Collagen Fibril Orientation In Vitro: From Formation to Advanced Biomaterial Development
Abstract
1. Introduction
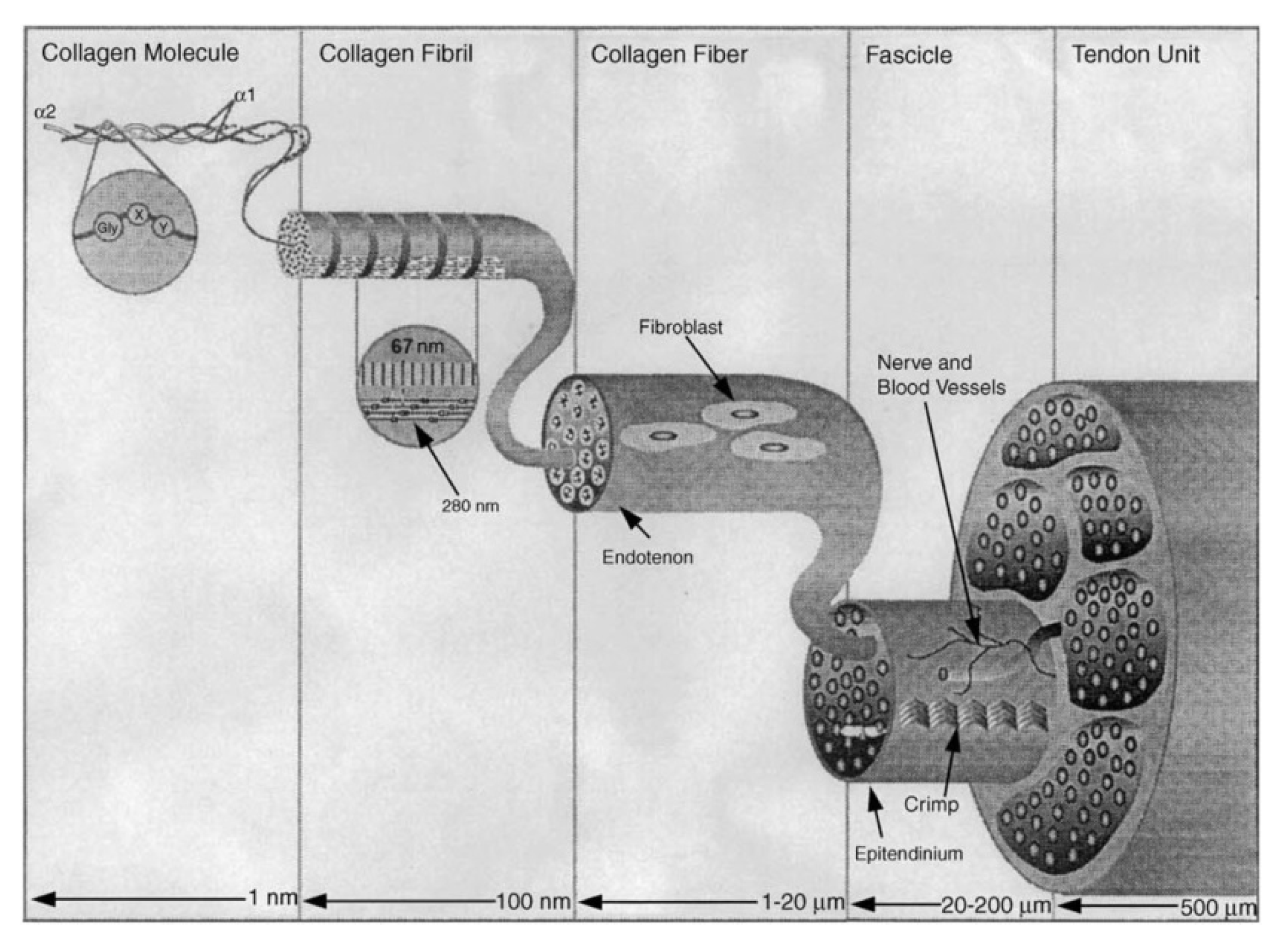
2. Structure of Collagen Type I
3. Collagen Fibril Orientation Theory
4. Mechanical Environment
5. Magnetic Orientation
6. Electrochemical Orientation
7. Biomedical Application of Oriented Collagen Fibrils
8. Conclusions and Perspectives
- The methods of obtaining oriented collagen fibrils are different, but it is still a difficult problem to obtain oriented collagen fibrils with a structure similar to the structure of the arrangement of collagen fibrils in native tissue. Therefore, it is still necessary to pay detailed attention to the study of the mechanisms of orientation of collagen fibers.
- Many factors affecting cell functions have been found, but the mechanism of factors affecting the secretion of directed collagen fibrils needs further study.
- There are many methods of obtaining collagen scaffolds for targeted regeneration, but the location of collagen fibrils in the scaffolds themselves is rarely studied. In future studies, we should pay more attention to promoting the restoration of the structure and function of the newly formed tissue.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Birk, D.E.; Trelstad, R.L. Extracellular compartments in matrix morphogenesis: Collagen fibril, bundle, and lamellar formation by corneal fibroblasts. J. Cell Biol. 1984, 99, 2024–2033. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Terzi, A.; Sannino, A.; Madaghiele, M. Mimicking the Hierarchical Organization of Natural Collagen: Toward the Development of Ideal Scaffolding Material for Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 644595. [Google Scholar] [CrossRef]
- Pawelec, K.M.; Best, S.M.; Cameron, R.E. Collagen: A network for regenerative medicine. J. Mater. Chem. B 2016, 4, 6484–6496. [Google Scholar] [CrossRef]
- Nashchekina, Y.; Nikonov, P.; Prasolov, N.; Sulatsky, M.; Chabina, A.; Nashchekin, A. The Structural Interactions of Molecular and Fibrillar Collagen Type I with Fibronectin and Its Role in the Regulation of Mesenchymal Stem Cell Morphology and Functional Activity. Int. J. Mol. Sci. 2022, 23, 12577. [Google Scholar] [CrossRef]
- Nashchekina, Y.; Chabina, A.; Konson, V.; Nashchekin, A.; Mikhailova, N. Carboxymethyl cellulose in composite collagen gel improves mesenchymal stromal cell proliferation and migration. Int. J. Biol. Macromol. 2025, 319, 145418. [Google Scholar] [CrossRef] [PubMed]
- Nimni, M.E.; Harkness, R.D. Molecular Structure and Functions of Collagen. In Collagen; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–78. [Google Scholar]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, V.; Barua, M.; Lennon, R. Collagen formation, function and role in kidney disease. Nat. Rev. Nephrol. 2024, 21, 200–215. [Google Scholar] [CrossRef]
- Gelse, K. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Thomopoulos, S.; Marquez, J.P.; Weinberger, B.; Birman, V.; Genin, G.M. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J. Biomech. 2006, 39, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, X.; Wu, X.; Liu, L.; Wang, S.; Sun, L.; Fan, Y. Effect of collagen fiber orientation on mechanical properties of bone and myofascia in hindlimb unloading rats. Acta Astronaut. 2023, 212, 261–269. [Google Scholar] [CrossRef]
- Holmes, D.F.; Gilpin, C.J.; Baldock, C.; Ziese, U.; Koster, A.J.; Kadler, K.E. Corneal collagen fibril structure in three dimensions: Structural insights into fibril assembly, mechanical properties, and tissue organization. Proc. Natl. Acad. Sci. USA 2001, 98, 7307–7312. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dong, Y. Collagen-Based Biomaterials for Tissue Engineering. ACS Biomater. Sci. Eng. 2023, 9, 1132–1150. [Google Scholar] [CrossRef]
- Nashchekina, Y.; Nikonov, P.; Mikhailova, N.; Nashchekin, A. Collagen Scaffolds Treated by Hydrogen Peroxide for Cell Cultivation. Polymers 2021, 13, 4134. [Google Scholar] [CrossRef] [PubMed]
- Delgado, L.M.; Shologu, N.; Fuller, K.; Zeugolis, D.I. Acetic acid and pepsin result in high yield, high purity and low macrophage response collagen for biomedical applications. Biomed. Mater. 2017, 12, 065009. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Lv, Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Friess, W. Collagen–biomaterial for drug delivery. Eur. J. Pharm. Biopharm. 1998, 45, 113–136. [Google Scholar] [CrossRef]
- Hauschka, S.D.; Konigsberg, I.R. The influence of collagen on the development of muscle clones. Proc. Natl. Acad. Sci. USA 1966, 55, 119–126. [Google Scholar] [CrossRef]
- Konigsberg, I.R. Clonal Analysis of Myogenesis: Its relevance to the general problem of the stability of cell-type in cultured animal cells is discussed. Science 1963, 140, 1273–1284. [Google Scholar] [CrossRef]
- Guilak, F.; Butler, D.L.; Goldstein, S.A.; Baaijens, F.P.T. Biomechanics and mechanobiology in functional tissue engineering. J. Biomech. 2014, 47, 1933–1940. [Google Scholar] [CrossRef]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-Dimensional Cell Culture Matrices: State of the Art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef]
- Ruberti, J.W.; Zieske, J.D. Prelude to corneal tissue engineering–Gaining control of collagen organization. Prog. Retin. Eye Res. 2008, 27, 549–577. [Google Scholar] [CrossRef]
- Caliari, S.R.; Harley, B.A.C. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 2011, 32, 5330–5340. [Google Scholar] [CrossRef]
- Torbet, J.; Malbouyres, M.; Builles, N.; Justin, V.; Roulet, M.; Damour, O.; Oldberg, Å.; Ruggiero, F.; Hulmes, D.J.S. Orthogonal scaffold of magnetically aligned collagen lamellae for corneal stroma reconstruction. Biomaterials 2007, 28, 4268–4276. [Google Scholar] [CrossRef]
- Lee, P.; Lin, R.; Moon, J.; Lee, L.P. Microfluidic alignment of collagen fibers for in vitro cell culture. Biomed. Microdevices 2006, 8, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shi, X.; Li, X.; Wang, J.; Wang, Y.; Luo, Y. Oriented collagen fiber membranes formed through counter-rotating extrusion and their application in tendon regeneration. Biomaterials 2019, 207, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Walters, B.D.; Stegemann, J.P. Strategies for directing the structure and function of three-dimensional collagen biomaterials across length scales. Acta Biomater. 2014, 10, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Rothrauff, B.B.; Yang, G.; Tuan, R.S. Chapter 2 Tendon Resident Cells—Functions and Features in Section I—Developmental Biology and Physiology of Tendons. Tendon Regen. 2015, 41–76. [Google Scholar] [CrossRef]
- Revell, C.K.; Jensen, O.E.; Shearer, T.; Lu, Y.; Holmes, D.F.; Kadler, K.E. Collagen fibril assembly: New approaches to unanswered questions. Matrix Biol. Plus 2021, 12, 100079. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, 1801651. [Google Scholar] [CrossRef]
- Rich, A.; Crick, F.H.C. The molecular structure of collagen. J. Mol. Biol. 1961, 3, 483–506. [Google Scholar] [CrossRef]
- Ramachandran, G.X.; Chandrasekharan, R. Interchain hydrogen bonds via bound water molecules in the collagen triple helix. Biopolymers 1968, 6, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Bächinger, H.P.; Boudko, S.P. Mysteries of the collagen triple helix. Matrix Biol. 2025, 137, 12–18. [Google Scholar] [CrossRef]
- Ignat’eva, N.Y.; Danilov, N.A.; Averkiev, S.V.; Obrezkova, M.V.; Lunin, V.V.; Sobol’, E.N. Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J. Anal. Chem. 2007, 62, 51–57. [Google Scholar] [CrossRef]
- Mizuno, K.; Boudko, S.P.; Engel, J.; Peter Bächinger, H. Kinetic hysteresis in collagen folding. Biophys. J. 2010, 98, 3004–3014. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.; Ramshaw, J.A.M. The collagen triple-helix structure. Matrix Biol. 1997, 15, 545–554. [Google Scholar] [CrossRef]
- Bella, J. Collagen structure: New tricks from a very old dog. Biochem. J. 2016, 473, 1001–1025. [Google Scholar] [CrossRef] [PubMed]
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The triple helix of collagens-An ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci. 2018, 131, jcs203950. [Google Scholar] [CrossRef]
- Khare, E.; Yu, C.H.; Gonzalez Obeso, C.; Milazzo, M.; Kaplan, D.L.; Buehler, M.J. Discovering design principles of collagen molecular stability using a genetic algorithm, deep learning, and experimental validation. Proc. Natl. Acad. Sci. USA 2022, 119, e2209524119. [Google Scholar] [CrossRef]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef]
- Collins, C.J.; Andriotis, O.G.; Nedelkovski, V.; Frank, M.; Katsamenis, O.L.; Thurner, P.J. Bone Micro- and Nanomechanics. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 22–44. [Google Scholar]
- Okuyama, K.; Xu, X.; Iguchi, M.; Noguchi, K. Revision of collagen molecular structure. Pept. Sci. 2006, 84, 181–191. [Google Scholar] [CrossRef]
- Capaldi, M.J.; Chapman, J.A. The C-terminal extrahelical peptide of type I collagen and its role in fibrillogenesis in vitro. Biopolymers 1982, 21, 2291–2313. [Google Scholar] [CrossRef]
- Canty, E.G.; Kadler, K.E. Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 2005, 118, 1341–1353. [Google Scholar] [CrossRef]
- Wood, G.C.; Keech, M.K. The formation of fibrils from collagen solutions 1. The effect of experimental conditions: Kinetic and electron-microscope studies. Biochem. J. 1960, 75, 588–598. [Google Scholar] [CrossRef]
- Noitup, P.; Morresey, M.T.; Garnjanagoonchorn, W. In vitro self-assembly of silver-line grunt type I collagen effects of collagen concentrations, pH and temperature on collagen collagen self-assembly. J. Food Biochem. 2006, 30, 547–555. [Google Scholar] [CrossRef]
- Yan, M.; Li, B.; Zhao, X.; Qin, S. Effect of concentration, pH and ionic strength on the kinetic self-assembly of acid-soluble collagen from walleye pollock (Theragra chalcogramma) skin. Food Hydrocoll. 2012, 29, 199–204. [Google Scholar] [CrossRef]
- Cisneros, D.A.; Friedrichs, J.; Taubenberger, A.; Franz, C.M.; Muller, D.J. Creating Ultrathin Nanoscopic Collagen Matrices For Biological And Biotechnological Applications. Small 2007, 3, 956–963. [Google Scholar] [CrossRef]
- Orgel, J.P.R.O.; Irving, T.C.; Miller, A.; Wess, T.J. Microfibrillar structure of type I collagen in situ. Proc. Natl. Acad. Sci. USA 2006, 103, 9001–9005. [Google Scholar] [CrossRef]
- Fang, M.; Goldstein, E.L.; Turner, A.S.; Les, C.M.; Orr, B.G.; Fisher, G.J.; Welch, K.B.; Rothman, E.D.; Banaszak Holl, M.M. Type i collagen D-spacing in fibril bundles of dermis, tendon, and bone: Bridging between nano- and micro-level tissue hierarchy. ACS Nano 2012, 6, 9503–9514. [Google Scholar] [CrossRef]
- Canty, E.G.; Lu, Y.; Meadows, R.S.; Shaw, M.K.; Holmes, D.F.; Kadler, K.E. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J. Cell Biol. 2004, 165, 553–563. [Google Scholar] [CrossRef]
- Giraud-Guille, M.M. Liquid crystallinity in condensed type I collagen solutions. A clue to the packing of collagen in extracellular matrices. J. Mol. Biol. 1992, 224, 861–873. [Google Scholar] [CrossRef]
- Giraud-Guille, M.M.; Mosser, G.; Belamie, E. Liquid crystallinity in collagen systems in vitro and in vivo. Curr. Opin. Colloid Interface Sci. 2008, 13, 303–313. [Google Scholar] [CrossRef]
- Hodge, A.J.; Petruska, J.A. Recent studies with the electron microscope on ordered aggregates of the tropocollagen macromolecules. In Aspects of Protein Structure; Academic Press: New York, NY, USA, 1963; pp. 289–300. [Google Scholar]
- Petruska, J.A.; Hodge, A.J. a Subunit Model for the Tropocollagen Macromolecule. Proc. Natl. Acad. Sci. USA 1964, 51, 871–876. [Google Scholar] [CrossRef]
- Sirotkina, M.Y.; Nashchekina, Y.A. Collagen Fibrils of Various Diameters: Formation Conditions and Principles of Functioning. Cell Tissue Biol. 2022, 16, 513–520. [Google Scholar] [CrossRef]
- Sirotkina, M.Y.; Chabina, A.S.; Lomert, E.V.; Nashchekina, Y.A. Effect of Telopeptides in Collagen on the Behavior of Mesenchymal Stromal Cells In Vitro. Cell Tissue Biol. 2024, 18, 699–706. [Google Scholar] [CrossRef]
- Xing, L.; Chen, B.; Qin, Y.; Li, X.; Zhou, S.; Yuan, K.; Zhao, R.; Qin, D. The role of neuropeptides in cutaneous wound healing: A focus on mechanisms and neuropeptide-derived treatments. Front. Bioeng. Biotechnol. 2024, 12, 1494865. [Google Scholar] [CrossRef]
- Terzi, A.; Gallo, N.; Bettini, S.; Sibillano, T.; Altamura, D.; Madaghiele, M.; De Caro, L.; Valli, L.; Salvatore, L.; Sannino, A.; et al. Sub- and Supramolecular X-Ray Characterization of Engineered Tissues from Equine Tendon, Bovine Dermis, and Fish Skin Type-I Collagen. Macromol. Biosci. 2020, 20, 2000017. [Google Scholar] [CrossRef]
- Birk, D.E.; Brückner, P. Collagens, Suprastructures, and Collagen Fibril Assembly. In The Extracellular Matrix: An Overview; Springer: Berlin/Heidelberg, Germany, 2011; pp. 77–115. [Google Scholar]
- Ostadi Moghaddam, A.; Arshee, M.R.; Lin, Z.; Sivaguru, M.; Phillips, H.; McFarlin, B.L.; Toussaint, K.C.; Wagoner Johnson, A.J. Orientation-dependent indentation reveals the crosslink-mediated deformation mechanisms of collagen fibrils. Acta Biomater. 2023, 158, 347–357. [Google Scholar] [CrossRef]
- Vicker, M.G. Eukaryotic Cell Locomotion Depends on the Propagation of Self-Organized Reaction–Diffusion Waves and Oscillations of Actin Filament Assembly. Exp. Cell Res. 2002, 275, 54–66. [Google Scholar] [CrossRef]
- Lin, J.; Shi, Y.; Men, Y.; Wang, X.; Ye, J.; Zhang, C. Mechanical Roles in Formation of Oriented Collagen Fibers. Tissue Eng. Part B Rev. 2020, 26, 116–128. [Google Scholar] [CrossRef]
- Silver, F.H.; Freeman, J.W.; Seehra, G.P. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 2003, 36, 1529–1553. [Google Scholar] [CrossRef]
- Cowin, S.C. Tissue Growth and Remodeling. Annu. Rev. Biomed. Eng. 2004, 6, 77–107. [Google Scholar] [CrossRef]
- Kjaer, M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef]
- Welch, M.P.; Odland, G.F.; Clark, R.A. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J. Cell Biol. 1990, 110, 133–145. [Google Scholar] [CrossRef]
- Kalson, N.S.; Starborg, T.; Lu, Y.; Mironov, A.; Humphries, S.M.; Holmes, D.F.; Kadler, K.E. Nonmuscle myosin II powered transport of newly formed collagen fibrils at the plasma membrane. Proc. Natl. Acad. Sci. USA 2013, 110, E4743–E4752. [Google Scholar] [CrossRef]
- Paten, J.A.; Siadat, S.M.; Susilo, M.E.; Ismail, E.N.; Stoner, J.L.; Rothstein, J.P.; Ruberti, J.W. Flow-Induced Crystallization of Collagen: A Potentially Critical Mechanism in Early Tissue Formation. ACS Nano 2016, 10, 5027–5040. [Google Scholar] [CrossRef]
- Trotter, J.; Kadler, K.; Holmes, D. Echinoderm collagen fibrils grow by surface-nucleation-and-propagation from both centers and ends. J. Mol. Biol. 2000, 300, 531–540. [Google Scholar] [CrossRef]
- Siadat, S.M.; Ruberti, J.W. Mechanochemistry of collagen. Acta Biomater. 2023, 163, 50–62. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef]
- Lanfer, B.; Freudenberg, U.; Zimmermann, R.; Stamov, D.; Körber, V.; Werner, C. Aligned fibrillar collagen matrices obtained by shear flow deposition. Biomaterials 2008, 29, 3888–3895. [Google Scholar] [CrossRef]
- Saeidi, N.; Sander, E.A.; Ruberti, J.W. Dynamic shear-influenced collagen self-assembly. Biomaterials 2009, 30, 6581–6592. [Google Scholar] [CrossRef]
- Lai, E.S.; Anderson, C.M.; Fuller, G.G. Designing a tubular matrix of oriented collagen fibrils for tissue engineering. Acta Biomater. 2011, 7, 2448–2456. [Google Scholar] [CrossRef]
- Hoogenkamp, H.R.; Bakker, G.-J.; Wolf, L.; Suurs, P.; Dunnewind, B.; Barbut, S.; Friedl, P.; van Kuppevelt, T.H.; Daamen, W.F. Directing collagen fibers using counter-rotating cone extrusion. Acta Biomater. 2015, 12, 113–121. [Google Scholar] [CrossRef]
- Wilson, D.L.; Martin, R.; Hong, S.; Cronin-Golomb, M.; Mirkin, C.A.; Kaplan, D.L. Surface organization and nanopatterning of collagen by dip-pen nanolithography. Proc. Natl. Acad. Sci. USA 2001, 98, 13660–13664. [Google Scholar] [CrossRef]
- Denis, F.A.; Pallandre, A.; Nysten, B.; Jonas, A.M.; Dupont-Gillain, C.C. Alignment and Assembly of Adsorbed Collagen Molecules Induced by Anisotropic Chemical Nanopatterns. Small 2005, 1, 984–991. [Google Scholar] [CrossRef]
- Voge, C.M.; Kariolis, M.; MacDonald, R.A.; Stegemann, J.P. Directional conductivity in SWNT-collagen-fibrin composite biomaterials through strain-induced matrix alignment. J. Biomed. Mater. Res. Part A 2008, 86A, 269–277. [Google Scholar] [CrossRef]
- Vader, D.; Kabla, A.; Weitz, D.; Mahadevan, L. Strain-Induced Alignment in Collagen Gels. PLoS ONE 2009, 4, e5902. [Google Scholar] [CrossRef]
- Iijima, M.; Sato, M.; Wakabayashi, H.; Kojima, K.; Togashi, K.; Oishi, S.; Misu, T.; Mukai, M.; Miyajima, H.; Maruo, S.; et al. Fabrication of Multiscale, Multidirectional Orientated Collagen Hydrogels with Guided Cell Alignment Using Fluidics and a Three-Dimensional Printing. ACS Biomater. Sci. Eng. 2025, 11, 2875–2887. [Google Scholar] [CrossRef]
- Houška, M.; Landfeld, A.; Novotná, P.; Strohalm, J.; Šupová, M.; Suchý, T.; Chlup, H.; Skočilas, J.; Štípek, J.; Žaloudková, M.; et al. Properties of Bovine Collagen as Influenced by High-Pressure Processing. Polymers 2023, 15, 2472. [Google Scholar] [CrossRef]
- Patrawalla, N.Y.; Raj, R.; Nazar, V.; Kishore, V. Magnetic Alignment of Collagen: Principles, Methods, Applications, and Fiber Alignment Analyses. Tissue Eng. Part B Rev. 2024, 30, 405–422. [Google Scholar] [CrossRef]
- Morikawa, H.; Sassa, K.; Asai, S. Control of Precipitating Phase Alignment and Crystal Orientation by Imposition of a High Magnetic Field. Mater. Trans. JIM 1998, 39, 814–818. [Google Scholar] [CrossRef]
- Tognato, R.; Bonfrate, V.; Giancane, G.; Serra, T. Fabrication of anisotropic collagen-based substrates for potential use in tissue engineering. Smart Mater. Struct. 2022, 31, 074001. [Google Scholar] [CrossRef]
- Guo, C.; Kaufman, L.J. Flow and magnetic field induced collagen alignment. Biomaterials 2007, 28, 1105–1114. [Google Scholar] [CrossRef]
- Kotani, H.; Iwasaka, M.; Ueno, S.; Curtis, A. Magnetic orientation of collagen and bone mixture. J. Appl. Phys. 2000, 87, 6191–6193. [Google Scholar] [CrossRef]
- Antman-Passig, M.; Shefi, O. Remote Magnetic Orientation of 3D Collagen Hydrogels for Directed Neuronal Regeneration. Nano Lett. 2016, 16, 2567–2573. [Google Scholar] [CrossRef]
- Hofmann-Amtenbrink, M.; Hofmann, H.; Montet, X. Superparamagnetic nanoparticles-a tool for early diagnostics. Swiss Med. Wkly. 2010, 140, w13081. [Google Scholar] [CrossRef]
- Jiang, Z.; Shan, K.; Song, J.; Liu, J.; Rajendran, S.; Pugazhendhi, A.; Jacob, J.A.; Chen, B. Toxic effects of magnetic nanoparticles on normal cells and organs. Life Sci. 2019, 220, 156–161. [Google Scholar] [CrossRef]
- Matos, A.M.; Gonçalves, A.I.; El Haj, A.J.; Gomes, M.E. Magnetic biomaterials and nano-instructive tools as mediators of tendon mechanotransduction. Nanoscale Adv. 2020, 2, 140–148. [Google Scholar] [CrossRef]
- Wright, A.L.; Righelli, L.; Broomhall, T.J.; Lamont, H.C.; El Haj, A.J. Magnetic Nanoparticle-Mediated Orientation of Collagen Hydrogels for Engineering of Tendon-Mimetic Constructs. Front. Bioeng. Biotechnol. 2022, 10, 797437. [Google Scholar] [CrossRef]
- Bongaerts, M.; Aizel, K.; Secret, E.; Jan, A.; Nahar, T.; Raudzus, F.; Neumann, S.; Telling, N.; Heumann, R.; Siaugue, J.-M.; et al. Parallelized Manipulation of Adherent Living Cells by Magnetic Nanoparticles-Mediated Forces. Int. J. Mol. Sci. 2020, 21, 6560. [Google Scholar] [CrossRef] [PubMed]
- Torbet, J.; Ronzière, M.C. Magnetic alignment of collagen during self-assembly. Biochem. J. 1984, 219, 1057–1059. [Google Scholar] [CrossRef]
- Cheng, X.; Gurkan, U.A.; Dehen, C.J.; Tate, M.P.; Hillhouse, H.W.; Simpson, G.J.; Akkus, O. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials 2008, 29, 3278–3288. [Google Scholar] [CrossRef]
- Gurkan, U.A.; Cheng, X.; Kishore, V.; Uquillas, J.A.; Akkus, O. Comparison of morphology, orientation, and migration of tendon derived fibroblasts and bone marrow stromal cells on electrochemically aligned collagen constructs. J. Biomed. Mater. Res. Part A 2010, 94A, 1070–1079. [Google Scholar] [CrossRef]
- Ficai, A.; Andronescu, E.; Trandafir, V.; Ghitulica, C.; Voicu, G. Collagen/hydroxyapatite composite obtained by electric field orientation. Mater. Lett. 2010, 64, 541–544. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Chi, Y.; Wang, Y.; Jiang, L.; Xu, N.; Wu, Q.; Feng, Q.; Sun, X. Preparation of oriented collagen fiber scaffolds and its application in bone tissue engineering. Appl. Mater. Today 2021, 22, 100902. [Google Scholar] [CrossRef]
- Kishore, V.; Bullock, W.; Sun, X.; Van Dyke, W.S.; Akkus, O. Tenogenic differentiation of human MSCs induced by the topography of electrochemically aligned collagen threads. Biomaterials 2012, 33, 2137–2144. [Google Scholar] [CrossRef]
- Younesi, M.; Goldberg, V.M.; Akkus, O. A micro-architecturally biomimetic collagen template for mesenchymal condensation based cartilage regeneration. Acta Biomater. 2016, 30, 212–221. [Google Scholar] [CrossRef]
- Abu-Rub, M.T.; Billiar, K.L.; Van Es, M.H.; Knight, A.; Rodriguez, B.J.; Zeugolis, D.I.; McMahon, S.; Windebank, A.J.; Pandit, A. Nano-textured self-assembled aligned collagen hydrogels promote directional neurite guidance and overcome inhibition by myelin associated glycoprotein. Soft Matter 2011, 7, 2770. [Google Scholar] [CrossRef]
- Nguyen, T.-U.; Bashur, C.A.; Kishore, V. Impact of elastin incorporation into electrochemically aligned collagen fibers on mechanical properties and smooth muscle cell phenotype. Biomed. Mater. 2016, 11, 025008. [Google Scholar] [CrossRef]
- Younesi, M.; Islam, A.; Kishore, V.; Panit, S.; Akkus, O. Fabrication of compositionally and topographically complex robust tissue forms by 3D-electrochemical compaction of collagen. Biofabrication 2015, 7, 035001. [Google Scholar] [CrossRef]
- Sapudom, J.; Karaman, S.; Quartey, B.C.; Mohamed, W.K.E.; Mahtani, N.; Garcia-Sabaté, A.; Teo, J. Collagen Fibril Orientation Instructs Fibroblast Differentiation Via Cell Contractility. Adv. Sci. 2023, 10, 2301353. [Google Scholar] [CrossRef]
- Evans, H.J.; Sweet, J.K.; Price, R.L.; Yost, M.; Goodwin, R.L. Novel 3D culture system for study of cardiac myocyte development. Am. J. Physiol. Circ. Physiol. 2003, 285, H570–H578. [Google Scholar] [CrossRef]
- Kato, Y.P.; Christiansen, D.L.; Hahn, R.A.; Shieh, S.-J.; Goldstein, J.D.; Silver, F.H. Mechanical properties of collagen fibres: A comparison of reconstituted and rat tail tendon fibres. Biomaterials 1989, 10, 38–42. [Google Scholar] [CrossRef]
- Keijzer, K.A.E.; Tsingos, E.; Merks, R.M.H. How cells align to structured collagen fibrils: A hybrid cellular Potts and molecular dynamics model with dynamic mechanosensitive focal adhesions. Front. Cell Dev. Biol. 2024, 12, 1462277. [Google Scholar] [CrossRef]
- Leighton, M.P.; Kreplak, L.; Rutenberg, A.D. Non-equilibrium growth and twist of cross-linked collagen fibrils. Soft Matter 2021, 17, 1415–1427. [Google Scholar] [CrossRef]
- Rezaei, N.; Lyons, A.; Forde, N.R. Environmentally Controlled Curvature of Single Collagen Proteins. Biophys. J. 2018, 115, 1457–1469. [Google Scholar] [CrossRef]
- Bell, J.S.; Hayes, S.; Whitford, C.; Sanchez-Weatherby, J.; Shebanova, O.; Vergari, C.; Winlove, C.P.; Terrill, N.; Sorensen, T.; Elsheikh, A.; et al. The hierarchical response of human corneal collagen to load. Acta Biomater. 2018, 65, 216–225. [Google Scholar] [CrossRef]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Raspanti, M.; Reguzzoni, M.; Protasoni, M.; Basso, P. Not only tendons: The other architecture of collagen fibrils. Int. J. Biol. Macromol. 2018, 107, 1668–1674. [Google Scholar] [CrossRef]
- Kagan, H.M.; Trackman, P.C. Properties and function of lysyl oxidase. Am. J. Respir. Cell Mol. Biol. 1991, 5, 206–210. [Google Scholar] [CrossRef]
- Eekhoff, J.D.; Fang, F.; Lake, S.P. Multiscale mechanical effects of native collagen cross-linking in tendon. Connect. Tissue Res. 2018, 59, 410–422. [Google Scholar] [CrossRef]
- Colabella, L.; Naili, S.; Le Cann, S.; Haiat, G. Effect of collagen fibril orientation on the anisotropic properties of peri-implant bone. Biomech. Model. Mechanobiol. 2024, 23, 879–891. [Google Scholar] [CrossRef]
- Milovanovic, P.; Busse, B. Phenomenon of osteocyte lacunar mineralization: Indicator of former osteocyte death and a novel marker of impaired bone quality? Endocr. Connect. 2020, 9, R70–R80. [Google Scholar] [CrossRef]
- Ascenzi, A.; Bonucci, E. The tensile properties of single osteons. Anat. Rec. 1967, 158, 375–386. [Google Scholar] [CrossRef]
- Giraud-Guille, M.M. Twisted plywood architecture of collagen fibrils in human compact bone osteons. Calcif. Tissue Int. 1988, 42, 167–180. [Google Scholar] [CrossRef]
- Reznikov, N.; Shahar, R.; Weiner, S. Bone hierarchical structure in three dimensions. Acta Biomater. 2014, 10, 3815–3826. [Google Scholar] [CrossRef]
- Ehrlich, H.; Deutzmann, R.; Brunner, E.; Cappellini, E.; Koon, H.; Solazzo, C.; Yang, Y.; Ashford, D.; Thomas-Oates, J.; Lubeck, M.; et al. Mineralization of the metre-long biosilica structures of glass sponges is templated on hydroxylated collagen. Nat. Chem. 2010, 2, 1084–1088. [Google Scholar] [CrossRef]
- Ehrlich, H.; Miksik, I.; Tsurkan, M.V.; Simon, P.; Porzucek, F.; Rybka, J.D.; Mankowska, M.; Galli, R.; Viehweger, C.; Brendler, E.; et al. Discovery of mammalian collagens I and III within ancient poriferan biopolymer spongin. Nat. Commun. 2025, 16, 2515. [Google Scholar] [CrossRef]
- Selvaraj, V.; Sekaran, S.; Dhanasekaran, A.; Warrier, S. Type 1 collagen: Synthesis, structure and key functions in bone mineralization. Differentiation 2024, 136, 100757. [Google Scholar] [CrossRef]
- McNally, E.; Nan, F.; Botton, G.A.; Schwarcz, H.P. Scanning transmission electron microscopic tomography of cortical bone using Z-contrast imaging. Micron 2013, 49, 46–53. [Google Scholar] [CrossRef]
- Zimmermann, E.A.; Busse, B.; Ritchie, R.O. The fracture mechanics of human bone: Influence of disease and treatment. Bonekey Rep. 2015, 4, 743. [Google Scholar] [CrossRef]
- Vom Scheidt, A.; Hemmatian, H.; Püschel, K.; Krause, M.; Amling, M.; Busse, B. Bisphosphonate treatment changes regional distribution of trabecular microstructure in human lumbar vertebrae. Bone 2019, 127, 482–487. [Google Scholar] [CrossRef]
- Willett, T.L.; Dapaah, D.Y.; Uppuganti, S.; Granke, M.; Nyman, J.S. Bone collagen network integrity and transverse fracture toughness of human cortical bone. Bone 2019, 120, 187–193. [Google Scholar] [CrossRef]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Martin, R.B.; Boardman, D.L. The effects of collagen fiber orientation, porosity, density, and mineralization on bovine cortical bone bending properties. J. Biomech. 1993, 26, 1047–1054. [Google Scholar] [CrossRef]
- Skedros, J.G.; Kiser, C.J.; Mendenhall, S.D. A weighted osteon morphotype score outperforms regional osteon percent prevalence calculations for interpreting cortical bone adaptation. Am. J. Phys. Anthropol. 2011, 144, 41–50. [Google Scholar] [CrossRef]
- Zhai, X.; Geng, X.; Li, W.; Cui, H.; Wang, Y.; Qin, S. Comprehensive Review on Application Progress of Marine Collagen Cross-Linking Modification in Bone Repairs. Mar. Drugs 2025, 23, 151. [Google Scholar] [CrossRef]
- Simon, P.; Grüner, D.; Worch, H.; Pompe, W.; Lichte, H.; El Khassawna, T.; Heiss, C.; Wenisch, S.; Kniep, R. First evidence of octacalcium phosphate@osteocalcin nanocomplex as skeletal bone component directing collagen triple–helix nanofibril mineralization. Sci. Rep. 2018, 8, 13696. [Google Scholar] [CrossRef]
- Hassell, J.R.; Birk, D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar] [CrossRef]
- Massoudi, D.; Malecaze, F.; Galiacy, S.D. Collagens and proteoglycans of the cornea: Importance in transparency and visual disorders. Cell Tissue Res. 2016, 363, 337–349. [Google Scholar] [CrossRef]
- Su, H.; Karin, M. Collagen architecture and signaling orchestrate cancer development. Trends Cancer 2023, 9, 764–773. [Google Scholar] [CrossRef]

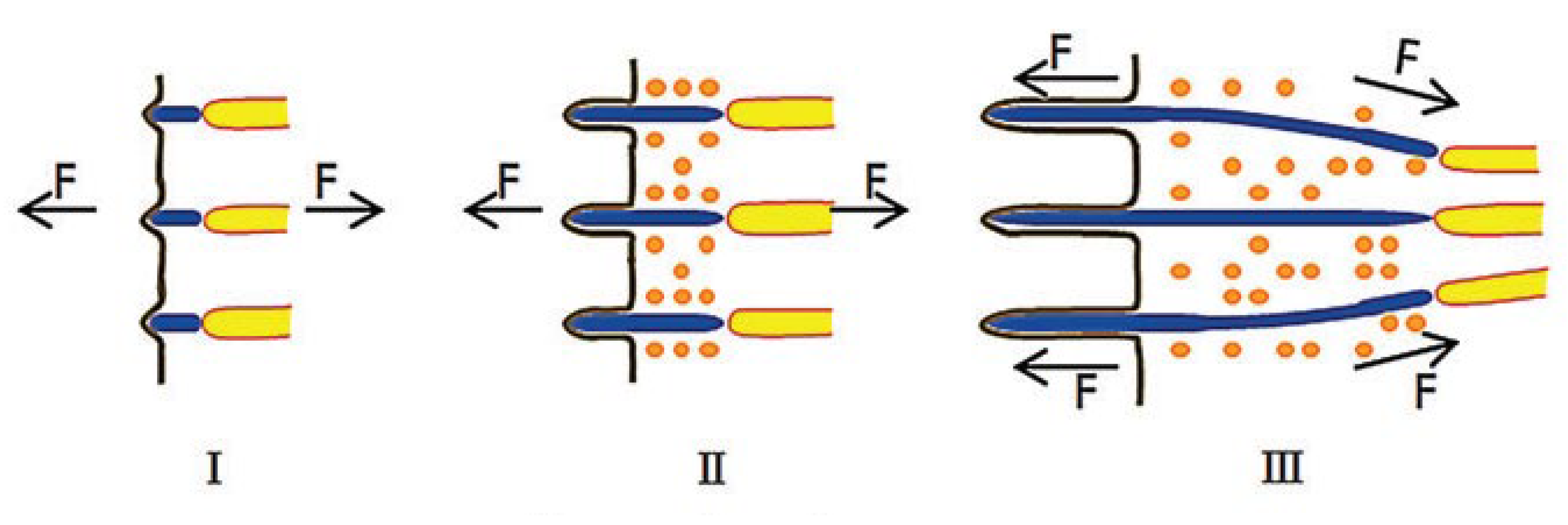
| Fabrication Methods | |||
|---|---|---|---|
| Mechanical Orientation | Magnetic Orientation | Electrochemical Orientation | |
| Orientation processes | The method mediated by shear is mainly divided into fluid displacement controlled by fluid flow and mechanical displacement created by the relative motion of the acting surfaces [74,75]. | Due to the magnetic anisotropy of collagen molecule, collagen fibrils can orient themselves under the influence of a magnetic field [87,88]. | The collagen molecule is an ampholite and under the action of electrostatic forces, the molecule is repelled from the similarly charged electrode and concentrated at the isoelectric point [97,98]. |
| Adjustable parameters | Flow rates and rotational speeds, concentration of collagen in solution. | Structure and quantity of magnetic particles, magnitude of magnetic field. | Concentration, voltage, current density, and time. |
| Limitations | Oriented fibers obtained do not have a striped pattern. The final products are small in size. It is impossible to obtain scaffolds with high packing density and elastic deformation. | The main limitation is the need for superconducting magnets of the Tesla order, since the collagen molecule itself has a low diamagnetic constant. | Currently, the largest limiting factor of electrochemical orientation collagen use is its insufficient mechanical strength. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nashchekina, Y.; Nashchekin, A. Collagen Fibril Orientation In Vitro: From Formation to Advanced Biomaterial Development. Biomimetics 2025, 10, 644. https://doi.org/10.3390/biomimetics10100644
Nashchekina Y, Nashchekin A. Collagen Fibril Orientation In Vitro: From Formation to Advanced Biomaterial Development. Biomimetics. 2025; 10(10):644. https://doi.org/10.3390/biomimetics10100644
Chicago/Turabian StyleNashchekina, Yuliya, and Alexey Nashchekin. 2025. "Collagen Fibril Orientation In Vitro: From Formation to Advanced Biomaterial Development" Biomimetics 10, no. 10: 644. https://doi.org/10.3390/biomimetics10100644
APA StyleNashchekina, Y., & Nashchekin, A. (2025). Collagen Fibril Orientation In Vitro: From Formation to Advanced Biomaterial Development. Biomimetics, 10(10), 644. https://doi.org/10.3390/biomimetics10100644







