Effects of rmBMP-7 on Osteoblastic Cells Grown on a Nanostructured Titanium Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Titanium (Ti) Surfaces
2.2. Osteogenic Cell Cultures
2.3. Epifluorescence Analyses
2.3.1. Cell Morphology and Cell Counting
2.3.2. Cell Proliferation by Ki-67 Labeling
2.3.3. In Situ ALP Activity by Fast Red Staining
2.4. mRNA Quantification by Real-Time PCR Analysis
2.5. Protein Detection by Western Blotting
2.6. Mineralized Matrix Formation/Calcium Content
2.7. Statistical Analysis
3. Results
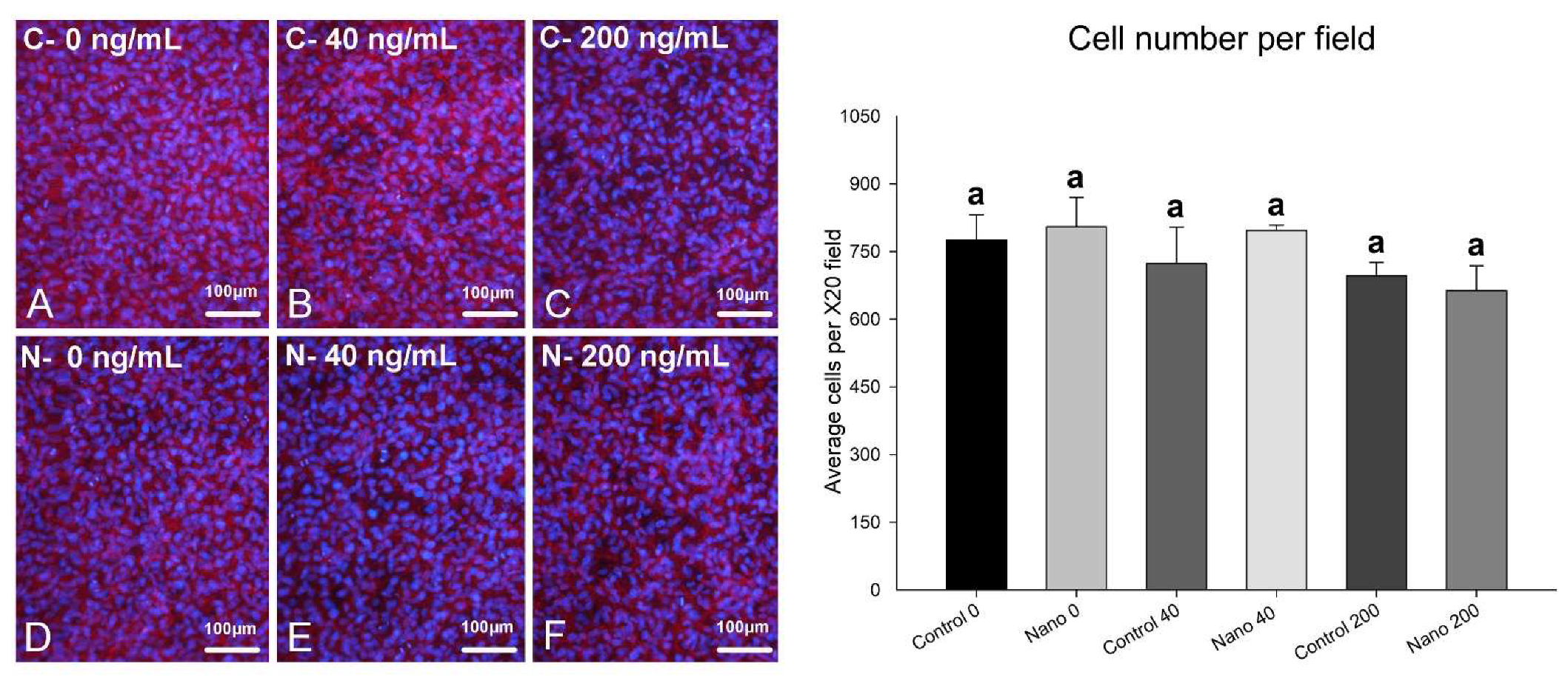
3.1. Epifluorescence Analyses: Cell Morphology and Cell Counting, Cell Proliferation by Ki-67 Labeling, and In Situ ALP Activity by Fast Red Staining
3.2. mRNA Quantification by Real-Time PCR Analysis
3.3. Protein Detection by Western Blotting
3.4. Mineralized Matrix Formation/Calcium Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Morra, M. Biochemical modification of titanium surfaces: Peptides and ECM proteins. Eur. Cells Mater. 2006, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, U.; Jaffery, H.; Salmeron-Sanchez, M.; Dalby, M.J. An ossifying landscape: Materials and growth factor strategies for osteogenic signalling and bone regeneration. Curr. Opin. Biotechnol. 2022, 73, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Ye, L.; Kobayashi, T.; Lucas, D.J.; Mochida, Y.; Yamauchi, M.; Kronenberg, H.M.; Feng, J.Q.; Mishina, Y. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J. Bone Miner. Res. 2008, 23, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Wu, J.; Xia, Y.; Yuan, Y.; Zhang, H.; Xu, S.; Lin, K. Loading BMP-2 on nanostructured hydroxyapatite microspheres for rapid bone regeneration. Int. J. Nanomed. 2018, 13, 4083–4092. [Google Scholar] [CrossRef]
- Goodman, S.B.; Lin, T. Modifying MSC Phenotype to Facilitate Bone Healing: Biological Approaches. Front. Bioeng. Biotechnol. 2020, 8, 641. [Google Scholar] [CrossRef]
- Huang, R.L.; Yuan, Y.; Tu, J.; Zou, G.M.; Li, Q. Exaggerated inflammatory environment decreases BMP-2/ACS-induced ectopic bone mass in a rat model: Implications for clinical use of BMP-2. Osteoarthr. Cartil. 2014, 22, 1186–1196. [Google Scholar] [CrossRef]
- Togashi, A.Y.; Cirano, F.R.; Marques, M.M.; Pustiglioni, F.E.; Lang, N.P.; Lima, L.A. Effect of recombinant human bone morphogenetic protein-7 (rhBMP-7) on the viability, proliferation and differentiation of osteoblast-like cells cultured on a chemically modified titanium surface. Clin. Oral Implant. Res. 2009, 20, 452–457. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, L.F.; Lin, H.S.; Yin, M.N.; Tong, Y.Q.; Shi, G.S. The optimal dose of recombinant human osteogenic protein-1 enhances differentiation of mouse osteoblast-like cells: An in vitro study. Arch. Oral Biol. 2012, 57, 460–468. [Google Scholar] [CrossRef]
- Cirano, F.R.; Togashi, A.; Marques, M.; Pustiglioni, F.; Lima, L. Role of rhBMP-2 and rhBMP-7 in the metabolism and differentiation of osteoblast-like cells cultured on chemically modified titanium surfaces. J. Oral Implantol. 2014, 40, 655–659. [Google Scholar] [CrossRef]
- Bais, M.V.; Wigner, N.; Young, M.; Toholka, R.; Graves, D.T.; Morgan, E.F.; Gerstenfeld, L.C.; Einhorn, T.A. BMP2 is essential for post natal osteogenesis but not for recruitment of osteogenic stem cells. Bone 2009, 45, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Castro-Raucci, L.M.S.; Francischini, M.S.; Teixeira, L.N.; Ferraz, E.P.; Lopes, H.B.; de Oliveira, P.T.; Hassan, M.Q.; Losa, A.L.; Beloti, M.M. Titanium With Nanotopography Induces Osteoblast Differentiation by Regulating Endogenous Bone Morphogenetic Protein Expression and Signaling Pathway. J. Cell. Biochem. 2016, 117, 1718–1726. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, S.; Roumans, N.; Honig, F.; Carlier, A.; Hebels, D.G.A.J.; Eren, A.D.; Dijke, P.T.; Vasilevich, A.; de Boer, J. Mechanotransduction is a context-dependent activator of TGF-β signaling in mesenchymal stem cells. Biomaterials 2020, 259, 120331. [Google Scholar] [CrossRef] [PubMed]
- Caneva, M.; Lang, N.P.; Calvo Guirado, J.L.; Spriano, S.; Iezzi, G.; Botticelli, D. Bone healing at bicortically installed implants with different surface configurations. An experimental study in rabbits. Clin. Oral. Implant. Res. 2015, 26, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Wähnert, D.; Greiner, J.; Brianza, S.; Kaltschmidt, C.; Vordemvenne, T.; Kaltschmidt, B. Strategies to Improve Bone Healing: Innovative Surgical Implants Meet Nano-/Micro-Topography of Bone Scaffolds. Biomedicines 2021, 9, 746. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.T.; Nanci, A. Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials 2004, 25, 403–413. [Google Scholar] [CrossRef]
- Bueno, R.d.B.; Adachi, P.; Castro-Raucci, L.M.; Rosa, A.L.; Nanci, A.; Oliveira, P.T. Oxidative nanopatterning of titanium surfaces promotes production and extracellular accumulation of osteopontin. Braz. Dent. J. 2011, 22, 179–184. [Google Scholar] [CrossRef]
- Wazen, R.M.; Kuroda, S.; Nishio, C.; Sellin, K.; Brunski, J.B.; Nanci, A. Gene expression profiling and histomorphometric analyses of the early bone healing response around nanotextured implants. Nanomedicine 2013, 8, 1385–1395. [Google Scholar] [CrossRef]
- Rosa, A.L.; Kato, R.B.; Castro Raucci, L.M.; Teixeira, L.N.; de Oliveira, F.S.; Bellesini, L.S.; de Oliveira, P.T.; Hassan, M.Q.; Beloti, M.M. Nanotopography drives stem cell fate toward osteoblast differentiation through α1β1 integrin signaling pathway. J. Cell. Biochem. 2014, 115, 540–548. [Google Scholar] [CrossRef]
- Kato, R.B.; Roy, B.; De Oliveira, F.S.; Ferraz, E.P.; De Oliveira, P.T.; Kemper, A.G.; Hassan, M.Q.; Rosa, A.L.; Beloti, M.M. Nanotopography directs mesenchymal stem cells to osteoblast lineage through regulation of microRNA-SMAD-BMP-2 circuit. J. Cell. Physiol. 2014, 229, 1690–1696. [Google Scholar] [CrossRef]
- Beutner, R.; Michael, J.; Schwenzer, B.; Scharnweber, D. Biological nano-functionalization of titanium-based biomaterial surfaces: A flexible toolbox. J. R. Soc. Interface 2010, 7, S93–S105. [Google Scholar] [CrossRef] [PubMed]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, Y.; Ma, A.; Li, C. Surface Immobilization of TiO2 Nanotubes with Bone Morphogenetic Protein-2 Synergistically Enhances Initial Preosteoblast Adhesion and Osseointegration. BioMed Res. Int. 2019, 2019, 5697250. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.T.; Zalzal, S.F.; Beloti, M.M.; Rosa, A.L.; Nanci, A. Enhancement of in vitro osteogenesis on titanium by chemically produced nanotopography. J. Biomed. Mater. Res. A 2007, 80, 554–564. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Truhlar, R.S.; Orenstein, I.H.; Morris, H.F.; Ochi, S. Distribution of bone quality in patients receiving endosseous dental implants. J. Oral Maxillofac. Surg. 1997, 55, 38–45. [Google Scholar] [CrossRef]
- Weng, D.; Hoffmeyer, M.; Hürzeler, M.B.; Richter, E.J. Osseotite vs. machined surface in poor bone quality. A study in dogs. Clin. Oral Implant. Res. 2003, 14, 703–708. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef]
- Wang, D.; Christensen, K.; Chawla, K.; Xiao, G.; Krebsbach, P.H.; Franceschi, R.T. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J. Bone Miner. Res. 1999, 14, 893–903. [Google Scholar] [CrossRef]
- Maliakal, J.C.; Asahina, I.; Hauschka, P.V.; Sampath, T.K. Osteogenic protein-1 (BMP-7) inhibits cell proliferation and stimulates the expression of markers characteristic of osteoblast phenotype in rat osteosarcoma (17/2.8) cells. Growth Factors 1994, 11, 227–234. [Google Scholar] [CrossRef]
- Baranowski, A.; Klein, A.; Ritz, U.; Ackermann, A.; Anthonissen, J.; Kaufmann, K.B.; Brendel, C.; Götz, H.; Rommens, P.M.; Hofmann, A. Surface Functionalization of Orthopedic Titanium Implants with Bone Sialoprotein. PLoS ONE 2016, 11, e0153978. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, G.; Mendonça, D.B.; Aragão, F.J.; Cooper, L.F. The combination of micron and nanotopography by H(2)SO(4)/H(2)O(2) treatment and its effects on osteoblast-specific gene expression of hMSCs. J. Biomed. Mater. Res. A 2010, 94, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Pereira, K.K.; Alves, O.C.; Novaes, A.B., Jr.; de Oliveira, F.S.; Yi, J.H.; Zaniquelli, O.; Wolf-Brandstetter, C.; Scharnweber, D.; Variola, F.; Nanci, A.; et al. Progression of osteogenic cell cultures grown on microtopographic titanium coated with calcium phosphate and functionalized with a type I collagen-derived peptide. J. Periodontol. 2013, 84, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Vitale, A.; Bertone, E.; Guastella, S.; Cassinelli, C.; Pan, J.; Spriano, S. Multifunctional commercially pure titanium for the improvement of bone integration: Multiscale topography, wettability, corrosion resistance and biological functionalization. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Kravanja, K.A.; Finšgar, M. A review of techniques for the application of bioactive coatings on metal-based implants to achieve controlled release of active ingredientes. Mater. Des. 2022, 217, 110653. [Google Scholar] [CrossRef]
- Ferraris, S.; Cazzola, M.; Zuardi, L.R.; Tambasco de Oliveira, P. Metal nanoscale systems functionalized with organic compounds. In Nanostructured Biomaterials for Regenerative Medicine; Woodhead Publishing Series in Biomaterials; Guarino, V., Iafisco, M., Spriano, S., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 407–436. ISBN 9780081025949. [Google Scholar] [CrossRef]
- Cheifetz, S.; Li, I.W.; McCulloch, C.A.; Sampath, K.; Sodek, J. Influence of osteogenic protein-1 (OP-1;BMP-7) and transforming growth factor-beta 1 on bone formation in vitro. Connect. Tissue Res. 1996, 35, 71–78. [Google Scholar] [CrossRef]
- Tang, J.; Qing, M.F.; Li, M.; Gao, Z. Dexamethasone inhibits BMP7-induced osteogenic differentiation in rat dental follicle cells via the PI3K/AKT/GSK-3β/β-catenin pathway. Int. J. Med. Sci. 2020, 17, 2663–2672. [Google Scholar] [CrossRef]
- Chen, F.; Bi, D.; Cheng, C.; Ma, S.; Liu, Y.; Cheng, K. Bone morphogenetic protein 7 enhances the osteogenic differentiation of human dermal-derived CD105+ fibroblast cells through the Smad and MAPK pathways. Int. J. Mol. Med. 2019, 43, 37–46. [Google Scholar] [CrossRef]
- Cao, X.; Chen, D. The BMP signaling and in vivo bone formation. Gene 2005, 357, 1–8. [Google Scholar] [CrossRef]
- Huang, W.; Carlsen, B.; Rudkin, G.; Berry, M.; Ishida, K.; Yamaguchi, D.T.; Miller, T.A. Osteopontin is a negative regulator of proliferation and differentiation in MC3T3-E1 pre-osteoblastic cells. Bone 2004, 34, 799–808. [Google Scholar] [CrossRef]
- Senger, D.R.; Perruzzi, C.A.; Papadopoulos-Sergiou, A.; Van de Water, L. Adhesive properties of osteopontin: Regulation by a naturally occurring thrombin-cleavage in close proximity to the GRGDS cell-binding domain. Mol. Biol. Cell 1994, 5, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Christensen, B.; Schack, L.; Kläning, E.; Sørensen, E.S. Osteopontin is cleaved at multiple sites close to its integrin-binding motifs in milk and is a novel substrate for plasmin and cathepsin D. J. Biol. Chem. 2010, 285, 7929–7937. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010, 147, 35–51. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.T.; de Oliva, M.A.; Maximiano, W.M.; Sebastião, K.E.; Crippa, G.E.; Ciancaglini, P.; Beloti, M.M.; Nanci, A.; Rosa, A.L. Effects of a mixture of growth factors and proteins on the development of the osteogenic phenotype in human alveolar bone cell cultures. J. Histochem. Cytochem. 2008, 56, 629–638. [Google Scholar] [CrossRef]
- Partridge, N.C.; Alcorn, D.; Michelangeli, V.P.; Ryan, G.; Martin, T.J. Morphological and biochemical characterization of four clonal osteogenic sarcoma cell lines of rat origin. Cancer Res. 1983, 43, 4308–4314. [Google Scholar]
- Kim, S.W.; Her, S.J.; Park, S.J.; Kim, D.; Park, K.S.; Lee, H.K.; Han, B.H.; Kim, M.S.; Shin, C.S.; Kim, S.Y. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone 2005, 37, 359–369. [Google Scholar] [CrossRef]
- Stanford, C.M.; Jacobson, P.A.; Eanes, E.D.; Lembke, L.A.; Midura, R.J. Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP). J. Biol. Chem. 1995, 270, 9420–9428. [Google Scholar] [CrossRef]
- Busuttil Naudi, K.; Ayoub, A.; McMahon, J.; Di Silvio, L.; Lappin, D.; Hunter, K.D.; Barbenel, J. Mandibular reconstruction in the rabbit using beta-tricalcium phosphate (β-TCP) scaffolding and recombinant bone morphogenetic protein 7 (rhBMP-7)—Histological, radiographic and mechanical evaluations. J. Cranio-Maxillofac. Surg. 2012, 40, e461–e469. [Google Scholar] [CrossRef]
- Mantripragada, V.P.; Jayasuriya, A.C. Bone regeneration using injectable BMP-7 loaded chitosan microparticles in rat femoral defect. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 596–608. [Google Scholar] [CrossRef]
- Zang, S.; Mu, R.; Chen, F.; Wei, X.; Zhu, L.; Han, B.; Yu, H.; Bi, B.; Chen, B.; Wang, Q.; et al. Injectable chitosan/β-glycerophosphate hydrogels with sustained release of BMP-7 and ornidazole in periodontal wound healing of class III furcation defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 919–928. [Google Scholar] [CrossRef]





| Genes | TaqMan Probe |
|---|---|
| Runx2 | Mm00501584_m1 |
| Osx | Mm04933803_m1 |
| Alp | Mm00475834_m1 |
| Bsp | Mm00492555_m1 |
| Opn | Mm00436767_m1 |
| Smad1 | Mm00484723_m1 |
| Gapdh | Mm99999915_g1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuardi, L.R.; de Oliveira, F.S.; Fernandes, R.R.; Gomes, M.P.O.; Spriano, S.; Nanci, A.; de Oliveira, P.T. Effects of rmBMP-7 on Osteoblastic Cells Grown on a Nanostructured Titanium Surface. Biomimetics 2022, 7, 136. https://doi.org/10.3390/biomimetics7030136
Zuardi LR, de Oliveira FS, Fernandes RR, Gomes MPO, Spriano S, Nanci A, de Oliveira PT. Effects of rmBMP-7 on Osteoblastic Cells Grown on a Nanostructured Titanium Surface. Biomimetics. 2022; 7(3):136. https://doi.org/10.3390/biomimetics7030136
Chicago/Turabian StyleZuardi, Leonardo Raphael, Fabíola Singaretti de Oliveira, Roger Rodrigo Fernandes, Maria Paula Oliveira Gomes, Silvia Spriano, Antonio Nanci, and Paulo Tambasco de Oliveira. 2022. "Effects of rmBMP-7 on Osteoblastic Cells Grown on a Nanostructured Titanium Surface" Biomimetics 7, no. 3: 136. https://doi.org/10.3390/biomimetics7030136
APA StyleZuardi, L. R., de Oliveira, F. S., Fernandes, R. R., Gomes, M. P. O., Spriano, S., Nanci, A., & de Oliveira, P. T. (2022). Effects of rmBMP-7 on Osteoblastic Cells Grown on a Nanostructured Titanium Surface. Biomimetics, 7(3), 136. https://doi.org/10.3390/biomimetics7030136









