The Use of Platelet-Rich Fibrin-Coated Three-Dimensionally (3D) Printed Scaffolds in Salvage of Complex Hindfoot Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative Planning
2.2. Surgical Technique
2.3. Postoperative Care
2.4. Data Collection
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shono, H.; Matsumoto, Y.; Kokubun, T.; Tsuruta, A.; Miyazawa, T.; Kobayashi, A.; Kanemura, N. Determination of Relationship between Foot Arch, Hindfoot, and Hallux Motion Using Oxford Foot Model: Comparison between Walking and Running. Gait Posture 2022, 92, 96–102. [Google Scholar] [CrossRef]
- Chatterton, B.D.; Muller, S.; Roddy, E. Epidemiology of Posterior Heel Pain in the General Population: Cross-Sectional Findings from the Clinical Assessment Study of the Foot. Arthritis Care Res. 2015, 67, 996–1003. [Google Scholar] [CrossRef]
- Adelaar, R.S.; Madrian, J.R. Avascular Necrosis of the Talus. Orthop. Clin. N. Am. 2004, 35, 383–395. [Google Scholar] [CrossRef]
- Jordan, R.K.; Bafna, K.R.; Liu, J.; Ebraheim, N.A. Complications of Talar Neck Fractures by Hawkins Classification: A Systematic Review. J. Foot Ankle Surg. 2017, 56, 817–821. [Google Scholar] [CrossRef]
- Stake, I.K.; Madsen, J.E.; Hvaal, K.; Johnsen, E.; Husebye, E.E. Surgically Treated Talar Fractures. A Retrospective Study of 50 Patients. Foot Ankle Surg. 2015, 22, 85–90. [Google Scholar] [CrossRef]
- Vints, W.; Matricali, G.; Geusens, E.; Nijs, S.; Hoekstra, H. Long-Term Outcome after Operative Management of Talus Fractures. Foot Ankle Int. 2018, 39, 1432–1443. [Google Scholar] [CrossRef]
- Clement, R.G.E.; Wong, S.J.; Hall, A.; Howie, S.E.M.; Simpson, A.H.R.W. The Long-Term Time Course of Septic Arthritis. Bone Jt. Open 2024, 5, 785–792. [Google Scholar] [CrossRef]
- Kim, H.; Bae, S.-Y. Talus Osteomyelitis by Candida Krusei with Multiple Huge Cystic Lesions: A Case Report and Review of Literatures. BMC Musculoskelet. Disord. 2022, 23, 687. [Google Scholar] [CrossRef]
- Winkler, E.; Schöni, M.; Krähenbühl, N.; Uçkay, I.; Waibel, F.W.A. Foot Osteomyelitis Location and Rates of Primary or Secondary Major Amputations in Patients with Diabetes. Foot Ankle Int. 2022, 43, 957–967. [Google Scholar] [CrossRef]
- Eschler, A.; Gradl, G.; Wussow, A.; Mittlmeier, T. Prediction of Complications in a High-Risk Cohort of Patients Undergoing Corrective Arthrodesis of Late Stage Charcot Deformity Based on the PEDIS Score. BMC Musculoskelet. Disord. 2015, 16, 349. [Google Scholar] [CrossRef]
- Leslie, M.D.; Schindler, C.; Rooke, G.M.J.; Dodd, A. CT-Verified Union Rate Following Arthrodesis of Ankle, Hindfoot, or Midfoot: A Systematic Review. Foot Ankle Int. 2023, 44, 665–674. [Google Scholar] [CrossRef]
- Miniaci-Coxhead, S.L.; Weisenthal, B.; Ketz, J.P.; Flemister, A.S. Incidence and Radiographic Predictors of Valgus Tibiotalar Tilt after Hindfoot Fusion. Foot Ankle Int. 2017, 38, 519–525. [Google Scholar] [CrossRef]
- Xu, W.; Liu, Z.; Lu, X.; Tian, J.; Chen, G.; Liu, B.; Li, Z.; Qu, X.; Wen, C. Porous Ti-10Mo Alloy Fabricated by Powder Metallurgy for Promoting Bone Regeneration. Sci. China Mater. 2019, 62, 1053–1064. [Google Scholar] [CrossRef]
- Mayfield, C.K.; Ayad, M.; Lechtholz-Zey, E.; Chen, Y.; Lieberman, J.R. 3D-Printing for Critical Sized Bone Defects: Current Concepts and Future Directions. Bioengineering 2022, 9, 680. [Google Scholar] [CrossRef]
- Hallman, M.; Driscoll, J.A.; Lubbe, R.; Jeong, S.; Chang, K.; Haleem, M.; Jakus, A.; Pahapill, R.; Yun, C.; Shah, R.N.; et al. Influence of Geometry and Architecture on the in Vivo Success of 3D-Printed Scaffolds for Spinal Fusion. Tissue Eng. Part A 2020, 27, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Schemitsch, E.H. Size Matters. J. Orthop. Trauma 2017, 31, S20–S22. [Google Scholar] [CrossRef]
- Dekker, T.J.; Steele, J.R.; Federer, A.E.; Hamid, K.S.; Adams, S.B. Use of Patient-Specific 3D-Printed Titanium Implants for Complex Foot and Ankle Limb Salvage, Deformity Correction, and Arthrodesis Procedures. Foot Ankle Int. 2018, 39, 916–921. [Google Scholar] [CrossRef]
- Abar, B.; Kwon, N.; Allen, N.B.; Lau, T.; Johnson, L.G.; Gall, K.; Adams, S.B. Outcomes of Surgical Reconstruction Using Custom 3D-Printed Porous Titanium Implants for Critical-Sized Bone Defects of the Foot and Ankle. Foot Ankle Int. 2022, 43, 750–761. [Google Scholar] [CrossRef]
- Wallace, N.; Schaffer, N.E.; Aleem, I.S.; Patel, R. 3D-Printed Patient-Specific Spine Implants: A Systematic Review. Clin. Spine Surg. 2020, 33, 400–407. [Google Scholar] [CrossRef]
- Pavlovic, V.; Ciric, M.; Jovanovic, V.; Trandafilovic, M.; Stojanovic, P. Platelet-Rich Fibrin: Basics of Biological Actions and Protocol Modifications. Open Med. 2021, 16, 446–454. [Google Scholar] [CrossRef]
- Beyzadeoğlu, T.; Pehlivanoğlu, T.; Yıldırım, K.; Buldu, H.; Tandoğan, R.N.; Tüzün, Ü. Does the Application of Platelet-Rich Fibrin in Anterior Cruciate Ligament Reconstruction Enhance Graft Healing and Maturation? A Comparative MRI Study of 44 Cases. Orthop. J. Sports Med. 2020, 8, 2325967120902013. [Google Scholar] [CrossRef] [PubMed]
- Certificate of Registration. Available online: https://meshworksimplants.com/wp-content/uploads/2024/08/MW_ISO13485-Certificate.pdf (accessed on 23 April 2025).
- Steele, J.R.; Kadakia, R.J.; Cunningham, D.J.; Dekker, T.J.; Kildow, B.J.; Adams, S.B. Comparison of 3D Printed Spherical Implants versus Femoral Head Allografts for Tibiotalocalcaneal Arthrodesis. J. Foot Ankle Surg. 2020, 59, 1167–1170. [Google Scholar] [CrossRef]
- Coughlin, M.J.; Grimes, J.S.; Traughber, P.D.; Jones, C.P. Comparison of Radiographs and CT Scans in the Prospective Evaluation of the Fusion of Hindfoot Arthrodesis. Foot Ankle Int. 2006, 27, 780–787. [Google Scholar] [CrossRef]
- Krause, F.; Younger, A.S.E.; Baumhauer, J.F.; Daniels, T.R.; Glazebrook, M.; Evangelista, P.T.; Pinzur, M.S.; Thevendran, G.; Donahue, R.M.J.; DiGiovanni, C.W. Clinical Outcomes of Nonunions of Hindfoot and Ankle Fusions. J. Bone Jt. Surg. Am. 2016, 98, 2006–2016. [Google Scholar] [CrossRef]
- Aubret, S.; Merlini, L.; Fessy, M.; Besse, J.-L. Poor Outcomes of Fusion with Trabecular Metal Implants after Failed Total Ankle Replacement: Early Results in 11 Patients. Orthop. Traumatol. Surg. Res. 2018, 104, 231–237. [Google Scholar] [CrossRef]
- Glazebrook, M.; Beasley, W.; Daniels, T.; Evangelista, P.T.; Donahue, R.; Younger, A.; Pinzur, M.S.; Baumhauer, J.F.; DiGiovanni, C.W. Establishing the Relationship between Clinical Outcome and Extent of Osseous Bridging between Computed Tomography Assessment in Isolated Hindfoot and Ankle Fusions. Foot Ankle Int. 2013, 34, 1612–1618. [Google Scholar] [CrossRef]
- Strydom, A.; Saragas, N.P.; Ferrao, P.N. The Use of a 3D Printed Titanium Implant for Arthrodesis in the Management of Large Osseous Defects in the Ankle. Foot Ankle Surg. 2023, 29, 576–583. [Google Scholar] [CrossRef]
- Kim, M.S.; Mann, T.; Kelly, C.; Palmer, R.C.; Abar, B.; Zhang, H.; Cush, G.J. Mid-Term Outcomes of Lower Limb Salvage with 3D-Printed Ankle Cages. Foot Ankle Surg. Tech. Rep. Cases 2024, 4, 100413. [Google Scholar] [CrossRef]
- Schick, V.D.; Zampogna, B.; Marrara, G.; Siracusano, L.; Larizza, L.; Calaciura, S.; Sanzarello, I.; Marinozzi, A.; Leonetti, D. Custom-Made 3D-Printed Titanium Implants for Managing Segmental Distal Tibial Bone Defects: A Systematic Literature Review. J. Clin. Med. 2025, 14, 1796. [Google Scholar] [CrossRef] [PubMed]
- Gamieldien, H.; Ferreira, N.; Birkholtz, F.F.; Hilton, T.; Campbell, N.; Laubscher, M. Filling the Gap: A Series of 3D-Printed Titanium Truss Cages for the Management of Large, Lower Limb Bone Defects in a Developing Country Setting. Eur. J. Orthop. Surg. Traumatol. 2022, 33, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.L.; Walker, R.; Alkhalfan, Y.; Latif, A.; Abbasian, A. Custom Patient-Specific 3D-Printed Titanium Truss Tibiotalocalcaneal Arthrodesis Implants for Failed Total Ankle Replacements: Classification, Technical Tips, and Treatment Algorithm. Foot Ankle Int. 2024, 45, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Ramhamadany, E.; Chadwick, C.; Davies, M.B. Treatment of Severe Avascular Necrosis of the Talus Using a Novel Keystone-Shaped 3D-Printed Titanium Truss Implant. Foot Ankle Orthop. 2021, 6, 24730114211043516. [Google Scholar] [CrossRef] [PubMed]
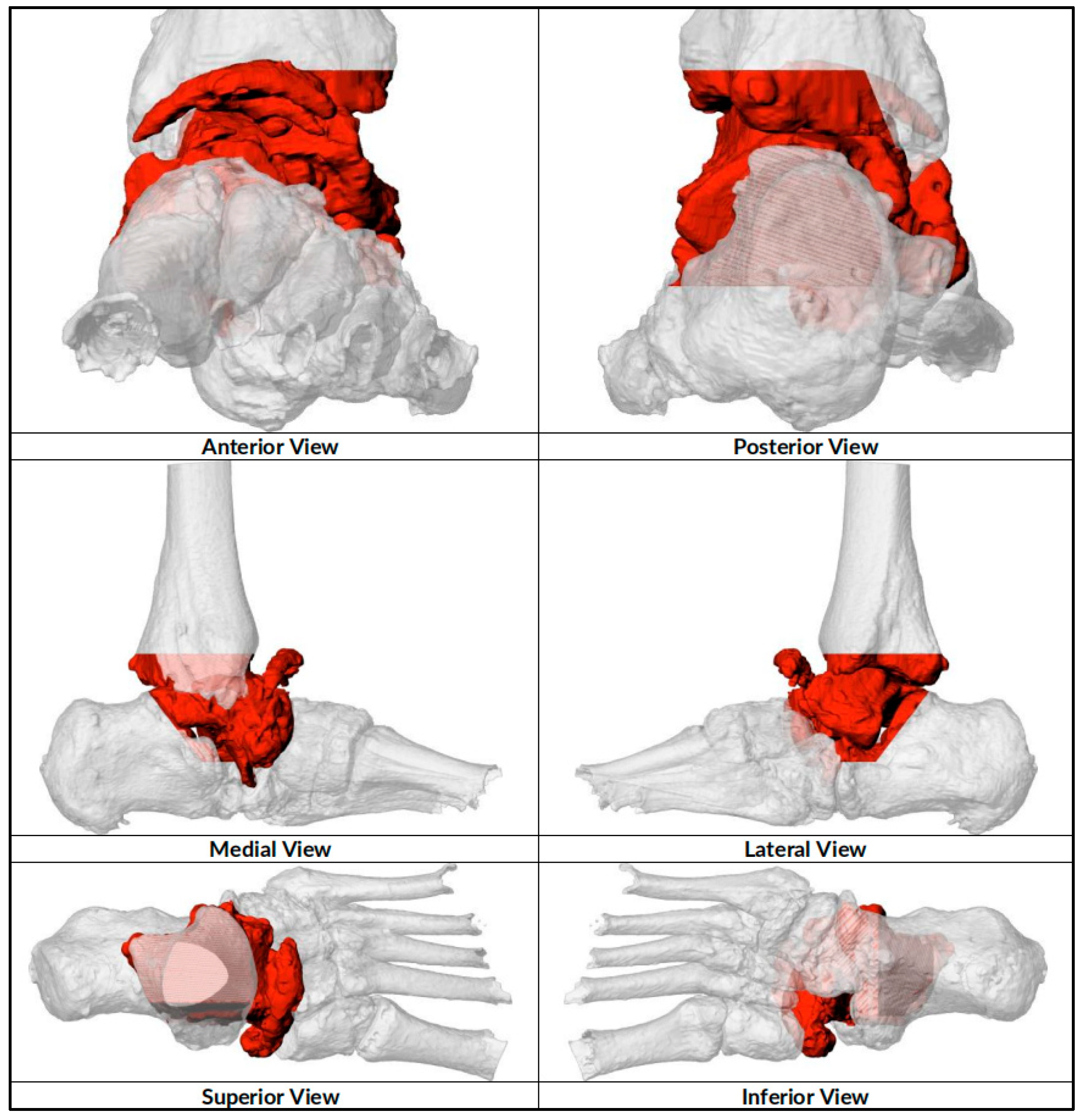
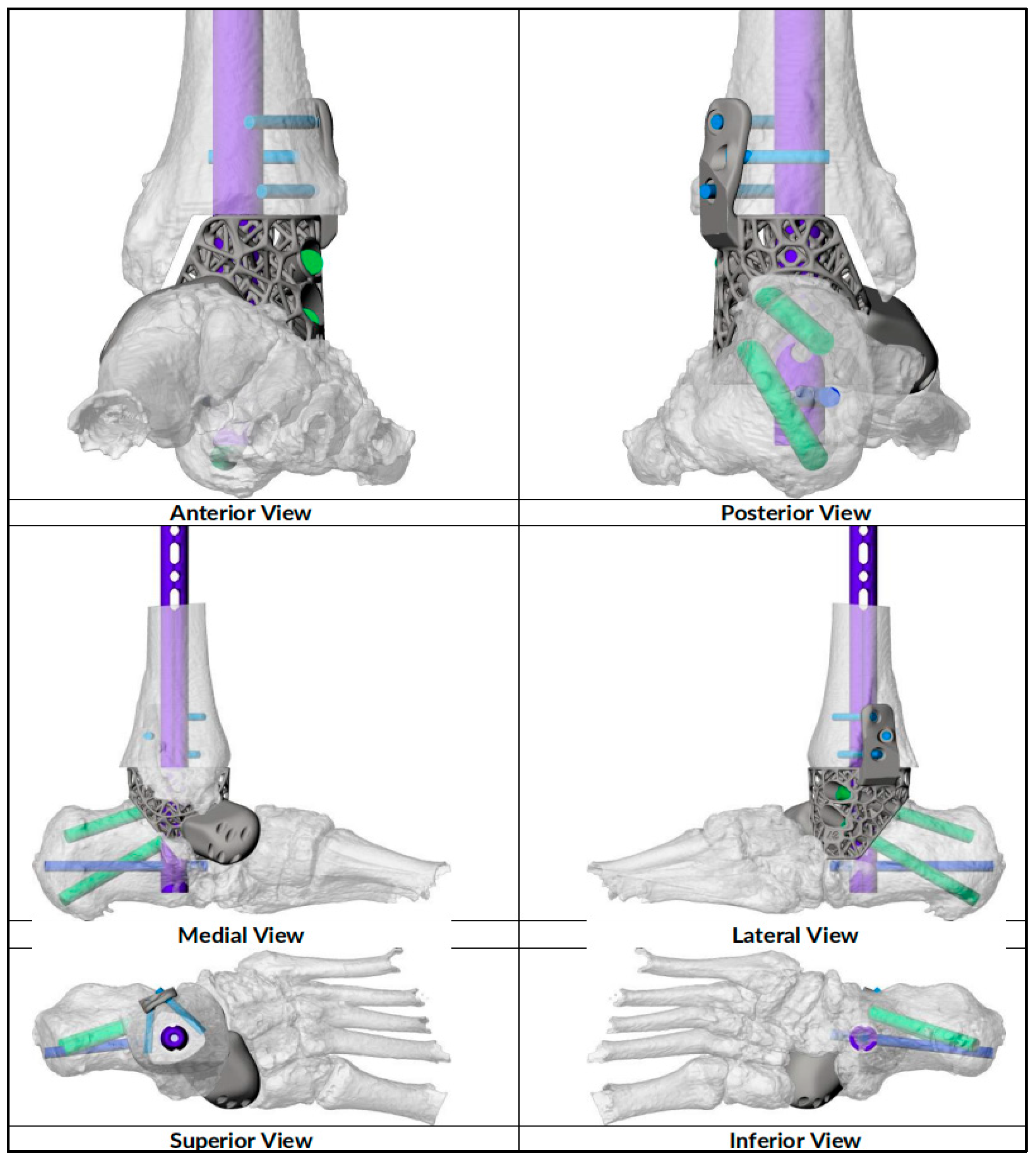
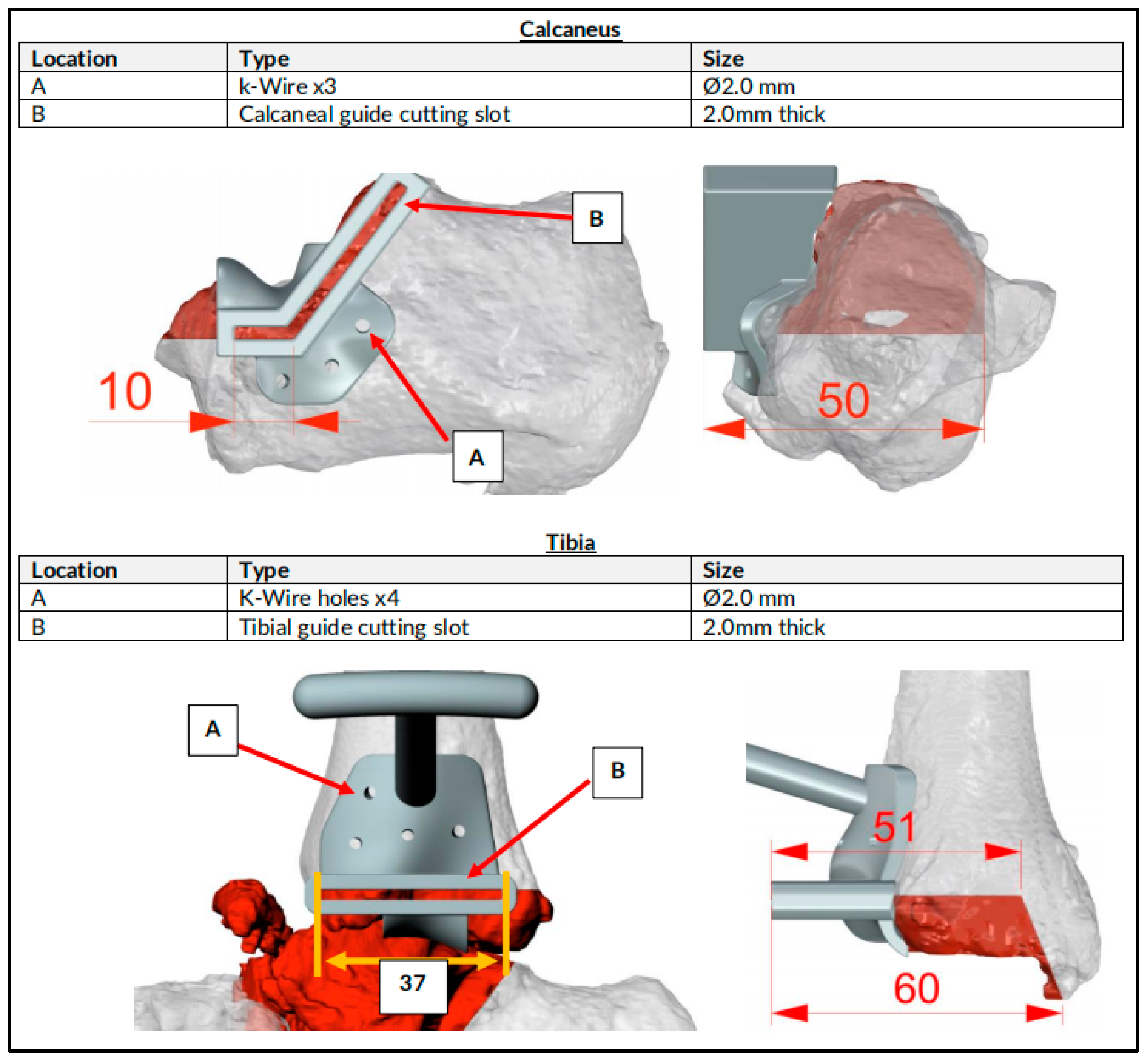
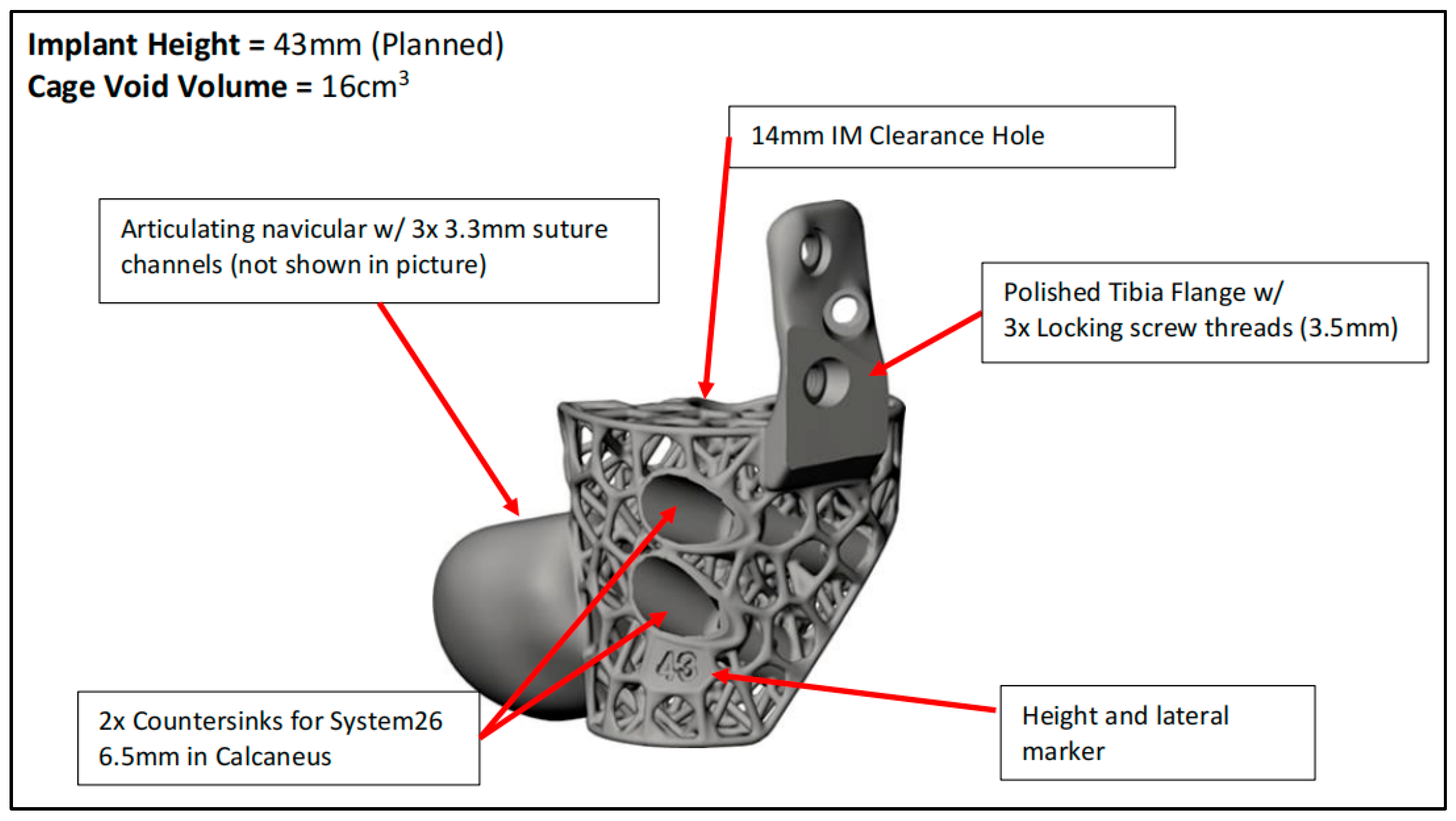

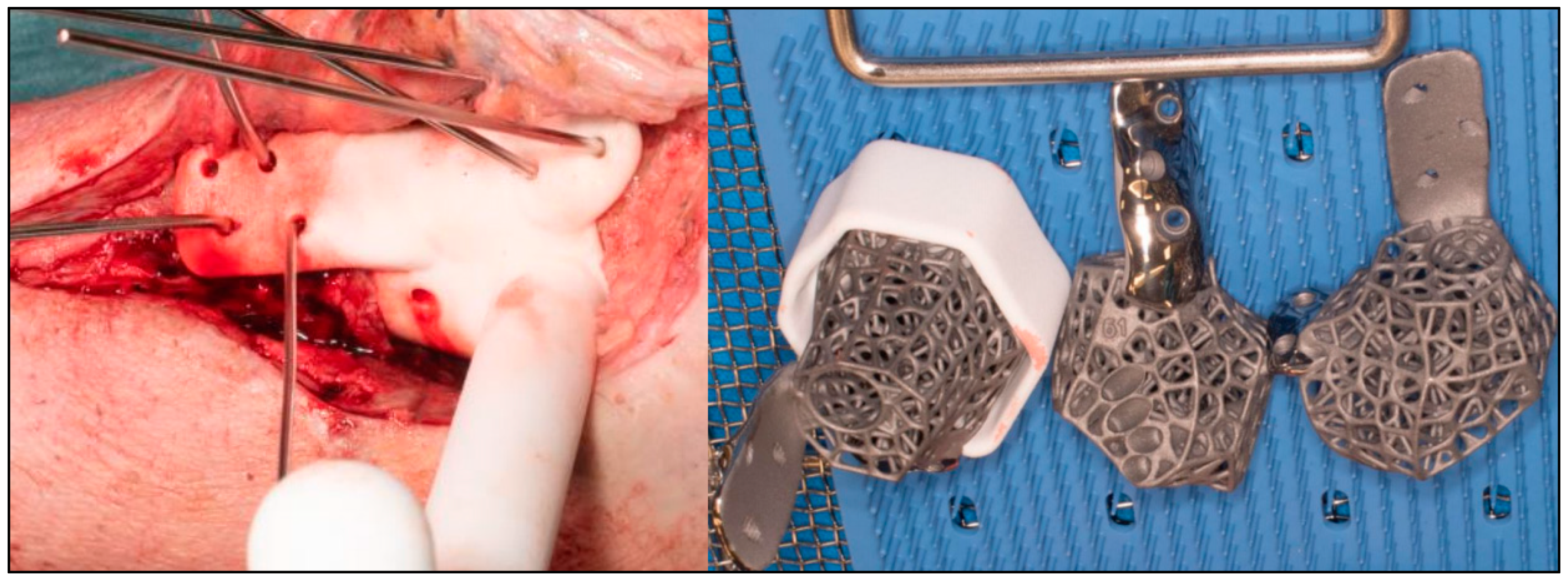
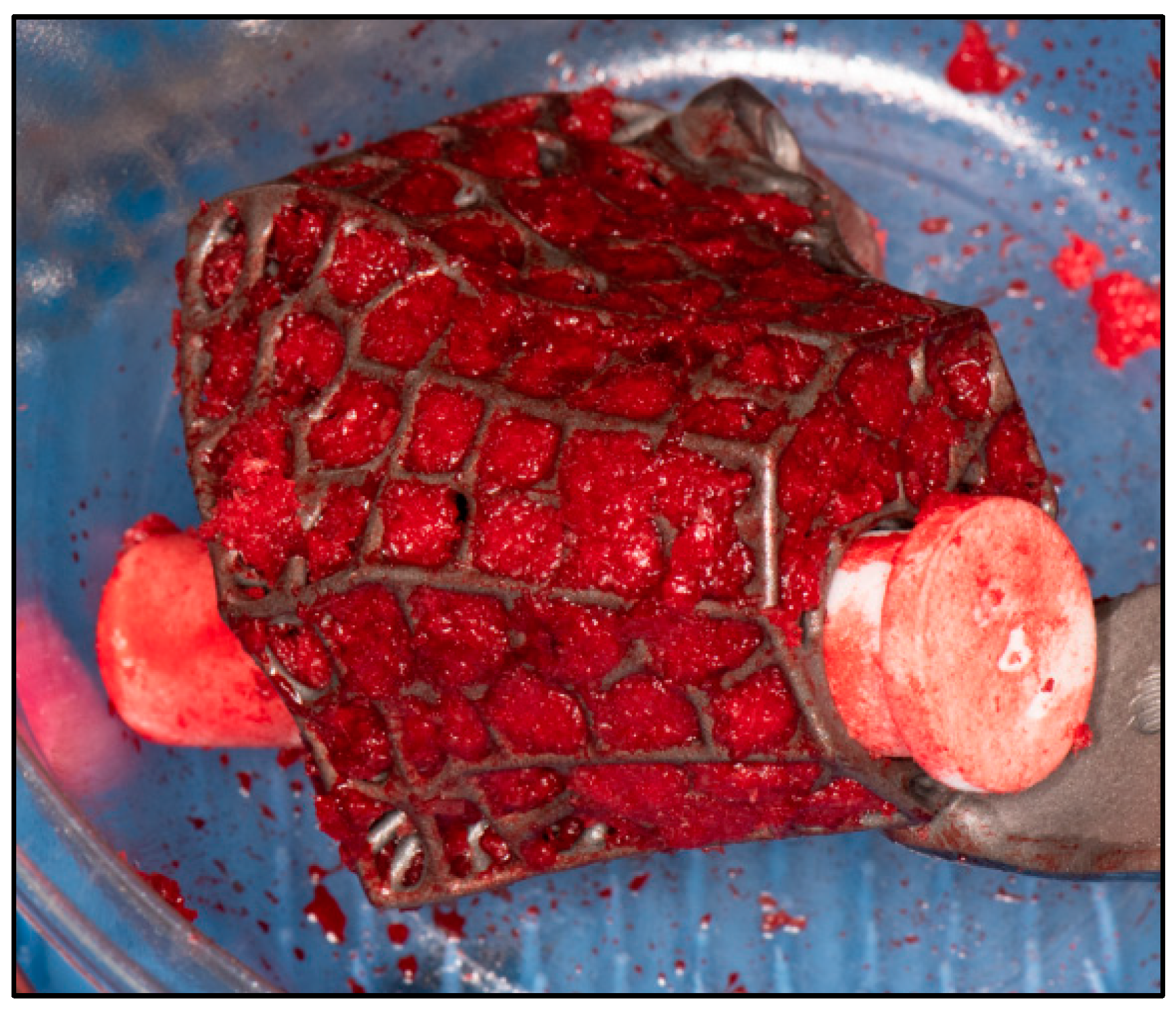

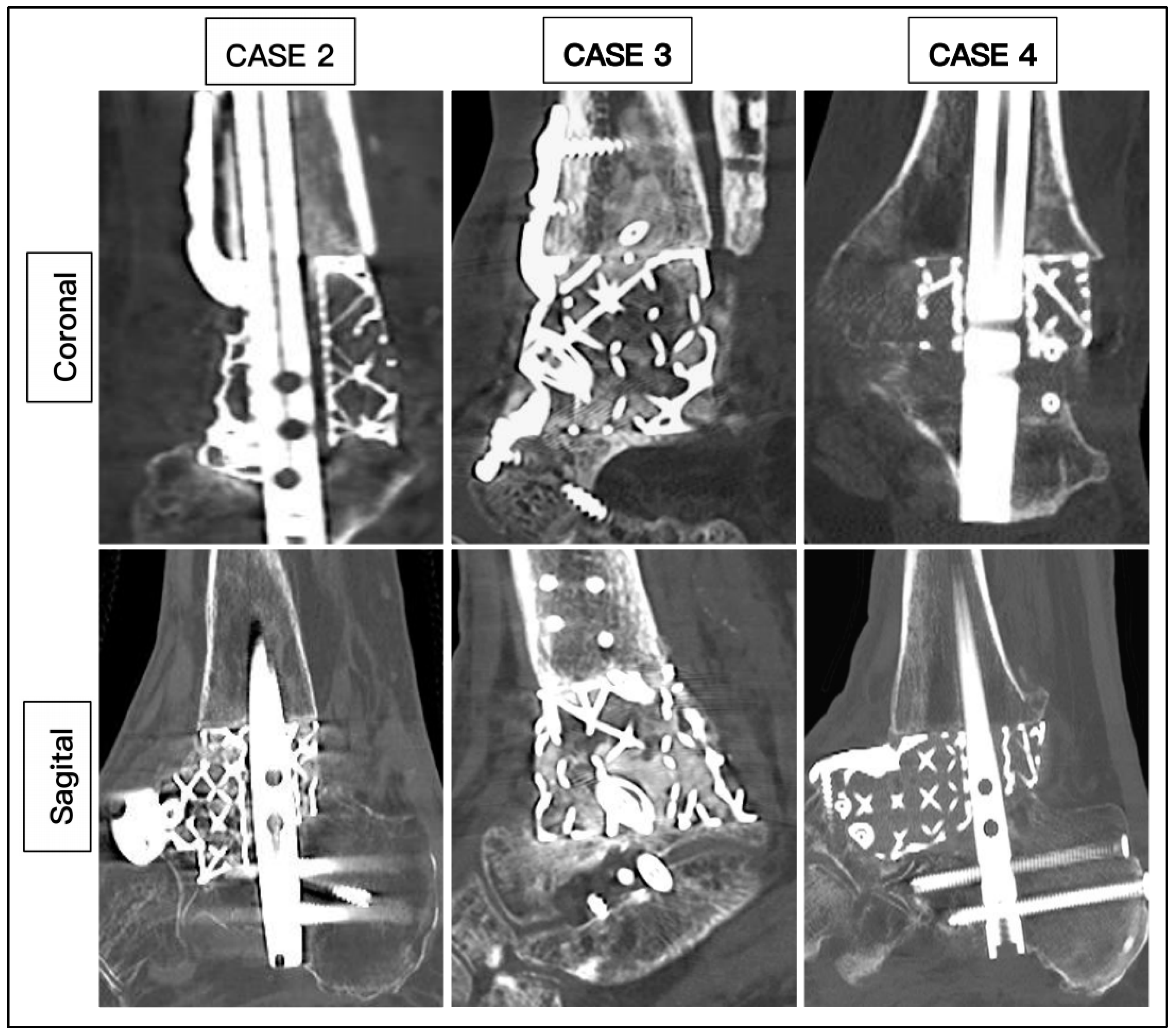
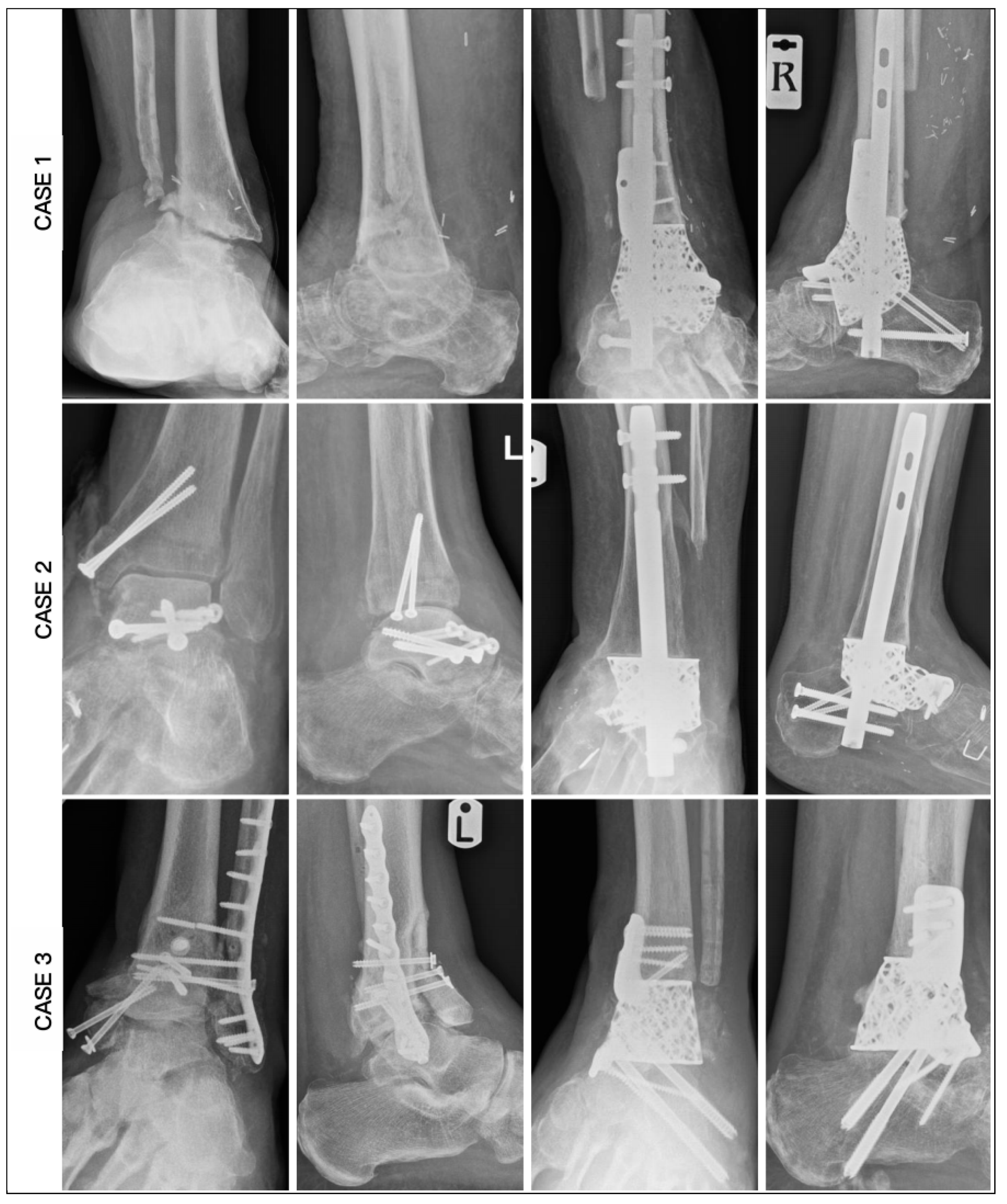

| CASE | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age | 62 | 59 | 60 | 60 | 78 | 67 |
| Diagnosis | OM | OM | OM | AVN | AVN | AVN |
| Smoking | No | Yes | Yes | No | Yes | No |
| BMI | 26.5 | 27.4 | 27.4 | 28.2 | 32.1 | 35.4 |
| Medical | Nil | IHD, CKD, PAD | CKD | CKD, PAD | DM | IHD, DM, CKD, CN |
| HbA1c | 36 | 39 | 39 | 41 | 49 | 70 |
| CRP | 8 | 7 | 16 | 9 | 10 | 6 |
| Hb (g/L) | 113 | 105 | 132 | 146 | 113 | 114 |
| Past surgery | 5 | 3 | 3 | 0 | 1 | 0 |
| Modification | Tibial and Navicular Flange, Nail | Navicular Flange, Nail | Tibial and Navicular Flange | Navicular Flange, Nail | Nail | Articulating Navicular Extension, Tibial Flange, Nail |
| Duration of surgery (min) | 255 | 220 | 162 | 210 | 190 | 205 |
| Wound Healing (days) | 42 | 40 | 20 | 29 | 32 | 45 |
| Union (weeks) | 25 | 19 | 16 | 14 | 27 | 20 |
| Ambulation (weeks) | 28 | 21 | 18 | 16 | 29 | 22 |
| CASE | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Tibiocalcaneal angle (pre) | 38.4 | 18.6 | 12.4 | −6.8 | 13.8 | 10.5 |
| Tibiocalcaneal angle (post) | 6.2 | 3.0 | 3.4 | 4.2 | 6.1 | 4.2 |
| Difference | 32.2 | 15.6 | 9.0 | 11 | 7.7 | 6.3 |
| Tibiotalar angle (pre) | 16.1 | 4.4 | 12.4 | −6.1 | 11.9 | 2.1 |
| Tibiotalar angle (post) | 1.4 | 1.2 | 0.9 | 0.2 | 0.5 | 0.7 |
| Difference | 14.7 | 3.2 | 11.5 | 6.3 | 11.4 | 2.0 |
| Tibiocalcaneal distance (pre) | 53.2 | 24.0 | 24.0 | 6.2 | 27.7 | 18.9 |
| Tibiocalcaneal distance (post) | 9.1 | 7.2 | 4.8 | 6.0 | 11.7 | 9.1 |
| Difference | 44.1 | 16.8 | 19.2 | 17.2 | 16.0 | 9.8 |
| Foot height (pre) | 43.8 | 58.1 | 54.8 | 54.1 | 52.8 | 38.3 |
| Foot height (post) | 51.9 | 58.3 | 74.3 | 51.5 | 54.6 | 50.1 |
| Difference | 8.1 | 0.2 | 19.5 | 2.6 | 1.8 | 11.8 |
| Lateral distal tibial angle (pre) | 81.2 | 87.5 | 76.6 | 97.7 | 101.5 | 87.8 |
| Lateral distal tibial angle (post) | 88.9 | 87 | 86.6 | 85.3 | 91.3 | 92.7 |
| Difference | 7.7 | 0.5 | 10.0 | 12.4 | 10.2 | 4.9 |
| Lateral tibiotalar angle (pre) | 87.8 | 67.1 | 107.6 | 81.5 | 103.6 | 59.1 |
| Lateral tibiotalar angle (post) | 69.9 | 71.1 | 67.3 | 72.1 | 70.8 | 71.2 |
| Difference | 17.9 | 4.0 | 40.3 | 9.4 | 32.8 | 12.1 |
| Meary angle (pre) | 8.0 | 10.1 | 2.3 | 3.2 | 24.1 | 11.4 |
| Meary angle (post) | 3.1 | 2.0 | 2.0 | 1.8 | 5.3 | 3.6 |
| Difference | 4.8 | 8.3 | 0.3 | 1.4 | 18.8 | 7.8 |
| Calcaneal inclination (pre) | 13.7 | 37.1 | 14.5 | 16.1 | 27.9 | 4.7 |
| Calcaneal inclination (post) | 22.5 | 21.1 | 14.8 | 22.6 | 22.1 | 16.7 |
| Difference | 8.8 | 16.0 | 0.3 | 6.2 | 5.8 | 12 |
| Navicular height (pre) | 33.1 | 39.3 | 44.8 | 37.5 | 33.4 | 17.4 |
| Navicular height (post) | 41.4 | 35.9 | 46.5 | 41.6 | 40.1 | 38.1 |
| Difference | 8.3 | 3.4 | 1.7 | 4.1 | 6.7 | 20.7 |
| Plantigrade angle (pre) | 85.1 | 93.7 | 86.9 | 90.0 | 89.8 | 87.4 |
| Plantigrade angle (post) | 86.9 | 88.7 | 89.4 | 89.5 | 89.5 | 92.6 |
| Difference | 1.8 | 5.0 | 2.5 | 0.5 | 0.3 | 5.2 |
| CASE | 1 | 2 | 3 | 4 | 5 | 6 | Mean Difference |
|---|---|---|---|---|---|---|---|
| Pain Score | |||||||
| VAS (pre) | 8 | 7 | 8 | 9 | 8 | 5 | 5.2 ± 0.8 |
| VAS (post) | 2 | 1 | 3 | 4 | 3 | 1 | |
| Functional Score | |||||||
| AOFAS (pre) | 20 | 27 | 8 | 10 | 9 | 7 | 55.5 ± 3.2 |
| AOFAS (post) | 71 | 86 | 63 | 66 | 62 | 66 | |
| FFI (pre) | 93 | 88 | 96 | 95 | 94 | 83 | 52.2 ± 9.1 |
| FFI (post) | 39 | 19 | 51 | 48 | 41 | 38 | |
| Pain (pre) | 84 | 60 | 88 | 100 | 94 | 46 | 55.0 ± 14.8 |
| Pain (post) | 26 | 6 | 28 | 32 | 32 | 20 | |
| Disability (pre) | 96 | 100 | 100 | 90 | 92 | 98 | 45.8 ± 13.4 |
| Disability (post) | 49 | 30 | 63 | 58 | 52 | 49 | |
| AL (pre) | 100 | 100 | 100 | 100 | 97 | 100 | 68.0 ± 15.0 |
| AL (post) | 30 | 10 | 53 | 43 | 20 | 33 | |
| Quality of Life (SF-36) | |||||||
| PF (pre) | 10 | 10 | 5 | 10 | 10 | 15 | 46.7 ± 14.0 |
| PF (post) | 55 | 85 | 45 | 50 | 50 | 55 | |
| RP (pre) | 0 | 0 | 0 | 0 | 0 | 0 | 54.2 ± 18.8 |
| RP (post) | 50 | 75 | 25 | 50 | 50 | 75 | |
| RE (pre) | 0 | 33.3 | 0 | 0 | 0 | 33.3 | 66.7 ± 21.1 |
| RE (post) | 66.7 | 100 | 66.7 | 66.7 | 100 | 66.7 | |
| EF (pre) | 5 | 35 | 10 | 20 | 10 | 35 | 45.8 ± 10.2 |
| EF (post) | 65 | 80 | 45 | 65 | 65 | 70 | |
| EW (pre) | 20 | 36 | 20 | 24 | 24 | 16 | 52.0 ± 10.1 |
| EW (post) | 80 | 84 | 60 | 72 | 72 | 84 | |
| SF (pre) | 0 | 25 | 0 | 25 | 12.5 | 12.5 | 54.0 ± 17.0 |
| SF (post) | 75 | 87.5 | 50 | 50 | 75 | 62.5 | |
| Pain (pre) | 22.5 | 32.5 | 0 | 0 | 10 | 45 | 60.8 ± 15.6 |
| Pain (post) | 67.5 | 87.5 | 77.5 | 77.5 | 77.5 | 87.5 | |
| GH (pre) | 30 | 35 | 5 | 15 | 15 | 25 | 49.0 ± 5.8 |
| GH (post) | 71 | 85 | 50 | 70 | 70 | 70 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, K.M.; Mooteeram, J.; Pillai, A. The Use of Platelet-Rich Fibrin-Coated Three-Dimensionally (3D) Printed Scaffolds in Salvage of Complex Hindfoot Cases. Biomimetics 2025, 10, 269. https://doi.org/10.3390/biomimetics10050269
Tai KM, Mooteeram J, Pillai A. The Use of Platelet-Rich Fibrin-Coated Three-Dimensionally (3D) Printed Scaffolds in Salvage of Complex Hindfoot Cases. Biomimetics. 2025; 10(5):269. https://doi.org/10.3390/biomimetics10050269
Chicago/Turabian StyleTai, Ken Meng, Justin Mooteeram, and Anand Pillai. 2025. "The Use of Platelet-Rich Fibrin-Coated Three-Dimensionally (3D) Printed Scaffolds in Salvage of Complex Hindfoot Cases" Biomimetics 10, no. 5: 269. https://doi.org/10.3390/biomimetics10050269
APA StyleTai, K. M., Mooteeram, J., & Pillai, A. (2025). The Use of Platelet-Rich Fibrin-Coated Three-Dimensionally (3D) Printed Scaffolds in Salvage of Complex Hindfoot Cases. Biomimetics, 10(5), 269. https://doi.org/10.3390/biomimetics10050269







