Arteriovenous Length Ratio: A Novel Method for Evaluating Retinal Vasculature Morphology and Its Diagnostic Potential in Eye-Related Diseases
Abstract
:1. Introduction
- We propose a unique automated method that combines vascular tortuosity assessment with AVLR analysis, which could be used to detect and grade HR. Since this is a novel approach and is not present in existing medical literature, our study represents a substantial development in the area.
- We provide a novel iterative approach to compute the AVLR inside the ROI (region of interest) around the optic disc using vessel caliber data. This technique improves the AVLR assessment’s precision and dependability.
- In the assessment of vessel AVLR, we employ a unique combination of nine features, which helps with accurately quantifying the severity of AVLR and effectively minimizing the subjectivity and variability associated with manual assessments conducted by ophthalmologists.
- The AV-Classification dataset has been expanded with new labels for AVLR metrics, creating the AV-Classification dataset extension. This extension includes vessel segments and image-level AVLR metrics, quantified using the proposed methodology.
2. Related Work
3. Methodologies and Materials
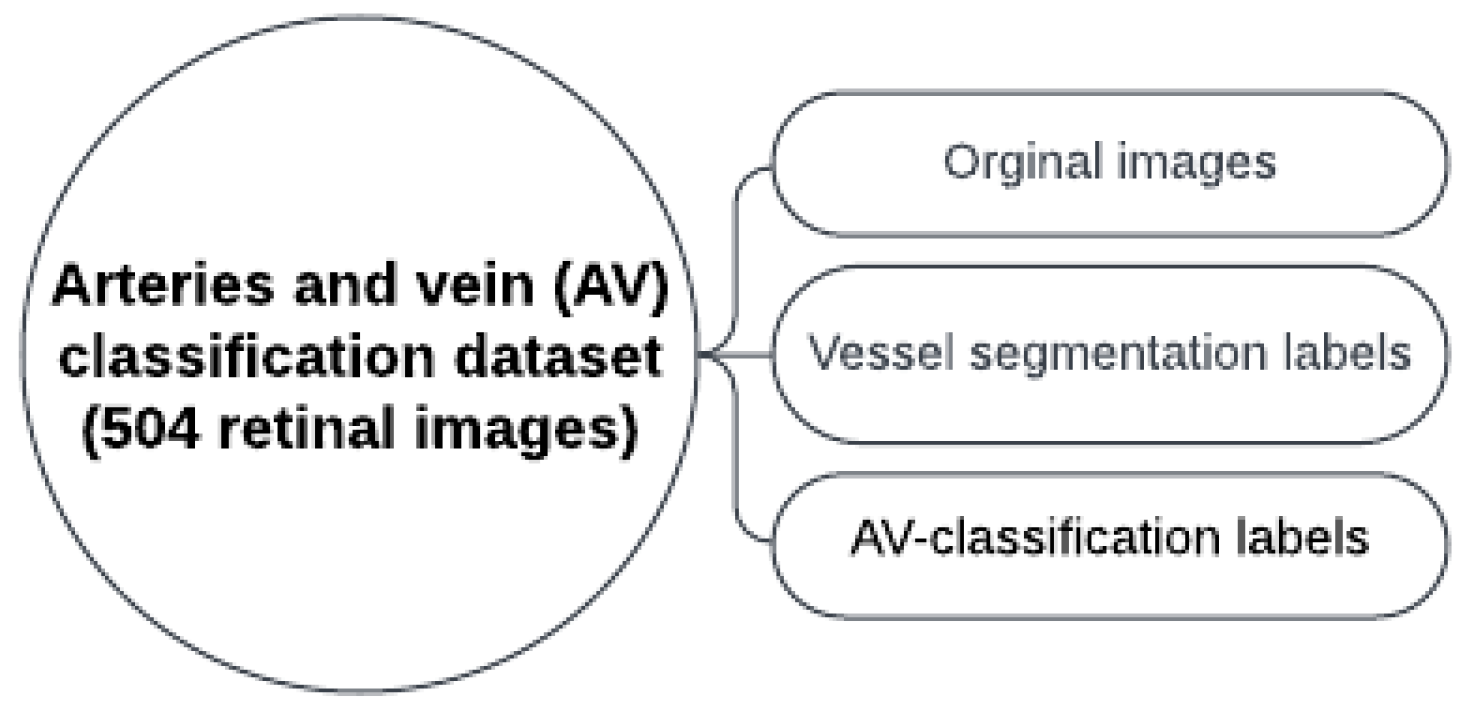
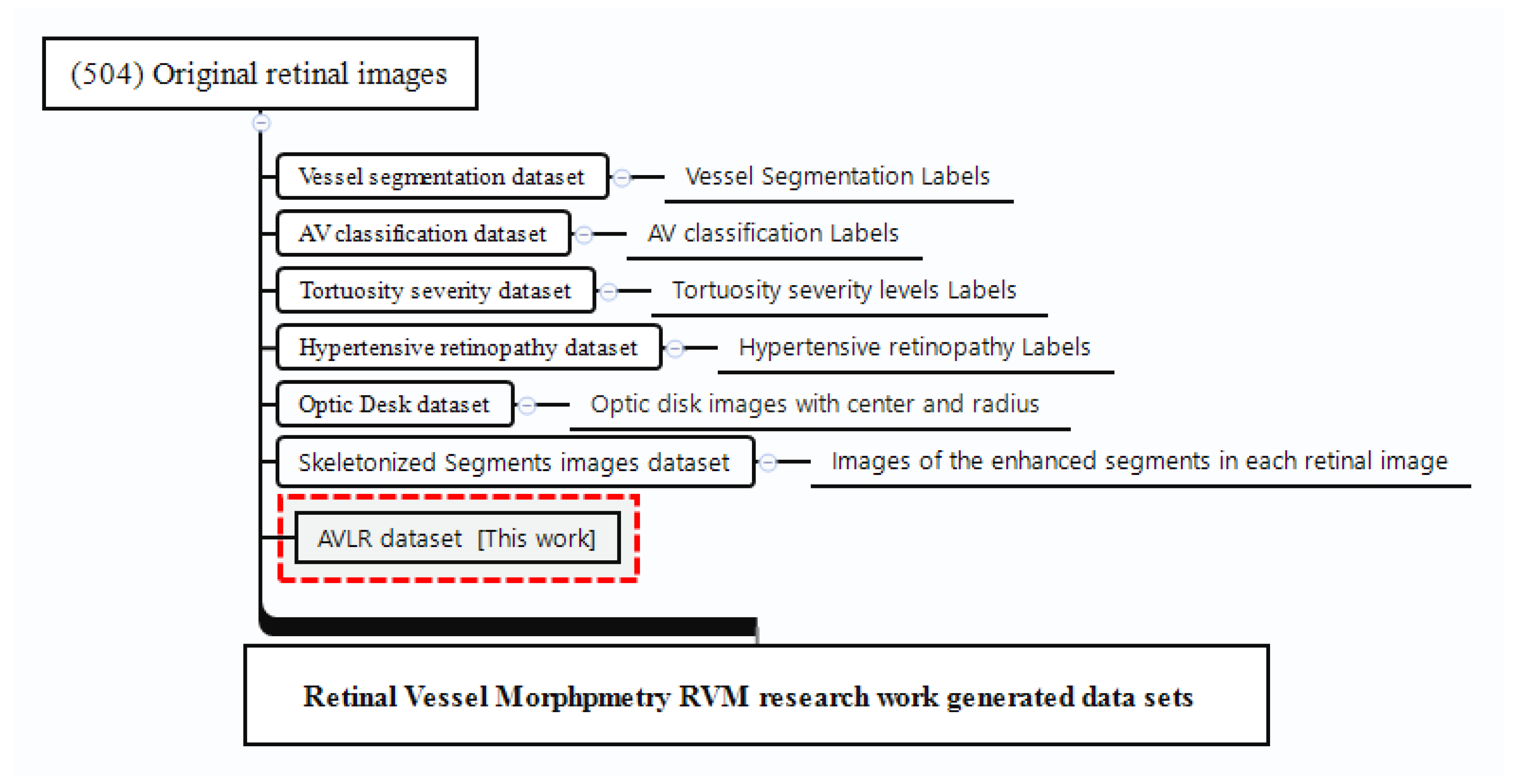
3.1. Used Materials
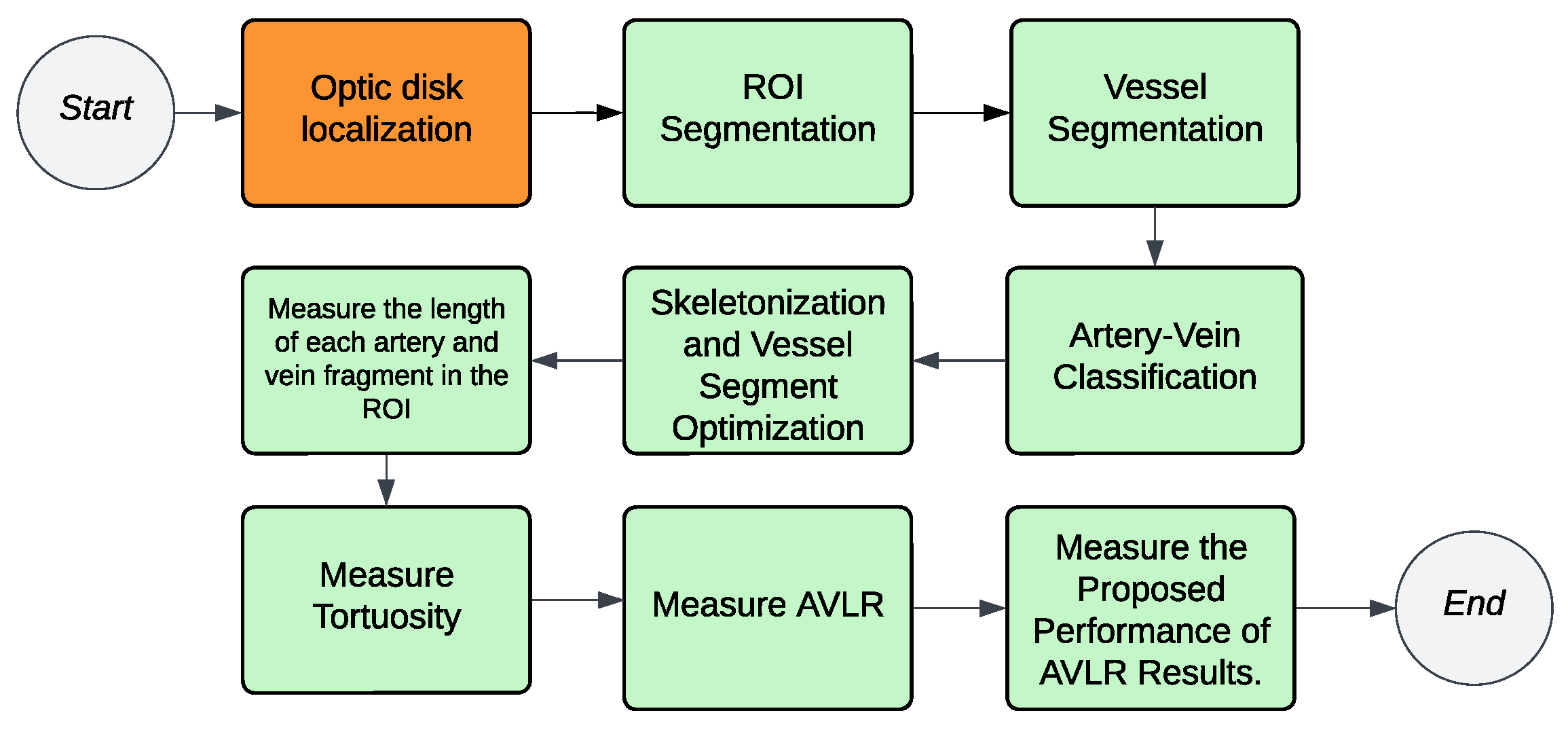
3.2. Methodology
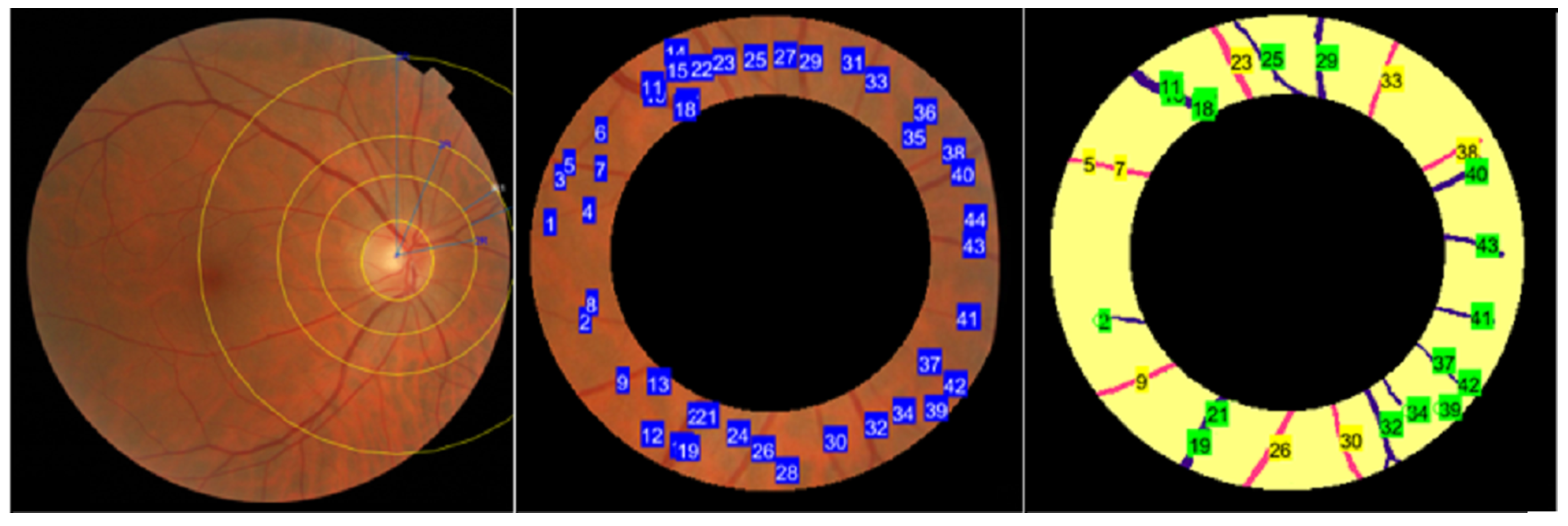
- Optic Desk Localization: The fundus image undergoes a process to detect the location of the optic disk.
- ROI Segmentation: The fundus images are subjected to ROI segmentation to detect and extract the ROI-2-3 ROD and 2-5 ROD from the rest of the image.
- Vessel segmentation: The retinal images are subjected to a vessel segmentation process to separate the blood vessels from the background.
- Artery–vein classification: The segmented vessels are analyzed to differentiate between arteries and veins in the vascular structure of the ROI.
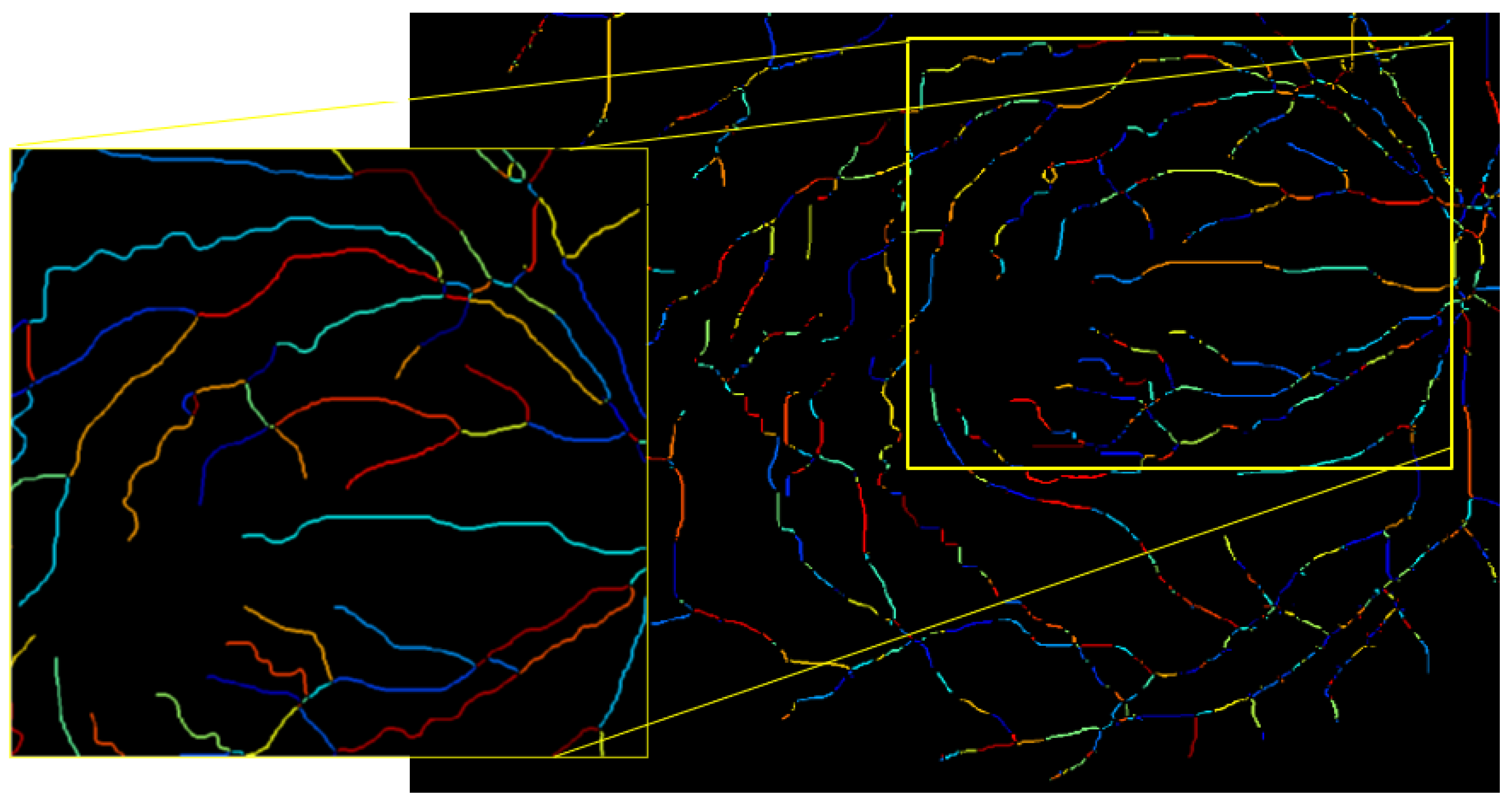
- Vessel skeletonization: The segmented vessels are further processed using skeletonization techniques to obtain a thin representation of the vascular structure.
- Identification of the bifurcation/intersection points: Key points along the skeletonized vessels are identified to extract the vessel segments between them.
- Generate the optimized vessel segments: Optimized vessel segments are generated based on the verified points, focusing on specific regions of interest.
- Calculate each of the nine metrics long feature vectors: The nine length/tortuosity metrics are calculated for each optimized vessel segment, providing quantitative measurements for AVLR assessment.
- Finalize the new AVLR feature set: The calculated AVLR values from each vessel segment are compiled to form a new feature set specifically for AVLR analysis.
3.3. Artery–Vein Classification
3.4. Vasculature Segmentation and Skeletonization
3.5. Optimized Retinal Images
3.6. AVLR Metrics
- Arteriovenous Chord Length Ratio: A ratio of the average Euclidean length of all veins to all arteries in the retinal picture is represented by this measure. It measures the proportional variations in length between veins and arteries.
- Arteriovenous Arc Length Ratio: The ratio of all the arteries’ mean geodesic distance to all the veins’ mean geodesic distance in the retinal picture is represented by this measure. It sheds light on the differences and curving routes of veins and arteries.
- Arteriovenous Distance Metric Ratio: The ratio of the average tortuosity length metric of all veins to all arteries in the retinal picture is represented by this measure. The overall tortuosity and variations in vein and artery curvature are quantified.
- Arteriovenous Inflection Count Metric Ratio: This measure shows the proportion of the average tortuosity. The total inflections measurement of each artery to the average tortuosity total inflections measurement of every vein in the retinal picture. The quantity of inflection points is counted, which represents the degree of vascular tortuosity.
- Arteriovenous Inflection Count Metric Binomial Ratio: The mean tortuosity inflection count measure binomial of all arteries divided by the average tortuosity inflection_Count_Metric_Binomial of all veins in the retinal picture is represented by this measure. It evaluates the veins’ and arteries’ binomial distribution of inflection sites.
- Arteriovenous Sum of Angles Metric Ratio: This metric is the average tortuosity inflection count divided by its ratio. Comparing the average inflection count metric of all arteries to the binomial of all veins in the retinal image; it assesses the binomial distribution of artery and vein inflection points.
- Arteriovenous Norm of Curvature Ratio: This measure represents the proportion of the average curvature of all vein segments to all artery segments in the retinal picture. It gauges the vessel segments’ curvature.
- Arteriovenous of Average Curvature Ratio Standard Deviation: This statistic calculates the difference between the average curvature of all arteries’ mean standard deviation and the average curvature of all veins’ mean standard deviation. the retinal picture. Quantification is used to the curvature variation along vessel segments.
- Arteriovenous of Centerline Length Ratio: This metric represents The mean centerline length of all the arteries divided by the mean centerline length of all the veins in the retinal image. It determines the distances between the arteries’ and veins’ centerlines.
3.7. The New Feature-Set Preparation
3.8. Right and Left Eyes Comparison
4. Results
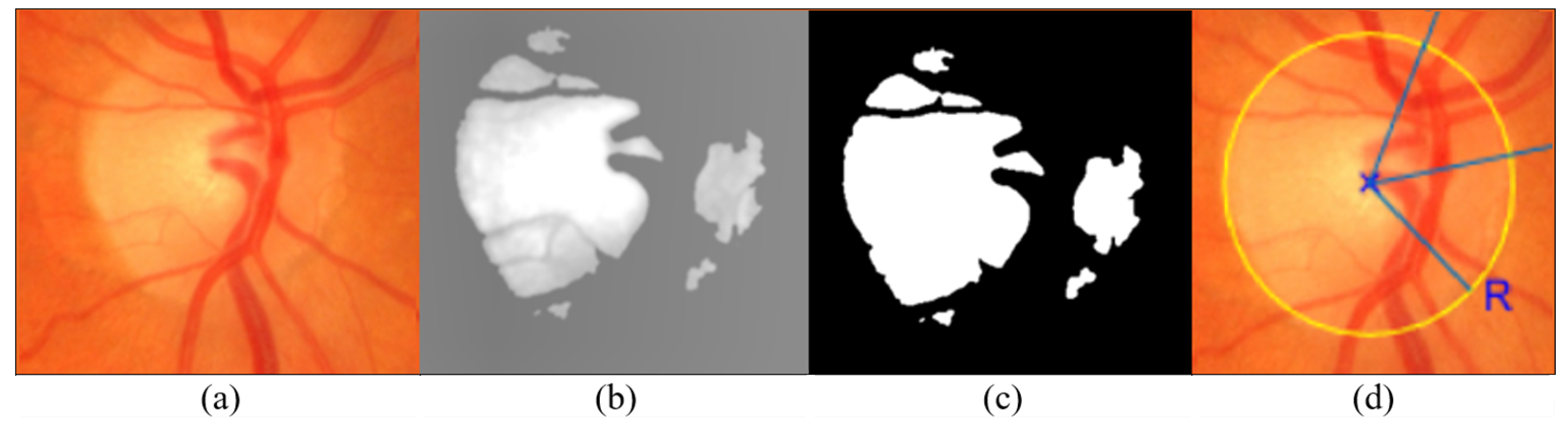
4.1. Optic Disc Localization
4.2. ROI Segmentation
4.3. Vessel Segmentation
4.4. Artery Vein Classification
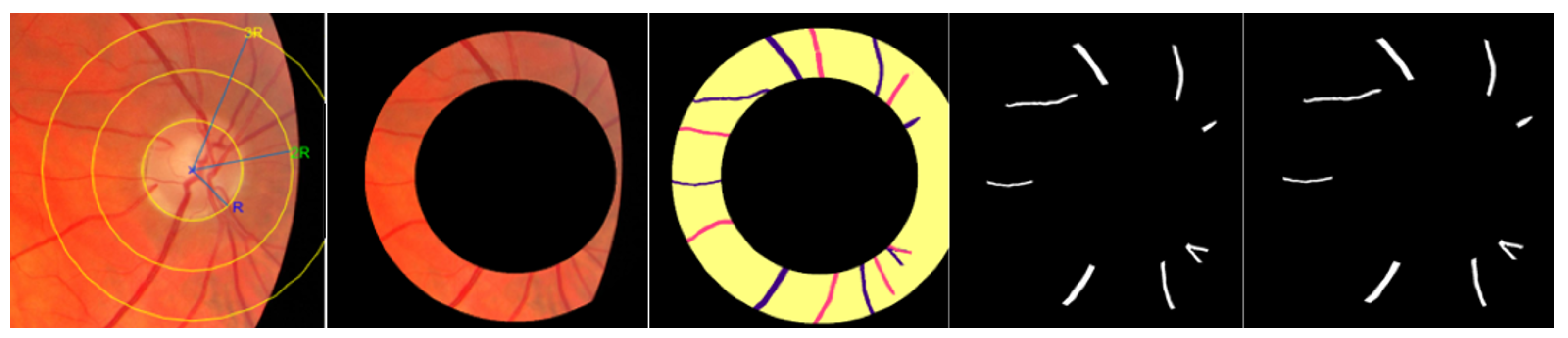
4.4.1. Skeletonization and Vessel Segment Optimization
4.4.2. Measure the Length of Each Artery and Vein Fragment in the ROI
4.5. Observations on Fundus Images and Their Corresponding Metric Values
4.5.1. Arteriovenous Length Analysis
4.5.2. An Intriguing Observation
- Atypically Elongated Arteries and Normal Veins: In a subset of retinal images within the AV-Classification dataset, we encountered instances where the arteries displayed significant elongation while the veins retained a relatively normal appearance. This divergence in vascular morphology raises intriguing questions about the potential factors driving this asymmetry and may help physicians to efficiently diagnose retinal abnormalities. Figure 14 showcases that.
- Atypically Elongated Veins and Normal Arteries: On the other hand, a contrasting pattern was evident in certain other fundus images. The veins showcased noticeable elongation, while the arteries preserved a normal appearance. This polarized pattern adds another layer of complexity to our understanding of retinal vascular abnormalities; such cases may be signs of specific further diagnoses.
- Mixed Elongation: A subset of images revealed that both the arteries and veins exhibited varying degrees of elongation or, conversely, both appeared to be normal. This variability within individual retinas underscores the intricate nature of vascular architecture and emphasizes the need for a more holistic investigation; Figure 15 showcases this.
4.5.3. Asymmetry in in Eye Pairs
4.5.4. Discussion
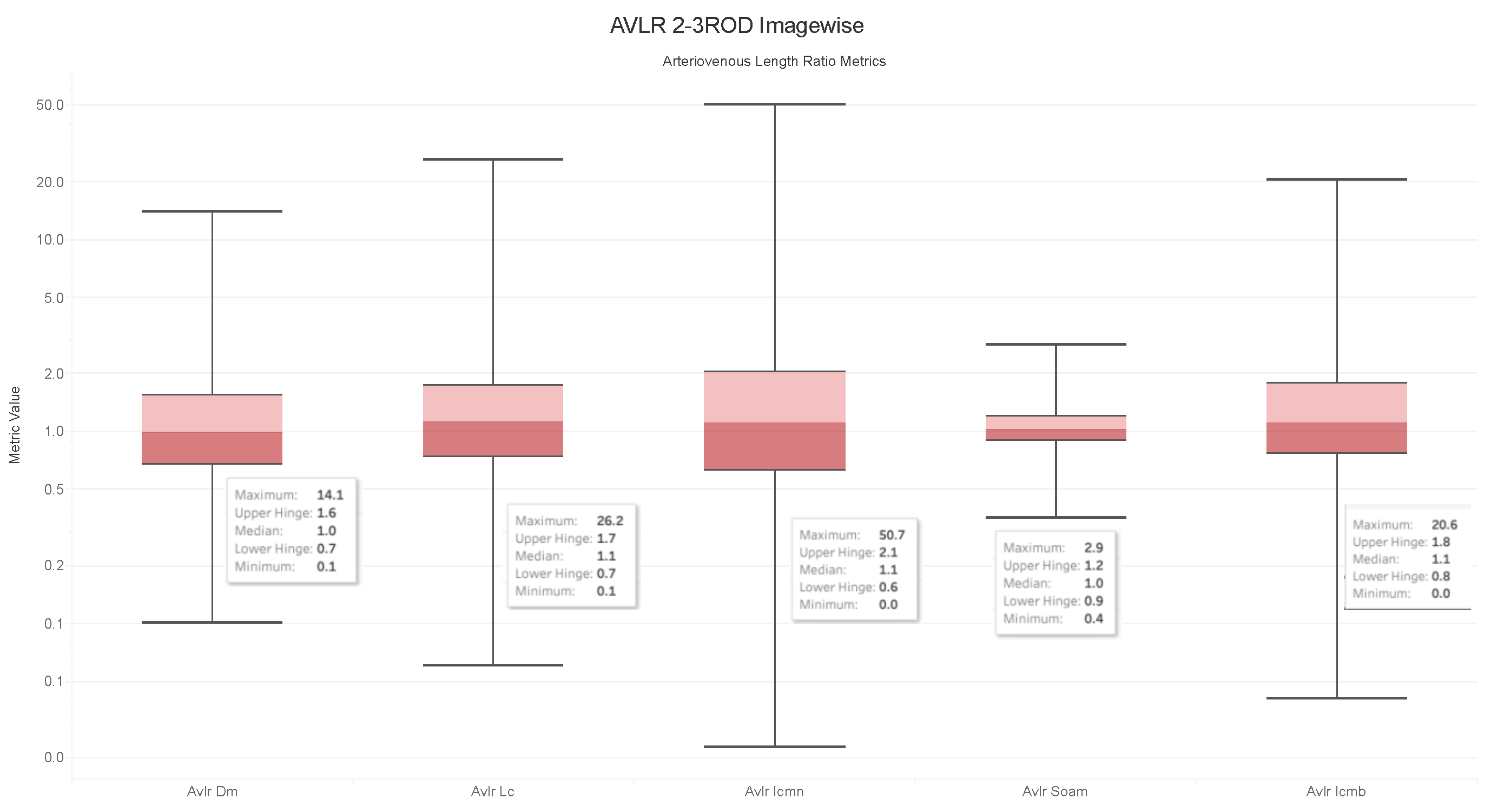
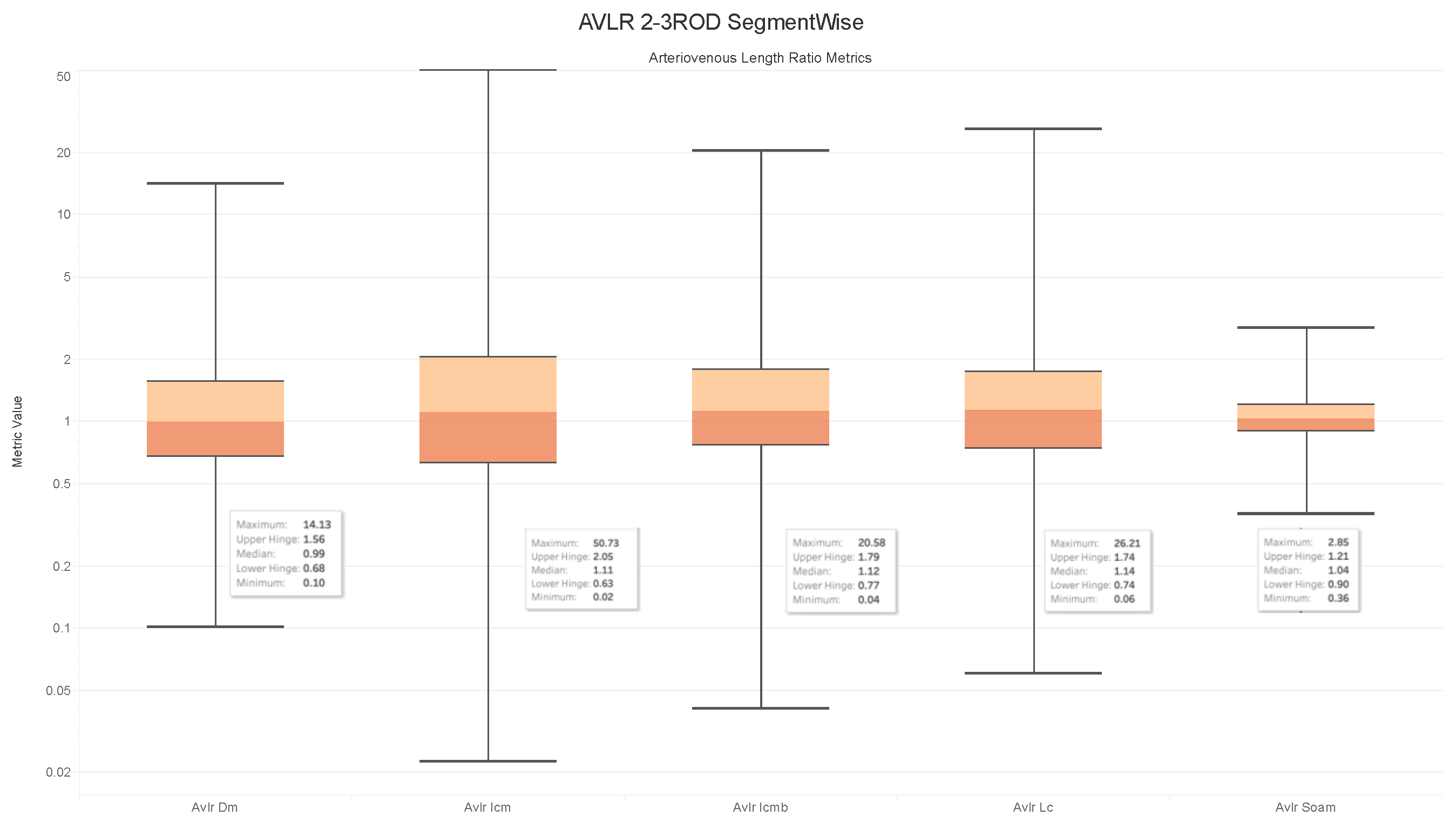
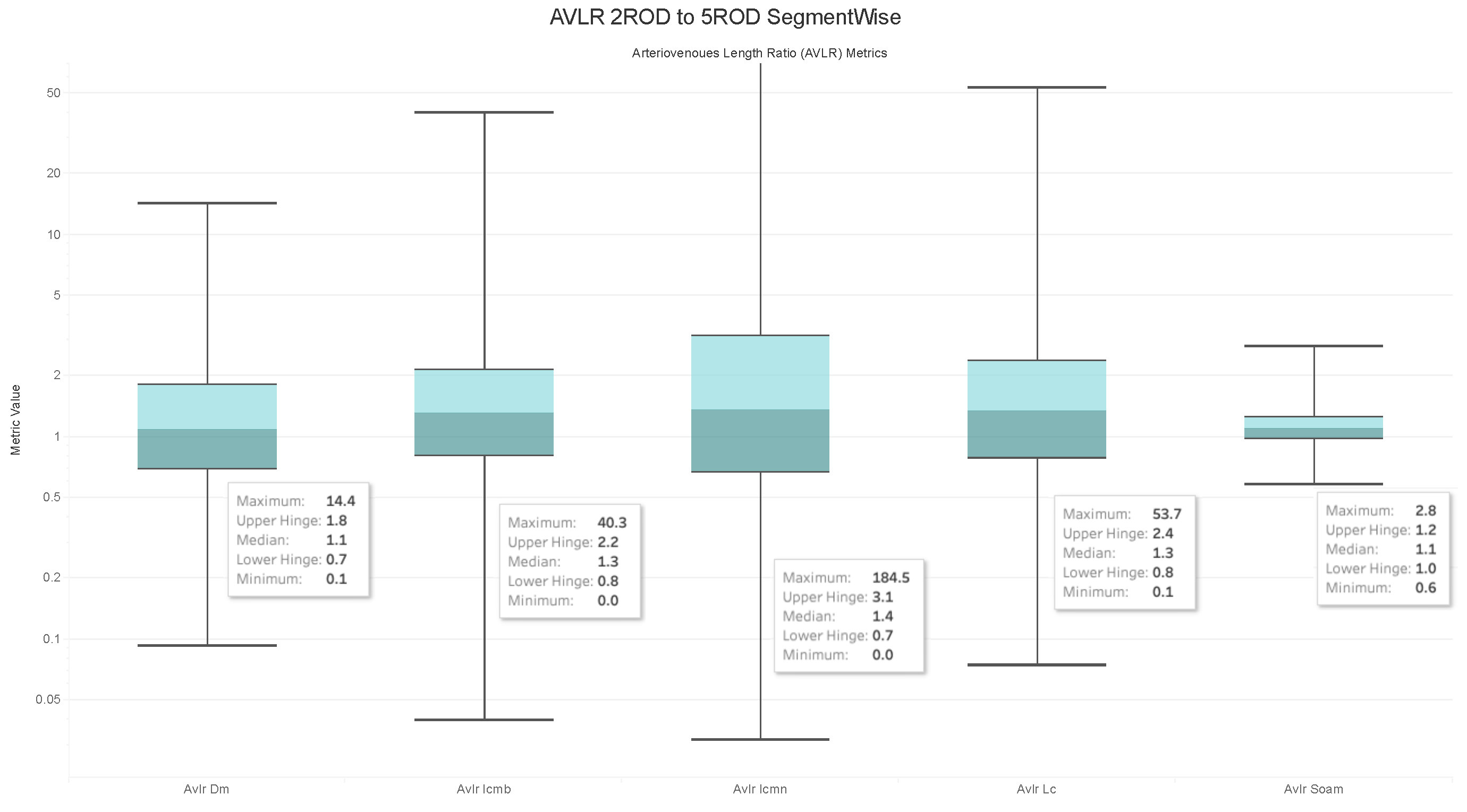
4.6. Observations on the Box Plot Graphs
Image Level Arterioveinous Length Ratios Analysis
- The ratios for veins and arteries often show a comparable length pattern, indicating that the morphological lengthiness of veins and arteries is almost the same, with a ratio approaching 1.
- The center value of the box plots, or the median, slightly surpasses 1, indicating that the mean length of a vein is shorter than the average length of the artery.
- The ratio moves toward the top of the box plot when the artery-length mean is higher than that of the veins. As a consequence, when the length difference grows, the retinal image point rises in the box plot to approximately 50% of the normal distribution data.
- On the other hand, the ratio veers toward the bottom of the box plot when the average length of both arteries is smaller than the average length of both veins. The retinal image point in the box plot moves below the median as the length difference decreases. This tendency is seen in around 50% of the data from the normal distribution plot.
- In order to evaluate the difference between the lengths of the arteries and veins, the arc and chord lengths may be substituted because of the normal distribution of both ratios. In the meanwhile, for certain pictures in the AV-Classification dataset, the box plot in Figure 20 shows that the arteriovenous tortuosity ratios in (4)–(9) are skewed above the mean and median. This observation leads us to the following conclusions:
- The arteriovenous SOAM ratio illustrates how the curvature angle influences the calculation and limits the findings within the constrained range of the y-axis (0 to 360) by displaying a regular distribution with a mean and median of 1. However, any possible distinctions between veins and arteries may not be readily apparent because of this narrow range.
- The lengths of the arteries and veins lengthen and the ratio becomes closer to one when they have the same degree of AVLR. While the mean and median of the other AVLR ratios range from 0.99 to 1.4, the AV SOAM ratio has both a mean and a median of 1. The variations in the artery and vein diameters and their visibility on the retinal surface in each picture may be ascribed to this deviation.
- The ratio moves to the top part of the box plot if the average length of veins is shorter than the average length of arteries. This implies that the tortuosity of arteries is much greater than that of veins in the retinal image.
- Conversely, in the event that the average length of the arteries is lower than the mean lengths of the veins, the ratio will shift towards the lower end of the box plot. Vein tortuosity does not deviate substantially from the median in the box plots shown in Figure 17, Figure 18, Figure 19 and Figure 20, suggesting that fewer cases of vein tortuosity arise in veins alone.
- The retinal images in the AV-Classification dataset display inconsistencies in the length and tortuosity of arteries and veins.
- Specifically, some images show tortuous arteries with normal veins, while others have tortuous veins but normal arteries.
- Additionally, there are images where both artery and vein lengths are either tortuous or normal.
4.7. AVLR New Dataset
- Arteriovenous chord length ratio;
- Arteriovenous arc length ratio;
- Arteriovenous distance metric ratio;
- Arteriovenous inflection count metric ratio;
- Arteriovenous inflection count metric binomial ratio;
- Arteriovenous sum of angles metric ratio;
- Arteriovenous norm of curvature ratio;
- Arteriovenous standard deviation of average curvature ratio;
- Arteriovenous centerline length ratio.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HR | Hypertensive Retinopathy |
| AVLR | Arteriovenous Length Ratio |
| AVR | Arteriovenous Ratio |
| ROI | Region of Interest |
| ROD | Radius of Optic disc |
| AV | Arteriovenous |
| DM | Distance Metric |
| SOAM | Sum of Angles Metric |
| ICM | Inflection Count Metric |
| ICMB | Inflection Count Metric Binomial |
| NC | Norm of Curvature |
| SDAC | Standard Deviation of Average Curvature |
| CL | Centerline Length |
| FCN | Fully Convolutional Neural Network |
| CNN | Convolutional Neural Network |
| OCT | Optical Coherence Tomography |
| RVM | Retinal Vessel Morphometry |
References
- Kanski, J.J.; Bowling, B. Clinical Ophthalmology: A Systematic Approach; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Dascalu, A.M.; Serban, D.; Tanasescu, D.; Vancea, G.; Cristea, B.M.; Stana, D.; Nicolae, V.A.; Serboiu, C.; Tribus, L.C.; Tudor, C.; et al. The Value of White Cell Inflammatory Biomarkers as Potential Predictors for Diabetic Retinopathy in Type 2 Diabetes Mellitus (T2DM). Biomedicines 2023, 11, 2106. [Google Scholar] [CrossRef] [PubMed]
- Fraz, M.; Badar, M.; Malik, A.; Barman, S. Computational methods for exudates detection and macular edema estimation in retinal images: A survey. Arch. Comput. Methods Eng. 2019, 26, 1193–1220. [Google Scholar] [CrossRef]
- Wegmann-Burns, M.; Gugger, M.; Goldblum, D. Hypertensive retinopathy. Lancet 2004, 363, 456. [Google Scholar] [CrossRef] [PubMed]
- Badawi, S.A.; Fraz, M.M.; Shehzad, M.; Mahmood, I.; Javed, S.; Mosalam, E.; Nileshwar, A.K. Detection and grading of hypertensive retinopathy using vessels tortuosity and arteriovenous ratio. J. Digit. Imaging 2022, 35, 281–301. [Google Scholar] [CrossRef] [PubMed]
- García-Sierra, R.; López-Lifante, V.M.; Isusquiza Garcia, E.; Heras, A.; Besada, I.; Verde Lopez, D.; Alzamora, M.T.; Forés, R.; Montero-Alia, P.; Ugarte Anduaga, J.; et al. Automated Systems for Calculating Arteriovenous Ratio in Retinographies: A Scoping Review. Diagnostics 2022, 12, 2865. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Akram, M.U.; Sharif, M.; Tariq, A.; ullah Yasin, U. Arteriovenous ratio and papilledema based hybrid decision support system for detection and grading of hypertensive retinopathy. Comput. Methods Programs Biomed. 2018, 154, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Agurto, C.; Joshi, V.; Nemeth, S.; Soliz, P.; Barriga, S. Detection of hypertensive retinopathy using vessel measurements and textural features. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 5406–5409. [Google Scholar] [CrossRef]
- Khitran, S.; Akram, M.U.; Usman, A.; Yasin, U. Automated system for the detection of hypertensive retinopathy. In Proceedings of the 2014 4th International Conference on Image Processing Theory, Tools and Applications (IPTA), Paris, France, 14–17 October 2014; pp. 1–6. [Google Scholar] [CrossRef]
- Mapayi, T.; Tapamo, J.R.; Viriri, S.; Adio, A. Automatic Retinal Vessel Detection and Tortuosity Measurement. Image Anal. Stereol. 2016, 35, 117–135. [Google Scholar] [CrossRef]
- Abbas, Q.; Qureshi, I.; Ibrahim, M.E.A. An Automatic Detection and Classification System of Five Stages for Hypertensive Retinopathy Using Semantic and Instance Segmentation in DenseNet Architecture. Sensors 2021, 21, 6936. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R.; Rab, S. Significance of machine learning in healthcare: Features, pillars and applications. Int. J. Intel. Netw. 2022, 3, 58–73. [Google Scholar] [CrossRef]
- Foulsham, W.; Chien, J.; Lenis, T.L.; Papakostas, T.D. Optical Coherence Tomography Angiography: Clinical Utility and Future Directions. J. Vitreoretin. Dis. 2022, 6, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, R.C.; Woods, R.E.; Eddins, S.L. Digital Image Processing Using MATLAB; Pearson Prentice Hall: Hoboken, NJ, USA, 2004. [Google Scholar]
- Badawi, S.M. Segmentation, Classification, and Morphometric Analysis of Retinal Vasculature. Ph.D. Thesis, National University for Sciences and Technology, Islamabad, Pakistan, 2020. [Google Scholar]
- Badawi, S.A.; Fraz, M.M. Multiloss function-based deep convolutional neural network for segmentation of retinal vasculature into arterioles and venules. BioMed Res. Int. 2019, 2019, 4747230. [Google Scholar] [CrossRef] [PubMed]
- Badawi, S.A.; Fraz, M.M. Optimizing the trainable B-COSFIRE filter for retinal blood vessel segmentation. PeerJ 2018, 6, e5855. [Google Scholar] [CrossRef] [PubMed]
- Badawi, S.A.; Takruri, M.; ElBadawi, I.; Chaudhry, I.A.; Mahar, N.U.; Nileshwar, A.K.; Mosalam, E. Enhancing Vessel Segment Extraction in Retinal Fundus Images Using Retinal Image Analysis and Six Sigma Process Capability Index. Mathematics 2023, 11, 3170. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badawi, S.A.; Takruri, M.; Al-Hattab, M.; Aldoboni, G.; Guessoum, D.; ElBadawi, I.; Aichouni, M.; Chaudhry, I.A.; Mahar, N.; Nileshwar, A.K. Arteriovenous Length Ratio: A Novel Method for Evaluating Retinal Vasculature Morphology and Its Diagnostic Potential in Eye-Related Diseases. J. Imaging 2023, 9, 253. https://doi.org/10.3390/jimaging9110253
Badawi SA, Takruri M, Al-Hattab M, Aldoboni G, Guessoum D, ElBadawi I, Aichouni M, Chaudhry IA, Mahar N, Nileshwar AK. Arteriovenous Length Ratio: A Novel Method for Evaluating Retinal Vasculature Morphology and Its Diagnostic Potential in Eye-Related Diseases. Journal of Imaging. 2023; 9(11):253. https://doi.org/10.3390/jimaging9110253
Chicago/Turabian StyleBadawi, Sufian A., Maen Takruri, Mohammad Al-Hattab, Ghaleb Aldoboni, Djamel Guessoum, Isam ElBadawi, Mohamed Aichouni, Imran Ali Chaudhry, Nasrullah Mahar, and Ajay Kamath Nileshwar. 2023. "Arteriovenous Length Ratio: A Novel Method for Evaluating Retinal Vasculature Morphology and Its Diagnostic Potential in Eye-Related Diseases" Journal of Imaging 9, no. 11: 253. https://doi.org/10.3390/jimaging9110253
APA StyleBadawi, S. A., Takruri, M., Al-Hattab, M., Aldoboni, G., Guessoum, D., ElBadawi, I., Aichouni, M., Chaudhry, I. A., Mahar, N., & Nileshwar, A. K. (2023). Arteriovenous Length Ratio: A Novel Method for Evaluating Retinal Vasculature Morphology and Its Diagnostic Potential in Eye-Related Diseases. Journal of Imaging, 9(11), 253. https://doi.org/10.3390/jimaging9110253






