Near-Infrared Fluorescence Imaging in Preclinical Models of Glioblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Cultures
2.3. Animals
2.4. Tumor Xenografts
2.5. C6 Cell Orthotopic Implantation in Wistar Rats
2.6. U87-MG Cell Orthotopic Implantation in Nude Mice
2.7. In Vivo and Ex Vivo Imaging
2.8. Histological Staining
2.9. Statistical Analysis
3. Results
3.1. IRDye 800CW RGD
3.2. IRDye 800CW PEG
3.3. IRDye 800CW 2-DG
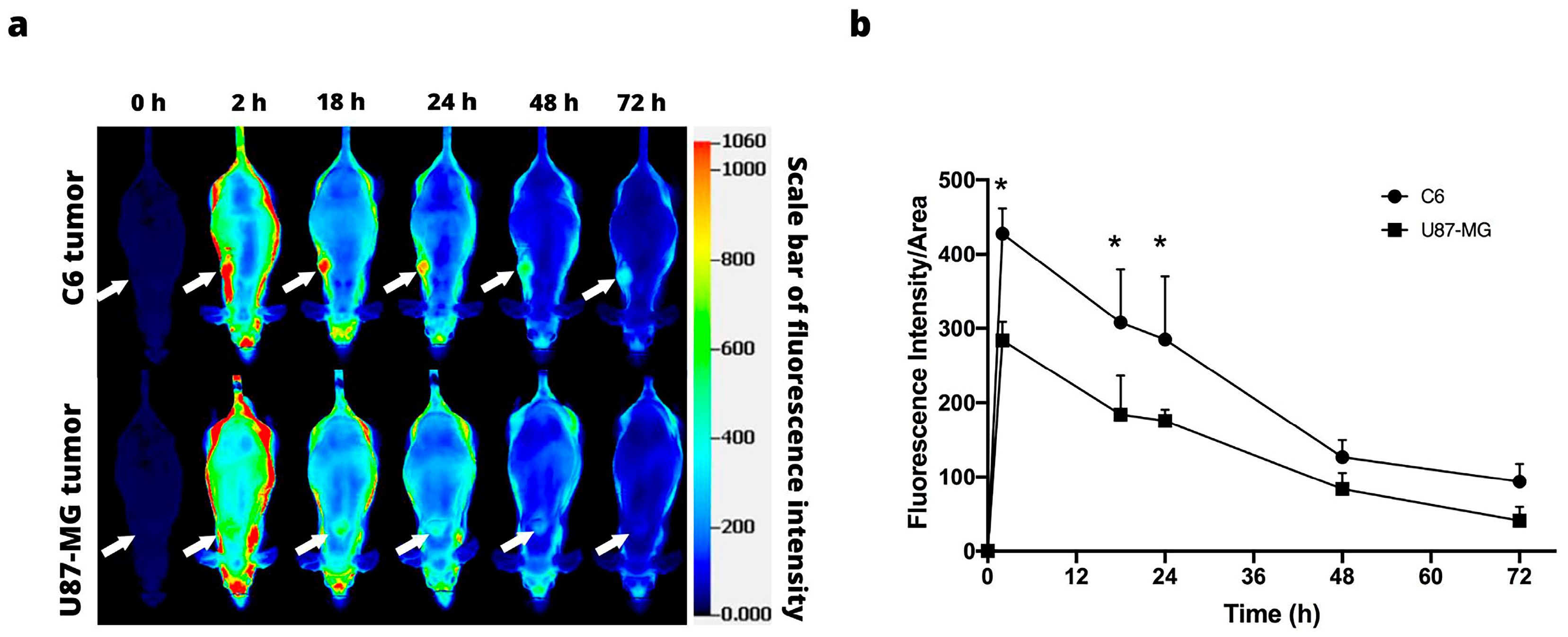
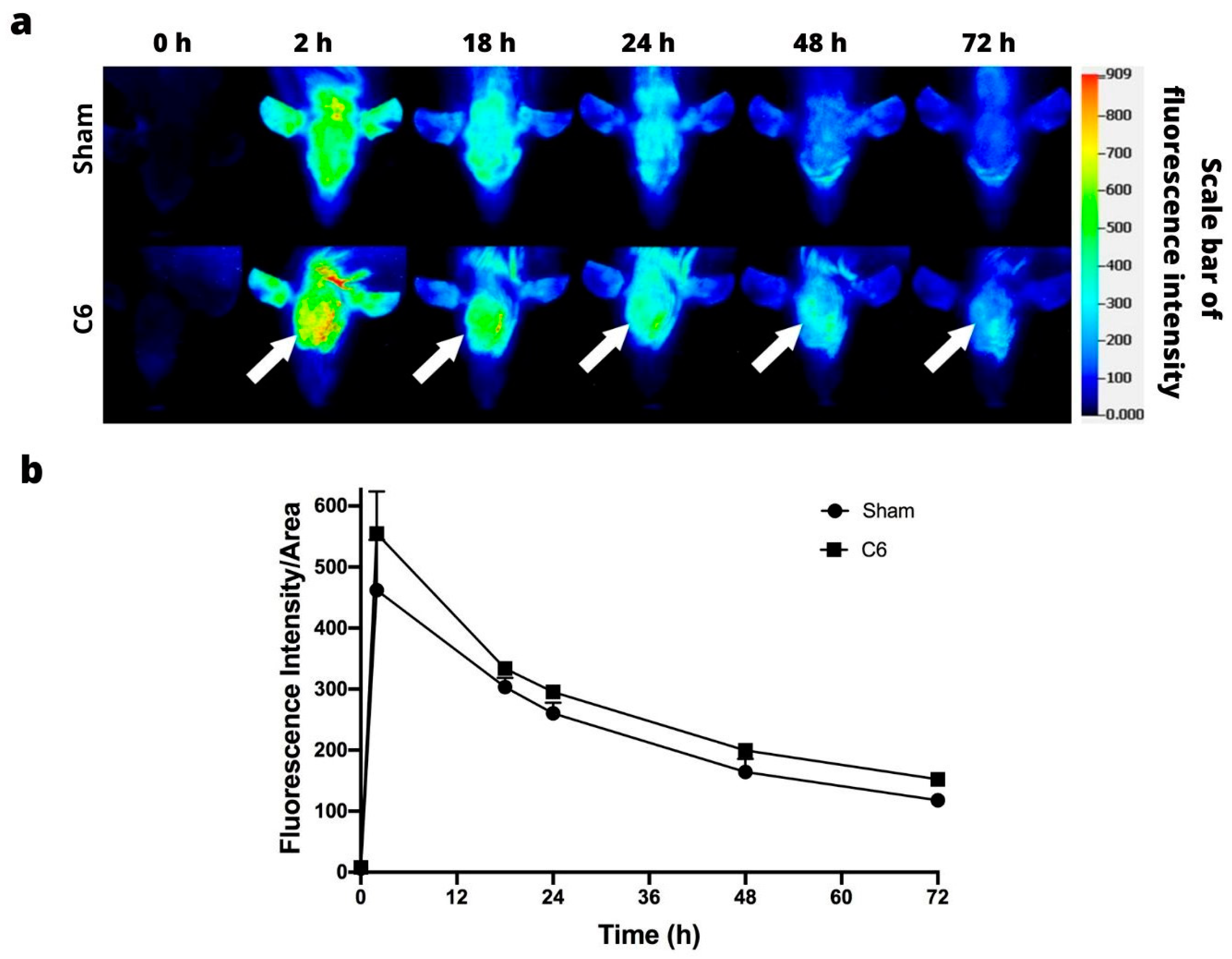
3.4. Orthotopic Models
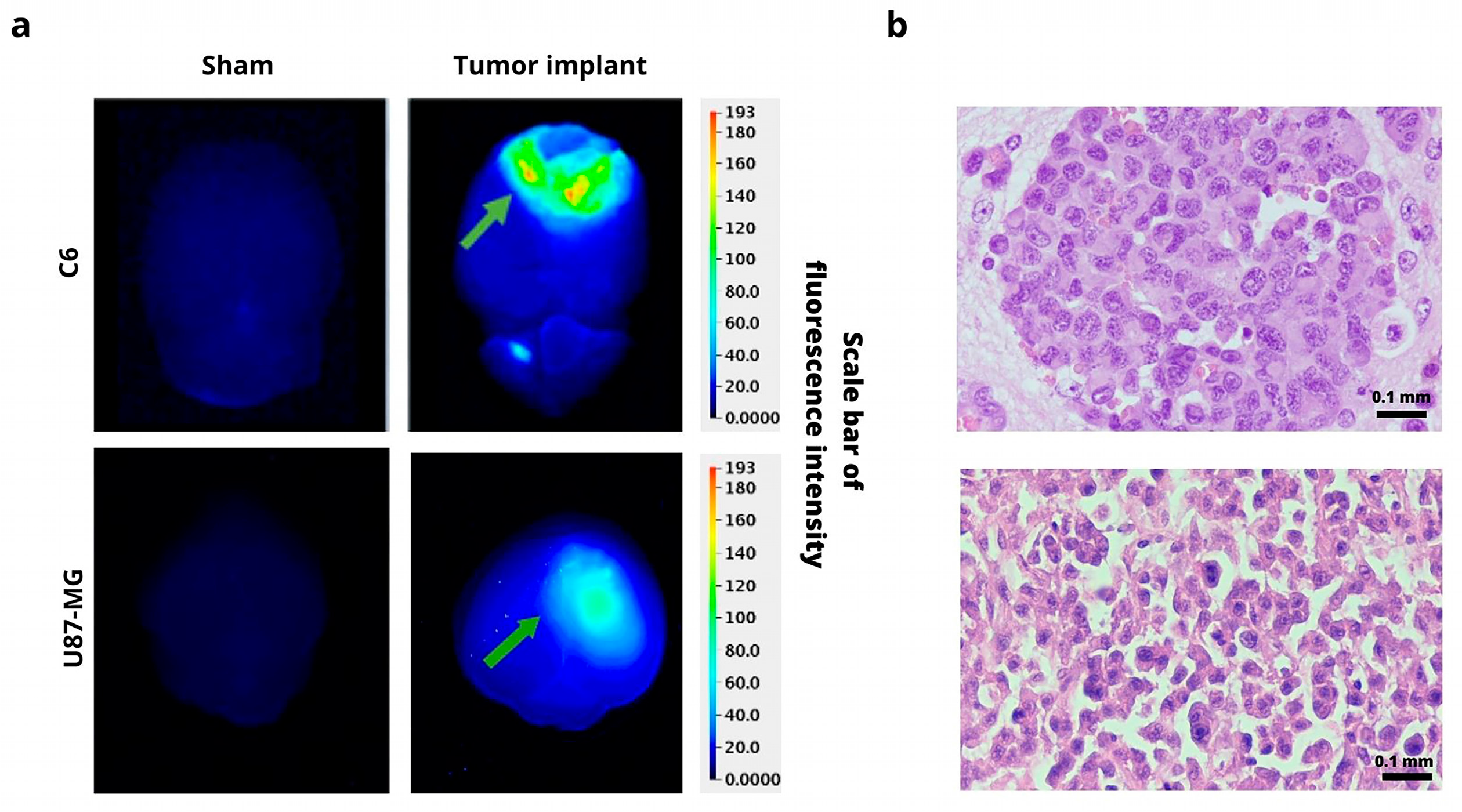
Ex Vivo Imaging and Histology Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thust, S.C.; van den Bent, M.J.; Smits, M. Pseudoprogression of brain tumors. J. Magn. Reson. Imaging 2018, 48, 571–589. [Google Scholar] [CrossRef]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Yang, G.; Jing, H.; Tan, Y.; Zhao, B.; Zhang, H. Tumor Progression and Treatment-Related Changes: Radiological Diagnosis Challenges for the Evaluation of Post Treated Glioma. Cancers 2022, 14, 3771. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.J.; Pomper, M.G. The evolution of imaging in cancer: Current state and future challenges. Semin. Oncol. 2011, 38, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.J.; Chang, T.-T.A.; Slauter, R. Chapter 32—Use of Imaging for Preclinical Evaluation. In A Comprehensive Guide to Toxicology in Preclinical Drug Development; Faqi, A.S., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 759–775. [Google Scholar]
- Orrit, M. Fluorescence as the Choice Method for Single-Molecule Detection. In Fluorescence of Supermolecules, Polymers, and Nanosystems; Berberan-Santos, M.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 105–113. [Google Scholar]
- Keereweer, S.; Van Driel, P.B.; Snoeks, T.J.; Kerrebijn, J.D.; Baatenburg de Jong, R.J.; Vahrmeijer, A.L.; Sterenborg, H.J.; Lowik, C.W. Optical image-guided cancer surgery: Challenges and limitations. Clin. Cancer Res. 2013, 19, 3745–3754. [Google Scholar] [CrossRef]
- Neijenhuis, L.K.A.; de Myunck, L.; Bijlstra, O.D.; Kuppen, P.J.K.; Hilling, D.E.; Borm, F.J.; Cohen, D.; Mieog, J.S.D.; Steup, W.H.; Braun, J.; et al. Near-Infrared Fluorescence Tumor-Targeted Imaging in Lung Cancer: A Systematic Review. Life 2022, 12, 446. [Google Scholar] [CrossRef]
- Kosaka, N.; Ogawa, M.; Choyke, P.L.; Kobayashi, H. Clinical implications of near-infrared fluorescence imaging in cancer. Future Oncol. 2009, 5, 1501–1511. [Google Scholar] [CrossRef]
- Hilderbrand, S.A.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef]
- Lwin, T.M.; Turner, M.A.; Amirfakhri, S.; Nishino, H.; Hoffman, R.M.; Bouvet, M. Fluorescence Molecular Targeting of Colon Cancer to Visualize the Invisible. Cells 2022, 11, 249. [Google Scholar] [CrossRef]
- Yang, R.Q.; Chen, M.; Zhang, Q.; Gao, Y.Y.; Lou, K.L.; Lin, T.T.; Huang, W.H.; Zeng, Y.Z.; Zhang, Y.Q.; Dang, Y.Y.; et al. Development and Preclinical Evaluation of a Near-Infrared Fluorescence Probe Based on Tailored Hepatitis B Core Particles for Imaging-Guided Surgery in Breast Cancer. Int. J. Nanomed. 2022, 17, 1343–1360. [Google Scholar] [CrossRef] [PubMed]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Li, J.; Zhao, J.; Gu, L.; Qian, X.; Yang, Y. A general approach to the design of high-performance near-infrared (NIR) D-π-A type fluorescent dyes. Chin. Chem. Lett. 2019, 30, 839–846. [Google Scholar] [CrossRef]
- Newton, A.; Predina, J.; Mison, M.; Runge, J.; Bradley, C.; Stefanovski, D.; Singhal, S.; Holt, D. Intraoperative near-infrared imaging can identify canine mammary tumors, a spontaneously occurring, large animal model of human breast cancer. PLoS ONE 2020, 15, e0234791. [Google Scholar] [CrossRef] [PubMed]
- Tipirneni, K.E.; Warram, J.M.; Moore, L.S.; Prince, A.C.; de Boer, E.; Jani, A.H.; Wapnir, I.L.; Liao, J.C.; Bouvet, M.; Behnke, N.K.; et al. Oncologic Procedures Amenable to Fluorescence-guided Surgery. Ann. Surg. 2017, 266, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; van den Berg, N.S.; Rosenthal, E.L.; Iv, M.; Zhang, M.; Vega Leonel, J.C.M.; Walters, S.; Nishio, N.; Granucci, M.; Raymundo, R.; et al. EGFR-targeted intraoperative fluorescence imaging detects high-grade glioma with panitumumab-IRDye800 in a phase 1 clinical trial. Theranostics 2021, 11, 7130–7143. [Google Scholar] [CrossRef]
- Gilmore, D.M.; Khullar, O.V.; Jaklitsch, M.T.; Chirieac, L.R.; Frangioni, J.V.; Colson, Y.L. Identification of metastatic nodal disease in a phase 1 dose-escalation trial of intraoperative sentinel lymph node mapping in non-small cell lung cancer using near-infrared imaging. J. Thorac. Cardiovasc. Surg. 2013, 146, 562–570; discussion 569–570. [Google Scholar] [CrossRef]
- Jin, H.; Varner, J. Integrins: Roles in cancer development and as treatment targets. Br. J. Cancer 2004, 90, 561–565. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Keereweer, S.; Mol, I.M.; Kerrebijn, J.D.; Van Driel, P.B.; Xie, B.; Baatenburg de Jong, R.J.; Vahrmeijer, A.L.; Lowik, C.W. Targeting integrins and enhanced permeability and retention (EPR) effect for optical imaging of oral cancer. J. Surg. Oncol. 2012, 105, 714–718. [Google Scholar] [CrossRef]
- Kovar, J.L.; Volcheck, W.; Sevick-Muraca, E.; Simpson, M.A.; Olive, D.M. Characterization and performance of a near-infrared 2-deoxyglucose optical imaging agent for mouse cancer models. Anal. Biochem. 2009, 384, 254–262. [Google Scholar] [CrossRef] [PubMed]
- [NOM-062- ZOO-1999]. Diario Oficial de la Federación, México. 15. Organización Mundial de Sanidad Animal. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 14 January 2023).
- Llaguno-Munive, M.; Romero-Pina, M.; Serrano-Bello, J.; Medina, L.A.; Uribe-Uribe, N.; Salazar, A.M.; Rodriguez-Dorantes, M.; Garcia-Lopez, P. Mifepristone Overcomes Tumor Resistance to Temozolomide Associated with DNA Damage Repair and Apoptosis in an Orthotopic Model of Glioblastoma. Cancers 2018, 11, 16. [Google Scholar] [CrossRef]
- Weissleder, R. Molecular imaging in cancer. Science 2006, 312, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Uselman, R.R.; Yee, D. Exogenous near-infrared fluorophores and their applications in cancer diagnosis: Biological and clinical perspectives. Expert. Opin. Med. Diagn. 2011, 5, 241–251. [Google Scholar] [CrossRef]
- Moreno, M.J.; Ling, B.; Stanimirovic, D.B. In vivo near-infrared fluorescent optical imaging for CNS drug discovery. Expert. Opin. Drug Discov. 2020, 15, 903–915. [Google Scholar] [CrossRef]
- Cheng, Z.; Wu, Y.; Xiong, Z.; Gambhir, S.S.; Chen, X. Near-infrared fluorescent RGD peptides for optical imaging of integrin alphavbeta3 expression in living mice. Bioconjug. Chem. 2005, 16, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Vider, J.; Kovar, J.L.; Olive, D.M.; Mellinghoff, I.K.; Mayer-Kuckuk, P.; Kircher, M.F.; Blasberg, R.G. Integrin alphavbeta3-targeted IRDye 800CW near-infrared imaging of glioblastoma. Clin. Cancer Res. 2012, 18, 5731–5740. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Razorenova, O.; McCabe, N.P.; O’Toole, T.; Qin, J.; Byzova, T.V. VEGF-integrin interplay controls tumor growth and vascularization. Proc. Natl. Acad. Sci. USA 2005, 102, 7589–7594. [Google Scholar] [CrossRef]
- Schnell, O.; Krebs, B.; Wagner, E.; Romagna, A.; Beer, A.J.; Grau, S.J.; Thon, N.; Goetz, C.; Kretzschmar, H.A.; Tonn, J.C.; et al. Expression of integrin alphavbeta3 in gliomas correlates with tumor grade and is not restricted to tumor vasculature. Brain Pathol. 2008, 18, 378–386. [Google Scholar] [CrossRef]
- Cheng, Z.; Levi, J.; Xiong, Z.; Gheysens, O.; Keren, S.; Chen, X.; Gambhir, S.S. Near-infrared fluorescent deoxyglucose analogue for tumor optical imaging in cell culture and living mice. Bioconjug. Chem. 2006, 17, 662–669. [Google Scholar] [CrossRef]
- Ma, Y.; Lee, Y.; Best-Popescu, C.; Gao, L. High-speed compressed-sensing fluorescence lifetime imaging microscopy of live cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2004176118. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.J.; Li, J.; Chaney, E.J.; Marjanovic, M.; Spillman, D.R., Jr.; Boppart, S.A. High-speed imaging of transient metabolic dynamics using two-photon fluorescence lifetime imaging microscopy. Optica 2018, 5, 1290–1296. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Xenograft Model | Orthotopic Model |
|---|---|---|
| Focus (mm) | 4.0 | 0.5 |
| Resolution (µm) | 337 | 337 |
| Quality | Medium | Medium |
| Depth of field (mm) | 4.0 | 4.0 |
| Penetration depth (mm) | 4.0 | 4.0 |
| Wavelength (nm) | 800 | 800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llaguno-Munive, M.; Villalba-Abascal, W.; Avilés-Salas, A.; Garcia-Lopez, P. Near-Infrared Fluorescence Imaging in Preclinical Models of Glioblastoma. J. Imaging 2023, 9, 212. https://doi.org/10.3390/jimaging9100212
Llaguno-Munive M, Villalba-Abascal W, Avilés-Salas A, Garcia-Lopez P. Near-Infrared Fluorescence Imaging in Preclinical Models of Glioblastoma. Journal of Imaging. 2023; 9(10):212. https://doi.org/10.3390/jimaging9100212
Chicago/Turabian StyleLlaguno-Munive, Monserrat, Wilberto Villalba-Abascal, Alejandro Avilés-Salas, and Patricia Garcia-Lopez. 2023. "Near-Infrared Fluorescence Imaging in Preclinical Models of Glioblastoma" Journal of Imaging 9, no. 10: 212. https://doi.org/10.3390/jimaging9100212
APA StyleLlaguno-Munive, M., Villalba-Abascal, W., Avilés-Salas, A., & Garcia-Lopez, P. (2023). Near-Infrared Fluorescence Imaging in Preclinical Models of Glioblastoma. Journal of Imaging, 9(10), 212. https://doi.org/10.3390/jimaging9100212






