Synthesis and Antimicrobial Activity of 3D Micro–Nanostructured Diatom Biosilica Coated by Epitaxially Growing Ag-AgCl Hybrid Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Diatom Biosilica
2.2. Instrumental Methods and Characterization
2.2.1. Elemental Composition Analysis
2.2.2. X-ray Diffraction Analysis
2.2.3. Transmission Electron Microscopy Studies
2.2.4. Zeta-Potential Measurements
2.2.5. Photoluminescence Analysis
2.3. Synthesis of 3D Micro–Nanostructured Composite
2.4. Antimicrobial Activity Study of the 3D Micro–Nanostructured Composite
3. Results
3.1. Characterization of the 3D Micro–Nanostructured Diatom Biosilica
3.2. Energy-Dispersive X-ray Spectroscopy (EDX) Studies
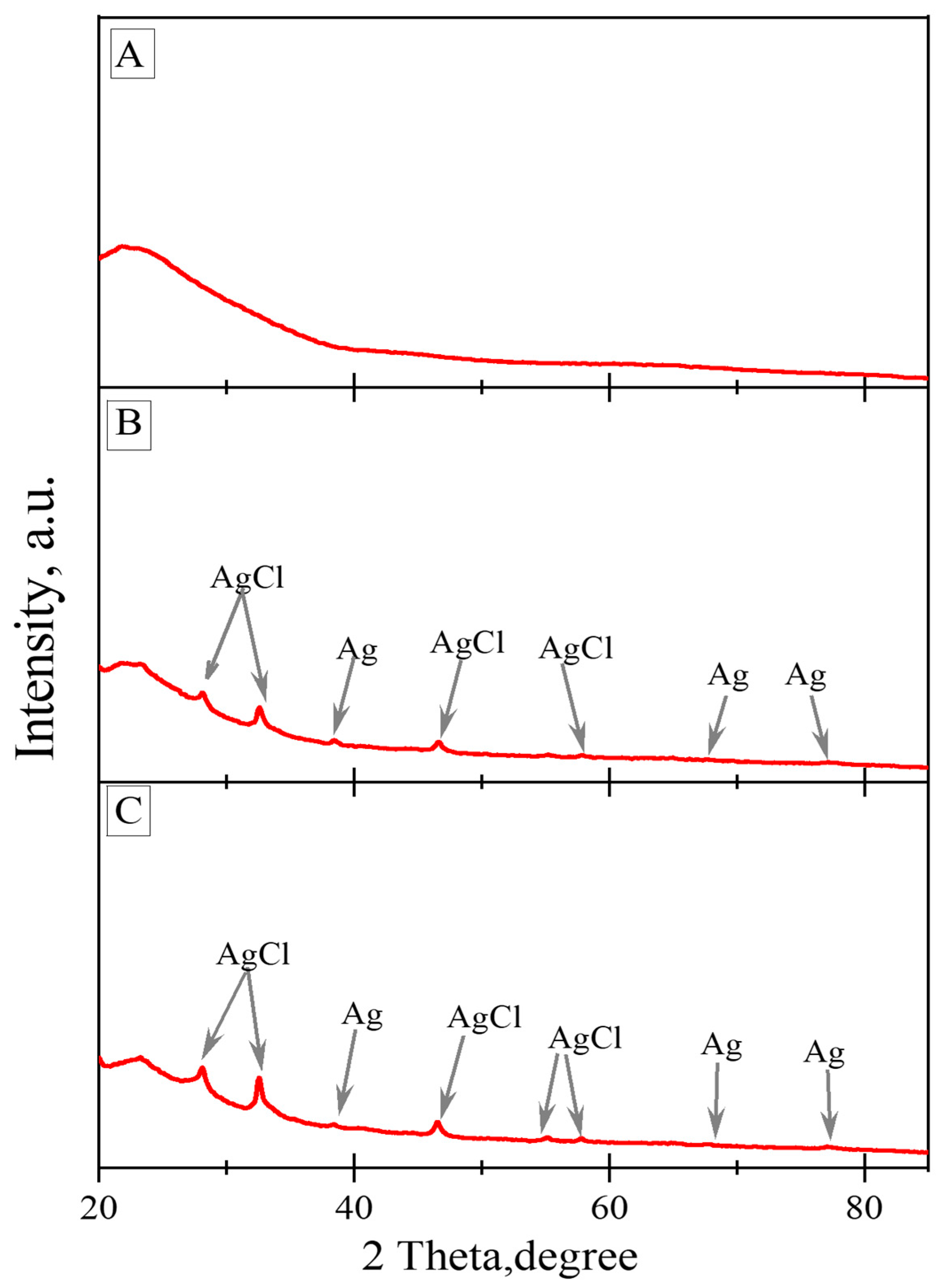
3.3. Powder X-ray Diffraction Results
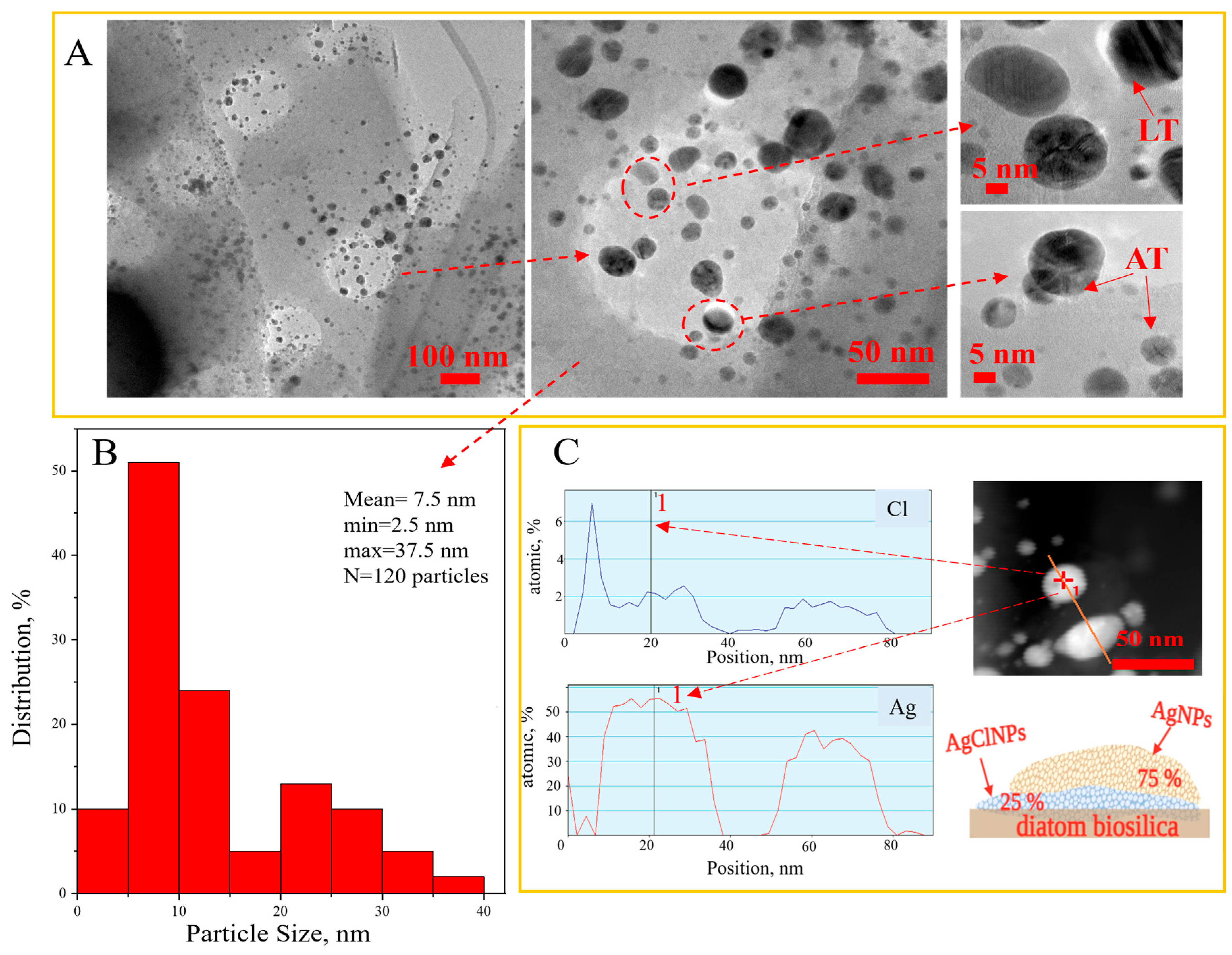
3.4. Transmission Electron Microscopy Results
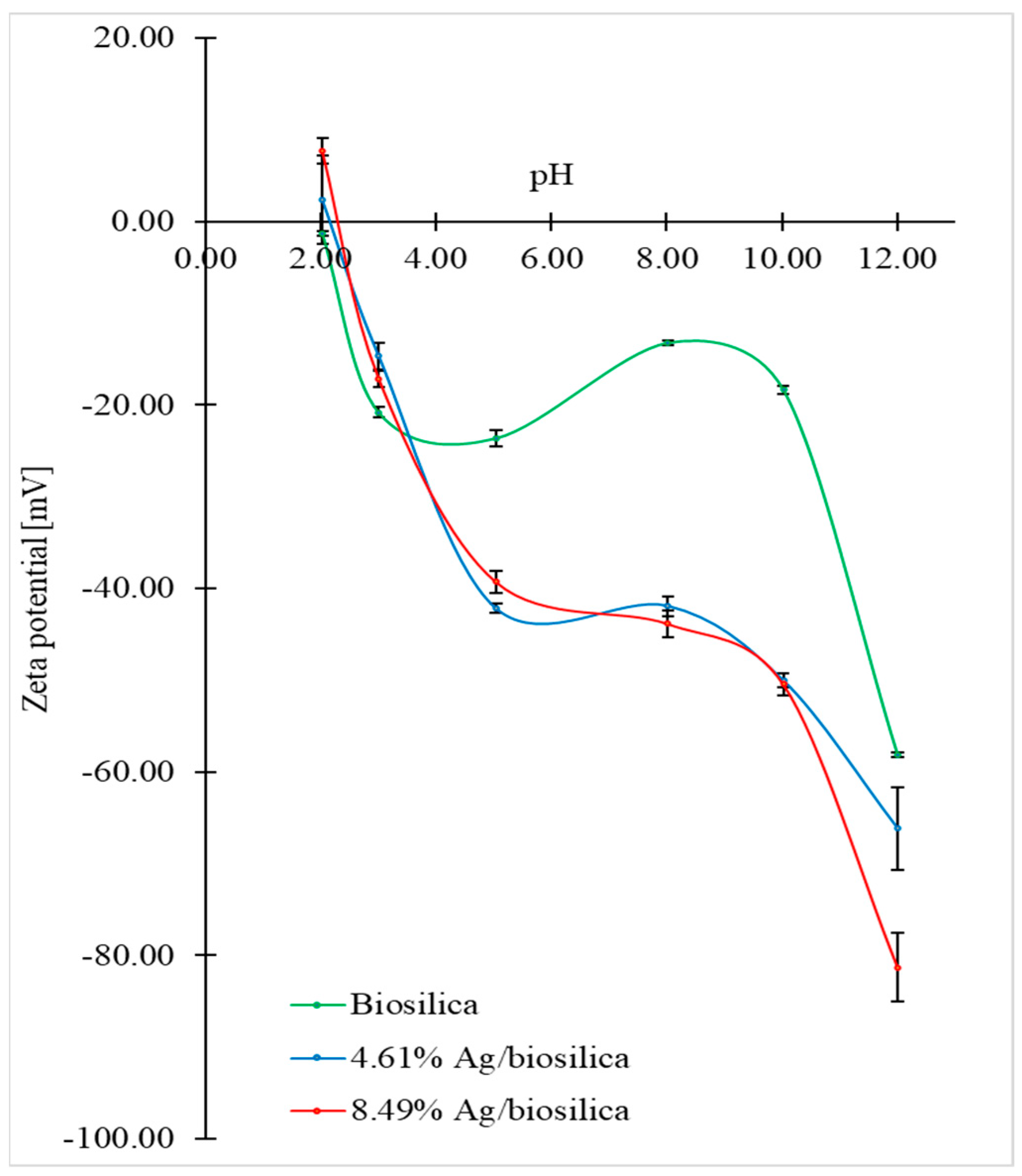
3.5. Zeta Potential
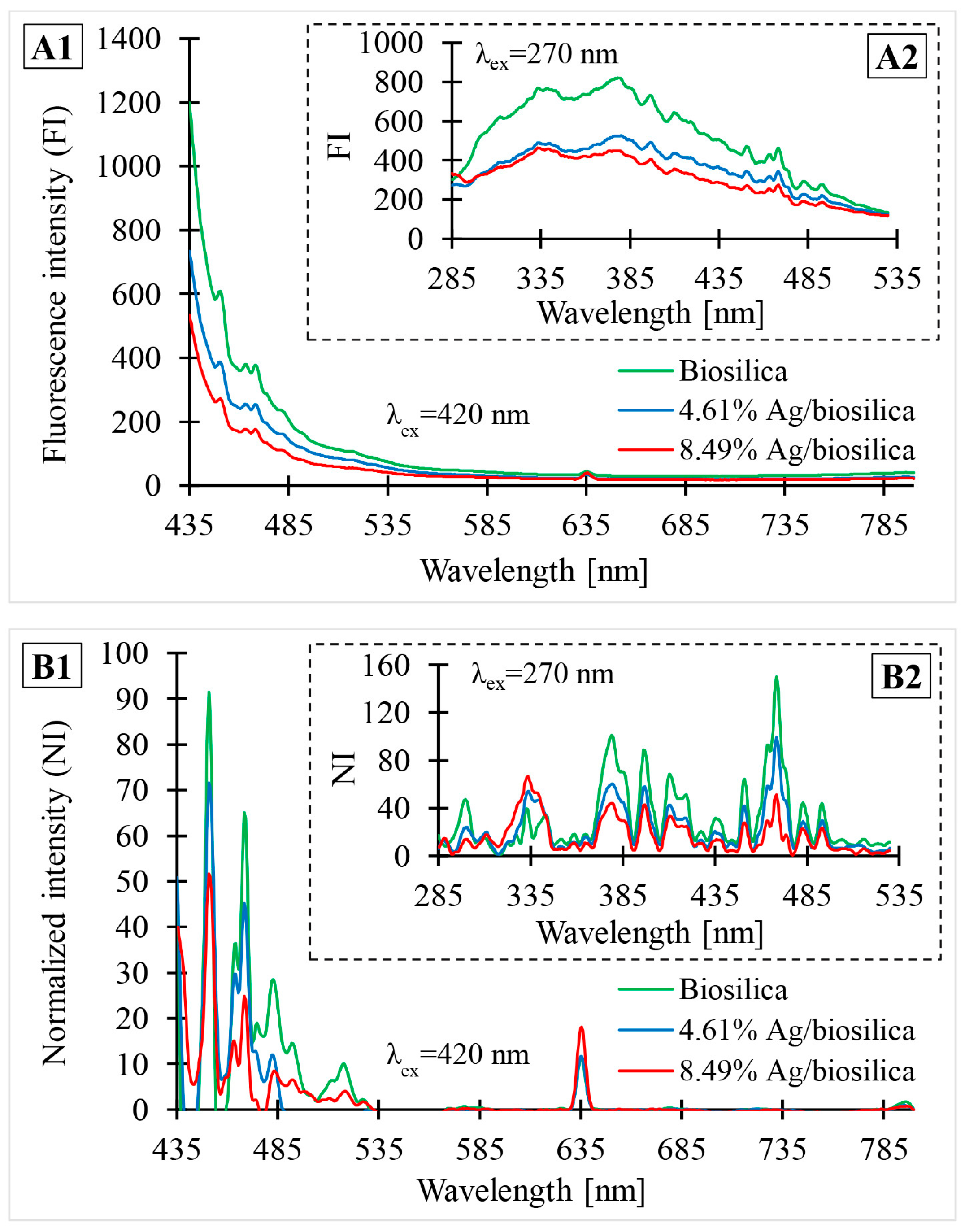
3.6. PL Spectroscopy
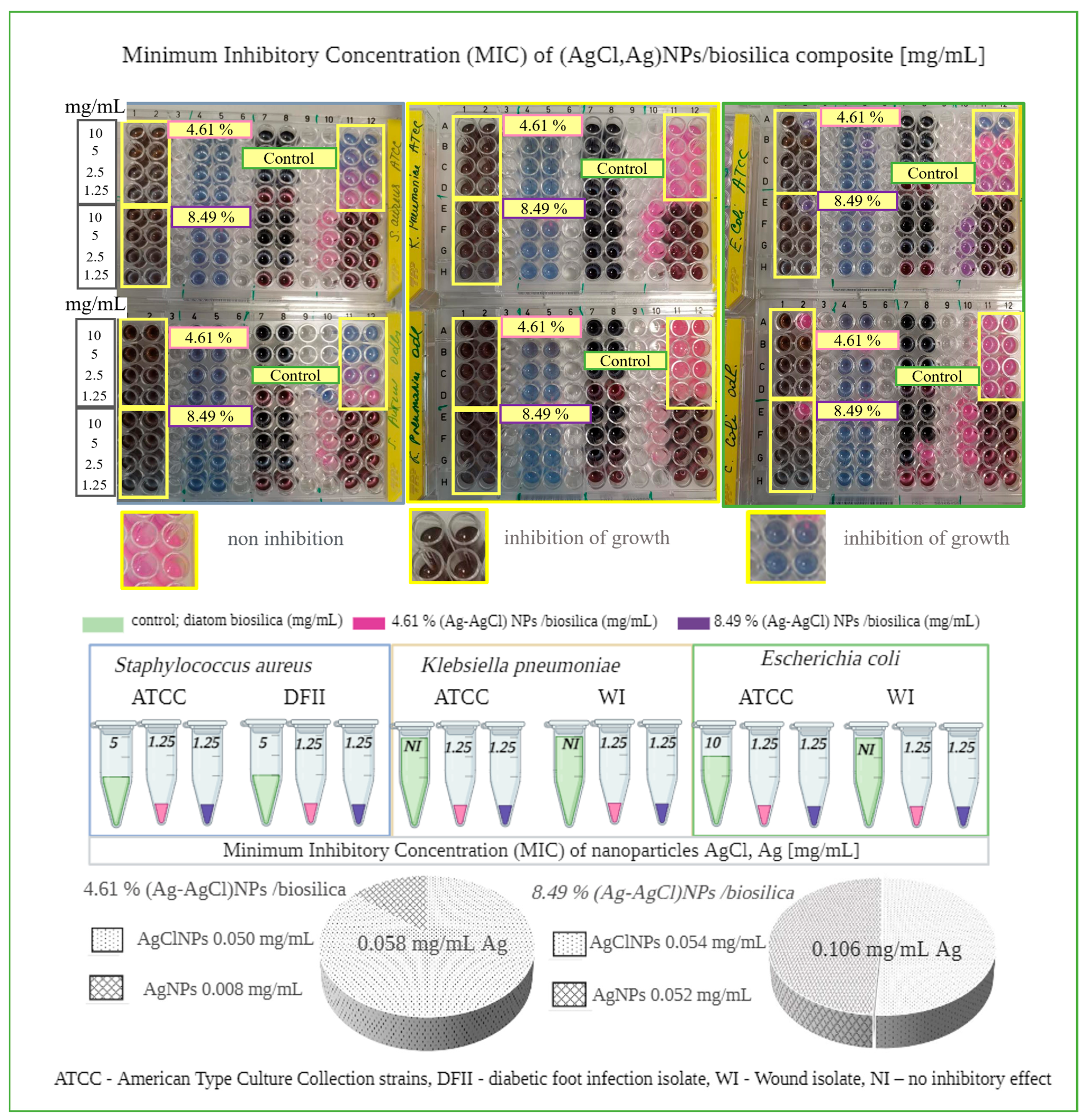
3.7. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pannico, M.; Rea, I.; Chandrasekaran, S.; Musto, P.; Voelcker, N.H.; De Stefano, L. Electroless Gold-Modified Diatoms as Surface-Enhanced Raman Scattering Supports. Nanoscale Res. Lett. 2016, 11, 315. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Kuang, M.; Li, F.; Liu, X.Y.; Zhang, Y.X.; Dong, F.; Losic, D. Engineering of Three Dimensional (3-D) Diatom@TiO2@MnO2 Composites with Enhanced Supercapacitor Performance. Electrochim. Acta 2016, 190, 159–167. [Google Scholar] [CrossRef]
- Brzozowska, W.; Sprynskyy, M.; Wojtczak, I.; Dąbek, P.; Markuszewski, M.J.; Witkowski, A.; Buszewski, B. Metabolically Doping of 3D Diatomaceous Biosilica with Titanium. Materials 2022, 15, 5210. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, C.; Chianese, G.; Terracciano, M.; de Stefano, L.; Rea, I. Nanostructured Biosilica of Diatoms: From Water World to Biomedical Applications. Appl. Sci. 2020, 10, 6811. [Google Scholar] [CrossRef]
- Panwar, V.; Dutta, T. Diatom Biogenic Silica as a Felicitous Platform for Biochemical Engineering: Expanding Frontiers. ACS Appl. Bio Mater. 2019, 2, 2295–2316. [Google Scholar] [CrossRef] [PubMed]
- Delasoie, J.; Zobi, F. Natural Diatom Biosilica as Microshuttles in Drug Delivery Systems. Pharmaceutics 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Sasirekha, R.; Sheena, T.S.; Sathiya Deepika, M.; Santhanam, P.; Townley, H.E.; Jeganathan, K.; Dinesh Kumar, S.; Premkumar, K. Surface Engineered Amphora Subtropica Frustules Using Chitosan as a Drug Delivery Platform for Anticancer Therapy. Mater. Sci. Eng. C 2019, 94, 56–64. [Google Scholar] [CrossRef]
- Uthappa, U.T.; Kigga, M.; Sriram, G.; Ajeya, K.V.; Jung, H.Y.; Neelgund, G.M.; Kurkuri, M.D. Facile Green Synthetic Approach of Bio Inspired Polydopamine Coated Diatoms as a Drug Vehicle for Controlled Drug Release and Active Catalyst for Dye Degradation. Microporous Mesoporous Mater. 2019, 288, 109572. [Google Scholar] [CrossRef]
- Delalat, B.; Sheppard, V.C.; Rasi Ghaemi, S.; Rao, S.; Prestidge, C.A.; McPhee, G.; Rogers, M.L.; Donoghue, J.F.; Pillay, V.; Johns, T.G.; et al. Targeted Drug Delivery Using Genetically Engineered Diatom Biosilica. Nat. Commun. 2015, 6, 8791. [Google Scholar] [CrossRef]
- Le, T.D.H.; Bonani, W.; Speranza, G.; Sglavo, V.; Ceccato, R.; Maniglio, D.; Motta, A.; Migliaresi, C. Processing and Characterization of Diatom Nanoparticles and Microparticles as Potential Source of Silicon for Bone Tissue Engineering. Mater. Sci. Eng. C 2016, 59, 471–479. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Szczyglewska, P.; Wojtczak, I.; Nowak, I.; Witkowski, A.; Buszewski, B.; Feliczak-Guzik, A. Diatom Biosilica Doped with Palladium(Ii) Chloride Nanoparticles as New Efficient Photocatalysts for Methyl Orange Degradation. Int. J. Mol. Sci. 2021, 22, 6734. [Google Scholar] [CrossRef] [PubMed]
- Gale, D.K.; Jeffryes, C.; Gutu, T.; Jiao, J.; Chang, C.H.; Rorrer, G.L. Thermal Annealing Activates Amplified Photoluminescence of Germanium Metabolically Doped in Diatom Biosilica. J. Mater. Chem. 2011, 21, 10658–10665. [Google Scholar] [CrossRef]
- Rea, I.; Terracciano, M.; Chandrasekaran, S.; Voelcker, N.H.; Dardano, P.; Martucci, N.M.; Lamberti, A.; De Stefano, L. Bioengineered Silicon Diatoms: Adding Photonic Features to a Nanostructured Semiconductive Material for Biomolecular Sensing. Nanoscale Res. Lett. 2016, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Rajabi-Abhari, A.; Lee, J.; Tabassian, R.; Kim, J.N.; Lee, H.; Oh, I.K. Antagonistically Functionalized Diatom Biosilica for Bio-Triboelectric Generators. Small 2022, 18, 2107638. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, A.; Sprynskyy, M.; Winkler, K.; Szymborski, T. Ultrasensitive SERS Immunoassay Based on Diatom Biosilica for Detection of Interleukins in Blood Plasma. Anal. Bioanal. Chem. 2017, 409, 6337–6347. [Google Scholar] [CrossRef] [PubMed]
- Zobi, F. Diatom Biosilica in Targeted Drug Delivery and Biosensing Applications: Recent Studies. Micro 2022, 2, 342–360. [Google Scholar] [CrossRef]
- Ragni, R.; Cicco, S.; Vona, D.; Leone, G.; Farinola, G.M. Biosilica from Diatoms Microalgae: Smart Materials from Bio-Medicine to Photonics. J. Mater. Res. 2017, 32, 279–291. [Google Scholar] [CrossRef]
- Rogato, A.; De Tommasi, E. Physical, Chemical, and Genetic Techniques for Diatom Frustule Modification: Applications in Nanotechnology. Appl. Sci. 2020, 10, 8738. [Google Scholar] [CrossRef]
- Bose, R.; Roychoudhury, P.; Pal, R. In-Situ Green Synthesis of Fluorescent Silica–Silver Conjugate Nanodendrites Using Nanoporous Frustules of Diatoms: An Unprecedented Approach. Bioprocess Biosyst. Eng. 2021, 44, 1263–1273. [Google Scholar] [CrossRef]
- Görlich, S.; Pawolski, D.; Zlotnikov, I.; Kröger, N. Control of Biosilica Morphology and Mechanical Performance by the Conserved Diatom Gene Silicanin-1. Commun. Biol. 2019, 2, 245. [Google Scholar] [CrossRef]
- Hildebrand, M.; Lerch, S.J.L.; Shrestha, R.P. Understanding Diatom Cell Wall Silicification-Moving Forward. Front. Mar. Sci. 2018, 5, 125. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, J.; Jiang, Y.; Jiang, X.; Zhang, D. Preparation of Biosilica Structures from Frustules of Diatoms and Their Applications: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Nowack, B.; Krug, H.F.; Height, M. Reply to Comments on ‘120 Years of Nanosilver History: Implications for Policy Makers’. Environ. Sci. Technol. 2011, 45, 7593–7595. [Google Scholar] [CrossRef]
- Lea, M.C. Allotropic forms of silver. Am. J. Sci. 1889, 3, 476–491. [Google Scholar] [CrossRef]
- Al-Rubaye, H.I.; Al-Rubaye, B.K.; Al-Abodi, E.E.; Yousif, E.I. Green Chemistry Synthesis of Modified Silver Nanoparticles. J. Phys. Conf. Ser. 2020, 1664, 012080. [Google Scholar] [CrossRef]
- Naik, B.; Desai, V.; Kowshik, M.; Prasad, V.S.; Fernando, G.F.; Ghosh, N.N. Synthesis of Ag/AgCl-Mesoporous Silica Nanocomposites Using a Simple Aqueous Solution-Based Chemical Method and a Study of Their Antibacterial Activity on E. coli. Particuology 2011, 9, 243–247. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Triangular Nanoplates of Silver: Synthesis, Characterization, and Use as Sacrificial Templates for Generating Triangular Nanorings of Gold. Adv. Mater. 2003, 15, 695–699. [Google Scholar] [CrossRef]
- Helmlinger, J.; Prymak, O.; Loza, K.; Gocyla, M.; Heggen, M.; Epple, M. On the Crystallography of Silver Nanoparticles with Different Shapes. Cryst. Growth Des. 2016, 16, 3677–3687. [Google Scholar] [CrossRef]
- Yu, N.; Peng, H.; Qiu, L.; Wang, R.; Jiang, C.; Cai, T.; Sun, Y.; Li, Y.; Xiong, H. New Pectin-Induced Green Fabrication of Ag@AgCl/ZnO Nanocomposites for Visible-Light Triggered Antibacterial Activity. Int. J. Biol. Macromol. 2019, 141, 207–217. [Google Scholar] [CrossRef]
- Dong, Y.Y.; Deng, F.; Zhao, J.J.; He, J.; Ma, M.G.; Xu, F.; Sun, R.C. Environmentally Friendly Ultrosound Synthesis and Antibacterial Activity of Cellulose/Ag/AgCl Hybrids. Carbohydr. Polym. 2014, 99, 166–172. [Google Scholar] [CrossRef]
- Khaligh, H.H.; Goldthorpe, I.A. A Simple Method of Growing Silver Chloride Nanocubes on Silver Nanowires. Nanotechnology 2015, 26, 381002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, P.; Meng, W.; Cui, E.X.; Zhang, Q.; Wang, Z.; Zheng, Z.; Liu, Y.; Cheng, H.; Dai, Y.; et al. Photococatalytic Anticancer Performance of Naked Ag/AgCl Nanoparticles. Chem. Eng. J. 2022, 428, 131265. [Google Scholar] [CrossRef]
- Puccetti, M.; Donnadio, A.; Ricci, M.; Latterini, L.; Quaglia, G.; Pietrella, D.; Di Michele, A.; Ambrogi, V. Alginate Ag/AgCl Nanoparticles Composite Films for Wound Dressings with Antibiofilm and Antimicrobial Activities. J. Funct. Biomater. 2023, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Mao, F.; Su, Y.; Sun, X.; Li, B.; Liu, P.F. Cu(I) Metal-Organic Framework Composites with AgCl/Ag Nanoparticles for Irradiation-Enhanced Antibacterial Activity against E. coli. ACS Omega 2022, 8, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, R.; He, T.; Xu, K.; Du, D.; Zhao, N.; Cheng, X.; Yang, J.; Shi, H.; Lin, Y. Biomedical Potential of Ultrafine Ag/AgCl Nanoparticles Coated on Graphene with Special Reference to Antimicrobial Performances and Burn Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 15067–15075. [Google Scholar] [CrossRef]
- Nocchetti, M.; Donnadio, A.; Ambrogi, V.; Andreani, P.; Bastianini, M.; Pietrella, D.; Latterini, L. Ag/AgCl Nanoparticle Decorated Layered Double Hydroxides: Synthesis, Characterization and Antimicrobial Properties. J. Mater. Chem. B 2013, 1, 2383–2393. [Google Scholar] [CrossRef]
- Furlan, P.Y.; Fisher, A.J.; Furlan, A.Y.; Melcer, M.E.; Shinn, D.W.; Warren, J.B. Magnetically Recoverable and Reusable Antimicrobial Nanocomposite Based on Activated Carbon, Magnetite Nanoparticles, and Silver Nanoparticles for Water Disinfection. Inventions 2017, 2, 10. [Google Scholar] [CrossRef]
- Malachová, K.; Praus, P.; Pavlíčková, Z.; Turicová, M. Activity of Antibacterial Compounds Immobilised on Montmorillonite. Appl. Clay Sci. 2009, 43, 364–368. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Pomastowski, P.; Hornowska, M.; Król, A.; Rafińska, K.; Buszewski, B. Naturally Organic Functionalized 3D Biosilica from Diatom Microalgae. Mater. Des. 2017, 132, 22–29. [Google Scholar] [CrossRef]
- Nowak, A.P.; Sprynskyy, M.; Brzozowska, W.; Lisowska-Oleksiak, A. Electrochemical Behavior of a Composite Material Containing 3D-Structured Diatom Biosilica. Algal Res. 2019, 41, 101538. [Google Scholar] [CrossRef]
- Kubasheva, Z.; Sprynskyy, M.; Railean-Plugaru, V.; Pomastowski, P.; Ospanova, A.; Buszewski, B. Synthesis and Antibacterial Activity of (AgCl, Ag)NPs/Diatomite Hybrid Composite. Materials 2020, 13, 3409. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Maiti, N.; Dhayagude, A.C.; Pathak, A.K.; Chadha, R.; Neogy, S.; Kapoor, S. A Study of Light Induced Surface Reactions of Sildenafil Citrate on Hybrid AgCl/Ag Nanoparticle Dimers by Surface Enhanced Raman Scattering and Pulse Radiolysis Techniques. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123864. [Google Scholar] [CrossRef]
- Temgire, M.K.; Joshi, S.S. Optical and Structural Studies of Silver Nanoparticles. Radiat. Phys. Chem. 2004, 71, 1039–1044. [Google Scholar] [CrossRef]
- Chen, S.; Carey, J.L.; Whitcomb, D.R.; Bühlmann, P.; Penn, R.L. Elucidating the Role of AgCl in the Nucleation and Growth of Silver Nanoparticles in Ethylene Glycol. Cryst. Growth Des. 2018, 18, 324–330. [Google Scholar] [CrossRef]
- Rakovan, J. Word of the wise: Hemimorphism. Rocks Miner. 2007, 82, 329–337. [Google Scholar] [CrossRef]
- Pohl, U.W. Epitaxy of Semiconductors: Introduction to Physical Principles; Springer: Berlin/Heidelberg, Germany, 2013; pp. 427–467. [Google Scholar] [CrossRef]
- Schuette, W.M.; Buhro, W.E. Silver Chloride as a Heterogeneous Nucleant for the Growth of Silver Nanowires. ACS Nano 2013, 7, 3844–3853. [Google Scholar] [CrossRef] [PubMed]
- Pedireddy, S.; Lee, H.K.; Tjiu, W.W.; Phang, I.Y.; Tan, H.R.; Chua, S.Q.; Troadec, C.; Ling, X.Y. One-Step Synthesis of Zero-Dimensional Hollow Nanoporous Gold Nanoparticles with Enhanced Methanol Electrooxidation Performance. Nat. Commun. 2014, 5, 4947. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zhang, J.; Song, G. Effect of Complexation on the Zeta Potential of Silica Powder. Powder Technol. 2003, 134, 218–222. [Google Scholar] [CrossRef]
- Szekeres, M.; Dékány, I.; De Keizer, A. Adsorption of Dodecyl Pyridinium Chloride on Monodisperse Porous Silica. Colloids Surf. A Physicochem. Eng. Asp. 1998, 141, 327–336. [Google Scholar] [CrossRef]
- Tzounis, L.; Contreras-Caceres, R.; Schellkopf, L.; Jehnichen, D.; Fischer, D.; Cai, C.; Uhlmann, P.; Stamm, M. Controlled Growth of Ag Nanoparticles Decorated onto the Surface of SiO2 Spheres: A Nanohybrid System with Combined SERS and Catalytic Properties. RSC Adv. 2014, 4, 17846–17855. [Google Scholar] [CrossRef]
- Ratirotjanakul, W.; Sioloetwong, T.; Suteewong, T.; Tangboriboonrat, P. Green Synthesis of AgNPs Coated Mesoporous Silica Nanoparticles Using Tyrosine as Reducing/Stabilising Agent. Mater. Sci. Forum 2018, 928, 89–93. [Google Scholar] [CrossRef]
- Pandian, A.M.K.; Karthikeyan, C.; Rajasimman, M.; Dinesh, M.G. Synthesis of Silver Nanoparticle and Its Application. Ecotoxicol. Environ. Saf. 2015, 121, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, Y.; Fang, Y. Spectroscopy Property of Ag Nanoparticles. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2006, 65, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Tri, P.N.; Rtimi, S.; Nguyen, T.A.; Vu, M.T. Physics, Electrochemistry, Photochemistry, and Photoelectrochemistry of Hybrid Nanoparticles; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128141342. [Google Scholar]
- Mooradian, A. Photoluminescence of Metals. Phys. Rev. Lett. 1969, 22, 185–187. [Google Scholar] [CrossRef]
- Bao, Y.; Chen, K. AgCl/Ag/g-C3N4 Hybrid Composites: Preparation, Visible Light-Driven Photocatalytic Activity and Mechanism. Nano-Micro Lett. 2016, 8, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Nakazato, G.; Seabra, A.B. Antimicrobial Activity of Biogenic Silver Nanoparticles, and Silver Chloride Nanoparticles: An Overview and Comments. Appl. Microbiol. Biotechnol. 2016, 100, 6555–6570. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.N.; Bhopate, D.P.; Kondalkar, V.V.; Majhi, S.M.; Bathula, C.D.; Tran, A.V.; Lee, S.W. Facile Synthesis of Ag-ZnO Core–Shell Nanostructures with Enhanced Photocatalytic Activity. J. Ind. Eng. Chem. 2018, 61, 78–86. [Google Scholar] [CrossRef]
- Huo, Y.; Han, Y.X.; Singh, P.; Kang, J.P.; Pu, J.Y.; Piao, C.H.; Yang, D.C. Antimicrobial, Antioxidant, and Anticancer Potentials of AgCl Nanoparticles Biosynthesized by Flavobacterium Panacis. Appl. Phys. A Mater. Sci. Process. 2021, 127, 227. [Google Scholar] [CrossRef]
- Conference, I.; Metallurgy, P.; Materials, A. Powder Metallurgy and Advanced Materials; Materials Research Forum LLC: Millersville, PA, USA, 2017; ISBN 9781945291982. [Google Scholar]
- Gui, F.; Mo, W.; Guo, X.; Cao, F.; Zhai, T.; Hong, C.; Guan, X.; Huang, B.; Pan, X. Biosynthesis of Nanocrystalline Silver Chloride with High Antibacterial Activity Using Bacterial Extracts. Adv. Agrochem 2023, 2, 88–96. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Jers, C.; Joshi, A.S.; Garnæs, J.; Mijakovic, I. Silver Nanoparticles Produced from Cedecea Sp. Exhibit Antibiofilm Activity and Remarkable Stability. Sci. Rep. 2021, 11, 12619. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.I.; Sportelli, M.C.; Picca, R.A.; Gentile, L.; Palazzo, G.; Ditaranto, N.; Cioffi, N. Green Synthesis and Characterization of Antimicrobial Synergistic AgCl/BAC Nanocolloids. ACS Appl. Bio Mater. 2022, 5, 3230–3240. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.T.; Ibraheem, I.J.; Hassan, O.M.; Obaid, A.S.; Ali, H.H.; Salih, T.A.; Kadhim, M.S. Facile Green Synthesis of Ag/AgCl Nanoparticles Derived from Chara Algae Extract and Evaluating Their Antibacterial Activity and Synergistic Effect with Antibiotics. J. Environ. Chem. Eng. 2021, 9, 105359. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, Y.; Teng, G.; Wang, L.; Jin, X.; Qiang, Z.; Ma, W. Fabrication of a Double-Shell Ag/AgCl/G-ZnFe2O4Nanocube with Enhanced Light Absorption and Superior Photocatalytic Antibacterial Activity. ACS Appl. Mater. Interfaces 2020, 12, 29883–29898. [Google Scholar] [CrossRef]

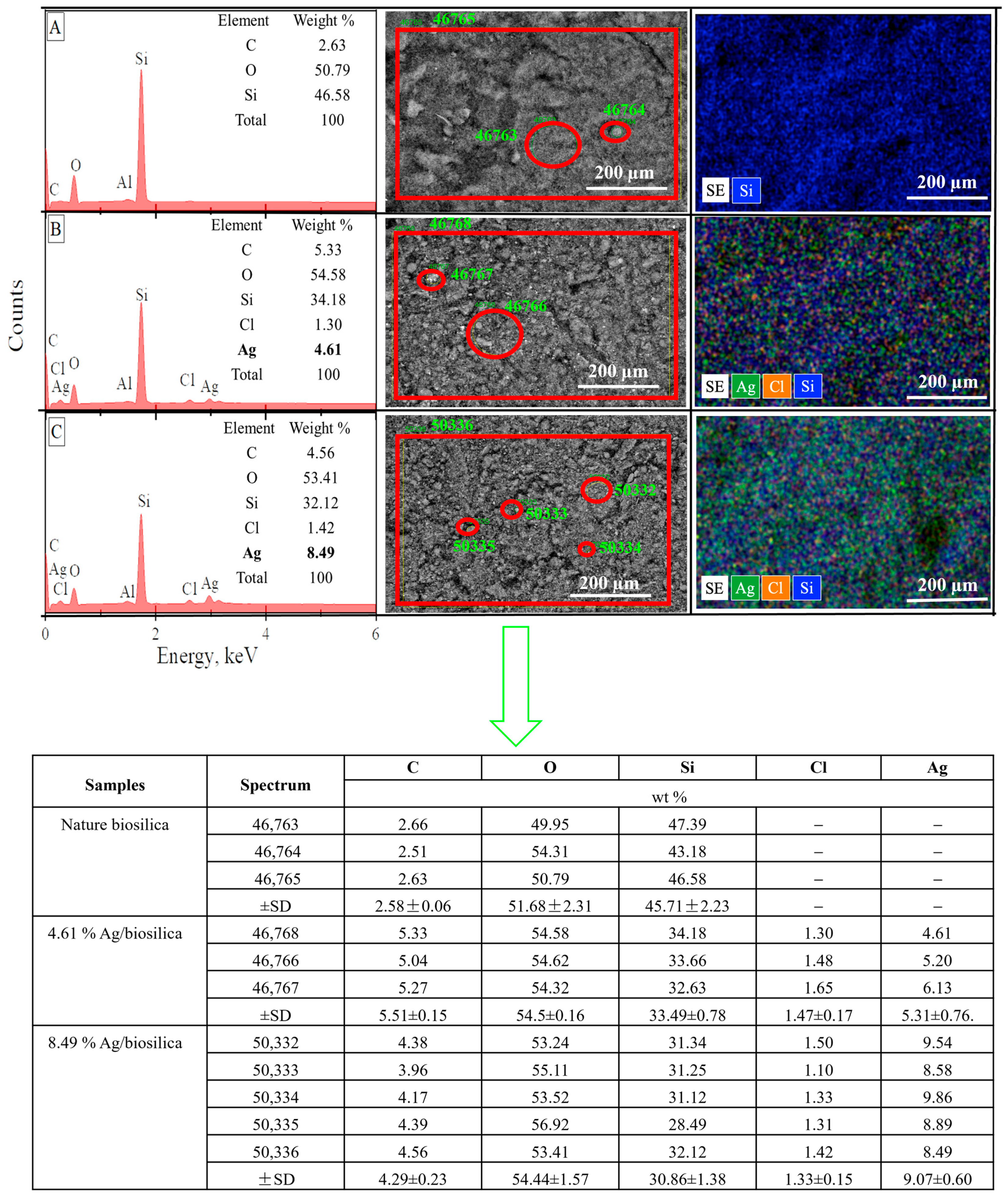
| Samples | Spectrum | Ag | Cl | AgClNPs | AgNPs | AgCl: AgNPs | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mass % | n, Mole | Mass % | n, Mole | n, Mole | % | n, Mole | % | % | ||
| 4.61% Ag/biosilica | 46,768 | 4.61 | 0.0426 | 1.30 | 0.0366 | 0.0366 | 86 | 0.006 | 14 | 86:14 |
| 46,766 | 5.20 | 0.0481 | 1.48 | 0.0416 | 0.0416 | 87 | 0.0065 | 13 | 87:13 | |
| 46,767 | 6.13 | 0.0567 | 1.65 | 0.0464 | 0.0464 | 82 | 0.0103 | 18 | 82:18 | |
| ±SD | 5.31 ± 0.76 | 1.47 ± 0.17 | 85 ± 3 | 15 ± 3 | ||||||
| 8.49% Ag/biosilica | 50,336 | 8.49 | 0.0786 | 1.42 | 0.040 | 0.040 | 51 | 0.0386 | 49 | 51:49 |
| 50,333 | 8.58 | 0.0794 | 1.10 | 0.030 | 0.0309 | 39 | 0.0494 | 61 | 39:61 | |
| 50,335 | 8.89 | 0.0823 | 1.31 | 0.0369 | 0.0369 | 45 | 0.0454 | 55 | 45:55 | |
| 50,332 | 9.54 | 0.0883 | 1.50 | 0.0422 | 0.0422 | 48 | 0.0461 | 52 | 48:52 | |
| 50,334 | 9.86 | 0.0912 | 1.33 | 0.0374 | 0.0374 | 41 | 0.0538 | 59 | 41:59 | |
| ±SD | 9.07 ± 0.60 | 1.33 ± 0.15 | 45 ± 5 | 55 ± 5 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekissanova, Z.; Railean, V.; Wojtczak, I.; Brzozowska, W.; Trykowski, G.; Ospanova, A.; Sprynskyy, M. Synthesis and Antimicrobial Activity of 3D Micro–Nanostructured Diatom Biosilica Coated by Epitaxially Growing Ag-AgCl Hybrid Nanoparticles. Biomimetics 2024, 9, 5. https://doi.org/10.3390/biomimetics9010005
Bekissanova Z, Railean V, Wojtczak I, Brzozowska W, Trykowski G, Ospanova A, Sprynskyy M. Synthesis and Antimicrobial Activity of 3D Micro–Nanostructured Diatom Biosilica Coated by Epitaxially Growing Ag-AgCl Hybrid Nanoparticles. Biomimetics. 2024; 9(1):5. https://doi.org/10.3390/biomimetics9010005
Chicago/Turabian StyleBekissanova, Zhanar, Viorica Railean, Izabela Wojtczak, Weronika Brzozowska, Grzegorz Trykowski, Alyiya Ospanova, and Myroslav Sprynskyy. 2024. "Synthesis and Antimicrobial Activity of 3D Micro–Nanostructured Diatom Biosilica Coated by Epitaxially Growing Ag-AgCl Hybrid Nanoparticles" Biomimetics 9, no. 1: 5. https://doi.org/10.3390/biomimetics9010005







