Infrared Macrothermoscopy Patterns—A New Category of Dermoscopy
Abstract
:1. Introduction
2. Materials and Methods
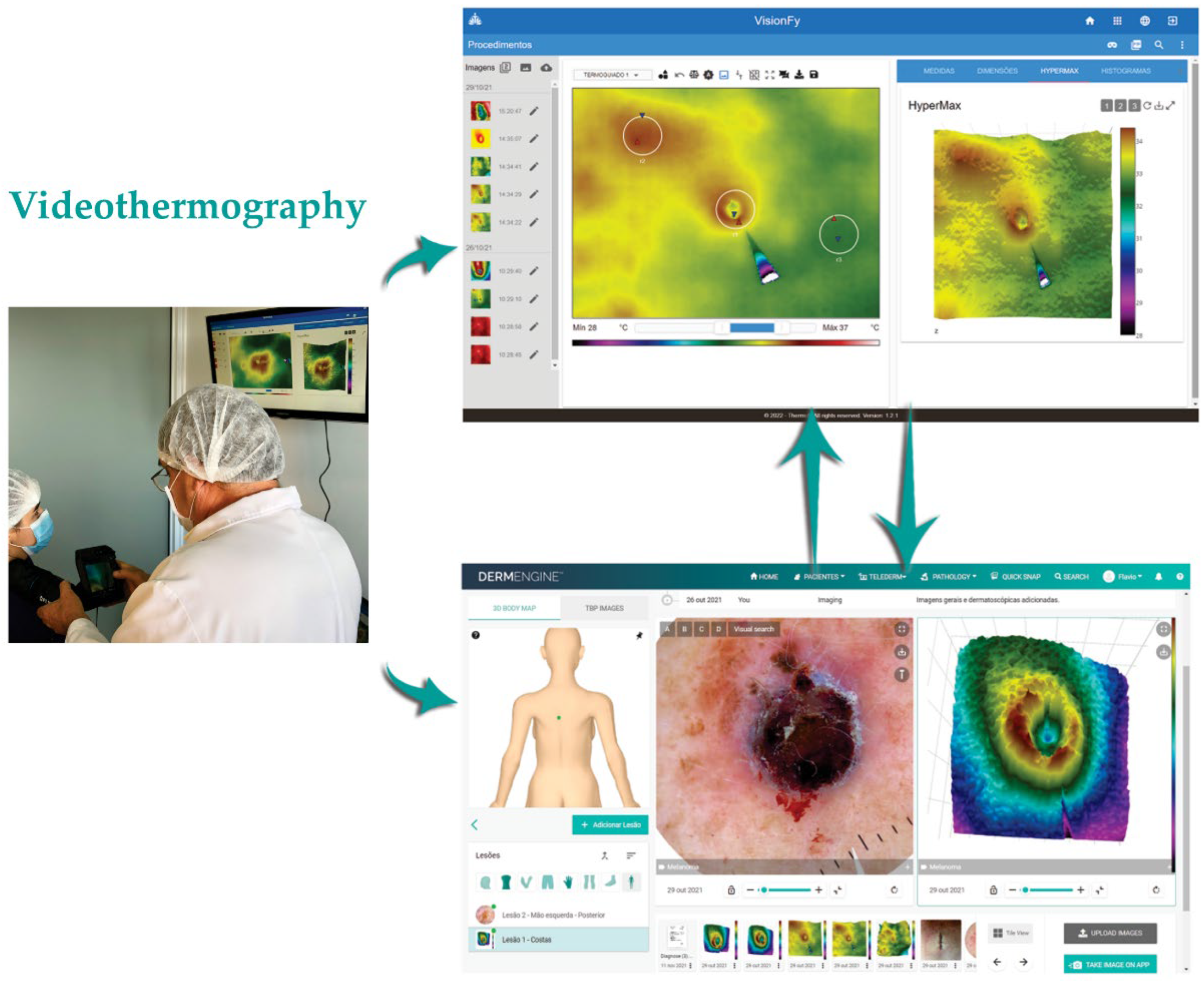
2.1. Equipment and Software
2.2. Steps to Perform Image Registration and Analysis
2.3. Malignant or Borderline Lesions
2.4. Benign Lesions
2.5. Evaluation Methods Comparison
3. Results
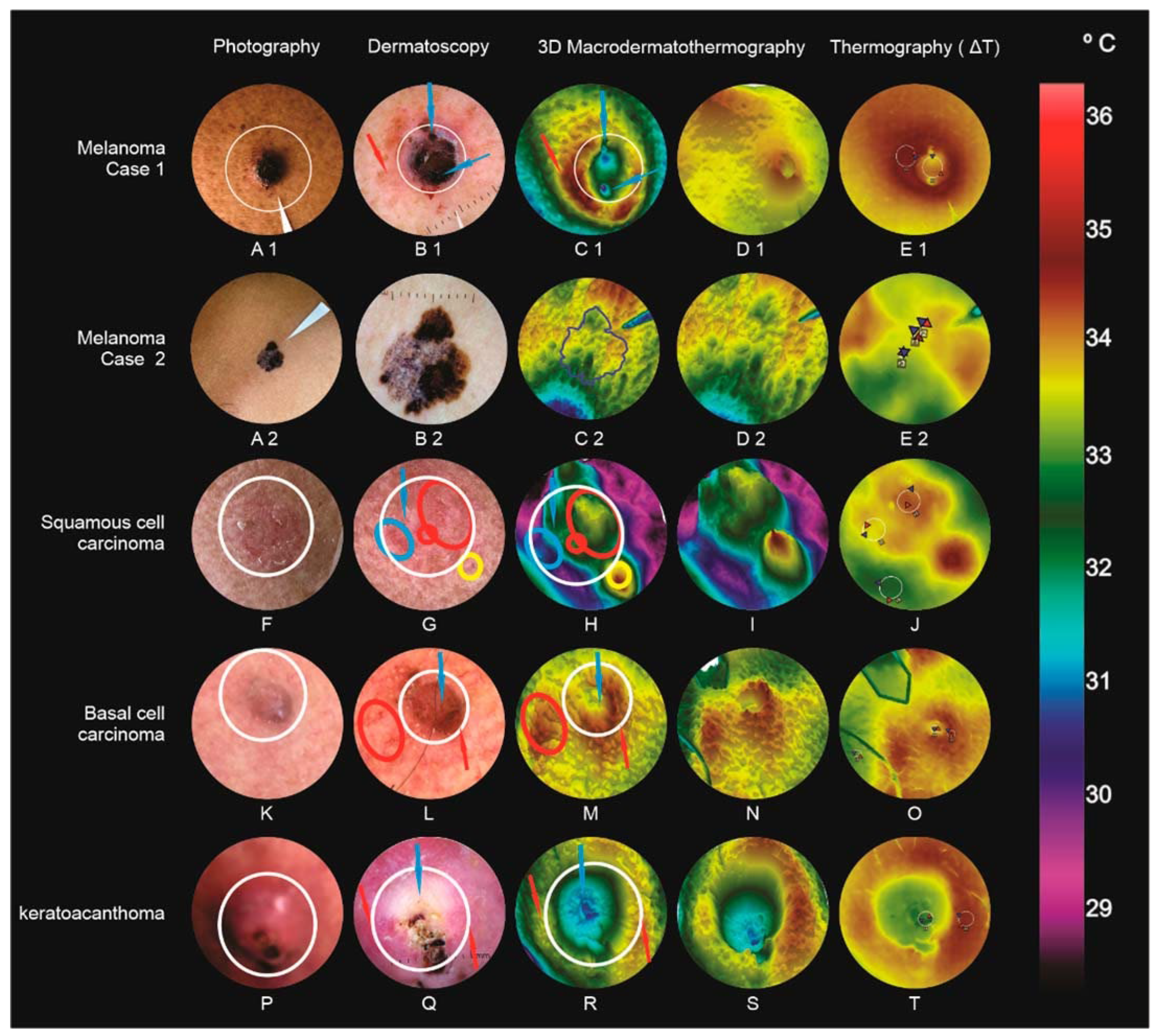
3.1. Malignant or Borderline Lesions (Figure 3)
3.1.1. Melanoma (Case 1)
3.1.2. Melanoma (Case 2)
3.1.3. Squamous Cell Carcinoma
3.1.4. Basal Cell Carcinoma
3.1.5. Keratoacanthoma
3.2. Benign Lesions (Figure 4)
3.2.1. Chronic Nodular Helix Chondrodermatitis (Case 1)
3.2.2. Chronic Nodular Helix Chondrodermatitis (Case 2)-(Winkler’s Disease)
3.2.3. Molluscum Contagiosum
3.2.4. Cutaneous Lichen Planus
3.2.5. Oral Lichen Planus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ring, C.; Cox, N.; Lee, J.B. Dermatoscopy. Clin. Dermatol. 2021, 39, 635–642. [Google Scholar] [CrossRef]
- Janssen, L.; Mylle, S.; Van Kelst, S.; De Smedt, J.; Diricx, B.; Kimpe, T.; Boone, M.; Verhaeghe, E.; Brochez, L.; Garmyn, M. Enhanced Visualization of Blood and Pigment in Multispectral Skin Dermoscopy. Ski. Res. Technol. 2020, 26, 708–712. [Google Scholar] [CrossRef]
- Schneider, S.L.; Kohli, I.; Hamzavi, I.H.; Council, M.L.; Rossi, A.M.; Ozog, D.M. Emerging Imaging Technologies in Dermatology. J. Am. Acad. Dermatol. 2019, 80, 1114–1120. [Google Scholar] [CrossRef]
- Phillips, M.; Marsden, H.; Jaffe, W.; Matin, R.N.; Wali, G.N.; Greenhalgh, J.; McGrath, E.; James, R.; Ladoyanni, E.; Bewley, A.; et al. Assessment of Accuracy of an Artificial Intelligence Algorithm to Detect Melanoma in Images of Skin Lesions. JAMA Netw. Open 2019, 2, e1913436. [Google Scholar] [CrossRef]
- Hoorens, I.; Vossaert, K.; Lanssens, S.; Dierckxsens, L.; Argenziano, G.; Brochez, L. Value of Dermoscopy in a Population-Based Screening Sample by Dermatologists. Dermatol. Pract. Concept. 2019, 9, 200–206. [Google Scholar] [CrossRef]
- Reiter, O.; Mimouni, I.; Gdalevich, M.; Marghoob, A.A.; Levi, A.; Hodak, E.; Leshem, Y.A. The Diagnostic Accuracy of Dermoscopy for Basal Cell Carcinoma: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2019, 80, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Errichetti, E.; Stinco, G. Dermoscopy in General Dermatology: A Practical Overview. Dermatol. Ther. 2016, 6, 471–507. [Google Scholar] [CrossRef] [PubMed]
- Lis-Święty, A.; Miziołek, B.; Ranosz-Janicka, I.; Bierzyńska-Macyszyn, G.; Brzezińska-Wcisło, L. Thermal Imaging and Dermoscopy for Detecting Inflammation in Frontal Fibrosing Alopecia. J. Cosmet. Dermatol. 2018, 17, 268–273. [Google Scholar] [CrossRef] [PubMed]
- FLIR FLIR T500-Series Macro Mode: Single-Lens Solution for Imaging Small Targets. Available online: https://www.flir.com/discover/rd-science/flir-t500-series-macro-mode-single-lens-solution-for-imaging-small-targets/ (accessed on 13 February 2022).
- Dziarski, K. Selection of the Observation Angle in Thermography Temperature Measurements with the Use of a Macro Lens. In Proceedings of the 2021 13th International Conference on Measurement, IEEE, Bratislava, Slovakia, 17–19 May 2021; pp. 101–104. [Google Scholar]
- Verstockt, J.; Verspeek, S.; Thiessen, F.; Tjalma, W.A.; Brochez, L.; Steenackers, G. Skin Cancer Detection Using Infrared Thermography: Measurement Setup, Procedure and Equipment. Sensors 2022, 22, 3327. [Google Scholar] [CrossRef]
- Buzug, T.M.; Schumann, S.; Pfaffmann, L.; Reinhold, U.; Ruhlmann, J. Skin-Tumour Classification with Functional Infrared Imaging. In Proceedings of the 8th IASTED International Conference Signal Image Processing SIP 2006, Honolulu, HI, USA, 14–16 August 2006; pp. 313–322. [Google Scholar]
- Pirtini Çetingül, M.; Alani, R.M.; Herman, C. Quantitative Evaluation of Skin Lesions Using Transient Thermal Imaging. In Proceedings of the 2010 14th International Heat Transfer Conference, ASMEDC, Washington, DC, USA, 8–13 August 2010; Volume 1, pp. 31–39. [Google Scholar]
- Pirtini Çetingül, M.; Herman, C. Using Dynamic Infrared Imaging to Detect Melanoma: Experiments on a Tissue-Mimicking Phantom. In Proceedings of the Biomedical and Biotechnology Engineering, ASMEDC, Vancouver, BC, Canada, 12–18 November 2010; Volume 2, pp. 139–147. [Google Scholar]
- Çetingül, M.P.; Alani, R.M.; Herman, C. Detection of Skin Cancer Using Transient/Thermal Imaging. In Proceedings of the ASME 2010 Summer Bioengineering Conference, Parts A and B, American Society of Mechanical Engineers, Farmington, PA, USA, 22–25 June 2010; pp. 601–602. [Google Scholar]
- Dermengine. Available online: https://www.dermengine.com/ (accessed on 3 December 2022).
- Thermofy Brasil. Available online: www.thermofy.com.br (accessed on 3 December 2022).
- Ring, E.F.J.; Ammer, K. The Technique Ofinfrared Imaging in Medicine. Thermol. Int. 2000, 10, 7–14. [Google Scholar]
- Brioschi, M.L.; Teixeira, M.J.; Silva, F.M.R.M.; Colman, D. Medical Thermography Textbook: Principles and Applications; Andreoli: São Paulo, Brazil, 2010. [Google Scholar]
- Fink, C.; Haenssle, H.A. Non-Invasive Tools for the Diagnosis of Cutaneous Melanoma. Ski. Res. Technol. 2017, 23, 261–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringasci, M.D.; Salvio, A.G.; Sbrissa Neto, D.; Vollet-Filho, J.D.; Bagnato, V.S.; Kurachi, C. Discrimination of Benign- versus -Malignant Skin Lesions by Thermographic Images Using Support Vector Machine Classifier. J. Appl. Phys. 2018, 124, 044701. [Google Scholar] [CrossRef]
- Moy, R.L. Clinical Presentation of Actinic Keratoses and Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2000, 42, S8–S10. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, G.; Zalaudek, I.; Corona, R.; Sera, F.; Cicale, L.; Petrillo, G.; Ruocco, E.; Hofmann-Wellenhof, R.; Soyer, H.P. Vascular Structures in Skin Tumors. Arch. Dermatol. 2004, 140, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Lallas, A.; Apalla, Z.; Ioannides, D.; Argenziano, G.; Castagnetti, F.; Moscarella, E.; Longo, C.; Palmieri, T.; Ramundo, D.; Zalaudek, I. Dermoscopy in the Diagnosis and Management of Basal Cell Carcinoma. Futur. Oncol. 2015, 11, 2975–2984. [Google Scholar] [CrossRef]
- Rosendahl, C.; Cameron, A.; Argenziano, G.; Zalaudek, I.; Tschandl, P.; Kittler, H. Dermoscopy of Squamous Cell Carcinoma and Keratoacanthoma. Arch. Dermatol. 2012, 148, 1386. [Google Scholar] [CrossRef]
- Kwiek, B.; Schwartz, R.A. Keratoacanthoma (KA): An Update and Review. J. Am. Acad. Dermatol. 2016, 74, 1220–1233. [Google Scholar] [CrossRef]
- Papageorgiou, V.; Apalla, Z.; Sotiriou, E.; Papageorgiou, C.; Lazaridou, E.; Vakirlis, S.; Ioannides, D.; Lallas, A. The Limitations of Dermoscopy: False-Positive and False-Negative Tumours. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 879–888. [Google Scholar] [CrossRef]
- Ianoși, S.; Forsea, A.; Lupu, M.; Ilie, M.; Zurac, S.; Boda, D.; Ianosi, G.; Neagoe, D.; Tutunaru, C.; Popa, C.; et al. Role of Modern Imaging Techniques for the in Vivo Diagnosis of Lichen Planus (Review). Exp. Ther. Med. 2018, 17, 1052–1060. [Google Scholar] [CrossRef]
- Leñero-Bardallo, J.A.; Serrano, C.; Acha, B.; Pérez-Carrasco, J.A.; Bernabeu-Wittel, J. Thermography for the Differential Diagnosis of Vascular Malformations. Clin. Exp. Dermatol. 2021, 46, 314–318. [Google Scholar] [CrossRef]
- Gautherie, M.; Grosshansm, E.; Fattal, M. Thermal Assessment of Malignant Melanomas and Other Skin Tumors Based on the Use of Flexibe Liquid Crystal Films and Standardized Protocol of Interpretation. Thermology 1985, 1, 20–25. [Google Scholar]


| Case | Main Dermoscopic Findings | Dermoscopy Compatible, Incongruous, or Indifferent towards the Final Diagnosis | Main MacroIR Findings | MacroIR Compatible, Incongruous, or Indifferent towards the Final Diagnosis | Largest Temperature Differences (ΔT) Founded between Regions of Interest (ROIs) |
|---|---|---|---|---|---|
| Melanoma (Case 1) |
| Dermoscopy left doubts with differential diagnosis with Basal Cell Carcinoma |
| ΔT and comet tail were points that helped to suspect more melanoma, aiding in dermoscopy | 1.62 °C |
| Melanoma (Case 2) |
| The structures found in dermoscopy were enough to indicate an excisional biopsy with high suspicion of melanoma. |
| Lack of high ΔTs, as well as small surface changes, suggests “in situ” or superficial expanding melanoma. Anatomopathological examination results showed to be expansive superficial with thickness (Breslow = 0.5 mm) | 0.21 °C |
| Squamous Cell Carcinoma |
| In the first dermoscopy view, the vascular changes (glomerular vessels) that are very common in Bowen were not noticed. When evaluating the MacroIR, the changes led to a review of dermoscopy |
| Visualization of the mountains in the MacroIR indicated the presence of vessels and forced a dermoscopy review. | 1.58 °C |
| Basal Cell Carcinoma |
| Dermoscopy compatible with basal cell carcinoma |
| MacroIR compatible with basal cell carcinoma | 1.39 °C |
| Keratoacanthoma |
| Dermoscopy is compatible with keratoacanthoma, but it always leaves doubts in the differential diagnosis with Squamous Cell Carcinoma |
| MacroIR compatible with dermoscopy and did not change the diagnostic hypothesis of keratoacanthoma nor the differential diagnosis with Squamous Cell Carcinoma | 1.01 °C |
| Chronic nodular helix chondrodermatitis (Case 1) |
| Dermoscopy very similar to keratoacanthoma |
| MacroIR findings were consistent with Chronic Nodular Helix Chondrodermatitis, ruling out keratoacanthoma. | −2.49 °C |
| Chronic nodular helix chondrodermatitis (Case 2) |
| Dermoscopy very similar to keratoacanthoma |
| MacroIR findings were consistent with Chronic Nodular Helix Chondrodermatitis, ruling out keratoacanthoma. In this second case it was decisive in the diagnosis | −3.82 °C |
| Molluscus Contagious |
| Compatible with the diagnosis of Molluscum Contagiosum |
| MacroIR compatible with dermoscopy. Indifferent in the diagnosis of Molluscum Contagiosum | 0.28 °C |
| Cutaneous lichen planus |
| Dermoscopy compatible with the diagnosis of Lichen Plane Cutaneous |
| MacroIR indifferent in the diagnosis of Lichen Plane Cutaneous Interesting to be able to follow the evolution after treatment | 0.27 °C |
| Oral lichen planus | - | Dermoscopy was not performed due to technical difficulties |
| MacroIR indifferent in diagnosis Compatible with the oral lesion and helps in the evaluation of the evolution after treatment. | 0.38 °C |
| Diagnostic | Temperature Range (°C) | |
|---|---|---|
| Minimum | Maximum | |
| Melanoma (Case 1) | 28.00 | 37.00 |
| Melanoma (Case 2) | 30.90 | 37.70 |
| Squamous cell carcinoma | 28.00 | 37.00 |
| Basal cell carcinoma | 28.00 | 35.40 |
| Keratoacanthoma | 28.00 | 34.80 |
| Chronic nodular helix chondrodermatitis (Case 1) | 28.60 | 37.00 |
| Chronic nodular helix chondrodermatitis (Case 2) | 26.20 | 37.00 |
| Molluscus contagious | 32.30 | 35.90 |
| Cutaneous lichen planus | 32.80 | 34.80 |
| Oral lichen planus | 28.00 | 37.00 |
| Diagnostic | Was the Isolated Method Sufficient to Reach the Histopathological Diagnosis? | ||
|---|---|---|---|
| Clinical Evaluation | Dermoscopic | MacroIR | |
| Melanoma (Case 1) | |||
| Melanoma (Case 2) | |||
| Squamous cell carcinoma | |||
| Basal cell carcinoma | |||
| Keratoacanthoma | |||
| Chronic nodular helix chondrodermatitis (Case 1) | |||
| Chronic nodular helix chondrodermatitis (Case 2) | |||
| Molluscus contagious | |||
| Cutaneous lichen planus | |||
| Oral lichen planus | |||
| The isolated method was sufficient to reach the histopathological diagnosis (%) | 50% | 40% | 90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, F.L.; Brioschi, M.L.; Dalmaso Neto, C.; Medeiros, C.R.d. Infrared Macrothermoscopy Patterns—A New Category of Dermoscopy. J. Imaging 2023, 9, 36. https://doi.org/10.3390/jimaging9020036
Ferrari FL, Brioschi ML, Dalmaso Neto C, Medeiros CRd. Infrared Macrothermoscopy Patterns—A New Category of Dermoscopy. Journal of Imaging. 2023; 9(2):36. https://doi.org/10.3390/jimaging9020036
Chicago/Turabian StyleFerrari, Flavio Leme, Marcos Leal Brioschi, Carlos Dalmaso Neto, and Carlos Roberto de Medeiros. 2023. "Infrared Macrothermoscopy Patterns—A New Category of Dermoscopy" Journal of Imaging 9, no. 2: 36. https://doi.org/10.3390/jimaging9020036
APA StyleFerrari, F. L., Brioschi, M. L., Dalmaso Neto, C., & Medeiros, C. R. d. (2023). Infrared Macrothermoscopy Patterns—A New Category of Dermoscopy. Journal of Imaging, 9(2), 36. https://doi.org/10.3390/jimaging9020036











