed Res. Int. 2015, 2015, 806368. [Google Scholar] [CrossRef] [PubMed]
- Son, E.J.; Kim, E.K.; Kim, J.A.; Kwak, J.Y.; Jeong, J. Diagnostic value of 3D fast low-angle shot dynamic MRI of breast papillomas. Yonsei Med. J. 2009, 50, 838–844. [Google Scholar] [CrossRef] [Green Version]
- Sarica, O.; Dokdok, M. Imaging Findings in Papillary Breast Lesions: An Analysis of Ductal Findings on Magnetic Resonance Imaging and Ultrasound. J. Comput. Assist. Tomogr. 2018, 42, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Bender, O.; Balci, F.L.; Yüney, E.; Akbulut, H. Scarless endoscopic papillomectomy of the breast. Oncol. Res. Treat. 2009, 32, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Kamali, S.; Bender, O.; Kamali, G.H.; Aydin, M.T.; Karatepe, O.; Yuney, E. Diagnostic and therapeutic value of ductoscopy in nipple discharge and intraductal proliferations compared with standard methods. Breast Cancer 2014, 21, 154–161. [Google Scholar] [CrossRef]
- Ling, H.; Liu, G.Y.; Lu, J.S.; Love, S.; Zhang, J.X.; Xu, X.L.; Xu, W.P.; Shen, K.W.; Shen, Z.Z.; Shao, Z.M. Fiberoptic ductoscopy-guided intraductal biopsy improve the diagnosis of nipple discharge. Breast J. 2009, 15, 168–175. [Google Scholar] [CrossRef]
- Liu, M.; Guo, G.; Xie, F.; Wang, S.; Yang, H.; Wang, S. Mammary ductoscopy and follow-up avoid unnecessary duct excision in patients with pathologic nipple discharge. J. Surg. Oncol. 2015, 112, 139–143. [Google Scholar] [CrossRef]
- Rageth, C.J.; O’Flynn, E.A.M.; Pinker, K.; Kubik-Huch, R.A.; Mundinger, A.; Decker, T.; Tausch, C.; Dammann, F.; Baltzer, P.A.; Fallenberg, E.M.; et al. Second International Consensus Conference on lesions of uncertain malignant potential in the breast (B3 lesions). Breast Cancer Res. Treat. 2019, 174, 279–296, Erratum in Breast Cancer Res. Treat. 2019, 176, 481–482. [Google Scholar] [CrossRef] [Green Version]
- Rosen, P.P.; Kimmel, M. Juvenile papillomatosis of the breast: A follow-up study of 41 patients having biopsies before 1979. Am. J. Clin. Pathol. 1990, 93, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Eberle, C.A.; Piscuoglio, S.; Rakha, E.A.; Ng, C.K.; Geyer, F.C.; Edelweiss, M.; Sakr, R.A.; Weigelt, B.; Reis-Filho, J.S.; Ellis, I.O. Infiltrating epitheliosis of the breast: Characterization of histological features, immunophenotype and genomic profile. Histopathology 2016, 68, 1030–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basavaiah, S.H.; Minal, J.; Sreeram, S.; Suresh, P.K.; Kini, H.; Adiga, D.; Sahu, K.K.; Pai, R.R. Diagnostic Pitfalls in Papillary Lesions of the Breast: Experience from a Single Tertiary Care Center. J. Clin. Diagn. Res. 2016, 10, EC18–EC21. [Google Scholar] [CrossRef] [PubMed]





| Study | Subjects | Clinical Findings | Imaging Findings | Upgrade Rate * | Follow-Up Time | Outcome | Additional Findings | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Design | Definition of MP | Number | Age (Years) | ||||||
| Carder 2008 [5] | Retrospective cohort, patients with B3/B4 lesions at CNB, with the words ‘‘papillary’’ or ‘‘papilloma’’ in the final diagnosis, followed by SE or mammotome excision | NR | 2 | NR | 48/61 Palpable breast mass (78%); 10/61 Nipple discharge (16%) | MX: 2/2 segmental indeterminate calcifications US: 1 negative; 1 altered echogenicity with nodules |
| |||
| Ali-Fehmi 2003 [6] | Retrospective cohort, patients with MP in all available pathologic materials (mastectomy/lumpectomy) | ≥5 papillomas in at least 2 non-consecutive tissue blocks |
| 36–84 (mean, 57.8) | NR | NR | NR |
|
|
|
| Carter 1977 [7] | Retrospective cohort, patients with MP in all available pathologic materials | Multiple discrete papillomas >3 mm without significant accompanying diffuse hyperplasia or papillomatosis | 6 | NR | NR | NR | NR | at least 5 years | 2/6 (33%) carcinoma (type not specified) | |
| Han 2018 [8] | Retrospective cohort, patients with IDPs without atypia at CNB (14G needle) | ≥2 lesions separated by normal breast tissue in imaging eventually proven to be benign MP on pathologic examination | 91 | NR | NR | NR | 3/91 (3.3%) upgraded to malignancy (all DCIS); 4/91 (4.4%) upgraded to high risk lesions (ADH/LIN) | NR | NR | |
| Harjit 2006 [9] | Retrospective cohort, patients with MP in all available pathologic materials (FNA/CNB/SE/mastectomy) | ≥5 papillomas in the same quadrant or in at least two consecutive surgical pathology tissue blocks | 23 total: 13 CNB (10 without atypia, 1 with ADH and 2 with DCIS); 9 excisional biopsy; 1 mastectomy | NR | 18 screen-detected lesions (non-palpable); 5 palpable lumps | NR |
| 4.1 years (range 1–10 years) | 3/23 recurrence of MP at the same site and 1/23 MP + DCIS in the contralateral breast after 1 year. | |
| Lewis 2006 [4] | Retrospective cohort, patients with IDPs on open excisional biopsy | ≥5 papillomas in 2 non-consecutive tissue blocks | 54: 41 without atypia and 13 with atypia (ADH/ALH within papilloma or surrounding parenchyma) | NR | NR | NR | NR | 16 years | RR of developing carcinoma: MP without atypia 3.01 (95%CI 1.10–6.55); MP with atypia 7.01 (95%CI 1.91–17.97). |
|
| Murad 1981 [10] | Retrospective cohort, patients with IDPs in surgical specimens | A lesion that involves many adjacent lactiferous ducts by a papillary process | 21 | NR | NR | NR | 6/21 (28.6%) showed malignancy changes within the area of MP (3/6 invasive) | NR | 50% recurrence rate after local excision | |
| Ohuchi 1984 [11] | Retrospective cohort, patients with MP in surgical specimens | NR | 15 | NR | NR | NR | 5/15 (33.3%) showed malignancy changes within the area of MP (all DCIS) | NR | NR | 100% of MP involved TDLUs (some confined within the TDLU and others extended to the subsegmental/segmental level) |
| Papotti 1984 [12] | Retrospective cohort, patients with MP + DCIS in surgical specimens | From a minimum of 5 to a maximum of 153 papillomas | 18 | mean 51.3 | 44.4 % nipple discharge | NR | NR | 17 months | 1/7 patients who underwent quadrantectomy recurred four years later (mastectomy was then performed) | Spatial distribution of papillomas and DCIS: MP only in the quadrant affected also by carcinoma in 6/11 mastectomy specimens; whole quadrant affected in 7/7 quadrantectomy cases |
| Pellettiere 1970 [13] | Retrospective cohort, patients with MP in surgical specimens | NR | 97 | mean 45.5 (range 18–71 years) |
| NR | NR | 5–18 years | 4/97 subsequently developed biologically invasive cancer: 2/4 ipsilateral developed in 1–3 years, while the 2/4 contralateral both developed in 4 years |
|
| Raju 1996 [14] | Retrospective cohort, patients with MP on open excisional biopsy | NR | 10 MP with ADH and 13 MP without atypia | NR | MP with ADH: 3/10 nipple discharge, 1/10 palpable mass (N.R. for MP without atypia) | MX: MP with ADH: 2/10 asymmetric density, 5/10 masses (NR for MP without atypia) | NR | NR |
| In 4/23 cases, MP was bilateral |
| Chang 2011 [15] | Prospective study, patients with SE of non-malignant papillary lesions diagnosed at US-guided 11-gauge VAB | NR | 7 | NR | NR | NR | 2/7 (28.6%) upgraded to MP + ADH | NR | NR | |
| Ciatto 1991 [16] | Retrospective cohort, patients with IDPs on surgical specimens (complete resection or mastectomy) | NR | 84 | NR | All patients self-referred for nipple discharge | NR | NR | 2 to 14 years (average, 6.62 years) | RR of developing carcinoma 1.40 (95%CI 0.04–7.79) | |
| Fu 2012 [17] | Retrospective cohort, CNB-diagnosed papillary lesions of the breast with subsequent excisional biopsy | NR | 109: 77 without atypia, 25 with atypia | NR | NR | NR |
| NR | NR | |
| Gendler 2004 [18] | Retrospective cohort, biopsy-diagnosed papillary lesions of the breast with subsequent SE | ≥5 papillomas in at least 2 consecutive surgical pathology tissue blocks | 11 | NR | NR | NR | 5/11 (45%) upgraded to breast cancer and 3/11 (27%) upgraded to MP + ADH | NR | NR | |
| Kabat 2010 [19] | Nested case-control study (Cases: women with biopsy for benign breast disease including IDP and who subsequently developed BC; controls: individually matched to cases women with biopsy for benign breast disease who did not develop breast cancer in the same FUP interval as that for the cases) | ≥3 papillomas | 11 | NR | NR | NR | NR | 15.4 years | Unadjusted OR 1.38 (95%CI 0.56–3.44) Adjusted OR 1.36 (95%CI 0.52–3.51) | |
| Koo 2013 [20] | Retrospective cohort, biopsy-diagnosed papillary lesions of the breast with subsequent SE | NR | 98 | NR | NR | NR | 10/98 (10.2%) papillary DCIS | NR | NR | Use of IHC may decrease upgrade-to-malignancy rate for benign papillary lesions on US-guided 14G CNB |
| Liberman 2006 [21] | Retrospective cohort, biopsy-diagnosed papillary lesions of the breast with subsequent SE or >2 years FUP | NR | 10: 7 surgically excised and 3 stable at FUP | NR | NR | NR | 2/10 (20.0%) upgraded to breast cancer and 3/10 (30.0%) upgraded to MP + ADH | NR | NR | In 4/7 surgically excised MP, other high-risk lesions were founded (3 ADH, 1 RS) |
| Sohn 2013 [22] | Retrospective cohort, 14G CNB-diagnosed papillary lesions of the breast with subsequent VAB or SE | NR | 17 | NR | NR | NR | 2/17 (11.8%) upgraded to atypical papillomas or papillomas with ADH | NR | NR | |
| Cardenosa 1991 [23] | Retrospective cohort, biopsy-diagnosed papillary lesions of the breast | NR | 14 peripheral MP and 12 central MP |
|
| MX:
| NR | NR | NR | The tissue adjacent to peripheral MP contained apocrine metaplasia, sclerosing adenosis, FEA, ADH, LCIS, RS |
| Manganaro 2015 [24] | Retrospective cohort, unilateral discharge patients who performed galactography and MRI | NR | 11 | NR | NR | MRI:
| NR | NR | NR |
|
| Son 2009 [25] | Retrospective cohort, patients who underwent surgery due to papillomas of the breast and performed 3D fast low-angle shot (FLASH) dynamic breast MRI | NR | 3 | 41.7 ± 12.9 (27–51) | 2/3 palpable mass, 1/3 bloody nipple discharge | MX: 2/3 microcalcifications US: 3/3 multiple masses MRI: 1/3 multiple nodular enhancement; 1 ductal non-mass enhancement, 1 segmental non-mass enhancement | NR | NR | NR | |
| Sarica 2018 [26] | Retrospective cohort, patients with a pathologic diagnosis of papillary lesion who performed MRI and US | NR | 11 | 41.45 ± 7.7 | 1/11 palpable mass, 3/11 unilateral nipple discharge | US: 3/11 dilated duct partially/completely filled with intraluminal content; 1/11 mass with ductal relation or intracystic mass; 6/11 heterogeneous tubular nonmass-like hypoechoic area or mass related to multiple dilated ducts; 1/11 occult MRI: 3/11 dilated duct and intraductal focal mass on T2; 2/11 Dilated duct and pre-contrast high T1 signal; 2/11 mass with crescentic peripheral fluid; 3/11 mass related with dilated duct-ductal contrast enhancement; 1/11 linear-ductal contrast enhancement; 3/11 segmental contrast enhancement | NR | NR | NR | |
| Bender 2009 [27] | Retrospective cohort, patients who underwent ductoscopy for pathologic nipple discharge | NR | 5 | NR | Nipple discharge | NR | NR | NR | NR | After endoscopic papillomectomy, nipple discharge stopped in all patients without recurrences |
| Kamali 2014 [28] | Prospective cohort, patients who underwent ductoscopy for pathologic nipple discharge and diagnosed with MP on final histology | NR | 14 | NR | Nipple discharge | MX: 4/14 soft tissue mass; 1/14 microcalcifications; 2/14 distortions | NR | NR | NR | Ductoscopy was diagnostic in 8/14 patients |
| Ling 2009 [29] | Retrospective cohort, patients who underwent ductoscopy for pathologic nipple discharge and subsequent MP diagnosis on final histology | NR | 12 | NR | Nipple discharge | NR | NR | NR | NR | All MP were underestimated as solitary papilloma (4/12), or ductal hyperplasia (8/12) by intraductal biopsy. |
| Liu 2015 [30] | Prospective cohort, patients who underwent ductoscopy for pathologic nipple discharge and diagnosed with MP on final histology | NR | 42 | NR | Nipple discharge | NR | NR | NR | NR | Ductoscopy was diagnostic in 24/42 patients |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rella, R.; Romanucci, G.; Arciuolo, D.; Scaldaferri, A.; Bufi, E.; Croce, S.; Caulo, A.; Tommasini, O. Multiple Papillomas of the Breast: A Review of Current Evidence and Challenges. J. Imaging 2022, 8, 198. https://doi.org/10.3390/jimaging8070198
Rella R, Romanucci G, Arciuolo D, Scaldaferri A, Bufi E, Croce S, Caulo A, Tommasini O. Multiple Papillomas of the Breast: A Review of Current Evidence and Challenges. Journal of Imaging. 2022; 8(7):198. https://doi.org/10.3390/jimaging8070198
Chicago/Turabian StyleRella, Rossella, Giovanna Romanucci, Damiano Arciuolo, Assunta Scaldaferri, Enida Bufi, Sebastiano Croce, Andrea Caulo, and Oscar Tommasini. 2022. "Multiple Papillomas of the Breast: A Review of Current Evidence and Challenges" Journal of Imaging 8, no. 7: 198. https://doi.org/10.3390/jimaging8070198
APA StyleRella, R., Romanucci, G., Arciuolo, D., Scaldaferri, A., Bufi, E., Croce, S., Caulo, A., & Tommasini, O. (2022). Multiple Papillomas of the Breast: A Review of Current Evidence and Challenges. Journal of Imaging, 8(7), 198. https://doi.org/10.3390/jimaging8070198






