The Role of the Pancreatic Extracellular Matrix as a Tissue Engineering Support for the Bioartificial Pancreas
Abstract
:1. Introduction
2. Fundamentals of Pancreatic Tissue Engineering
2.1. Anatomy, Physiology, and Endocrine/Exocrine Functions of the Pancreas
2.2. Challenges Related to Manufacturing Bioartificial Pancreases, Including the Precise Reproduction of Cellular Architecture and the Maintenance of Secretory Function
2.3. Traditional and Emerging Tissue Engineering Strategies Used to Build Bioartificial Organs, with an Emphasis on Specific Approaches Applied to the Pancreas
3. Extracellular Matrix: Composition and Role in the Pancreas
3.1. The Critical Role of the ECM in Regulating Cell Differentiation, Migration, and Proliferation, as Well as in Maintaining Tissue Architecture
3.2. Complex Interactions between Pancreatic Endocrine and Exocrine Cells and ECM Components, and How These Interactions Influence Normal and Pathological Pancreatic Function
4. Extracellular Matrix Engineering for Bioartificial Pancreas
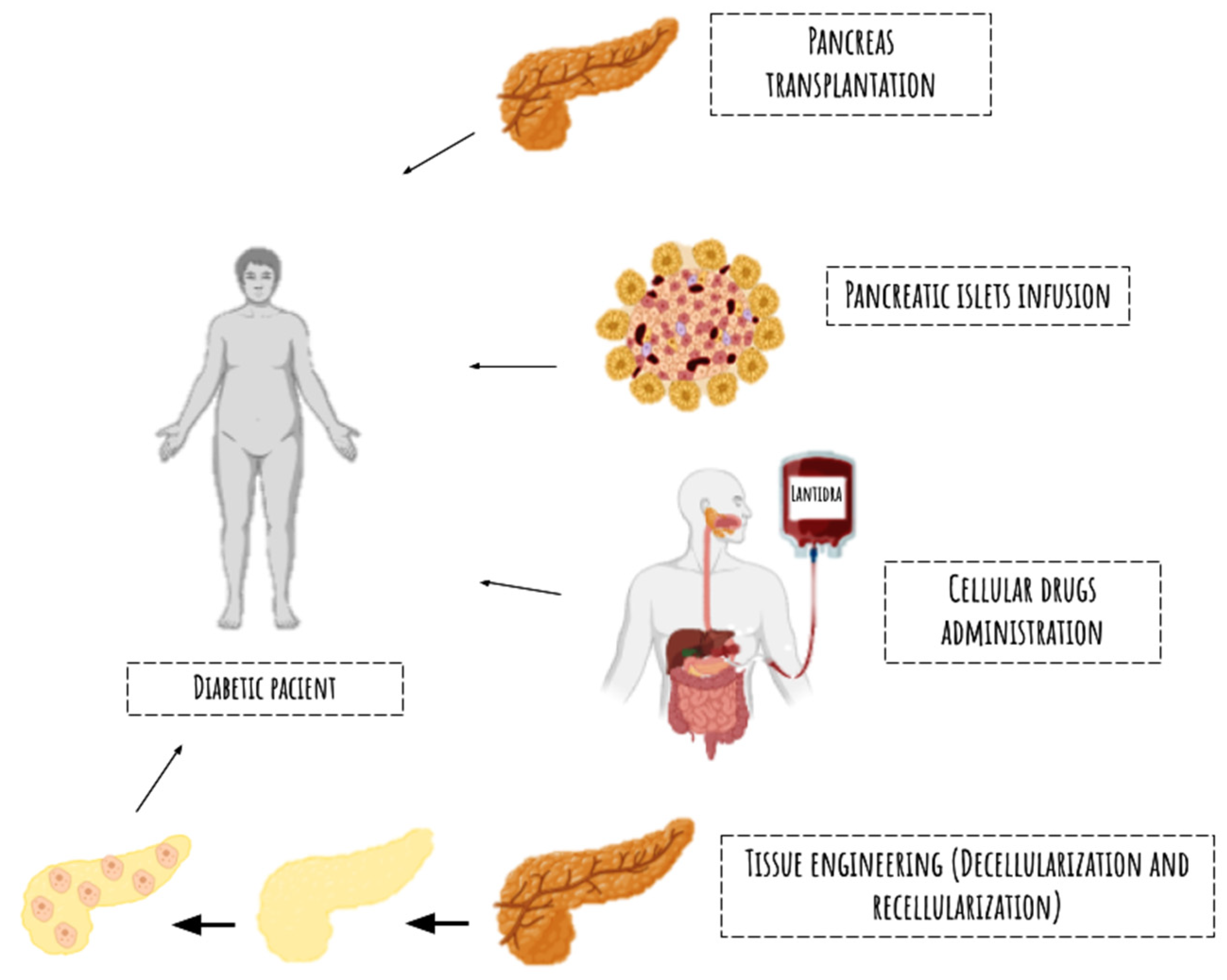
5. Clinical Applications and Future Perspectives
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Mota, R.I.; Morgan, S.E.; Bahnson, E.M. Diabetic Vasculopathy: Macro and Microvascular Injury. Curr. Pathobiol. Rep. 2020, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- von Scholten, B.J.; Kreiner, F.F.; Gough, S.C.L.; von Herrath, M. Current and future therapies for type 1 diabetes. Diabetologia 2021, 64, 1037–1048. [Google Scholar] [CrossRef]
- Sagoo, M.K.; Gnudi, L. Diabetic Nephropathy: An Overview. Methods Mol. Biol. 2020, 2067, 3–7. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Nalbach, L.; Schmitt, B.M.; Becker, V.; Scheller, A.; Laschke, M.W.; Menger, M.D.; Ampofo, E. Nerve/glial antigen 2 is crucially involved in the revascularization of freely transplanted pancreatic islets. Cell Tissue Res. 2019, 378, 195–205. [Google Scholar] [CrossRef]
- Bowers, D.T.; Song, W.; Wang, L.H.; Ma, M. Engineering the Vasculature for Islet Transplantation. Acta Biomater. 2019, 95, 131. [Google Scholar] [CrossRef]
- Farney, A.C.; Sutherland, D.E.R.; Opara, E.C. Evolution of Islet Transplantation for the Last 30 Years. Pancreas 2016, 45, 8–20. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Kong, H.; He, Q.; Sun, H.; Bhugul, P.A.; Zhang, Q.; Chen, B.; Zhou, M. The rat pancreatic body tail as a source of a novel extracellular matrix scaffold for endocrine pancreas bioengineering. J. Biol. Eng. 2018, 12, 6. [Google Scholar] [CrossRef]
- Wan, J.; Huang, Y.; Zhou, P.; Guo, Y.; Wu, C.; Zhu, S.; Wang, Y.; Wang, L.; Lu, Y.; Wang, Z. Culture of iPSCs Derived Pancreatic β-Like Cells In Vitro Using Decellularized Pancreatic Scaffolds: A Preliminary Trial. BioMed Res. Int. 2017, 2017, 4276928. [Google Scholar] [CrossRef]
- Bi, H.; Karanth, S.S.; Ye, K.; Stein, R.; Jin, S. Decellularized Tissue Matrix Enhances Self-Assembly of Islet Organoids from Pluripotent Stem Cell Differentiation. ACS Biomater. Sci. Eng. 2020, 6, 4155–4165. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wu, G.; Li, H.; Qiao, Y.; Gao, S. Type 1 and type 2 diabetes mortality burden: Predictions for 2030 based on Bayesian age-period-cohort analysis of China and global mortality burden from 1990 to 2019. J. Diabetes Investig. 2024, 15, 623. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, Y.; Huang, Y.; Xiong, Y.; Xu, Y.; Li, X.; Lu, J.; Wang, L.; Wang, Y.; Lu, Y.; et al. Constructing heparin-modified pancreatic decellularized scaffold to improve its re-endothelialization. J. Biomater. Appl. 2018, 32, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Chaimov, D.; Baruch, L.; Krishtul, S.; Meivar-levy, I.; Ferber, S.; Machluf, M. Innovative encapsulation platform based on pancreatic extracellular matrix achieve substantial insulin delivery. J. Control. Release 2017, 257, 91–101. [Google Scholar] [CrossRef]
- Hogrebe, N.J.; Ishahak, M.; Millman, J.R. Developments in stem cell-derived islet replacement therapy for treating type 1 diabetes. Cell Stem Cell 2023, 30, 530. [Google Scholar] [CrossRef]
- Berger, C.; Bjørlykke, Y.; Hahn, L.; Mühlemann, M.; Kress, S.; Walles, H.; Luxenhofer, R.; Ræder, H.; Metzger, M.; Zdzieblo, D. Matrix decoded—A pancreatic extracellular matrix with organ specific cues guiding human iPSC differentiation. Biomaterials 2020, 244, 119766. [Google Scholar] [CrossRef]
- Klak, M.; Wszoła, M.; Berman, A.; Filip, A.; Kosowska, A.; Olkowska-Truchanowicz, J.; Rachalewski, M.; Tymicki, G.; Bryniarski, T.; Kołodziejska, M.; et al. Bioprinted 3D Bionic Scaffolds with Pancreatic Islets as a New Therapy for Type 1 Diabetes—Analysis of the Results of Preclinical Studies on a Mouse Model. J. Funct. Biomater. 2023, 14, 371. [Google Scholar] [CrossRef]
- Klak, M.; Łojszczyk, I.; Berman, A.; Tymicki, G.; Adamiok-Ostrowska, A.; Sierakowski, M.; Olkowski, R.; Szczepankiewicz, A.A.; Kamiński, A.; Dobrzyń, A.; et al. Impact of Porcine Pancreas Decellularization Conditions on the Quality of Obtained dECM. Int. J. Mol. Sci. 2021, 22, 7005. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Faulk, D.M.; Wildemann, J.D.; Badylak, S.F. Decellularization and cell seeding of whole liver biologic scaffolds composed of extracellular matrix. J. Clin. Exp. Hepatol. 2015, 5, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, F. Pancreatic Decellularization Aiming at Recellularization as an Alternative Treatment for Type I Diabetes mellitus. Ph.D. Thesis, University of São Paulo, São Paulo, Brazil, 2019. [Google Scholar]
- Napierala, H.; Hillebrandt, K.H.; Haep, N.; Tang, P.; Tintemann, M.; Gassner, J.; Noesser, M.; Everwien, H.; Seiffert, N.; Kluge, M.; et al. Engineering an endocrine Neo-Pancreas by repopulation of a decellularized rat pancreas with islets of Langerhans. Sci. Rep. 2017, 7, 41777. [Google Scholar] [CrossRef]
- Fang, J.Y.; Lin, C.H.; Huang, T.H.; Chuang, S.Y. In Vivo Rodent Models of Type 2 Diabetes and Their Usefulness for Evaluating Flavonoid Bioactivity. Nutrients 2019, 11, 530. [Google Scholar] [CrossRef]
- Lutz, T.A. Mammalian models of diabetes mellitus, with a focus on type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2023, 19, 350–360. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, F.; Bi, Y.; Ji, B. Animal Models of Acute and Chronic Pancreatitis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 311, G343–G355. [Google Scholar] [CrossRef]
- Gilpin, A.; Yang, Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. BioMed Res. Int. 2017, 2017, 9831534. [Google Scholar] [CrossRef] [PubMed]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-Specific Decellularization Methods: Rationale and Strategies to Achieve Regenerative Compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Khajouei, F.; Rodriguez, J.; Kim, S.; Lee, E.J.A.; Nebe, B.; Sartore, L.; Hao, L.; Khajouei, F.; Rodriguez, J.; et al. Unlocking the Promise of Decellularized Pancreatic Tissue: A Novel Approach to Support Angiogenesis in Engineered Tissue. Bioengineering 2024, 11, 183. [Google Scholar] [CrossRef]
- Katsuki, Y.; Yagi, H.; Okitsu, T.; Kitago, M.; Tajima, K.; Kadota, Y.; Hibi, T.; Abe, Y.; Shinoda, M.; Itano, O.; et al. Endocrine pancreas engineered using porcine islets and partial pancreatic scaffolds. Pancreatology 2016, 16, 922–930. [Google Scholar] [CrossRef]
- Peloso, A.; Urbani, L.; Cravedi, P.; Katari, R.; Maghsoudlou, P.; Fallas, M.E.A.; Sordi, V.; Citro, A.; Purroy, C.; Niu, G.; et al. The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas. Ann. Surg. 2016, 264, 169–179. [Google Scholar] [CrossRef]
- Akester, A.R.; Nickel, R.; Schummer, A.; Seiferle, E.; Frewein, J.; Wilkens, H.; Translated, K.-H.W.; Siller, W.G.; Stokoe, W.M. The Anatomy of the Domestic Animals. Volume 1. The Locomotor System of the Domestic Mammals. J. Anat. 1987, 150, 289. [Google Scholar]
- Talathi, S.S.; Zimmerman, R.; Young, M. Anatomy, Abdomen and Pelvis, Pancreas; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Center, S.A. The Liver, Biliary Tract, and Exocrine Pancreas. Small Anim. Pediatr. 2011, 368–390. [Google Scholar] [CrossRef]
- Lim, L.Y.; Ding, S.S.L.; Muthukumaran, P.; Teoh, S.H.; Koh, Y.; Teo, A.K.K. Tissue engineering of decellularized pancreas scaffolds for regenerative medicine in diabetes. Acta Biomater. 2023, 157, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Guruswamy Damodaran, R.; Vermette, P. Decellularized pancreas as a native extracellular matrix scaffold for pancreatic islet seeding and culture. J. Tissue Eng. Regen. Med. 2018, 12, 1230–1237. [Google Scholar] [CrossRef]
- Hashemi, J.; Pasalar, P.; Soleimani, M.; Arefian, E.; Khorramirouz, R.; Akbarzadeh, A.; Ghorbani, F.; Enderami, S.E.; Kajbafzadeh, A.M. Decellularized Pancreas Matrix Scaffolds for Tissue Engineering Using Ductal or Arterial Catheterization. Cells Tissues Organs 2018, 205, 72–84. [Google Scholar] [CrossRef]
- Srokowski, E.M.; Woodhouse, K.A. 2.20 Decellularized Scaffolds. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 452–470. [Google Scholar] [CrossRef]
- Sevastianov, V.I.; Ponomareva, A.S.; Baranova, N.V.; Kirsanova, L.A.; Basok, Y.B.; Nemets, E.A.; Kruglov, D.N.; Miloserdov, I.A.; Gautier, S.V. Decellularization of Human Pancreatic Fragments with Pronounced Signs of Structural Changes. Int. J. Mol. Sci. 2022, 24, 119. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Y.Z.; Jiang, Z.; Wang, Z.; Wang, Q.; Kou, L.; Yao, Q. Immune-Protective Formulations and Process Strategies for Improved Survival and Function of Transplanted Islets. Front. Immunol. 2022, 13, 923241. [Google Scholar] [CrossRef]
- Vigersky, R.A. The benefits, limitations, and cost-effectiveness of advanced technologies in the management of patients with Diabetes mellitus. J. Diabetes Sci. Technol. 2015, 9, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Peters, T.M.; Haidar, A. Dual-hormone artificial pancreas: Benefits and limitations compared with single-hormone systems. Diabet. Med. 2018, 35, 450–459. [Google Scholar] [CrossRef]
- Christiansen, S.C.; Fougner, A.L.; Stavdahl, Ø.; Kölle, K.; Ellingsen, R.; Carlsen, S.M. A Review of the Current Challenges Associated with the Development of an Artificial Pancreas by a Double Subcutaneous Approach. Diabetes Ther. 2017, 8, 489. [Google Scholar] [CrossRef]
- Esposito, S.; Santi, E.; Mancini, G.; Rogari, F.; Tascini, G.; Toni, G.; Argentiero, A.; Berioli, M.G. Efficacy and safety of the artificial pancreas in the paediatric population with type 1 diabetes. J. Transl. Med. 2018, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, C.; Perrone, V.G.; Amorese, G.; Vistoli, F.; Baronti, W.; Marchetti, P.; Boggi, U. Update on Pancreatic Transplantation in the Management of Diabetes. In Learning Surgery: The Surgery Clerkship Manual; Springer: New York, NY, USA, 2021; pp. 718–734. [Google Scholar] [CrossRef]
- Ho, B.X.; Teo, A.K.K.; Ng, N.H.J. Innovations in bio-engineering and cell-based approaches to address immunological challenges in islet transplantation. Front. Immunol. 2024, 15, 1375177. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.; Alberti, P.; Demartines, N.; Phillips, M.; Casey, J.; Sutherland, A. Whole-Organ Pancreas and Islets Transplantations in UK: An Overview and Future Directions. J. Clin. Med. 2023, 12, 3245. [Google Scholar] [CrossRef]
- Bahar, S.G.; Devulapally, P. Pancreas Transplantation; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Akashi, Y.; Anazawa, T.; Fujikura, J.; Park, C.G. Islet Transplantation. In Pancreas Transplantation—The Asian Experience, A Registry Report; Springer: Berlin/Heidelberg, Germany, 2022; pp. 227–239. [Google Scholar] [CrossRef]
- Takaki, T.; Shimoda, M. Pancreatic islet transplantation: Toward definitive treatment for diabetes mellitus. Glob. Health Med. 2020, 2, 200. [Google Scholar] [CrossRef]
- Gruessner, A.C. 2011 Update on Pancreas Transplantation: Comprehensive Trend Analysis of 25,000 Cases Followed Up Over the Course of Twenty-Four Years at the International Pancreas Transplant Registry (IPTR). Rev. Diabet. Stud. 2011, 8, 6. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves first cell therapy for type 1 diabetes. Nat. Rev. Drug Discov. 2023, 22, 611. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: First Regulatory Approval for Allogeneic Pancreatic Islet Beta Cell Infusion for Adult Patients with Type 1 Diabetes Mellitus. Med. Sci. Monit. 2023, 29, e941918-1–e941918-2. [Google Scholar] [CrossRef] [PubMed]
- Giri, O.; Goldman, J.D. Donislecel: First Cellular Therapy to Treat Patients with Brittle Type 1 Diabetes. Clin. Diabetes 2024, 42, 337–340. [Google Scholar] [CrossRef]
- Orlando, G.; Farney, A.C.; Iskandar, S.S.; Mirmalek-Sani, S.H.; Sullivan, D.C.; Moran, E.; AbouShwareb, T.; Paolo, D.C.; Wood, K.J.; Stratta, R.J.; et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann. Surg. 2012, 256, 363–370. [Google Scholar] [CrossRef]
- Lim, F.; Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef]
- Goh, S.K.; Bertera, S.; Olsen, P.; Candiello, J.E.; Halfter, W.; Uechi, G.; Balasubramani, M.; Johnson, S.A.; Sicari, B.M.; Kollar, E.; et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 2013, 34, 6760. [Google Scholar] [CrossRef] [PubMed]
- Westenfelder, C.; Hu, Z.; Zhang, P.; Gooch, A. Intraperitoneal administration of human “Neo-Islets”, 3-D organoids of mesenchymal stromal and pancreatic islet cells, normalizes blood glucose levels in streptozotocin-diabetic NOD/SCID mice: Significance for clinical trials. PLoS ONE 2021, 16, e0259043. [Google Scholar] [CrossRef] [PubMed]
- Llacua, L.A.; Faas, M.M.; de Vos, P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia 2018, 61, 1261–1272. [Google Scholar] [CrossRef]
- Ma, F.; Tremmel, D.M.; Li, Z.; Lietz, C.B.; Sackett, S.D.; Odorico, J.S.; Li, L. In Depth Quantification of Extracellular Matrix Proteins from Human Pancreas. J. Proteome Res. 2019, 18, 3156–3165. [Google Scholar] [CrossRef]
- Sackett, S.D.; Tremmel, D.M.; Ma, F.; Feeney, A.K.; Maguire, R.M.; Brown, M.E.; Zhou, Y.; Li, X.; O’Brien, C.; Li, L.; et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci. Rep. 2018, 8, 10452. [Google Scholar] [CrossRef]
- Asthana, A.; Tamburrini, R.; Chaimov, D.; Gazia, C.; Walker, S.J.; Van Dyke, M.; Tomei, A.; Lablanche, S.; Robertson, J.; Opara, E.C.; et al. Comprehensive characterization of the human pancreatic proteome for bioengineering applications. Biomaterials 2021, 270, 120613. [Google Scholar] [CrossRef]
- Enck, K.; Tamburrini, R.; Deborah, C.; Gazia, C.; Jost, A.; Khalil, F.; Alwan, A.; Orlando, G.; Opara, E.C. Effect of alginate matrix engineered to mimic the pancreatic microenvironment on encapsulated islet function. Biotechnol. Bioeng. 2021, 118, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Stendahl, J.C.; Kaufman, D.B.; Stupp, S.I. Extracellular Matrix in Pancreatic Islets: Relevance to Scaffold Design and Transplantation. Cell Transplant. 2009, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Kruegel, J.; Miosge, N. Basement membrane components are key players in specialized extracellular matrices. Cell. Mol. Life Sci. C. 2010, 67, 2879. [Google Scholar] [CrossRef]
- Meier, R.P.H.; Meyer, J.; Muller, Y.D.; Szot, G.L.; Bédat, B.; Andres, A.; Massé, N.; Lablanche, S.; Puppa, G.; Bosco, D.; et al. Pancreas collagen digestion during islet of Langerhans isolation-a prospective study. Transpl. Int. 2020, 33, 1516–1528. [Google Scholar] [CrossRef]
- Exposito, J.Y.; Valcourt, U.; Cluzel, C.; Lethias, C. The fibrillar collagen family. Int. J. Mol. Sci. 2010, 11, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Hlund, D.; Lundin, C.; Ardnor, B.; Man, M.; Naredi, P.; Sund, M. Type IV collagen is a tumour stroma-derived biomarker for pancreas cancer. Br. J. Cancer 2009, 101, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sakata, N.; Yoshimatsu, G.; Koadama, S. The Roles of Collagen in Islet Transplantation. OBM Transplant. 2020, 4, 1–17. [Google Scholar] [CrossRef]
- Topalovski, M.; Brekken, R.A. Matrix control of pancreatic cancer: New insights into fibronectin signaling. Cancer Lett. 2016, 381, 252. [Google Scholar] [CrossRef]
- Ansari, D.; Aronsson, L.; Sasor, A.; Welinder, C.; Rezeli, M.; Marko-Varga, G.; Andersson, R. The role of quantitative mass spectrometry in the discovery of pancreatic cancer biomarkers for translational science. J. Transl. Med. 2014, 12, 87. [Google Scholar] [CrossRef]
- Hiram-Bab, S.; Katz, L.S.; Shapira, H.; Sandbank, J.; Gershengorn, M.C.; Oron, Y. Platelet-Derived Growth Factor BB Mimics Serum-Induced Dispersal of Pancreatic Epithelial Cell Clusters. J. Cell. Physiol. 2014, 229, 743. [Google Scholar] [CrossRef]
- Principe, D.R.; DeCant, B.; Mascariñas, E.; Wayne, E.A.; Diaz, A.M.; Akagi, N.; Hwang, R.; Pasche, B.; Dawson, D.W.; Fang, D.; et al. TGFβ Signaling in the Pancreatic Tumor Microenvironment Promotes Fibrosis and Immune Evasion to Facilitate Tumorigenesis. Cancer Res. 2016, 76, 2525–2539. [Google Scholar] [CrossRef]
- Jiang, F.-X.; Naselli, G.; Harrison, L.C. Distinct Distribution of Laminin and Its Integrin Receptors in the Pancreas. J. Histochem. Cytochem. 2002, 50, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2002; ISBN 0-8153-3218-1. [Google Scholar]
- Li, J.; Peng, L.; Chen, Q.; Ye, Z.; Zhao, T.; Hou, S.; Gu, J.; Hang, Q. Integrin β1 in Pancreatic Cancer: Expressions, Functions, and Clinical Implications. Cancers 2022, 14, 3377. [Google Scholar] [CrossRef]
- Ley, K. Functions of selectins. Results Probl. Cell Differ. 2001, 33, 177–200. [Google Scholar] [CrossRef]
- Mia, M.S.; Jarajapu, Y.; Rao, R.; Mathew, S. Integrin β1 Promotes Pancreatic Tumor Growth by Upregulating Kindlin-2 and TGF-β Receptor-2. Int. J. Mol. Sci. 2021, 22, 10599. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Dandara, C. The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis. Biomimetics 2023, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D Extracellular Matrix Mimics: Fundamental Concepts and Role of Materials Chemistry to Influence Stem Cell Fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Tran, R.; Moraes, C.; Hoesli, C.A. Developmentally-Inspired Biomimetic Culture Models to Produce Functional Islet-Like Cells From Pluripotent Precursors. Front. Bioeng. Biotechnol. 2020, 8, 583970. [Google Scholar] [CrossRef]
- Cirulli, V.; Beattie, G.M.; Klier, G.; Ellisman, M.; Ricordi, C.; Quaranta, V.; Frasier, F.; Ishii, J.K.; Hayek, A.; Salomon, D.R. Expression and function of alpha(v)beta(3) and alpha(v)beta(5) integrins in the developing pancreas: Roles in the adhesion and migration of putative endocrine progenitor cells. J. Cell Biol. 2000, 150, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, O.; Berman, D.M.; Kenyon, N.S.; Ricordi, C.; Berggren, P.O.; Caicedo, A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl. Acad. Sci. USA 2006, 103, 2334. [Google Scholar] [CrossRef]
- Galli, A.; Algerta, M.; Marciani, P.; Schulte, C.; Lenardi, C.; Milani, P.; Maffioli, E.; Tedeschi, G.; Perego, C. Shaping Pancreatic β-Cell Differentiation and Functioning: The Influence of Mechanotransduction. Cells 2020, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Tunggal, P.; Smyth, N.; Paulsson, M.; Ott, M.-C. Laminins: Structure and genetic regulation. Microsc. Res. Technol. 2000, 51, 214–227. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Felgueiras, J.; Fardilha, M. Signaling pathways in anchoring junctions of epithelial cells: Cell-to-cell and cell-to-extracellular matrix interactions. J. Recept. Signal Transduct. Res. 2015, 35, 67–75. [Google Scholar] [CrossRef]
- Gannon, M. Pancreas Development and Stem Cells; S. A. Mood: Burlington, MA, USA, 2007; pp. 946–971. [Google Scholar]
- Lumelsky, N. Pancreatic Differentiation of Pluripotent Stem Cells. In Human Embryonic Stem Cells; Humana Press: Totowa, NJ, USA, 2003; pp. 161–179. [Google Scholar] [CrossRef]
- Iglesias, L.P.; Favaron, P.O.; Borghesi, J.; Oliveira Carreira, A.C.; Miglino, M.A.; de Melo, A.P.F. Trend Toward Individualization of the Endocrine and Exocrine Portions of the Giant Anteater Pancreas (Myrmecophaga Tridactyla, Xenarthra). Anat. Rec. 2017, 300, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Makar, S.; Ghosh, A.; Divya; Shivhare, S.; Kumar, A.; Singh, S.K. Molecular Processes Involved in Pancreatic Cancer and Therapeutics. Curr. Chem. Biol. 2020, 15, 85–108. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Tremmel, D.M.; Sackett, S.D.; Feeney, A.K.; Mitchell, S.A.; Schaid, M.D.; Polyak, E.; Chlebeck, P.J.; Gupta, S.; Kimple, M.E.; Fernandez, L.A.; et al. A human pancreatic ECM hydrogel optimized for 3-D modeling of the islet microenvironment. Sci. Rep. 2022, 12, 7188. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Xavier, G. The Cells of the Islets of Langerhans. J. Clin. Med. 2018, 7, 54. [Google Scholar] [CrossRef]
- Pandiri, A.R. Overview of Exocrine Pancreatic Pathobiology. Toxicol. Pathol. 2014, 42, 207. [Google Scholar] [CrossRef] [PubMed]
- Sarles, H. The Exocrine Pancreas. Int. Rev. Physiol. 2010, 12, 173–221. [Google Scholar] [CrossRef]
- El Sayed, S.A.; Mukherjee, S. Physiology, Pancreas; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Valente, R.; Coppola, A.; Scandavini, C.M.; Halimi, A.; Magnusson, A.; Lauro, A.; Sotirova, I.; Arnelo, U.; Franklin, O. Interactions between the Exocrine and the Endocrine Pancreas. J. Clin. Med. 2024, 13, 1179. [Google Scholar] [CrossRef]
- Overton, D.L.; Mastracci, T.L. Exocrine-Endocrine Crosstalk: The Influence of Pancreatic Cellular Communications on Organ Growth, Function and Disease. Front. Endocrinol. 2022, 13, 904004. [Google Scholar] [CrossRef]
- Zhen, G.D.; Zhao, L.B.; Wu, S.S.; Chen, M.Y.; Li, Z.H.; Zhou, S.Z.; Li, Z.F. Associations of MMP-2 and MMP-9 gene polymorphism with ulinastatin efficacy in patients with severe acute pancreatitis. Biosci. Rep. 2017, 37, 20160612. [Google Scholar] [CrossRef]
- Elebring, E.; Kuna, V.K.; Kvarnström, N.; Sumitran-Holgersson, S. Cold-perfusion decellularization of whole-organ porcine pancreas supports human fetal pancreatic cell attachment and expression of endocrine and exocrine markers. J. Tissue Eng. 2017, 8, 2041731417738145. [Google Scholar] [CrossRef] [PubMed]
- Kuna, V.K.; Kvarnström, N.; Elebring, E.; Holgersson, S.S. Isolation and Decellularization of a Whole Porcine Pancreas. J. Vis. Exp. 2018, 2018, 58302. [Google Scholar] [CrossRef]
- Neuzillet, C.; de Gramont, A.; Tijeras-Raballand, A.; de Mestier, L.; Cros, J.; Faivre, S.; Raymond, E. Perspectives of TGF-β inhibition in pancreatic and hepatocellular carcinomas. Oncotarget 2014, 5, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Böttinger, E.P.; Jakubczak, J.L.; Roberts, I.S.D.; Mumy, M.; Hemmati, P.; Bagnall, K.; Merlino, G.; Wakefield, L.M. Expression of a dominant-negative mutant TGF-beta type II receptor in transgenic mice reveals essential roles for TGF-beta in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997, 16, 2621–2633. [Google Scholar] [CrossRef]
- Shakoor, S.; Kibble, E.; El-Jawhari, J.J. Bioengineering Approaches for Delivering Growth Factors: A Focus on Bone and Cartilage Regeneration. Bioengineering 2022, 9, 223. [Google Scholar] [CrossRef]
- Gresham, R.C.H.; Bahney, C.S.; Leach, J.K. Growth factor delivery using extracellular matrix-mimicking substrates for musculoskeletal tissue engineering and repair. Bioact. Mater. 2020, 6, 1945–1956. [Google Scholar] [CrossRef]
- Sadeghi-Ardebili, M.; Hasannia, S.; Dabirmanesh, B.; Khavari-Nejad, R.A. Functional characterization of the dimeric form of PDGF-derived fusion peptide fabricated based on theoretical arguments. Sci. Rep. 2024, 14, BSR20160612. [Google Scholar] [CrossRef]
- Gorla Junior, J.A.; Fagundes, D.J.; Parra, O.M.; Zaia, C.T.B.V.; Bandeira, C.O.P. Fatores hepatotróficos e regeneração hepática. Parte II: Fatores de crescimento. Acta Cirúrgica Bras. 2001, 16, 261–266. [Google Scholar] [CrossRef]
- Silva, I.B.B.; Kimura, C.H.; Colantoni, V.P.; Sogayar, M.C. Stem cells differentiation into insulin-producing cells (IPCs): Recent advances and current challenges. Stem Cell Res. Ther. 2022, 13, 309. [Google Scholar] [CrossRef]
- Paz, J.C. Acute Care Handbook for Physical Therapists; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9781455728961. [Google Scholar]
- Hu, L.; Wang, H.X.; Li, J.F.; Su, L.P.; Yan, M.; Li, C.; Yang, Q.; Liu, B.; Zhu, Z. gang Biglycan stimulates VEGF expression in endothelial cells by activating the TLR signaling pathway. Mol. Oncol. 2016, 10, 1473–1484. [Google Scholar] [CrossRef]
- Glorieux, L.; Vandooren, L.; Derclaye, S.; Pyr dit Ruys, S.; Oncina-Gil, P.; Salowka, A.; Herinckx, G.; Aajja, E.; Lemoine, P.; Spourquet, C.; et al. In-Depth Analysis of the Pancreatic Extracellular Matrix during Development for Next-Generation Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 10268. [Google Scholar] [CrossRef] [PubMed]
- Peloso, A.; Citro, A.; Oldani, G.; Brambilla, S.; Piemonti, L.; Cobianchi, L.; Peloso, A.; Citro, A.; Oldani, G.; Brambilla, S.; et al. Bioengineering the Pancreas: Cell-on-Scaffold Technology. Scaffolds Tissue Eng. Mater. Technol. Clin. Appl. 2017, 293. [Google Scholar] [CrossRef]
- Salg, G.A.; Giese, N.A.; Schenk, M.; Hüttner, F.J.; Felix, K.; Probst, P.; Diener, M.K.; Hackert, T.; Kenngott, H.G. The emerging field of pancreatic tissue engineering: A systematic review and evidence map of scaffold materials and scaffolding techniques for insulin-secreting cells. J. Tissue Eng. 2019, 10, 2041731419884708. [Google Scholar] [CrossRef] [PubMed]
- Paolillo, M.; Schinelli, S. Extracellular Matrix Alterations in Metastatic Processes. Int. J. Mol. Sci. 2019, 20, 4947. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wan, J.; Huang, Y.; Guo, Y.; Xu, T.; Zhu, M.; Fan, X.; Zhu, S.; Ling, C.; Li, X.; et al. 3D Culture of MIN-6 Cells on Decellularized Pancreatic Scaffold: In Vitro and In Vivo Study. BioMed Res. Int. 2015, 2015, 432645. [Google Scholar] [CrossRef]
- Youngblood, R.L.; Sampson, J.P.; Lebioda, K.R.; Shea, L.D. Microporous scaffolds support assembly and differentiation of pancreatic progenitors into β-cell clusters. Acta Biomater. 2019, 96, 111–122. [Google Scholar] [CrossRef]
- Huhtaniemi, I.T. Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Ed.; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128122006. [Google Scholar]
- Salvatori, M.; Katari, R.; Patel, T.; Peloso, A.; Mugweru, J.; Owusu, K.; Orlando, G. Extracellular Matrix Scaffold Technology for Bioartificial Pancreas Engineering: State of the Art and Future Challenges. J. Diabetes Sci. Technol. 2014, 8, 159. [Google Scholar] [CrossRef]
- Kumar, N.; Joisher, H.; Ganguly, A. Polymeric Scaffolds for Pancreatic Tissue Engineering: A Review. Rev. Diabet. Stud. 2018, 14, 334–353. [Google Scholar] [CrossRef]
- de Jongh, D.; Thom, R.L.; Cronin, A.J.; Bunnik, E.M.; Massey, E.K. Clinical Translation of Bio-Artificial Pancreas Therapies: Ethical, Legal and Psychosocial Interdisciplinary Considerations and Key Recommendations. Transpl. Int. 2023, 36, 11705. [Google Scholar] [CrossRef]
- Raoufinia, R.; Rahimi, H.R.; Saburi, E.; Moghbeli, M. Advances and challenges of the cell-based therapies among diabetic patients. J. Transl. Med. 2024, 22, 435. [Google Scholar] [CrossRef]
- Calafiore, R.; Basta, G. Artificial pancreas to treat type 1 diabetes mellitus. Methods Mol. Med. 2007, 140, 197–236. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, G.C.A.; Teramura, Y.; Iwata, H. Cryopreserved agarose-encapsulated islets as bioartificial pancreas: A feasibility study. Transplantation 2009, 87, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Wu, C.C.; Sumi, S.; Tseng, C.L.; Wu, Y.H.S.; Kuo, T.F.; Lin, F.H. Calcium phosphate cement chamber as an immunoisolative device for bioartificial pancreas: In vitro and preliminary in vivo study. Pancreas 2010, 39, 444–451. [Google Scholar] [CrossRef]
- Ludwig, B.; Rotem, A.; Schmid, J.; Weir, G.C.; Colton, C.K.; Brendel, M.D.; Neufeld, T.; Block, N.L.; Yavriyants, K.; Steffen, A.; et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc. Natl. Acad. Sci. USA 2012, 109, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Sanyal, A.; Mallick, A.; Mallick, A. Recent advances in the development of bioartificial pancreas using 3D bioprinting for the treatment of type 1 diabetes: A review. Open Explor. 2023, 4, 886–922. [Google Scholar] [CrossRef]
- Li, H.; Shang, Y.; Feng, Q.; Liu, Y.; Chen, J.; Dong, H. A novel bioartificial pancreas fabricated via islets microencapsulation in anti-adhesive core-shell microgels and macroencapsulation in a hydrogel scaffold prevascularized in vivo. Bioact. Mater. 2023, 27, 362–376. [Google Scholar] [CrossRef]

| Method | Advantages | Disadvantages |
|---|---|---|
| Tissue engineering through decellularization | Preserves the native structure of the pancreas; retains vascular networks, facilitating regeneration | Challenges in recellularizing the scaffold; potential residual immune response |
| Optimization of culture conditions for cell functionality | Greater control over culture conditions; can enhance cell longevity and functionality | Does not fully replicate the natural tissue environment; difficulty in maintaining optimal conditions over time |
| Study Author and Year | Method | Main Results |
|---|---|---|
| Goh et al. (2013) [57] | Perfusion method to decellularize the entire pancreas, followed by recellularization with β-cells | Demonstrated insulin expression after recellularization, supporting potential for diabetes treatment |
| Theocharis et al. (2016) [20] | Detailed the structure of pancreatic ECM and its role in cell function | Highlighted ECM’s importance in cell survival, growth, and communication through adhesion receptors |
| Chaimov et al. (2017) [14] | Encapsulation of insulin-producing cells in porcine-derived ECM | Created a 3D fibrous niche that supports cell viability and differentiation for insulin delivery |
| Elebring et al. (2017) [101] | Cold perfusion of porcine pancreas, followed by human fetal pancreatic cell adherence | Achieved expression of endocrine (C-peptide, PDX1) and exocrine markers (glucagon, alpha amylase) |
| Wan et al. (2017) [10] | Use of induced pluripotent stem cells in a placental scaffold | Accelerated insulin expression compared to traditional plate cultures |
| Sackett et al. (2018) [61] | Production of hydrogel from decellularized human pancreas for cell culture | Developed a hydrogel for transplantation and cell culture, addressing vascular integration challenges |
| Xu et al. (2018) [13] | Incorporation of heparin in the decellularization process for reendothelialization | Enhanced scaffold stability and circulation of secreted factors, aiding in vascular regeneration |
| Technique | Advantages | Disadvantages |
|---|---|---|
| Decellularized ECM for 3D printing | Allows customization of tissue architecture; greater control over cell and ECM deposition, adapting the shape of the tissue | Creating a microenvironment as complex as that of natural tissue remains a challenge; it may require large quantities of decellularized ECM to create effective scaffolds |
| Decellularized tissue followed by direct recellularization | Maintains the native three-dimensional structure of the organ; provides greater support for cell adhesion due to the preservation of the ECM and key proteins such as collagen and elastin | It can be difficult to adequately recellularize the entire tissue; the process may be more time-consuming and requires advanced techniques for uniformity control |
| Study Author and Year | Method | Main Results |
|---|---|---|
| Agudelo et al., 2009 [124] | Microencapsulation of islets in agarose hydrogel | The data indicated that cryopreserved Mic-islets transplanted as a bioartificial pancreas in diabetic mice restored normoglycemia and successfully controlled blood glucose levels for extended periods. |
| Yang et al., 2010 [125] | Mouse insulinoma cells encapsulating in agarose gel were enclosed in a calcium phosphate cement chamber to create a bioartificial pancreas (BAP) | The case report revealed that the BAPs implanted in the bone marrow cavity of a spontaneous diabetic feline were effective. The implanted BAPs provided therapeutic benefits despite sustained hyperglycemia. |
| Ludwig et al., 2012 [126] | Encapsulation of islet using a bioartificial microchamber | The work showed a minimally invasive implantable chamber normalized blood glucose in streptozotocin-induced diabetic rodents for up to 3 months. As a result of hypervascularization of the tissue surrounding the device, no relevant delay in insulin response to glucose changes has been observed. |
| Peloso et al., 2016 [31] | Decellularization with triton-based solution | The human pancreatic acellular extracellular matrix (hpaECM) scaffolds maintained the molecular and spatial template of the intact pancreas, were cytocompatible, and were able to modulate the immune response. |
| Salvatori et al., 2014 [119] | Decellularization using ionic and nonionic detergents, enzymatic nucleases and antimicrobials | The study outlines emergent technologies in regenerative medicine that may overcome the limitations of conventional diabetes therapies. Among them, novel decellularization protocols have allowed researchers to discover the advantages afforded by the native pancreatic extracellular matrix, proven to be an optimal platform for recellularization and whole-organ pancreas bioengineering to resolve the dire shortage of transplantable organs. |
| Ghosh et al., 2023 [127] | Three-dimensional (3D) bioprinting by extrusion | The review highlighted advances in islet encapsulation and 3D bioprinting for bioartificial pancreas development. Extrusion-based bioprinting (EBB) is noted for its versatility and ability to print high cell densities and various cell types. Coaxial extrusion bioprinting is particularly suited for pancreatic islet bioprinting, addressing immunoisolation and hypoxia through vascular network formation. |
| Li et al., 2023 [128] | Encapsulation of pancreatic islet in core–shell microgels and a pre-vascularized scaffold | As a result of the synergistic effect between anti-adhesive core–shell microgels and prevascularized hydrogel scaffold, the bioartificial pancreas can reverse the blood glucose levels of diabetic mice from hyperglycemia to normoglycemia for at least 90 days. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos da Silva, T.; Silva-Júnior, L.N.d.; Horvath-Pereira, B.d.O.; Valbão, M.C.M.; Garcia, M.H.H.; Lopes, J.B.; Reis, C.H.B.; Barreto, R.d.S.N.; Buchaim, D.V.; Buchaim, R.L.; et al. The Role of the Pancreatic Extracellular Matrix as a Tissue Engineering Support for the Bioartificial Pancreas. Biomimetics 2024, 9, 598. https://doi.org/10.3390/biomimetics9100598
Santos da Silva T, Silva-Júnior LNd, Horvath-Pereira BdO, Valbão MCM, Garcia MHH, Lopes JB, Reis CHB, Barreto RdSN, Buchaim DV, Buchaim RL, et al. The Role of the Pancreatic Extracellular Matrix as a Tissue Engineering Support for the Bioartificial Pancreas. Biomimetics. 2024; 9(10):598. https://doi.org/10.3390/biomimetics9100598
Chicago/Turabian StyleSantos da Silva, Thamires, Leandro Norberto da Silva-Júnior, Bianca de Oliveira Horvath-Pereira, Maria Carolina Miglino Valbão, Matheus Henrique Herminio Garcia, Juliana Barbosa Lopes, Carlos Henrique Bertoni Reis, Rodrigo da Silva Nunes Barreto, Daniela Vieira Buchaim, Rogerio Leone Buchaim, and et al. 2024. "The Role of the Pancreatic Extracellular Matrix as a Tissue Engineering Support for the Bioartificial Pancreas" Biomimetics 9, no. 10: 598. https://doi.org/10.3390/biomimetics9100598








